Abstract
Objectives
To determine if supra-threshold measures of auditory function, such as distortion-product otoacoustic emissions (DPOAEs) and auditory brainstem responses (ABRs), are correlated with noise exposure history in normal-hearing human ears. Recent data from animal studies have revealed significant deafferentation of auditory nerve fibers following full recovery from temporary noise-induced hearing loss (NIHL). Furthermore, these data report smaller ABR wave I amplitudes in noise-exposed animal ears when compared to non-noise exposed control animals or pre-noise exposure amplitudes in the same animal. It is unknown if a similar phenomenon exists in the normal-hearing, noise-exposed human ear.
Design
Thirty normal-hearing human subjects with a range of noise exposure backgrounds (NEBs) participated in this study. NEB was quantified by the use of a noise exposure questionnaire that extensively queried loud sound exposure over the previous 12 months. DPOAEs were collected at three f2’s (1, 2, and 4 kHz) over a range of L2’s. DPOAE stimulus level began at 80 dB FPL (forward-pressure level) and decreased in 10 dB steps. Two-channel ABRs were collected in response to click stimuli and 4 kHz tone bursts; one channel utilized an ipsilateral mastoid electrode and the other an ipsilateral tympanic membrane (TM) electrode. ABR stimulus level began at 90 dB nHL and was decreased in 10 dB steps. Amplitudes of waves I and V of the ABR were analyzed.
Results
A statistically significant relationship between ABR wave I amplitude and NEB was found for clicked-evoked ABRs recorded at a stimulus level of 90 dB nHL using a mastoid recording electrode. For this condition, ABR wave I amplitudes decreased as a function of NEB. Similar systematic trends were present for ABRs collected in response to clicks and 4 kHz tone bursts at additional supra-threshold stimulation levels (≥ 70 dB nHL). The relationship weakened and disappeared with decreases in stimulation level (≤ 60 dB nHL). Similar patterns were present for ABRs collected using a TM electrode. However, these relationships were not statistically significant and were weaker and more variable than those collected using a mastoid electrode. In contrast to the findings for ABR wave I, wave V amplitude was not significantly related to NEB. Furthermore, there was no evidence of a systematic relationship between supra-threshold DPOAEs and NEB.
Conclusions
A systematic trend of smaller ABR wave I amplitudes was found in normal-hearing human ears with greater amounts of voluntary NEB in response to supra-threshold clicks and 4 kHz tone bursts. These findings are consistent with data from previous work completed in animals, where the reduction in supra-threshold responses was a result of deafferentation of high-threshold/low-spontaneous rate auditory nerve fibers. These data suggest a similar mechanism might be operating in human ears following exposure to high sound levels. However, evidence of this damage is only apparent when examining supra-threshold wave I amplitude of the ABR. In contrast, supra-threshold DPOAE level was not significantly related to NEB. This was expected, given noise-induced auditory damage findings in animal ears did not extend to the outer hair cells, the generator for the DPOAE response.
Keywords: noise exposure, auditory brainstem response, distortion-product otoacoustic emissions
INTRODUCTION
Noise-induced hearing loss (NIHL) affects approximately 15 percent of Americans 20 to 69 years of age (NIDCD, 2001). While the detrimental effects of noise on the auditory system have been well documented (e.g., Spoendlin, 1971; Saunders et al., 1985; Bohne & Harding, 2000; Nordmann et al., 2000; Kujawa & Liberman, 2009), the relationship between the amount of acoustic exposure and the resulting anatomic and physiologic damage is variable, even in highly controlled studies.
Clinical protocols for assessing NIHL have traditionally relied on the evaluation of behavioral thresholds, where the hallmark of NIHL is a high frequency 3–6 kHz notching audiometric pattern. It is widely accepted that permanent thresholds shifts (PTS) following noise exposure are a result of permanent damage to auditory structures. An assumption underlying the concept of a temporary threshold shift (TTS) is that following full recovery of threshold(s), no residual anatomical damage is present and the temporary decrease in hearing has been essentially harmless (Humes et al., 2005; Kujawa & Liberman, 2009).
Recent investigations in mice (Kujawa & Liberman, 2009) and guinea pigs (Lin et al., 2011; Furman et al., 2013) challenge the view that temporary NIHL does not result in permanent auditory damage and also suggest current clinical testing protocols may be insensitive to detecting evidence of early auditory damage. Kujawa and colleagues induced a temporary NIHL (up to a 40 dB loss) in mice and guinea pig ears. Following threshold recovery, auditory function was assessed via auditory brainstem responses (ABRs) and distortion-product otoacoustic emissions (DPOAEs). Anatomical damage was evaluated by examining the outer hair cells (OHCs), the inner hair cells (IHCs) and their nerve terminal connections (i.e., synaptic ribbons). Results demonstrated an abrupt, permanent loss of up to 50% of afferent nerve terminal connections between IHCs and auditory nerve fibers in the frequency region of maximum TTS. Despite substantial deafferentation, ABR thresholds demonstrated full recovery to pre-noise exposure levels. Threshold responses have been shown to be relatively insensitive to large changes in the auditory nerve fiber population (Earl & Chertoff, 2010) because they are dependent upon synchronous firing of neuronal fibers but at a criterion response only slightly above the noise floor. Therefore, in recent findings from noise-exposed animals (Kujawa & Liberman, 2009; Lin et al., 2011; Furman et al., 2013), the remaining undamaged IHC afferent connections proved sufficient to preserve the threshold response, even in the presence of a substantial loss of synaptic ribbons.
At supra-threshold stimulation levels (> 40 dB SPL), ABR wave I amplitude was significantly smaller in the noise-exposed animals relative to either control animals or pre-exposure baselines. Furman et al. (2013) reported specific loss of auditory nerve fiber populations with low spontaneous rates and high thresholds, further suggesting that temporary NIHL selectively damages auditory nerve fibers that contribute to high-level amplitude responses. Therefore, ABR supra-threshold responses demonstrated better sensitivity at revealing auditory damage than threshold responses.
In contrast to the damage seen in the IHC afferent connections, the OHCs appeared undamaged upon anatomical assessment. Not surprisingly, there were no differences in DPOAE threshold or supra-threshold responses in noise-exposed animal ears relative to controls (Kujawa & Liberman, 2009; Lin et al., 2011; Furman et al., 2013).
These investigations (Kujawa & Liberman, 2009; Lin et al., 2011; Furman et al., 2013) contradict the assumption that no permanent anatomical damage occurs with TTS. Permanent anatomical damage likely does not occur with all episodes of TTS (Fernandez et al., 2014). It is not clear what specific factors distinguish TTS episodes that yield permanent damage from those that do not. However, when permanent anatomical damage is present, the data from Kujawa and colleagues suggest the use of supra-threshold stimuli may provide evidence of early-onset noise-induced auditory damage that is not yet evident in threshold assessment. This is of clinical importance because the current gold standard for NIHL assessment is based on the determination of threshold. These data are also provocative because high-threshold, low-spontaneous rate auditory nerve fibers may be important for hearing in noisy environments, partially due to their resistance to masking in background noise (Costalupes et al., 1984; Lopez-Poveda & Barrios, 2013). Individuals with damage to these fibers might report difficulty hearing in noise, even in the presence of normal behavioral thresholds. Difficulty hearing in noise, tinnitus, and hyperacusis have all been reported in noise-exposed human ears with normal behavioral thresholds (Sanchez et al., 2005; Muhr & Rosenhall, 2010). Based on the work of Kujawa and colleagues, it appears possible to have normal behavioral thresholds in the absence of “normal” auditory function.
Several investigations have described the use of OAEs in humans as an early indicator of noise-induced damage or as a method to determine if an individual is at risk for developing NIHL (Attias et al., 2001; Lapsley Miller et al., 2006; Marshall et al., 2009). Other reports have not found evidence to support OAEs in this context (Seixas et al., 2012). While the data reported by Kujawa and colleagues (as discussed above) suggest that OAEs (and OHCs) are affected in the TTS stage, the location of permanent damage appears to be in the ribbon synapses between IHCs and auditory nerve fibers and not in the OHCs. This suggests that OAE assessment may not be sensitive to the damage that would be present after TTS has recovered and before behavioral threshold is permanently affected.
There is some evidence of reduced wave I amplitude in human ears not related to changes in behavioral threshold in aging ears (Konrad-Martin et al., 2012) and in normal-hearing ears with tinnitus (Schaette & McAlpine, 2011). Variability is commonly seen in ABR response amplitude, even in normal-hearing ears (Schwartz et al., 1994). In light of the recent animal data relative to wave I amplitude, it is possible some variability could be due to differences in noise exposure history. A concern regarding ABR wave I assessment is that it can be difficult to visualize at low stimulus levels. Enhancement of wave I can be achieved by using a slower presentation rate and an ear canal or TM electrode (Ferraro & Ferguson, 1989; Schwartz et al., 1994; Hall, 2007b; Gaddam & Ferraro, 2008). Further investigation of ABR responses, particularly wave I, in normal-hearing, noise-exposed human ears is warranted.
In order to investigate the auditory responses in noise-exposed human ears, it is necessary to have some metric to quantify the amount of noise exposure an individual has encountered in their daily life. However, defining the noise exposure background (NEB) in human subjects is not a simple undertaking and there is no universally accepted approach. Questionnaires can be helpful in quantifying the amount of NEB based on the type and duration of noise exposures reported.
A questionnaire was developed by Neitzel and colleagues to quantify the annual amount of non-occupational noise exposure in construction workers (Neitzel et al., 2004a,b; Reeb-Whitaker et al., 2004). This questionnaire was used in conjunction with daily activity logs and personal dosimetry to provide a description of the total occupational and non-occupational noise exposure in construction workers. Estimates of noise exposure through the questionnaire were shown to be no different from estimates obtained with daily activity logs and dosimetry measurements, indicating that construction workers were able to accurately recall their exposure to noise (Reeb-Whitaker et al., 2004).
The questionnaire included questions pertaining to the frequency (‘how often’) of noise exposures during ‘routine’ (home, travel, shopping, etc.) and ‘episodic’ activities (using power tools, attending sporting events, etc.) over 6760 h (the number of hours spent annually in non-occupational activities, assuming a 40 h work week for 50 wks/y). The questionnaire by Neitzel and colleagues was expanded by Megerson (2010) to include questions pertaining to musical activities (musical instrument playing, music listening via personal earphones, and music listening via audio speakers) and occupational noise exposures. The modifications by Megerson also included specific queries regarding the duration of each exposure (‘how long’). The Megerson noise exposure questionnaire (NEQ) yields a LAeq8760h value which can be interpreted as an annual estimate of noise exposure. While self-report questionnaires are imperfect in nature by being subject to recall accuracy and recall bias (Coughlin, 1990), they can be helpful in describing the noise exposure history for an individual.
In summary, recent evidence suggests exposure to loud sound may be more dangerous than previously believed and that current assessment protocols may be insensitive to detecting evidence of early noise-induced auditory damage (Kujawa & Liberman, 2009; Lin et al., 2011; Furman et al., 2013). However, as these studies were completed in the mouse and guinea pig ear, further investigation is needed prior to generalizing findings to the human ear.
The purpose of the present study was to characterize supra-threshold cochlear and auditory nerve function among a group of normal-hearing human ears with different amounts of NEB, defined as the value obtained on the Megerson NEQ (in LAeq8760h). It was hypothesized that supra-threshold ABR wave I amplitude would decrease as the level of NEB increases. It was also hypothesized that supra-threshold DPOAE level would not differ across ears with varying amounts of NEB.
METHODS
Subjects
Data were collected from one ear of 30 normal-hearing subjects (20 female) ranging in age from 19 to 28 years of age (mean = 22.8 years). While all subjects had normal hearing, specific attention was directed at recruiting individuals with varying amounts of NEB. Many subjects who reported higher NEB were recruited from music departments of local universities. Normal hearing was defined as pure-tone behavioral thresholds ≤ 20 dB HL (re: ANSI, 2004) for the octave and interoctave frequencies between 0.25 and 8 kHz. Normal middle-ear function was defined as a normal 226-Hz tympanogram. Each subject’s pure-tone thresholds were assessed at the first test session and middle-ear function was assessed (and required to be normal) at both data-collection sessions. Behavioral thresholds and middle-ear function were assessed in both ears of each subject. If the test ear could not be selected based on audiometric or tympanometric results, the test ear was selected as the ear that had the greatest amount of noise exposure or was chosen at random if noise exposure was similar between the two ears.
Subject Sample Size
Since it was unknown if a relationship between NEB and ABR amplitude existed in human ears, the decision was made to power the study based on the expected findings for a single, high stimulus level. If the mechanism of damage observed in the mouse and guinea pig ear operates in a similar fashion in human ears, evidence of damage would be apparent at this high stimulus level. Specifically, the study was powered based on the expected relationship between NEB and ABR wave I amplitude to click stimuli presented at 90 dB nHL. A power analysis was performed (Statistical Analysis Software, v. 9.3, Cary, NC) and indicated, for a significance level of α=0.05, 30 subjects were sufficient to achieve a power of approximately 80%.
Procedures
Data were collected in two testing sessions. Both sessions occurred within 1 or 2 wk(s) of each other and subjects were asked to refrain from participating in any high-noise activity the night prior to the testing session. The first testing session (1 h) consisted of consenting procedures, audiometric evaluation, and assessment of NEB. The second session (4 h) consisted of DPOAE and ABR testing. At the second session, subjects reclined in a comfortable chair and slept or relaxed during data collection. This study was approved by the Institutional Review Board/Human Subjects Committee at the University of Kansas Medical Center and signed consents were obtained from all participants.
Assessment of Noise Exposure Background
NEB was assessed via a self-report questionnaire (the NEQ) developed by Megerson (2010). The NEQ yields a value that is an estimate of the annual amount of noise exposure in LAeq8760h. Here, “L” represents sound pressure level in dB, “A” represents use of an A-weighted frequency response, “eq” represents a 3-dB exchange rate for calculation of the time/level relationship, and “8760h” represents the total duration of the noise exposure in hours over 1 y (365 d/y × 24 h/d). The NEQ assesses nine specific known high-noise situations (power tools, heavy equipment/machinery, commercial sporting/entertainment events, motorized vehicles, small/private aircraft, musical instrument playing, music listening via personal earphones, music listening via audio speakers, occupational). The frequency (i.e., how often) and duration (i.e., how long) of noise exposures were queried. See supplemental Appendix for further detail on the questionnaire and computation of LAeq8760h.
NEB was assessed at the first data collection session (prior to any DPOAE and ABR testing) and was not re-evaluated during or after the second data collection session. This was done to ensure knowledge of auditory response behavior did not influence the determination of NEB. In other words, NEQ values were never changed after DPOAE/ABR testing was complete and no subject was excluded after DPOAE/ABR data were collected.
DPOAE Testing Protocol
All DPOAE data were collected using custom-designed software (EMAV, Neely & Liu, 1993) that controlled a 24-bit soundcard (CardDeluxe, Digital Audio Labs, Chanhassen, MN) housed in a PC. A probe microphone (ER-10C, Etymotic Research, Elk Grove, IL) was used to calibrate and present stimuli and to record emissions. Calibration was completed using the forward-pressure level (FPL) technique outlined by Scheperle et al. (2008, 2011) which has been shown to reduce variability across repeated testing sessions. The FPL calibration technique estimates the Thévenin-equivalent acoustic properties of the probe microphone (i.e., the source) and allows for isolation of the incident from the reflected components of the calibration signal. Prior to each subject’s testing session, a wideband chirp stimulus was presented to five brass tubes. The pressure response in each tube was measured and used to estimate source impedance and pressure. Source impedance and pressure are needed to estimate load impedance, which is necessary to convert SPL to FPL.
DPOAEs were recorded as level functions in response to pairs of primary tones (f1, f2; f1 < f2) for f2 frequencies of 1, 2 and 4 kHz. The level of f2(L2) began at 80 dB FPL and descended in 10 dB steps down to 0 dB FPL. The level of f1(L1) and the f2/f1 ratio were determined based on stimulus parameters shown to result in increased test performance from Kirby et al. (2011):
Measurement-based stopping rules were used during data collection such that, for each condition, averaging continued until the noise level was ≤ −25 dB SPL or a maximum of 32 s of artifact-free averaging had occurred. Noise levels were estimated from the 2f1-f2 frequency bin plus five bins on either side of this frequency. Estimation of noise level in the 2f1-f2 bin was accomplished by alternately storing 0.25-s samples of the response in one of two buffers. The buffers were summed to provide an estimate of the response level (at 2f1-f2) and were subtracted to provide the noise estimate. The use of measurement-based stopping rules allowed the averaging time to increase whenever the noise level exceeded −25 dB SPL. As a result, we were able to achieve consistent noise levels across subjects and conditions, while maximizing the efficiency with which data were collected for conditions where the noise level was ≤ −25 dB SPL.
ABR Protocol
ABR testing was performed using a commercial system (System 3, Tucker-Davis Technologies, Alachua, FL) in a single-walled, sound attenuated booth. Stimulus level calibration in dB nHL was achieved by measuring behavioral thresholds to each stimulus in 10 normal-hearing adults. ABR stimuli were presented with alternating polarity at a rate of 11.3/s via insert earphones (E-A-RTONE 3A, 3M, St. Paul, MN). ABRs were collected using 100-μs clicks (0 dB nHL = 31 dB ppe SPL) and Blackman-gated tones at 4000 Hz (1-ms rise/fall, 0 dB nHL = 21 dB ppe SPL). Presentation level began at 90 dB nHL and decreased in 10 dB steps down to 10 dB below threshold.
Recording parameters included a gain of 5100 (the use of a battery powered preamplifier and a fiber optic cable allowed for the use of less amplification than is typically seen in ABR recordings) and band-pass filtering from 100 to 3000 Hz. ABRs were collected during a 20-ms time window. Noninverting electrodes were placed on the ipsilateral TM (commercially available electrode from Sanibel Supply, Eden Prairie, MN) and ipsilateral mastoid. An inverting electrode was placed on the subject’s high forehead (Fz) and the ground electrode was placed on the contralateral mastoid. Prior to analysis, all waveforms were inverted to allow for the typical vertex positive orientation. At each stimulus level, two replications of 2000 stimulus repetitions were collected and the averaged waveform was used for analysis.
Two independent judges separately identified peak to trough amplitudes of waves I and V using visual overlay cursors on a computer screen. The first judge analyzed the waveforms during data collection and was therefore not blinded to subject information (i.e., NEB). The second judge was blinded to subject information during ABR waveform analyses. Any inter-scorer disagreements between the two judges were resolved by reviewing the data together.
Data Analyses
Linear regression techniques were used to characterize relationships between DPOAE and ABR data and NEB. All statistical procedures were completed using commercially available software (IBM SPSS Statistics v. 20, Armonk, NY; Microsoft Excel 2010, Redmond, WA).
RESULTS
Variation of NEB Across Subjects
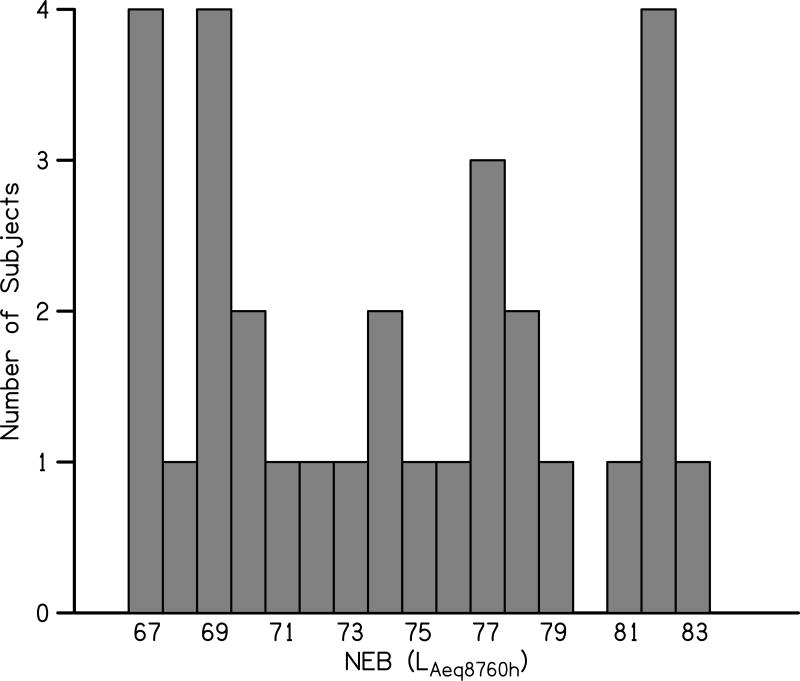
A histogram displaying the range of NEQ values (rounded to the nearest integer) obtained across the 30 study participants is shown in Fig. 1. The NEQ has a theoretical range of 64 to 95.5 LAeq8790h. In the present study, NEQ values ranged from 67 to 83 LAeq8790h. This demonstrates that the NEQ values in the present study span a wide range and indicates variation in NEB across subjects. While no subjects had NEQs close to the upper theoretical limit, it is unlikely that an individual scoring near the theoretical upper end would have normal hearing.
Figure 1.
Variation in NEB across study participants.
Table 1 displays subject NEQ values along with additional subject characteristics such as behavioral threshold and ABR amplitude. Here, subjects are arranged by their NEQ value, with NEQ increasing from top to bottom in the table. The relationship between NEQ value, behavioral threshold, and ABR amplitude will be discussed later in this paper.
Table 1.
Characteristics of Study Participants. Subjects are ordered by NEQ value (rounded to the nearest integer). Subject number and ABR amplitudes correspond to waveforms displayed in Fig. 3.
| NEQ | Age | Behavioral Threshold (dB HL) | ABR Amplitude (nV) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| .25 | .5 | .75 | 1k | 1.5k | 2k | 3k | 4k | 6k | 8k | Wave I | Wave V | |||
| 1 | 67 | 23 | 5 | 10 | 5 | 10 | 0 | 5 | 15 | 10 | 10 | 10 | 518.36 | 789.59 |
| 2 | 67 | 22 | 5 | 0 | −5 | 0 | 0 | 0 | −5 | 0 | 0 | −5 | 572.48 | 989.03 |
| 3 | 67 | 26 | 0 | 0 | 5 | 0 | 10 | 0 | 10 | 5 | 0 | 0 | 561.14 | 595.92 |
| 4 | 67 | 23 | 0 | 0 | 5 | 15 | 10 | 10 | 5 | 15 | 15 | 15 | 586.01 | 733.96 |
| 5 | 68 | 24 | 5 | 5 | 15 | 5 | 5 | 0 | 5 | 10 | 5 | 0 | 401.20 | 909.18 |
| 6 | 69 | 21 | 5 | 5 | 5 | 0 | 5 | 0 | 5 | 5 | 5 | 5 | 611.00 | 641.02 |
| 7 | 69 | 23 | 5 | 10 | 5 | 5 | 0 | 10 | 5 | −5 | 5 | 0 | 554.06 | 512.33 |
| 8 | 69 | 23 | 10 | 10 | 10 | 15 | 10 | 5 | 10 | 10 | 5 | 5 | 529.70 | 500.27 |
| 9 | 69 | 23 | 5 | 5 | 5 | 5 | 10 | 15 | 15 | 5 | 10 | 5 | 781.23 | 535.76 |
| 10 | 70 | 25 | 10 | 15 | 20 | 15 | 10 | 10 | 15 | 10 | 10 | 10 | 344.90 | 723.62 |
| 11 | 70 | 22 | 5 | 10 | 5 | 0 | 0 | 10 | 0 | 5 | 5 | 5 | 224.68 | 771.96 |
| 12 | 71 | 22 | 10 | 5 | 5 | 0 | 10 | 15 | 5 | 5 | 5 | 10 | 336.70 | 453.97 |
| 13 | 72 | 19 | 0 | 0 | 0 | 0 | 0 | −5 | −5 | 0 | 0 | −10 | 671.68 | 519.09 |
| 14 | 73 | 21 | 0 | 0 | 0 | 0 | −5 | 0 | 5 | 0 | 0 | 5 | 411.65 | 709.63 |
| 15 | 74 | 21 | 15 | 5 | 10 | 10 | 10 | 5 | 5 | 5 | 5 | 5 | 299.87 | 1110.99 |
| 16 | 74 | 26 | 10 | 10 | 5 | 10 | 0 | 5 | 10 | 5 | 5 | 10 | 555.25 | 788.44 |
| 17 | 75 | 23 | 0 | 0 | 0 | 5 | 5 | 5 | 10 | 0 | −10 | −10 | 488.12 | 568.29 |
| 18 | 76 | 21 | 5 | 5 | 5 | 5 | −5 | −5 | 5 | 5 | 10 | −5 | 416.18 | 654.78 |
| 19 | 77 | 23 | 10 | 10 | 5 | 5 | 0 | 5 | 0 | 5 | 0 | −5 | 167.47 | 457.04 |
| 20 | 77 | 19 | 5 | 5 | 0 | 0 | 5 | 5 | 10 | 0 | −10 | −10 | 372.08 | 673.21 |
| 21 | 77 | 24 | 5 | 5 | 5 | 5 | 0 | 0 | 0 | 5 | 0 | 10 | 308.55 | 626.42 |
| 22 | 78 | 21 | 5 | 10 | 5 | 0 | 0 | 0 | −5 | −5 | −5 | −5 | 249.22 | 765.50 |
| 23 | 78 | 21 | 0 | 5 | 10 | 10 | 10 | 0 | 5 | 5 | 15 | 5 | 372.90 | 755.49 |
| 24 | 79 | 20 | 5 | 5 | 10 | 5 | 0 | 5 | 10 | 20 | 20 | 10 | 485.81 | 287.33 |
| 25 | 81 | 28 | 5 | 10 | 5 | 10 | 0 | 0 | 5 | 5 | 10 | 10 | 387.80 | 868.44 |
| 26 | 82 | 23 | 15 | 10 | 5 | 5 | 5 | 0 | 0 | 5 | 20 | 15 | 308.14 | 638.80 |
| 27 | 82 | 21 | 0 | 0 | 0 | 0 | 10 | 0 | 5 | 5 | 5 | 0 | 335.14 | 723.79 |
| 28 | 82 | 20 | 10 | 5 | 10 | 5 | 5 | 0 | 0 | 0 | −5 | −5 | 564.29 | 760.78 |
| 29 | 82 | 27 | 0 | 0 | −5 | 0 | 5 | 0 | −5 | 0 | 5 | 10 | 313.61 | 219.43 |
| 30 | 83 | 28 | 10 | 10 | 10 | 5 | 5 | 5 | 0 | 5 | 5 | 5 | 447.64 | 553.45 |
Influence of NEB on Auditory Function
DPOAE Level
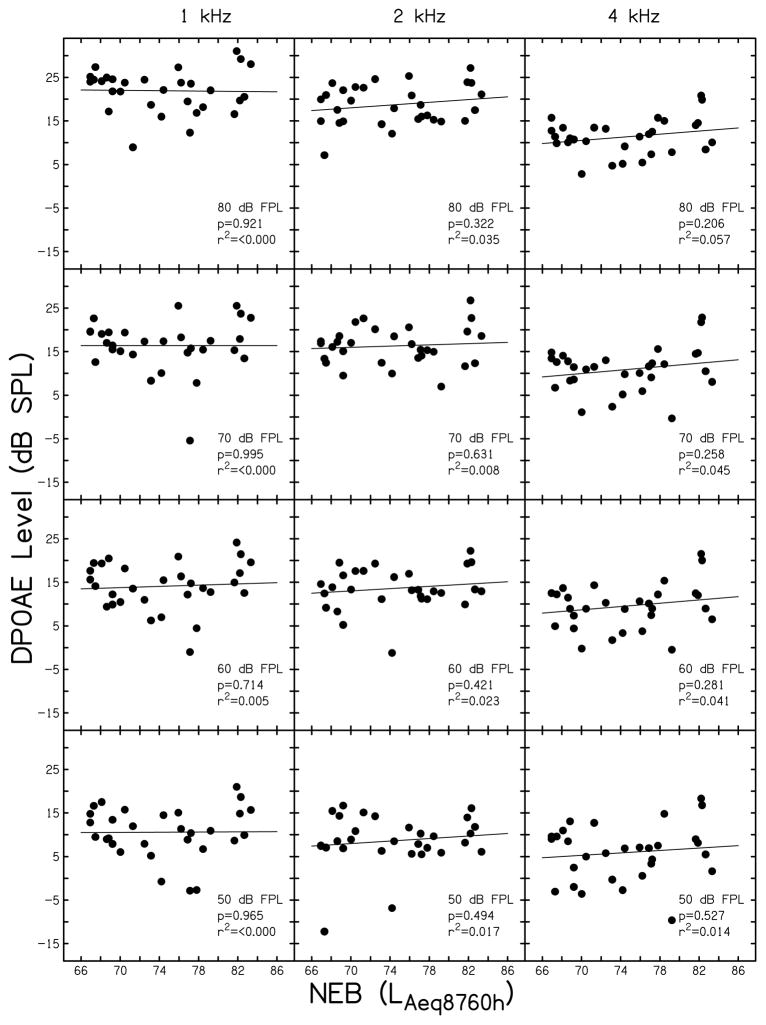
Supra-threshold DPOAE level (Ld) is plotted as a function of NEB in Fig. 2. Data are provided for L2’s of 80 dB FPL (top row), 70 dB FPL (second row), 60 dB FPL (third row) and 50 dB FPL (bottom row) for each of the f2’s assessed (1 kHz, left column; 2 kHz, middle column; 4 kHz, right column). Data were available from all participants for each condition shown (n=30). All data shown in Fig. 2 met a signal-to-noise ratio (SNR) criterion of ≥ 6 dB.
Figure 2.
Supra-threshold DPOAE level. DPOAE level (in dB SPL) is plotted is a function of NEB (as defined by the NEQ score) for f2’s of 1 kHz (left column), 2 kHz (middle column), and 4 kHz (right column). The level (L2) is inset in each panel (80 to 50 dB FPL). Linear regression analysis was completed in each panel and the resulting regression line, p-value, and r2 are shown.
Linear regression analyses for the data shown in Fig. 2 (results shown as insets in each panel) did not reveal a systematic relationship between NEB and DPOAE level at any stimulus condition assessed (p-values were > 0.05 and r2 was < 0.06 for all comparisons). Although not shown in Fig. 2, the relationship between DPOAE level and NEB was assessed for L2 < 50 dB FPL (as low as 10–20 dB FPL, the point where SNRs typically fell below 6 dB). The results for L2 < 50 dB FPL were similar to those shown in Fig. 2. Specifically, no systematic trends were observed and no statistically significant relationships were found. These data suggest that DPOAE level for L2’s between 20 and 80 dB FPL did not vary as a function of NEB, at least for the noise exposures reported by the subjects tested here.
ABR Amplitudes
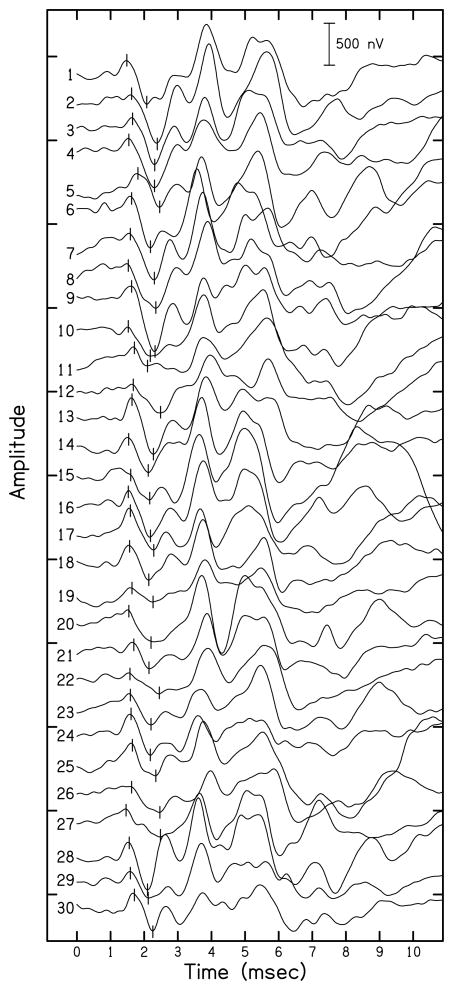
ABR waveforms recorded with a mastoid electrode for each study participant in response to click stimuli presented at 90 dB nHL are shown in Fig. 3. Waveforms are ordered according to NEB (with NEB increasing from top to bottom in the figure) and the numbers correspond to those listed in Table 1. Vertical lines indicate ABR wave I peaks and troughs for each waveform. Wave I and V amplitudes for the waveforms displayed in Fig. 3 are listed in Table 1. In general, wave I amplitude decreased with increasing NEB, although variability across participants was present. In contrast, wave V does not appear to change systematically with NEB. A more detailed analysis of these relationships is presented below.
Figure 3.
ABR waveform series for click-evoked ABRs presented at 90 dB nHL collected with a mastoid electrode. Vertical lines indicate wave I peak and trough for each subject. Amplitudes are shown in nV with a scale bar present in the upper right corner.
Table 2 lists means and standard deviations (SD) for amplitude and latency of ABR waves I and V recorded in response to click and 4 kHz tone burst stimuli presented at 80 and 90 dB nHL. Data were available from 30 subjects at each condition. The coefficient of variation (CV), or the ratio of the SD to the mean amplitude (i.e., 100 × [SD/mean amplitude]), is also displayed in Table 2 for ABR amplitude. The latencies and amplitudes of the ABR responses from the present study are in agreement with ABR data available in the literature for normal-hearing human ears (e.g., Hall, 2007b; Konrad-Martin et al., 2012). As expected, wave I amplitude measured with a TM electrode was larger when compared to wave I amplitude collected with a mastoid electrode. Additionally, the SD and CV of wave I amplitude collected with a TM electrode was larger when compared to responses collected with a mastoid electrode; this indicates greater variability in ABR wave I amplitudes measured with a TM electrode. In contrast to wave I amplitude, there were slightly larger wave V amplitudes measured with a mastoid recording electrode when compared to a TM electrode. Electrode recording site did not appear to have any influence on the latency of wave I or wave V.
Table 2.
Descriptive statistics for ABR latency and amplitude. Mean and standard deviations (SD) are shown for ABR latency (ms) and amplitude (nV) for each ABR recording condition at 90 and 80 dB nHL. For ABR amplitude (bottom panel), the coefficient of variation (CV) is denoted in parentheses.
| ABR Recording Condition
|
||||
|---|---|---|---|---|
| 4 kHz tone bursts | Click | |||
|
| ||||
| Latency | Mean | SD | Mean | SD |
| Wave I 90 dB nHL | ||||
| TM | 1.99 | 0.08 | 1.58 | 0.07 |
| Mastoid | 2.00 | 0.09 | 1.60 | 0.10 |
|
| ||||
| Wave I 80 dB nHL | ||||
| TM | 2.16 | 0.09 | 1.64 | 0.08 |
| Mastoid | 2.16 | 0.10 | 1.65 | 0.09 |
|
| ||||
| Wave V 90 dB nHL | ||||
| TM | 5.95 | 0.22 | 5.49 | 0.17 |
| Mastoid | 5.96 | 0.17 | 5.52 | 0.18 |
|
| ||||
| Wave V 80 dB nHL | ||||
| TM | 6.03 | 0.20 | 5.57 | 0.17 |
| Mastoid | 6.04 | 0.18 | 5.59 | 0.17 |
| 4 kHz tone bursts | Click | |||
|---|---|---|---|---|
|
| ||||
| Amplitude | Mean | SD (CV) | Mean | SD (CV) |
| Wave I 90 dB nHL | ||||
| TM | 816.97 | 334.26 (40.91) | 870.29 | 314.56 (36.14) |
| Mastoid | 386.88 | 141.30 (36.52) | 428.01 | 153.73 (35.92) |
|
| ||||
| Wave I 80 dB nHL | ||||
| TM | 738.76 | 316.14 (42.79) | 769.13 | 329.61 (42.85) |
| Mastoid | 367.66 | 134.84 (36.68) | 397.29 | 136.77 (34.43) |
|
| ||||
| Wave V 90 dB nHL | ||||
| TM | 359.07 | 108.59 (36.68) | 540.49 | 135.38 (25.05) |
| Mastoid | 459.43 | 149.53 (30.24) | 661.25 | 188.89 (28.57) |
|
| ||||
| Wave V 80 dB nHL | ||||
| TM | 324.48 | 115.82 (35.69) | 494.75 | 142.04 (28.71) |
| Mastoid | 395.39 | 130.58 (33.03) | 583.78 | 187.37 (32.10) |
Wave I
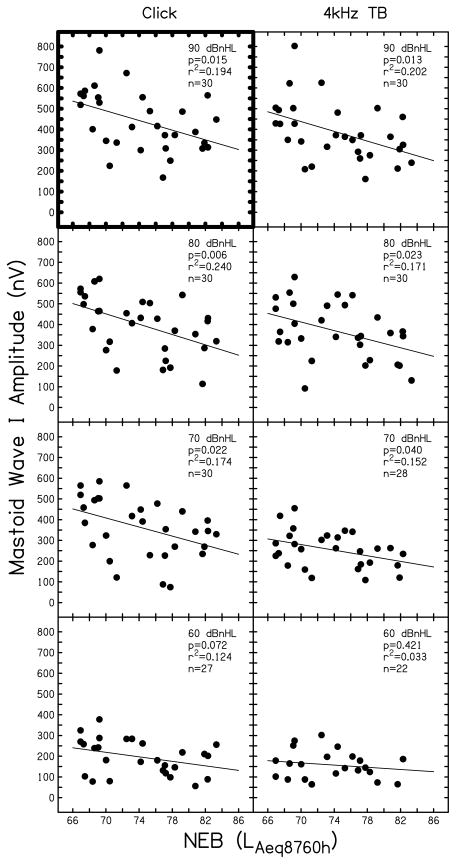
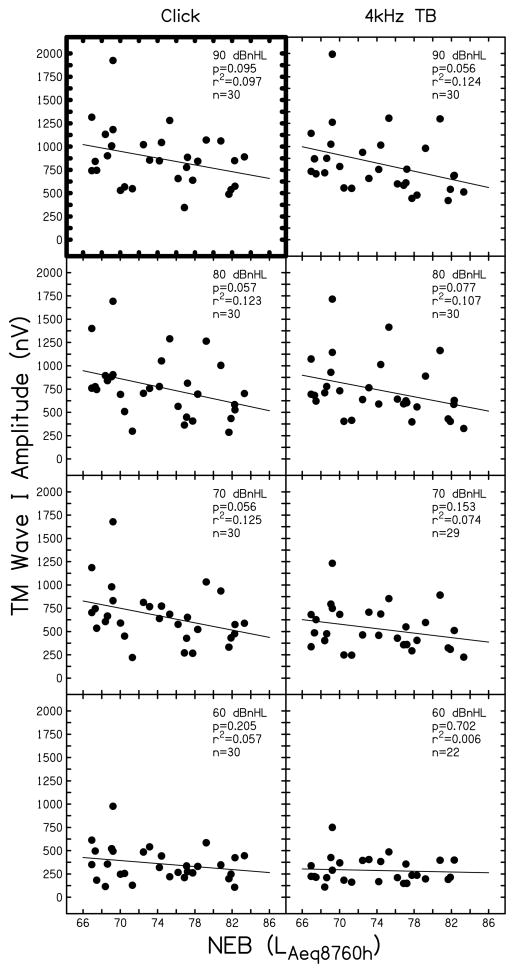
Supra-threshold ABR wave I amplitude is displayed as a function of NEB in Figs. 4 (mastoid electrode site) and 5 (TM electrode site) for click (left column) and 4 kHz tone burst (right column) stimuli. Signal level (from 90 to 60 dB nHL) is indicated in the upper right portion of each panel. At each condition, linear regression analysis was completed; the results are inset in each panel (p-value and r2) along with the number of subjects that contributed data (n). As stated previously, this study was powered (using α=.05) to evaluate the relationship between NEB and ABR wave I amplitude to click stimuli at 90 dB nHL. The two comparisons available for this condition (data collected using a mastoid and TM electrode) are highlighted in Figs. 4 and 5 by dark outlines on the plot axes. Table 3 displays the regression coefficients and 95% confidence intervals for these two comparisons. Results for stimulus levels < 90 dB nHL for click stimuli and at all levels for the 4 kHz stimulus in Figs. 4 and 5 can be viewed as trends, but statistical significance should be interpreted with caution due to the multiple comparisons that are presented.
Figure 4.
Supra-threshold ABR wave I amplitude recorded with a mastoid electrode. Wave I amplitude (in nV) is shown as a function of NEB for ABRs in response to clicks (left column) and 4 kHz tone bursts (right column). Stimulus level is denoted in each panel (90 to 60 dB nHL). Symbols represent individual responses. Linear regression results are inset in each panel along with the number of subjects contributing data (n).
Figure 5.
Supra-threshold ABR wave I amplitude recorded with a TM electrode. Data are shown following the conventions used in Fig. 4.
Table 3.
Regression coefficients for ABRs collected in response to 90 dBnHL click stimuli shown in Figs. 4 and 5.
| 95% Confidence Interval
|
||||||
|---|---|---|---|---|---|---|
| Electrode Site | Predictor | Unstandardized Coefficients (B) | Std. Error | p-value | Upper Bound | Lower Bound |
| Mastoid | Intercept | 1305.176 | 333.963 | 0.001 | 621.083 | 1989.268 |
| NEB | −11.652 | 4.483 | 0.015 | −20.835 | −2.470 | |
|
| ||||||
| TM | Intercept | 2221.114 | 782.570 | 0.008 | 618.091 | 3824.136 |
| NEB | −18.177 | 10.504 | 0.095 | −39.694 | 3.339 | |
For data collected using a mastoid electrode (Fig. 4), results for a click stimulus presented at 90 dB nHL indicated a statistically significant relationship between wave I amplitude and NEB, where wave I amplitude decreased as NEB increased (p-value=0.015, r2=0.194). At this condition, nearly 20% of the variance in wave I amplitude was explained by NEB. The regression coefficients shown in Table 3 for this condition suggest that for every unit increase in NEB, the amplitude of wave I decreases by approximately 11.6 nV. ABRs collected at 70 and 80 dB nHL using a click stimulus, and at 70–90 dB nHL using a 4 kHz tone burst stimulus, revealed similar systematic relationships of decreasing wave I amplitude with increasing NEB. Across these conditions, NEB explained approximately 15 to 24% of the variance in wave I amplitude, indicating a medium to large effect size (Cohen, 1992). However, when the stimulus level decreased to 60 dB nHL, these relationships were weaker (r2 = 0.03 to 0.12). At 50 dB nHL and lower (data not shown in Fig. 4), many subjects (typically more than half) did not have an identifiable wave I. Therefore, the relationship between NEB and ABR wave I amplitude at lower stimulus levels could not be determined.
ABR data collected using a TM electrode, shown in Fig. 5, are less systematic than those collected using a mastoid electrode (Fig. 4). Statistical significance was not met for click-evoked ABRs collected at 90 dB nHL (p-value=0.095, r2=0.097, B=−18.177, 95% CI [−39.694, 3.339]). For 4 kHz tone bursts presented at 90 dB nHL and for stimulation levels of 70 and 80 dB nHL to either stimulus, there was a trend of decreasing ABR wave I amplitude with increasing NEB. For these comparisons, the relationships were not as strong as those observed for the mastoid recording electrode, with NEB explaining only 7 to 12% of the variance in wave I amplitude. At stimulation levels of 60 dB nHL, r2 values were lower (<0.06). Although not shown in Fig. 5, similar r2 values were present for ABRs collected at 50 dB nHL (4 kHz tone bursts: r2=0.082, clicks: r2=0.094). Subjects typically had an identifiable wave I at lower stimulation levels for ABRs collected with a TM electrode compared to a mastoid electrode. However, for stimulation levels of 40 dB nHL and lower, an identifiable wave I was absent for more than half of the subjects, preventing an evaluation of the relationship between NEB and wave I amplitude for those conditions.
ABRs collected using a TM electrode were more variable than responses collected using a mastoid electrode (note the difference in the amplitude ranges displayed in Figs. 4 and 5). Recall that the standard deviation of response amplitude for data collected with a TM electrode was roughly double that of the variability seen for responses collected with a mastoid electrode (Table 2). Furthermore, the CV was larger for ABRs collected with a TM electrode compared to those collected with a mastoid recording site.
In summary, for ABR responses collected using a mastoid recording site, there was a systematic trend for ABR wave I amplitude to decrease as NEB increased for high-level (≥ 70 dB nHL) stimuli. The pattern was seen in ABR responses to both click and 4 kHz tone burst stimuli. For ABRs collected using a TM recording site, a weaker trend of smaller wave I amplitude in individuals with greater amounts of NEB was present. It appears that the higher variability associated with the TM recording site obscured any systematic trends in wave I amplitude as a function of NEB.
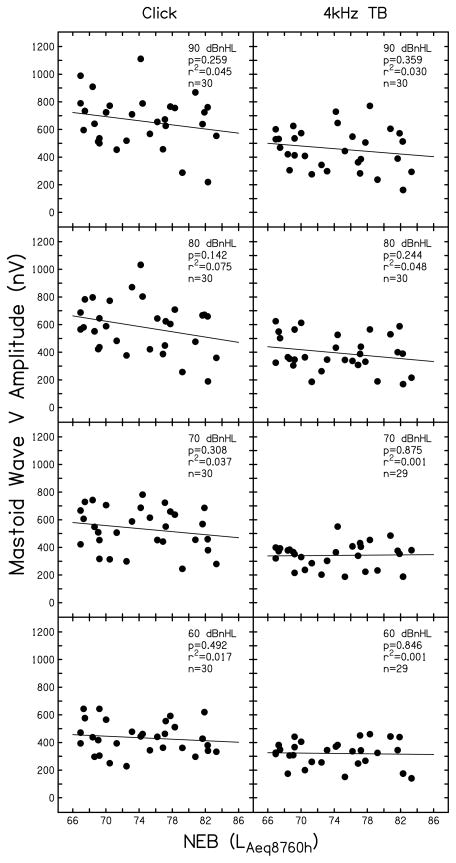
Wave V
The relationship between ABR wave V amplitude and NEB was similar for responses collected using both a TM electrode and a mastoid electrode. Therefore, findings are only presented for ABRs collected with a mastoid recording electrode. Figure 6 displays supra-threshold ABR wave V amplitude as a function of NEB and follows the plotting conventions used in Figs. 4 and 5.
Figure 6.
Supra-threshold ABR wave V amplitude recorded with a mastoid electrode. Data are shown following the conventions used in Figs. 4 and 5.
Results displayed in Fig. 6 do not support the existence of a systematic relationship between supra-threshold ABR wave V amplitude and NEB. In general, less than 5% of the variance in wave V amplitude was attributed to NEB. This indicates that although there was a trend for ABR responses generated in the cochlear nerve (i.e., wave I) to be smaller in normal-hearing ears with larger amounts of NEB, this effect was not present for ABR responses generated at the level of the auditory midbrain (i.e., wave V).
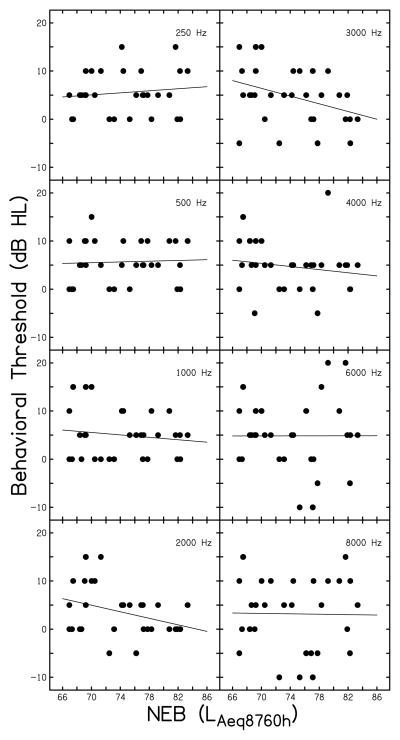
Influence of Behavioral Threshold on Auditory Responses
All study participants had normal hearing (individual thresholds are shown in Table 1); however, thresholds considered normal span a 30 dB range (−10 dB HL to 20 dB HL). It was necessary to consider the relationship between NEB and behavioral threshold(s). If individuals with higher NEB had higher behavioral thresholds (i.e., poorer hearing), then behavioral threshold might be a confounding factor when examining the influence of NEB on DPOAEs and ABRs. Figure 7 shows the relationship between NEB and behavioral threshold at eight frequencies. In each panel, the filled symbols represent individual subjects and a regression line fit to the data is shown. In general, no systematic trends are present, indicating a lack of relationship between behavioral threshold and NEB. Linear regression analyses revealed the strongest relationships were present at 2000 (p=0.052, r2=0.128) and 3000 Hz (p=0.046, r2=0.135). However, at these conditions, better hearing thresholds were associated greater NEB. These results indicate that behavioral threshold is likely not a confounding variable in the previously described relationship between NEB and ABR wave I amplitude.
Figure 7.
Behavioral threshold variation across participants. Individual behavioral thresholds are plotted as a function of NEB. The frequency (Hz) is noted in each panel.
While behavioral threshold was not found to be correlated with NEB, entering behavioral threshold as an additional predictor variable into a regression model with NEB could potentially explain more of the variance in the measured auditory response than what was explained by NEB alone. Therefore, a stepwise multiple linear regression analysis (using 7agr;=0.05 level of significance) was completed using behavioral threshold and NEB as independent variables. This analysis was conducted on the high level (≥70 dB nHL) ABR wave I amplitudes displayed in Fig. 4, the six stimulus conditions where a systematic relationship was revealed between ABR amplitude and NEB. The behavioral threshold at 4 kHz was used as a predictor variable for the ABRs collected in response to 4 kHz tone bursts. As behavioral thresholds were not available for click stimuli, the average of behavioral thresholds from 2–4 kHz was used as a substitute for click threshold.
Stepwise linear regression results completed with ABR responses collected using a mastoid electrode indicated that NEB was the sole significant predictor for all test conditions. Stated another way, behavioral threshold was excluded from the model at all test conditions, a result that was not surprising given the relationships between behavioral threshold and NEB shown in Fig. 7. Thus, behavioral threshold was not found to significantly contribute to a multiple linear regression model for click-evoked or 4 kHz tone burst ABRs collected with a mastoid recording site for stimulation levels of 90 to 70 dB nHL.
DISCUSSION
To summarize, ABR wave I amplitudes collected with a mastoid electrode to 90 dB nHL click stimuli were significantly smaller in ears with greater amounts of NEB. For other high-level (≥ 70 dB nHL) testing conditions, there was a systematic trend for wave I amplitude to decrease with increasing NEB. This trend weakened and disappeared with decreases in stimulus level (< 70 dB nHL). Although similar trends were observed for responses recorded with a TM electrode, the responses were more variable across subjects and the relationship was weaker. In contrast to the results observed for wave I, there was no apparent relationship between supra-threshold wave V amplitude and NEB for either recording electrode. Furthermore, supra-threshold DPOAE level did not appear to be systematically correlated with NEB.
Defining NEB in a Human Population
The amount of NEB reported by the subjects in the current study are very different compared to the highly controlled noise exposures of animals tested by Kujawa and colleagues (Kujawa & Liberman, 2009; Lin et al., 2011; Furman et al., 2013). For ethical reasons, exposing human subjects to a controlled noise source for the purposes of this study was not feasible. Therefore, a convenience sample of individuals with varying NEB (as defined by the NEQ score) was used. For the majority of individuals reporting greater amounts of NEB, noise exposure was attributable to music listening (e.g., concert attendance, playing a musical instrument, listening to music via speakers or personal headphones, etc.). Musicians have been identified as a population frequently exposed voluntarily to high levels of noise (Chasin, 1996; O’Brien et al., 2008; Zhao et al., 2010; Cook-Cunningham et al., 2012), making this population a reasonable choice for subject recruitment.
A limitation of using the NEQ score to quantify NEB is that the questionnaire focuses on exposure to loud sound in the past year; noise exposures beyond this time frame are not specifically probed. An effort was made in the current study to recruit subjects whose lifetime noise exposure was accurately reflected in their NEQ score. This was done by obtaining a detailed case history. For example, if a subject reported not playing a musical instrument in the past year via the NEQ, they were asked if they had ever played a musical instrument. If the subject stated playing a musical instrument in high school but not since, the annual NEQ score was determined to be therefore not reflective of the lifetime noise exposure reported in the detailed case history. When a discrepancy existed between the NEQ score and the detailed case history, the subject was not enrolled.
Influence of NEB on Auditory Responses Measured via the ABR
ABR Wave I Amplitude
Results of the present study support the hypothesis that supra-threshold ABR wave I amplitude is smaller in normal-hearing subjects with greater amounts of NEB when compared to normal-hearing subjects with lesser amounts of NEB (Figs. 4 and 5). Additionally, these findings are in agreement with data from mice and guinea pig ears (Kujawa & Liberman, 2009; Lin et al., 2011; Furman et al., 2013). In the human ear, it is unknown how much noise exposure must be encountered before the anatomical and physiological patterns described by Kujawa and colleagues might develop. Although an association between supra-threshold ABR wave I amplitudes and NEB was revealed in the present study, it was not possible to identify a specific wave I amplitude value that indicated whether a response originated from an individual with high NEB or low NEB (Figs. 4 and 5). Linear regression analyses indicated approximately 15 to 24% of the variance in wave I amplitude could be explained by NEB (Fig. 4). Therefore, NEB is not the only factor influencing ABR amplitude. Although behavioral threshold variation was considered, it did not emerge as a contributing predictor of wave I amplitude in a multiple linear regression model. Prior to supra-threshold ABR wave I amplitude being utilized clinically as a method to potentially identify early evidence of noise-induced auditory damage before a permanent hearing loss is present, additional pathological and non-pathological sources of amplitude variability must be understood.
An additional approach to determining when potential deafferentation is present in the human ear would be to inspect differences between pre-noise exposure ABR wave I amplitude relative to post-noise exposure amplitude. The design of this study did not allow for comparison between pre- and post-noise exposure auditory responses in the same subject. It is difficult to obtain this type of data in humans as it is necessary to test subjects prior to being voluntarily exposed to loud noise and again after the noise exposure. Many of the subjects in the present study had initiated voluntary noise exposure years prior to being enrolled in the study. A prospective, longitudinal study design would be better suited for this approach.
ABR Wave V Amplitude
ABR wave V amplitude was also measured in the present study and was not found to be systematically related to NEB (Fig. 6). This contrasts findings related to supra-threshold wave I amplitude and NEB. There are several possible reasons for the different findings for wave V compared to wave I that can be considered.
Wave I of the ABR is generated in the distal portion of the auditory nerve (Melcher & Kiang, 1996) while wave V of the ABR is generated at the level of the auditory midbrain (Møller et al., 1995; Hall, 2007a). Since there was a trend toward smaller wave I amplitudes to be seen in subjects with greater NEB, the data suggest the presence of a mechanism between the auditory nerve and the auditory midbrain that might compensate for the reduced output from the auditory nerve. This idea is in agreement with data from Schaette and McAlpine (2011) where reduced ABR wave I amplitudes were found in normal-hearing human ears with tinnitus when compared to normal-hearing ears without tinnitus, but no differences were reported in wave V amplitude for the two groups. The authors argued their findings suggested the existence of a “homeostatic gain control” mechanism. Additional support for the idea of a “gain control” mechanism can be found in data from Mulders and Robertson (2009, 2011). These two studies reported evidence of a significant increase in the spontaneous firing rate of inferior colliculus neurons in animal ears following noise exposure when compared to control ears. While the data in the present study were not designed to specifically address the existence of a homeostatic mechanism along the auditory pathway, the data presented here follow a similar pattern in ABR amplitude responses as that described by Schaette and McAlpine. If exposure to loud sound does lead to an increase in the spontaneous firing rates of inferior colliculus neurons, this might explain, at least in part, why differences were seen in wave I amplitude but not in wave V amplitude across subjects with different NEB in the present study.
An alternate explanation for the contrasting findings of wave I and wave V amplitude can be found in Don and Eggermont (1978). In their study, a high pass masker was used to determine frequency contributions to the click-evoked ABR. The data by Don and Eggermont suggest that wave I is generated mainly by neurons with characteristic frequencies greater than 2 kHz while wave V includes contributions from along the entire cochlear partition. Therefore, it can be postulated that if damage to auditory structures is only present in structures responsible for encoding the higher frequencies (i.e., the 3 to 6 kHz region commonly affected by NIHL), it is possible that evidence of this damage would be revealed in smaller wave I amplitudes due to a reduction in the number of neurons contributing to the response. In contrast, if the auditory structures responsible for encoding the lower frequencies (i.e., less than 2 kHz) remain unaffected, wave V amplitude would be expected to be altered to a lesser degree or not at all, even when the high frequency neurons are compromised.
Differences Between ABR Recording Electrodes
The data collected with the TM electrode differed in a number of ways from the data collected with the mastoid electrode. As expected, the TM electrode resulted in larger wave I amplitudes (Table 2) and the ability to identify wave I down to a lower stimulation level when compared to responses collected with a mastoid electrode. These findings indicate the use of a TM electrode was successful in enhancing visualization of wave I of the ABR.
However, variability in ABR wave I amplitude was larger for responses collected with a TM electrode compared to responses obtained with a mastoid electrode (Table 2). Increased variability (larger SDs) in wave I amplitude across subjects for a TM electrode compared to a mastoid electrode recording site has been reported in previous investigations (Ferraro & Ferguson, 1989). The larger SDs may essentially reflect the overall larger amplitudes that are recorded when utilizing a TM electrode. Rather than looking at the absolute value of the SD, a proportional measure of variability, the CV, can be examined (see bottom panel of Table 2). In general, although the CV values were similar for the two recording sites, the CV was consistently smaller for the mastoid recording site. It is possible that the proportionally larger variability at the TM electrode was responsible for the similar, but weaker, trends observed with the TM electrode. Replication of the present findings with a larger number of subjects is necessary to determine if the reason for the lack of significant findings with the TM electrode is due to greater between-subject variability.
Influence of NEB on Auditory Responses Arising from the OHCs
NEB was not systematically correlated with DPOAE level at f2’s of 1, 2, and 4 kHz across a range of L2’s. These findings are in agreement with recent results from animal ears where DPOAE levels were unchanged following full recovery from TTS and there was an absence of permanent noise-induced OHC damage (Kujawa & Liberman, 2009; Lin et al., 2011; Furman et al., 2013). While a lack of OHC damage following noise exposure resulting in TTS is in agreement with recent reports and some older findings (Robertson & Johnstone, 1980; Liberman & Mulroy, 1982), it contradicts other reports that have described noise-induced damage to the OHCs. These have including swelling of the OHCs (Liberman & Dodds, 1987) and damage to OHC stereocilia (Dunn et al., 1979; Gao et al., 1992). However, in these reports, variability among animals in terms of the absence or presence of OHC damage existed (Dunn et al., 1979) and some investigations included data from animals experiencing active TTS (Liberman & Dodds, 1987; Gao et al., 1992).
For the human ears assessed in the present study, it is not feasible to complete anatomical assessment of the OHCs. Therefore, the status of the OHCs must be inferred from looking at a functional measure of the OHCs, such as DPOAEs. As no difference in DPOAE level was found across the 30 subjects tested here with varying NEB, it is postulated that the current study participants’ noise exposure did not result in permanent anatomical damage to the OHCs. If this study were repeated in an additional group of individuals with different amounts or types of NEB, it is possible that a different outcome could have been observed.
Cross-sectional studies have shown that OAEs are a good assessment approach in noise-exposed individuals who have elevated behavioral thresholds (Attias et al., 2001). Furthermore, numerous studies have demonstrated the effectiveness of OAEs in identifying the presence or absence of hearing loss (Gorga et al., 1993, 1997; Stover et al., 1996); the cause of hearing loss in these studies included NIHL along with a multitude of other causes. In ears with normal behavioral thresholds (typically defined as ≤ 20 dB nHL), the literature has not strongly supported the use of DPOAEs as a method for detecting earlier evidence of noise damage than that garnered by behavioral threshold assessment (Lapsley Miller et al., 2006; Marshall et al., 2009; Seixas et al., 2012). The results of the current study do not support the use of DPOAE assessment as an effective method to detect early evidence of noise-induced damage before increases in behavioral thresholds are apparent.
While the results of the present study did not reveal any systematic difference in DPOAEs as a function of NEB, data were collected at a single test session and pre-noise exposure DPOAE levels were not available for comparison. It is unknown how DPOAE levels in the normal-hearing, noise-exposed ears tested here would compare relative to DPOAE levels obtained from the same ear prior to the onset of noise exposure. Furthermore, in an ear with active temporary hearing loss due to noise exposure, it is likely smaller DPOAE levels would be recorded. However, these comparisons could not be investigated in the present study as baseline DPOAEs not were available and no subject was actively experiencing TTS.
CONCLUSIONS
The results reported here describe a pattern of smaller high-level ABR wave I amplitude in normal-hearing human subjects reporting greater amounts of NEB when compared to subjects reporting lesser amounts of NEB. Other measures of auditory function (supra-threshold DPOAEs and ABR wave V amplitude) were not found to be systematically correlated to NEB. These findings are in agreement with recent data from noise-exposed animals (Kujawa & Liberman, 2009; Lin et al., 2011; Furman et al., 2013) and are consistent with the idea that noise exposures that do not result in permanent hearing loss might permanently damage high-threshold/low-spontaneous rate auditory nerve fibers in human ears. Furthermore, the data suggest evidence of this damage is only apparent when examining supra-threshold (≥ 70 dB nHL) ABR wave I amplitude.
Supplementary Material
Acknowledgments
This investigation was supported by a Student Investigator Research Grant from the American Academy of Audiology/American Academy of Audiology Foundation and by a grant from the NIH NIDCD (R03 DC011367). Portions of this work were presented at the 2014 MidWinter Meeting of the Association for Research in Otolaryngology. The authors would like to thank Susan Cooper (Megerson) for her assistance and helpful comments.
Footnotes
Financial Disclosures/Conflicts of Interest:
This research was funded by the NIH-NIDCD and by the American Academy of Audiology/American Academy of Audiology Foundation.
References
- ANSI. ANSI Report No. S3.6-2004. New York: American National Standards Institute; 2004. Specifications for Audiometers. [Google Scholar]
- Attias J, Horovitz G, El-Hatib N, et al. Detection and Clinical Diagnosis of Noise-Induced Hearing Loss by Otoacoustic Emissions. Noise Health. 2001;3:19–31. [PubMed] [Google Scholar]
- Bohne BA, Harding GW. Degeneration in the cochlea after noise damage: primary versus secondary events. Am J Otol. 2000;21:505–509. [PubMed] [Google Scholar]
- Chasin M. Clinical assessment of musicians – audiologist as a detective. In: Chasin M, editor. Musicians and the Prevention of Hearing Loss. San Diego: Singular Publishing Group, Inc; 1996. pp. 103–119. [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cook-Cunningham SL, Grady ML, Nelson H. Hearing dose and perceptions of hearing and singing effort among university choir singers in varied rehearsal and performance settings. Int J Res Choral Singing. 2012;4:19–35. [Google Scholar]
- Costalupes JA, Young ED, Gibson DJ. Effects of continuous noise backgrounds on rate response of auditory nerve fibers in cat. J Neurophysiol. 1984;51:1326–1344. doi: 10.1152/jn.1984.51.6.1326. [DOI] [PubMed] [Google Scholar]
- Coughlin SS. Recall bias in epidemiologic studies. J Clin Epidemiol. 1990;43:87–91. doi: 10.1016/0895-4356(90)90060-3. [DOI] [PubMed] [Google Scholar]
- Don M, Eggermont JJ. Analysis of the click-evoked brainstem potentials in man using high-pass noise masking. J Acoust Soc Am. 1978;63:1084–1092. doi: 10.1121/1.381816. [DOI] [PubMed] [Google Scholar]
- Dunn DE, Ferraro JA, Lim DJ. Electro-physiological and morphological correlates of TTS in chinchilla. Abstr Assoc Res Otolaryngol. 1979;2:37. [Google Scholar]
- Earl BR, Chertoff ME. Predicting auditory nerve survival using the compound action potential. Ear Hear. 2010;31:7–21. doi: 10.1097/AUD.0b013e3181ba748c. [DOI] [PubMed] [Google Scholar]
- Ferraro JA, Ferguson R. Tympanic ECochG and conventional ABR: a combined approach for the identification of wave I and the I-V interwave interval. Ear Hear. 1989;10:161–166. [PubMed] [Google Scholar]
- Fernandez K, Lall K, Jeffers PWC, et al. Acceleration of sensory and neural cochlear aging after TTS. 2014 Association for Research in Otolaryngology MidWinter Meeting; San Diego, CA. 2014. [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110:577–586. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddam A, Ferraro JA. ABR recordings in newborns using an ear canal electrode. Int J Audiol. 2008;47:499–504. doi: 10.1080/14992020802116268. [DOI] [PubMed] [Google Scholar]
- Gao WY, Ding DL, Zheng XY, et al. A comparison of changes in the stereocilia between temporary and permanent hearing losses in acoustic trauma. Hear Res. 1992;62:27–41. doi: 10.1016/0378-5955(92)90200-7. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Bergman B, et al. Otoacoustic emissions from normal-hearing and hearing-impaired subjects. J Acoust Soc Am. 1993;93:2050–2060. doi: 10.1121/1.406691. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Ohlrich B, et al. From laboratory to clinic: a large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear. 1997;18:440–455. doi: 10.1097/00003446-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Hall J. Anatomy and physiology principles of auditory evoked potentials. In: Hall J, editor. New Handbook of Auditory Evoked Responses. Boston: Pearson Education, Inc; 2007a. pp. 35–57. [Google Scholar]
- Hall J. ABR analysis and interpretation. In: Hall J, editor. New Handbook of Auditory Evoked Responses. Boston: Pearson Education, Inc; 2007b. pp. 171–211. [Google Scholar]
- Humes LE, Joellenbeck LM, Durch JS. Noise and Military Service: Implications for Hearing Loss and Tinnitus. Washington DC: National Academies Press; 2005. pp. 33–47. [Google Scholar]
- Kirby BJ, Kopun JG, Tan H, et al. Do “optimal” conditions improve distortion product otoacoustic emission test performance? Ear Hear. 2011;32:230–237. doi: 10.1097/AUD.0b013e3181fa5da2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad-Martin D, Dille MF, McMillan G, et al. Age-related changes in the auditory brainstem response. J Am Acad Audiol. 2012;23:18–35. doi: 10.3766/jaaa.23.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapsley Miller JA, Marshall L, Heller LM, et al. Low-level otoacoustic emissions may predict susceptibility to noise-induced hearing loss. J Acoust Soc Am. 2006;120:280–296. doi: 10.1121/1.2204437. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW. Acute ultrastructural changes in acoustic trauma: serial-section reconstruction of sterocilia and cuticular plates. Hear Res. 1987;26:65–88. doi: 10.1016/0378-5955(87)90035-9. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Mulroy MJ. Acute and chronic effects of acoustic trauma: cochlear pathology and auditory nerve pathophysiology. In: Hamernick RP, Henderson D, Salvi R, editors. New Perspectives on Noise-Induced Hearing Loss. New York: Raven Press; 1982. pp. 105–135. [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, et al. Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12:605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Barrios P. Perception of stochastically undersampled sound waveforms: a model of auditory deafferentation. Front Neurosci. 2013;7:124. doi: 10.3389/fnins.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Lapsley Miller JA, Heller LM, et al. Detecting incipient inner-ear damage from impulse noise with otoacoustic emissions. J Acoust Soc Am. 2009;125:995–1013. doi: 10.1121/1.3050304. [DOI] [PubMed] [Google Scholar]
- Megerson SC. Published Dissertation. University of Kansas; Ann Arbor: ProQuest; 2010. Development of a screening tool for identifying young people at risk for noise-induced hearing loss. [Google Scholar]
- Melcher JR, Kiang NY. Generators of the brainstem auditory evoked potential in cat. III: Identified cell populations. Hear Res. 1996;93:52–71. doi: 10.1016/0378-5955(95)00200-6. [DOI] [PubMed] [Google Scholar]
- Møller AR, Jho HD, Yokota M, et al. Contribution from crossed and uncrossed brainstem structures to the brainstem auditory evoked potentials: a study in humans. Laryngoscope. 1995;105:596–605. doi: 10.1288/00005537-199506000-00007. [DOI] [PubMed] [Google Scholar]
- Muhr P, Rosenhall U. Self-assessed auditory symptoms, noise exposure, and measured auditory function among healthy young Swedish men. Int J Audiol. 2010;49:317–325. doi: 10.3109/14992020903431280. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity. Neuroscience. 2009;164:733–746. doi: 10.1016/j.neuroscience.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Progressive centralization of midbrain hyperactivity after acoustic trauma. Neuroscience. 2011;192:753–760. doi: 10.1016/j.neuroscience.2011.06.046. [DOI] [PubMed] [Google Scholar]
- National Institute on Deafness and Other Communication Disorders (NIDCD) Fact Sheet: Work Related Hearing Loss. Washington, DC: National Institute on Deafness and Other Communication Disorders, Health and Human Services; 2001. [Google Scholar]
- Neely ST, Liu Z. Tech Memo 17. Boys Town National Research Hospital; Omaha, NE: 1993. EMAV: Otoacoustic emission average. [Google Scholar]
- Neitzel R, Seixas N, Olson J, et al. Nonoccupational noise: exposures associated with routine activities. J Acoust Soc Am. 2004a;115:237–245. doi: 10.1121/1.1615569. [DOI] [PubMed] [Google Scholar]
- Neitzel R, Seixas N, Goldman B, et al. Contributions of non-occupational activities to total noise exposure of construction workers. Ann Occup Hyg. 2004b;48:463–473. doi: 10.1093/annhyg/meh041. [DOI] [PubMed] [Google Scholar]
- Nordmann AS, Bohne BA, Harding GW. Histopathological differences between temporary and permanent threshold shift. Hear Res. 2000;139:13–30. doi: 10.1016/s0378-5955(99)00163-x. [DOI] [PubMed] [Google Scholar]
- O’Brien I, Wilson W, Bradley A. Nature of orchestral noise. J Acoust Soc Am. 2008;124:926–939. doi: 10.1121/1.2940589. [DOI] [PubMed] [Google Scholar]
- Reeb-Whitaker CK, Seixas NS, Sheppard L, et al. Accuracy of task recall for epidemiological exposure assessment to construction noise. Occup Environ Med. 2004;61:135–142. doi: 10.1136/oem.2002.000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Johnstone BM. Acoustic trauma in the guinea pig cochlea: early changes in ultrastructure and neural threshold. Hear Res. 1980;3:167–179. doi: 10.1016/0378-5955(80)90044-1. [DOI] [PubMed] [Google Scholar]
- Sanchez TG, Medeiros IR, Levy CP, et al. Tinnitus in normally hearing patients: clinical aspects and repercussions. Braz J Otorhinolaryngol. 2005;71:427–431. doi: 10.1016/S1808-8694(15)31194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JC, Dear SP, Schneider ME. The anatomical consequences of acoustic injury: A review and tutorial. J Acoust Soc Am. 1985;78:833–860. doi: 10.1121/1.392915. [DOI] [PubMed] [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31:13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperle RA, Neely ST, Kopun JG, et al. Influence of in situ, sound-level calibration on distortion-product otoacoustic emission variability. J Acoust Soc Am. 2008;124:288–300. doi: 10.1121/1.2931953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperle RA, Goodman SS, Neely ST. Further assessment of forward pressure level for in situ calibration. J Acoust Soc Am. 2011;130:3882–3892. doi: 10.1121/1.3655878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DM, Morris MD, Jacobson JT. The normal auditory brainstem response and its variants. In: Jacobson JT, editor. Principles & Applications in Auditory Evoked Potentials. Needham Heights: Allyn and Bacon; 1994. pp. 123–153. [Google Scholar]
- Seixas NS, Neitzel R, Stover B, et al. 10-Year prospective study of noise exposure and hearing damage among construction workers. Occp Environ Med. 2012;69:643–650. doi: 10.1136/oemed-2011-100578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H. Primary structural changes in the organ of Corti after acoustic overstimulation. Acta Otolaryngol. 1971;71:166–176. doi: 10.3109/00016487109125346. [DOI] [PubMed] [Google Scholar]
- Stover L, Gorga MP, Neely ST, et al. Toward optimizing the clinical utility of distortion product otoacoustic emissions. J Acoust Soc Am. 1996;100:956–967. doi: 10.1121/1.416207. [DOI] [PubMed] [Google Scholar]
- Zhao F, Manchaiah VK, French D, et al. Music exposure and hearing disorders: an overview. Int J Audiol. 2010;49:54–64. doi: 10.3109/14992020903202520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.