Abstract
Context:
Tobacco abuse is a well-known risk factor for potentially malignant disorders as well as oral squamous cell carcinoma (SCC). Factors that influence tobacco-exposed individuals developing a malignancy may include a combination of total tobacco exposure and genetic susceptibility.
Aim:
This study was undertaken to determine the prevalence of the glutathione S-transferase M1 (GSTM1) null polymorphism in oral leukoplakia and oral SCC patients in South Indian population.
Settings and Design:
This case-control study was conducted in hospital setting on South Indian population.
Materials and Methods:
Totally, 280 subjects with a history of tobacco use, oral leukoplakia, oral SCC were included in this study. Three milliliter of blood was collected and transported under cold cycle and taken for evaluation of GSTM1 null polymorphism using Multiplex Polymerase Chain Reaction.
Results and Discussion:
On comparing the prevalence of GSTM1 null polymorphism among the group with subjects with habits and no oral lesions, oral leukoplakia and oral SCC, it was observed that there was a statistically significant association between GSTM1 null polymorphism and the different groups (P < 0.01).
Conclusion:
The lack of GSTM1 activity would make the oral tissues more susceptible to action of tobacco carcinogens and to the development of a high-grade level of dysplasia in oral leukoplakia and thereby increases the susceptibility of lesion to undergo malignant changes.
Keywords: Genetic polymorphism, glutathione S-transferase M1, oral cancer
Introduction
Tobacco use is a well-known risk factor for potentially malignant disorders as well as oral squamous cell carcinoma (SCC).[1,2] Despite the risk of tobacco exposure, the majority of patients who smoke or chew tobacco do not get oral cancer. Factors that influence tobacco-exposed individuals developing a malignancy may thus include a combination of total tobacco exposure and genetic susceptibility.[3] The environment–gene interaction in carcinogenesis is well reflected by Phase 1 and Phases 2 enzymes that are involved in the metabolism of carcinogens. Glutathione S-transferases (GSTs) are a group of Phase 2 enzymes that are primarily involved in detoxifying carcinogenic metabolites.[3] Numerous polymorphisms occur in the genes encoding GSTs. Among them, GSTM1, GSTT1, and GSTP1 enzymes play an important role in the detoxification of metabolites of carcinogens in tobacco smoke.[4,5,6,7] The GSTM1 gene exhibits a deletion polymorphism, which in case of homozygosity (GSTM1 null) leads to the absence of phenotypic enzyme activity. The GSTM1 (null) genotype has been found to be significantly associated with an increased risk of oral SCC.[3,8,9,10,11,12] Therefore through this study, it is intended to determine the prevalence of GSTM1 null polymorphism in oral leukoplakia and oral SCC and thereby to show that GSTM1 genotype may increase the risk of development of oral SCC in subjects with oral leukoplakia and tobacco users.
Materials and Methods
The study was conducted in the Department of Oral Medicine and Radiology and Jain Institute of Vocational and Advanced Studies, Bengaluru. A total number of 280 subjects with history of smoking, oral leukoplakia, oral SCC were included in the study and were grouped as:
Group A: 100 subjects with habit of tobacco or alcohol use without oral lesions
Group B: 100 subjects with habit of tobacco or alcohol use with oral leukoplakia
Group C: 80 subjects with habit of tobacco or alcohol use with oral SCC.
After obtaining informed consent, information regarding demograhic data, type of tobacco used, daily frequency and duration of use were recorded. Information on smoking and alcohol habits were obtained in a structured questionnaire and tabulated. After an explanation of the study design, the potential risks and benefits, an informed consent was obtained from each patient. Study was conducted in accordance with the Helsinki's declaration of 1975, as revised in 2008. The study was approved by the Ethical committee of the institution. Subjects with history of diseases that are associated with GSTM1 null polymorphism such as bladder cancer, lung cancer, cervical cancer or any hematological malignancies, history of uncontrolled diabetes and bleeding disorders, pregnant and lactating women were also excluded from the study.
Method of collection of data
Intra-oral examination was carried out under adequate illumination and aseptic condition to evaluate the presence of oral leukoplakia and oral SCC lesions.
Biopsy and histopathological examination
The incisional or excisional biopsy was carried out under utmost aseptic precautions, using autoclaved set of instruments and disposable gloves, syringe, needles and surgical blade. The biopsy site for leukoplakia/oral SCC was selected by clinical examination and intravital staining using toluidine blue solution. The biopsy sample was immediately fixed in 10% formalin and carried to Department of Oral Pathology of our institution, where the histopathological examination was carried out. The leukoplakia biopsy specimens were histopathologically divided into those, which exhibited keratosis without dysplasia, and those, which exhibited features of dysplasia. The dysplastic lesions were graded according to a degree of dysplastic features exhibited into mild, moderate and severe dysplasia. Oral SCC biopsy samples were graded histopathologically into well-differentiated, moderately-differentiated, or poorly-differentiated based on Broders classification criteria.
Collection of the blood sample
Three milliliter of blood was collected and transported under cold cycle and taken for evaluation of GSTM1 null polymorphism using Multiplex Polymerase Chain Reaction (PCR) to the Jain Institute of Vocational and Advanced Studies, Bangalore. Evaluation of GSTM 1 null polymorphism using Multiplex PCR was done based on the method described by Gronau et al.[3]
Interpreting the results
The albumin band was present in all samples since it was the internal positive control that indicates DNA was present in the sample. Homozygote (+) samples will show double band for GSTM1 of the size 273 bp fragment. The homozygote null samples will be missing the band for the gene that is deleted (one or both bands). In this PCR assay, the absence of a 273-bp fragment indicated the GSTM1 null genotype which was denoted by presence of single band for the gene GSTM1 instead of two bands for the gene GSTM1 [Figure 1].
Figure 1.
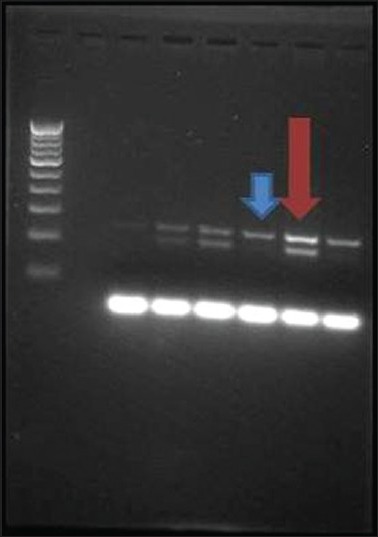
Gel electrophoresis of Multiplex Polymerase Chain Reaction amplified products showing individuals for glutathione S-transferase M1 (GSTM1) polymorphism. Blue arrowhead indicating single band suggestive of GSTM1 null polymorphism and red arrowhead indicating double band suggestive of GSTM1 polymorphism present
Method of statistical analysis
MS Excel and SPSS (SPSS Inc., Chicago, IL, USA) software packages were used for data entry and analysis was done using Z-test. The results were averaged (mean ± standard deviation) for each parameter were presented in Tables 3–5.
Table 3.
GSTM1 null polymorphism in Broder's classification of oral squamous cell carcinoma (Group C)
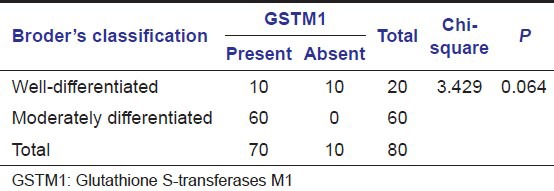
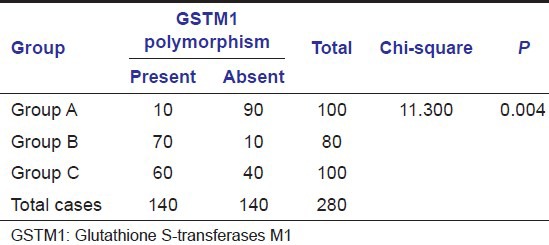
Table 5.
Comparison of prevalance of GSTM1 null polymorphism in the groups
Formula used (Z-test for proportions) in the analysis:
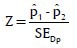
Where:
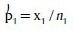 and
and 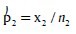
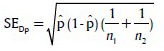 and
and 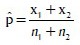
In all the above test, a P < 0.01 was accepted as indicating statistical significance.
Results
The subjects include in our study were within age group ranging between 49 and 59 years. The gender proportion was represented in the present study as in subjects with habits and no lesions (Group A), 10 subjects (10%) were females and 90 subjects (90%) were males. Among the leukoplakia subjects (Group B) 10 (10%) were females and 90 (90%) were males and among the subjects with oral SCC (Group C) 10 (25%) were females and 70 (75%) were males.
A detailed history of the tobacco or alcohol habits in our study group revealed that all the subjects included in the study had habit of using smoking form of tobacco either in the form of cigarettes, and/or bidis and some in combination with smokeless form of tobacco and alcohol.
In Group B, the duration of smoking ranged between 20 and 40 years and frequency of the smoked form of tobacco ranged between 8 and 30 cigarettes or bidis per day. Among the subjects in Group B with mixed habits, the duration of smokeless form of tobacco ranged from 20 to 40 years and frequency of the habit ranged from 12 to 25 packets per day. In Group B, the duration of alcohol use ranged between 15 and 50 years with a frequency that ranged between 300 and 1000 ml/day.
In subjects with oral SCC (Group C), the duration of smoking ranged between 25 and 50 years and frequency of the smoked form of tobacco ranged between 25 and 50 cigarettes or bidis per day. Among the subjects in Group C with mixed habits, the duration of smokeless form of tobacco ranged from 30 to 40 years and frequency of the habit ranged from 20 to 40 packets per day. In Group C subjects, the duration of alcohol use ranged between 25 and 50 years and frequency ranged between 200 and 1000 ml/day.
In our study, the histopathological examination of Group B lesions was done to evaluate the degree of dysplasia. A mild degree of dysplasia was present in 60 subjects (60%), and a moderate degree of dysplasia was present in 10 subjects (10%). Thirty subjects (30%) did not show any dysplastic features on histopathological examination. The histopathological examination of Group C subjects were done in our study and subjects were categorized using Broder's classification as well differentiated, moderately differentiated and poorly differentiated SCC. Twenty subjects (25%) had well-differentiated type of SCC, and 60 subjects (75%) had moderately differentiated type of SCC.
Glutathione S-transferase M1 null polymorphism was assessed using multiplex PCR in study as well as a control group. Subjects homozygous for the wild-type GSTM1 (+/+) were categorized as polymorphism present and those who were heterozygous (+/0) were categorized as polymorphism absent or null polymorphism. GSTM1 null genotype denotes the absence of GSTM1 genotype. In this PCR assay, the absence of a 273-bp fragment indicates the GSTM1 null genotype which is denoted by presence of single band for the gene GSTM1 instead of two bands for the gene GSTM1 [Figure 1].
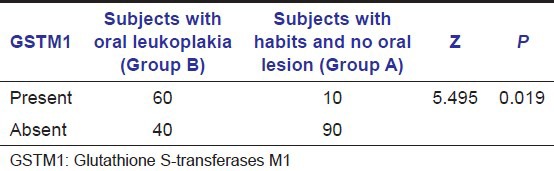
In our study, we observed that GSTM1 polymorphism was absent in 60 subjects (60%) with oral leukoplakia (Group B) [Graph 1]. Within the Group B, GSTM1 null genotype was found in 50 subjects with mild dysplastic lesions and 10 subjects with moderate dysplastic lesions [Table 1]. On comparing the proportion of subjects with GSTM1 null polymorphism, it was found to be higher in Group B when compared with the Group A [Table 2 and Graph 1]. A Statistically significant difference was also found between the proportion of leukoplakia subjects (Group B) with GSTM1 null polymorphism and Group A (P < 0.05) [Table 2].
Graph 1.
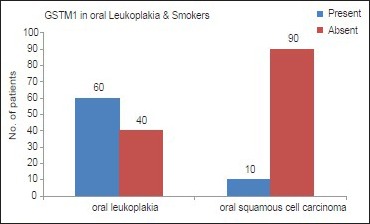
Representation of the presence of glutathione S-transferase M1 null polymorphism in leukoplakia and Subjects with habits and no oral lesions
Table 1.
GSTM1 null polymorphism in oral leukoplakia subjects (Group B)
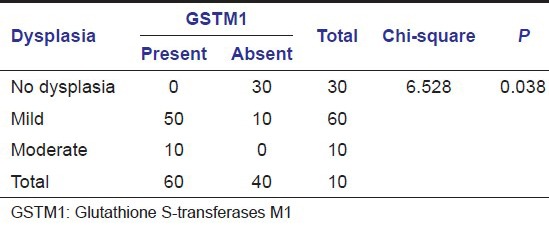
Table 2.
Analysis of the presence of GSTM1 null polymorphism in leukoplakia and subjects with habits and no oral lesions
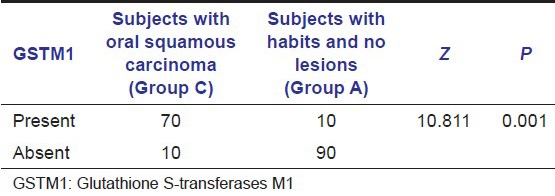
In subjects with oral SCC (Group C), GSTM1 null genotype was found in 70 subjects (87%) in our study. Within Group C, GSTM1 null genotype was found in 60 subjects (75%) with histological moderately differentiated oral SCC and 10 subjects with well-differentiated oral SCC [Table 3]. The proportion of subjects with GSTM1 null polymorphism is higher in SCC group (Group C) compared to subjects with habits and no lesions group (Group A) [Table 4 and Graph 2]. A statistically significant difference was found between subjects in Groups C and A with GSTM1 null polymorphism (P < 0.01) in our study.
Table 4.
Analysis of the presence of GSTM1 null polymorphism in squamous carcinoma patients and subjects with habits and no oral lesions
Graph 2.
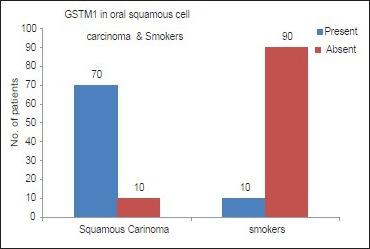
Representation of the presence of glutathione S-transferase M1 null polymorphism in oral squamous cell carcinoma and subjects with habits and no oral lesions
In our study, on comparing the prevalence of GSTM1 null polymorphism among the group with subjects with habits and no oral lesions (Group A), subjects with leukoplakia (Group B), and subjects with oral SCC (Group C), it was observed that there was a statistically significant difference between the prevalence of GSTM1 null polymorphism and the different groups (P < 0.01) [Table 5 and Graph 3].
Graph 3.
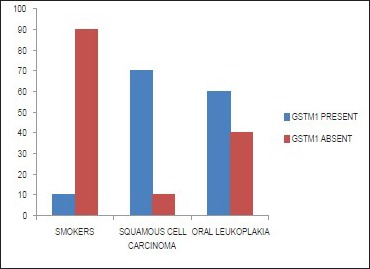
Representation of the presence of glutathione S-transferase M1 null polymorphism in leukoplakia, oral squamous cell carcinoma and Subjects with habits and no oral lesions
Discussion
Few recent studies suggested an association between the GSTM1 null genotype with oral cavity or head and neck cancer,[11,12] whereas other working groups did not show any, or a weak relationship between the null genotype and increased cancer risk.[7,9,10] The association with GSTM1 null genotype and the oral cancer risk has been assessed in various ethnic groups. Ethnic differences in prevalence of the GSTM1 null genotypes have been reported to vary between 23 and 35% in Africans, 38–67% in Caucasians and 33–63% in East Asian population.[13] In a Brazilian study, a positive association between the GSTM1 null genotype and oral leukoplakia was observed. No statistical difference was observed when the samples were stratified based on the location of the lesion.[6]
The GSTM1 null polymorphism was also demonstrated to be a risk factor for developing oral leukoplakia in betel quid/tobacco chewers in study based on ethnic Indian population.[10] In the study, it was concluded that null genotype of GSTM1 is a high penetrance genetic factor for developing oral leukoplakia that consequently modulate the risk of developing cancerous oral lesions in habituated betel quid/tobacco chewers of Indian ethnicity. In the Brazilian study, the frequency of the GSTM1 null genotype in the group with oral leukoplakia (57.7%) was statistically higher as compared to the controls (34.6%) (P < 0.05).[6]
In the present study, statistically significant difference was found between the proportion of leukoplakia subjects (Group B) with GSTM1 null polymorphism and Group A (P < 0.05) [Table 2]. The stratification of the samples according to the level of dysplasia showed increased prevalence of GSTM1 null genotype on lesions with moderate/severe histological dysplasia in subjects with leukoplakia in the study. This deletion of the GSTM1 gene may cause the loss of detoxification and may thus result in a higher oral cancer risk, especially in tobacco users.
The GSTM1 null genotypes (0/0) polymorphism has been linked to an increased susceptibility to oral SCC development. In addition, this polymorphism seems to be a risk factor for developing multiple primary neoplasms in the upper aerodigestive tract.[9,10,11,12] In a study based on Japanese population it was found that those who had null genotypes of GSTM1 had an increased oral cancer risk compared with those who had nonnull genotypes of GSTM1, particularly if associated with moderate cigarette smoking.[14] Investigation in German population[15] was conducted to determine the association of genetic polymorphisms in genes such as CYP1A1, GSTM1 and NAT2 and susceptibility to oral cavity cancer. The results showed no association between CYP1A1, GSTM1 and NAT2 genotypes and susceptibility to the oral cavity squamous cancer and suggested that it is unlikely that these polymorphisms cause a predisposition to oral cavity SCC. In a study based on the American population[16] the absence of both the GSTM1 gene and the GSTT1 gene were found to be associated with elevated odds ratios for head and neck cancer. The study concluded that genetically determined factors of carcinogen metabolism may be associated with increased risk for head and neck cancer. Another study was done in Germany to investigate concomitant polymorphisms in genes encoding for various detoxification enzymes in patients with head and neck SCC (HNSCC). The study concluded that detoxification enzymes are functionally redundant, and only the simultaneous deficiency of several detoxification enzymes increase the risk for HNSCC in alcohol- and tobacco-exposed individuals.[3] In Japanese population a case-control study was done and it was concluded patients with GSTM1 null polymorphism was present in patients with HNSCC after fewer cigarettes as compared to those with other genotypes and individual differences in polymorphism of GSTM1 gene is one of the important factors in the estimate of risk of oral SCC at low dose level of cigarette smoking.[9] In a study based on Indian population concluded that polymorphism in CYP1A1 m2 or GSTM1 null genotype in Indian subjects may confer an increased risk of cancers of the oral cavity.[10] Our results showed a statistically significant difference between subjects in Groups C and A with GSTM1 null polymorphism (P < 0.01) and this data is in agreement with above-mentioned studies.
Furthermore in our study, on comparisons of GSTM1 null polymorphism in between the three defined groups, it was observed that there was a statistically significant difference between the prevalence of GSTM1 null polymorphism and the different groups (P < 0.01) [Table 5 and Graph 3]. In the present study, it was also noted that the prevalence of GSTM1 null polymorphism was higher as the severity of the disease progressed histologically from normal to oral precancerous lesion to oral cancer.
The limitations of this study were small sample size in study and null polymorphism was studied in expression of GSTM 1 gene only while several genes perform same function which implies the need to study several genetic polymorphism to identify individual risk.
Conclusion
Within the limitation of the study, the following conclusions were drawn:
The prevalence of GSTM1 null polymorphism was maximum in subjects with oral SCC (Group C) followed by subjects with oral leukoplakia (Group B) and followed by subjects with habits and no lesions (Group A)
The prevalence of GSTM1 null polymorphism increased as the severity of the lesions progressed from precancerous lesion to the cancerous lesion
There was statistical difference in prevalence of GSTM1 null polymorphism within the groups
With the progression of severity of the levels of dysplasia in leukoplakia subjects, there was statistically significant increase in prevalence of GSTM1 null polymorphism.
The lack of GSTM1 activity would make the oral tissues more susceptible to the action of tobacco carcinogens and to the development of severe grade of dysplasia in oral leukoplakia and thereby increases the susceptibility of lesion to undergo malignant changes. To draw a substantial conclusion as to whether GSTM1 null polymorphism in individuals predisposes to oral SCC even at low doses of carcinogens awaits further longitudinal studies with larger sample sizes.
Acknowledgments
Dr. Prashanti Rao, Senior Scientist, JIVAS, Bengaluru, Karnataka, India.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Sciubba JJ. Oral cancer and its detection, history taking and the diagnostic phase of management. J Am Dent Assoc. 2001;132:152. [PubMed] [Google Scholar]
- 2.Van der Waal I, Axéll T. Oral leukoplakia: A proposal for uniform reporting. Oral Oncol. 2002;38:521–6. doi: 10.1016/s1368-8375(01)00125-7. [DOI] [PubMed] [Google Scholar]
- 3.Gronau M, Koenig-Greger D, Jerg M, Riechelmann H. Gene polymorphisms in detoxification enzymes as susceptibility factor for head and neck cancer? Otolaryngol Head Neck Surg. 2003;128:674–80. doi: 10.1016/S0194-59980300176-1. [DOI] [PubMed] [Google Scholar]
- 4.Drummond SN, De Marco L, Noronha JC, Gomez RS. GSTM1 polymorphism and oral squamous cell carcinoma. Oral Oncol. 2004;40:52–5. doi: 10.1016/s1368-8375(03)00132-5. [DOI] [PubMed] [Google Scholar]
- 5.Duarte EC, da Silva MS, Gomez MV, Gomez RS. GSTM1 polymorphism and oral leukoplakia. J Oral Pathol Med. 2006;35:202–5. doi: 10.1111/j.1600-0714.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 6.Matthias C, Bockmühl U, Jahnke V, Jones PW, Hayes JD, Alldersea J, et al. Polymorphism in cytochrome P450 CYP2D6, CYP1A1, CYP2E1 and glutathione S-transferase, GSTM1, GSTM3, GSTT1 and susceptibility to tobacco-related cancers: Studies in upper aerodigestive tract cancers. Pharmacogenetics. 1998;8:91–100. [PubMed] [Google Scholar]
- 7.Matthias C, Bockmühl U, Jahnke V, Jones PW, Hayes JD, Alldersea J, et al. Polymorphism in cytochrome P450 CYP2D6, CYP1A1, CYP2E1 and glutathione S-transferase, GSTM1, GSTM3, GSTT1 and susceptibility to tobacco-related cancers: Studies in upper aerodigestive tract cancers. Pharmacogenetics. 1998;8:91–100. [PubMed] [Google Scholar]
- 8.Sato M, Sato T, Izumo T, Amagasa T. Genetic polymorphism of drug-metabolizing enzymes and susceptibility to oral cancer. Carcinogenesis. 1999;20:1927–31. doi: 10.1093/carcin/20.10.1927. [DOI] [PubMed] [Google Scholar]
- 9.Amtha R, Ching CS, Zain R, Razak IA, Basuki B, Roeslan BO, et al. GSTM1, GSTT1 and CYP1A1 polymorphisms and risk of oral cancer: A case-control study in Jakarta, Indonesia. Asian Pac J Cancer Prev. 2009;10:21–6. [PubMed] [Google Scholar]
- 10.Purnendu R, Kumbhare SP, Sathawane RS, Kumar P, Athawale RP, Husain S. GSTM1 polymorphism as risk factor in oral carcinoma in central Indian population: A pilot study. J Res Adv Dent. 2014;3:251–7. [Google Scholar]
- 11.Zhang ZJ, Hao K, Shi R, Zhao G, Jiang GX, Song Y, et al. Glutathione S-transferase M1 (GSTM1) and glutathione S-transferase T1 (GSTT1) null polymorphisms, smoking, and their interaction in oral cancer: A HuGE review and meta-analysis. Am J Epidemiol. 2011;173:847–57. doi: 10.1093/aje/kwq480. [DOI] [PubMed] [Google Scholar]
- 12.Zhao SF, Yang XD, Lu MX, Sun GW, Wang YX, Zhang YK, et al. GSTM1 null polymorphisms and oral cancer risk: A meta-analysis. Tumour Biol. 2014;35:287–93. doi: 10.1007/s13277-013-1037-z. [DOI] [PubMed] [Google Scholar]
- 13.Blot W, Mclaughlin J, Devesa SS, Fraumani JF, Jr, Schottenfeld D. Cancers of oral cavity and pharynx. In: Schottenfeld D, Fraumani JF Jr, editors. Cancer Epidemiology and Prevention. London, England: Oxford University Press; 1996. pp. 666–80. [Google Scholar]
- 14.Hung HC, Chuang J, Chien YC, Chern HD, Chiang CP, Kuo YS, et al. Genetic polymorphisms of CYP2E1, GSTM1, and GSTT1; environmental factors and risk of oral cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:901–5. [PubMed] [Google Scholar]
- 15.Hahn M, Hagedorn G, Kuhlisch E, Schackert HK, Eckelt U. Genetic polymorphisms of drug-metabolizing enzymes and susceptibility to oral cavity cancer. Oral Oncol. 2002;38:486–90. doi: 10.1016/s1368-8375(01)00086-0. [DOI] [PubMed] [Google Scholar]
- 16.Trizna Z, Clayman GL, Spitz MR, Briggs KL, Goepfert H. Glutathione s-transferase genotypes as risk factors for head and neck cancer. Am J Surg. 1995;170:499–501. doi: 10.1016/s0002-9610(99)80339-0. [DOI] [PubMed] [Google Scholar]


