Abstract
The protozoan parasite Trypanosoma cruzi causes Chagas disease. Cardiac and adipose tissues are among the early targets of infection and are sites of persistent infection. In the heart and adipose tissue, T. cruzi infection results in an upregulation of pro-inflammatory mediators. In the heart, infection is associated with an increase in the markers of oxidative stress. To date, markers of oxidative stress have not been evaluated in adipose tissue in this infection. Brown and white adipose tissues were obtained from CD-1 mice infected with the Brazil strain of T. cruzi for 15, 30, and 130 days post infection. Protein carbonylation and lipid peroxidation assays were performed on these samples. There was an upregulation of these markers of oxidative stress at all time-points in both white and brown adipose tissue. Determinants of anti-oxidative stress were downregulated at similar time-points. This increase in oxidative stress during T. cruzi infection most likely has a deleterious effect on host metabolism and on the heart.
Keywords: Trypanosoma cruzi, Chagas disease, Adipose tissue, Oxidative stress, Protein carbonylation, Lipid peroxidation
Introduction
For over a century research in Chagas disease, caused by Trypanosoma cruzi, the heart and the gastrointestinal tract have understandably received the greatest scrutiny. However, T. cruzi also has significant effects on other organs such as adipose tissue and the pancreas (Combs et al. 2005; Nagajyothi et al. 2008, 2010, 2012a, b, 2013). Adipose tissue is both an early target as well as a reservoir of latent T. cruzi infection (Combs et al. 2005; Nagajyothi et al. 2012a, b). Our laboratory group has demonstrated that T. cruzi infection of adipose tissue leads to an intense inflammatory reaction that extends into the chronic phase of infection (Combs et al. 2005). This, in some aspects, is similar to the obese state where adipose tissue displays a chronic inflammatory phenotype (Ferrante 2007) which contributes, in part, to heart disease (Turer et al. 2012) and other host metabolic disorders, such as insulin resistance.
Adipose tissue is the largest endocrine organ in the body accounting for 10 to 50 % of body composition, depending on the host, and contributes to energy homeostasis and fulfills critical roles in host immune responses (Halberg et al. 2008). Among the first experimental observations examining the relationship of infection and adipose tissue was the demonstration that injection of LPS into mice that were rendered fatless did not result in immediate death, as is observed in control mice with a normal component of adipose tissue (Pajvani et al. 2005). The major component of adipose tissue is the adipocyte which exerts its influence through the synthesis and release of cytokine-like proteins known as adipokines. It is now appreciated that the intense pro-inflammatory potential of adipose tissue indicates that it plays an important role both in the innate and adaptive immune responses during infection and that its absence reduces inflammatory markers (Kaminski and Randall 2010; Sell et al. 2012; Bondia-Pons et al. 2012).
Adipose tissue obtained from T. cruzi-infected mice and T. cruzi-infected cultured adipocytes displays an upregulation in traditional markers of inflammation such as cytokines, chemokines, Toll-like receptors, and components of the mitogen-activated protein kinase (MAPK) pathway (Combs et al. 2005; Nagajyothi et al. 2008, 2010, 2012a, b).
In T. cruzi-infected adipose tissue samples and cultured adipocytes, there is a reduction in the adipokine, adiponectin, and in peroxisome proliferator-activated receptor (PPAR-γ) (Combs et al. 2005; Nagajyothi et al. 2008). T. cruzi-infected mice display a reduction in serum adiponectin levels (Combs et al. 2005). Both adiponectin and PPAR-γ are inversely associated with upregulation of inflammation; a situation reminiscent of the obese state (Ferrante 2007; Shetty et al. 2009; Hotamisligil 2006; Weisberg et al. 2003; Bondia-Pons et al. 2012).
The accumulating data strongly suggests that there are some similarities between adipose tissue in the obese state and in adipose tissue in Chagas disease. For example, in both disease states, there is an influx of macrophages into adipose tissue and an upregulation of pro-inflammatory pathways. In the obese state, markers of oxidative stress are increased (Murdolo et al. 2013; Crujeiras et al. 2013; Furukawa et al. 2004; Bondia-Pons et al. 2012). Oxidative stress is also increased in the myocardium of mice infected with T. cruzi (Wen et al. 2008, 2010). To date, however, no study has been performed in adipose tissue obtained from T. cruzi-infected mice or patients. Therefore, we examined the level of oxidative stress in white (WAT) and brown (BAT) adipose tissue of mice infected with T. cruzi. Markers of oxidative stress were increased during both the acute and chronic stages of infection, and these observations are consistent with and likely involved in the host metabolic changes observed in infected mice and similar changes seen in humans (Bondia-Pons et al. 2012).
Materials and methods
Parasitology
In all experiments, 8 to 10-week-old male CD-1 mice (Charles River, Wilmington, MA) were used. They were injected IP with 5×104 trypomastigotes of the Brazil strain of T. cruzi. After the initial injection, there is a gradual increase in parasitemia which peaked at 30 to 40 days post infection (dpi). During this time, the mortality rate is approximately 50 to 60 %. The parasitemia then wanes, and by 90 to 100 dpi, there is morphological and functional evidence of cardiomyopathy (Jelicks and Tanowitz 2011). Both WAT and BAT were removed 15, 30, 130, and 160 dpi. Epididymal WAT and interscapular BAT were collected and stored at −80 °C, until used. These animal protocols were approved by the Institutional Animal Care and Use Committees of the Albert Einstein College of Medicine and of the University of Texas Medical Branch.
Protein carbonylation
Protein carbonyls were measured as described (Levine et al. 2000) and modified by Wen et al. (2006a, 2008). Briefly, 20 μg proteins was denatured and derivatized in 3 % sodium dodecyl sulfate (SDS), 10 mM 2,4-dinitrophenylhydrazine (DNPH) dissolved in 10 % trifluoroacetic acid (20-μl volume). After neutralization with 7.5 μl of 2 M Tris, 30 % glycerol, DNP-derivatized protein samples were resolved by 10 % of SDS–PAGE. Gels were transferred to PVDF membranes. Carbonylized proteins were probed with rabbit anti-DNP antibody (1:2,000, Sigma-Aldrich, St Louis, MO), followed by HRP-conjugated goat anti-rabbit IgG (1:5,000, Sigma-Aldrich), and signal was developed by using an enhanced chemiluminescence detection system (GE Healthcare, Pittsburgh, PA). Images were visualized, digitized, and quantified by densitometry using a FluorChem 8800 (Alpha Innotech, San Jose, CA) image analyzing system.
Lipid peroxidation
We evaluated the extent of lipid peroxidation products, i.e., malonyldialdehyde (MDA) contents, by a thiobarbituric acid reactive substances (TBARS) assay as described (Ohkawa et al. 1979) and modified by Wen et al. (2008). Tissue lysates (1:10w/v) were mixed with 0.2 ml of 8.1 % SDS, 1.5 ml of 20 % acetic acid, pH 3.5, and 1.5 ml of 0.8 % thiobarbituric acid (TBA). The mixture was diluted to 4 ml with distilled H2O and heated at 95 °C for 60 min. After cooling on ice, samples were extracted with 5 ml n-butanol:pyridine (15:1, v/v), and the concentration of lipid peroxides was calculated as an MDA equivalent using the extinction coefficient for the MDA–TBA complex of 1.56× 105 M−1 cm−1 at 532 nm.
Quantitative real-time PCR
RNA was isolated from adipose tissue using TRIzol (Invitrogen). Total RNA was purified using the RNeasy Mini Kit (Qiagen, Valencia, CA). First-strand complementary DNA (cDNA) was synthesized from 1 μg of total RNA in a final volume of 50 μl using SuperScript III transcriptase (Invitrogen). A real-time PCR was performed on an iQ5 thermal cycler (Bio-Rad) with SYBR Green Supermix (Qiagen) using Mouse Oxidative Stress PCR Array (PAM 065Z, SA Biosciences) and cDNA template. The threshold cycle (Ct) values for each target messenger RNA (mRNA) were normalized to 18S rRNA mRNA, and the relative expression level of each target gene was calculated with the formula n-fold change=2−ΔCt, where ΔCt represents Ct (infected) − Ct (control).
Results
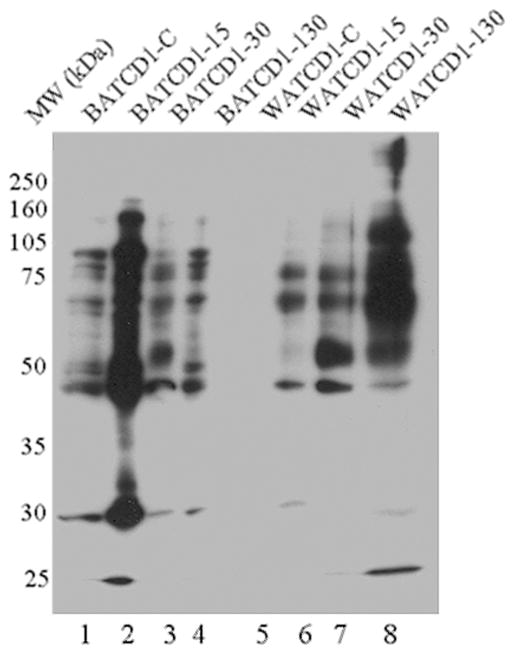
The Western blot shown in Fig. 1 demonstrates that the level of protein carbonylation, a biomarker of oxidative stress, was increased in BAT and WAT of mice harvested at the early, 15 days post infection (dpi); acute (30 dpi); and chronic (130 dpi) stages of T. cruzi infection and disease development. Similarly, there was an upregulation in lipid peroxidation in both BAT and WAT of chagasic mice at all time-points post infection (Fig. 2). Together, these data suggest that oxidative stress is increased in the BAT and WAT of mice during the course of infection and chronic disease development.
Fig. 1.
Protein carbonylation in adipose tissue of chagasic mice. CD1 mice were sacrificed at 15, 30, and 130 dpi corresponding to early, acute, and chronic phases, respectively, of T. cruzi infection and disease development. Carbonylated proteins in the adipose tissue homogenates were derivatized with DNPH, and the DNP-reactive proteins were identified by Western blot analysis using DNP-specific antibody. A representative Western blot is shown. Lane 1: BAT CD1-control; Lane 2: BAT CD1-D15; Lane 3: BAT CD1-D30; Lane 4: BAT CD1-D130; Lane 5: WAT CD1-control; Lane 6: WAT CD1-D15; Lane 7: WAT CD1-D30; Lane 8: WAT CD1-D130 (BAT brown adipose tissue, WAT white adipose tissue)
Fig. 2.
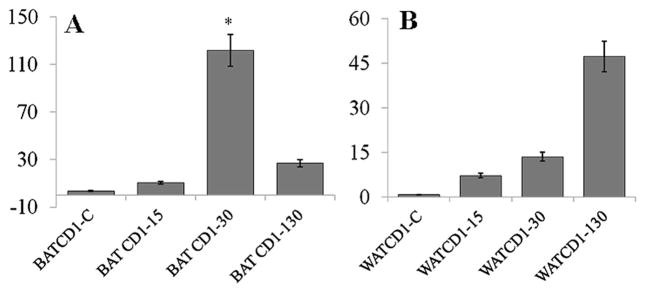
Bar graphs showing the levels of malonyldialdehydes (MDA) in adipose tissue of chagasic mice. Mice were harvested as in Fig. 1. MDA contents, indicative of lipid peroxidation products in BAT (a) and WAT (b) tissue of infected mice at 15, 30, and 130 dpi was determined by a TBARS assay as described in “Materials and methods”. Data are presented as n mlo/mg protein and represents mean±SD (*p<0.05; **p<0.01)
We evaluated the mRNA levels of the genes involved in the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) to determine the source of oxidative stress (Fig. 3). Testing of a ROS-generating enzyme NADPH oxidase (NOX) revealed different expression levels of NOX1 and NOX4 between BAT and WAT during infection. We did not observe significant differences in the expression of NOX1 in BAT during acute and chronic infection (data not shown); however, BAT displayed an increased expression of neutrophil cytosolic factor (a subunit of neutrophil NADPH oxidase) and NOX4 during both acute and chronic infection. In WAT tissue, we observed a higher expression of NOX1 (5-fold) during acute infection which significantly decreased (−2.8-fold) during chronic infection. WAT revealed no significant changes in NOX4 expression. These data suggested that ROS production, most likely due to activation of NADPH oxidase, is enhanced in BAT and WAT of T. cruzi-infected mice.
Fig. 3.
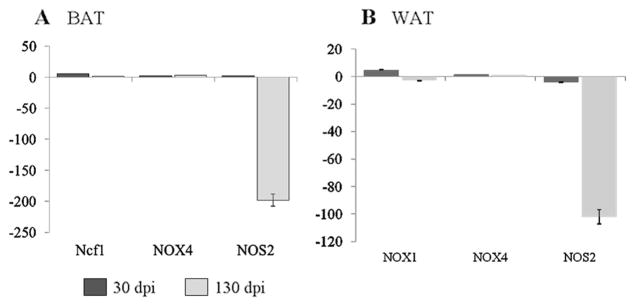
The mRNA level for subunits of NADPH oxidase complex is enhanced in adipose tissue of mice infected by T. cruzi. Adipose tissue (a BAT and b WAT) samples from normal and infected mice were harvested at acute and chronic stages of infection and disease development, and total RNA isolated as discussed in “Materials and methods”. A quantitative real-time PCR was performed to evaluate the mRNA levels of selected genes. Transcript levels were normalized against 18S rRNA, and derived from at least four mice per group. Data are presented as fold change (*p<0.05, normal vs. infected; SD<12 %)
Previously, we demonstrated that WAT undergoes significant lipolysis during acute infection compared to BAT (Nagajyothi et al. 2012a, b). It has been shown that NOS2 modulates lipolysis in adipocytes (Penfornis and Marette 2005). NOS2 mediates the synthesis of nitric oxide, a reactive free radical which acts as a biologic mediator in several processes, including neurotransmission and antimicrobial and anti-tumoral activities. NOS2 expression was downregulated in both BAT and WAT of chronically infected mice which could contribute to increased lipolysis during infection (Fig. 3).
Finally, we examined if the antioxidant capacity of the BAT and WAT in chagasic mice is altered (Fig. 4). The expression levels of glutathione peroxidase (GPX) and superoxide dismutase (SOD) isoforms was significantly reduced in BAT (Fig. 4a) and WAT (Fig. 4b) of acutely and chronically infected mice. The mRNA levels of eosinophil peroxidase (EPX) antioxidant in BAT and WAT was increased by 2-fold and >50-fold, respectively, during the acute phase of infection. With the progression to chronic phase, the expression level of EPX in BAT and WAT was decreased by 4-fold or became non-responsive, respectively. These data suggest that despite an increase in oxidative stress, the antioxidant response is compromised in BAT and WAT of chagasic mice.
Fig. 4.
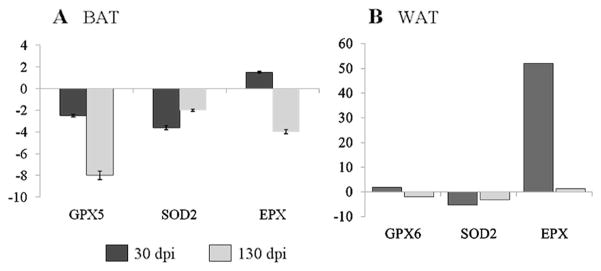
The expression level of genes encoding antioxidants is compromised in adipose tissue of mice infected by T. cruzi (a BAT and b WAT). A quantitative real-time PCR was performed as in Fig. 3 to evaluate the mRNA levels for antioxidants. The data are presented as fold change in expression (*p<0.05, normal vs. infected; SD<12 %)
Discussion
Many human diseases have been linked to the generation of oxidative stress such as neurodegenerative diseases, obesity, and heart disease (Bondia-Pons et al. 2012; Enns 2003; Luczak and Anderson 2014). In recent years, oxidative stress has been linked to T. cruzi-associated heart disease (reviewed in Machado et al. 2012; Nagajyothi et al. 2012a, b). The potential role of adipose tissue in the pathogenesis of Chagas disease including cardiomyopathy has only recently received attention (Nagajyothi et al. 2009). Herein, we demonstrate that both BAT and WAT obtained from T. cruzi-infected mice during acute and chronic infection displayed significant up-regulation of markers of oxidative stress including protein carbonylation and lipid peroxidation. The quantitative PCR (qPCR) analysis demonstrated that increased oxidative stress was associated with an increase in the expression of genes that encode components of NADPH oxidase complex, indicating that the production of ROS might be enhanced in BAT and WAT during infection. In addition, some antioxidant markers were reduced in BAT and WAT, consistent with the increase in oxidative stress in acutely and chronically infected mice.
In general, oxidative stress is defined as a steady-state condition where ROS (free radicals) flux is not balanced by antioxidant defense. It is now accepted that inflammation of adipose tissue and the upregulation of oxidative stress play an important role in the pathogenesis of obesity, atherosclerosis, and diabetes and insulin resistance. Adipose tissue is also considered a major source of free radicals and of ROS. This has led to the notion that the increase in oxidative stress may result in the obesity-associated complications. However, it is noteworthy that the mechanistic basis for the linkage of oxidative stress-associated tissue damage to adipose tissue dysfunction is not entirely known.
ROS are critical signaling intermediates linking the innate and adaptive immune systems by triggering the production of pro-inflammatory cytokines (TNF-α, IL-1β) by macrophages and dendritic cells (DCs) of the innate immune system. Inhibition studies with cultured and primary macrophages revealed that NOX/ROS was a critical regulator of cytokine production in response to T. cruzi infection (Dhiman and Garg 2011). In vivo studies using splenocytes of T. cruzi-infected mice, with or without in vitro stimulation with parasite antigens, validated the above observations and demonstrated that the inhibition of NOX by apocynin or use of ROS scavengers substantially blocked the activation and proliferation of phagocytes and inflammatory mediators such as IL-1, IL-6, IFN-γ, and TNF-α (Dhiman and Garg 2011). Subsequently, inhibition of NOX/ROS resulted in an increased susceptibility to T. cruzi, a finding suggesting that redox status plays an important role in immune activation and control of T. cruzi (Garg, unpublished).
A chronic upregulation of pro-oxidants affects the cardiac function in the setting of Chagas disease. Interestingly, studies in experimental models and infected humans demonstrate that an infected host sustains oxidative stress due to T. cruzi-elicited splenic NOX/ROS and the enhanced mitochondrial release of ROS in the myocardium (Gupta et al. 2011). Our studies demonstrated that the host responds to acute T. cruzi infection by upregulating its glutathione antioxidant defense constituted by GPX, GSR, and GSH. However, in the chronic phase, the pro-oxidant milieu of the heart was evidenced by increased ROS levels, decreased activity of MnSOD, insensitivity of glutathione defense to oxidative stress and increased GSSG, and lipid (MDA) and protein (carbonyl) oxidation products (Wen et al. 2004). A similar pro-oxidant status in seropositive humans has been reported and demonstrated by increased GSSG and MDA contents; decreased MnSOD, GPX activity, and GSH contents (Perez-Fuentes et al. 2003; de Oliveira et al. 2007); and inhibition of CIII activity (Wen et al. 2006b). Importantly, the treatment of T. cruzi-infected animals with an antioxidant tipped the balance in favor of preserving mitochondrial and cardiac function. T. cruzi--infected mice and rats, treated with an antioxidant, exhibited a significant decline in the myocardial accumulation of oxidative adducts concurrent with improved mitochondrial function as evidenced by increased ATP synthesis and decreased ROS production (Wen et al 2006b). Thus, preventing oxidative injuries during chronic infection preserved the cardiac hemodynamic state that otherwise was compromised in chagasic rats Wen et al (2010), and others have demonstrated a decline in oxidative stress in chagasic patients given vitamin A (Macao et al. 2007). Collectively, these observations support the idea that antioxidant depletion and inefficient scavenging of ROS, resulting in sustained oxidative stress, are important in the pathogenesis of Chagas disease and in the progression to chagasic cardiomyopathy.
Oxidative stress in adipose tissue has been examined in the setting of obesity and as noted, in the chagasic heart. It has not been investigated in adipose tissue in T. cruzi infection either in animals or humans. Herein, we demonstrate that both in BAT and in WAT obtained from infected mice, markers of oxidative stress were significantly increased as determined by protein carbonylation and lipid peroxidation studies. Previously, we reported that at 15 dpi, when mice are clinically well and there is often no visible parasitemia, pro-inflammatory markers are already elevated and associated with presence of parasites in adipose tissue (Nagajyothi et al. 2012a, b). Similarly, during the chronic stage of the disease in infected CD-1 mice, pro-inflammatory markers as well as markers of oxidative stress are upregulated. Therefore, these findings are associated with evidence of parasite persistence as determined by qPCR (Combs et al. 2005). We believe that the persistence of the parasite in adipose tissue even into the chronic stage is responsible, in part, for the persistence of inflammation and oxidative stress, similar to what has been reported in the obese state.
The relationship between adipose tissue and host metabolism and heart disease has been well-described (Turer et al. 2012). Well-functioning adipose tissue is important to the host. For example, adiponectin, synthesized by healthy adipose tissue, has anti-inflammatory properties. Low levels of adiponectin in adipose tissue and in the blood is associated with an increased pro-inflammatory phenotype as observed in the obese state and in the serum and adipose tissue of T. cruzi-infected mice. Low levels of adiponectin are associated with heart disease and insulin resistance (Turer and Scherer 2010). It is noteworthy that impairment of insulin release has been reported in individuals with chronic Chagas disease (dos Santos et al. 1999; Oliveira et al. 1993; Guariento et al. 1993; Long et al. 1980). Although inflammation and oxidative stress are observed in chagasic heart disease, it is likely that the increased levels of inflammation and oxidative stress in adipose tissue contribute to the cardiac dysfunction observed in Chagas disease.
Acknowledgments
This work was supported in part by the grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases to NJG (AI-054578) and HBT (AI-076248) and from the National Heart Lung Blood Institute to FN (HL-112099). This work was also supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 308980/2011-5; 483168/2011-4) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG; APQ-01738-11) to FSM.
Contributor Information
Jian-Jun Wen, Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX, USA.
Fnu Nagajyothi, Department of Pathology, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461, USA.
Fabiana S. Machado, Program in Health Sciences: Infectious Diseases and Tropical Medicine/Interdisciplinary Laboratory of Medical Investigation, Faculty of Medicine and Department of Biochemistry and Immunology, Institute of Biological Science, Federal University of Minas Gerais, Belo Horizonte, MG, Brazil
Louis M. Weiss, Department of Pathology, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461, USA. Department of Medicine, Albert Einstein College of Medicine, Bronx, NY, USA
Philipp E. Scherer, Departments of Internal Medicine and Cell Biology and the Touchstone Diabetes Center, University of Texas Southwestern, Dallas, TX, USA
Herbert B. Tanowitz, Email: herbert.tanowitz@einstein.yu.edu, Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX, USA. Department of Pathology, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461, USA. Department of Medicine, Albert Einstein College of Medicine, Bronx, NY, USA
Nisha Jain Garg, Email: nigarg@utmb.edu, Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX, USA.
References
- Bondia-Pons I, Ryan L, Martinez JA. Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem. 2012;68:701–711. doi: 10.1007/s13105-012-0154-2. [DOI] [PubMed] [Google Scholar]
- Combs TP, Nagajyothi, Mukherjee S, de Almeida CJ, Jelicks LA, Schubert W, Lin Y, Jayabalan DS, Zhao D, Braunstein VL, Landskroner-Eiger S, Cordero A, Factor SM, Weiss LM, Lisanti MP, Tanowitz HB, Scherer PE. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem. 2005;280:24085–24094. doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- Crujeiras AB, Díaz-Lagares A, Carreira MC, Amil M, Casanueva FF. Oxidative stress associated to dysfunctional adipose tissue: a potential link between obesity, type 2 diabetes mellitus and breast cancer. Free Radic Res. 2013;47:243–256. doi: 10.3109/10715762.2013.772604. [DOI] [PubMed] [Google Scholar]
- de Oliveira TB, Pedrosa RC, Filho DW. Oxidative stress in chronic cardiopathy associated with Chagas disease. Int J Cardiol. 2007;116:357–363. doi: 10.1016/j.ijcard.2006.04.046. [DOI] [PubMed] [Google Scholar]
- Dhiman M, Garg NJ. NADPH oxidase inhibition ameliorates Trypanosoma cruzi-induced myocarditis during Chagas disease. J Pathol. 2011;225:583–596. doi: 10.1002/path.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos VM, da Cunha SF, de Teixeira VP, Monteiro JP, dos Santos JA, dos Santos TA, dos Santos LA, da Cunha DF. Frequency of diabetes mellitus and hyperglycemia in chagasic and non-chagasic women. Rev Soc Bras Med Trop. 1999;32:489–496. doi: 10.1590/s0037-86821999000500004. [DOI] [PubMed] [Google Scholar]
- Enns GM. The contribution of mitochondria to common disorders. Mol Genet Metab. 2003;80:11–26. doi: 10.1016/j.ymgme.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Ferrante AW., Jr Obesity-induced inflammation; a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guariento ME, Saad MJ, Muscelli EO, Gontijo JZ. Heterogeneous insulin response to an oral glucose load by patients with the indeterminate clinical form of Chagas’ disease. Braz J Med Biol Res. 1993;26:491–495. [PubMed] [Google Scholar]
- Gupta S, Dhiman M, Wen JJ, Garg NJ. ROS signaling of inflammatory cytokines during Trypanosoma cruzi infection. Adv Parasitol. 2011;76:153–170. doi: 10.1016/B978-0-12-385895-5.00007-4. [DOI] [PubMed] [Google Scholar]
- Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab. 2008;37:753–768. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Jelicks LA, Tanowitz HB. Advances in imaging of animal models of Chagas disease. Adv Parasitol. 2011;75:193–208. doi: 10.1016/B978-0-12-385863-4.00009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski DA, Randall TD. Adaptive immunity and adipose tissue biology. Trends Immunol. 2010;31:384–390. doi: 10.1016/j.it.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Wehr N, Williams JA, Stadtman ER, Shacter E. Determination of carbonyl groups in oxidized proteins. Methods Mol Biol. 2000;99:15–24. doi: 10.1385/1-59259-054-3:15. [DOI] [PubMed] [Google Scholar]
- Long RG, Albuquerque RH, Prata A, Barnes AJ, Adrian TE, Christofides ND, Bloom SR. Response of plasma pancreatic and gastrointestinal hormones and growth hormone to oral and intravenous glucose and insulin hypoglycaemia in Chagas’ disease. Gut. 1980;21:772–777. doi: 10.1136/gut.21.9.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak ED, Anderson ME. CaMKII oxidative activation and the pathogenesis of cardiac disease. J Mol Cell Cardiol. 2014 doi: 10.1016/j.yjmcc.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macao LB, Filho DW, Pedrosa RC, Pereira A, Backes P, Torres MA, Fröde TS. Antioxidant therapy attenuates oxidative stress in chronic cardiopathy associated with Chagas’ disease. Int J Cardiol. 2007;123:43–49. doi: 10.1016/j.ijcard.2006.11.118. [DOI] [PubMed] [Google Scholar]
- Machado FS, Dutra WO, Esper L, Gollob KJ, Teixeira MM, Factor SM, Weiss LM, Nagajyothi F, Tanowitz HB, Garg NJ. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin Immunopathol. 2012;34:753–770. doi: 10.1007/s00281-012-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdolo G, Piroddi M, Luchetti F, Tortoioli C, Canonico B, Zerbinati C, Galli F, Iuliano L. Oxidative stress and lipid peroxidation byproducts at the crossroad between adipose organ dysregulation and obesity-linked insulin resistance. Biochimie. 2013;95:585–594. doi: 10.1016/j.biochi.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Nagajyothi F, Desruisseaux M, Niranjan T, Weiss LM, Braunstein V, Albanese C, Teixeira M, de Almeida C, Lisanti MP, Scherer PE, Tanowitz HB. Trypanosoma cruzi infection of cultured adipocytes results in an inflammatory phenotype. Obesity. 2008;16:992–997. doi: 10.1038/oby.2008.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagajyothi F, Desruisseaux MS, Jelicks LA, Machado FS, Chua S, Scherer PE, Tanowitz HB. Perspectives on adipose tissue, Chagas disease and implications for the metabolic syndrome. Interdiscip Perspect Infect Dis. 2009;2009:824324. doi: 10.1155/2009/824324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagajyothi FNU, Zhao D, Machado FS, Weiss LM, Schwartz GJ, Desruisseaux MS, Zhao Y, Factor SM, Huang H, Albanese C, Teixeira MM, Scherer PE, Chua SC, Jr, Tanowitz HB. Crucial role of the central leptin receptor in murine Trypanosoma cruzi infection. J Inf Dis. 2010;202:1104–1113. doi: 10.1086/656189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagajyothi F, Desruisseaux MS, Machado FS, Upadhya R, Zhao D, Schwartz GJ, Teixeira MM, Albanese C, Chua SCJ, Weiss LM, Scherer PE, Tanowitz HB. Response of adipose tissue to early infection with Trypanosoma cruzi (Brazil strain) J Inf Dis. 2012a;205:830–840. doi: 10.1093/infdis/jir840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagajyothi F, Machado FS, Burleigh BA, Jelicks LA, Scherer PE, Mukherjee S, Lisanti MP, Weiss LM, Garg NJ, Tanowitz HB. Mechanisms of Trypanosoma cruzi persistence in Chagas disease. Cell Microbiol. 2012b;14:634–643. doi: 10.1111/j.1462-5822.2012.01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagajyothi F, Kuliawat R, Kusminski CM, Machado FS, Desruisseaux MS, Zhao D, Schwartz GJ, Huang H, Albanese C, Lisanti MP, Singh R, Li F, Weiss LM, Factor SM, Pessin JE, Scherer PE, Tanowitz HB. Alterations in glucose homeostasis in a murine model of Chagas disease. Am J Pathol. 2013;182:886–894. doi: 10.1016/j.ajpath.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Oliveira LC, Juliano Y, Novo NF, Neves MM. Blood glucose and insulin response to intravenous glucose by patients with chronic Chagas’ disease and alcoholism. Braz J Med Biol Res. 1993;26:1187–1190. [PubMed] [Google Scholar]
- Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, Roth KA, Kitsis RN, Scherer PE. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med. 2005;11:2005–2803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]
- Penfornis P, Marette A. Inducible nitric oxide synthase modulates lipolysis in adipocytes. J Lipid Res. 2005;46:135–142. doi: 10.1194/jlr.M400344-JLR200. [DOI] [PubMed] [Google Scholar]
- Perez-Fuentes R, Guegan JF, Barnabe C, Lopez-Colombo A, Salgado-Rosas H, et al. Severity of chronic Chagas disease is associated with cytokine/antioxidant imbalance in chronically infected individuals. Int J Parasitol. 2003;33:293–299. doi: 10.1016/s0020-7519(02)00283-7. [DOI] [PubMed] [Google Scholar]
- Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709–716. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: evaluation of adiponectin targeted drug development strategies. Trends Pharmacol Sci. 2009;30:234–239. doi: 10.1016/j.tips.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2010;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- Turer AT, Hill JA, Elmquist JK, Scherer PE. Adipose tissue biology and cardiomyopathy: translational implications. Circ Res. 2012;111:1565–1577. doi: 10.1161/CIRCRESAHA.111.262493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J-J, Vyatkina G, Garg NJ. Oxidative damage during chagasic cardiomyopathy development: role of mitochondrial oxidant release and inefficient antioxidant defense. Free Radic Biol Med. 2004;37:1821–1833. doi: 10.1016/j.freeradbiomed.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Wen JJ, Bhatia V, Popov VL, Garg NJ. Phenyl-alpha-tert-butyl nitrone reverses mitochondrial decay in acute Chagas’ disease. Am J Pathol. 2006a;169:1953–1964. doi: 10.2353/ajpath.2006.060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J-J, Yachelini PC, Sembaj A, Manzur RE, Garg NJ. Increased oxidative stress is correlated with mitochondrial dysfunction in chagasic patients. Free Radic Biol Med. 2006b;41:270–276. doi: 10.1016/j.freeradbiomed.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Wen JJ, Dhiman M, Whorton EB, Garg NJ. Tissue-specific oxidative imbalance and mitochondrial dysfunction during Trypanosoma cruzi infection in mice. Microbes Infect. 2008;10:1201–1209. doi: 10.1016/j.micinf.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen JJ, Gupta S, Guan Z, Dhiman M, Condon D, Lui C, Garg NJ. Phenyl-alpha-tert-butyl-nitrone and benzonidazole treatment controlled the mitochondrial oxidative stress and evolution of cardiomyopathy in chronic chagasic rats. J Am Coll Cardiol. 2010;55:2499–2508. doi: 10.1016/j.jacc.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]