Abstract
Replication-competent oncolytic viruses hold great potential for the clinical treatment of many cancers. Importantly, many oncolytic virus candidates, such as reovirus and myxoma virus, preferentially infect cancer cells bearing abnormal cellular signaling pathways. Reovirus and myxoma virus are highly responsive to activated Ras and Akt signaling pathways, respectively, for their specificity for viral oncolysis. However, considering the complexity of cancer cell populations, it is possible that other tumor-specific signaling pathways may also contribute to viral discrimination between normal versus cancer cells. Because carcinogenesis is a multistep process involving the accumulation of both oncogene activations and the inactivation of tumor suppressor genes, we speculated that not only oncogenes but also tumor suppressor genes may have an important role in determining the tropism of these viruses for cancer cells. It has been previously shown that many cellular tumor suppressor genes, such as p53, ATM and Rb, are important for maintaining genomic stability; dysfunction of these tumor suppressors may disrupt intact cellular antiviral activity due to the accumulation of genomic instability or due to interference with apoptotic signaling. Therefore, we speculated that cells with dysfunctional tumor suppressors may display enhanced susceptibility to challenge with these oncolytic viruses, as previously seen with adenovirus. We report here that both reovirus and myxoma virus preferentially infect cancer cells bearing dysfunctional or deleted p53, ATM and Rb tumor suppressor genes compared to cells retaining normal counterparts of these genes. Thus, oncolysis by these viruses may be influenced by both oncogenic activation and tumor suppressor status.
Keywords: reovirus, myxoma virus, tumor suppressor, oncolytic virus, anticancer therapy
Reovirus is a ubiquitous, non-enveloped virus containing 10 segments of double-stranded RNA as its genome. In humans, infections are generally mild and restricted to the upper respiratory and gastrointestinal tracts. Importantly, reovirus has been recognized for many years as displaying striking cytocidal activity when it infects certain types of transformed cells (Hashiro et al., 1977; Duncan et al., 1978). It has been shown that reovirus preferentially infects Ras-transformed cells in vitro and in vivo (Coffey et al., 1998; Strong et al., 1998; Norman et al., 2004). As Ras gene mutations are observed in over 30% of all human cancers (Duursma and Agami, 2003), these findings have led to the current use of reovirus in clinical trials (Norman and Lee, 2005; Forsyth et al., 2008; Vidal et al., 2008). Myxoma virus (Myx) is a rabbit-specific poxvirus that is also considered a promising oncolytic virus (Stanford and McFadden, 2007). Its natural tropism is highly restricted to European rabbits and it is nonpathogenic for all other vertebrate species tested, including humans (McFadden, 2005). Despite this narrow host specificity, Myx is capable of infecting and killing a wide variety of human tumor cell lines in vitro (Sypula et al., 2004). It has recently been shown that cellular Akt signaling pathways that are often dysregulated in various cancer cells also have an important role in determining the oncolytic potential of Myx (Wang et al., 2006). Furthermore, a murine xenograft model of human malignant glioma, medulloblastoma and acute myeloid leukemia showed the efficacy and safety of Myx oncolysis in vivo (Lun et al., 2005, 2007; Kim et al., 2009). Myx also exhibits potent oncolytic activity against metastatic melanoma in immunocompetent mice (Stanford et al., 2008).
Tumorigenesis is a multistep process involving the combined accumulation of oncogenic pathway activations and the inactivation of tumor suppressor genes by mutational events affecting proto-oncogenes and tumor suppressor genes. Interestingly, the oncolytic viruses described above use different oncogene-driven cellular signaling pathways to selectively replicate and kill the cells that bear them. In addition to oncogene-dependent oncolysis, we speculated that some tumor suppressor genes may also have an important role in facilitating reoviral and myxoma viral oncolysis, as previously shown for adenovirus (McCormick, 2005). Relevant to this speculation, tumor suppressor activity can modulate genomic stability, cell-cycle progression, sensitivity to apoptosis and interferon-based responses to viral infection. Thus, we tested various cancer cell lines whose tumor suppressor genes are defective due to genetic knockdown or spontaneous deletion to examine whether tumor suppressor gene functionality can affect both reovirus and myxoma viral oncolysis.
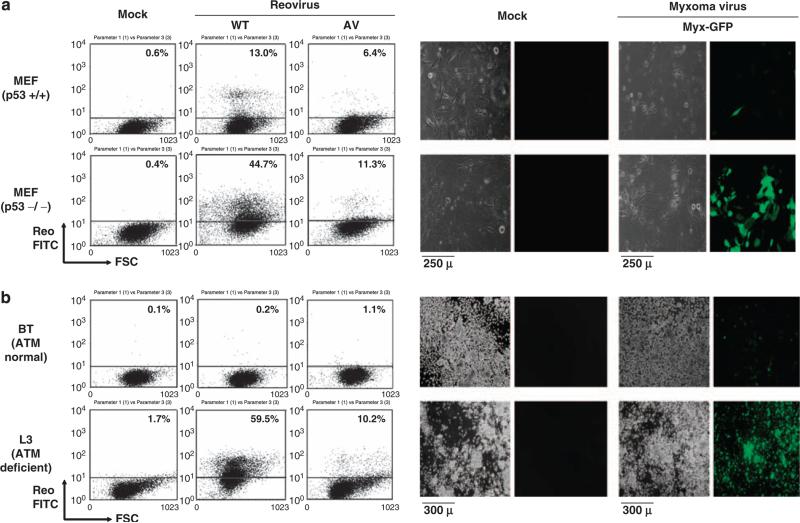
To examine whether modulation of tumor suppressor genes can affect oncolytic virus susceptibility, we selected p53 and ATM tumor suppressor genes that are frequently mutated in various cancers. p53 is the prototypical tumor suppressor gene and most commonly mutated in various cancer cells, and it has been shown that over 50% of cancers display p53 mutations (White, 1994; Morris, 2002). ATM (ataxia telangiectasia mutated) is a serine threonine protein kinase that is activated in response to DNA damage. Both copies of the ATM gene are dysfunctional in ataxia telangiectasia, a rare syndrome characterized by cancer predisposition, radiosensitivity and neurodegeneration (Kitagawa and Kastan, 2005). Thus we used p53−/− (mouse embryonic fibroblasts) and MEFs ATM-deficient L3 lymphoblastoid cells (Kozlov et al., 2003) to examine oncolytic virus susceptibility. As shown in Figure 1, reoviruses (wild-type (WT) and attenuated (AV) reovirus (Kim et al., 2007a, b)) and Myx each preferentially infected and replicated in p53−/− cells MEF or L3 (ATM-deficient) as shown by fluorescence-activated cell sorting (FACS) or microscopic detection of fluorescence-positive cells (FITC + or GFP + cells) compared to the MEF (p53 normal) and BT (ATM normal) control cells.
Figure 1.
Reoviral and myxoma viral preferential infection of p53−/− MEF and ATM-defective L3 cells. (a) p53−/− and+/+ MEF (p53 knockout or p53 wild-type murine embryonic fibroblasts, respectively) were infected with WT/AV reovirus (multiplicity of infection (MOI) of 40) or GFP-expressing myxoma virus (Myx-GFP, which is a GFP-expressing version of the Lausanne (American Type Culture Collection, ATCC, Manassas, VA, USA) strain of MYXV, as described by Johnston et al. (2003); MOI of 5). GFP expression in the myxoma virus is driven by the poxviral synthetic E/L promoter; the GFP expression cassette is located between M135R and M136R by intergenic insertion (Johnston et al., 2003). At 3 days after infection with reovirus, cells were fixed/permeabilized and analyzed by FACS using reovirus antiserum and secondary FITC antiserum. The results show that both wild-type and attenuated reovirus were able to preferentially infect p53−/− MEF. For the myxoma infection study, cells were infected for 24 h and visualized under phase-contrast and fluorescence microscopy. GFP-expressing cells represent myxoma virus infection. Mock, mock infection. (b) BT (ATM normal lymphoblastoid C3ABR cells; Kozlov et al., 2003) and L3 (ATM-deficient lymphoblastoid cells; Kozlov et al., 2003) were infected with WT/AV reovirus or myxoma virus (Myx-GFP). At 3 days after infection with reovirus, cells were fixed/ permeabilized and analyzed by FACS using reovirus antiserum and secondary FITC antiserum. The results show that both wild-type and attenuated reovirus were able to preferentially infect ATM-deficient L3 cells. For myxoma infection, cell were infected for 48 h and visualized under phase-contrast and fluorescence microscopy. GFP-expressing cells represent myxoma virus infection. C35ABR (B3) and L3 cell lines were kindly provided by Dr M Lavin (Queensland Institute of Medical Research) and Dr Y Shiloh (Tel Aviv University), respectively. Mock, mock infection.
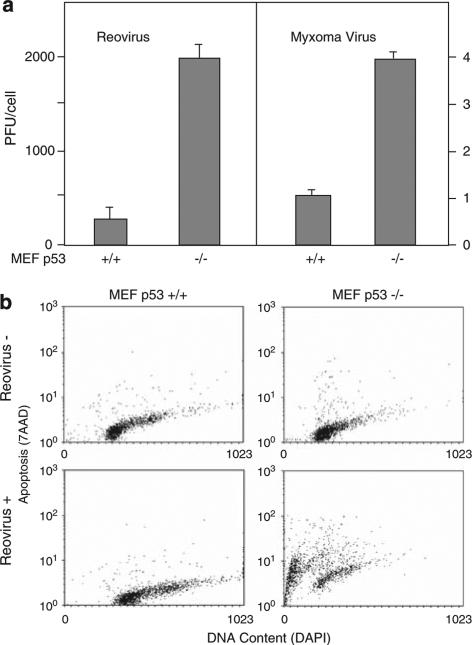
To further evaluate relative susceptibility to these viruses, we measured viral production through burst assays (Figure 2a). MEF p53+/+ or −/− cells were infected with either WT reovirus or Myx, and the production of infectious viral particles was followed over 72 h. We observed that overall viral yield was much greater for reovirus than for Myx (in parallel studies we have found that the latter virus shows enhanced yield with increased passaging of primary normal cells as they become immortalized and reduce their synthesis of type I interferon; Wang et al., 2009), but in each case p53−/− cells produced significantly more virus (7.9- and 3.5- fold, respectively) than did p53+/+ cells. We also conducted assays of apoptosis (Figure 2b) in MEF cells exposed to reovirus and observed that after 9 days of viral exposure cell death was extensive in the p53−/− cells but not in the p53+/+ cells (in these experiments we did not measure staining with Annexin V as an indicator of apoptosis as we have previously found that reovirus infection causes an anomalous elevation in Annexin V labeling even in non-dying cells; MK and RNJ, unpublished results). Taken together these results show that loss of p53 activity confers enhanced susceptibility to reoviral and myxoma viral infectivity, replication and cytolysis.
Figure 2.
p53 deficiency enhances viral replication and virusinduced apoptosis. (a) MEF p53+/+ and −/− cells were infected with reovirus at multiplicity of infection (MOI) of 40 or myxoma virus at MOI of 5. At 3 days after infection, cells and supernatant are harvested and frozen and thawed three times. Viral titer was determined by plaque assays using L929 cells (reovirus) or RK13 cells (myxoma) and expressed as PFU per cell. (b) MEF p53+/+ and −/− cells were either mock infected or exposed to reovirus for 9 days, then stained for DNA content with DAPI and for apoptotic activity with 7AAP. MEF p53−/− cells showed extensive cellular fragmentation and apoptosis in response to reovirus, whereas MEF p53+/+ cells did not.
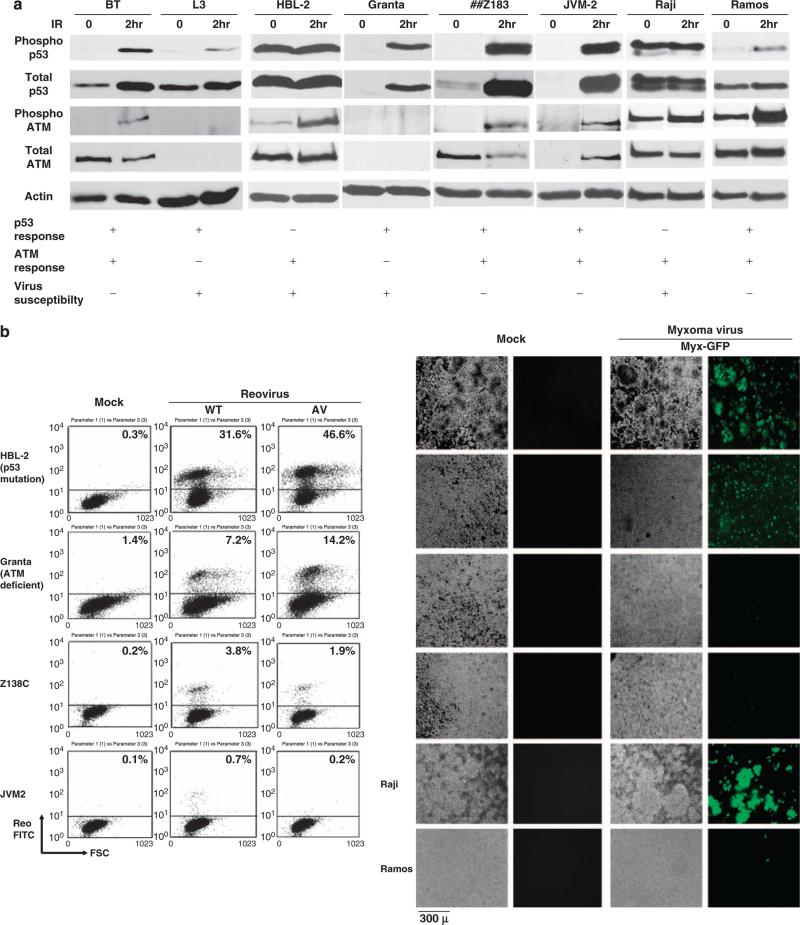
Because reovirus and Myx preferentially infect p53-or ATM-deficient cells that are otherwise normal, we then examined whether reovirus and Myx can preferentially infect human lymphomas that carry p53 or ATM deficiencies. We tested six different lymphoma cell lines (HBL-2, Granta-519, Z138, JVM2, Raji and Ramos) that exhibit variable cellular responses arising from p53-and ATM-dependent pathways on genotoxic challenge (Figure 3a). The functional status of p53 and ATM is critical in these experiments and was evaluated by irradiation and detection of activated p53 and ATM phosphorylation by western blotting using phosphospecific antibodies. BT (ATM normal) and L3 (ATM deficient) were used as controls for ATM responsiveness to IR. HBL-2 and Raji showed a dysfunctional p53 response on genotoxic stress as evidenced by the constitutive phosphorylation of Ser15 of p53 (Li et al., 2006; Figure 3a). Granta-519 cells exhibited a dysfunctional ATM response on genotoxic stress as ATM was not phosphorylated on Ser1981 on IR exposure (Figure 3a). In the case of Z138, JVM2 and Ramos cells, p53 and ATM responses were normal after genotoxic stress as shown by hyperphosphorylation of p53 and ATM Ser1981 phosphorylation after IR exposure (Figure 3a). Interestingly, p53 and/or ATM dysfunctional lymphomas (HBL-2 and Raji showed constitutive p53 activation, and Granta-519 had reduced ATM protein and no detectable IR-induced phosphor-ylation) are preferentially sensitive to both reovirus and Myx infection as shown by FACS or microscopic detection of fluorescent-positive cells (FITC+ or GFP + cells) compared to ATM- and p53-responsive lymphomas (Z183C, JVM-2, Ramos) (Figure 3b). These results strongly suggest that both ATM and p53 are important in establishing cellular resistance to viral infections whereas defects in function of either gene or its response pathway can confer viral susceptibility.
Figure 3.
Reovirus and myxoma virus preferential infection of p53 and ATM dysfunctional human lymphomas. (a) p53 and ATM phosphorylation in response to IR. Mantle cell lymphomas (Granta-519, HBL-2 (p53 deletion, Tucker et al., 2006 and/or mutation, Beà et al., 2009), Z138, JVM-2) and Burkitt's lymphomas (Raji and Ramos) were irradiated at 2 Gy for 2 h and cells were harvested. Whole-cell lysates were produced by treatment with NET-N lysis buffer (1% NP-40) followed by sonication. 50 μg of protein was then separated on either 8% or 10% SDS–PAGE, transferred to nitrocellulose and probed with the indicated antibodies (Phospho-ATM; pSer1981 ATM and Phospho-p53; pSer15, and p53 were purchased from Rockland Immunochemicals (Philadelphia, PA, USA) and Cell Signaling Technology (Danvers, MA, USA); the ATM-specific rabbit polyclonal antibody 4BA was a kind gift from Dr M Lavin). BT and L3 cells were used as controls. (b) Cells were infected with WT/AV reovirus at multiplicity of infection (MOI) of 40 or myxoma virus (Myx-GFP) at MOI of 5. Previous work showed that Raji cells are susceptible and Ramos cells are resistant to reovirus (Alain et al., 2002). At 3 days after infection, cells were fixed/permeabilized and analyzed by FACS using reovirus antiserum and secondary FITC antiserum. The results showed that both reovirus and myxoma virus preferentially infect p53 and/or ATM nonresponsive lymphomas. For the myxoma infection study, cell were infected for 48h and visualized under phase-contrast and fluorescence microscopy. GFP-expressing cells represent myxoma virus infection. Mock, mock infection.
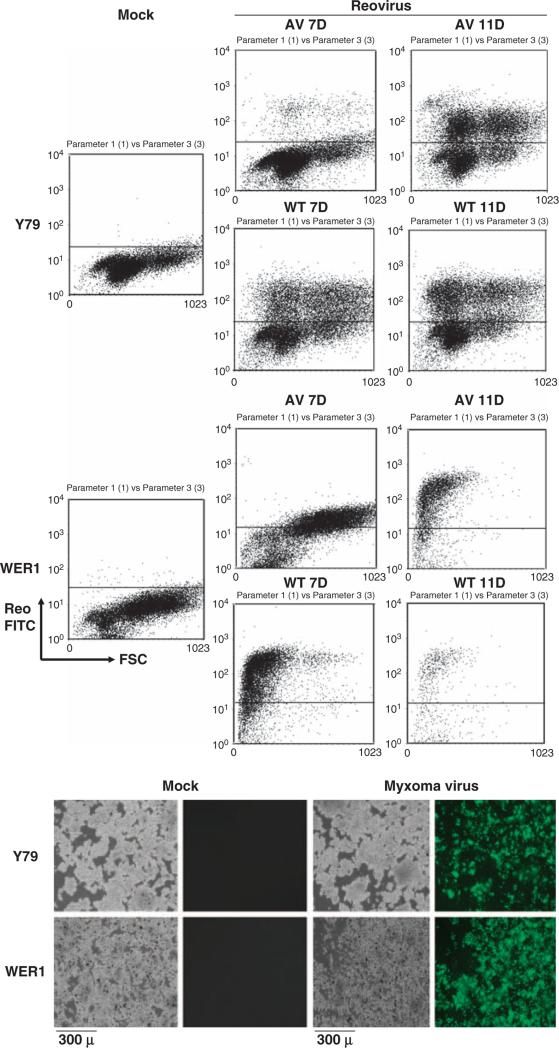
Because reovirus and Myx preferentially infect p53 and ATM dysfunctional cancer cells, we also examined whether RB-defective cancer cells (Reid et al., 1974) are susceptible to reovirus and Myx infection. As shown in Figure 4, two different human retinoblastoma cell lines are susceptible to reovirus and Myx infection as shown by FACS or by microscopic detection of fluorescence-positive cells (FITC + or GFP+ cells). it appears that activity of at Thus, least three different tumor suppressor genes (p53, ATM and Rb) can modulate responses to these oncolytic viruses.
Figure 4.
Reovirus and myxoma virus infection of retinoblastoma cells. Retinoblastoma cells (Y79 and WERI-Rb-1, purchased from ATCC) were infected with WT/AV reovirus at multiplicity of infection (MOI) of 40 or myxoma virus (Myx-GFP) at MOI of 5. At designated days after infection, cells were fixed/permeabilized and analyzed by FACS using reovirus antiserum and secondary FITC antiserum. For the myxoma infection study, cell were infected for 48 h and visualized under phase-contrast and fluorescence microscopy. GFP-expressing cells represent myxoma infection. Mock, mock infection.
The molecular basis of reoviral oncolysis was initially advanced through efforts to characterize cellular receptors for reovirus. In earlier work, we reported that two mouse cell lines expressing no epidermal growth factor receptors were relatively resistant to reovirus infection, whereas the same cell lines transfected with the gene encoding epidermal growth factor receptor manifested significantly higher susceptibility (Strong et al., 1993). This suggested that reovirus exploits activated cellular signal transduction pathways conferred by the presence of functional epidermal growth factor receptor on the host cell. Later, we were able to show that NIH3T3 cells, which are resistant to reovirus infection, became susceptible when transformed with activated Sos, Ras or v-erbB (Strong and Lee, 1996; Strong et al., 1998), and the exploitation of the oncogenic Ras signaling pathway is now considered a critical step in reoviral oncolysis. Many human cancers show enhanced Ras pathway activation, emphasizing the potent effect of this signaling network on tumorigenesis, and suggesting that reovirus may exert broad oncolytic potential in various tumor types. Indeed, initial experiments found that over 80% of cell lines originating from a variety of tumor types were sensitive to reovirus-mediated killing (Coffey et al., 1998). Some recent work has argued that Ras pathway activation is not the critical factor determining reoviral permissiveness (Song et al., 2009), but this interpretation has been challenged (Shmulevitz et al., 2010). As we have earlier shown that reovirus resistance can arise in Ras-transformed cells (Kim et al., 2007b), we argue that Ras remains as an important factor establishing susceptibility to viral oncolysis, but that the degree of susceptibility may be further modulated by other pathways, including tumor suppressor function as shown here.
Myx is also capable of infecting and killing a wide variety of human tumor cell lines (Sypula et al., 2004). Myx tropism at the cellular level is largely regulated by intracellular events downstream of virus binding and entry, rather than at the level of specific host receptors (McFadden, 2005). We have earlier shown that the viral M-T5 protein interacts with cellular Akt and this interaction enhances myxoma oncolytic potential in human cancer cells (Wang et al., 2006). While M-T5 knockout Myx exerts an attenuated viral oncolysis in some cancer cells, constitutively Akt-activated cancer cells are still permissive to this virus, suggesting that upregulation of Akt, frequently found in many cancers, has an important role in determining Myx oncolytic potential (Wang et al., 2006).
The connection between viral oncolysis and tumor suppressor activity was first established through the study of adenovirus. The human adenovirus E1B gene encodes a 55 kDa protein that binds and inactivates the cellular tumor suppressor protein p53. It has been shown that a mutant adenovirus that does not express E1B can replicate in and kill p53-dysfunctional human tumor cells but not cells with functional p53 (Bischoff et al., 1996). Later studies, however, showed that adenoviral oncolysis is not fully explained by dependence on p53 function in various models (Abou El Hassan et al., 2004; O'shea et al., 2004; Royds et al., 2006), and it is possible that p53 dysfunctional cancer cells may also have undergone further disruption of cellular antiviral mechanisms (Carroll et al., 1999). Although some work has argued (Huang et al., 2004) that p53 dysfunction does not enhance reoviral susceptibility further in cells already highly responsive to the virus, other studies have shown that p53 contributes to innate immunity by enhancing interferon-dependent antiviral activity independent of its functions as a proapoptotic and tumor suppressor gene (Takaoka et al., 2003; Munñoz-Fontela et al., 2008). In particular, p53 can activate transcription of interferon regulatory factor 9 on viral challenge (Munñoz-Fontela et al., 2008). Moreover, significantly higher levels of viral replication were observed when p53 expression was reduced in cancer cells (Dharel et al., 2008). These findings show an important antiviral activity of p53 and are consistent with our own observations above. This relationship may also be true in the case of other tumor suppressor genes that are important in DNA repair and genomic stability.
In summary, we have shown here that cancer cells dysfunctional in p53, ATM or Rb show enhanced susceptibility to both reoviral and myxoma viral challenge. This observation may help to delineate how diverse RNA- and DNA-based oncolytic viruses can discriminate between normal versus cancer cells, in addition to susceptibility that may be elevated by the activation of specific oncogene signaling pathways. The identification of a link between these genetic abnormalities and oncolytic virus susceptibility greatly increases the potential for applying oncolytic viral therapy to those cancers with abnormalities in specific pathways that confer elevated responsiveness.
Acknowledgements
This work was supported by funding from the Canadian Institutes of Health Research and the Canadian Breast Cancer Foundation (to RNJ) and a start-up grant from the University of Florida College of Medicine and NIH grant R01 CA138541 (to GM). GM is an International Scholar of the Howard Hughes Medical Institute.
Abbreviations
- WT reovirus
wild-type reovirus
- AV reovirus
S1 attenuated reovirus
- Myx
myxoma virus
- Myx-GFP
GFP-expressing myxoma virus
- MOI
multiplicity of infection
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Abou El Hassan MA, van der Meulen-Muileman I, Abbas S, Kruyt FA. Conditionally replicating adenoviruses kill tumor cells via a basic apoptotic machinery-independent mechanism that resembles necrosis-like programmed cell death. J Virol. 2004;78:12243–12251. doi: 10.1128/JVI.78.22.12243-12251.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain T, Hirasawa K, Pon KJ, Nishikawa SG, Urbanski SJ, Auer Y, et al. Reovirus therapy of lymphoid malignancies. Blood. 2002;100:4146–4153. doi: 10.1182/blood-2002-02-0503. [DOI] [PubMed] [Google Scholar]
- Beà S, Salaverria I, Armengol L, Pinyol M,V, Hartmann EM, et al. Uniparental disomies, homozygous deletions, amplifications, and target genes in mantle cell lymphoma revealed by integrative high-resolution whole-genome profiling. Blood. 2009;113:3059–3069. doi: 10.1182/blood-2008-07-170183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- Carroll PE, Okuda M, Horn HF, Biddinger P, Stambrook PJ, Gleich LL, et al. Centrosome hyperamplification in human cancer: chromosome instability induced by p53 mutation and/or Mdm2 overexpression. Oncogene. 1999;18:1935–1944. doi: 10.1038/sj.onc.1202515. [DOI] [PubMed] [Google Scholar]
- Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- Dharel N, Kato N, Muroyama R, Taniguchi H, Otsuka M, Wang Y, et al. Potential contribution of tumor suppressor p53 in the host defense against hepatitis C virus. Hepatology. 2008;47:1136–1149. doi: 10.1002/hep.22176. [DOI] [PubMed] [Google Scholar]
- Duursma AM, Agami R. Ras interference as cancer therapy. Semin Cancer Biol. 2003;13:267–273. doi: 10.1016/s1044-579x(03)00040-3. [DOI] [PubMed] [Google Scholar]
- Duncan MR, Stanish SM, Cox DC. Differential sensitivity of normal and transformed human cells to reovirus infection. J Virol. 1978;28:444–449. doi: 10.1128/jvi.28.2.444-449.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth P,G, George D, Wallace C, Palmer CA, Morris D, et al. Phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- Hashiro G, Loh PC, Yau JT. The preferential cytotoxicity of reovirus for certain transformed cell lines. Arch Virol. 1977;54:307–315. doi: 10.1007/BF01314776. [DOI] [PubMed] [Google Scholar]
- Huang S, Qu LK, Koromilas AE. Induction of p53-dependent apoptosis in HCT116 tumor cells by RNA viruses and possible implications in virus mediated oncolysis. Cell Cycle. 2004;3:1043–1045. [PubMed] [Google Scholar]
- Johnston JB, Barrett JW, Chang W, Chung CS, Zeng W, Masters J, et al. Role of the serine-threonine kinase PAK-1 in myxoma virus replication. J Virol. 2003;77:5877–5888. doi: 10.1128/JVI.77.10.5877-5888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Alain T, Urbanski SJ, Kossakowska AE, Lee PWK, Forsyth PA, et al. An attenuated reovirus isolated from persistent reovirus infection exerts viral oncolysis with reduced pathogenicity; 2007 Annual Meeting; July 2007; Corvallis, OR.: American Society for Virology (ASV); Oregon State University; 2007a. [Google Scholar]
- Kim M, Egan C, Alain T, Urbanski SJ, Lee PW, Forsyth PA, et al. Acquired resistance to reoviral oncolysis in Ras-transformed fibrosarcoma cells. Oncogene. 2007b;26:4124–4134. doi: 10.1038/sj.onc.1210189. [DOI] [PubMed] [Google Scholar]
- Kim M, Madlambayan GJ, Rahman MM, Smallwood SE, Meacham AM, Hosaka K, et al. Myxoma virus targets primary human leukemic stem and progenitor cells while sparing normal hematopoietic stem and progenitor cells. Leukemia. 2009;23:2313–2317. doi: 10.1038/leu.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa R, Kastan MB. The ATM-dependent DNA damage signaling pathway. Cold Spring Harb Symp Quant Biol. 2005;70:99–109. doi: 10.1101/sqb.2005.70.002. [DOI] [PubMed] [Google Scholar]
- Kozlov S, Gueven N, Keating K, Ramsay J, Lavin MF. ATP activates ataxia-telangiectasia mutated (ATM) in vitro. Importance of autophosphorylation. J Biol Chem. 2003;278:9309–9317. doi: 10.1074/jbc.m300003200. [DOI] [PubMed] [Google Scholar]
- Li DW, Liu JP, Schmid PC, Schlosser R, Feng H, Liu WB, et al. Protein serine/threonine phosphatase-1 dephosphorylates p53 at Ser-15 and Ser-37 to modulate its transcriptional and apoptotic activities. Oncogene. 2006;25:3006–3022. doi: 10.1038/sj.onc.1209334. [DOI] [PubMed] [Google Scholar]
- Lun X, Yang W, Alain T, Shi ZQ, Muzik H, Barrett JW, et al. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 2005;65:9982–9990. doi: 10.1158/0008-5472.CAN-05-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun XQ, Zhou H, Alain T, Sun B, Wang L, Barrett JW, et al. Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res. 2007;67:8818–8827. doi: 10.1158/0008-5472.CAN-07-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F. Future prospects for oncolytic therapy. Oncogene. 2005;24:7817–7819. doi: 10.1038/sj.onc.1209064. [DOI] [PubMed] [Google Scholar]
- McFadden G. Poxvirus tropism. Nat Rev Microbiol. 2005;3:201–213. doi: 10.1038/nrmicro1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM. A role for p53 in the frequency and mechanism of mutation. Mutat Res. 2002;511:45–62. doi: 10.1016/s1383-5742(01)00075-8. [DOI] [PubMed] [Google Scholar]
- Muñoz-Fontela C, Macip S,L, Brown L, Ashour J,A, et al. Transcriptional role of p53 in interferon-mediated antiviral immunity. J Exp Med. 2008;205:1929–1938. doi: 10.1084/jem.20080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KL, Hirasawa K, Yang AD, Shields MA, Lee PW. Reovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infection. Proc Natl Acad Sci USA. 2004;101:11099–11104. doi: 10.1073/pnas.0404310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KL, Lee PW. Not all viruses are bad guys: the case for reovirus in cancer therapy. Drug Discov Today. 2005;10:847–855. doi: 10.1016/S1359-6446(05)03483-5. [DOI] [PubMed] [Google Scholar]
- O'Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Reid TW, Albert DM, Rabson AS, Russell P, Craft J, Chu EW, et al. Characteristics of an established cell line of retinoblastoma. J Natl Cancer Inst. 1974;53:347–360. doi: 10.1093/jnci/53.2.347. [DOI] [PubMed] [Google Scholar]
- Royds JA, Hibma M, Dix BR, Hananeia L, Russell IA, Wiles A, et al. p53 promotes adenoviral replication and increases late viral gene expression. Oncogene. 2006;25:1509–1520. doi: 10.1038/sj.onc.1209185. [DOI] [PubMed] [Google Scholar]
- Shmulevitz M, Marcato P, Lee PW. Activated Ras signaling significantly enhances reovirus replication and spread. Cancer Gene Ther. 2010;17:69–70. doi: 10.1038/cgt.2009.46. [DOI] [PubMed] [Google Scholar]
- Song L, Ohnuma T, Gelman IH, Holland JF. Reovirus infection of cancer cells is not due to activated Ras pathway. Cancer Gene Ther. 2009;16:382. doi: 10.1038/cgt.2008.84. [DOI] [PubMed] [Google Scholar]
- Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong JE, Lee PWK. The v-erbB oncogene confers enhanced cellular susceptibility to reovirus infection. J Virol. 1996;70:612–616. doi: 10.1128/jvi.70.1.612-616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong JE, Tang D, Lee PWK. Evidence that the epidermal growth factor receptor on host cells confers reovirus infection efficiency. Virology. 1993;197:405–411. doi: 10.1006/viro.1993.1602. [DOI] [PubMed] [Google Scholar]
- Stanford M, Shaban M, Barrett JW, Gilbert PA, Bondy-Denomy J, Mackenzie L, et al. Myxoma virus oncolysis of primary and metastatic B16F10 mouse tumors in vivo. Mol Ther. 2008;16:52–59. doi: 10.1038/sj.mt.6300348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford MM, McFadden G. Myxoma virus and oncolytic virotherapy: a new biologic weapon in the war against cancer. Expert Opin Biol Ther. 2007;7:1415–1425. doi: 10.1517/14712598.7.9.1415. [DOI] [PubMed] [Google Scholar]
- Sypula J, Wang F, Ma Y, Bell J, McFadden G. Myxoma virus tropism in human tumor cells. Gene Ther Mol Biol. 2004;8:103–114. [Google Scholar]
- Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, et al. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- Tucker CA, Bebb G, Klasa RJ, Chhanabhai M, Lestou V, Horsman DE, et al. Four human t(11;14)(q13;q32)-containing cell lines having classic and variant features of mantle cell lymphoma. Leuk Res. 2006;30:449–457. doi: 10.1016/j.leukres.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–7137. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- Wang F, Barrett JW, Ma Y, Dekaban GA, McFadden G. Induction of alpha/beta interferon by myxoma virus is selectively abrogated when primary mouse embryo fibroblasts become immortalized. J Virol. 2009;83:5928–5932. doi: 10.1128/JVI.02587-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Barrett JW, Stanford M, Werden SJ, Johnston JB, Gao X, et al. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci USA. 2006;103:4640–4645. doi: 10.1073/pnas.0509341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E. Tumour biology. p53, guardian of Rb. Nature. 1994;371:21–22. doi: 10.1038/371021a0. [DOI] [PubMed] [Google Scholar]