Abstract
Purpose:
The aim of the study was to evaluate salivary flow rate, pH, buffering capacity, calcium, total protein content and total antioxidant capacity in relation to dental caries, age and gender.
Materials and Methods:
The study population consisted of 120 healthy children aged 7–15 years that was further divided into two groups: 7–10 years and 11–15 years. In this 60 children with DMFS/dfs = 0 and 60 children with DMFS/dfs ≥5 were included. The subjects were divided into two groups; Group A: Children with DMFS/dfs = 0 (caries-free) Group B: Children with DMFS/dfs ≥5 (caries active). Unstimulated saliva samples were collected from all groups. Flow rates were determined, and samples analyzed for pH, buffer capacity, calcium, total protein and total antioxidant status. Salivary antioxidant activity is measured with spectrophotometer by an adaptation of 2,2’-azino-di-(3-ethylbenzthiazoline-6-sulphonate) assays.
Results:
The mean difference of the two groups; caries-free and caries active were proved to be statistically significant (P < 0.05) for salivary calcium, total protein and total antioxidant level for both the sexes in the age group 7–10 years and for the age 11–15 years the mean difference of the two groups were proved to be statistically significant (P < 0.05) for salivary calcium level for both the sexes. Salivary total protein and total antioxidant level were proved to be statistically significant for male children only.
Conclusions:
In general, total protein and total antioxidants in saliva were increased with caries activity. Calcium content of saliva was found to be more in caries-free group and increased with age.
Keywords: Antioxidant, dental caries, saliva
Introduction
Saliva, a heterogeneous fluid comprising proteins, glycoproteins, electrolytes, small organic molecules and compounds transported from the blood, constantly bathes the teeth and oral mucosa. Whole saliva represents a mixture of the secretions of the major (submandibular, sublingual, parotid) and minor (accessory) salivary glands, together with the gingival fluid.[1] There are many and varied biological factors in saliva that protect enamel, dentin and cementum from caries development and facilitate the remineralization. The ability of saliva to affect caries development is dependent upon the quantity and composition of the secretions.[2]
Saliva possesses antimicrobial components and a buffering agent that act to protect and maintain oral tissues. Proteins that are found in saliva, such as lactoferrin, lysozyme, peroxidase, defensins and histatins, can destroy or inhibit the growth of microorganisms in the oral cavity.[3]
Oxidative stress is defined as a disturbance in the pro-oxidant – antioxidant balance in favor of the former, leading to potential damage. Antioxidant is defined as those substances which when present at low concentrations compared to those of an oxidizable substrate will significantly delay or inhibit oxidation of that substrate. e.g.: Uric acid, superoxide dismutase, glutathione peroxidase, carotenoids, etc.[1] In normal physiology there is a dynamic equilibrium between reactive oxygen species activity and antioxidant defense capacity and when that equilibrium shifts in favor of reactive oxygen species, either by a reduction in antioxidant defense or an increase in reactive oxygen species production or activity, oxidative stress results.[1] The antioxidant defense systems are highly complex. Their most important function is to control oral bacteria that form dental plaque that lead to dental caries and chronic inflammatory periodontal diseases.[4]
There are very few studies on the salivary flow rate, pH, buffer capacity, calcium, protein content and relationship between oxidant-antioxidant defense systems of the saliva and their relations with dental caries. So this study is designed in the Department of Pedodontics and Preventive Dentistry, Rajah Muthiah Dental College and Hospital, Chidambaram.
Materials and Methods
The present study was conducted in the Department of Pedodontics and Preventive Dentistry, Rajah Muthiah Dental College and Hospital, Annamalai University in association with Department of Biochemistry, Annamalai University to compare and evaluate salivary flow rate, pH, buffer capacity, calcium, total protein content and total antioxidant capacity with dental caries severity in 7–15 year old school going children of Chidambaram with DMFS/dfs ≥5. Inclusion criteria included 120-healthy children aged 7–15 years and divided into two groups on the basis of DMFS/defs score: Caries-free (Group A) and caries active (Group B). Exclusion criteria were on any medication for current and past illness and oral status other than dental caries like ulcers, oral tumors, herpetic lesions. Prior consent was obtained from the respective school authorities and from the parents through the school to conduct the study. The examinations were carried out in the subjects own surroundings that is, the school. The examination for dental caries was made according to the dentition status, and treatment needs as described by WHO (1997).
An organizing clerk at the examination site maintained a constant flow of subjects to the examiner and also entered general descriptive information on the survey form. The ages of the children were obtained from their records. A trained dental surgeon was involved to enter the codes on the survey form. The instruments were kept in Dettol solution for disinfection and sterilized using a water bath. Dettol was diluted by adding potable water in the ratio of 1:9 dilutions. The subjects were examined in the corridor of the school. The subjects were allowed to sit on a chair or stool, where sufficient natural daylight was available. A table to place the instruments was placed within easy reach of the examiner. The recording person was allowed to sit close enough to the examiner so that instructions and codes could be easily heard, and the examiner could see that findings were being recorded correctly.
Oral examination was conducted using No. 5 dental mouth mirrors and 0.5 mm diameter explorer, under natural light. Radiograph was not obtained. The type III clinical examination was carried out during the survey by the investigator himself. The data were entered on a standard proforma. Approximately 2–3 ml of unstimulated whole saliva was collected in the beaker following examination. The samples were then frozen and stored at −80°C until analyzed.
Methods
Early morning at least 2 h after breakfast, unstimulated saliva samples collected from all the groups by spitting the method [Figure 1]. Saliva samples are collected into a preweighed tube during a 5-min period. After collection, the tube is weighed again, and the flow rate calculated. Immediately after collection, pH is measured by a manual pH meter.
Figure 1.
Saliva sample collection
Buffer capacity is determined by quantitative test using a hand-held pH meter method [Figure 2]. This method involves the addition of 0.5 ml of saliva to 1.5 ml of 5 mmol/L Hcl. Mixture was vigorously shaken. Then stream of Nitrogen was passed through the mixture for 20 min to eliminate carbon dioxide from the sample and allowed to stand for 10 min when the final pH is measured.
Figure 2.

Measuring pH
The total protein and calcium levels of the samples are measured by autoanalyzer [Figure 3]. Measurement of protein content is based on biuret method [Figure 4], and alkaline copper reagent is used. The protein in saliva produces an alkaline copper-protein chelate when combined with the reagent. The resulting increase in absorbency is monitored by a detector at 545 nm. The observed rate of chelate formation is directly proportional to the total protein concentration in the sample.
Figure 3.

Measuring calcium
Figure 4.

Measuring total protein
Salivary calcium concentration is measured by Arsenazo-III method. The absorbency of the reagent is measured bichromatically at 650 and 700 nm. The change in absorbency is directly proportional to the amount of calcium in the sample.
Salivary antioxidant activity is measured with a spectrophotometer. Antioxidants of salivary samples are estimated by an adaptation of 2,2’-azino-di (3-ethylbenzthiazoline-6-sulphonate) (ABTS) assays, which involves the interaction of ferrylmyoglobin radical produced from activation of methmyoglobin, with the ABTS forming ABTS radical cation. The suppression of blue/green color production is proportional to the concentration. Ferric reducing antioxidant potential (FRAP) was used to determine the antioxidant potential in a given sample. FRAP utilizes the reducing potential of the antioxidant to react with a ferric tripyridyltriazine complex. This produces a colored ferrous tripyridyltriazine form. Then change in absorbance at 600 nm can then be compared with a standard to determine the antioxidant potential in a given sample.
Results
Statistical analysis
The results obtained were tabulated and statistically analyzed by independent sample t-test, anova test and Whitney–Mann U-test and software used is SPSS (IBM corporation) [Tables 1–6]. The mean difference of the two groups were proved to be statistically significant (P < 0.05) for salivary calcium, total protein and total antioxidant level for both the sexes in age group 7–10 years (n = 15) [Table 7]. The mean difference of the two groups were proved to be statistically significant (P < 0.05) for salivary calcium level for both the sexes and salivary total protein and total antioxidant level were proved to be statistically significant for male children only in the age group 11–15 years (n = 15) [Table 8].
Table 1.
Salivary flow rate (mL/min) in caries-free and caries active groups of children of both the sexes
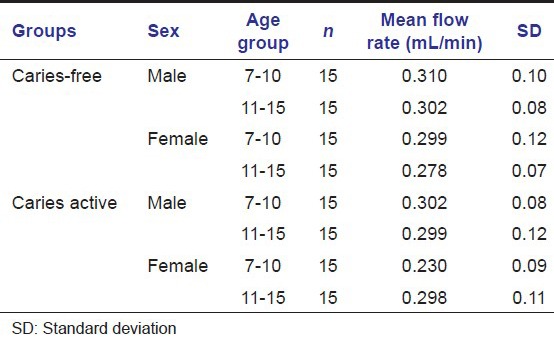
Table 6.
Salivary total antioxidant level (mmol/L) in caries-free and caries active groups of children of both the sexes
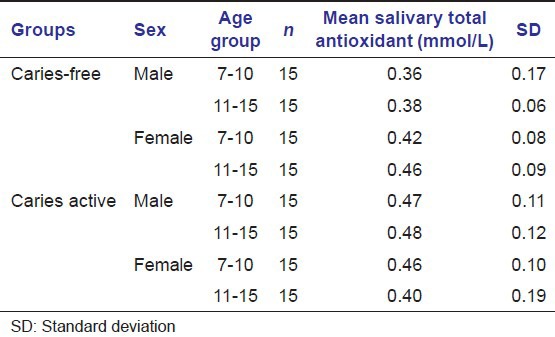
Table 7.
Comparison of salivary flow rate, pH, buffer capacity, calcium, total protein and total antioxidant levels in caries-free and caries active children in age group 7-10 years (n=15)
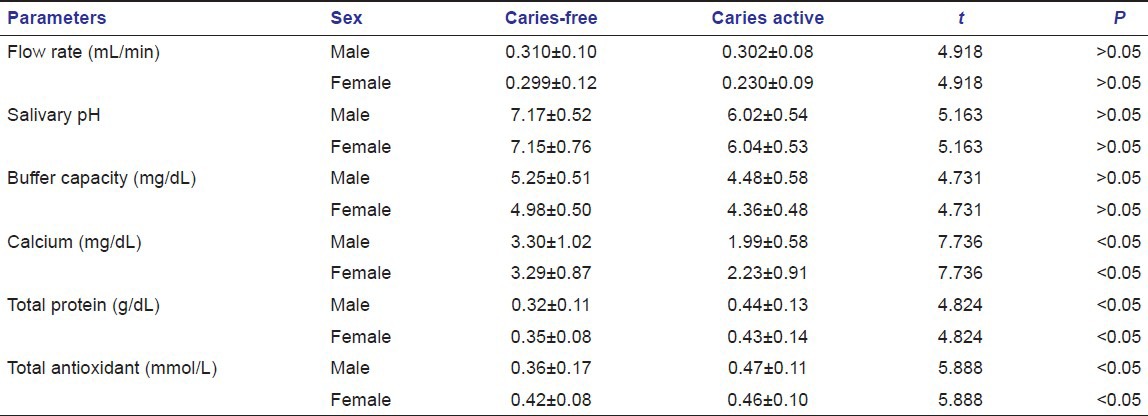
Table 8.
Comparison of salivary flow rate, pH, buffer capacity, calcium, total protein and total antioxidant levels in caries-free and caries active children in age group 11-15 years (n=15)
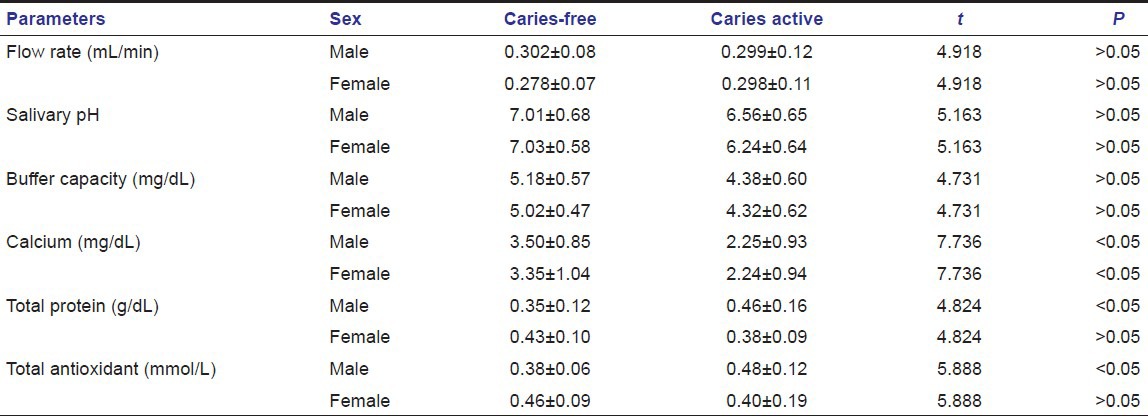
Table 2.
Salivary pH value in caries-free and caries active groups of children of both the sexes
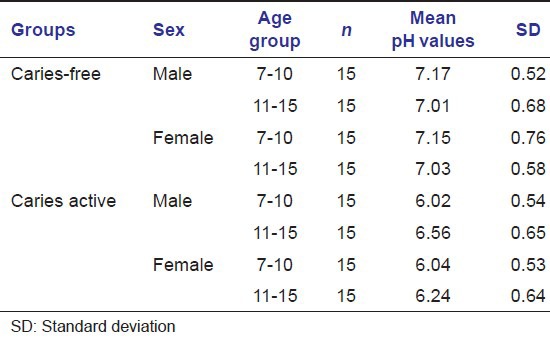
Table 3.
Salivary buffer capacity (mg/dL) in caries-free and caries active groups of children of both the sexes
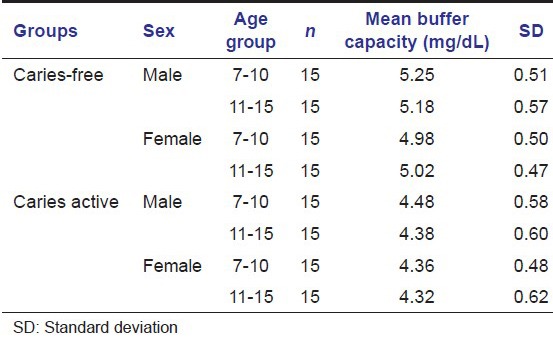
Table 4.
Salivary calcium level (mg/dL) in caries-free and caries active groups of children of both the sexes
Table 5.
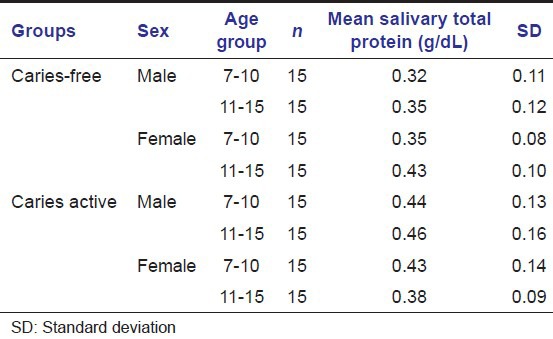
Salivary total protein level (g/dL) in caries-free and caries active groups of children of both the sexes
Discussion
My aim of selecting this study was because it would demonstrate the salivary factors that may in the future prove to be useful measures of caries activity in children and allow dentists to target preventive measures appropriately.[4]
Dawes, 1987[5] described the terms “unstimulated” saliva when no exogenous or pharmacological stimulation is used and “stimulated” saliva when secretion is promoted by mechanical or gustatory stimuli or by pharmacological agents. In our study unstimulated saliva sample was collected as Stookey, 2008[6] reported that stimulating the flow of saliva can alter its composition. Kaufman and Lamster, 2002[7] reported that salivary stimulation affects the quantity of saliva, concentration of some constituents and pH of the fluid.
Our study included the collection of whole saliva. Whole saliva is most frequently studied when salivary analysis is used. Whole saliva is a mixture of oral fluids, in addition to several constituents of nonsalivary origin, such as gingival crevicular fluid, expectorated bronchial and nasal secretions, serum and blood derivatives from oral wounds, bacteria and bacterial products, viruses and fungi, desquamated epithelial cells, and other cellular components and food debris.[7]
Unstimulated saliva sample was collected in our study by spitting method that appeared to be the most reproducible. Dawes, 1987[5] reported several different ways of collecting unstimulated whole saliva. They are:
Draining method
Spitting method
Suction method
Swab method.
Navazesh, 1993 reported the best two ways to collect whole saliva are the draining method, in which saliva is allowed to drip off the lower lip, and the spitting method, in which the subject expectorates saliva into a test tube.
Mandel, 1990[8] reported unstimulated or resting saliva is usually collected by passive drooling into a graduated tube or preweighed vial so that flow rate per unit time can be measured.
In our study, we measured salivary flow rate by the method given by Navazesh and Kumar, 2008 which was found to be more convenient.[9]
In our study, no significant correlation between caries activity and salivary flow rate were established. The effect of age, gender and salivary flow rate was not entirely clear. In our study, salivary flow rate were higher in boys compared with girls.
Heintze et al., 1983, Parvinen and Larmas, 1981 have also reported lower salivary flow rates in females. Heff and Baum, 1984 have noted no effect of gender on salivary flow rate.[5]
Dawes, 2008[5] reported that unstimulated flow rate averages 0.3–0.4 ml/min, but the range is wide. Unstimulated flow rates of <0.1 ml/min are considered as evidence of hyposalivation.
In our study, salivary pH values were found to be higher in caries-free group. pH values decreased with age in caries-free group and in caries active group pH values increased with age. In our study, there were no significant correlation found between pH values and caries activity, age and gender.
Saliva pH was measured directly using a small hand-held pH meter. Dimension of this compact hand-held pH meter was 165 × 29 × 19 mm; weight 53 g. This compact pH meter can determine the pH value of a single drop of saliva (0.1 ml) using a flat-surface sensor that is, flat glass electrodes.[10]
After the pH of saliva sample was measured using a hand-held pH meter, the saliva samples were titrated with 0.1 N Hcl to evaluate the buffering capacity.[11]
According to Neil, 1978,[12] the normal range of buffer capacity in saliva is 3–30 mg/100 ml.
Salivary buffer capacity can also be measured by other methods given by Kitasako et al., 2008[13] like Modified Ericsson test, quantitative test using a hand-held pH meter, Colorimetric paper strip test and liquid colorimetric test. In our study, we used quantitative test using a hand-held pH meter that was found to be more convenient and can also be used as a chair side test.
As reviewed by Ericsson, salivary buffer capacity has an inverse relationship with human caries incidence. In our study, buffer capacity values were significantly lower in caries active group than in caries-free group. There were no significant differences when the groups were compared related to age, except only in caries active group where buffer capacity values were higher in boys than in girls.[4]
Karshan[14] had also reported that in caries-free group, mean values for buffer capacity were more.
In our study calcium content of saliva was found to be more in caries-free group. With age advancement, calcium values were found to be increased.
Karshan[14] had also reported that in caries-free group, mean values for calcium content were more.
Study done by Tulunoglu et al., 2006 also reported increased calcium level in caries-free group.[4]
Helmerhorst and Oppenheim, 2007[15] reported as many as 309 proteins in whole saliva. More than 95% of salivary protein is from the major salivary protein families, which include acidic and basic proline rich proteins, amylases, high and low molecular weight mucous glycoproteins, agglutinins, cystatins, histatins and statherin.
In our study, there was an increase in total protein level in caries active children of both age groups that is total protein level was higher in those with dental caries.
Karshan[14] also reported that mean values for total protein were less in the caries-free than in the caries active group.
Study done by Tulunoglu et al., 2006 also reported increased protein level in caries active group.[4]
In our study, total antioxidant capacity was detected using FRAP assay.[16]
In this protocol, aqueous and lipid soluble antioxidants are not separated, so we get combined antioxidant activity of all its constituents including vitamins, protein, lipid, uric acid and glutathione. This assay inhibits the oxidation of ABTS to ABTS radical by the metmyoglobin, so the amount produced can be monitored by reading absorbance at 750 nm or 405 nm.
Schlesier et al., 2002[16] measured antioxidant activity using different in vitro methods like Trolox equivalent antioxidant capacity I-III assay, 2,2-diphenyl-1-picrylhydrazyl assay, N, N-dimethyl-p-phenylendiamine assay, photochemiluminescence assay and ferric reducing ability of plasma assay (FRAP assay).
Antioxidant system include enzymes such as superoxide dismutase, catalase and glutathione peroxidase; macromolecules such as albumin, ceruloplasmin and ferritin and an array of small molecules including ascorbic acid, alpha-tocopherol, beta-carotene, reduced glutathione, uric acid and bilirubin.[17]
The sum of endogenous and food derived antioxidant represent the total antioxidant capacity of the system.[17]
The cooperation among different antioxidants provide greater protection against attack by reactive oxygen or nitrogen species, than any single compound alone. Thus, the overall antioxidant capacity may provide more relevant biological information compared to that obtained by the measurement of individual components, as it considers the cumulative effect of all antioxidants present in body fluids.[17]
In our study, there was an increase in total antioxidant capacity in caries active children of both age groups.
Study done by Tulunoglu et al., 2006 also reported increased antioxidant level in caries active group.[4]
The increase in antioxidant in caries active group can be attributed to neutralize the effect of oxidant level that was supposed to be increased during pathological process.
The higher total antioxidant values in caries active children can be attributed to elevated protein levels. So it can be concluded that salivary antioxidant levels must be in a linear association with total protein levels.[4]
In caries active girls in 11–15 years of age groups, total protein and total antioxidants were found to be decreased (not statistically significant). This age group of 11–15 years are known to be related with an increase sexual hormone levels mainly in girls. So hormonal relationship with these parameters of saliva should be investigated in details.
Reactive oxygen species cause tissue damage through numerous ways that include DNA damage, protein damage, lipid peroxidation and stimulation of proinflammatory cytokines. The antioxidant micronutrients are important not only for limiting oxidative and tissue damage, but also in preventing increased cytokine production, which is a result of prolonged activation of the immune response.[18]
Summary and Conclusion
The findings of our study can be summarized as follows:
No significant correlation between caries activity and salivary flow rate were established. In our study, salivary flow rate was higher in boys compared with girls
Salivary pH values were found to be higher in caries-free group. pH values decreased with age in caries-free group and in caries active group pH values increased with age. In our study, there were no significant correlation found between pH values and caries activity, age and gender
Buffer capacity values were significantly lower in caries active group than in caries-free group. There were no significant differences when the groups were compared related to age, except only in caries active group where buffer capacity values were higher in boys than in girls
Calcium content of saliva was found to be more in caries-free group. With age advancement, calcium values were found to be increased
There was an increase in protein level in caries active children of both age groups
There was an increase in total antioxidant capacity in caries active children of both age groups
In caries active girls in 11–15 years of age groups, total protein and total antioxidants were found to be decreased (not statistically significant).
A further elaborate and in depth investigation has to be carried out with more sample measures and more clinical and laboratory studies are needed to determine the exact relationship between the physicochemical properties of saliva such as flow rate, pH, buffer capacity, calcium, total protein content and total antioxidant status, and dental caries, age and gender.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Battino M, Ferreiro MS, Gallardo I, Newman HN, Bullon P. The antioxidant capacity of saliva. J Clin Periodontol. 2002;29:189–94. doi: 10.1034/j.1600-051x.2002.290301x.x. [DOI] [PubMed] [Google Scholar]
- 2.Dowd FJ. Saliva and dental caries. Dent Clin North Am. 1999;43:579–97. [PubMed] [Google Scholar]
- 3.Lamey PJ, Lewis MA. Oral medicine in practice: Salivary gland disease. Br Dent J. 1990;168:237–43. doi: 10.1038/sj.bdj.4807149. [DOI] [PubMed] [Google Scholar]
- 4.Tulunoglu O, Demirtas S, Tulunoglu I. Total antioxidant levels of saliva in children related to caries, age, and gender. Int J Paediatr Dent. 2006;16:186–91. doi: 10.1111/j.1365-263X.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- 5.Dawes C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. J Dent Res. 1987;66:648–53. doi: 10.1177/00220345870660S107. [DOI] [PubMed] [Google Scholar]
- 6.Stookey GK. The effect of saliva on dental caries. J Am Dent Assoc. 2008;139:272–85. doi: 10.14219/jada.archive.2008.0347. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman E, Lamster IB. The diagnostic applications of saliva - A review. Crit Rev Oral Biol Med. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 8.Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19:119–25. doi: 10.1111/j.1600-0714.1990.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 9.Navazesh M, Kumar SK. Measuring salivary flow challenges and opportunities. J Am Dent Assoc. 2008;139:309–26. doi: 10.14219/jada.archive.2008.0353. [DOI] [PubMed] [Google Scholar]
- 10.Dawes C. Salivary flow patterns and the health of hard and soft oral tissues. J Am Dent Assoc. 2008;139:172–90. doi: 10.14219/jada.archive.2008.0351. [DOI] [PubMed] [Google Scholar]
- 11.Moritsuka M, Kitasako Y, Burrow MF, Ikeda M, Tagami J. The pH change after HCl titration into resting and stimulated saliva for a buffering capacity test. Aust Dent J. 2006;51:170–4. doi: 10.1111/j.1834-7819.2006.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 12.Neil JG. J Oral Pathol Med. 4th ed. 1978. The Physiology and Biochemistry of the Mouth; pp. 284–359. [Google Scholar]
- 13.Kitasako Y, Burrow MF, Stacey M, Huq L, Reynolds EC, Tagami J. Comparative analysis of three commercial saliva testing kits with a standard saliva buffering test. Aust Dent J. 2008;53:140–4. doi: 10.1111/j.1834-7819.2008.00023.x. [DOI] [PubMed] [Google Scholar]
- 14.Karshan M, Rosebury T, Waugh LM. Factors in Saliva Correlated with Dental Caries. Am J Dis Child. 1939;57:1026. [Google Scholar]
- 15.Helmerhorst EJ, Oppenheim FG. Saliva: A dynamic proteome. J Dent Res. 2007;86:680–93. doi: 10.1177/154405910708600802. [DOI] [PubMed] [Google Scholar]
- 16.Schlesier K, Harwat M, Böhm V, Bitsch R. Assessment of antioxidant activity by using different in vitro methods. Free Radic Res. 2002;36:177–87. doi: 10.1080/10715760290006411. [DOI] [PubMed] [Google Scholar]
- 17.Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–61. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carnelio S, Khan SA, Rodrigues G. Definite, probable or dubious: Antioxidant triology in clinical dentistry. Br Dent J. 2008:204. doi: 10.1038/bdj.2007.1186. [DOI] [PubMed] [Google Scholar]


