Abstract
The thymus is a vital organ for T lymphocyte development. Of thymic stromal cells, thymic epithelial cells (TECs) are particularly crucial at multiple stages of T cell development: T cell commitment, positive selection and negative selection. However, the function of TECs in the thymus remains incompletely understood. In the article, we provide a method to isolate TEC subsets from fresh mouse thymus using a combination of mechanical disruption and enzymatic digestion. The method allows thymic stromal cells and thymocytes to be efficiently released from cell-cell and cell-extracellular matrix connections and to form a single-cell suspension. Using the isolated cells, multiparameter flow cytometry can be applied to identification and characterization of TECs and dendritic cells. Because TECs are a rare cell population in the thymus, we also describe an effective way to enrich and purify TECs by depleting thymocytes, the most abundant cell type in the thymus. Following the enrichment, cell sorting time can be decreased so that loss of cell viability can be minimized during purification of TECs. Purified cells are suitable for various downstream analyses like Real Time-PCR, Western blot and gene expression profiling. The protocol will promote research of TEC function and as well as the development of in vitro T cell reconstitution.
Keywords: Immunology, Issue 90, Immunology; Thymus; T cell development; Thymic epithelial cell; Isolation; Positive selection
Introduction
Early in T cell development, bone marrow hematopoietic stem cell-derived multipotent progenitors are recruited to the cortex of the thymus, undergo commitment to T lineage and become T cell precursors1. In the cortex, T cell precursor CD4 and CD8 double negative (DN) thymocytes expand and differentiate into immature CD4 and CD8 double positive (DP) thymocytes, forming a large pool of progenitors with highly variable T cell receptors1. Only a select MHC-restricted subset of DP cells will become CD4 or CD8 single positive (SP) thymocytes, migrate to the medulla of the thymus, and differentiate into functionally competent mature T cells, an event that is referred to as positive selection2-6. In contrast, clones of auto-reactive thymocytes undergo negative selection and are removed via apoptosis, converted to regulatory T cells for self-tolerance, or diverted to intraepithelial lymphocytes for purposes that are not yet clear3,7-10.
In the thymus, thymic stromal cells form a unique microenvironment providing signals for these various T cell development fates5,11,12. Thymic stromal cells are composed of thymic epithelial cells (TECs) - including cortical TECs (cTECs) and medullary TECs (mTECs), dendritic cells, macrophages, fibroblasts, endothelial cells, neural crest-derived pericytes and other mesenchymal cells13-15. Among these, TECs are crucial at the various stages of T cell development1,2,16,17. However, lack of a robust way to isolate TECs has hampered a comprehensive understanding of their functions16. In particular, cTECs, which form a three-dimensional network surrounding progenitors in the cortex, are essential for positive selection13,18,19 for reasons that are not yet clear. Earlier studies provided clues as to the heterogeneity and role of TECs, mostly relying on morphological and histological tools13. Recently the unique roles of TEC subsets were addressed by genetic approaches in mouse models12,20. A robust and reproducible way to isolate TECs is fundamental to achieve unbiased characterization of TEC subsets, quantitative and qualitative assessment of TEC functions, and clarification of mechanisms how cTECs support positive selection.
Due to the rarity of TECs in the thymus and the tight interactions they form in the intact organ, the isolation of TECs has been challenging. The protocol described here is based on previous discoveries, currently available reagents, techniques, and knowledge of thymus structure and stromal composition. Nearly two decades ago, several procedures were reported to disaggregate thymus tissues21-27, in which different enzymes were used during digestion, including Trypsin, Collagenase and Dispase. Gray et al. compared those enzymes in their precedure28, and reported an improved method with a multiple-step digestion of enzyme cocktails29 that became widely used20,30. However, this method involves a long preparation time and complex digestion steps and results in variable final cell numbers and proportions of TECs even in the same murine cohort29,30. Several years ago, Liberase research grade enzyme, containing highly purified Collagenase and neutral protease started to be used in thymus tissue dissociation30. Here, we describe a Liberase digestion-based protocol, with optimized mechanical separation procedures, that yields a high number of viable TECs from mouse thymus tissues. .
To enrich dissociated stromal cells, previous studies have used either density gradients or magnetic bead separation21,29. However, both methods cause severe lost of certain populations of thymic stromal cells, particularly the population of cTECs29. Cytotoxic elimination and panning techniques31,32 have widely been used for depletion or separation of lymphocytes in the immunological field12,31. After comparison of these techniques, we established the current panning protocol for enrichment of TECs. The gentle condition during the enrichment procedure leads to less cell death and unbiased and increased TEC recovery.
The isolated thymus cell suspension described in Section 3 can be directly applied to flow cytometric analysis for identification and characterization of TEC subsets and dendritic cells. Section 4 describes a simple and useful way to identify TEC subsets using multiparameter flow cytometer. For experiments that seek to obtain purified cTECs or mTECs, TEC enrichment and cell sorting procedures can be found in Section 5 and 6.
Protocol
In this study, adult (6 - 8 weeks) female C57Bl/6 mice were used. Mice were purchased from the National Cancer Institute and maintained under specific pathogen free conditions. The University of Minnesota Institutional Animal Care and Use Committee (IACUC) approved all animal experimentation.
1. Preparation of Instruments and Buffers
Prepare enzyme solution (RPMI1640 medium with 0.05% [w/v] of Liberase TH and 100 U/ml of DNase I), albumin-rich buffer (1X PBS [Ca2+/Mg2+-free] with 0.5% Bovine serum albumin and 2 mM Ethylenediamine Tetraacetic acid [EDTA]), FACS buffer (1X PBS [Ca2+/Mg2+-free] containing 1% Fetal bovine serum [FBS] and 0.02% NaN3), FACS sorting buffer (1X PBS [Ca2+/Mg2+-free] containing 2% FBS), and RP10 medium (RPMI-1640 medium supplied with 10% FBS).
Prepare 10 panning plates. Prepare 40 ml coating solution (0.01 mg/ml Goat anti-Rat IgG in 0.1 M NaHCO3 Buffer), and add 4 ml of coating solution into each 100 x 15 mm plate, swirl and incubate at 4 °C O/N. Pour off antibody solution and rinse the plate using 5 ml of 1x PBS for 2 times, swirl vigorously between rinses. Store the prepared plates at 4 °C.
2. Harvest of Thymus Tissue
Euthanize mouse in a CO2 chamber. Put the animal on its back on a dissection mat, pin the feet on the mat and wet the fur by spraying with 70% ethanol.
Make a midline incision through the skin, starting from above the urethral opening straight up to the lower jaw with scissors and extend the incision downward to the knees on both sides. The incision looks like an upside down “Y”. Pull the loose skin back on the sides, and pin it to the dissection mat.
Puncture the diaphragm from the xiphoid, cut the ribs on each side up to about the clavicle, then lift up the ribs with forceps and pin it to the dissection mat. The thymus is just under the ribs, and looks like two thin white lobes overlying the heart (Figure 1). Disconnect connective tissue surrounding the thymus, gently pull and remove the pair of thymus lobes with curved serrated forceps. Place thymus lobes into a 6 well plate containing 5 ml RPMI-1640.
3. Preparation of Thymic Stromal Cells
Trim any surrounding fat and connective tissue and transfer the thymus lobes to a new well of the 6 well plate containing 5 ml freshly prepare enzyme solution. Make small incisions in the thymus lobes with fine scissors, and incubate the plate at 37 °C for 20 min.
Gently pipette digesting thymus tissue up and down 2 - 3 times using 5 ml pipette for tissue dissociation. Collect supernatant fraction in a 50 ml tube containing 10 ml cold albumin-rich buffer to neutralize enzymes. Keep the collection tube on ice. Add 2.5 ml enzyme solution to the remaining tissue and incubate the plate at 37 °C for 15 min.
Perform gentle mechanical agitation with a 3 ml syringe and 18 G needle to break up aggregates. Pool supernatant into the collection tube. Add 2.5 ml enzyme solution to the remaining tissue and incubate the plate at 37 °C for 15 min.
Repeat gentle mechanical agitation with a 3 ml syringe and a 25 G needle, and incubate the plate additional 5 - 10 min.
After complete digestion, pool the supernatant into the collection tube. Centrifuge the collection tube at 400 x g, 4 °C for 8 min to collect the cells. Aspirate the liquid and suspend the pelleted cells in 10 ml albumin-rich buffer and filter the sample through 100 mm mesh. Count cells using a hemocytometer. Then perform flow cytometic analysis (Section 4) or TECs enrichment (Section 5).
4. Identification of TECs by Flow Cytometry
Prepare cells to a final concentration of 4 x 106 cells per 100 µl in FACS buffer for staining in a 96 well round-bottom test plate and keep the plate on ice. Add 20 µl Fc block solution (anti-CD16/CD32 antibody at 10 µg/ml in FACS buffer) to the cells and incubate the cells for 10 min on ice. Spin the plate at 400 x g, 4 °C for 3 min. Discard liquid from wells by flicking the plate face down into a sink.
Suspend the pelleted cells in 100 µl antibody cocktail (anti-CD45, -EpCAM, -MHC class II, and -Ly51 antibodies, and the UEA-1 lectin labeled with different fluorochromes at manufacturer recommended or tested optimal concentrations of each antibody in FACS buffer), and incubate the cells on ice for 20 min in the dark. For compensation, stain 1 x 106 cells with each individual fluorochrome.
Add 100 µl of FACS buffer to each well and spin the plate at 400 x g, 4 °C for 3 min. Repeat the wash step one time, and then resuspend cells in 100 µl FACS buffer. Transfer the cells to a 12 x 15 mm round-bottom FACS tube filled with 300 µl FACS buffer. Acquire samples on flow cytometer and analyze data using FACS analysis software. TEC gating strategy is shown in Figure 2.
5. Enrichment of TECs by Panning
Spin down the thymus cells (prepared in Section 3) at 400 x g, 4 °C for 5 min. Aspirate the liquid and suspend the pelleted cells in FACS sorting buffer at a concentration of 2 x 108 cells per ml in a 15 ml or 50 ml conical tube. Add anti-CD90.2 antibody (Clone 30-H12) (or anti-CD45) at a final concentration of 2.5 µg/ml of cell suspension and incubate cells gently on a rotating mixer at 4 °C for 30 min. Following the incubation, wash with a 5 - 10x volume of RP10 medium and centrifuge the tube at 400 x g, 4 °C for 5 min.
Aspirate the liquid and resuspend the pelleted cells in RP10 medium at a concentration of 1 x 107 per ml. Add 5 ml cell suspension to each coated panning plate (prepared in Step 1.3), swirl and incubate for 30 min at RT. Swirl vigorously; transfer the supernatants into a conical tube. Rinse the plate two times using RP10 medium and pool the supernatants into the collection tube.
Centrifuge the collection tube at 400 x g, 4 °C for 5 min. Aspirate the liquid and resuspend the pelleted cells in 5 ml RP10 medium. Add the cell suspension to a new panning plate, swirl and incubate for 30 min at RT. Collect the cells rinse the plate and pool them into a conical tube. Once TECs are enriched, spin down the cells at 400 x g, 4 °C for 5 min and suspend cells in 5 ml FACS sorting buffer.
6. Purification of TECs by Cell Sorting
Count cells using a hemocytometer and prepare cell suspension at a concentration of 4 x 107 cells in FACS sorting buffer. Add antibodies described in Section 4.4 into cell solution and incubate cells gently on a rotating mixer at 4 °C for 30 min. Following staining, wash and suspend cells to a concentration of 10 x 106 cells per ml of FACS sorting buffer.
Sort TEC subsets through a 100 µM nozzle using fluorescence activated cell sorting. Sorted cell are collected in 30% (v/v) FBS in RPMI-1640). Once cell sorting is done, centrifuge cells at 400 x g, 4 °C for 5 min and prepare for downstream analysis.
Representative Results
Using this protocol, a thymus organ was removed from an adult mouse (Section 2) and a thymic cell suspension was prepared as outlined in Section 3. The obtained cell suspension consisted of thymocytes, hematopoietic-derived stromal cells and non-hematopoietic stromal cells. CD45 is a hematopoietic pan-marker expressed on both thymocytes and hematopoietic stromal cells such as macrophages and dendritic cells. In contrast, TECs are not of hematopoietic origin and do not express CD4515. Cell staining and flow cytometric analysis were performed as outlined in Section 4.
Two types of TEC subsets, cTECs and mTECs, originate from a common bi-potent TEC precursor15. Both cTECs and mTECs express the EpCAM molecule on their surface28. Thus TECs can be gated according to EpCAM-positive and CD45-negative by flow cytometry (Figure 2A). cTECs and mTECs can be distinguished by two reagents: the Ly51 antibody that recognizes glutamyl aminopeptidase expressed by cTEC, and the lectin Ulex Europaeus agglutinin-1 (UEA-1)28 that binds to mTEC (Figure 2B). Both mTECs and cTECs express MHC class II molecules on their surface (Figure 2C and 2D). mTECs can be further classified into to mTEClow and mTEChigh depending upon the level of MHC II molecules (Figure 2D). The average number of TEC per thymus is about 9 x 105. In direct comparisons, we recovered approximately 8 times more TECs using this Liberase enzyme based protocol compared to a multi-step protocol with collagenase/dispase29 (Figure 3).
In order to decrease sorting time and increase viability of recovered stromal cells, we developed a panning method to deplete thymocytes because the thymus cell suspension consists of over 95% thymocytes. Cells were incubated with anti-CD90 antibody and captured by precoated plates. Compared to that in the whole prepared thymus cells (pre-enriched), the proportion of TECs was increased from less than 0.5% to over 15% in post-enriched cells (Figure 4A and 4B). Panning with anti-CD45 can also be used if, for example, one does not need to recover dendritic cells as well. In this case, TEC enrichment increases to about 80% (data not shown). After stromal cell enrichment by panning, the proportions of TEC subsets were not altered (Figure 4C and 4D), suggesting that one subset or the other is not selectively lost during panning. Compared to the pre-enrichment sample, the recovery rate of TECs was about 85% (Figure 4E).
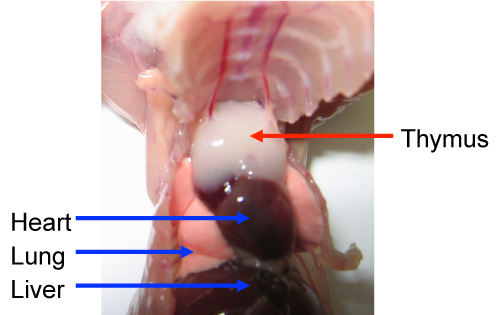 Figure 1. The mouse thymus during dissection. The thymus is located above the heart in the anterior superior thorax. After cutting and lifting the ribs, the two white lobes of the thymus are exposed. Please click here to view a larger version of this figure.
Figure 1. The mouse thymus during dissection. The thymus is located above the heart in the anterior superior thorax. After cutting and lifting the ribs, the two white lobes of the thymus are exposed. Please click here to view a larger version of this figure.
 Figure 2. Identification of cTEC and mTEC with flow cytometric data analysis. Stained thymus cell samples were analyzed using flow cytometry. Representative FACS profiles are shown. The numbers indicate the percentage of each population. (A) Of live cells, a gate of CD45-negative and EpCAM-positive events represents TEC cells. (B) Within the TEC gate, two populations are separated by level of Ly51 and UEA-1. cTECs have higher expression of Ly51; mTECs have higher level of UEA-1. (C) MHC II molecules are highly expressed on cTECs. (D) mTECs consists of MHC IIhigh and MHC IIlow sub-populations, which are separately referred to as mTEChigh and mTEClow. Please click here to view a larger version of this figure.
Figure 2. Identification of cTEC and mTEC with flow cytometric data analysis. Stained thymus cell samples were analyzed using flow cytometry. Representative FACS profiles are shown. The numbers indicate the percentage of each population. (A) Of live cells, a gate of CD45-negative and EpCAM-positive events represents TEC cells. (B) Within the TEC gate, two populations are separated by level of Ly51 and UEA-1. cTECs have higher expression of Ly51; mTECs have higher level of UEA-1. (C) MHC II molecules are highly expressed on cTECs. (D) mTECs consists of MHC IIhigh and MHC IIlow sub-populations, which are separately referred to as mTEChigh and mTEClow. Please click here to view a larger version of this figure.
 Figure 3. A liberase-based protocol allowed higher cell yields from thymus than using a multi-step collagenase/dispase protocol. Thymus cells were prepared using a previously published multi-step collagenase/dispase protocol or this liberase-based protocol. The two groups of samples were analyzed by flow cytometry. (A) Representative FACS profiles are shown. The numbers indicate the percentages of TEC cells in each group. (B) Total cell numbers of TEC per mouse thymus are shown in the bar graph. Error bars indicate the standard deviation (n >3). A two-tailed, unpaired T test was performed using software Prism. P <0.0001. Please click here to view a larger version of this figure.
Figure 3. A liberase-based protocol allowed higher cell yields from thymus than using a multi-step collagenase/dispase protocol. Thymus cells were prepared using a previously published multi-step collagenase/dispase protocol or this liberase-based protocol. The two groups of samples were analyzed by flow cytometry. (A) Representative FACS profiles are shown. The numbers indicate the percentages of TEC cells in each group. (B) Total cell numbers of TEC per mouse thymus are shown in the bar graph. Error bars indicate the standard deviation (n >3). A two-tailed, unpaired T test was performed using software Prism. P <0.0001. Please click here to view a larger version of this figure.
 Figure 4. Enrichment of TECs by the panning method. FACS profiles of pre- and post-enriched thymic stromal cells are shown. Prepared thymus cells were stained with anti-CD90 antibody. CD90-positive thymocytes compose over 95% of thymus cells. During the TEC enrichment procedure, thymocytes are captured and depleted on the pre-coated panning plate, thus TECs are enriched in the supernatant. (A) and (B), TEC gates are shown. The numbers show the proportion of TECs amongst total live cells in the starting population (Pre-enrichment) or after enrichment (Post-enrichment). (C) and (D), Cells within cTEC and mTEC gates are shown. The numbers display the percentages of cTEC and mTEC in the TEC populations from each group. (E) Cell numbers of cTECs and mTECs per mouse thymus are shown in the graph. Data are representative of 5 experiments. “Pre” stands for pre-enrichment sample; “Post” stands for post-enrichment sample; small horizontal lines indicate the mean. Please click here to view a larger version of this figure.
Figure 4. Enrichment of TECs by the panning method. FACS profiles of pre- and post-enriched thymic stromal cells are shown. Prepared thymus cells were stained with anti-CD90 antibody. CD90-positive thymocytes compose over 95% of thymus cells. During the TEC enrichment procedure, thymocytes are captured and depleted on the pre-coated panning plate, thus TECs are enriched in the supernatant. (A) and (B), TEC gates are shown. The numbers show the proportion of TECs amongst total live cells in the starting population (Pre-enrichment) or after enrichment (Post-enrichment). (C) and (D), Cells within cTEC and mTEC gates are shown. The numbers display the percentages of cTEC and mTEC in the TEC populations from each group. (E) Cell numbers of cTECs and mTECs per mouse thymus are shown in the graph. Data are representative of 5 experiments. “Pre” stands for pre-enrichment sample; “Post” stands for post-enrichment sample; small horizontal lines indicate the mean. Please click here to view a larger version of this figure.
Discussion
In the protocol, critical steps are the preparation of thymus stromal cells (Section 3) and the enrichment of TECs (Section 4). It is strongly recommended that fresh enzyme solution is prepared each time and the tissue is treated as soon as possible. For pooled thymi, optimizing the volume of enzyme solution is required depending upon the number of thymi. If any tissue residue is left after the processing, adding more enzyme solution and extension of incubation time could increase cell yield. During enrichment of TECs, completing collection of the supernatant and repeating the plate rinse outlined in Section 5.3 will decrease cell loss.
In 1980, Wekerle et al. first reported a type of TECs - ”thymic nurse cells” (TNCs)33, which are large cortical epithelial cells that completely envelop many viable lymphoid cells within intracellular vesicles. In 1982, Kyewski and Kaplan described various isolation conditions that influence the yield of TNCs34. Recently, Takahama and colleagues revisited the isolation and characteristics of TNCs. They identified such cells on the basis of preferential staining for CD45 only after membrane permeabilization35. They also described “broken” TNC in their preparation, which consisted of a cTEC and multiple bound thymocytes. Such broken TNC were positive for extracellular CD45 and for the cTEC marker β5t and comprised 70% of total cTECs in their preparation. Indeed, we also observed a similar cell population, which was CD45high EpCAM+ and Ly51+ in our prepared thymus cell suspension, although comprised less than 40% of total cTECs with our isolation procedure. These broken TNC are removed from TEC enrichment protocols, like ours, that utilize depletion with either CD90 or CD45. Thus, a future challenge will be to minimize the loss of this population.
Compared to the existing multi-step digestion procedure29, the Liberase digestion protocol allows a significantly higher cell yield of TECs (Figure 3). Other groups have also reported their new methods for thymic stromal cell isolation by modifying Gray’s method30,36, although cell yield was not significantly increased. Recently, Chidgey and colleagues independently reported a TEC protocol using Liberase digestion and they also obtained effective release of TECs37. However, the first thymocyte release step of their protocol has the potential to discard about 1.6 x 104 TECs from each thymus29, most of which are cTECs. Due to density gradient enrichment causing loss of smaller stromal cells, an AutoMACS-based CD45-microbead depletion is popularly used for enrichment of TECs prior to FACS purification29,36,37. Compared to the AutoMACS-based enrichment using CD45-microbead depletion protocols29, the panning enrichment obtained higher recovery rates of TECs. Overall, the protocol presented here aggregates a number of previously reported advantages. TEC subsets purified with this protocol (Section 6) have been successfully used for further analysis, such as Real Time-PCR and Western blot analysis12.
This protocol is also suitable for isolation and study of other thymic stromal cells, such as dendritic cells. We believe that robust isolation of thymic stromal cells, particularly TECs, will accelerate understanding of how the thymus functions for T cell development and achieving in vitro T cell constitution. Furthermore, characterization of these cells has great practical value for the medical community, since a common problem with recovery from therapies (such as those used in transplantation and cancer treatment) is poor reconstitution of epithelial cells in the thymus leading to immunodeficiency.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 AI088209 (to K.A.H.). We also thank the University of Minnesota Flow Cytometry Resource.
References
- Rothenberg EV, Moore JE, Yui MA. Launching the T-cell-lineage developmental programme. Nat. Rev. Immunol. 2008;8:9–21. doi: 10.1038/nri2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Takahama Y. Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol. 2012;33:256–263. doi: 10.1016/j.it.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat. Rev. Immunol. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- Takahama Y, Takada K, Murata S, Tanaka K. beta5t-containing thymoproteasome: specific expression in thymic cortical epithelial cells and role in positive selection of CD8. T cells. Curr. Opin. Immunol. 2012;24:92–98. doi: 10.1016/j.coi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Xing Y, Wang X, Igarashi H, Kawamoto H, Sakaguchi N. Protein phosphatase subunit G5PR that regulates the JNK-mediated apoptosis signal is essential for the survival of CD4 and CD8 double-positive thymocytes. Mol. Immunol. 2008;45:2028–2037. doi: 10.1016/j.molimm.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Benoist C, Mathis D. Treg cells, life history, and diversity. Cold Spring Harb. Perspect. Biol. 2012;4:a007021. doi: 10.1101/cshperspect.a007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobezinsky LA, et al. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat. Immunol. 2012;13:569–578. doi: 10.1038/ni.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu. Rev. Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Hogquist KA. T-cell tolerance: central and peripheral. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Lane PJ, Jenkinson EJ. Generating intrathymic microenvironments to establish T-cell tolerance. Nat. Rev. Immunol. 2007;7:954–963. doi: 10.1038/nri2187. [DOI] [PubMed] [Google Scholar]
- Xing Y, Jameson SC, Hogquist KA. Thymoproteasome subunit-beta5T generates peptide-MHC complexes specialized for positive selection. Proc. Natl. Acad. Sci. U. S. A. 2013;110:6979–6984. doi: 10.1073/pnas.1222244110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu. Rev. Immunol. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- Rezzani R, Bonomini F, Rodella LF. Histochemical and molecular overview of the thymus as site for T-cells development. Prog. Histochem. Cytochem. 2008;43:73–120. doi: 10.1016/j.proghi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Rodewald HR. Thymus organogenesis. Annu. Rev. Immunol. 2008;26:355–388. doi: 10.1146/annurev.immunol.26.021607.090408. [DOI] [PubMed] [Google Scholar]
- Griffith AV, et al. Spatial mapping of thymic stromal microenvironments reveals unique features influencing T lymphoid differentiation. Immunit. 2009;31:999–1009. doi: 10.1016/j.immuni.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerder S, Viret C, Luche H, Ardouin L, Malissen B. Differential processing of self-antigens by subsets of thymic stromal cells. Curr. Opin. Immunol. 2012;24:99–104. doi: 10.1016/j.coi.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Xing Y. Why CD8+ T cells need diversity when growing up. Immunit. 2010;32:5–6. doi: 10.1016/j.immuni.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Jenkinson EJ, Parnell S, Shuttleworth J, Owen JJ, Anderson G. Specialized ability of thymic epithelial cells to mediate positive selection does not require expression of the steroidogenic enzyme p450scc. J. Immunol. 1999;163:5781–5785. [PubMed] [Google Scholar]
- Murata S, et al. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science. 2007;316:1349–1353. doi: 10.1126/science.1141915. [DOI] [PubMed] [Google Scholar]
- Chidgey AP, Pircher H, MacDonald HR, Boyd RL. An adult thymic stromal-cell suspension model for in vitro positive selection. Dev. Immunol. 1998;6:157–170. doi: 10.1155/1998/10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izon DJ, Nieland JD, Godfrey DI, Boyd RL, Kruisbeek AM. Flow cytometric analysis reveals unexpected shared antigens between histologically defined populations of thymic stromal cells. Int. Immunol. 1994;6:31–39. doi: 10.1093/intimm/6.1.31. [DOI] [PubMed] [Google Scholar]
- Jenkinson EJ, Anderson G, Owen JJ. Studies on T cell maturation on defined thymic stromal cell populations in vitro. J. Exp. Med. 1992;176:845–853. doi: 10.1084/jem.176.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L, Klugmann M, Nave KA, Tuohy VK, Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat. Med. 2000;6:56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- Shortman K, Vremec D, D'Amico A, Battye F, Boyd R. Nature of the thymocytes associated with dendritic cells and macrophages in thymic rosettes. Cell. Immunol. 1989;119:85–100. doi: 10.1016/0008-8749(89)90226-8. [DOI] [PubMed] [Google Scholar]
- Volkmann A, Zal T, Stockinger B. Antigen-presenting cells in the thymus that can negatively select MHC class II-restricted T cells recognizing a circulating self antigen. J. Immunol. 1997;158:693–706. [PubMed] [Google Scholar]
- Yang SJ, Ahn S, Park CS, Choi S, Kim MG. Identifying subpopulations of thymic epithelial cells by flow cytometry using a new specific thymic epithelial marker, Ly110. J. Immunol. Method. 2005;297:265–270. doi: 10.1016/j.jim.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Gray DH, Chidgey AP, Boyd RL. Analysis of thymic stromal cell populations using flow cytometry. J. Immunol. Method. 2002;260:15–28. doi: 10.1016/s0022-1759(01)00493-8. [DOI] [PubMed] [Google Scholar]
- Gray DH, et al. Unbiased analysis, enrichment and purification of thymic stromal cells. J. Immunol. Method. 2008;329:56–66. doi: 10.1016/j.jim.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Williams KM, et al. Single cell analysis of complex thymus stromal cell populations: rapid thymic epithelia preparation characterizes radiation injury. Clin. Transl. Sci. 2009;2:279–285. doi: 10.1111/j.1752-8062.2009.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanof ME. Purification of T cell subpopulations. Curr. Protoc. Immunol. 2001;7(Unit 7.3) doi: 10.1002/0471142735.im0703s00. [DOI] [PubMed] [Google Scholar]
- Mage MG. Fractionation of T cells and B cells using panning techniques. Curr. Protoc. Immunol. 1991;3(Unit 3.5) [Google Scholar]
- Wekerle H, Ketelsen UP, Ernst M. Thymic nurse cells. Lymphoepithelial cell complexes in murine thymuses: morphological and serological characterization. The Journal of experimental medicin. 1980;151:925–944. doi: 10.1084/jem.151.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyewski BA, Kaplan HS. Lymphoepithelial interactions in the mouse thymus: phenotypic and kinetic studies on thymic nurse cells. J Immuno. 1982;128:2287–2294. [PubMed] [Google Scholar]
- Nakagawa Y, et al. Thymic nurse cells provide microenvironment for secondary T cell receptor alpha rearrangement in cortical thymocytes. Proc. Natl. Acad. Sci. U. S. A. 2012;109:20572–20577. doi: 10.1073/pnas.1213069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland BT, Gravano D, Castillo J, Montoy S, Manilay JO. Enhanced isolation of adult thymic epithelial cell subsets for multiparameter flow cytometry and gene expression analysis. J. Immunol. Method. 2011;367:85–94. doi: 10.1016/j.jim.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Seach N, Wong K, Hammett M, Boyd RL, Chidgey AP. Purified enzymes improve isolation and characterization of the adult thymic epithelium. Journal of immunological method. 2012;385:23–34. doi: 10.1016/j.jim.2012.07.023. [DOI] [PubMed] [Google Scholar]


