Abstract
Aims and Objectives:
Estimation of changes in C-reactive protein (CRP) level and pregnancy outcome after nonsurgical supportive periodontal therapy in pregnant women affected with Periodontitis.
Materials and Methods:
A total of 100 pregnant females with periodontitis were assigned to treatment and control groups. All the details about previous and current pregnancies were obtained. Full-mouth periodontal examination was done at baseline, which included oral hygiene index simplified plaque index, gingival index, and clinical attachment loss. CRP level was also measured from collected blood sample initially at baseline and later after the delivery in both the group. Subjects in the treatment group received nonsurgical periodontal treatment during the second trimester of gestational period, and those in the control group did not receive any periodontal therapy during this period. Periodontal therapy included mechanical plaque control instructions and scaling and root planning. Outcome measures assessed were changes in CRP levels, gestational age, and birth weight of the infants. When delivery occurred at <37 weeks of gestation, it was considered as preterm birth (PTB), and low birth weight (LBW) was recorded when the infant weighed <2500 g.
Results:
In the treatment group, 32% of PTB and 68% of Normal term birth (NTB) delivery whereas in the control group 72% PTB and 28% of NTB were recorded. Infants measured with LBW were 36% in the treatment group and 52% in the control group. Mean birth weight was 2644.44 ± 450.53 g in the treatment group and 2447.82 ± 368.02 g in the control group (P < 0.05). Mean gestational age in the treatment group was 35.57 ± 2.40 weeks and 34.17 ± 2.92 weeks in the control group (P < 0.05). The treatment group showed statistically significant reduction in mean values of CRP level after delivery in comparison to baseline values (P < 0.05), whereas control group showed no significant reduction in values (P > 0.05).
Conclusion:
Nonsurgical supportive periodontal therapy may lower the risk of preterm delivery in females affected with periodontitis by reducing CRP level.
Keywords: Low birth weight, periodontitis, preterm birth
Introduction
Periodontal disease (PD) is chronic infection induced by multiple bacteria and aggravated by host inflammatory response leading to a loss of periodontal connective attachment tissue and tooth support.[1] The concept of PD influencing systemic health has been discussed over several decades. Miller[2] published his “focal infection theory” in 1981 suggesting that “mainly microorganisms or their waste products enter other parts of the body from the mouth.” Several studies[3,4] and systematic review[5] have suggested that chronic periodontal infection serves as a pool for micro-organism, endotoxins, and inflammatory mediators such as interleukin-1β (IL-1β), IL-6, prostaglandin E2 (PGE2), and tumor necrosis factor-alpha (TNF-α), C-reactive protein (CRP), etc., providing the hematogenic translocation of these products to the feto-placental unit resulting into adverse pregnancy outcomes. CRPs are acute phase reactant produced by liver in response to diverse inflammatory response. CRP level is the markers for systemic inflammation and is also associated with chronic bacterial infection like periodontitis.[6] Evidence also shows that treating periodontal diseases seems to reduce serum CRP levels. Raised CRP level has also been associated with adverse pregnancy outcome such as preterm birth (PTB),[7] low birth weight (LBW), preeclampsia, intrauterine growth restriction.[8] Controlling the PD could prevent this hematogenous spread of bacteria, inflammatory cytokines and mediators to feto-placental unit, thus minimizing the chances of adverse pregnancy outcomes.[9,10,11]
Considering, periodontal infection as one of the risk factor in preterm LBW (PTLBW), this study was performed to determine the effect of periodontal treatment on reduction of the incidence of PTB and LBW infants and associated raised serum CRP level in pregnant females affected with periodontitis.
Aims and Objectives
To evaluate the association between PD and CRP levels in pregnant women
To assess the effect of nonsurgical supportive periodontal therapy on pregnancy outcome like PTB and PLBW
To assess the effect of nonsurgical supportive periodontal therapy on serum CRP level in pregnant women.
Materials and Methods
Study design
The study population consisted of 100 pregnant women affected with periodontitis visiting the Department of Gynecology and Obstetrics, Rural Medical College and Hospital, Pravara Institute of Medical Sciences, Loni. After receiving ethical clearance, institutional approval and consent of the subjects, a single blinded, single centered, and randomized controlled trial was performed on 100 pregnant female subjects. Every participant was assigned either to treatment or control group randomly. The detail of subjects assigned to a particular group was concealed to examiner and operator.
Inclusion criteria
Healthy pregnant women within age range of 17-35 years with single gestation between 11 and 20 weeks; subject with at least >20 erupted teeth, excluding third molars with >2 mm of clinical attachment loss (CAL) at >50% examine sites.
Exclusion criteria
H/o congenital heart disease, diabetes mellitus, corticosteroid therapy, asthmatics, kidney disorder, hyperthyroidism, multiple gestation, +Rh factor, systemic infection, alcoholic, tobacco chewers, smokers, and one with H/o periodontal therapy in last 6 months and on antibiotic or antifungal medication therapy.
Prenatal care
The women in the present study had easy access to prenatal care program to minimize known risk factors for PTB and to provide healthcare to a pregnant female and growing fetus. Women were cared for immunization against tetanus, anemia prophylaxis, and antenatal checkup at least 3 times during pregnancy, birth timing and spacing recording, and care at birth - promotion of clean delivery.
Data collection
Obstetric and maternal data
Demographic factors such as age, race, educational level, and detailed history about previous pregnancies were obtained from the gynecology and obstetrics case records and personal interview.
Following information were recorded for subjects: Maternal age at the time of entry into the study, nutritional status, onset of prenatal care, tobacco use, details of previous pregnancy history, consumption of alcohol, sexually transmitted diseases, urinary tract infections or any other maternal infectious disease, number of prenatal visits, intrauterine growth restriction, gestational age, birth weight, and fetal death. Thus, patient not matching selection criteria were excluded from the study.
Prenatal care included urine tests, blood tests, recording of blood pressure, maternal weight gain, physical and pelvic examination. All women had undergone ultrasonographic examination between 9 and 32 weeks for gestational age dating and to rule out congenital anomalies. Subjects with inadequate prenatal care were excluded from the study. Socioeconomic status was classified according to the occupation as follows: Class I (professionals), class II (intermediate), class III (skilled worker), class IV (partly skilled worker), and class V (unskilled worker).
Clinical examination
Plaque index (PI),[12] gingival index (GI),[13] and oral hygiene index-simplified (OHIS)[14] were recorded by examiner for each subject at baseline. Periodontal status was measured as CAL at four different site of each tooth.
Periodontal therapy
Totally 50 patients were randomly assigned to the treatment group and received periodontal therapy during the second trimester of gestational period and remaining 50 in the control group did not receive periodontal treatment after during the gestational period. Periodontal therapy included scaling and root planning, which was performed under local anesthesia. At the beginning of treatment, each subject was instructed to rinse once a day with 0.2% chlorhexidine.
Serum C-reactive protein levels measurement
A volume of 10 ml of the blood sample was collected from each of the subjects from the antecubital vein and transferred to an appropriately labeled test tube. Samples were sent immediately for biochemical analysis. Immunoturbidimetry method was used to detect CRP level in the blood sample (Aggagape Diagnostic Ltd., Kerala) using ERBA Chem 7 semiautoanalyzer (ERBA, Germany). It had a detection limit of 0.015 mg/dL. CRP levels were measured initially at baseline and after the delivery in both the groups. In the treatment group, periodontal treatment was carried only after collecting blood sample for CRP measurement.
Assessment of pregnancy outcomes
Outcomes measured were PTB delivery, LBW infant. PTB was defined as spontaneous delivery at <37 completed weeks of gestation that followed spontaneous labor or spontaneous rupture of membranes. Infant <2500 g were considered as LBW infant. The birth outcome which occurred ≥37 weeks of gestation or with a weight of infant ≥2500 g was defined normal. Estimation of gestational age was based on the last date of menstrual period, along with ultrasound, physical and postnatal examination.
Data analysis
Chi-square (χ2) test was used to find differences in the distribution of age, socioeconomic status, and the number of previous pregnancies among subjects between treatment and control groups. The Z-test of significance was used to find a significant difference between the prevalence of PTB and LBW infants in treatment and control group. The Student's t-test was used find a significant difference between mean values of periodontal characteristic, gestational age, birth weight in treatment and control group. Two-tailed t-test was used to find significant changes in the level of CRP at baseline and postpregnancy in both groups.
Results
Using χ2 test, it was found that there was no significant difference in the distribution of age, socioeconomic status, and number of previous pregnancies of subjects between treatment and control groups (P > 0.05). Thus, both the groups were matched for demographic factors [Table 1; Figures 1–3].
Table 1.
General characteristic in treatment and control group (n=100)
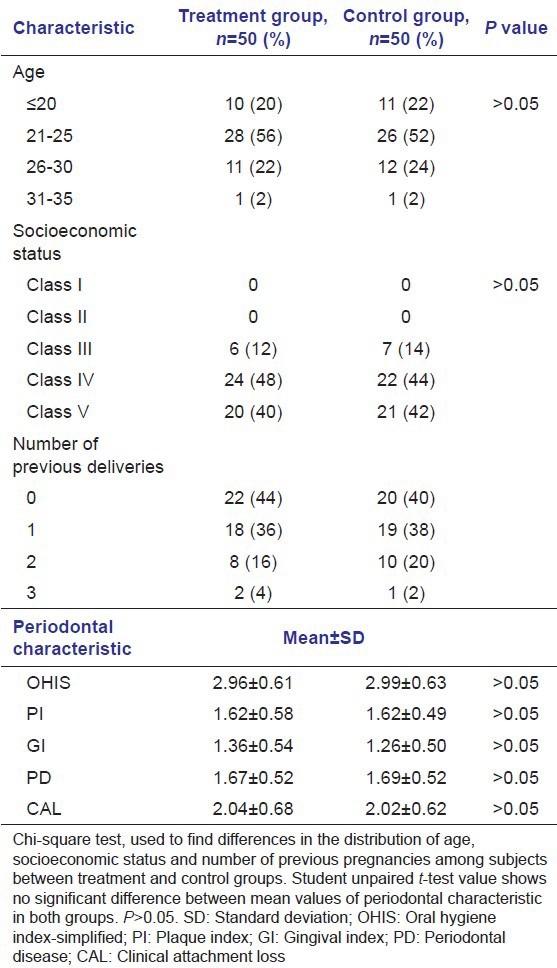
Figure 1.
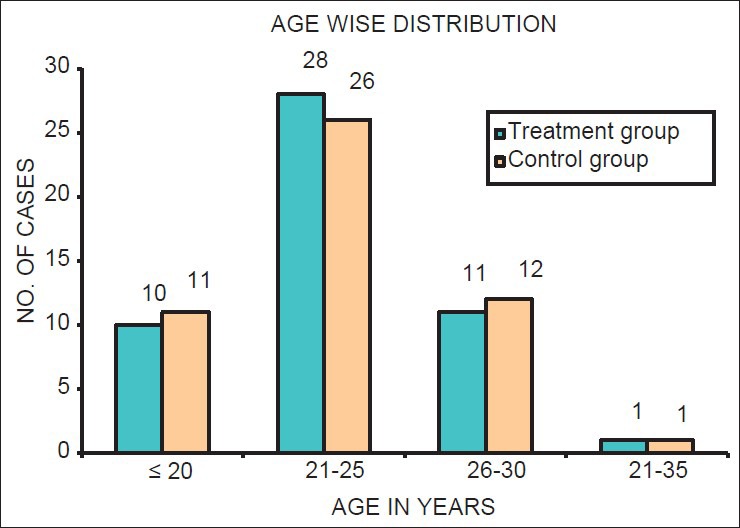
Age-wise distribution of sample in both treatment and control group
Figure 3.
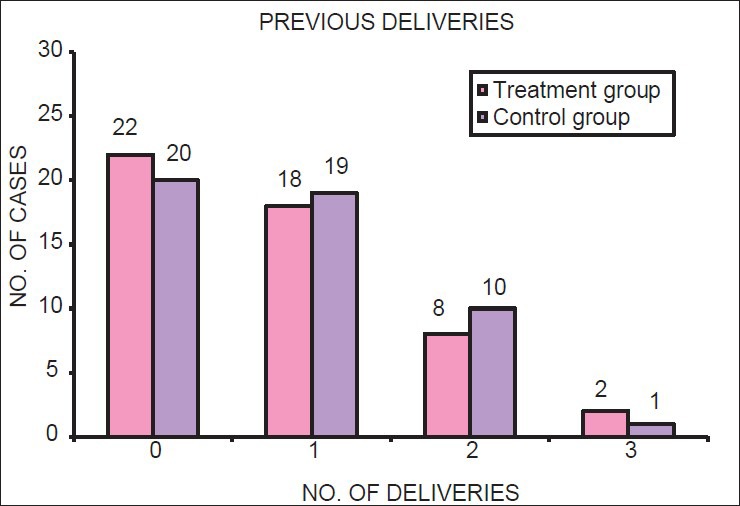
Parity of mothers in treatment and control group
Figure 2.
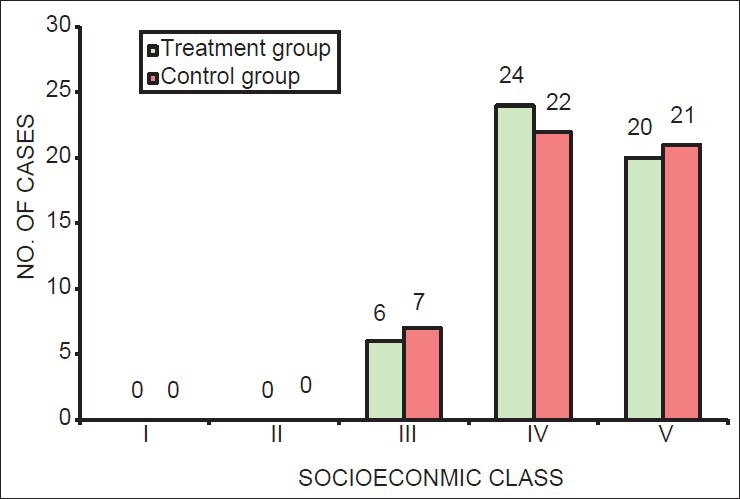
Distribution of socioeconomic status of the mother in treatment and control group
Student's t-test showed there were no significant differences in mean values of periodontal characteristic in the treatment and control group (P > 0.05). Women in both groups had mild to moderate periodontitis, mean values of CAL, PD, OHIS, PI, and GI did not differ significantly between the two groups. This show subjects in both groups has similar severity of PD [Table 1; Figure 4].
Figure 4.
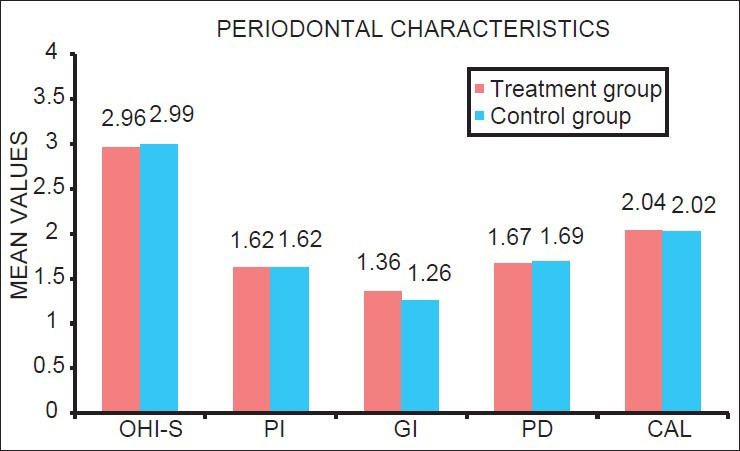
Mean values of each periodontal characteristic among the treatment and control group
The prevalence of PTB and LBW was lower in the treatment group than the control group. There were 16 PTBs (32%) in treatment group and 36 PTBs (72%) in the control group (P < 0.05). There were 18 (36%) cases of LBW in treatment group and 26 (52%) in control group (P < 0.05) [Tables 2a and b; Figures 5 and 6].
Table 2a.
Pregnancy outcomes: Distribution of gestational period among treatment and control group
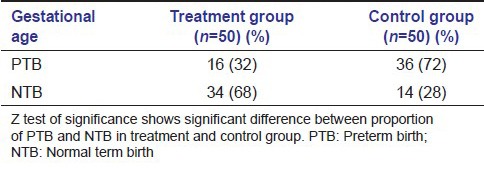
Table 2b.
Pregnancy outcomes: Distribution of weight of the neonates among treatment and control group
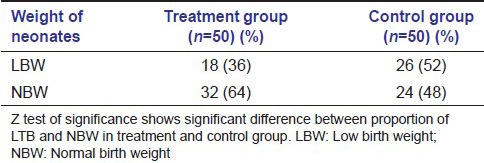
Figure 5.
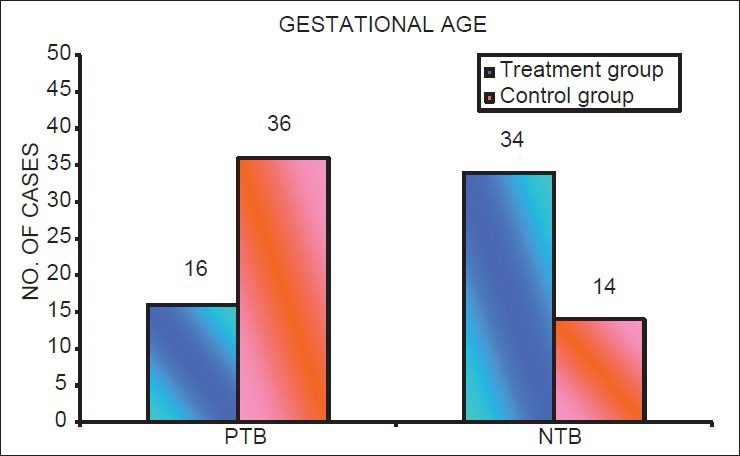
Distribution of number of cases with preterm birth and normal term birth in mothers in treatment group and control group
Figure 6.
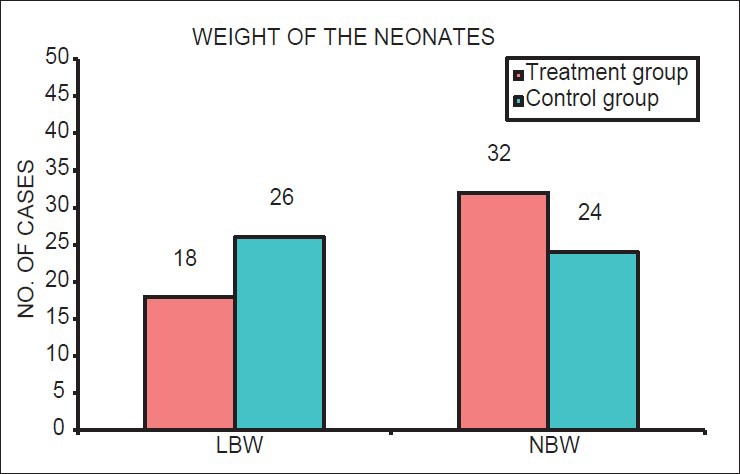
Distribution of number of cases with low birth weight and normal birth weight neonates in mothers in treatment group and control group
Mean gestational age in the treatment group was 36.57 weeks (SD ± 2.40) and 34.17 weeks (SD ± 2.92) in the control group (P < 0.05) [Table 3; Figure 7]. Mean birth weight was 2644.44 g (SD ± 450.53) in the treatment group and 2447.82 g (SD ± 368.02) in the control group with the difference being statistically significant at P < 0.05 [Table 3; Figure 8].
Table 3.
Outcome measures
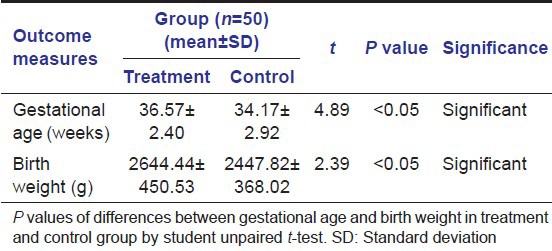
Figure 7.
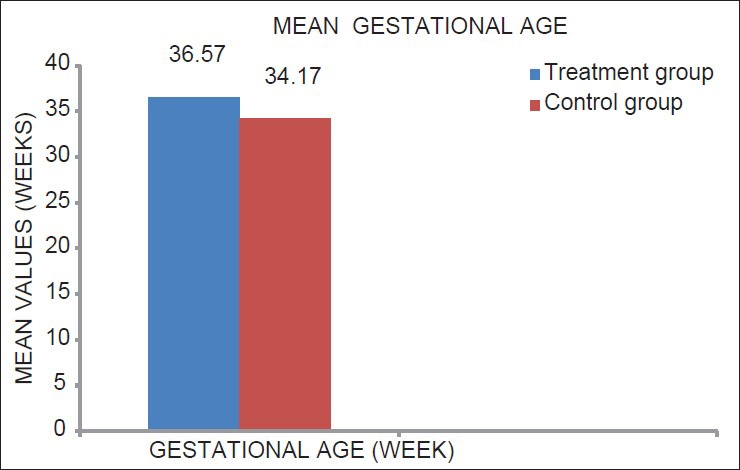
Mean value of gestational age of mothers in treatment and control group
Figure 8.
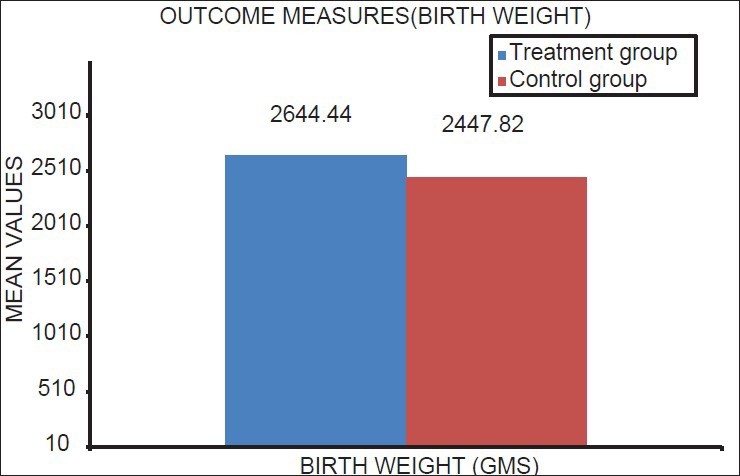
Mean value of birth weight of neonates of mothers in treatment and control group
Serum CRP level measurement was done after delivery showed significant reduction in the level of CRP compared to the baseline value in the treatment group (P < 0.05). Whereas in the control group, no significant reduction in CRP levels were found in comparison to baseline CRP levels (P > 0.05) [Table 4; Figure 9].
Table 4.
Mean values of serum CRP level measured at baseline and postdelivery in treatment group and control group
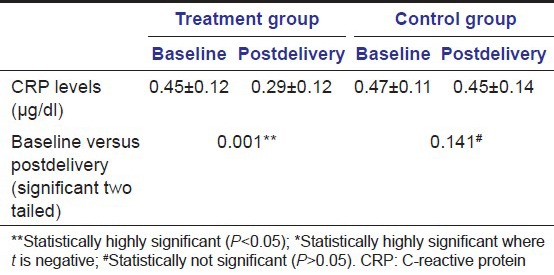
Figure 9.
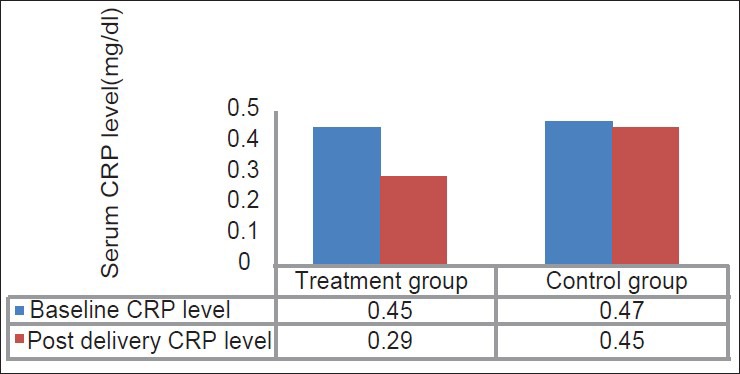
Mean values of serum C-reactive protein level measured at baseline and postdelivery in treatment group and control group
Discussion
To establish a relationship between two conditions, different levels of evidence have been examined. The strongest form of evidence is randomized controlled clinical trial. A randomized, controlled clinical trial is the best method to establish a cause-and-effect relationship between exposure and disease. The present study was designed to determine whether nonsurgical periodontal therapy can reduce raised serum CRP level and decrease the risk for PTB and LBW infants in women affected by periodontitis.
Subjects in this study were relatively similar based on the social and demographic factors. The distributions of other known risk factors for PLBW were similar in the treatment and control groups. Women aged age <18 years or >35 years is a risk factor for PLBW. Multiple gestations are also closely associated with preterm labor.[15] Hence, subjects with only singleton gestation within age range of 18–35 years were included in the study.
The study population was drawn from a rural set up based community belonging to the similar socioeconomic status mostly classes III, IV and V; most of the subjects had a similar education level and similar oral hygiene practices and awareness. Among rural Indian women, prevalence of smoking is low. However, the use of tobacco as pan chewing or for tooth cleansing (Mishri) is very common. The use of alcohol is not rare among groups with a low socioeconomic status.[16] Thus, subjects with a history of or current use of tobacco chewing and/or smoking and alcohol were excluded from the study.
Any infection, especially genitourinary tract infections is considered as an important risk factor in the premature rupture of membranes.[17] Thus, subjects who developed symptoms of any genital or systemic infection or who required any antibiotic therapy during pregnancy were also excluded from the study.
An increase in number of previous delivery is associated with greater risk for PTB.[18] In our study, there was no significant difference in the distribution of a number of previous delivery between treatment and control groups. Inadequate prenatal care and low maternal weight gain are also reported to be associated with PTB.
In women with a low socioeconomic status, inadequate prenatal care is one of major a risk factor for adverse pregnancy outcomes.[19] Studies have shown that adequate prenatal care lower risk for PTB and are associated improved birth weights infant delivery.[19] All women in the study received adequate prenatal care and gain optimum weight throughout their pregnancy.
Anemia is one of the risk factors for preterm delivery.[20] Every subject was evaluated periodically for hemoglobin levels and was supplied with iron supplements if found to be deficient.
There was no significant difference in the mean values of periodontal characteristics OHIS, PI, GI, PD, and CAL between the treatment and control groups at baseline. Thus, the distribution of severity of periodontitis was similar in treatment and control group. Thus, study had characteristically matched treatment and controls.
Pitiphat et al. in prospective cohort study showed periodontitis increases CRP levels in pregnancy. CRP could mediate association of the periodontitis with adverse pregnancy outcomes.[21] Studies have shown that nonsurgical periodontal therapy decreases the incidence of PTB and LBW delivery,[22,23] and also levels of inflammatory mediators such as CRP[22] and other pro-inflammatory cytokine IL-1β,[23] IL-6.[23] Hence, nonsurgical mechanical debridement was provided to the subjects in the treatment group which included plaque control instructions, scaling and root planning.
Outcome measures considered in the study were gestational age and weight of an infant at delivery. The birth outcome which occurred after 37 completed weeks of gestation or, birth of an infant with a weight ≥ 2500 g was defined as normal. Estimation of gestational age was based on the last menstrual period, ultrasound examination, sequential physical examinations, and postnatal examination. All examinations were done under the supervision of a gynecologist.
Considering, exposure of major risk factor were similar in both the group, decrease in the incidence of preterm and LBW infants in the treatment group could be attributed to periodontal intervention. The results are in agreement with findings of Tarannum and Faizuddin[24] that states significant reduction in the incidence of PTB and LBW in 67.03% and 35.16% in women who received periodontal therapy respectively in contrast to incidence of 83.15% and 58.43% in the control group, respectively.
C-reactive protein is an acute phase reactant produced in response to inflammatory stimuli, trauma, heat, infection, and hypoxia. CRP has been associated with adverse pregnancy outcomes. Furthermore, PD has also been associated with increased risk of preterm delivery and LBW infants.[25] Therefore, CRP might be one of the plausible mediators of the association between periodontitis and adverse pregnancy outcomes. Raised CRP levels could multiply inflammatory response through activation of complement system, release of inflammatory cytokines and resulting into premature rupture feto-placental membrane. Therefore, may mediate the relationship between periodontitis and adverse pregnancy outcomes.[22,26]
Periodontal therapy at least in the form of mechanical debridement can be helpful to reduce bacterial load and associated bacteremia. Thus, reducing the systemic effect of raised inflammatory mediators. Very few studies have shown the effect of periodontal therapy on CRP level and adverse pregnancy outcome in women suffering from periodontitis. Sharma et al.[22] showed statistically significant reduction in CRP level and PTB after periodontal therapy. However, experimental and control group were not matched for other exposure or risk factor.
In the present study, all attempts are made to match treatment and control group for other risk factors such as age, socioeconomic status, parity, single gestation, prenatal care, adequate hemoglobin, evidence of other infection and severity of periodontal disease. Nonsurgical supportive periodontal therapy by mean of thorough mechanical debridement and root planning carried out in the second trimester of pregnancy showed a significant reduction in serum CRP level compared with control the group. Reduction of CRP and other inflammatory mediator may be a possible reason for reducing incidence of PTB and LBW infants in the treatment group.
The potential mechanisms that are put forward to explain the relationship between PD and PTB and LBW infant delivery is that a periodontal infection serves as a chronic reservoir of lipopolysaccharide, which are responsible for the production of IL-1β, PGE2 and TNF-α and CRP that are in turn associated with PTB and fetotoxicity.
Limitation of study
Since study was carried in rural areas of India, higher socioeconomic status women were not enrolled in the study. Only low socioeconomic status women participated study
The exclusion of subjects with systemic infections was based on clinical presentation; hence, subjects with subclinical infection must have been included
Subjects with a history of alcohol/tobacco use were excluded from the study. Hence, the results of the study do not apply to the entire rural population of India
Larger, Multi-centered trial with more sample size and evaluation of the effect of periodontal therapy on different inflammatory mediator on pregnancy outcome is required.
Conclusion
This randomized controlled clinical trial provides evidence that:
Nonsurgical supportive periodontal therapy can significantly reduce the risk of PTB and LBW deliveries
Nonsurgical supportive periodontal therapy can reduce raised serum CRP levels in pregnant females affected with periodontitis.
From the results of this study, it seems likely that PD can influence the adverse pregnancy outcome by modulating levels of inflammatory mediators. Nonsurgical supportive periodontal therapy reduces the microbial load and inflammatory response, and thereby reduces the risk of adverse pregnancy outcomes. The potential relationship between PD and adverse birth outcomes, if proven to be causative, could be significant for increasing oral health awareness among pregnant women in the community.
Acknowledgments
The authors would like to thank Dr. Prashant Kharde, Department of Gynecology for active support and express gratitude to Dr. Ishwardas, Dr. H. Sheetal, Dr. Larissa, and Dr. Sagar for their assistance. Great appreciation to Mr. Hemant Pawar for his support in statistical analysis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Page RC, Kornman KS. The pathogenesis of human periodontitis: An introduction. Periodontol 2000. 1997;14:9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 2.Miller WD. The human mouth as a focus of infection. Dent Cosm. 1891;33:687–713. [Google Scholar]
- 3.Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 1996;67:1103–13. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- 4.López NJ, Smith PC, Gutierrez J. Higher risk of preterm birth and low birth weight in women with periodontal disease. J Dent Res. 2002;81:58–63. doi: 10.1177/002203450208100113. [DOI] [PubMed] [Google Scholar]
- 5.Scannapieco FA, Bush RB, Paju S. Periodontal disease as a risk factor for adverse pregnancy outcomes. A systematic review. Ann Periodontol. 2003;8:70–8. doi: 10.1902/annals.2003.8.1.70. [DOI] [PubMed] [Google Scholar]
- 6.Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35:277–90. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 7.Moghaddam Banaem L, Mohamadi B, Asghari Jaafarabadi M, Aliyan Moghadam N. Maternal serum C-reactive protein in early pregnancy and occurrence of preterm premature rupture of membranes and preterm birth. J Obstet Gynaecol Res. 2012;38:780–6. doi: 10.1111/j.1447-0756.2011.01804.x. [DOI] [PubMed] [Google Scholar]
- 8.Tjoa ML, van Vugt JM, Go AT, Blankenstein MA, Oudejans CB, van Wijk IJ. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol. 2003;59:29–37. doi: 10.1016/s0165-0378(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 9.Siqueira FM, Cota LO, Costa JE, Haddad JP, Lana AM, Costa FO. Intrauterine growth restriction, low birth weight, and preterm birth: Adverse pregnancy outcomes and their association with maternal periodontitis. J Periodontol. 2007;78:2266–76. doi: 10.1902/jop.2007.070196. [DOI] [PubMed] [Google Scholar]
- 10.Newnham JP, Newnham IA, Ball CM, Wright M, Pennell CE, Swain J, et al. Treatment of periodontal disease during pregnancy: A randomized controlled trial. Obstet Gynecol. 2009;114:1239–48. doi: 10.1097/AOG.0b013e3181c15b40. [DOI] [PubMed] [Google Scholar]
- 11.Michalowicz BS, Hodges JS, DiAngelis AJ, Lupo VR, Novak MJ, Ferguson JE, et al. Treatment of periodontal disease and the risk of preterm birth. N Engl J Med. 2006;355:1885–94. doi: 10.1056/NEJMoa062249. [DOI] [PubMed] [Google Scholar]
- 12.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 13.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 14.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 15.Berkowitz GS, Papiernik E. Epidemiology of preterm birth. Epidemiol Rev. 1993;15:414–43. doi: 10.1093/oxfordjournals.epirev.a036128. [DOI] [PubMed] [Google Scholar]
- 16.Shiono PH, Klebanoff MA, Rhoads GG. Smoking and drinking during pregnancy. Their effects on preterm birth. JAMA. 1986;255:82–4. [PubMed] [Google Scholar]
- 17.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333:1737–42. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 18.Meis PJ, Goldenberg RL, Mercer BM, Iams JD, Moawad AH, Miodovnik M, et al. The preterm prediction study: Risk factors for indicated preterm births. Maternal-Fetal Medicine Units Network of the National Institute of Child Health and Human Development. Am J Obstet Gynecol. 1998;178:562–7. doi: 10.1016/s0002-9378(98)70439-9. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg RS. The impact of prenatal care in different social groups. Am J Obstet Gynecol. 1983;145:797–801. doi: 10.1016/0002-9378(83)90681-6. [DOI] [PubMed] [Google Scholar]
- 20.Klebanoff MA, Shiono PH, Selby JV, Trachtenberg AI, Graubard BI. Anemia and spontaneous preterm birth. Am J Obstet Gynecol. 1991;164:59–63. doi: 10.1016/0002-9378(91)90626-3. [DOI] [PubMed] [Google Scholar]
- 21.Pitiphat W, Joshipura KJ, Rich-Edwards JW, Williams PL, Douglass CW, Gillman MW. Periodontitis and plasma C-reactive protein during pregnancy. J Periodontol. 2006;77:821–5. doi: 10.1902/jop.2006.050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma A, Ramesh A, Thomas B. Evaluation of plasma C-reactive protein levels in pregnant women with and without periodontal disease: A comparative study. J Indian Soc Periodontol. 2009;13:145–9. doi: 10.4103/0972-124X.60227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Offenbacher S, Lin D, Strauss R, McKaig R, Irving J, Barros SP, et al. Effects of periodontal therapy during pregnancy on periodontal status, biologic parameters, and pregnancy outcomes: A pilot study. J Periodontol. 2006;77:2011–24. doi: 10.1902/jop.2006.060047. [DOI] [PubMed] [Google Scholar]
- 24.Tarannum F, Faizuddin M. Effect of periodontal therapy on pregnancy outcome in women affected by periodontitis. J Periodontol. 2007;78:2095–103. doi: 10.1902/jop.2007.060388. [DOI] [PubMed] [Google Scholar]
- 25.Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: Results of a prospective study. J Am Dent Assoc. 2001;132:875–80. doi: 10.14219/jada.archive.2001.0299. [DOI] [PubMed] [Google Scholar]
- 26.Cermak J, Key NS, Bach RR, Balla J, Jacob HS, Vercellotti GM. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82:513–20. [PubMed] [Google Scholar]


