Abstract
Over the past several years, the field of cancer research has directed increased interest towards subsets of obesity-associated tumours, which include mammary, renal, oesophageal, gastrointestinal and reproductive cancers in both men and women. The increased risk of breast cancer that is associated with obesity has been widely reported; this has drawn much attention and as such, warrants investigation of the key mechanisms that link the obese state with cancer aetiology. For instance, the obese setting provides a unique adipose tissue microenvironment with concomitant systemic endocrine alterations that favour both tumour initiation and progression. Major metabolic differences exist within tumours that distinguish them from non-transformed healthy tissues. Importantly, considerable metabolic differences are induced by tumour cells in the stromal vascular fraction that surrounds them. The precise mechanisms that underlie the association of obesity with cancer and the accompanying metabolic changes that occur in the surrounding microenvironment remain elusive. Nonetheless, specific therapeutic agents designed for patients with obesity who develop tumours are clearly needed. This Review discusses recent advances in understanding the contributions of obesity to cancer and their implications for tumour treatment.
Introduction
In the context of metabolism, the basic principles of conservation of energy dictate that excess energy intake and decreased physical activity result in an increased accumulation of adipose tissue, leading to obesity. Excess adipose tissue accrual is associated with dysfunction of this tissue, which can predispose indivi duals to develop type 2 diabetes mellitus (T2DM) and cardiovascular disease.1 Additionally, indivi duals who are overweight (BMI 25.0–29.9 kg/m2) or who have obesity (BMI ≥30 kg/m2) are at increased risk of developing several types of cancer. Growing evidence from both clinical and preclinical studies indicates that increased adiposity is associated with increases in cancer incidence, morbidity and mortality.2,3 However, despite strong epidemiological evidence linking obesity to increased cancer risk and mortality,4,5 mechanistic insights explaining these data are notably lacking. Results from preclinical studies suggest that components of chronic inflammation (which is often associated with obesity), as well as the unique nature of cancer stem cells (CSCs) derived from adipose tissue in indivi duals with obesity, might contribute to the increased cancer risk of such patients.6–8 Moreover, prediagnosis BMI is an important indicator of survival in patients who develop colorectal cancer,9 and poorer prognosis and higher recurrence rates of cancer after radiotherapy or chemo-therapy are more frequently observed in patients with obesity than in lean ones.10,11 Together, these observations highlight the need to focus research on the contributions of obesity to cancer incidence and outcomes. Understanding the key pathological features of dys-functional adipose tissue might, therefore, offer important mechanistic insights into the relationship between obesity and cancer.3
Three main factors are considered to connect obesity and cancer: the insulin–IGF-1 axis, sex hormones, and the adipocyte-derived cytokines (termed adipokines). Each of these three factors is intimately linked to endocrine and paracrine dysregulation of adipose tissue in obesity.3 For example, local metabolic alterations in adipose tissue in individuals with obesity result in multiple systemic metabolic alterations, such as insulin resistance, hyperglycaemia, dyslipi daemia and chronic inflammation.12,13 Tumours invade stromal compartments that are rich in adipose tissue, and adipo cytes function as endocrine cells to critically shape the tumour microenvironment and contribute to tumour development and progression.3 These changes contribute to the increased cancer risk and poor outcomes in individuals with obesity. In this Review, we will discuss the metabolic symbiosis between adipocytes and tumour cells with respect to subtypes of lipid metabolites, hyperglycaemic memory effects and adipocyte progenitor cells. We will also consider how these factors are associated with metabolic abnormalities in the adipose tissue of individuals with obesity, and ways in which they could influence obesity-related cancer risk and mortality.
The link between obesity and cancer
Adipose tissue and the tumour microenvironment
The survival of cancer cells is critically dependent on their interactions with neighbouring nonmalignant cells of the tumour stroma. These cells include fibro-blasts, endothelial cells and pericytes, all of which are embedded in a unique extracellular matrix.14 The contributions of the tumour stroma to cancer cell survival have been widely studied. A primary focus of investigations has been on cancer-associated fibroblasts and tumour-associated macrophages, both of which are well- established tumour-promoting cell types.15,16 The adipocytes surrounding tumours are another major component of the tumour stroma. Moreover, adipose tissue within the tumour microenvironment actively contributes to tumour growth and metastasis by functioning as an endocrine organ, through secretion of signalling molecules (such as adipokines, proinflammatory cytokines, proangiogenic factors and extracellular matrix constituents) and acting as an energy reservoir for embedded cancer cells.17–19 Thus, the importance of adipose tissue as an integral contributor to cancer progression and r ecurrence is increasingly appreciated.20
Hypertrophic expansion of adipose tissue in the context of obesity shares many features with solid tumour growth. For example, tumour hypoxia is a hallmark of cancer that is associated with poor patient outcomes and resistance to chemotherapy.21,22 The rapid cellular proliferation and expansion of adipose tissue also induces hypoxia, which triggers compensatory angio genesis, so that limitations in nutrient and oxygen supply can be overcome.23 Similar to that seen in tumour growth, the hypoxia in adipose tissue that occurs in the context of obesity induces expression of the transcription factor HIF-1α, which upregulates a profibrotic pathway involving extracellular matrix proteins (such as collagens, matrix metallo proteinases and tissue inhibitors of metalloproteinases) and proinflammatory cytokines (such as IL-6, tumour necrosis factor [TNF] and C-C motif chemokine 2 [CCL2, also known as monocyte chemo tactic protein 1]).24–26 The micro environment within local adipose deposits clearly provides a tumour-permissive niche for transformed, infiltrating cells. Importantly, fibrotic and proinflammatory cytokines that are characteristic of adipose tissue in individuals with obesity, such as endotrophin (a C-terminal cleavage fragment of collagen 3α(VI) chain), IL-6, IL-8 and PAI-1, have also been directly implicated in tumour growth and metastasis.20,27,28 However, the molecular basis of the interactions between these key mediators, cancer cells and adipocytes, which create the tumour-permissive microenvironment, remains elusive.
Cancer-associated adipocytes
Adipose tissue comprises a heterogeneous cell population, which mostly consists of adipocytes but also contains various other stromal cells, including endothelial cells, pericytes, macrophages and adipocyte progenitor cells. Both adipocytes and the stromal vascu lar cells in adipose tissue contribute to tumour development and progression.29 In a subset of tumours, such as breast, prostate, ovarian, gastric, renal and colon cancers, growth and/or metastasis predominantly occur as a result of the adipocyte-rich micro environments in which these tumours are found, and reflect a role for adipo cytes in tumour maintenance and progression.17,20 For many of these cancers, the degree of adipose tis sue invasion is a reflection of the aggressiveness of the tumour, and extensive invasion is indicative of poor prognosis.30,31 Emergence of rapidly proliferat ing stromal cells is also a feature of the hypertrophic and hyper-plastic expansion of adipose tissue in obesity. As such, adipocyte progenitors in stromal vascular fractions rapidly proliferate and differ entiate into mature adipocytes.32 Given this simi larity, it is plausible that multi-potent adipocyte progenitors in the stromal vascu lar fraction might contribute to cancer development and progression. Several reports show that adipocyte progenitors in adipose tissues from individuals with obesity contribute to tumour progression through increasing the number of endothelial precursors, pericytes and adipocytes, thereby resulting in angiogenesis and cancer cell pr oliferation in vivo.33,34
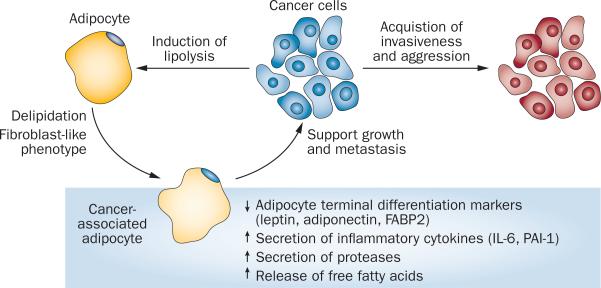
Cancer-associated adipocytes (CAAs) undergo considerable morphological and functional alterations dur ing cancer progression. In the presence of cancer cells, especial ly at the tumour invasive front, CAAs undergo delipidation and acquire a fibroblast-like phenotype. This phenotypic change is accompanied by loss of expression of adipocyte terminal differentiation markers, such as adiponectin, leptin and fatty acid binding protein, intestinal (FABP2) and by increased secretion of proinflammatory cytokines such as IL-6 and PAI-1.35,36 In vitro experiments, in which no direct contact is permitted between differentiated adipocytes and tumour cells, demonstrate that secreted paracrine signals from cancer cells can induce lipolysis in adipocytes, causing them to release free fatty acids.36 CAA delipidation and the accompanying increase in secretion of inflammatory cytokines and proteases creates an environ ment that encourages a shift in tumour cell pheno type towards increased invasiveness and a ggression (Figure 1).36
Figure 1.
Reciprocal signalling between cancer-associated adipocytes and cancer cells. Crosstalk between cancer-associated adipocytes and cancer cells within the tumour microenvironment is crucial for creation of a niche that is permissive for cancer cell growth and metastasis. Cancer cells stimulate lipolysis in adipocytes, which leads to delipidation and acquisition of a fibroblast-like phenotype in adipocytes. These alterations in cancer-associated adipocytes are associated with functional changes in the cells, such as loss of adipogenic markers, increased secretion of inflammatory cytokines and proteases, and increased release of free fatty acids, all of which support aggressive tumour growth and invasiveness.
The adipocyte-rich tissue surrounding tumour cells offers an easily accessible reservoir of lipids, and the pheno typic transition of CAAs is likely to reflect the high energy requirements of cancer cells. Cancer cells can use free fatty acids as a fuel source, particularly if they metasta size to the abdominal cavity where there is easy access to adipocytes.17 Cancer cells also reprogram adipocytes into CAAs to support tumour growth and survival. Nevertheless, the mechanisms that dictate the heterotypic crosstalk between cancer cells and adipocytes need to be further studied.
The source of the increased population of fibroblast-like cells among CAAs remains unclear. Cancer cells might induce nonmalignant stromal cells, such as fibro-blasts and macrophages, to become cancer-associated fibroblasts and tumour-associated macrophages.14,37 The question of whether these cells dedifferentiate from mature adipocytes, are newly recruited cells of mesenchymal origin, or are simply delipidated mature adipocytes that still express most of the characteristic adipocyte markers, remains unanswered.
Cancer stem cells
Stromal cell populations that have tumorigenic potential and drive tumour growth are known as CSCs.38 These cells are commonly isolated from tumours using a combi nation of positive and negative selection markers; for example, breast cancer stem cells are represented by the CD44+CD22low subset within a given tumour.39 Many other solid tumours contain CSCs, which can drive metastasis, cancer recurrence and drug resistance;40 CSCs are, therefore, a key target of anticancer therapeutic agents.41 Various types of CSCs will only proliferate rapidly in a specific permissive tumour microenvironment, indicating a crucial role of environmental stimuli to support tumour growth and survival.38
Various cytokines released from adipose tissue in obese states stimulate CSC growth and survival. High levels of proinflammatory cytokines, such as IL-6 and IL-8, stimulate CSCs in breast cancer models.42,43 Furthermore, TGF-β confers stem-cell-like traits to cancer cells as well as inducing epithelial–mesenchymal transition (EMT),44,45 and leptin has a key role in stimulation and maintenance of certain populations of CSCs in breast cancer models, as discussed above.46,47 Our group also reported that endotrophin potentiates TGF-β-dependent EMT, which results in high levels of metastasis and resistance to chemotherapy.19,48 Clinically, individuals with obesity display more resistance to chemo therapy or radiotherapy than do lean individuals, which is in part due to the increased number of CSCs present in the adipose tissue of individuals with obesity.
Obesity-related inflammation
Chronic inflammation, which is exemplified by elevated circulating levels of IL-6 and TNF, is associated with obesity.49 Additionally, an accumulating body of clinical data suggests that individuals with obesity are at an elevated risk of developing several types of cancer. Many preclinical studies have, therefore, focused attention on inflammation as a possible mechanism linking obesity with increased tumour burden.8,50,51
Liver cancer
Exposure of male C57BL/6J mice to a high-fat diet (HFD) resulted in increased incidence of spon taneous hepato cellular carcinoma (HCC), although this effect was not observed in other inbred mouse strains.52 Interest ingly, notable differences were observed between the weights of the different inbred strains of mice fed a HFD: male C57BL/6J mice fed a HFD were on average 60–70% heavier than inbred male A/J mice receiving the same diet, suggesting that genetic background contributes to both diet-induced obesity and development of HCC.
The link between obesity and cancer was also explored using another model of HCC, in which diethylnitrosamine was administered by injection 14 days after birth, which resulted in hepatic adenoma formation by 8 months of age. In these experiments, both obese mice fed a HFD and leptin-deficient Lepob/ob mice (which are genetically predisposed to obesity) demonstrated an increased tumour burden compared with lean mice after challenge with diethylnitrosamine.8 Female C57BL/6 mice, which show attenuated inflammation in res ponse to a HFD, as well as male IL-6-knockout and TNF-knockout mice, were protected from increased HCC incidence under conditions of HFD-induced obesity,8 suggesting that elevated circulating levels of IL-6 and TNF in the obese animals were responsible for this d ifference in tumour initiation.8
Another research group subsequently showed that acyclic retinoids can inhibit diethylnitrosamine-induced hepatic neoplasia in obese mice.53 Treatment with these agents decreased levels of Stat3 protein, which is a downstream mediator of IL-6 signalling. Total serum levels of both IL-6 and TNF were decreased in the acyclic-retinoid-treated mice, suggesting that these compounds prevent HCC tumourigenesis in HFD-fed mice through inhibition of the IL-6–Stat3 axis.34
Colon cancer
Individuals with obesity are at an increased risk of developing colon cancer,54 and these findings have been readily recapitulated in rodent models. Specifically, Zucker obese rats, KK-Ay mice and HFD-fed mice all show an increased incidence of colon cancer following treatment with azoxymethane as compared to controls.55,56
A key finding that implicated inflammation in colon cancer initiation was that Lepob/ob mice fed a bean-based diet had a considerably lower tumour burden following azoxymethane treatment than did Lepob/ob mice fed a standard diet.57 The researchers attributed this observation to the fact that consumption of the bean diet resulted in considerably lower serum levels of IL-6. Treatment of leptin-receptor-deficient Leprdb/db mice with the ch olesterol-lowering drug pitavastatin resulted in decreased levels of circulating inflammatory cytokines, as well as reduced tumour initiation.58 Additionally, administration of angiotensin- converting enzyme inhibitors or angiotensin-receptor blockers reduced chronic inflammation in the Leprdb/db mouse, thereby attenuating tumour initi ation.59 Although all of these studies have reached similar conclusions, the field still lacks the neces sary preclinical models to demon strate direct causality and, therefore, these findings remain correlative at best. Cell-type-specific elimination of the IL-6 receptor in these models might, however, provide important insights into the relationship between obesity, inflammation and tumourigenesis.
Some direct evidence that inflammatory signalling has a role in colitis-associated cancer does exist. Results in a dextran-sodium-sulphate–azoxymethane model of intestinal inflammation in global IL-6-knockout mice, or mice with specific ablation of Stat3 from the gut, showed that the IL-6–Stat3 inflammatory axis contributed to tumour formation, and loss of IL-6 signalling resulted in significantly attenuated tumour development and progression.60 However, these studies did not provide evidence linking HFD-induced inflammation with increased tumour risk.
Emerging evidence suggests that TNF is a key mediator of both obesity-induced inflammation and colon cancer development. TNF is highly elevated in the colons of C57/BL6 mice fed a HFD.61 Treatment with infliximab, a TNF-neutralizing monoclonal antibody, decreased growth of colon cancer xenografts and decreased tumour incidence in azoxymethane-treated Lepob/ob mice.51 These data provide compelling evidence that local inflammation, primarily mediated by TNF, has a key role in tumour initiation in obese rodents.
Adipokines
Leptin
Leptin is a 16 kD hormone produced by adipocytes, and is known primarily for its role in the mammalian central nervous system, where it regulates food intake. Leptin receptors are expressed in almost every tissue and have a dynamic role in many organ systems, including the regulation of cancer growth. Leptin levels rise dramatically during states of obesity, and as a result, this adipokine is thought to have a pivotal role at the interface of obesity and cancer development.
Role in colon cancer
A case–control study conducted in the USA demonstrated that men with colorectal adenomas had elevated circulating leptin levels.62 However, the findings of this study have since been challenged. Other researchers demonstrated that leptin levels were not significantly correlated with increased colorectal cancer burden when factors such as BMI and waist circumference were taken into consideration.63 Regardless of the contrary outcomes reported, studies such as these have prompted investigations into the role of leptin in the development of colorectal cancer in both human-derived cell lines and in mouse models.
In the human colorectal cancer cell line HCT-116, leptin activates the PI3K–AKT pathway, resulting in increased cancer cell proliferation.64 This growth-stimulatory effect of leptin was mitigated by treatment with the PI3K inhibitor LY294002. Notably, other groups have argued that the Jak2–STAT3-activating capacity of leptin is responsible for stimulating colon cancer cell proliferation.65 However, these pathways are clearly not mutually exclusive, and these findings could simply imply that therapies targeting multiple pathways might be needed in certain patient populations.64,66 Lepob/ob mice are significantly less sensitive to azoxymethane-induced polyp formation than are wild-type controls,66 suggesting a role for leptin in stimulating initiation of colon cancer. Furthermore, a comparison of wild-type and Lepob/ob mice fed a HFD (a challenge that is strongly associated with increased polyp formation) showed that Lepob/ob mice were strongly protected from polyp f ormation compared with wild-type mice.66
Role in ovarian cancer
A study of epithelial ovarian cancers in Middle Eastern women revealed that up to 60% of tumours showed overexpression of the leptin receptor, and that this upregulation correlated with reduced progression-free survival.67 These findings stimulated further research to identify a mechanism whereby leptin might influence the growth of ovarian cancer cells. Investigations in the OVCAR-3 ovarian cancer cell line showed that leptin signalling inhibited apoptosis and stimulated cell division, via inhibition of p21 and increased expression of cyclin D1 and myeloid cell leukaemia sequence 1.68,69 Interestingly, exposure to bisphenol A, a common molecule found in many plastics, increased leptin receptor expression and inhibited caspase-3 expression and activity in ovarian cancer cell lines.51,70 Moreover, the inhibition of caspase-3 activity was augmented by exposure to estrogen.71 Together, these observations demonstrate a direct role for leptin in the growth and survival of ovarian cancer cells, but further in vivo studies are clearly needed to gain a thorough understanding of the relevance of these findings to the clinical setting.
Role in breast cancer
A meta-analysis showed that women with breast cancer frequently exhibit significantly elevated circulating levels of leptin, which suggests a role for this adipokine in disease progression.72 Furthermore, patients with breast cancer who had increased levels of leptin receptor mRNA transcripts in their tumours, concomitant with elevated serum leptin levels, had a poorer prognosis than did their counterparts without these factors.73
Studies in breast cancer cell lines showed that leptin induced growth of breast tumour cells through activation of the JAK–STAT and PI3K signalling pathways.74 Leptin also potently activated the migration and motility of breast cancer cells.75 Moreover, leptin signalling maintained CSC-like properties in triple-negative breast cancer cells (that is, lacking expression of the estrogen receptor, progesterone receptor and HER2), which enabled CSCs with self-renewal capacity to mediate tumour recurrence and metastasis.7 Contributions of leptin to mammary tumour development have also been assessed in a rodent model. In the MMTV-PyMT mammary- tumour-prone model, hypothalamus-specific restoration of functional leptin receptors in female Leprdb/db mice normalized their ductal development, and also ameliorated the obesity phenotype of these animals.76 Peripheral leptin-receptor-deficiency in these MMTV-PyMT mice attenuated tumour growth and metastasis, indicating that these characteristics are indepen dent of the tumour and reflect the metabolic state of the host. The leptin-receptor-deficient tumour cells also had higher mitochondrial respiration capacity and attenuated activation of ERK and Stat3.76 Overall, these observations demonstrate that leptin-receptor signalling has a crucial role in mammary cancer progression, and that inhibition of this pathway could hold substantial therapeutic benefit.
Adiponectin
Adiponectin is primarily known for its role in insulin sensitization of tissues such as muscle and liver. Long thought to act through an AMP kinase (AMPK)-dependent signalling pathway, emerging evidence now suggests that adiponectin modulates the activity of a ceramidase, which leads to decreased intracellular levels of ceramides, improved insulin sensitivity and inhibition of apoptosis.77 In the past 20 years, substantial efforts have been directed toward identifying the functions of adiponectin in various tissues, including its effects on tumour growth.
Role in HCC
In experiments using a choline-deficient l-amino-acid-defined diet, which predisposes mice to nonalcoholic steatohepatitis, adiponectin-knockout mice bore significantly more tumours in their livers after 24 weeks than did control mice. These findings demonstrated that loss of adiponectin renders the liver more susceptible to hepatic adenoma formation.78 Adiponectin also increased caspase-3 activation and apoptosis in HCC cell lines, via inhibition of serine–threonine-protein kinase mTOR (mTOR), in a c-JNK dependent manner.79 These tumour-inhibiting effects of adponectin were corrobor ated by in vivo observations showing that overexpression of adiponectin resulted in inhibition of tumour growth and metastasis in nude mice.80 Interestingly, these experiments also found decreased macrophage and hepatic stellate cell infiltration into the tumour grafts, as well as decreased angiogenesis, suggest ing that these functions are the primary mechanisms by which adiponectin exerts its anticancer effects in the context of HCC.
In states of obesity, adiponectin levels decrease and leptin levels rise. In a study assessing this dynamic relation ship between the adipokines and their effects on tumour progression, adiponectin inhibited the leptin-induced proliferation of HCC tumour cells via decreased activation of Stat3.81 These findings were confirmed in a xenograft model with nude mice, in which adiponectin treatment was associated with a significantly reduced leptin-induced tumour burden.
Role in colon cancer
A prospective study has demonstrated that low levels of plasma adiponectin are correlated with an increased risk of colon cancer;82 however, the mechanisms through which adiponectin protects against colon cancer formation remain unknown. Adiponectin receptors are expressed on both normal and malignant intestinal epithelial cells, suggesting that adiponectin could exert its antitumour effects in either a cell autonomous or non-autonomous fashion.83 This hypothesis has been tested by several groups, who have determined that adiponectin activates AMPK signalling, and the consequent suppression of mTOR and S6 kinase phosphorylation results in inhibition of cell proliferation.84,85 Several in vivo studies have demonstrated that adiponectin deficiency promotes intestinal carcinogenesis in both APCMin mice (which spontaneously develop intestinal tumours) and wild-type mice treated with azoxy-methane.86 By contrast, other groups showed that adi ponectin deficiency alone did not promote polyp formation in mice fed standard chow, but did significantly increase polyp formation in mice subjected to azoxymethane challenge and fed a HFD.87 These studies clearly demonstrate a role for adiponectin as a repressor of intestinal tumour initiation.
Role in breast cancer
Strong evidence supports an epidemiological relationship between obesity, low levels of adiponectin and an increased incidence of breast cancer;88 however, pre-clinical studies from our own and other groups suggest that a nuanced assessment of the contribution of adiponectin to this relationship needs to be applied if we go beyond the use of this hormone as an obesity marker. Using the MMTV-PyMT mouse model of breast cancer, we and others have demonstrated that delayed tumour development occurs in adiponectin-null mice,89,90 and that the proangiogenic effects of adiponectin also enhance tumour neovascularization.70 These findings are consistent with observations of enhanced accumulation of sphingosine-1-phosphate, which is a potent pro angiogenic stimulus, in the presence of elevated ad iponectin levels.77
Metabolic symbiosis—cancer and obesity
Warburg and reverse Warburg effects
The Warburg effect is the most widely appreciated metabolic phenotype in cancer cells. This term refers to a metabolic shift in ATP generation from oxidative phosphorylation to glycolysis, which occurs even under aerobic conditions.91 Emerging insights also emphasize the importance of metabolites from tumour stromal cells, with a special focus on those secreted by cancer-associated fibroblasts, which are utilized by cancer cells for growth and metastasis.15 This phenomenon is referred to as the reverse Warburg effect, which posits that aerobic glycolysis occurs in stromal cells rather than cancer cells, and provides lactate and pyruvate to cancer cells through a paracrine exchange of these nutrients.92 The transferred nutrients are used by cancer cells to generate energy by mitochondrial oxidative metabolism.93 Similarly, increased lipolytic activity in stromal adipocytes provides free fatty acids to tumour cells, which are then utilized as an energy source to facilitate β-oxidation in the cancer cell mitochondria.17 Collectively, cancer cells are metabolically very flexible and actively direct the metabolism of tumour stromal cells, such as fibroblasts and adipocytes, to take advantage of the unique tumour microenvironment. In other words, catabolic pathways in these tumour stromal cells fuel anabolic tumour growth by transferring high-energy metabolites such as lactate, ketones, glutamine and fatty acids to cancer cells.
Hyperglycaemia
The results of clinical studies and meta-analyses indicate that an important association exists between diabetes mellitus and a subset of cancers, including liver, pancreas, endometrium, colorectal, breast and renal cancers; however, little is known about the molecular mechanisms underlying these connections.94 Hyperglycaemia is a hallmark of both type 1 diabetes mellitus and T2DM. Furthermore, hyperglycaemia frequently occurs in patients with other chronic diseases, such as obesity and cardiovascular disease.95 The increased prevalence of diabetes mellitus in patients with cancer has drawn attention to the effects of hyperglycaemia on cancer initiation and progression.96,97
The study of glucose metabolism in tumours has been intensively studied since Warburg's observation in the 1950s that tumours face hypoglycaemia in a tumour-mass-dependent manner.98,99 These metabolic observations prompted a number of groups to look at tumour growth characteristics in hyperglycaemic environments using animal models. Severe hypoinsulinaemia was induced in mice by chemical destruction of pancreatic β cells using alloxan, thereby achieving hyper-glycaemic conditions in vivo. These mice demonstrated decreased tumour growth owing to the lack of insulin, which functions as a potent mitogenic factor.100–102 This observation is also consistent with the current understanding that even though many tumour cells utilize glycolytic mecha nisms, the basic availability of glucose is rarely rate- limiting for cancer growth. This experimental limitation in early studies meant that the effects of h yperglycaemia on cancer cells could not be properly investigated.
Our own studies, in which we induced hyperglycaemia in mice by prompting apoptosis of pancreatic β cells, convinced us that a ‘hyperglycaemic memory effect’ in cancer cells contributes to aggressive tumour growth.103,104 Hyperglycaemic conditions do induce epi-genetic modification of components of some oncogenic pathways, such as the EGFR, HER2 and HER3 signalling cascades and their corresponding ligands (such as ne uregulin-1), thereby stimulating tumour growth even after the cells have been returned to a euglycemic environ ment.103,104 Chronic exposure to elevated glucose levels, therefore, induces epigenetic modifications at the level of DNA and chromatin. Identifying hyperglycaemic conditions in patients at the time of diagnosis could help to direct early intervention efforts towards key targets, such as HER3, in the context of breast cancer.
Hyperinsulinaemia and IGF-1 signalling
Obesity is associated with several risk factors for cancer development, specifically hyperinsulinaemia and low serum levels of insulin-like growth factor-binding protein (IGFBP)-1.15,105–109 Overweight individuals also have elevated circulating levels of insulin-like growth factor 1 (IGF-1),110 which could also confer increased susceptibility to cancer development. Insulin is exclusively produced and secreted by pancreatic β cells, whereas IGF-1 is mainly produced in the liver. IGF-1 and insulin share sequence similarities,105 and both can activate mitogenic pathways and inhibit cell apoptosis. Obesity can contribute to carcinogenesis by activating the IGF-1–insulin pathway, which tradition ally stimulates intracellular signalling through mitogen-activated protein kinases (MAPKs) or the PI3K–AKT cascade.105,111–113
In general, insulin-receptor-mediated signalling regulates metabolic pathways, whereas proliferation and cell growth are stimulated by IGF-1-receptor-mediated signalling, which suggests that the mitogenic effects of insulin might be mediated by IGF-1. Insulin increases IGF-1 levels through direct regulation of IGF1 transcription. Importantly, in addition to insulin, other factors also regulate IGF bioavailability, such as IGFBPs. Six iso-forms of IGFBPs have been identified, of which the most abundant is IGFBP-3. Levels of IGFBP-3 are regulated by nutrients.114 Hyperinsulinaemia can reduce levels of IGFBP-1,115 suggesting that insulin might have a critical role in obesity-aggravated cancer development by r egulating IGFs and IGFBPs.
The insulin receptor and the IGF-1 receptor (IGF-1R) are integral to insulin–IGF-1 signalling pathways. Insulin and bioavailable IGF-1 can crossreact with each other's receptor, and IGF-1R and the insulin receptor can form heterodimers. Insulin receptor–IGF-1R hybrids, the insulin receptor, IGF-1R and IGF-2R phosphorylate insulin receptor substrates, which can then activate MAPKs and ultimately activate the PI3K– AKT oncogenic pathway. In mouse studies, Igf and Igf1r transgenic mice develop a variety of cancers.116–120 Therefore, preventing the obesity-induced activation of the insulin–IGF-1 axis could be a viable therapeutic target for cancer.
Sex hormones
Cytochrome P450 aromatase, which is encoded by the CYP19 gene, converts androgens to estrogens121 and, therefore, has a critical role in determining the balance between the levels of these sex hormones. The ovarian follicle is a major site for producing aromatase in premenopausal women; however, in postmenopausal women aromatase is predominantly produced in adipose tissues and skin.122 The rate of conversion of androgens to estrogens is elevated in postmenopausal women with obesity,123,124 and an increased level of estrogens in these women is associated with an increased risk of breast cancer.125 Fibroblasts in adipose tissue have long been thought to be critical for aromatase expression, and consequently for estrogen production.126,127 Some researchers have also proposed that cytokines, prostaglandins and hormones regulate aromatase gene expression in obesity; for example, levels of prostaglandin E2 are elevated in obesity and promote aromatase expression in breast adipose tissues.128
Obesity is associated with increased activity and expression of aromatase in certain cancers. Moreover, obesity is a chronic inflammatory state in which circulating levels of proinflammatory cytokines such as TNF and IL-6 are elevated, and TNF can induce aromatase expression in human adipose stromal cells.129
Blocking aromatase to inhibit estrogen production is an effective initial therapeutic strategy for estrogen-receptor-positive breast cancers until drug resistance develops. Whole-genome analysis of breast cancer samples identified 18 mutations that were associated with differential survival after treatment with an aroma-tase inhibitor;130 however, currently little is known about the mechanisms and factors that contribute to responses to aromatase inhibitor therapy. Results from a study that assessed chromatin-binding on a genome-wide scale suggested that the binding pattern of estrogen receptor α to target promoters and epigenetic modifications of H3K27me3 regulate drug resistance to aromatase i nhibitors in breast cancer.131
Prevention and treatment
Metformin
In the past decade, a series of publications reported that patients with T2DM who were treated with the oral biguanide metformin had a lower incidence of cancer than did their counterparts taking other medications for diabetes mellitus, including insulin and sulfonylureas.132,133 A number of studies have since attempted to determine the molecular mechanisms mediating this reduction in cancer incidence.
Metformin requires activation of AMPK to elicit its antidiabetic effects.134 AMPK-dependent inhibition of insulin-stimulated growth has been demonstrated in several breast cancer cell lines.135 Metformin-induced AMPK activation in these cell lines led to growth inhibition, and siRNA-mediated knockdown of AMPK was sufficient to restore the proliferative response to insulin stimulation. Metformin blocked protein translation via inhibition of mTOR, and this effect was specifically dependent on the serine–threonine-protein kinase STK11 (LKB1).136 Emerging data have demonstrated that metformin can selectively kill CSCs, as determined by decreased numbers of CD44highCD24low cells.137 This effect was especially strong following combination therapy with doxorubicin, and this treatment regime caused prolonged remission in a xenograft model. Metformin is highly active against cancer growth, and clinical trials are currently being conducted for a number of different malignancies.
Thiazolidinediones
The thiazolidinediones (a class of peroxisome proliferator-activated receptor γ agonists) have growth-inhibitory effects on transformed cells.138 When these com pounds were tested in combination treatment regimes, an unexpectedly potent synergy was observed between the platinum- based agent cisplatin and thiazolidinediones.139,140 Initial analysis revealed that treatment with rosiglitazone reduced metallothionine expression, which was at least partially responsible for the increased efficacy of cisplatin.140 However, our group's data present a different view of how thiazolidinediones might sensitize tumours to cisplatin-based chemotherapy.48 Our analysis of the tumour micro environment showed that treatment with cisplatin robustly increased the production of collagen α3(VI) chain, and that thiazolidinediones very effectively suppressed this alteration. In the MMTV-PyMT breast cancer model, tumours in collagen α3(VΙ) knockout mice are highly sensitized to cisplatin, and reconstitution with endotrophin restored tumour resistance to cisplatin. Inhibition of endotrophin with a monoclonal antibody also enhanced tumour sensitivity to cisplatin, suggesting that targeting this pathway might provide new possibilities for treatment in patients with tumour r esistance to platinum-based therapies.48
Exercise
For decades, debates have been waged over the effects of exercise on tumour initiation and progression. Despite heated controversy, this aspect of cancer physiology has proven particularly difficult to study, owing to the lack of long-term controlled trials and variability between exercise regimes. Taking these factors into consideration, a large comprehensive meta-analysis reported reasonable benefits, in terms of quality of life, cardiorespiratory fitness and overall patient well-being, in response to exercise therapy during breast cancer treatment.141 However, few studies have positively correlated survival in breast cancer patients with exercise regimes. An 18 year longitudinal study of 2,900 participants linked increased aerobic exercise following breast cancer diagnosis with improved survival; however, although trends were a pparent, no data in the study reached statistical significance.142
Many studies have used carcinogen-induced tumours in rodent models to test the potential of exercise to protect against cancer initiation and progression. A major confounding variable in these experiments is that the carcino gen used in most breast cancer studies, 2,7- dimethylbenzanthracene, is a procarcinogen that is converted to its active form in the liver, a metabolic process that might possibly be affected by exercise. Despite this concern, a comprehensive review on the subject reported a positive benefit from exercise on reducing tumour development in both 2,7- dimethylbenzanthracene and N-methyl-N-nitrosourea-induced mammary tumours.143 Whilst exercise slowed the progression of N-methyl-N-nitrosourea-induced mammary cancers in some studies, it potentiated 2,7-dimethylbenzanthracene-induced carcino genesis in other studies.144,145 Although these results are intriguing they are most likely not directly applicable to understanding human tumour initiation and progression. The limited available evidence does suggest that exercise may have beneficial effects, such as an improved prognosis, in patients with cancer. As individual responses to exercise are difficult to predict, and alterations in whole body metabolism, including changes in levels of circulating adipokines, may be different, more research is needed to identify the patient cohorts that are most likely to benefit from exercise.
Weight loss
The effect of weight loss on tumour initiation and progression is an important issue that offers a means for testing the reversibility of some mechanisms triggered by obesity. In a study of 2,437 women who underwent surgical resection of early-stage breast cancer, participants who were assigned to an intervention group with decreased dietary fat intake experienced concomitant weight loss and an improved rate of relapse-free survival compared with women in the control group; however, the mechanisms underlying this effect are unclear.146 As previously discussed, inflammation in adipose tissues is regarded as a critical mediator of both obesity and cancer. C-reactive protein (CRP) levels, a serum marker of inflammation, are increased in individuals who are overweight or have obesity147 and decline with weight loss.113,148 In a randomized controlled trial in postmenopausal women, weight loss achieved by caloric restriction was associated with a reduction in levels of several inflammatory markers, such as serum amyloid A, CRP and IL-6. Exercise combined with caloric restriction did not result in differences in levels of inflammatory markers when compared with caloric restriction alone.149 Although these findings are promising, whether the reductions in the levels of inflammatory markers were caused by caloric restriction itself, or by its weight-loss-associated metabolic benefits, is currently not clear. Furthermore, hypoxia increases levels of vascular endothelial growth factor (VEGF) in adipocytes and has a crucial role in tumour angiogenesis. Elevated BMI is also associated with increased levels of VEGF,150 which are also associated with poor outcomes in patients with obesity.146 Despite accumulating evidence for an anti-cancer role for both weight loss and caloric restriction, long-term studies with larger cohorts and better controls than have so far been conducted are still needed.
Conclusions and future directions
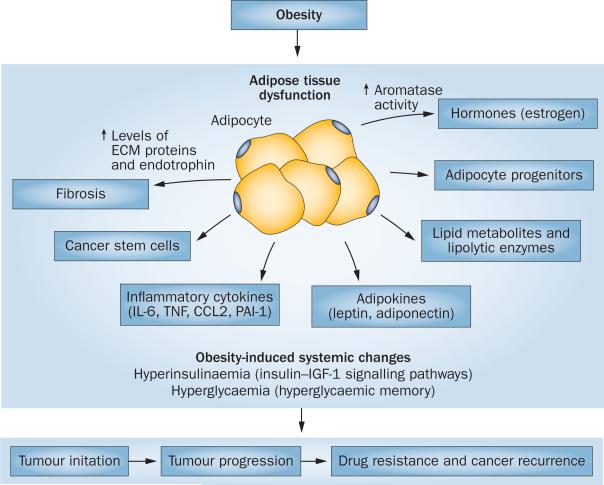
Patients with obesity are widely accepted to be at an increased risk of initiation, growth and progression of certain cancers; however, the molecular mechanisms that underlie these associations remain elusive. A model in which cancer cells reprogram adipocytes into CAAs to support tumour growth is emerging. Subsequent to being reprogrammed, CAAs provide a tumour- permissive microenvironment by releasing inflammatory, fibrotic and angiogenic factors, in addition to various lipids and other metabolites that promote tumour growth and metastasis (Figure 2).
Figure 2.
Potential consequences of obesity-induced dysfunction of adipose tissue on tumour initiation, progression and recurrence. Obesity leads to development of dysfunctional adipose tissue, which produces abundant levels of proinflammatory cytokines, sex hormones and lipid metabolites, along with altered adipokine profiles. The altered adipose tissue is a source of various ECM proteins, cancer stem cells, cancer-associated adipocytes and adipocyte progenitors. Each of these factors contributes to various stages of tumour progression, including initiation, growth and recurrence. Obesity-associated systemic metabolic changes, such as hyperinsulinaemia and hyperglycaemia, also contribute to a tumour-permissive environment. Abbreviation: ECM, extracellular matrix.
In the context of this model, it is not surprising that targeting adipose tissues with antiobesity and/or anti-diabetic agents, such as metformin and thiazolidinediones, might be a promising option to treat patients with cancer who are overweight or have obesity. Understanding the heterotypic interactions between adipocytes and cancer cells, which occur through paracrine signals, could lead to the identification of further novel targets for cancer therapies. Lifestyle interventions, such as exercise and weight loss, might also be considered as basic public health strategies for cancer prevention and treatment. The effects of exercise and/or weight loss on cancer mortality and recurrence are not yet clear, but this area is intuitively relevant for further studies.
A provocative question is whether reducing obesity is the only viable therapeutic approach to effectively decrease risk and improve outcomes for patients with cancer who are overweight or have obesity. Developing a mechanistic understanding of the contribution of dysfunctional adipose tissue to cancer progression will certainly reveal direct molecular targets for treating cancer in patients with high BMIs. In an age of individualized cancer therapy, an unmet need clearly remains for develop ment of targeted therapeutic approaches for patients with obesity and cancer.
Key points.
■ The current notion that obesity is a major risk factor for development of, and mortality associated with a subset of cancers is well appreciated; however, detailed mechanistic insights underlying this relationship are still lacking
■ Adipose tissues that influence functions of cancer cells, such as growth, metastasis and recurrence, are an integral part of the tumour microenvironment
■ Heterotypic signals between cancer-associated adipocytes and cancer cells provide a permissive niche for the growth and metastasis of tumours
■ Obesity-related inflammation is a plausible link between obesity and cancer
■ Metabolic symbiosis between stromal adipocytes and cancer cells may account, in part, for the link between obesity and cancer
■ The effects of obesity on cancer progression might be specifically curbed through weight-loss interventions, such as exercise or medication
Review criteria.
PubMed was searched using the terms “adiponectin breast cancer, leptin receptor, leptin breast cancer, colon cancer leptin STAT3, high fat diet mice, Il-6 colon cancer, obesity colon cancer, STAT3 colon cancer, prospective incidence cancer obesity, cancer treatment obese patients, prospective, BMI breast cancer, DMBA mice breast cancer, metformin cancer, adiponectin colon cancer, improved survival cancer exercise, leptin colorectal cancer ob/ob” both alone and in combination to identify suitable articles for reference literature. Original articles, reviews, editorial and their reference lists were considered. There were no language restrictions. The literature search was performed in October 2013 and updated in April 2014.
Acknowledgements
The authors’ research was supported by NIH Grants R01-DK55758, R01-DK099110 (to P.E.S.) and P01-DK088761 (to P.E.S. and D.J.C.). J.P.'s research was supported by a fellowship from the US Department of Defence (USAMRMC BC085909) and 2014 research fund (1.130088.01) of Ulsan National Institute of Science and Technology. T.S.M.'s work was supported by NIH Training Grant T32-GM083831.
Footnotes
Competing interests
The authors declare no competing interests.
J.P., T.S.M., D.J.C. and P.E.S undertook discussions of the article content, writing, and review or editing of the manuscript before submission. In addition, M.K. contributed to discussions of the content and writing the manuscript.
References
- 1.Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol. Metab. 2010;21:345–352. doi: 10.1016/j.tem.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parekh N, Chandran U, Bandera EV. Obesity in cancer survival. Annu. Rev. Nutr. 2012;32:311–342. doi: 10.1146/annurev-nutr-071811-150713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park J, Euhus DM, Scherer PE. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr. Rev. 2011;32:550–570. doi: 10.1210/er.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlow WE, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J. Natl Cancer Inst. 2006;98:1204–1214. doi: 10.1093/jnci/djj331. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N. Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, et al. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012;72:5198–5208. doi: 10.1158/0008-5472.CAN-12-0294. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Q, et al. Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocr. Relat. Cancer. 2013;20:797–808. doi: 10.1530/ERC-13-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park EJ, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell PT, et al. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study–II Nutrition Cohort. J. Clin. Oncol. 2012;30:42–52. doi: 10.1200/JCO.2011.38.0287. [DOI] [PubMed] [Google Scholar]
- 10.Bastarrachea J, Hortobagyi GN, Smith TL, Kau SW, Buzdar AU. Obesity as an adverse prognostic factor for patients receiving adjuvant chemotherapy for breast cancer. Ann. Intern. Med. 1994;120:18–25. doi: 10.7326/0003-4819-120-1-199401010-00004. [DOI] [PubMed] [Google Scholar]
- 11.Meyerhardt JA, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J. Clin. Oncol. 2004;22:648–657. doi: 10.1200/JCO.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 12.Semenkovich CF. Insulin resistance and atherosclerosis. J. Clin. Invest. 2006;116:1813–1822. doi: 10.1172/JCI29024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth—bystanders turning into key players. Curr. Opin. Genet. Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 17.Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tessitore L, et al. Adipocyte expression and circulating levels of leptin increase in both gynaecological and breast cancer patients. Int. J. Oncol. 2004;24:1529–1535. [PubMed] [Google Scholar]
- 19.Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. J. Clin. Invest. 2012;122:4243–4256. doi: 10.1172/JCI63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim. Biophys. Acta. 2013;1831:1533–1541. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 22.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 23.Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J. 2009;276:5738–5746. doi: 10.1111/j.1742-4658.2009.07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halberg N, et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan T, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell. Metab. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park J, Scherer PE. Endotrophin—a novel factor linking obesity with aggressive tumor growth. Oncotarget. 2012;3:1487–1488. doi: 10.18632/oncotarget.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iyengar P, et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/ stroma microenvironment. J. Clin. Invest. 2005;115:1163–1176. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyengar P, et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408–6423. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- 30.Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE. Selective inhibition of hypoxiainducible factor 1α ameliorates adipose tissue dysfunction. Mol. Cell Biol. 2013;33:904–917. doi: 10.1128/MCB.00951-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kusminski CM, et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 2012;18:1539–1549. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neels JG, Thinnes T, Loskutoff DJ. Angiogenesis in an in vivo model of adipose tissue development. FASEB J. 2004;18:983–985. doi: 10.1096/fj.03-1101fje. [DOI] [PubMed] [Google Scholar]
- 33.Brown JM, McIntosh MK. Conjugated linoleic acid in humans: regulation of adiposity and insulin sensitivity. J. Nutr. 2003;133:3041–3046. doi: 10.1093/jn/133.10.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol. Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Andarawewa KL, et al. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell–adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res. 2005;65:10862–10871. doi: 10.1158/0008-5472.CAN-05-1231. [DOI] [PubMed] [Google Scholar]
- 36.Dirat B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 37.Wyckoff J, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 38.Zhang XH, et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell. 2013;154:1060–1073. doi: 10.1016/j.cell.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cufi S, et al. Metformin-induced preferential killing of breast cancer initiating CD44+CD24−/low cells is sufficient to overcome primary resistance to trastuzumab in HER2+ human breast cancer xenografts. Oncotarget. 2012;3:395–398. doi: 10.18632/oncotarget.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J. Clin. Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korkaya H, et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol. Cell. 2012;47:570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinohara K, Gotoh N. Inflammatory signaling pathways in self-renewing breast cancer stem cells. Curr. Opin. Pharmacol. 2010;10:650–654. doi: 10.1016/j.coph.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Asiedu MK, Ingle JN, Behrens MD, Radisky DC, Knutson KL. TGFβ/TNFα-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res. 2011;71:4707–4719. doi: 10.1158/0008-5472.CAN-10-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheel C, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng Q, et al. Leptin deficiency suppresses MMTV–Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr. Relat. Cancer. 2011;18:491–503. doi: 10.1530/ERC-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feldman DE, Chen C, Punj V, Tsukamoto H, Machida K. Pluripotency factor-mediated expression of the leptin receptor (OB-R) links obesity to oncogenesis through tumor-initiating stem cells. Proc. Natl Acad. Sci. USA. 2012;109:829–834. doi: 10.1073/pnas.1114438109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park J, Morley TS, Scherer PE. Inhibition of endotrophin, a cleavage product of collagen VI, confers cisplatin sensitivity to tumours. EMBO Mol. Med. 2013;5:935–948. doi: 10.1002/emmm.201202006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Res. Clin. Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 50.He G, et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell. 2013;155:384–396. doi: 10.1016/j.cell.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flores MB, et al. Obesity-induced increase in tumor necrosis factor-α leads to development of colon cancer in mice. Gastroenterology. 2012;143:741–753. e1–e4. doi: 10.1053/j.gastro.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 52.Hill-Baskin AE, et al. Diet-induced hepatocellular carcinoma in genetically predisposed mice. Hum. Mol. Genet. 2009;18:2975–2988. doi: 10.1093/hmg/ddp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimizu M, et al. Acyclic retinoid inhibits diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BLKS/J-+(db)/+Lepr(db) mice. Cancer Prev. Res. (Phila.) 2011;4:128–36. doi: 10.1158/1940-6207.CAPR-10-0163. [DOI] [PubMed] [Google Scholar]
- 54.Yehuda-Shnaidman E, Schwartz B. Mechanisms linking obesity, inflammation and altered metabolism to colon carcinogenesis. Obes. Rev. 2012;13:1083–1095. doi: 10.1111/j.1467-789X.2012.01024.x. [DOI] [PubMed] [Google Scholar]
- 55.Jain SS, Bird RP. Elevated expression of tumor necrosis factor-α signaling molecules in colonic tumors of Zucker obese (fa/fa) rats. Int. J. Cancer. 2010;127:2042–2050. doi: 10.1002/ijc.25232. [DOI] [PubMed] [Google Scholar]
- 56.Teraoka N, et al. High susceptibility to azoxymethane-induced colorectal carcinogenesis in obese KK-Ay mice. Int. J. Cancer. 2011;129:528–535. doi: 10.1002/ijc.25711. [DOI] [PubMed] [Google Scholar]
- 57.Mentor-Marcel RA, et al. Inflammation-associated serum and colon markers as indicators of dietary attenuation of colon carcinogenesis in ob/ob mice. Cancer Prev. Res. (Phila.) 2009;2:60–69. doi: 10.1158/1940-6207.CAPR-08-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yasuda Y, et al. Pitavastatin inhibits azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Cancer Sci. 2010;101:1701–7. doi: 10.1111/j.1349-7006.2010.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kubota M, et al. Renin–angiotensin system inhibitors suppress azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/ db obese mice. Biochem. Biophys. Res. Commun. 2011;410:108–113. doi: 10.1016/j.bbrc.2011.05.115. [DOI] [PubMed] [Google Scholar]
- 60.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Z, et al. Diet-induced obesity elevates colonic TNF-α in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer. J. Nutr. Biochem. 2012;23:1207–1213. doi: 10.1016/j.jnutbio.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chia VM, et al. Leptin concentrations, leptin receptor polymorphisms, and colorectal adenoma risk. Cancer Epidemiol. Biomarkers Prev. 2007;16:2697–2703. doi: 10.1158/1055-9965.EPI-07-0467. [DOI] [PubMed] [Google Scholar]
- 63.Aleksandrova K, et al. Leptin and soluble leptin receptor in risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Cancer Res. 2012;72:5328–5337. doi: 10.1158/0008-5472.CAN-12-0465. [DOI] [PubMed] [Google Scholar]
- 64.Wang D, et al. Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J. Biosci. 2012;37:91–101. doi: 10.1007/s12038-011-9172-4. [DOI] [PubMed] [Google Scholar]
- 65.Ogunwobi OO, Beales IL. The anti-apoptotic and growth stimulatory actions of leptin in human colon cancer cells involves activation of JNK mitogen activated protein kinase, JAK2 and PI3 kinase/Akt. Int. J. Colorectal Dis. 2007;22:401–409. doi: 10.1007/s00384-006-0181-y. [DOI] [PubMed] [Google Scholar]
- 66.Endo H, et al. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut. 2011;60:1363–1371. doi: 10.1136/gut.2010.235754. [DOI] [PubMed] [Google Scholar]
- 67.Uddin S, et al. Overexpression of leptin receptor predicts an unfavorable outcome in Middle Eastern ovarian cancer. Mol. Cancer. 2009;8:74. doi: 10.1186/1476-4598-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen C, Chang YC, Lan MS, Breslin M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of the MEK/ ERK1/2 and PI3K/Akt signaling pathways. Int. J. Oncol. 2013;42:1113–1119. doi: 10.3892/ijo.2013.1789. [DOI] [PubMed] [Google Scholar]
- 69.Ptak A, Kolaczkowska E, Gregoraszczuk EL. Leptin stimulation of cell cycle and inhibition of apoptosis gene and protein expression in OVCAR-3 ovarian cancer cells. Endocrine. 2013;43:394–403. doi: 10.1007/s12020-012-9788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ptak A, Gregoraszczuk EL. Bisphenol A induces leptin receptor expression, creating more binding sites for leptin, and activates the JAK/ Stat., MAPK/ERK and PI3K/Akt signalling pathways in human ovarian cancer cell. Toxicol. Lett. 2012;210:332–337. doi: 10.1016/j.toxlet.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 71.Ptak A, Rak-Mardyla A, Gregoraszczuk EL. Cooperation of bisphenol A and leptin in inhibition of caspase-3 expression and activity in OVCAR-3 ovarian cancer cells. Toxicol. In Vitro. 2013;27:1937–1943. doi: 10.1016/j.tiv.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 72.Niu J, et al. The association between leptin level and breast cancer: a meta-analysis. PLoS ONE. 2013;8:e67349. doi: 10.1371/journal.pone.0067349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyoshi Y, et al. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int. J. Cancer. 2006;118:1414–1419. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- 74.Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: an overview. J. Cell Biochem. 2008;105:956–964. doi: 10.1002/jcb.21911. [DOI] [PubMed] [Google Scholar]
- 75.Andó S, Catalano S. The multifactorial role of leptin in driving the breast cancer microenvironment. Nat. Rev. Endocrinol. 2012;8:263–275. doi: 10.1038/nrendo.2011.184. [DOI] [PubMed] [Google Scholar]
- 76.Park J, Kusminski CM, Chua SC, Scherer PE. Leptin receptor signaling supports cancer cell metabolism through suppression of mitochondrial respiration in vivo. Am. J. Pathol. 2010;177:3133–3144. doi: 10.2353/ajpath.2010.100595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holland WL, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamada Y, et al. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J. Hepatol. 2007;47:556–564. doi: 10.1016/j.jhep.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 79.Saxena NK, et al. Adiponectin modulates C-jun N-terminal kinase and mammalian target of rapamycin and inhibits hepatocellular carcinoma. Gastroenterology. 2010;139:1762–1773. doi: 10.1053/j.gastro.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Man K, et al. Suppression of liver tumor growth and metastasis by adiponectin in nude mice through inhibition of tumor angiogenesis and downregulation of Rho kinase/IFN-inducible protein 10/matrix metalloproteinase 9 signaling. Clin. Cancer Res. 2010;16:967–977. doi: 10.1158/1078-0432.CCR-09-1487. [DOI] [PubMed] [Google Scholar]
- 81.Sharma D, et al. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology. 2010;52:1713–1722. doi: 10.1002/hep.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J. Natl Cancer Inst. 2005;97:1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 83.Yoneda K, et al. Expression of adiponectin receptors, AdipoR1 and AdipoR2, in normal colon epithelium and colon cancer tissue. Oncol. Rep. 2008;20:479–483. [PubMed] [Google Scholar]
- 84.Kim AY, et al. Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol. Endocrinol. 2010;24:1441–1452. doi: 10.1210/me.2009-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sugiyama M, et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/ mTOR pathway. Int. J. Oncol. 2009;34:339–344. [PubMed] [Google Scholar]
- 86.Mutoh M, et al. Loss of adiponectin promotes intestinal carcinogenesis in Min and wild-type mice. Gastroenterology. 2011;140:2000–2008. doi: 10.1053/j.gastro.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 87.Fujisawa T, et al. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut. 2008;57:1531–1538. doi: 10.1136/gut.2008.159293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tworoger SS, et al. Plasma adiponectin concentrations and risk of incident breast cancer. J. Clin. Endocrinol. Metab. 2007;92:1510–1516. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]
- 89.Denzel MS, et al. Adiponectin deficiency limits tumor vascularization in the MMTV-PyV-mT mouse model of mammary cancer. Clin. Cancer Res. 2009;15:3256–3264. doi: 10.1158/1078-0432.CCR-08-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Landskroner-Eiger S, et al. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin. Cancer Res. 2009;15:3265–3276. doi: 10.1158/1078-0432.CCR-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 92.Pavlides S, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 93.Migneco G, et al. Glycolytic cancer associated fibroblasts promote breast cancer tumor growth, without a measurable increase in angiogenesis: evidence for stromal–epithelial metabolic coupling. Cell Cycle. 2010;9:2412–2422. doi: 10.4161/cc.9.12.11989. [DOI] [PubMed] [Google Scholar]
- 94.Gallagher EJ, LeRoith D. Diabetes, antihyperglycemic medications and cancer risk: smoke or fire? Curr. Opin. Endocrinol. Diabetes Obes. 2013;20:485–494. doi: 10.1097/01.med.0000433065.16918.83. [DOI] [PubMed] [Google Scholar]
- 95.Brand-Miller JC. Glycemic load and chronic disease. Nutr. Rev. 2003;61:S49–S55. doi: 10.1301/nr.2003.may.S49-S55. [DOI] [PubMed] [Google Scholar]
- 96.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr. Relat. Cancer. 2009;16:1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 97.Ferguson RD, Gallagher EJ, Scheinman EJ, Damouni R, LeRoith D. The epidemiology and molecular mechanisms linking obesity, diabetes, and cancer. Vitam. Horm. 2013;93:51–98. doi: 10.1016/B978-0-12-416673-8.00010-1. [DOI] [PubMed] [Google Scholar]
- 98.Warburg O. The reaction of ascites tumor cells to oxygen under high pressure. Arch. Geschwulstforsch. 1953;6:7–11. [PubMed] [Google Scholar]
- 99.Shapot VS, Blinov VA. Blood glucose levels and gluconeogenesis in animals bearing transplantable tumors. Cancer Res. 1974;34:1827–1832. [PubMed] [Google Scholar]
- 100.Pavelic K, et al. Growth and treatment of Ehrlich tumor in mice with alloxan-induced diabetes. Cancer Res. 1979;39:1807–1813. [PubMed] [Google Scholar]
- 101.Gullino PM, Grantham FH, Courtney AH. Glucose consumption by transplanted tumors in vivo. Cancer Res. 1967;27:1031–1040. [PubMed] [Google Scholar]
- 102.Goranson ES, Tilser GJ. Studies on the relationship of alloxan-diabetes and tumor growth. Cancer Res. 1955;15:626–631. [PubMed] [Google Scholar]
- 103.Park J, Sarode VR, Euhus D, Kittler R, Scherer PE. Neuregulin 1–HER axis as a key mediator of hyperglycemic memory effects in breast cancer. Proc. Natl Acad. Sci. USA. 2012;109:21058–21063. doi: 10.1073/pnas.1214400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang ZV, et al. PANIC-ATTAC: a mouse model for inducible and reversible β-cell ablation. Diabetes. 2008;57:2137–2148. doi: 10.2337/db07-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 106.Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat. Rev. Endocrinol. 2011;7:11–24. doi: 10.1038/nrendo.2010.171. [DOI] [PubMed] [Google Scholar]
- 107.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin–IGF axis. Trends Endocrinol. Metab. 2006;17:328–336. doi: 10.1016/j.tem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 108.Renehan AG, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 109.Parekh N, et al. Lifestyle, anthropometric, and obesity-related physiologic determinants of insulin-like growth factor-1 in the Third National Health and Nutrition Examination Survey (1988–1994). Ann. Epidemiol. 2010;20:182–193. doi: 10.1016/j.annepidem.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 110.Crowe FL, et al. A cross-sectional analysis of the associations between adult height, BMI and serum concentrations of IGF-I and IGFBP-1 -2 and -3 in the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann. Hum. Biol. 2011;38:194–202. doi: 10.3109/03014460.2010.507221. [DOI] [PubMed] [Google Scholar]
- 111.Fogarty AW, et al. A prospective study of weight change and systemic inflammation over 9 y. Am. J. Clin. Nutr. 2008;87:30–35. doi: 10.1093/ajcn/87.1.30. [DOI] [PubMed] [Google Scholar]
- 112.Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes. Rev. 2009;10:610–616. doi: 10.1111/j.1467-789X.2009.00607.x. [DOI] [PubMed] [Google Scholar]
- 113.O’Brien KD, et al. Diet-induced weight loss is associated with decreases in plasma serum amyloid A and C-reactive protein independent of dietary macronutrient composition in obese subjects. J. Clin. Endocrinol. Metab. 2005;90:2244–2249. doi: 10.1210/jc.2004-1011. [DOI] [PubMed] [Google Scholar]
- 114.Tran CD, Diorio C, Berube S, Pollak M, Brisson J. Relation of insulin-like growth factor (IGF) I and IGF-binding protein 3 concentrations with intakes of fruit, vegetables, and antioxidants. Am. J. Clin. Nutr. 2006;84:1518–1526. doi: 10.1093/ajcn/84.6.1518. [DOI] [PubMed] [Google Scholar]
- 115.Heald AH, et al. Close relation of fasting insulin-like growth factor binding protein-1 (IGFBP-1) with glucose tolerance and cardiovascular risk in two populations. Diabetologia. 2001;44:333–339. doi: 10.1007/s001250051623. [DOI] [PubMed] [Google Scholar]
- 116.Bol DK, Kiguchi K, Gimenez-Conti I, Rupp T, DiGiovanni J. Overexpression of insulin-like growth factor-1 induces hyperplasia, dermal abnormalities, and spontaneous tumor formation in transgenic mice. Oncogene. 1997;14:1725–1734. doi: 10.1038/sj.onc.1201011. [DOI] [PubMed] [Google Scholar]
- 117.DiGiovanni J, et al. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc. Natl Acad. Sci. USA. 2000;97:3455–3460. doi: 10.1073/pnas.97.7.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carboni JM, et al. Tumor development by transgenic expression of a constitutively active insulin-like growth factor I receptor. Cancer Res. 2005;65:3781–3787. doi: 10.1158/0008-5472.CAN-04-4602. [DOI] [PubMed] [Google Scholar]
- 119.Moorehead RA, Sanchez OH, Baldwin RM, Khokha R. Transgenic overexpression of IGF-II induces spontaneous lung tumors: a model for human lung adenocarcinoma. Oncogene. 2003;22:853–857. doi: 10.1038/sj.onc.1206188. [DOI] [PubMed] [Google Scholar]
- 120.Pravtcheva DD, Wise TL. Metastasizing mammary carcinomas in H19 enhancers Igf2 transgenic mice. J. Exp. Zool. 1998;281:43–57. [PubMed] [Google Scholar]
- 121.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr. Rev. 2009;30:343–375. doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]
- 122.Simpson ER, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 123.Chen J. Multiple signal pathways in obesity-associated cancer. Obes. Rev. 2011;12:1063–1070. doi: 10.1111/j.1467-789X.2011.00917.x. [DOI] [PubMed] [Google Scholar]
- 124.Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol. Metab. 2012;23:83–89. doi: 10.1016/j.tem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Key T, et al. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J. Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 126.Bulun SE, Price TM, Aitken J, Mahendroo MS, Simpson ER. A link between breast cancer and local estrogen biosynthesis suggested by quantification of breast adipose tissue aromatase cytochrome P450 transcripts using competitive polymerase chain reaction after reverse transcription. J. Clin. Endocrinol. Metab. 1993;77:1622–1628. doi: 10.1210/jcem.77.6.8117355. [DOI] [PubMed] [Google Scholar]
- 127.Oneill JS, Elton RA, Miller WR. Aromatase activity in adipose tissue from breast quadrants: a link with tumour site. Br. Med. J. 1988;296:741–743. doi: 10.1136/bmj.296.6624.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen D, et al. Prostaglandin E(2) induces breast cancer related aromatase promoters via activation of p38 and c-Jun NH(2)-terminal kinase in adipose fibroblasts. Cancer Res. 2007;67:8914–8922. doi: 10.1158/0008-5472.CAN-06-4751. [DOI] [PubMed] [Google Scholar]
- 129.Zhao Y, Nichols JE, Valdez R, Mendelson CR, Simpson ER. Tumor necrosis factor-α stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Mol. Endocrinol. 1996;10:1350–1357. doi: 10.1210/mend.10.11.8923461. [DOI] [PubMed] [Google Scholar]
- 130.Ellis MJ, et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486:353–360. doi: 10.1038/nature11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jansen MP, et al. Hallmarks of aromatase inhibitor drug resistance revealed by epigenetic profiling in breast cancer. Cancer Res. 2013;73:6632–6641. doi: 10.1158/0008-5472.CAN-13-0704. [DOI] [PubMed] [Google Scholar]
- 132.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou G, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 136.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 137.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Satoh T, et al. Activation of peroxisome proliferator-activated receptor-γ stimulates the growth arrest and DNA-damage inducible 153 gene in non-small cell lung carcinoma cells. Oncogene. 2002;21:2171–2180. doi: 10.1038/sj.onc.1205279. [DOI] [PubMed] [Google Scholar]
- 139.Girnun GD, et al. Regression of drug-resistant lung cancer by the combination of rosiglitazone and carboplatin. Clin. Cancer Res. 2008;14:6478–6486. doi: 10.1158/1078-0432.CCR-08-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Girnun GD, et al. Synergy between PPARγ ligands and platinum-based drugs in cancer. Cancer Cell. 2007;11:395–406. doi: 10.1016/j.ccr.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McNeely ML, et al. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 143.Thompson HJ. Effect of exercise intensity and duration on the induction of mammary carcinogenesis. Cancer Res. 1994;54:1960s–1963. [PubMed] [Google Scholar]
- 144.Thompson HJ, Westerlind KC, Snedden J, Briggs S, Singh M. Exercise intensity dependent inhibition of 1-methyl-1-nitrosourea induced mammary carcinogenesis in female F-344 rats. Carcinogenesis. 1995;16:1783–1786. doi: 10.1093/carcin/16.8.1783. [DOI] [PubMed] [Google Scholar]
- 145.Thompson HJ, Ronan AM, Ritacco KA, Tagliaferro AR, Meeker LD. Effect of exercise on the induction of mammary carcinogenesis. Cancer Res. 1988;48:2720–2723. [PubMed] [Google Scholar]
- 146.Chlebowski RT, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J. Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 147.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 148.Tchernof A, Nolan A, Sites CK, Ades PA, Poehlman ET. Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation. 2002;105:564–569. doi: 10.1161/hc0502.103331. [DOI] [PubMed] [Google Scholar]
- 149.Imayama I, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res. 2012;72:2314–2326. doi: 10.1158/0008-5472.CAN-11-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int. J. Obes. (Lond.) 2005;29:1308–1314. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]