Abstract
Background
Facial allotransplantation is a revolutionary operation that has at last introduced the possibility of near-normal facial restoration to patients afflicted by the most severe cases of facial disfigurement.
Methods
The facial transplantation team at Brigham and Women’s Hospital (BWH) evaluated more than 20 patients as potential face transplant recipients; of these, 6 became face transplant candidates and underwent full screening procedures. The team performed facial allotransplantations in four of these patients between April of 2009 and May of 2011. This is the largest clinical volume of facial transplant recipients in the United States to date.
Results
We have learned important lessons from each of these four unique cases, as well as from the more than 20 patients that we have evaluated as potential face transplant recipients. We have translated lessons learned through direct experience into a set of fundamental surgical principles of the operation.
Conclusions
Our surgical principles emphasize safety, technical feasibility, preservation of functional facial units, and return of motor and sensory functions. This article describes each of these principles along with their rationale and, in some instances illustrates their application.
Introduction
Severe facial deformity often leads to impairments in facial function and social interactions, as well as discrimination, disability, depression, and body image and self-perception issues, all of which are detrimental to quality of life.1–6 Facial deformity may be consequential to trauma, burns, high voltage injury, malignancy or congenital disease. Reconstructive surgery provides only partial restoration of appearance and function, thus falling short on its ability to restore quality of life to patients with severe facial defects. Through many decades, researchers have tried to develop therapies to bestow a more normal appearance and function to disfigured patients, which if successful, would improve quality of life. Up until the recent advent of facial allotransplantation, these research efforts had not met with outstanding success. Facial allotransplantation entered the clinical arena almost 7 years ago7 as the only therapy to replace missing or damaged facial units such as the nose, lips, maxilla, and/or eyelids with functional and aesthetic equivalents. Reported outcomes clearly surpass those expected or achieved with conventional reconstruction.8–12
Planning a face transplant operation requires careful analysis of the recipient’s facial defect, as well as meticulous design of the facial allograft. A facial transplantation is a highly dynamic, prolonged operation requiring multiple teams communicating seamlessly through its duration.13–15 The multidisciplinary members of our team, along with their roles in the screening, planning, peri-operative and post-operative phases of facial transplantation have been previously described in detail.15 Our team has evaluated over 20 patients interested in undergoing facial transplantation; of these, 6 have been selected as candidates for the procedure based on criteria previously described,16 and we have performed facial transplantations in four of these candidates over the past 3 years.10, 11 Thus, we are currently the institution with the most clinical experience in the United States. Through these four successful experiences, we have identified the set of surgical principles described below. These principles serve as the guidelines for our processes of planning and execution of face transplant operations.
Surgical Principle #1: Safety
Face transplantation is an elective operation aimed at enhancing quality of life and potentially bringing severely disfigured patients back to a near normal appearance and functional status, productive lives, and active participation in family and society. The clinical volume of face transplantations to date is small, and it is only expected to increase if the inclusion criteria can be safely broadened16 based on positive outcomes, one of which is safety. With regards to safety, we have identified two main factors that must be addressed comprehensively during the planning stages:
1. Preserving salvage options in case of facial allograft failure
The longest follow-up peer-reviewed publication available on face transplantation dates to 5 years after the operation, and reports some manageable complications not unlike those reported after solid organ transplantation, as well as excellent function, patient satisfaction and social reintegration.8 In spite of these encouraging results, the long-term outcomes of face transplantation remain unknown, and although unlikely, allograft loss is always possible. It is important to have a robust salvage plan to execute in the unfortunate event of facial allograft loss; death is not an acceptable outcome of face transplant failure. The salvage plan must address safe coverage of the defect left after loss of the allograft, and it typically involves autologous skin graft or flap reconstruction. Discussions regarding the salvage plan must be carried out during the pre-transplantation evaluation, and must include the patient as well as the physicians. These discussions must address the possibility that the outcomes of salvage might leave the patient in a functional and aesthetic state worse than the pre-transplantation state. An example of a case where we applied the principle of preserving salvage options is the gentleman depicted in Figure 1a, who had suffered electrical burn injuries to the entire face 1.5 years prior to presenting for face transplantation consideration. During acute post-injury care, extensive debridement left the patient with exposed bone, which was then covered by bilateral muscle free flaps. The surgical plan we executed consisted on maintaining this muscle (Figure 1b) which can be re-grafted in one operation should the transplanted face fail. Of note, burn patients present the most challenges for salvage: those with burns covering large surface areas or having a history of multiple reconstructive efforts may have depleted their donor sites. Within this context, embarking on extensive reconstructive efforts for the treatment of facial defects that have no known acceptable clinical solution can be unwise. Our recommended approach is to present the option of transplantation following basic wound control in this patient population.
Figure 1.
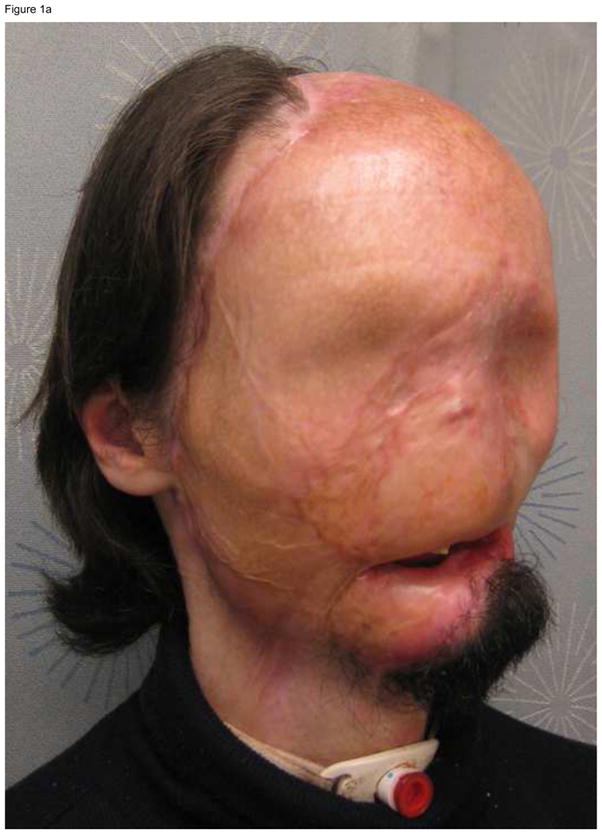
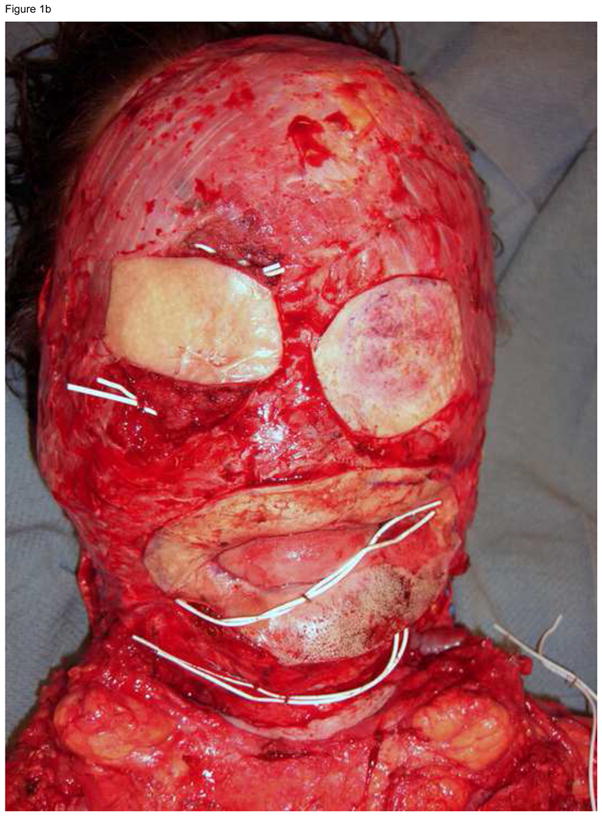
(A) A full facial transplantation candidate at the time of initial evaluation. (B) Orbital islands of skin were maintained to provide for lining behind planned ocular prostheses. Periorbital skin was maintained to substitute missing intraoral lining.
2. Life-long immune suppression and follow-up coverage
Although not a surgical factor, ensuring that a plan is in place for the provision and financial coverage of life-long immunosuppression and follow-up is imperative for the survival of the allograft. Prior to each face transplant operation and as an absolute criterion for inclusion, the Brigham and Women’s Hospital team secured written authorization from the medical insurance providers of its four face transplant recipients in regards to life-long coverage of post-transplant immunosuppression and follow-up. To date, coverage of transplant-related follow-up and immunosuppressive medications has proceeded without any issues for all of these patients. At the present time, compliance with immunosuppresive medications is absolutely necessary to reduce the incidence and intensity of rejection episodes; our team’s psychiatry and social work members assess the patient’s likelihood of compliance during the screening phase for facial transplantation,15 patients who are deemed likely non-compliant are excluded from the intervention. The team has not observed issues of compliance with immunosuppressive medications in any of its four transplanted patients. If rejection presents, medical treatment must be provided to prevent allograft loss. Finally, periodic monitoring is needed to minimize and address the serious side effects of immunosuppression.17–19 Of the four current Brigham and Women’s Hospital face transplant recipients, three have experienced single episodes of acute rejection in the months following the operation, all of which were successfully managed with methyl prednisolone boluses administered in an inpatient setting and followed by steroid taper.10, 11 None of the recipients have developed symptoms of chronic rejection. Finally, the team at Brigham and Women’s Hospital, as well as other teams across the globe are currently actively pursuing research aimed at safely minimizing or completely withdrawing immunosuppression in face transplant recipients by inducing donor specific tolerance.
Surgical Principle #2: Technical feasibility
Once started, the face transplant operation is subject to significant time constraints. From the instant the donor’s facial allograft is removed from physiologic blood flow until the moment of anastomosis to the recipient’s circulatory system and re-establishment of blood flow, there is only a four-hour window of safety. Beyond this 4-hour window, the recovery of facial muscles is unclear. Only a decade ago, it was thought that a full facial flap including portions of the lateral cheek, ears, scalp and forehead had to be anastomosed to multiple arteries on each side of the face in order to obtain adequate perfusion (Figure 2).20 Dissection of this much vascular territory is time-consuming, and requires inclusion of the parotid gland, which further enhances the complexity of inset. The time needed for extensive dissection may interfere with the recovery of life-saving organs and may eliminate the future option of facial allograft recovery from donors deceased by cardiac death, where the reperfusion window is even narrower. Therefore, in our patients, we pursued considerable simplification on the design of the anastomoses. In our recent series of full facial transplants, following extensive preoperative planning, we performed only single arterial anastomosis on each side of the face for three patients.10 We observed that only one sided anastomosis was adequate to achieve full perfusion of the full face allograft, but due to the high stakes of the operation, both sides were reconnected. In all three patients, we observed excellent blood flow without significant bleeding complications, and full revascularization within 5–10 minutes after retiring the clamps. One of the reasons we were confident on the success of this simplified approach was our pre-operative imaging with computerized tomography angiography (CTA) and magnetic resonance angiogram (MRA), which allowed us to understand the intricacies of the vascular anatomy of each recipient21, 22 and identify the recipient’s vessels that were suitable for anastomosis, thus minimizing the risks of critical blood loss and increased ischemia time, and minimizing venous outflow problems by preparing back-up options, such as vein grafts. In addition, we had a plan should the allograft face not perfuse completely based on facial vessels alone. Our plan was not transplanting the recipient’s forehead (the likely site of ischemia), but rather insetting the allograft at or above the level of the eyebrows. We therefore did not remove the skin from the forehead of any of our patients until after we observed excellent perfusion of the entire allograft and bleeding on its edges following anastomosis.
Figure 2.
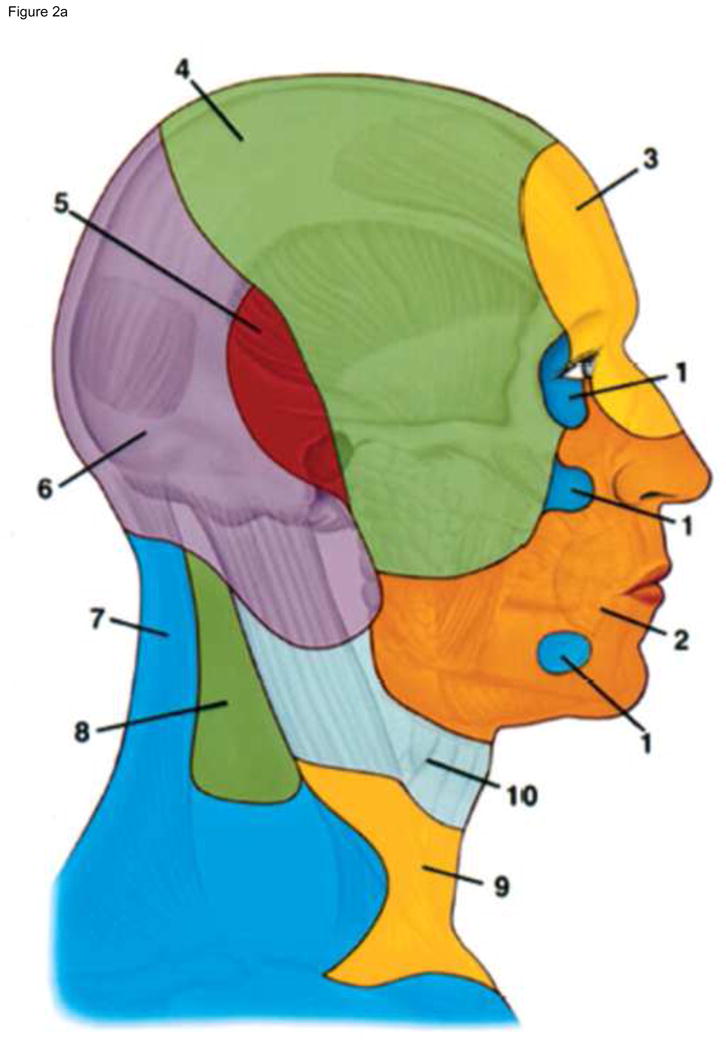
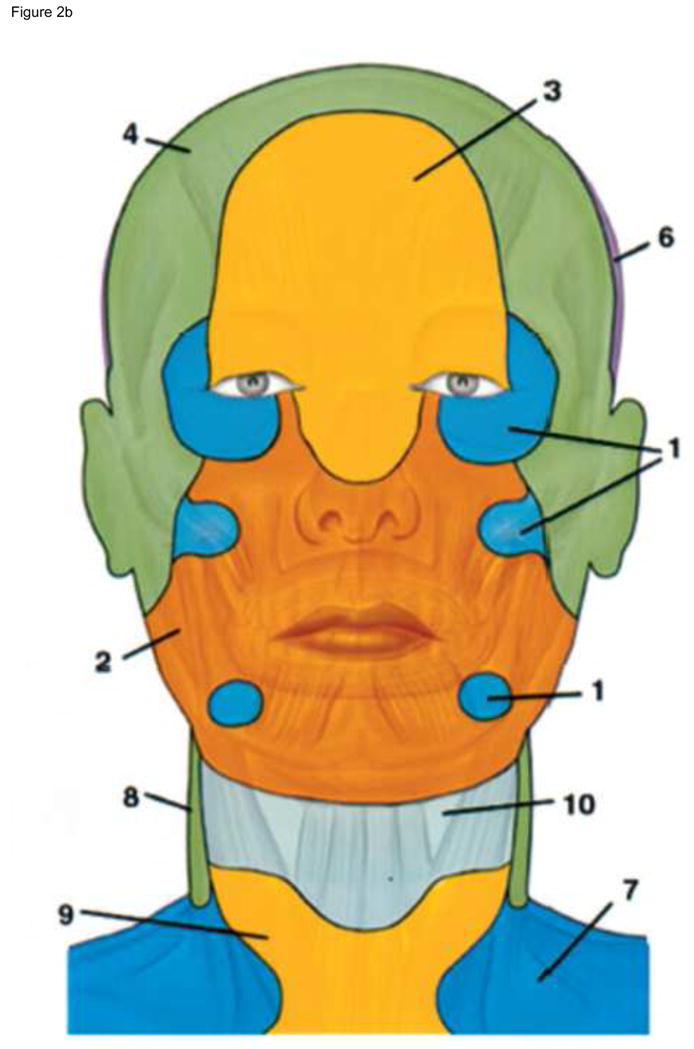
* From Housemann ND et al. The Angiosomes of the Head and Neck: Anatomic Study and Clinical Applications, Plastic Reconst Surg 2000 (105):2287–2313.
The angiosome territories of the facial artery (2) are shown in frontal and profile view. The angiosome territories of the: internal maxillary (1), facial (2), ophthalmic and internal carotid (3), superficial temporal (4), posterior auricular (5), occipital (6), transverse cervical (7), deep cervical (8), inferior thyroid (9) and superior thyroid (10) arteries are depicted in frontal and lateral views. Based on these angiosomes, it was thought that in order to perfuse a full facial flap including portions of the lateral cheek, ears, scalp and forehead, multiple arteries had to be anastomosed on each side. We demonstrated that single anastomosis of facial artery (2) on each side is sufficient to perform a full facial flap containing full cheeks, forehead, and partial scalp.
* will be reprinted with permission.
Surgical Principle #3: Preservation of Functional Units
Although unprecedented to date, failure of facial allografts can occur. Failure may be acute and due to vascular compromise, or secondarily due to acute or chronic rejection. Failure of the facial allograft would mandate surgical removal. Removal should preserve the patient’s life, and if possible it should lead to the same (i.e. not worse) post-injury, pre-transplant state of function. However, as discussed earlier, return to the pre-transplant state can be deemed unfeasible in some cases. This must be carefully discussed and disclosed to the patient as part of the informed consent process.
An attempt should be made to preserve the pre-transplant functional units of the face, even if preservation increases the degree of complexity of the operations. For example, two out of our four face transplant recipients had functional facial units that, if removed, would significantly simplify their operation. Yet, we strived to preserve these functional units in case the transplants ever fail. In one of the patients, we preserved the functional soft and hard tissues of the chin, forehead, eyebrows, and eyelids, and designed a mid-face allograft containing the nose, cheeks and maxilla.11 In another, we designed a full face allograft where the functional musculature of the forehead, cheeks, and eyelids was preserved, but the entire face was resurfaced, and the muscles and nerves that provide function to the lips were restored (Figure 3).10 If the allografts of either of these two patients fail and are removed at any point in time, conventional reconstruction techniques can return these patients to a state of disfigurement and function similar to that post-injury, pre-transplant, but not significantly worse.
Figure 3.
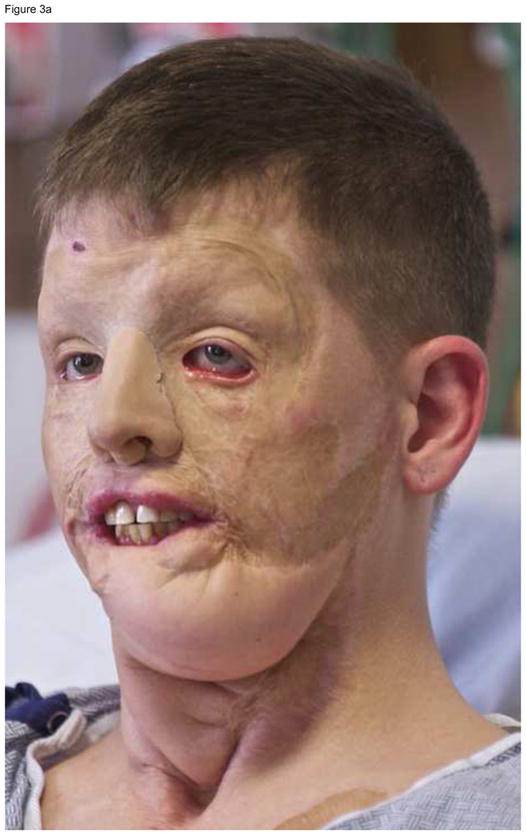
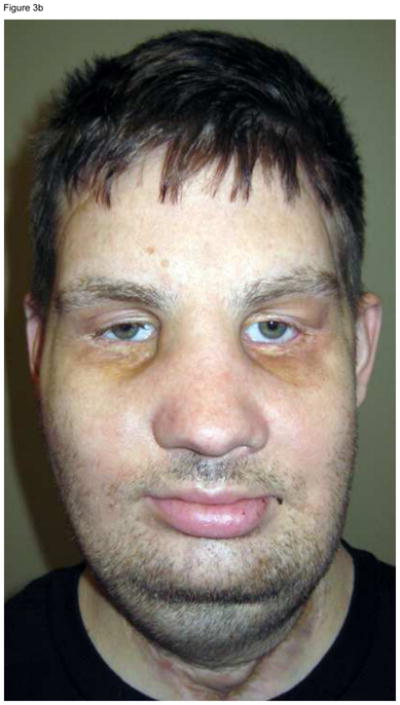
This patient suffered high voltage burn injuries to the face 11 years prior. Extensive conventional reconstruction yielded the results observed in (A). Note that the patient is wearing a prosthetic nose. Oral competence could not be restored. The facial muscles of the forehead, cheeks, and eyelids were functional and therefore preserved. The allograft face was placed over the functioning facial bed, and only nerves that provide function to the lips were reconnected, yielding the results observed in (B), where 4 months after the transplant operation the patient is capable of facial expression, limited by receding swelling.
Surgical Principle #4: Functional and Aesthetic Reintegration
Recovery of facial sensation is faster when coaptation of proper sensory nerves is performed.10 With the exception of direct neurotization of the most central facial muscle-to-muscle connections, motor recovery is dependent on facial nerve coaptation. The speed of sensory and motor recovery depends on axonal re-growth of the recipient’s nerve past the coaptation site. This type of nerve regeneration proceeds slowly, at a rate of approximate 1 mm per day following an initial delay of 1 month,23 and appears to be accelerated by tacrolimus immunosuppression.24, 25 Neurorrhaphies should therefore be performed as close to the effector muscles as the anatomy allows, by minimizing the length of the donor portion of the nerves, and/or maximizing the length of the recipient portion of the nerves. The previously described simplicity of the facial artery-based allograft fits well in the context of coapting individual branches of the facial nerve as close to the effector muscles as possible, thus facilitating targeted innervation of effector muscles.
We advocate for attempting neurorrhaphies in every instance when nerve stumps are present and healthy. If they are not, nerve grafts can be used to bridge distances. If nerves are not present and usable, and nerve grafting is not possible, nerve transfers based on our knowledge of head and neck reconstruction can be utilized. There has been substantial clinical experience with the use of cross-facial nerve grafts and nerve transfers (or crossovers) for facial reanimation in patients who lack proximal facial nerve segments suitable for coaptation but have intact distal neuromuscular pathways – patients whose anatomy mimics those of face transplant recipients, who have irrecoverable native facial nerves but pristine donor allografts. These techniques have been used for the treatment of both congenital and acquired facial paralysis, but best results have been demonstrated in patients who undergo reanimation within one year of onset of paralysis.26 Cross-facial nerve grafting depends on intact distal branches of facial nerve to provide the donor nerve for contralateral musculature. In transplant recipients with intact native buccal or mandibular branches, this remains an option. However, for patients with significant damage to native anatomy requiring coaptation of facial nerve at the level of the trunk, these distal segments would be incorporated within donor allograft tissues, and would themselves be downstream of facial nerve coaptations. In all cases, cross-facial nerve grafting involves long-segment nerve regeneration, given the length of graft required to reach from ipsilateral to contralateral face. As such, facial transplantation lends itself more readily to nerve transfers that utilize intact motor nerves from other tissues to power the facial nerve territory. When intact, the motor nerve to the masseter is used due to its proximity, and produces the best results. In patients with damaged masseteric nerves, extra-facial nerves may be used, such as the glossopharyngeal, accessory, phrenic, or – most commonly – hypoglossal nerve. These coaptations can be performed using partial or complete proximal nerves; the entire nerve may be used or, after intraneural dissection, 20% to 50% of proximal nerve axons may be coapted to allograft facial nerve to restore function to facial nerve territory muscles. Functionally, results are suboptimal. There is loss of function of the donor nerve, with the majority of patients undergoing hypoglossal nerve transfer demonstrating some degree of ipsilateral tongue atrophy.27 In addition, this technique does not easily allow for targeted reconnection of distal nerves, relying only on a single proximal nerve segment to innervate all desired facial nerve territory musculature. If the donor nerve is coapted at the level of the facial nerve trunk, movement that is generated postoperatively is therefore characterized by significant synkinesis. Spontaneous and undesired movements, such as eye-closing with attempted smile, require further interventions and therefore make this avenue of treatment undesirable. Nonetheless, in facial transplant recipients with absent facial nerves, these techniques represent plausible treatment options when incorporating and reinnervating donor tissues.
Finally, integration of the facial allograft with the best aesthetic result is often in direct competition with the preservation of functional units, and of unscarred facial skin. Better aesthetic results are generally accomplished when larger areas following aesthetic units are resurfaced.28 As we do not know at the present time what the future may bring with regard to overall survival of the allograft, we believe that the patient has to be consulted to make an informed decision. The level of risk acceptance can be quite different in various patients, and ultimately each individual should have the autonomy to decide.
Conclusions
Facial allotransplantation is a single operation that can provide near-normal restoration to severely disfigured patients that, prior to the advent of this revolutionary intervention, were limited to multiple staged reconstructions and suboptimal functional and aesthetic results. Experience with four operations and over twenty patients evaluated for facial transplantation has highlighted the importance of surgical safety, which must include planning for exit options (i.e. salvage plans in case of allograft failure), simplification of vascular anastomoses, preservation of uninjured, functional facial units over aesthetic considerations, and advocating for functional reintegration of the allograft through maximized attempts at proximal neurorraphies, and use of nerve grafts and/or nerve transfers. Finally, we acknowledge that the outcomes of the first series of face transplant interventions in the world are still young (less than 7 years), and that much is yet to be learned about the long term outcomes of this intervention. As our experiential knowledge in this field increases, we vouch to modify our principles and protocols towards optimum patient safety, satisfaction and functionality.
Table 1.
| Recipient | Date Transplanted | Description of Facial Defect at the Time of Face Transplant Evaluation | Salvage Plan |
|---|---|---|---|
| 1 | April 2009 | Complex bony and soft tissue defect of the mid-face. Loss of nose, maxilla and upper lip. Reconstructed with anterolateral thigh fasciocutaneous free flap to separate the oral and nasal cavities. |
Repeat anterolateral thigh flap. |
| 2 | March 2011 | Loss of most soft tissues of the face, eyelids, left eye, nose, lips, teeth and large portion of temporo-parietal scalp. Reconstructed with bilateral latissimus serratus muscles and skin grafts. |
Split thickness skin grafting of underlying latissimus and serratus muscles. |
| 3 | April 2011 | Loss of nose, facial skin over the entire face, upper and lower lips (with remnants of orbicularis in the upper lip), bilateral lower lid ectropion. Reconstructed with skin grafting over forehead and eyelids, and lateral arm flap for the neck, mandible and lower lip. |
Split thickness skin grafting of facial wound, with preservation of motor function |
| 4 | May 2011 | Loss of central facial tissues including the nose, eyelids, both eyes, maxilla, and both lips, with extensive scarring of the remaining face. Reconstructed with anterolateral free flap for wound control, and nasal reconstruction with rib cartilage. |
Repeat anterolateral tight flap to separate the oral and nasal cavities |
Acknowledgments
The authors wish to thank the sponsor of the Brigham and Women’s Hospital face transplantation study, the United States Department of Defense’s Biomedical Translational Initiative (Research Contract # W911QY-09-C-0216). The authors also acknowledge the support of Clinical Translational Science Award UL1RR025758 to Harvard University and Brigham and Women’s Hospital from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Institutes of Health or the United States Department of Defense. The authors also express special gratitude to the donor families and the New England Organ Bank.
References
- 1.Bjordal K, Kaasa S, Mastekaasa A. Quality of Life in Patients Treated for Head and Neck Cancer: A Follow-up Study 7 to 11 Years after Radiotherapy. Int J Radiat Oncol Biol Phys. 1994;28:847–856. doi: 10.1016/0360-3016(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 2.Gamba A, Romano M, Grosso IM, et al. Psychosocial Adjustment of Patients Surgically Treated for Head and Neck Cancer. Head Neck. 1992;14:218–223. doi: 10.1002/hed.2880140309. [DOI] [PubMed] [Google Scholar]
- 3.van der Wouden JC, Greaves-Otte JG, Greaves J, Kruyt PM, van Leeuwen O, van der Does E. Occupational Reintegration of Long-Term Cancer Survivors. J Occup Med. 1992;34:1084–1089. doi: 10.1097/00043764-199211000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Bonanno A, Esmaeli B, Fingeret MC, Nelson DV, Weber RS. Social Challenges of Cancer Patients with Orbitofacial Disfigurement. Ophthal Plast Reconstr Surg. 2010;26:18–22. doi: 10.1097/IOP.0b013e3181b8e646. [DOI] [PubMed] [Google Scholar]
- 5.Tartaglia A, McMahon BT, West SL, Belongia L. Workplace Discrimination and Disfigurement: The National Eeoc Ada Research Project. Work. 2005;25:57–65. [PubMed] [Google Scholar]
- 6.Kauvar DS, Wolf SE, Wade CE, Cancio LC, Renz EM, Holcomb JB. Burns Sustained in Combat Explosions in Operations Iraqi and Enduring Freedom (Oif/Oef Explosion Burns) Burns. 2006;32:853–857. doi: 10.1016/j.burns.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Devauchelle B, Badet L, Lengele B, et al. First Human Face Allograft: Early Report. Lancet. 2006;368:203–209. doi: 10.1016/S0140-6736(06)68935-6. [DOI] [PubMed] [Google Scholar]
- 8.Petruzzo P, Testelin S, Kanitakis J, et al. First Human Face Transplantation: 5 Years Outcomes. Transplantation. 2012;93:236–240. doi: 10.1097/TP.0b013e31823d4af6. [DOI] [PubMed] [Google Scholar]
- 9.Lantieri L, Meningaud JP, Grimbert P, et al. Repair of the Lower and Middle Parts of the Face by Composite Tissue Allotransplantation in a Patient with Massive Plexiform Neurofibroma: A 1-Year Follow-up Study. Lancet. 2008;372:639–645. doi: 10.1016/S0140-6736(08)61277-5. [DOI] [PubMed] [Google Scholar]
- 10.Pomahac B, Pribaz J, Eriksson E, et al. Three Patients with Full Facial Transplantation. N Engl J Med. 2012;366:715–722. doi: 10.1056/NEJMoa1111432. [DOI] [PubMed] [Google Scholar]
- 11.Pomahac B, Pribaz J, Eriksson E, et al. Restoration of Facial Form and Function after Severe Disfigurement from Burn Injury by a Composite Facial Allograft. Am J Transplant. 2011;11:386–393. doi: 10.1111/j.1600-6143.2010.03368.x. [DOI] [PubMed] [Google Scholar]
- 12.Siemionow MZ, Papay F, Djohan R, et al. First U.S. Near-Total Human Face Transplantation: A Paradigm Shift for Massive Complex Injuries. Plast Reconstr Surg. 2010;125:111–122. doi: 10.1097/PRS.0b013e3181c15c4c. [DOI] [PubMed] [Google Scholar]
- 13.Pomahac B, Bueno EM, Siemionow M. Donor Procurement for Facial Allograft Transplantation. 2011. [Google Scholar]
- 14.Pomahac B, Nowinski D, Diaz-Siso JR, et al. Face Transplantation. Curr Probl Surg. 2011;48:293–357. doi: 10.1067/j.cpsurg.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Bueno EM, Diaz-Siso JR, Pomahac B. A Multidisciplinary Protocol for Face Transplantation at Brigham and Women’s Hospital. J Plast Reconstr Aesthet Surg. 2011;64:1572–1579. doi: 10.1016/j.bjps.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Pomahac B, Diaz-Siso JR, Bueno EM. Evolution of Indications for Facial Transplantation. J Plast Reconstr Aesthet Surg. 2011;64:1410–1416. doi: 10.1016/j.bjps.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Vicari-Christensen M, Repper S, Basile S, Young D. Tacrolimus: Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics to Facilitate Practitioners’ Understanding and Offer Strategies for Educating Patients and Promoting Adherence. Prog Transplant. 2009;19:277–284. doi: 10.1177/152692480901900315. [DOI] [PubMed] [Google Scholar]
- 18.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after Kidney Transplantation in the United States. Am J Transplant. 2004;4:905–913. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 19.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes Mellitus after Kidney Transplantation in the United States. Am J Transplant. 2003;3:178–185. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 20.Houseman ND, Taylor GI, Pan WR. The Angiosomes of the Head and Neck: Anatomic Study and Clinical Applications. Plast Reconstr Surg. 2000;105:2287–2313. doi: 10.1097/00006534-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Soga S, Pomahac B, Mitsouras D, et al. Preoperative Vascular Mapping for Facial Allotransplantation: Four-Dimensional Computed Tomographic Angiography Versus Magnetic Resonance Angiography. Plast Reconstr Surg. 2011;128:883–891. doi: 10.1097/PRS.0b013e3182268b43. [DOI] [PubMed] [Google Scholar]
- 22.Soga S, Ersoy H, Mitsouras D, et al. Surgical Planning for Composite Tissue Allotransplantation of the Face Using 320-Detector Row Computed Tomography. J Comput Assist Tomogr. 2010;34:766–769. doi: 10.1097/RCT.0b013e3181e9c133. [DOI] [PubMed] [Google Scholar]
- 23.Tinel J. Les Blessures Des Nerfs; Sémiologie Des Lésions Nerveuses Périphériques Par Blessures De Guerre. Paris: Masson et cie; 1916. [Google Scholar]
- 24.Gold BG, Katoh K, Storm-Dickerson T. The Immunosuppressant Fk506 Increases the Rate of Axonal Regeneration in Rat Sciatic Nerve. J Neurosci. 1995;15:7509–7516. doi: 10.1523/JNEUROSCI.15-11-07509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons WE, George EB, Dawson TM, Steiner JP, Snyder SH. Immunosuppressant Fk506 Promotes Neurite Outgrowth in Cultures of Pc12 Cells and Sensory Ganglia. Proc Natl Acad Sci U S A. 1994;91:3191–3195. doi: 10.1073/pnas.91.8.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inigo F, Ysunza A, Rojo P, Trigos I. Recovery of Facial Palsy after Crossed Facial Nerve Grafts. Br J Plast Surg. 1994;47:312–317. doi: 10.1016/0007-1226(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 27.Shipchandler TZ, Seth R, Alam DS. Split Hypoglossal-Facial Nerve Neurorrhaphy for Treatment of the Paralyzed Face. Am J Otolaryngol. 2011;32:511–516. doi: 10.1016/j.amjoto.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Ulloa M, Castillo A, Stevens E, Alvarez Fuertes G, Leonelli F, Ubaldo F. Preliminary Study of the Total Restoration of the Facial Skin. Plast Reconstr Surg (1946) 1954;13:151–161. doi: 10.1097/00006534-195403000-00001. [DOI] [PubMed] [Google Scholar]


