Abstract
The present review assesses the current state of literature defining integrative autonomic-immune physiological processing, focusing on studies that have employed electrophysiological, pharmacological, molecular biological and central nervous system experimental approaches. Central autonomic neural networks are informed of peripheral immune status via numerous communicating pathways, including neural and non-neural. Cytokines and other immune factors affect the level of activity and responsivity of discharges in sympathetic and parasympathetic nerves innervating diverse targets. Multiple levels of the neuraxis contribute to cytokine-induced changes in efferent parasympathetic and sympathetic nerve outflows, leading to modulation of peripheral immune responses. The functionality of local sympathoimmune interactions depends on the microenvironment created by diverse signaling mechanisms involving integration between sympathetic nervous system neurotransmitters and neuromodulators; specific adrenergic receptors; and the presence or absence of immune cells, cytokines and bacteria. Functional mechanisms contributing to the cholinergic anti-inflammatory pathway likely involve novel cholinergic-adrenergic interactions at peripheral sites, including autonomic ganglion and lymphoid targets. Immune cells express adrenergic and nicotinic receptors. Neurotransmitters released by sympathetic and parasympathetic nerve endings bind to their respective receptors located on the surface of immune cells and initiate immune-modulatory responses. Both sympathetic and parasympathetic arms of the autonomic nervous system are instrumental in orchestrating neuroimmune processes, although additional studies are required to understand dynamic and complex adrenergic-cholinergic interactions. Further understanding of regulatory mechanisms linking the sympathetic nervous, parasympathetic nervous, and immune systems is critical for understanding relationships between chronic disease development and immune-associated changes in autonomic nervous system function.
INTRODUCTION
Autonomic Nervous System Regulation and Integrative Physiology: An Evolving State of Cooperation
The autonomic nervous system (ANS), composed of two primary branches, the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS), plays a critical role in regulating processes required for maintaining physiological homeostasis and responding to acute stressors, and has often been considered to function rather independently of other adaptive systems. However, recent lines of inquiry have expanded the functional repertoire of the ANS by establishing an essential role for this system in regulating, integrating, and orchestrating processes between diverse physiological systems (49, 51, 71, 96, 112). Specifically, the results of many studies (71, 96, 97, 120, 121, 134, 135, 136, 150, 152, 181, 200, 256, 281, 296, 297, 298) have established a critical role for the ANS in mediating interactions between the nervous and immune systems, two important adaptive systems that were originally considered to function independently of each other. The physiology of ANS function and regulation involves numerous complex, dynamic, and integrated steps (e.g., neural outflow, transmitter synthesis, release and degradation, ganglionic regulation, receptor-mediated effects), many of which are likely involved in mediating neural-immune interactions.
A key working principle for defining integrative autonomic-immune physiological processing is determining how signaling components of the immune system engage central autonomic neural circuits and regulate the level of activity in sympathetic and parasympathetic nerves, and how changes in autonomic regulation influence target immune organ and cell function. This review focuses on these physiological relationships with an emphasis on the results of studies focused on adult physiology that have used central microinjection and electrophysiological approaches, direct peripheral nerve recordings, and pharmacological and molecular biological techniques at both central and peripheral sites to investigate fundamental autonomic-immune interactions.
AUTONOMIC NERVOUS SYSTEM OVERVIEW
Sympathetic Nervous System and Parasympathetic Nervous System Regulatory Components
Sympathetic nerves innervating many target organs are tonically active. Direct recordings of the discharges of sympathetic nerves provide an output measure of central sympathetic neural circuits (148). The activity in sympathetic nerves contains multiple oscillations and, as reviewed by Barman and Kenney (12) and Gilbey (100), the sympathetic nerve discharge (SND) bursting pattern influences multiple physiological functions, including; regulating the level of efferent sympathetic nerve outflow, synchronizing or desynchonizing the activity in nerves innervating different targets, regulating target organ function, and generating differential patterns of sympathetic nerve outflow. SND pattern transformation is a consistent feature of SNS regulation. A fundamental regulatory strategy of the SNS involves selectively controlling the level and frequency components of SND bursts in nerves innervating different targets in response to a number of physiological conditions (148, 201). By altering important characteristics of efferent SND, including the level of activity, the bursting pattern, and the relationships between discharges in nerves innervating different targets, central sympathetic neural circuits regulate target organ responses.
The PNS innervates multiple organ systems and plays an important role in regulating a diverse array of physiological functions, including; heart rate, hormone secretion, gastrointestinal peristalsis, digestion, inflammation, and immune function. The vagus nerve (10th cranial nerve) and its branches are extensively distributed through the body and are composed of approximately 80% afferent fibers (sensory) and 20% efferent fibers (motor) (69), thereby providing critical communicating pathways for relaying sensory information to central neural circuits and conveying efferent activity to peripheral targets. As reviewed by Tonhajzerova et al. (295), vagal nerve outflow can exhibit oscillatory fluctuations, indexed often by oscillations in cardiac vagal nerve activity and heart rate.
Multiple sites in the central nervous system (CNS), including forebrain, brainstem and spinal neural circuits, regulate efferent sympathetic nerve outflow. The cell bodies of brainstem neurons whose axons project to sympathetic preganglionic neurons in the spinal cord are primarily confined to the rostral ventral lateral medulla (RVLM), midline medulla (rostral raphe pallidus, rRPa), and the A5 cell group in the pons (11, 58, 59, 179, 282, 283). The RVLM and rRPa play critical roles in SND regulation (11, 58, 59, 179, 282, 283). Forebrain nuclei (hypothalamic regions, anteroventral third ventricle region) also contribute to SND regulation (58, 59, 158, 179, 282). The paraventricular nucleus (PVN) of the hypothalamus is a major source of input to sympathetic preganglionic neurons and PVN neurons project to brainstem nuclei (e.g., RVLM) involved in SND regulation (58, 59, 158, 179, 282). It is well-established that other hypothalamic nuclei, including the dorsomedial hypothalamus (DMH), ventromedial hypothalamus (VMH), and the preoptic area (POA) of the anterior hypothalamus, as well as the subfornical organ (SFO), are involved in SND regulation (58, 59, 158, 179, 282). The primary neurotransmitter released at SNS ganglionic sites is acetylcholine (ACh), whereas norepinephrine (NE) is the predominant neurotransmitter released from postganglionic SNS neurons at target organ sites.
Peripheral vagal afferents terminate in the nucleus tractus solitarius (NTS), and projection neurons from the NTS make direct and indirect connections to a wide range of brain areas. Preganglionic parasympathetic neurons arise primarily from the caudal medulla, including the dorsal motor nucleus of the vagus (DMV) and the nucleus ambiguus (NA). The primary neurotransmitter released at both ganglionic and postganglionic sites in the parasympathetic system is ACh. It is generally considered that multiple CNS sites, including forebrain and brainstem nuclei, are involved in regulating the background level of activity and the responsivity of efferent parasympathetic nerve outflow (179), although central mechanisms remain to a large extent undefined.
Multiple experimental methods have been employed for assessing SNS and PNS contributions to physiological states and pathophysiological conditions including: pharmacological blockade of nicotinic, adrenergic, and muscarinic receptors; molecular approaches involving genomic and proteomic analyses; direct recordings of afferent and efferent activity in sympathetic and parasympathetic nerves; and the use of time-domain, frequency-domain, and nonlinear methods to analyze specific components of directly-recorded nerve activity, as well as physiological indices of nerve activity, such as heart rate variability (46). Regarding the latter, analysis of heart rate variability provides an important noninvasive, clinically-relevant technique for studying ANS regulation (1, 46, 64, 111, 286).
CYTOKINES, IMMUNE SYSTEM MEDIATORS AND AUTONOMIC NERVE RESPONSES
Introduction
Cytokines are signaling proteins that are synthesized and secreted by numerous types of cells, including; immune cells (e.g., macrophages, monocytes, lymphocytes), CNS neurons, microglia and astrocytes, and vascular endothelial cells (95). Cytokines and other immune cell products influence regulation of numerous physiological processes (e.g., sleep, fever production, neuroendocrine function, neuronal development, ANS responsiveness). Investigations studying interactions of immune mediators with the ANS have not been limited by the choice of cytokines or mediators to study. Indeed, interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interferon-α (IFN-α), bacterial products such as lipopolysaccharide (LPS), and other inflammatory mediators, such as those from the cyclooxygenase pathway have been studied. This section reviews studies that have documented the effects of cytokines and other immune mediators on basal SNS and PNS activity, and autonomic neural responsiveness to acute experimental interventions.
Interleukin-1β
The cytokine IL-1β mediates many of the diverse physiological responses of the acute-phase reaction. The intravenous IL-1β injection paradigm has been used to identify central pathways involved in cytokine-induced effects on stress-related neuroendocrine neurons (72, 73). The results of previous studies demonstrate that IL-1β influences SND regulation in several ways. First, IL-1β administration (intravenous, intracerebroventricular, intracisternal) alters the level of activity in sympathetic nerves innervating visceral organs (kidney, spleen, adrenal gland) (129, 137, 210, 213, 246, 247). Central microinjections of IL-1β in the PVN increase renal SND (268). IL-1β receptors are located in the brain, cells in the CNS express mRNA for IL-1 receptors, and IL-1β mRNA is expressed in brainstem and hypothalamic areas under basal or nonactivated physiological conditions (56, 74, 77, 99, 324). Renal and lumbar SND responses to intravenous IL-1β are dependent on the integrity of forebrain neural circuits (152). Second, IL-1β produces nonuniform changes in the level of sympathetic nerve outflow (210, 247). Specifically, intravenous IL-1β increases lumbar and splenic SND without significantly altering the level of renal and BAT SND in chloralose-anesthetized rats (247). Moreover, Niijima et al. (210) reported that intravenous IL-1β increased adrenal and splenic SND, but produced only transient increases followed by sustained reductions in renal SND in urethane-anesthetized rats. Third, the frequency-domain coupling of SND bursts can be influenced by IL-1β alone and in response to combined IL-1β and mild hypothermia (150), suggesting an additional mechanism via which IL-1β can influence sympathetic nerve outflow. Fourth, IL-1β can modulate responses to other physiological interventions. For example, IL-1β (intra-arterial administration) sensitizes the activity of visceral afferents recorded in the right thoracic sympathetic chain to ischemia and histamine (91), PVN IL-1β microinjection enhances the cardiac sympathetic afferent reflex as evaluated by renal SND responsiveness to epicardial application of bradykinin (268), and IL-1β markedly enhances BAT but not renal SND responses to hypothermia (151). These findings suggest that an important mechanism via which IL-1β influences SNS regulation is to modulate SND responses to acute physical stress. Fifth, Saindon et al. (247) reported renal, splenic and lumbar SND responses to IL-1β were augmented in vagotomized rats compared to rats with intact vagus nerves, suggesting the vagus nerve provides a tonic inhibition to visceral and peripheral SND in response to intravenous IL-1β, supporting the existence of complex interactions between IL-1β and SNS and PNS regulation.
Interleukin-6
This cytokine belongs to a group of structurally related IL-6-type cytokines that includes IL-11, leukemia inhibitory factor, oncostatin M, ciliary neurotrophic factor, cardiotrophin-1, and cardiotrophin-like cytokine (119). Although IL-6 modulates numerous centrally-mediated physiological responses (e.g., fever, activation of the hypothalamic-pituitary-adrenal axis, immune regulation), the effect of either peripheral or central IL-6 on SND regulation remains poorly understood. Helwig et al. (120) reported central IL-6 administration (intracerebroventricular; icv) activates splenic SND, and is associated with co-localization of IL-6 with the IL-6 receptor (IL-6R) in periventricular tissue at the level of the third ventricle, but is not widely distributed in brain parenchyma. IL-6 mRNA and IL-6 receptor mRNA have been reported to be localized in several medial hypothalamic areas, including the dorsomedial, ventromedial, and medial preoptic nuclei (262). Vallieres and Rivest (301) reported low levels of IL-6 receptor mRNA under basal conditions in the ependymal cells of the ventricles, and several sites in the CNS, including the circumventricular organs (CVOs). In addition, central IL-6 administration induces cellular activation in numerous areas of the rat brain, including the ependymal cell layer, the ventricular walls, and CVOs (300).
Haensel et al. (111) reviewed the results of studies examining relationships between heart rate variability and markers of inflammation in various cardiovascular diseases, whereas Huston and Tracey (128) reviewed the literature regarding interactions between the cholinergic anti-inflammatory pathway, heart rate variability, and potential therapeutic interventions. The existing literature provides support for the idea that R-R interval variability, interpreted in many studies as an index of cardiac vagal modulation, is inversely related to IL-6 and other inflammatory markers, including C-reactive protein, in both healthy subjects and patients with cardiovascular disease (10, 111, 128). These data suggest the existence of interactions between inflammation and ANS physiology and regulation in human subjects. As discussed by Haensel (111), measures of heart rate variability reflect PNS as well as SNS modulation of the heart, and the interpretation of correlations between inflammatory markers and autonomic function should consider both arms of the ANS.
Tumor necrosis factor-α and Interferon-α
TNF-α has been shown to modulate SND regulation (213, 246, 331). Intravenous TNF-α reduces ear temperature (indicative of increased cutaneous SND) in anesthetized (246) and conscious rabbits (213), reduces renal SND in anesthetized rabbits (246), but increases renal SND in conscious rabbits (213). SND responses to TNF-α in conscious rabbits were blocked by indomethacin administration (213). Intravenous and intracarotid artery TNF-α administration in anesthetized rats increases renal SND, mean arterial blood pressure, and neuronal firing of RVLM and PVN neurons (331). Responses to intravenous TNF-α were reduced following mid-collicular transection but not vagotomy (331). ICV administration of the cyclooxygenase inhibitor ketorolac reduced renal SND and PVN neuronal activation to intracarotid artery TNF-α administration, icv PGE2 increased the activity of PVN neurons and elicited renal sympathoexcitation, and PVN PGE2 microinjection increased RVLM neuronal activity and renal SND (331). These data suggest the excitatory influences of systemically administered TNF-α on renal SND are mediated by central actions of prostaglandins acting, at least in part, in the PVN (331). Additional central effects of TNF-α on SND regulation have been identified. For example, pentoxifylline (PTX, inhibitor of proinflammatory cytokine synthesis) administration attenuated the renal sympathoexcitation and decreased the PVN production of TNF-α in rats with congestive heart failure (109), whereas Shi et al. (268) reported that PVN TNF-α microinjection increased renal SND and enhanced the cardiac sympathetic afferent reflex.
Several lines of evidence indicate that the cytokine IFN-α can affect the regulatory status of central sympathetic neural circuits. The icv administration of IFN-α in rodents increases splenic SND (141) and microinjection of IFN-α into the medial preoptic area (MPO) of the hypothalamus increases splenic sympathetic nerve outflow in anesthetized rats (140).
Granulocyte-Colony Stimulating Factor
A novel regulatory axis connecting the endogenous growth factor and hematopoietic cytokine granulocyte-colony stimulating factor (G-CSF) and the SNS has recently been characterized (142, 168). G-CSF is a hematopoietic growth factor that was initially identified for its role in mediating the proliferation and differentiation of cells of the myeloid lineage (260, 261, 273, 274, 294). Functional effects are primarily dependent on the binding of G-CSF to its specific receptor (G-CSFR) (260, 261, 273, 274, 294), which is expressed in a variety of hematopoietic cells as well as on neurons, endothelial cells, glial cells, and numerous rat brain regions including forebrain, cerebellum, and the brainstem (260, 261).
Several lines of evidence support the existence of regulatory interactions between G-CSF and the SNS. Yujiri et al. (330) reported that G-CSF administration significantly altered plasma levels of norepinephrine (NE) and epinephrine in human subjects. Hematopoietic stem and progenitor cell mobilization from bone marrow is enhanced following subcutaneous G-CSF administration in animals with an intact SNS (142). Importantly, G-CSF-induced mobilization of hematopoietic stem and progenitor cells from bone marrow was significantly reduced in animals that received 6-hydroxydopamine or were pretreated with the non-selective β-adrenergic receptor antagonist propranolol (142). Moreover, Katayama et al. (142) reported bone NE levels were markedly reduced whereas cardiac NE levels were only mildly reduced after the subcutaneous administration of G-CSF, suggesting that peripheral administration of G-CSF can produce rapid and possibly nonuniform effects on sympathetic nerve outflow.
Prostaglandin E2
PGE2 is an important effector molecule for central neural inflammatory responses. Proinflammatory cytokines induce COX-2 expression in central neural sites, including blood-brain barrier microvascular and endothelial cells, which in turn induces production of PGE2 that influences centrally-mediated physiological responses, including activation of the hypothalamic-pituitary-adrenal (HPA) axis, fever, and activation of the SNS (333). ICV PGE2 administration increases PVN neuronal activity and renal SND (331, 333), and RVLM neuronal activity is enhanced in response to microinjection of PGE2 in the PVN (331). Renal sympathoexcitation to icv PGE2 is reduced by icv pretreatment with a prostaglandin E receptor 3 (EP3) antagonist (333). PVN microinjection of PGE2 activates renal SND, an effect that is reduced by the PVN microinjection of an EP3 receptor antagonist (333). Central (both icv administration and microinjection into the medial preoptic area of the hypothalamus) PGE2 increases brown adipose tissue (BAT) SND (186, 202). Consistent with this response, Ootsuka et al. (216) reported that intravenous PGE2 increased BAT SND, an effect that was prevented by inhibition of the medullary raphe region but not by acute bilateral cervical vagotomy or truncal subdiaphragmatic vagotomy.
Lipopolysaccharide
The bacterial endotoxin LPS, a key ligand for toll-like receptors (182, 224, 239, 272, 276), has been used widely in experimental studies to enhance the systemic and CNS synthesis of various cytokines. Intravenous LPS or endotoxin administration increases renal and splenic SND in conscious and anesthetized animals (184, 185, 213, 217, 307). LPS-induced splenic sympathoexcitation is delayed by the icv administration of the nonsteroidal anti-inflammatory indomethacin (185). Sayk et al. (257) reported LPS administration in human subjects reduced muscle SND, blunted SND responsiveness to changes in arterial blood pressure, and increased systemic levels of TNF-α and IL-6. LPS-induced elevations in plasma levels of TNF-α were positively correlated to the relative reductions in muscle SND whereas plasma IL-6 levels were negatively correlated to the relative reductions in muscle SND (257). These data suggest that systemic inflammatory responses secondary to LPS administration may influence central SND regulation.
Vayssettes-Courchay et al. (307) investigated the origin of SND responses to intravenous LPS administration in a rodent model of sepsis. Intravenous LPS administration produced renal sympathoexcitation in anesthetized rats, an effect that was not substantially altered after lesion of the NTS. Intracisternal LPS directed to the surface of the ventral medulla produced renal sympathoexcitation that was similar in magnitude to that observed after intravenous LPS, suggesting a role for the ventral medulla in mediating SND responses to LPS.
The expression of central cytokines and signaling pathways mediating renal sympathoexcitation to central (icv) LPS administration has been studied by Zhang et al. (332). LPS administration increased hypothalamic synthesis of TNF-α and cyclooxygenase-2 (COX-2), levels of TNF-α and prostaglandin E2 (PGE2) in cerebrospinal fluid, PVN production of superoxide, arterial blood pressure, and renal SND. LPS-induced renal sympathoexcitation was reduced by blocking central NAD(P)H oxidase activity, scavenging superoxide, or inhibiting p38 MAPK, and was eliminated by inhibiting COX-2 activity. In addition, LPS stimulated the hypothalamic expression of angiotensin II type 1 receptor mRNA. These results demonstrate that central LPS administration stimulates the central production of pro-inflammatory cytokines, resulting in PGE2 synthesis, which in turn activates sympathetic neural circuits and increases renal sympathetic nerve outflow.
PATHWAYS OF IMMUNE TO CENTRAL NERVOUS SYSTEM COMMUNICATION
Multiple communication pathways and/or processes are known to transmit information regarding peripheral immune status to the CNS. These physiological communication pathways have been extensively researched, studied, and previously reviewed (10, 229, 230). There are generally two major pathways of communication relaying peripheral immune status to the CNS, neural (sensory nerves) and non-neural.
The vagus nerve has been implicated as a component of the neural pathway transmitting signals from the peripheral immune system to the brain (98, 103, 114, 229, 315). However, as discussed by Saper et al. (256), the physiological relevance of the vagus nerve in immune signaling to central neural circuits is not entirely clear and requires additional inquiry. Vagal paraganglia demonstrate chemosensitive properties (212) and IL-1β receptors are present on abdominal paraganglia (101). The intravenous administration of IL-1β increases the level of afferent gastric vagal nerve activity in anesthetized rats (70), a response that is antagonized by pretreatment with indomethacin (non-steroidal anti-inflammatory drug). Moreover, cutaneous sensory afferents have also been implicated in providing a communication pathway to central neural circuits regarding local immune cytokine formation (244). MacGrory and colleagues (183) recently tested the hypothesis that vagal paraganglia play a role in facilitating communication regarding peripheral levels of proinflammatory cytokines and central nervous sites via changes in the discharge rate of vagal nerve ganglia. These investigators reported that TNF-α and IL-1β administration did not acutely alter the discharge rate of rat vagal paraganglia in vitro (183), results that question an immune role for vagal paraganglia as an afferent signaling pathway to central neural circuits.
Mechanisms mediating immune to CNS communication via the non-neural/blood brain barrier pathway have been extensively reviewed by Banks (10). Three mechanisms are reviewed. First, the transport of cytokines can occur via saturable transport systems across the blood brain barrier via complementary receptors, e.g., TNF/IL-1 transport has been found to be mediated by TNF/IL-1 receptors (7, 48, 218). Second, the induction of blood brain barrier secretions, such as prostaglandins, nitric oxide, and cytokines can occur following systemic LPS or IL-1 challenge (76), and these molecules can subsequently gain access to the brain parenchyma. For example, cyclooxygenase expression occurs in perivascular macrophages (a subset of brain-resident macrophages) and endothelial cells of the brain microvasculature in response to immune activation (36, 258, 265). Third, selected brain structures lining the third and fourth ventricles are devoid of a blood brain barrier and are collectively termed CVOs (132). These areas contain blood vessels with fenestrated capillaries that lack tight junctions, allowing circulating molecules in the blood to enter into CVOs (106, 166). Their anatomical location and unique structural morphology establish CVOs as a point of communication between brain parenchyma, blood, and cerebrospinal fluid (106). It is hypothesized that sensory CVOs, including the SFO, organum vasculosum lamina terminalis, and area postrema (269), provide communication processes via the presence of receptors and ligand binding sites for hormones, neurotransmitters, and immune mediators (132). In support of the above studies, Maness et al. (193) and Peruzzo et al. (226) demonstrated the tanycytic barrier in CVOs prevents cytokine leakage, and suggested that immune to brain signaling at the CVOs may be linked to activation and release of local cytokines from the adjacent immune cells (102). Consistent with this view, Komaki (163) proposed that IL-1signaling involves specific receptors located in the CVOs.
In summary, both neural and non-neural pathways are likely involved in mediating peripheral immune to brain communication, and each pathway may be activated individually or together based on numerous factors including; the type of antigen or immune stimulant, dose or concentration of the immune stimulant, and the route of antigen administration (104, 113, 115).
CENTRAL NERVOUS SYSTEM-IMMUNE SYSTEM-SYMPATHETIC NERVOUS SYSTEM SIGNALING INTERACTIONS
Introduction
Much of the contemporary understanding of central regulation of SND has emanated from studies using specific central neural microinjection and electrophysiological techniques employed during a variety of physiological and pathophysiological conditions. Similar experimental approaches have been used to investigate the role of the CNS in mediating immune system-SNS interactions. The following sections highlight selected central SNS-immune system and immune system-SNS lines of inquiry that provide a template for establishing a conceptual framework to further understand fundamental strategies used by these systems to regulate physiological function and sympathetic nerve outflow.
Activation of Central Sympathetic Nerve Outflow and Peripheral Immune Responses
Acute heat stress is an environmental stressor that markedly increases the level of activity in numerous sympathetic nerves, including visceral SND (renal, splanchnic, splenic, adrenal) (54, 125, 149, 153, 154, 155, 156, 157, 165, 211). Experimental interruption of RVLM synaptic activation and axonal transmission during acute heating produce significant reductions in hyperthermia-induced SND activation (125), suggesting the functional integrity of RVLM neural circuits is required for sustaining a substantial degree of visceral sympathoexcitation to increased internal body temperature (125).
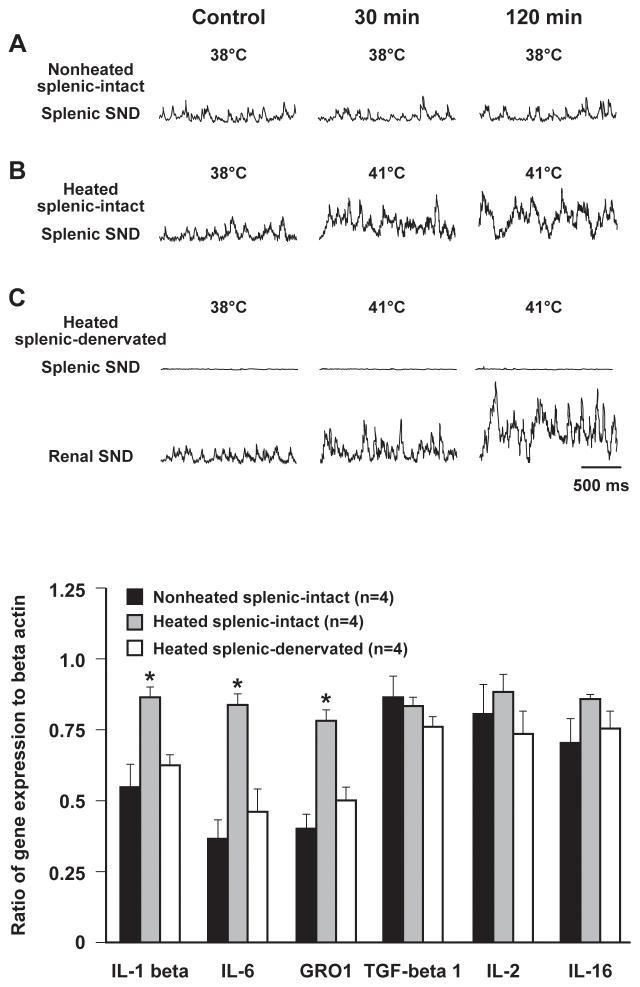
The SNS innervation to the spleen provides a link between central sympathetic neural circuits and splenic immunocompetent cells (81, 84), providing rationale for determining the effect of heating-induced splenic SND activation on splenic cytokine gene expression. Ganta et al. (96) reported the splenic sympathoexcitation to acute heating was associated with increased splenic expression of IL-1β, IL-6, and growth-regulated oncogene 1 mRNA, levels that were significantly higher than those observed in nonheated rats (Figure 1, adapted from Ganta et al.; 96). Heating-induced increases in splenic IL-1β, IL-6, and GRO 1 mRNA expression were significantly attenuated in splenic nerve-denervated compared with splenic nerve-intact rats (Figure 1), an effect that was not secondary to differences in splenic blood flow during heating (96). These data suggest that splenic sympathoexcitation, mediated via activation of RVLM presympathetic neurons in response to increased internal body temperature (125), modulates splenic cytokine gene expression, supporting the hypothesis that physiological stimuli that increase splenic nerve outflow can affect peripheral immune responses.
Figure 1.
Traces of splenic sympathetic nerve discharge (SND) recorded during nonheated (A) and heated (B) experimental conditions in rats with intact splenic nerves. Note the heating-induced increase in splenic SND (B). As expected, splenic-denervation abolished splenic SND but not renal SND responses to heating (C). Expression of splenic interleukin-1β (IL-1β), interleukin-6 (IL-6), and growth-regulated oncogene 1 (GRO1) genes was significantly higher in heated splenic-intact compared with nonheated splenic-intact, and in heated splenic-intact compared with heated splenic-denervated rats (bottom). Black box, non-heated splenic-intact; gray box, heated splenic-intact; white box, heated splenic-denervated. Adapted with permission from Ganta et al. (96).
Angiotension II (Ang II) is known to exert prominent effects on central regulation of cardiovascular function and sympathetic nerve outflow (9, 42, 191, 293, 334). Activation of central sympathetic neural circuits, produced by icv administration of Ang II, and the accompanying splenic sympathoexcitation, modulate splenic cytokine gene expression (97). Splenic cytokine (IL-1β, IL-2, IL-5, IL-16, and transforming growth factor-β1) gene expression was increased in response to central Ang II-induced increases in splenic SND in rats with intact splenic nerves, responses that were significantly attenuated in splenic nerve-denervated rats. Collectively, these findings provide experimental support for a functional relationship between activation of central sympathetic neural circuits, increased levels of splenic sympathetic nerve outflow, and modulation of splenic cytokine gene expression, although the role of vagal contributions cannot be discounted.
A pathophysiological extension of this tenet, supported by the results of studies reviewed by Catania et al. (44), is that acute brain injury exerts detrimental effects on peripheral immune tissues and cells, responses that are, at least in part, secondary to activation of central sympathetic nerve outflow. These authors highlighted a contributing role for brain injury induced-central sympathoactivation in diverse immunological responses, including; immunodepression, inflammatory responses in peripheral organs, alterations in intestinal permeability, and acute-phase responses (44). Moreover, as reviewed by Dirnagl et al. (68), there is emerging evidence that the SNS may contribute to the development of stroke-induced immunodepression.
Central Inflammation, Sympathetic Neural Circuits, and Neurogenic Hypertension
Inflammation has been implicated as a contributing factor in numerous cardiovascular diseases, including hypertension (89, 267). In a recent study, Kang et al. (135) established that enhanced expression of nuclear factor kappaB (NFkB), activation of proinflammatory cytokines, and production of reactive oxygen species contribute to hypertension produced by chronic infusions of Ang II (135). Shi et al. (267) reported that chronic Ang II infusions increased mean arterial pressure (MAP) and plasma NE levels, activated microglia in the PVN, increased PVN mRNA expression of IL-1β, IL-6, and TNF-α, and reduced PVN mRNA expression of IL-10. Moreover, central administration of an anti-inflammatory antibiotic attenuated arterial blood pressure and NE responses, decreased activation of PVN microglia, reduced PVN expression of mRNAs for proinflammatory cytokines, and increased PVN expression of IL-10 mRNA in response to chronic Ang II. These data support the hypothesis that activation of PVN microglial cells and the subsequent generation of proinflammatory cytokines in the PVN play a critical role in mediating neurogenic hypertension produced by chronic Ang II infusions.
The SFO, a forebrain circumventricular structure that lies outside the blood-brain barrier, has been implicated in mediating Ang II-induced hypertension (86, 335, 336). The SFO is considered a gateway for circulating Ang II to access AT1 receptors and activate neural signaling pathways to areas of the brain involved in regulation of SND, including the PVN (327). Young and colleagues (327) reported that in response to chronic Ang II, cells in the subfornical region demonstrate enhanced biomarkers of endoplasmic reticulum stress, alterations that can disrupt cellular function and contribute to chronic pathophysiological states. Additional experiments demonstrated that genetic supplementation of the endoplasmic reticulum chaperon protein 78-kDa glucose-regulated protein in the SFO prevented the hypertension produced by peripheral administration of Ang II; supporting the hypothesis that endoplasmic reticulum stress plays a role in the development of neurogenic hypertension in this experimental model (327). Furthermore, arterial blood pressure and renal SND were significantly increased following central administration of the endoplasmic reticulum stress inducer, thapsigargin, indicating that central neural endoplasmic reticulum stress can activate brain sympathetic neural circuits and increase arterial blood pressure (327). Studies by Lob et al. (177, 178) have identified activation of NADPH oxidases, and increased oxidant formation in the SFO as a contributing factor mediating the hypertension induced by chronic Ang II infusion. Moreover, SND activation, mediated via the effects of reactive oxygen species on central sympathetic neural signaling, may activate peripheral T-cells thereby contributing to pathophysiological complications in vascular and renal tissues (60, 177, 178), as well as providing a signal back to the SFO to further facilitate this cycle (60).
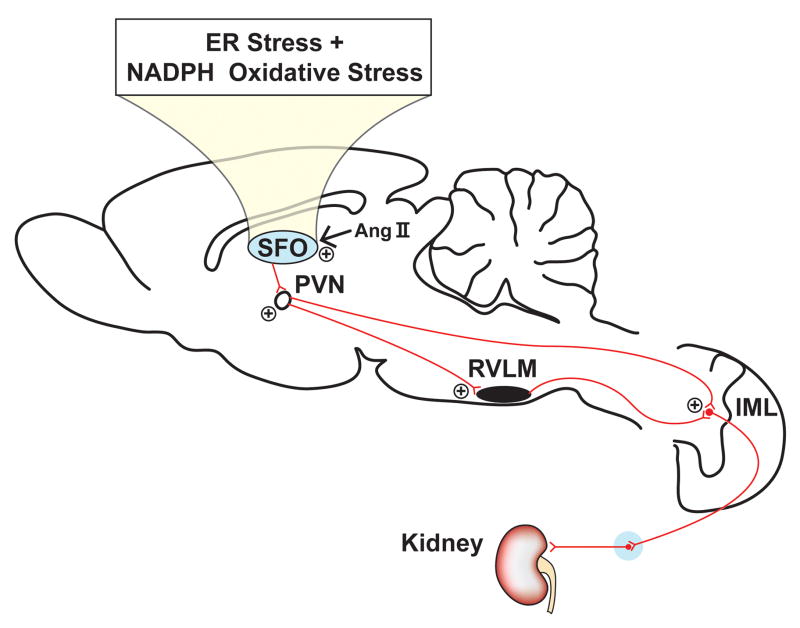
Taken together the results of these studies suggest that circulating Ang II may activate AT1 receptors in the SFO, or other CVOs that lie outside the blood-brain barrier. Chronic activation of cells in the subfornical organ can induce endoplasmic reticulum/oxidant stress, thereby signaling neural projections to the PVN, which in turn may activate local PVN microglia to enhance the expression and release of proinflammatory cytokines which provides an excitatory signal to sympathetic premotor neurons to increase sympathetic nerve outflow (Figure 2, adapted from Davisson and Zimmerman; 60).
Figure 2.
Schematic diagram illustrating how the subfornical organ (SFO) may link angiotension II (Ang II) to visceral sympathoexcitation. Ang II-mediated activation of NADPH oxidases and increased oxidative stress in the SFO, along with resulting endoplasmic reticulum stress, leads to stimulation of neural projections to the paraventricular nucleus (PVN), which signals downstream sympathetic pathways mediating increases in renal sympathetic nerve discharge. Adapted with permission from Davisson and Zimmerman (60).
Prostaglandin E2, Perivascular Macrophages, Paraventicular Nucleus Signaling, Heart Failure and Sympathetic Nerve Discharge Regulation
PGE2 plays a key role mediating the effects of proinflammatory cytokines (e.g., IL-6, IL-1β, TNF-α) on the CNS. Cyclooxygenase expression occurs in perivascular (a subset of brain-resident macrophages) and endothelial cells of the brain microvasculature in response to immune activation (36, 258, 265). Schiltz and Sawchenko (258) reported an enhanced sensitivity for COX-2 in response to IL-1 or endotoxin treatment in perivascular macrophages compared with endothelial cells. PGE2 can gain entrance to the brain and activate neurohumoral systems (214, 237, 325, 333). PGE2 acts on at least four subtypes of E-class prostanoid receptors to elicit physiological responses (8, 209, 214, 237, 325, 333).
Mechanisms involved in mediating PGE2 effects on central regulation of SND have been the focus of several recent lines of inquiry (331, 333). Zhang et al. (333) reported icv PGE2 administration in anesthetized rats increased the discharge of PVN neurons and renal SND, and PVN PGE2 microinjections increased renal sympathetic nerve activity. Renal sympathoexcitatory responses to icv PGE2 administration and PVN PGE2 microinjections were substantially attenuated by pretreatment with a selective EP3 receptor antagonist, providing support for the hypothesis that EP3 receptors, including those within the PVN, are involved in mediating central PGE2-induced sympathoexcitatory effects (333).
Recent lines of inquiry have established that myocardial infarction and heart failure induce inflammation in central neural sites (90, 136, 328). Using a rat model of ligation-induced myocardial infarction, Francis et al. (90) reported increased hypothalamic synthesis of TNF-α within minutes following induction of myocardial infarction, a response that was sustained for at least four weeks. Treatment with PTX, an inhibitor of proinflammatory cytokine synthesis, prevented the increase in hypothalamic TNF-α. Consistent with these findings, Kang et al. (136) reported that inhibition of central neural proinflammatory cytokine synthesis in rats with heart failure reduced multiple indicators of hypothalamic activation, including; proinflammatory cytokines, components of the renin-angiotensin system, superoxide, and PGE2. The increased expression of COX-2 in the PVN of rats with heart failure, as well as several proinflammatory cytokines and other indices of inflammatory activation, are prevented by treatment with an NF-kappaB inhibitor (328), suggesting that this transcription factor plays a key role in cytokine-mediated upregulation of PVN PGE2 in rats with ischemia-induced heart failure (328).
Activation of centrally-mediated sympathetic nerve outflow is a hallmark of heart failure (50, 66, 67, 85, 87, 88, 116, 126, 171, 288, 302, 314, 337). Rats with heart failure demonstrate increased levels of plasma proinflammatory cytokines (110), NE, epinephrine, and directly-recorded renal SND (134). Numerous indices of central neural excitation are upregulated in heart failure rats, including; PVN levels of glutamate, NE, and tyrosine hydroxylase (134). Moreover, levels of GABA, nNOS, GAD67 are reduced in the PVN of heart failure rats (134). Importantly, icv treatment with PTX and etanercept (recombinant TNF-alpha receptor fusion protein) prevented increases in PVN glutamate, NE, tyrosine hydroxylase, and decreases in PVN GABA, nNOS, and GAD67 in heart failure rats (134). In addition, PTX and etanercept prevented heart failure-induced increases in renal SND (134). These data provide evidence that proinflammatory cytokines modulate PVN neurotransmitters and contribute to the activation of renal SND in heart failure (134).
Perivascular macrophages have been implicated as providing an important link between central cytokine-induced COX-2 activity, PGE2 production, and activation of SND. Yu et al. (329) reported that elimination of perivascular macrophages in rats with increased circulating levels of cytokines, secondary to an acute episode of myocardial infarction, normalized expression of COX-2 in the PVN, reduced levels of PGE2 in cerebrospinal fluid, and renal sympathetic nerve activity. Moreover, increases in arterial blood pressure and renal SND in response to intracarotid TNF-α administration in normal rats were prevented by the selective depletion of perivascular macrophages (329). These data suggest that perivascular macrophages may provide a critical link between systemic inflammation, circulating cytokines or immune mediators, and activation of central sympathetic neural outflow (Figure 3, adapted in part from Serrats et al.; 265). In a similar manner, Helwig et al. (120) reported icv IL-6 administration activates splenic SND, is associated with co-localization of IL-6 with the IL-6 receptor (IL-6R) in periventricular tissue at the level of the third ventricle, but is not widely distributed in brain parenchyma, suggesting that periventricular tissue may be involved in transducing the IL-6 sympathoexcitatory signal to sympathetic neural circuits, possibly at the level of the PVN.
Figure 3.
Perivascular macrophages may provide a critical link between systemic inflammation, circulating cytokines, and sympathetic nerve discharge (SND) activation. Cyclooxygenase expression and synthesis of prostaglandin E2 (PGE2) occurs in perivascular macrophages and endothelial cells of the brain microvasculature in response to immune activation. PGE2 can gain access to the brain parenchyma, activate presympathetic neurons in sympathetic nuclei, including the paraventricular nucleus (PVN), and signal downstream sympathetic nuclei (rostral ventral lateral medulla, RVLM; intermediolateral nucleus of the spinal cord, IML) and pathways to increase renal SND. Adapted with permission from Serrats et al. (265).
Medial Preoptic Neurons Contribute to Cytokine- and Prostaglandin E2-induced Changes in Sympathetic Nerve Discharge
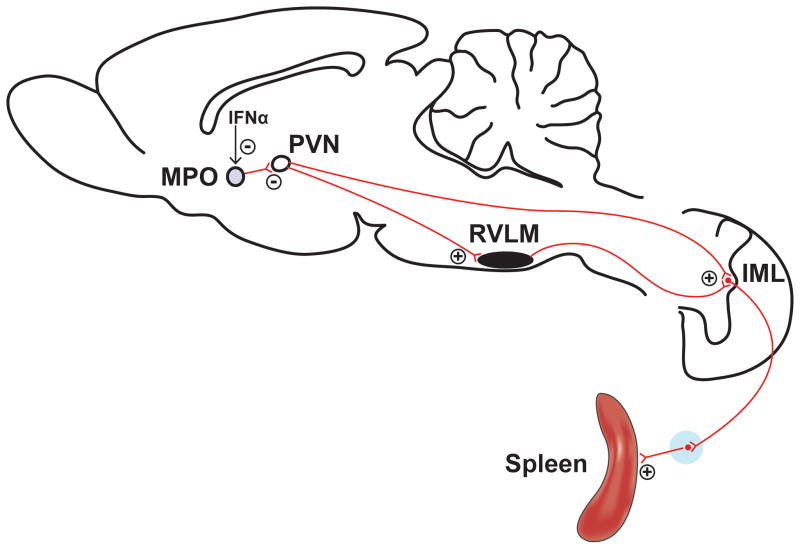
The icv administration of IFN-α in rodents reduces splenic natural killer (NK) cell activity, an effect that is not observed in splenic sympathetic nerve-denervated animals (139, 284), supporting a critical role for the SNS in central IFN-α-induced modulation of peripheral NK cell activity. ICV IFN-α administration increases splenic SND and electrical stimulation of the peripheral end of the cut splenic nerve suppresses splenic natural killer cytotoxicity (141). Microinjection of IFN-α into the hypothalamic MPO increases splenic SND whereas activation of MPO neurons by glutamate microinjections or electrical stimulation of this hypothalamic area reduces splenic SND (140). MPO microinjections of IFN-α suppress the activity of MPO neurons (139), possibly via a pathway that involves PGE2, and the neural projection pathway from MPO to PVN is considered to be primarily inhibitory to PVN cells (139). Therefore, suppression of MPO neurons secondary to microinjection of IFN-α mediates an increase in splenic SND resulting from disinhibition of PVN neurons from the inhibitory MPO projection (139). Increased splenic SND reduces splenic NK cell activity via activation of β-adrenergic receptors in the spleen (141). The physiological connectivity linking central IFN-α, activation of sympathetic neural pathways (splenic SND in the context of these studies) (Figure 4, adapted from Katafuchi et al.; 139), and reduced splenic NK cell activity may be a functional component of the adaptive immune response that limits overactivation of proinflammatory pathways and responses (139).
Figure 4.
Schematic diagram illustrating how central neural administration of interferon-α (IFN-α) may modulate splenic sympathetic nerve discharge (SND). The neural projection pathway from the medial preoptic area (MPO) to the paraventricular nucleus (PVN) is considered to be primarily inhibitory, and MPO neurons are suppressed by IFN-α microinjections. Therefore, MPO IFN-α administration leads to activation of PVN neurons secondary to disinhibition. The PVN has direct excitatory projections to the RVLM and the IML nucleus leading to activation of splenic SND. Adapted with permission from Katafuchi et al. (139).
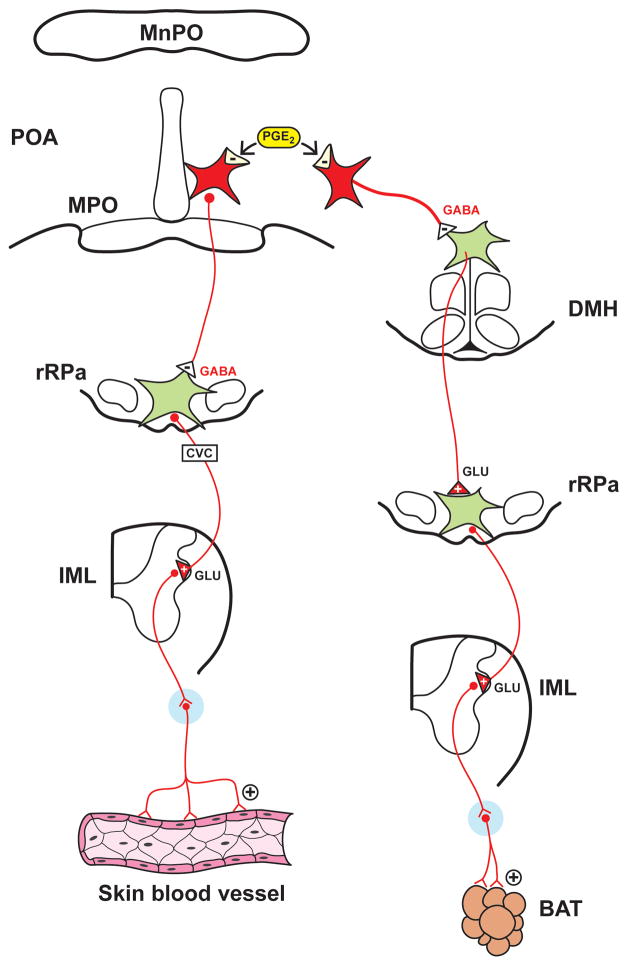
As has been extensively reviewed (94, 200, 205, 256), PGE2 in the POA of the hypothalamus plays a critical role in mediating sympathoexcitatory responses that contribute to fever development (Figure 5, adapted from Morrison; 200). PGE2 in the POA influences the activation state of POA neurons that project to the DMH and the rRPa, thereby providing a critical substrate for activation of BAT SND and cutaneous vasoconstriction sympathetic outflow (94, 200, 205, 206, 256, 285, 326). It is likely that POA neurons that express EP3 receptors tonically inhibit DMH and rRPa neurons via GABAergic projections, and inhibition of POA neurons secondary to binding of PGE2 to the EP3 receptor produces disinhibition of DMH and rRPa neurons (200, 205, 206). The use of anatomical and functional experimental approaches reveal that increased BAT SND is mediated by activation of rRPa neurons secondary to disinhibition of DMH neurons, indicating that BAT thermogenesis responses are dependent on activation of neurons in the dorsal hypothalamus (94, 200, 202, 205, 206, 256, 285, 326). Cutaneous vasoconstriction produced by POA PGE2 microinjection is mediated by inhibition of POA projection neurons that bypass the dorsal hypothalamus and project directly to the rRPa (94, 200, 202, 205, 206, 256, 285, 326).
Figure 5.
Schematic diagram illustrating how central prostaglandin E2 (PGE2) in the preoptic area (POA) of the hypothalamus mediates sympathetic activation. PGE2 acts on EP3 receptors to inhibit POA neurons that are inhibitory to neurons in the dorsal medial hypothalamus (DMH) and rostral raphe pallidus (rRPa), thus activating sympathetic outflow mediating cutaneous vasoconstriction and sympathetic neural pathways to adrenergic receptors (ARs). Adapted with permission from Morrison (200).
Summary
The results of studies using central microinjection and electrophysiological approaches, peripheral nerve recordings, and molecular biological and pharmacological techniques have advanced the understanding of mechanisms mediating sympathetic-immune interactions and central regulation of sympathetic nerve outflow in several prominent ways. First, and consistent with previous reviews regarding communication between the immune system and the CNS (10, 230), sympathetic neural circuits are apprised of peripheral immune status, and alterations in this status, via multiple pathways, including but not limited to; activation of peripheral afferents with subsequent neural communication to central neural nuclei, access of immune cell products and other immune mediators to central sympathetic sites via CVOs, and induction of COX-2 activity and the synthesis of PGE2 in endothelial cells and perivascular macrophages in the brain microvasculature. Second, the capability of cytokines and other immune cell products to alter the level of activity in sympathetic nerves innervating multiple targets (kidney, spleen, brown adipose tissue, tail) supports an extensive integrative influence of immune system mediators on SND regulation and physiological function. Third, cytokines and other inflammatory mediators produce changes in sympathetic nerve activity, at least in part, via specific neural pathways. For example, PGE2 in the POA contributes to fever generation by influencing the activation state of POA neurons that project to the DMH, which in turn activate cell bodies in the rRPa, with subsequent increases in the level of BAT SND (200). Fourth, SNS-immune system interactions can influence peripheral immune responses to acute physiological stimuli. For example, heating-induced activation of central sympathetic nerve outflow can modulate splenic cytokine gene expression secondary to increased splenic SND. Fifth, immune system mediators have been investigated as contributors to sympathetic neural alterations associated with several pathophysiological states, including, but not limited to neurogenic hypertension, heart failure, and fever (89, 90, 134, 135, 200, 267). Consistent with many physiological processes and interactions, an appropriate level of cooperation and physiological balance between immune cell mediators and sympathetic neural circuits is important for maintaining homeostasis, and alterations in this balance can contribute to the development and maintenance of pathophysiological conditions. Sixth, bacterial disease in living organisms influences numerous sites and progresses in diverse ways, therefore, a variety of experimental approaches and interventions have been used to characterize and identify fundamental regulatory components relating the immune system and central sympathetic neural circuits. For example, intravenous cytokine administration models systemic bacterial infections, brain intraparenchymal cytokine microinjections model the release of cytokines from CNS astrocytes, microglial cells, and neurons, and icv administration of cytokines into the lateral ventricles has been used as an experimental model for cytokines released from choroid plexus. Supporting the use of diverse administrative approaches, Vallieres et al. (300) reported that systemic injection of IL-6 strongly activated cells in the CVOs, whereas a different pattern of cellular activation of brain sites was observed after intracerebral injection of IL-6, suggesting that the response of brain areas to IL-6 and other cytokines may be dependent on the origin of the cytokine during an immune challenge.
PERIPHERAL SYMPATHETIC NERVOUS SYSTEM-IMMUNE SYSTEM SIGNALING INTERACTIONS
Introduction
Complex interactions exist between the SNS and the immune system and it has been shown that the SNS can both enhance and inhibit immune responses, depending on; experimental conditions, the type of stress paradigm used, the specific pathophysiological state or disease condition, activation state of the SNS, and types of immune cells activated. In addition, and as reviewed previously, immune cell products can influence the functional state of the SNS. This section reviews studies that have documented peripheral SNS-immune system signaling interactions.
Sympathetic Innervation of Lymphoid Organs
Neuroanatomical and neurochemical studies using retrograde tracers and immunocytochemical techniques have demonstrated that both primary and secondary lymphoid organs, including; bone marrow, thymus, spleen, lymph nodes, and gut and bronchiolar associated lymphoid tissue, are innervated by sympathetic nerve fibers (18, 19, 41, 79, 80, 82, 83, 208, 236, 299, 305, 320, 321). The sympathetic innervation of primary and secondary lymphoid organs contributes to the maturation, development and regulation of immune cells throughout the life of the animal (19). The sympathetic innervation to the spleen is well-characterized (19). Post-ganglionic sympathetic nerves enter the spleen along with the splenic artery after arising primarily from the superior mesenteric plexus and the celiac ganglionic plexus (16, 47, 207). Sympathetic neural projections to the spleen include a prominent innervation to the splenic white pulp concentrated around the central arteriole and its branches (4, 15, 43, 78, 81, 84, 176). Studies using immunocytochemistry identified that the sympathetic projections ultimately enter the periarterial lymphoid sheets ending near T cells and surrounding supporting interstitial cells (4, 14, 15, 17, 19, 78, 81, 84, 320, 321). The sympathetic innervation to the spleen also includes projections to the splenic red pulp (4, 78, 81, 84, 176). High resolution studies of the splenic white pulp reveal the presence of sympathetic nerve endings in close proximity (4–6 nm) to splenic T cells, B cells and dendritic cells, forming synapses that have been termed neuroimmune junctions (78, 84, 278).
Adrenergic Receptor Expression on Immune Cells
Adrenergic receptors, as well as receptors for the binding of other ligands, including; neuropeptide Y, β-endorphin, adenosine-3-phosphate and adenosine, are expressed on the cell membranes of macrophages, T lymphocytes, B lymphocytes and NK cells (3, 28, 29, 33, 52, 55, 92, 122, 124, 131, 159, 164, 173, 180, 197, 198, 199, 227, 228, 231, 232, 243, 250, 275, 304, 306, 318, 322). Adrenergic receptors are seven-transmembrane, guanine nucleotide-binding protein-coupled receptors, and ligand binding leads to activation of adenylyl cyclase, intracellular accumulation of 3′-5′-cyclic adenosine monophosphate (cAMP), and increased concentrations of protein kinase A (160, 196) or protein kinase C (146). The protein kinases phosphorylate various transcription factors [e.g., nuclear factor κB (NF-κB), mitogen activated protein kinase, Ras, Src] resulting in altered (increase or decrease, based on the type of transcription factor activated) immune cell function, including changes in cytokine secretion and adrenergic receptor expression (53, 57, 146, 167).
Functional and Regulatory Mechanisms Mediating Sympathoimmune Interactions
The presence of intimate anatomical connections between sympathetic nerve fibers and immune cells, coupled with the expression of adrenergic receptors on immune cells, set the stage for studying functional and regulatory mechanisms mediating sympathoimmune interactions. A number of investigations have studied sympathoimmune interactions using the spleen as the target organ (161, 190, 251, 280). Activation of post-ganglionic splenic sympathetic nerve fibers leads to the release of NE at the neuroimmune junction (225). The amount of NE released is dependent on several factors including: the level of sympathetic nerve activity; expression of α/β adrenergic receptors on presynaptic nerve terminals and immune cells (117, 310); and neuromodulators released by immune cells, such as cytokines and neuropeptides. (6, 25, 30, 118, 161, 219, 245, 280).
Molecular mechanisms mediating sympathoimmune interactions have been extensively studied (248, 249, 250, 251, 252, 253, 254). NE released from sympathetic nerve endings binds to α and/or β adrenergic receptors (AR) expressed on immune cells (T cells, B cells, NK cells and macrophages). Macrophages express α2ARs and β2ARs (3, 122, 124, 173, 243, 275, 304). Stimulation of α2ARs enhances macrophage activity via activation of phospholipase C and G-protein dependent mechanisms, whereas stimulation of β2ARs suppresses macrophage activity secondary to elevations in cAMP (3, 123, 130, 169, 235, 243, 304, 308, 317). T lymphocytes predominantly express β2ARs (28, 29, 33, 52, 92, 159, 164, 180, 197, 227, 228, 231, 232, 250, 306, 318, 322), and these receptors are expressed on naïve T cells and T helper (Th)1 cells, but not on Th2 cells (232). Stimulation of β2ARs on T cells increases cAMP and protein kinase A and inhibits T cell proliferation (13, 29, 33, 37, 52, 63, 65, 75, 159, 170, 192, 227, 250, 271, 309, 316, 322). Although not commonly expressed under normal conditions, αARs can be expressed by T cells under certain pathophysiological conditions states (203), and activation of T cell αARs often results in negative regulatory function of T lymphocytes, including alterations in T cell maturation in the thymus (105). β2ARs are predominantly expressed on B lymphocytes (28, 55, 92, 162, 164, 198, 199, 227, 306). Stimulation of these receptors increases cAMP and protein kinase A (138) which in turn influences (increase or decrease, dependent on the type of mitogen used for stimulation) the proliferation of B lymphocytes and subsequent antibody production (19). NK cells express α1-, α2- and β2-ARs and activation of these receptors influences the migration pattern of NK cells and their functional ability to cause cellular lysis. Activation of NK cell β2ARs stimulates recruitment of these cells from the marginating pool to circulating pools (21), and in vivo stimulation of the β2ARs receptors causes suppression of NK cell activity (133, 266). Activation of α1- and α2-ARs has been shown to enhance the cytotoxic potential of NK cells (323).
Previous studies have established a role for changes in the level of SND in regulating the functional status of the immune system. Ganta et al. (96) provided support for the hypothesis that central activation of splenic SND can modulate peripheral cytokine gene expression. These investigators reported that whole body hyperthermia (96) and central angiotensin II infusion (96) increased splenic SND and the expression of selective splenic cytokine genes in rats with intact splenic nerves. Splenic cytokine gene expression responses to these experimental interventions were significantly reduced in splenic nerve-denervated compared with splenic nerve-intact rats (96). These studies linked direct recordings of splenic SND and splenic cytokine gene expression responses during experimental conditions simulating non-infectious, physiological stress (96). Studies employing 6-hydroxydopamine to produce chemical denervation of the SNS provided additional support for the functional contributions of the splenic sympathetic innervation in T and B cell proliferation, migration, and maturation (5, 187, 189), NK cell activity (187), and splenic production of immunoglobulin M (20, 188). Together these findings support the functional significance of the sympathetic innervation to lymphoid organs and its role in immune system modulation.
The functionality of sympathoimmune interactions depends on the microenvironment created by the release of factors during normal and altered physiological conditions, including infectious states (278). Straub et al. (278) provides an excellent example of complex sympathoimmune interactions. These investigators reported that electrical stimulation-induced release of NE from spleen slices inhibited secretion of IL-6 via α2ARs under bacteria-free experimental conditions, whereas NE-induced inhibition of IL-6 secretion was mediated via βARs under experimental conditions in which bacteria were present (278). This physiological set of events has been termed the α-to-β adrenoswitch of NE-induced inhibition of IL-6 secretion (278). Additional experiments revealed that TNF-α secretion plays a role in IL-6 secretion, which is more pronounced in experimental conditions with bacteria present, and that prior TNF-α secretion is critical for the α-to-β adrenoswitch of the inhibition of IL-6 secretion by the SNS when conditions are changed from bacteria-free medium to a medium containing bacteria (278). These experiments highlight complex local interactions involving NE release, cytokines (IL-6 and TNF-α), specific types of adrenergic receptors, and the presence versus the absence of bacteria in mediating intimate sympathoimmune interactions.
Hypothalamic-Pituitary-Adrenal Axis and Sympathetic Nervous System Interactions in Neuroimmune Modulation
The combined activities of the SNS and the hypothalamic-pituitary-adrenal (HPA) axis are crucial for regulating immune system function and assisting in maintaining homeostasis (278). Activation of the HPA-axis and the subsequent release of cortisol influences the number of β-adrenergic receptors expressed on immune cells (204). In addition, activation of β2-adrenergic receptors potentiates the rate of glucocorticoid receptor gene expression through the activation of the downstream phosphoinositide 3-kinase pathway (259), resulting in controlled and enhanced immune responses to disease. Generally, during an acute inflammatory state, both the HPA axis and the SNS act in concert, or are coupled, resulting in enhanced release of cortisol and SNS neurotransmitters that contribute to regulation of immune cell activity (26, 32, 210, 246, 287). However, during various chronic disease conditions such as hepatic cirrhosis (319), inflammatory bowel disease (279), systemic lupus erythematosus and rheumatoid arthritis (279), there is often a sustained activation of the SNS (indicated by increased levels of neuropeptide Y) that occurs simultaneously with reduced activation of the HPA-axis (indicated by decreased reduced levels of cortisol). Straub et al. (281) termed this physiological phenomenon “uncoupling of SNS and HPA-axis”, and suggested this sequence of events may contribute to the progression of chronic pathophysiological conditions.
Effect of Aging on Sympathoimmune Interactions
Advancing age is associated with marked alterations in SNS regulation, including age-related changes in basal levels of SND, autonomic support of arterial blood pressure, and SND responses to acute physiological stress (147). The intimate anatomical and physiological sympathoimmune connections make the immune system susceptible to altered SND regulation. For example, increased levels of SND may alter immune cell trafficking (234), host defense mechanisms, and contribute to immune-related diseases (62). Neuroanatomical studies reveal a progressive, age-associated loss in the sympathetic neural innervation to lymphoid organs (14, 17, 19, 291), and advancing age is associated with altered βAR expression and cAMP production in immune cells (263). Thyagarajan et al. (291, 292) reported that administration of L-depreynl (monoamine oxidase-B inhibitor) in aged rats produced a partial recovery of age-associated declines in splenic NE and INF-γ, and increased levels of IL-2 secretion. In addition, Perez et al. (223) reported that administration of rilmenidine (a centrally-acting, anti-hypertensive drug) in 15-month old rats reduced sympathetic tone (demonstrated by reduced plasma and splenic NE concentrations and NE turnover in the spleen), produced a partial reversal of the loss of splenic sympathetic nerve fibers, increased splenic β-AR density, and increased cAMP levels in splenic cells. These data support the existence of aging-associated changes in sympathoimmune physiological interactions and suggest pharmacological strategies may be useful in mitigating age-related alterations in neural-immune relationships.
CENTRAL NERVOUS SYSTEM- PARASYMPATHETIC NERVOUS SYSTEM-IMMUNE SYSTEM-SIGNALING INTERACTIONS
Recent investigations have been initiated to determine CNS mechanisms mediating immune system-PNS interactions. These studies have provided a framework to further understand fundamental strategies to regulate parasympathetic nerve outflow and peripheral immune function. If central neural circuits are critically involved in PNS-immune system interactions, it would be expected that central modulation of efferent vagal nerve outflow would influence peripheral immune responses.
CNS administration of CNI-1493 (also known as Semapimod), a tetravalent guanyl hydrazine, on centrally-mediated changes in efferent vagal nerve regulation and peripheral immune function has been the focus of several recent lines of inquiry (22, 31, 215, 220, 290). Borovikova et al. (31) reported icv administration of CNI-1493 suppressed carrageenan-induced paw edema in rats, an effect that was dependent on the presence of an intact CNS-vagus nerve pathway. In addition, peripheral CNI-1493 administration markedly increased directly-recorded vagus nerve activity (31). Bernik et al. (22) demonstrated that icv CNI-1493 suppressed endotoxin-induced peripheral TNF release and hypotension, effects that were dependent on a functional efferent vagal nerve pathway. Moreover, vagotomy eliminated the anti-inflammatory effect of central CNI-1493 administration in an experimental model of post-operative ileus (290). In this latter study, The et al. (290) reported that icv CNI-1493 administration increased c-fos expression in specific forebrain (PVN) and brainstem (NTS and DMV) nuclei.
CNI-1493 is a multifunctional anti-inflammatory agent that may influence physiological function via multiple, potential pathways, including; preventing the phosphorylation of p38 mitogen-activated protein kinase, deactivating macrophages and inhibiting the synthesis of proinflammatory mediators, inhibiting macrophage arginine transport and nitric oxide production, and activating M1 muscarinic receptors (27, 31, 215, 220, 290). Although identification of central neural receptors for CNI-1493 and specific CNS areas and neural pathways involved in mediating the effects of this drug on regulation of centrally-mediated parasympathetic nerve outflow remain to be determined, these results provide support for the hypothesis that central modulation of efferent vagal nerve outflow modulates peripheral immune function.
PERIPHERAL PARASYMPATHETIC NERVOUS SYSTEM-IMMUNE SYSTEM SIGNALING INTERACTIONS
Parasympathetic Innervation and Immunomodulation
The vagus nerve is comprised primarily of afferent fibers (23, 69, 107), and the sensory component of the peripheral vagus is thought to play a role in the detection of peripheral antigens (32, 103, 115, 194, 315). The presence of vagal efferent connections to lymphoid organs and the source of ACh in these organs has been vigorously studied and debated. It has been considered that lymphoid organs may (2, 38, 47) or may not (18, 35, 207, 238, 240) be innervated by the vagus nerve, and the functional relevance of the potential cholinergic innervation to the spleen has also been called into question (241, see below for further details). There are several factors that may contribute to the evolving understanding regarding the potential for anatomical and/or functional cholinergic innervations to lymphoid organs including: the presence of multiple neurotransmitters released from nerve endings resulting in cross-talk and co-transmission interactions; the presence of dynamic neuroimmune junctions (due to moving immune cells) thereby posing a challenge for identifying direct autonomic innervations to the lymphoid organs; and changes in the conceptual understanding of neurotransmission (40, 289).
Studies in the past several decades have identified a role for the PNS in regulation of peripheral inflammatory responses; an interaction termed the cholinergic anti-inflammatory pathway (221). Using an in vitro preparation, Borovikova et al. (32) reported that ACh attenuated proinflammatory cytokine release induced by LPS stimulation of human macrophages, although secretion of the anti-inflammatory cytokine IL-10 was not affected. In addition, electrical stimulation of the peripheral vagus nerve has been shown to decrease serum and liver TNF levels (32). In addition, activation of the cholinergic nervous system in specific animal models was shown to produce remission of symptoms in ischemia–reperfusion injury, hemorrhagic shock, experimental arthritis, pancreatitis, peritonitis and experimental colitis (22, 108, 172). Together these findings support the idea that activated efferent cholinergic nerves modulate peripheral immune responses. In addition and as previously discussed, cytokines released from peripheral immune cells (e.g., splenic macrophages) bind to glomus cells that are anatomically located adjacent to the vagus nerve (315), which could activate afferent vagal nerve fibers and relay signals to the medullary NTS. The NTS in turn communicates with the DMN and the NA providing the potential to activate efferent parasympathetic nerve outflow. In this scenario both vagal afferents and efferents contribute to the cholinergic anti-inflammatory response, thereby characterizing what has been termed an inflammatory reflex. In addition, the inflammatory reflex mechanism also likely involves CNS nuclei that control the HPA-axis and central regulation of the SNS.
Molecular Mechanisms Mediating the Cholinergic Anti-Inflammatory Pathway
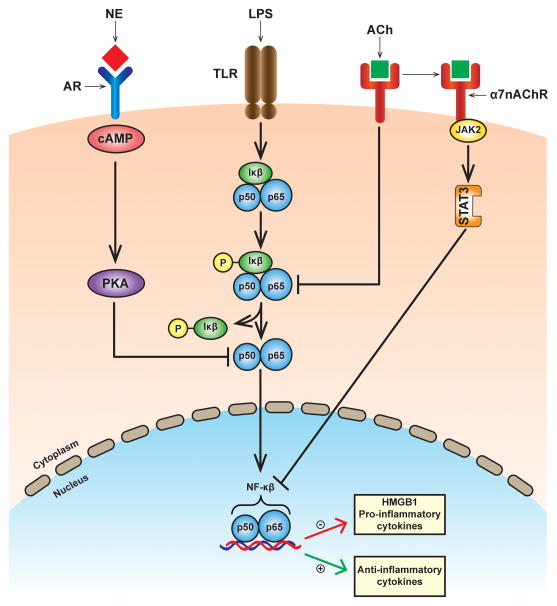
Molecular studies have identified the presence of muscarinic (metabotropic) and nicotinic (ionotropic) ACh receptors on cells that synthesize and secrete cytokines (93, 296). Muscarinic acetylcholine receptors (mAChRs) are guanine nucleotide-binding protein (G protein) coupled receptors that are characterized by the presence of multiple subtypes (45). Nicotinic acetylcholine receptors (nAChRs) are comprised of homo- and heteropentameric structures with multiple subunit compositions including α1–α10, β1–β4, δ, γ, and ε (174). The expression of ACh receptors on immune cells was initially identified on rodent T lymphocytes (93) and macrophages (296). Macrophages are highly sensitive to modulation by ACh (296). Receptor binding studies demonstrated that nicotine had a dose-dependent inhibitory effect on TNF release in endotoxin-stimulated primary human macrophages (32). On the other hand, the inhibitory effect was minimal with muscarine, an AChR agonist with high affinity for muscarinic receptors, suggesting a potential role for nAChRs in the cholinergic anti-infammatory pathway in the peripheral nervous system. Muscarine administered intracerebrally (but not intravenously) inhibited serum TNF levels following endotoxemia, suggesting that the cholinergic anti-inflammatory pathway does not use peripheral mAChR signaling (220). Receptor knock-out studies in mice have provided additional evidence supporting a critical role for the α7 subunit of the nAChr on macrophages in the cholinergic anti-inflammatory pathway (312). ACh binding to the α7 subunit of nAChR leads to activation of the tyrosine kinase, Jak2. Activated Jak2 phosphorylates STAT3 and SOCS3 transcription factors which bind to DNA and transactivates an anti-inflammatory gene response (61), decreases the nuclear translocation of the proinflammatory cytokine transcription factor NF-κB (264, 313), and reduces the transcription of the high mobility group box 1 (HMGB1) DNA-binding protein (311) (Figure 6, adapted from Sternberg; 277).
Figure 6.
Schematic diagram illustrating intracellular signaling pathways in a macrophage involved in cytokine regulation by adrenergic and cholinergic agonists and receptors following lipopolysaccharide challenge. Norepinephrine (NE) binds to adrenergic receptors (ARs) and induces cyclic AMP and protein Kinase A (PKA) activation which inhibits pro-inflammatory cytokine production by inhibition of NF-κB nuclear translocation. Acetylcholine (ACh) binds to the α7nACh receptor and inhibits phosphorylation and nuclear translocation of NF-κB. In addition, ACh activates the JAK2/STAT3 pathway leading to increased transactivation of anti-inflammatory cytokines, and decreased transcription of high mobility group box 1 (HMGB1) and proinflammatory cytokines. LPS, lipopolysaccharide; TLR, toll like receptor; α7nACh, α7 nicotinic acetylcholine receptor. Adapted with permission from Sternberg (277).
Role of the Spleen in the Cholinergic Anti-Inflammatory Pathway
Anatomical, immunological and molecular approaches have been used to demonstrate a role for the spleen in vagally-mediated regulation of inflammation (127). The spleen is the major source of serum TNF levels during sepsis (127), and NE released by sympathetic nerve endings binds to adrenergic receptors on splenic immune cells (255) and attenuates serum TNF levels (145). Activation of vagal nerve efferents reduces serum TNF levels, a response not observed following splenectomy (127, 298), suggesting an obligatory role for the spleen in vagally-mediated modulation of TNF. Vagal nerve stimulation, completed after splenic nerve denervation or reserpine-induced depletion of NE, failed to attenuate endotoxemia-induced elevations in serum TNF levels (240). Moreover, vagal nerve stimulation failed to reduce serum TNF levels in α7nACh receptor knockout mice (222, 312), suggesting a role for ACh and α7nACh receptor expression in suppressing elevated serum TNF levels to systemic endotoxemia. Based on these findings, Rosas-Ballina and Tracey (241, 242) speculated that the efferent arm of the inflammatory reflex may function by binding of ACh to α7nACh receptors expressed on neurons in the celiac-superior mesenteric plexus (24, 175, 270) which in turn activates splenic sympathetic nerve outflow and causes release of NE from splenic nerve endings. In this scenario norepinephrine released from activated splenic nerve endings may bind to β2ARs on memory T lymphocytes resulting in ACh release (145, 233, 241). Consistent with this idea, splenic ACh levels are markedly increased following electrical stimulation of splenic nerve efferents (34, 39, 236). ACh released by memory T lymphocytes binds to α7nACh receptors on macrophages and attenuates the release of proinflammatory cytokines, such as TNF (241, 296). In contrast, Bratton et al. (35) employed neuroanatomical and electrophysiological techniques to detect if vagal nerve efferents synapse with sympathetic neurons in the celiac ganglion. These investigators reported that splenic sympathetic nerve activity was not altered in response to electrical stimulation of vagal nerve efferents, suggesting the lack of sympathovagal interactions in the celiac ganglion. Additional studies are required to clarify peripheral neural mechanisms regulating the cholinergic anti-inflammatory pathway.
Bellinger et al. (18) probed the spleen for acetylcholinesterase staining and choline acetyltransferase activity as an experimental means to determine the extent of the splenic cholinergic innervation. Acetylcholinesterase neural-like profiles were observed along the splenic vasculature, and acetylcholinesterase staining was present in splenic lymphoid cells and in neuronal cell bodies in the superior mesenteric-coeliac ganglion (18). Surgical removal of the superior mesenteric-coeliac ganglion as well as chemical sympathectomy produced by 6-hydroxydopamine treatment, but not vagal nerve transections, resulted in loss of splenic acetylcholinesterase and noradrenergic nerve profiles (18). These findings support the hypothesis that the spleen does not receive a robust cholinergic innervation, although, it is known that lymphocytes are an important source of non-neuronal ACh and express most of the cholinergic components intrinsic to the cholinergic nervous system (143, 144). In addition to the spleen, there is evidence that the cholinergic anti-inflammatory pathway modulates the gut immune system (195, 303). The gut receives direct vagal innervation and electrical stimulation of the vagus nerve results in release of ACh, which binds to α7nAChRs on macrophages present in the gut wall (303).
CONCLUSIONS
Studies employing neuroanatomical, pharmacological, electrophysiological, and molecular biological approaches have substantially shaped the contemporary understanding of mechanisms involved in regulating and integrating physiological interactions between the autonomic nervous and immune systems. From a historical perspective it had often been considered that these two adaptive systems functioned independently. However, it is now well-established that prominent bidirectional communication pathways exist between the immune system and both arms of the ANS, the SNS and the PNS. The current topical review assessed integrative autonomic-immune physiological processing by reviewing studies that have contributed to understanding how signaling components of the immune system engage central autonomic neural circuits and regulate efferent sympathetic and parasympathetic nerve outflows, and how changes in autonomic regulation influence immune organ function.
Several points are summarized (Figure 7). First, multiple pathways of communication, including non-neural and neural (several lines of evidence suggest the physiological significance of the neural pathway requires additional investigation), are available to convey information regarding local and systemic immune status to central neural circuits (left side, red lines and blood vessel). Second, nuclei contained in or associated with autonomic neural circuits are present at many levels of the supraspinal neuraxis and neural-immune interactions can occur at numerous sites in these distributed systems (brain and selective nuclei). Receptors for multiple proinflammatory cytokines and immune cell products are located in the CNS, and these immune mediators affect neurotransmitters and neuromodulators contained in central autonomic neural networks to elicit changes in centrally-mediated sympathetic and parasympathetic nerve outflows via multiple neural communicating pathways. Third, the ANS is highly responsive to the administration of cytokines and immune cell factors administered via a number of different routes, including; intravenous, intra-arterial, icv, and central microinjections in selected nuclei. Because of well-developed SND recording techniques used in studies involving human and animal subjects, the majority of information regarding the effects of cytokines on autonomic nerve outflow has emanated from studies associated with the SNS. By altering fundamental characteristics of sympathetic nerve outflow, including the level of activity, the bursting pattern, and the responsiveness of neural circuits to various stimuli, cytokines and other immune factors influence target organ function. Fourth, the SNS (middle, black lines) innervates the spleen and other secondary lymphoid organs, and alterations in splenic SND can influence peripheral immune responses, including changes in splenic cytokine gene expression. NE released from sympathetic nerve terminals binds to either α/β adrenergic receptors expressed on immune cells triggering a series of intracellular downstream signaling events resulting in activation or inhibition of transcription factors that regulate gene expression of various immune cell-derived factors. Local splenic sympathetic-immune interactions involve complex integrative processes including NE release, cytokines (e.g., IL-6 and TNF-α), the activation state of specific adrenergic receptors, and the presence of bacteria in local tissue (bottom, left side). In addition, cytokine-induced changes in SND can modulate physiological function in nonlymphoid targets (e.g., kidney, skin blood vessels, BAT tissue) (bottom, right side), which likely communicate information regarding changes in their physiological status with the CNS via afferent signaling mechanisms (right side, red line). Fifth, the efferent PNS, identified primarily by the vagus nerve (green line), markedly influences peripheral inflammatory responses, a physiological process identified as the cholinergic anti-inflammatory pathway. The α7 subunit of the nAChr plays a critical role in this pathway. ACh binds to nAChRs expressed on immune cells (macrophages) and activates downstream signaling events resulting in suppression of proinflammatory cytokines and activation of anti-inflammatory cytokines. The cholinergic innervation of the spleen and other lymphoid organs is still debated (depicted schematically by the hatched vagus nerve projecting from the ganglia to the spleen). However, the functional effects of the cholinergic anti-inflammatory pathway are mediated via ACh released by activated memory T cells (Figure 7; bottom left), and may not require an intact vagus innervation to the spleen.
Figure 7.
Schematic diagram highlighting the critical roles that both arms of the autonomic nervous system (i.e., sympathetic nervous system and parasympathetic nervous system) play in regulating central neural and peripheral neuroimmune interactions. See text for details. (CNS, central nervous system; HPA, hypothalamic pituitary adrenal axis CVOs, circumventricular organs; BBB, Blood Brain Barrier; ANS, autonomic nervous system; SNS, sympathetic nervous system; PNS, parasympathetic nervous system; NO, Nitric Oxide; Ach, acetylcholine; NE, norepinephrine; ARs, adrenergic receptors; α7nACh, α7 nicotinic acetylcholine receptor; M, macrophage; T, T cell (memory type); B, B cell, NK, natural killer cell; PGE2, prostaglandin E2; IML, spinal intermediolateral nucleus; SFO, subfornical organ; MPO, medial preoptic nucleus; DMH, dorsomedial hypothalamus; PVN, paraventicular nucleus; NTS, nucleus tractus solitarius; DMV, dorsal motor nucleus of the vagus; NA, nucleus ambiguous; rRPa, rostral raphe pallidus; RVLM, rostral ventral lateral medulla; BAT, brown adipose tissue).
Studies completed during the past several decades have provided novel perspectives regarding physiological interactions between the nervous system and the immune system. It is now well-established that the sympathetic and parasympathetic arms of the ANS play key roles in orchestrating neuroimmune interactions, although additional studies are required to more fully understand the dynamic and complex adrenergic-cholinergic interactions that comprise the regulatory tenets of neuroimmune processes. Further understanding of regulatory mechanisms linking these two important adaptive systems may contribute to understanding relationships between chronic disease development and immune-associated changes in ANS function.
Acknowledgments
MJK is supported by NIH grant AG-041948. The authors thank Dr. Barbara Lutjemeier for her insightful comments and critical review of the manuscript, Mal Hoover for assistance with preparation of figures, and Bryan Larsen for assistance with preparation of the manuscript.
References
- 1.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 2.PHARMACOLOGY AND NERVE ENDINGS: FIRST DIXON MEMORIAL LECTURE BY SIR HENRY DALE. Br Med J. 1934;2:1161–1163. [PMC free article] [PubMed] [Google Scholar]
- 3.Abrass CK, O’Connor SW, Scarpace PJ, Abrass IB. Characterization of the beta-adrenergic receptor of the rat peritoneal macrophage. J Immunol. 1985;135:1338–1341. [PubMed] [Google Scholar]
- 4.Ackerman KD, Felten SY, Bellinger DL, Felten DL. Noradrenergic sympathetic innervation of the spleen: III. Development of innervation in the rat spleen. J Neurosci Res. 1987;18:49–54. 123–125. doi: 10.1002/jnr.490180109. [DOI] [PubMed] [Google Scholar]
- 5.Ackerman KD, Madden KS, Livnat S, Felten SY, Felten DL. Neonatal sympathetic denervation alters the development of in vitro spleen cell proliferation and differentiation. Brain Behav Immun. 1991;5:235–261. doi: 10.1016/0889-1591(91)90021-2. [DOI] [PubMed] [Google Scholar]
- 6.Akiyoshi M, Shimizu Y, Saito M. Interleukin-1 increases norepinephrine turnover in the spleen and lung in rats. Biochem Biophys Res Commun. 1990;173:1266–1270. doi: 10.1016/s0006-291x(05)80923-4. [DOI] [PubMed] [Google Scholar]
- 7.Anthony DC, Bolton SJ, Fearn S, Perry VH. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood-brain barrier permeability in rats. Brain. 1997;120 ( Pt 3):435–444. doi: 10.1093/brain/120.3.435. [DOI] [PubMed] [Google Scholar]
- 8.Ariumi H, Takano Y, Masumi A, Takahashi S, Hirabara Y, Honda K, Saito R, Kamiya HO. Roles of the central prostaglandin EP3 receptors in cardiovascular regulation in rats. Neurosci Lett. 2002;324:61–64. doi: 10.1016/s0304-3940(02)00174-x. [DOI] [PubMed] [Google Scholar]
- 9.Averill DB, Tsuchihashi T, Khosla MC, Ferrario CM. Losartan, nonpeptide angiotensin II-type 1 (AT1) receptor antagonist, attenuates pressor and sympathoexcitatory responses evoked by angiotensin II and L-glutamate in rostral ventrolateral medulla. Brain Res. 1994;665:245–252. doi: 10.1016/0006-8993(94)91344-7. [DOI] [PubMed] [Google Scholar]
- 10.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Barman SM. Brainstem control of Cardiovascular Function. In: Klemm W, Vertes R, editors. Brainstem Mechanisms of Behavior. New York: Wiley; 1990. pp. 353–381. [Google Scholar]
- 12.Barman SM, Kenney MJ. Methods of analysis and physiological relevance of rhythms in sympathetic nerve discharge. Clin Exp Pharmacol Physiol. 2007;34:350–355. doi: 10.1111/j.1440-1681.2007.04586.x. [DOI] [PubMed] [Google Scholar]
- 13.Bartik MM, Bauman GP, Brooks WH, Roszman TL. Costimulatory signals modulate the antiproliferative effects of agents that elevate cAMP in T cells. Cell Immunol. 1994;158:116–130. doi: 10.1006/cimm.1994.1261. [DOI] [PubMed] [Google Scholar]
- 14.Bellinger DL, Ackerman KD, Felten SY, Felten DL. A longitudinal study of age-related loss of noradrenergic nerves and lymphoid cells in the rat spleen. Exp Neurol. 1992;116:295–311. doi: 10.1016/0014-4886(92)90009-f. [DOI] [PubMed] [Google Scholar]
- 15.Bellinger DL, Felten SY, Collier TJ, Felten DL. Noradrenergic sympathetic innervation of the spleen: IV. Morphometric analysis in adult and aged F344 rats. J Neurosci Res. 1987;18:55–63. 126–129. doi: 10.1002/jnr.490180110. [DOI] [PubMed] [Google Scholar]
- 16.Bellinger DL, Felten SY, Lorton D, Felten DL. Origin of noradrenergic innervation of the spleen in rats. Brain Behav Immun. 1989;3:291–311. doi: 10.1016/0889-1591(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 17.Bellinger DL, Lorton D, Felten SY, Felten DL. Innervation of lymphoid organs and implications in development, aging, and autoimmunity. Int J Immunopharmacol. 1992;14:329–344. doi: 10.1016/0192-0561(92)90162-e. [DOI] [PubMed] [Google Scholar]
- 18.Bellinger DL, Lorton D, Hamill RW, Felten SY, Felten DL. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav Immun. 1993;7:191–204. doi: 10.1006/brbi.1993.1021. [DOI] [PubMed] [Google Scholar]
- 19.Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, Molinaro C, Lubahn C, Lorton D. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 2008;252:27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellinger DL, Stevens SY, Thyaga Rajan S, Lorton D, Madden KS. Aging and sympathetic modulation of immune function in Fischer 344 rats: effects of chemical sympathectomy on primary antibody response. J Neuroimmunol. 2005;165:21–32. doi: 10.1016/j.jneuroim.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Benschop RJ, Oostveen FG, Heijnen CJ, Ballieux RE. Beta 2-adrenergic stimulation causes detachment of natural killer cells from cultured endothelium. Eur J Immunol. 1993;23:3242–3247. doi: 10.1002/eji.1830231230. [DOI] [PubMed] [Google Scholar]
- 22.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, Sudan S, Czura CJ, Ivanova SM, Tracey KJ. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 24.Berthoud HR, Powley TL. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc Res Tech. 1996;35:80–86. doi: 10.1002/(SICI)1097-0029(19960901)35:1<80::AID-JEMT7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 25.Besedovsky HO, del Rey A, Sorkin E, Da Prada M, Keller HH. Immunoregulation mediated by the sympathetic nervous system. Cell Immunol. 1979;48:346–355. doi: 10.1016/0008-8749(79)90129-1. [DOI] [PubMed] [Google Scholar]
- 26.Besedovsky HO, del Rey AE, Sorkin E. Immune-neuroendocrine interactions. J Immunol. 1985;135:750s–754s. [PubMed] [Google Scholar]
- 27.Bianchi M, Ulrich P, Bloom O, Meistrell M, 3rd, Zimmerman GA, Schmidtmayerova H, Bukrinsky M, Donnelley T, Bucala R, Sherry B, et al. An inhibitor of macrophage arginine transport and nitric oxide production (CNI-1493) prevents acute inflammation and endotoxin lethality. Mol Med. 1995;1:254–266. [PMC free article] [PubMed] [Google Scholar]
- 28.Bidart JM, Motte P, Assicot M, Bohuon C, Bellet D. Catechol-O-methyltransferase activity and aminergic binding sites distribution in human peripheral blood lymphocyte subpopulations. Clin Immunol Immunopathol. 1983;26:1–9. doi: 10.1016/0090-1229(83)90167-8. [DOI] [PubMed] [Google Scholar]
- 29.Bishopric NH, Cohen HJ, Lefkowitz RJ. Beta adrenergic receptors in lymphocyte subpopulations. J Allergy Clin Immunol. 1980;65:29–33. doi: 10.1016/0091-6749(80)90173-6. [DOI] [PubMed] [Google Scholar]
- 30.Bognar IT, Albrecht SA, Farasaty M, Schmitt E, Seidel G, Fuder H. Effects of human recombinant interleukins on stimulation-evoked noradrenaline overflow from the rat perfused spleen. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:497–502. doi: 10.1007/BF00169139. [DOI] [PubMed] [Google Scholar]
- 31.Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, Ombrellino M, Tracey KJ. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci. 2000;85:141–147. doi: 10.1016/S1566-0702(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 32.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 33.Bourne HR, Melmon KL. Adenyl cyclase in human leukocytes: evidence for activation by separate beta adrenergic and prostaglandin receptors. J Pharmacol Exp Ther. 1971;178:1–7. [PubMed] [Google Scholar]
- 34.Brandon KW, Rand MJ. Acetylcholine and the sympathetic innervation of the spleen. J Physiol. 1961;157:18–32. doi: 10.1113/jphysiol.1961.sp006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bratton BO, Martelli D, McKinley MJ, Trevaks D, Anderson CR, McAllen RM. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp Physiol. 2012;97:1180–1185. doi: 10.1113/expphysiol.2011.061531. [DOI] [PubMed] [Google Scholar]
- 36.Breder CD, Saper CB. Expression of inducible cyclooxygenase mRNA in the mouse brain after systemic administration of bacterial lipopolysaccharide. Brain Res. 1996;713:64–69. doi: 10.1016/0006-8993(95)01474-8. [DOI] [PubMed] [Google Scholar]
- 37.Brodde OE, Daul A, O’Hara N. Beta-adrenoceptor changes in human lymphocytes, induced by dynamic exercise. Naunyn Schmiedebergs Arch Pharmacol. 1984;325:190–192. doi: 10.1007/BF00506201. [DOI] [PubMed] [Google Scholar]
- 38.Buijs RM, van der Vliet J, Garidou ML, Huitinga I, Escobar C. Spleen vagal denervation inhibits the production of antibodies to circulating antigens. PLoS One. 2008;3:e3152. doi: 10.1371/journal.pone.0003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bulloch K, Damavandy T, Badamchian M. Characterization of choline O-acetyltransferase (ChAT) in the BALB/C mouse spleen. Int J Neurosci. 1994;76:141–149. doi: 10.3109/00207459408985999. [DOI] [PubMed] [Google Scholar]
- 40.Burnstock G. Autonomic neurotransmission: 60 years since sir Henry Dale. Annu Rev Pharmacol Toxicol. 2009;49:1–30. doi: 10.1146/annurev.pharmtox.052808.102215. [DOI] [PubMed] [Google Scholar]
- 41.Calvo W. The innervation of the bone marrow in laboratory animals. Am J Anat. 1968;123:315–328. doi: 10.1002/aja.1001230206. [DOI] [PubMed] [Google Scholar]
- 42.Campese VM, Ye S, Zhong H. Downregulation of neuronal nitric oxide synthase and interleukin-1beta mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension. 2002;39:519–524. doi: 10.1161/hy0202.102815. [DOI] [PubMed] [Google Scholar]
- 43.Carlson SL, Felten DL, Livnat S, Felten SY. Noradrenergic sympathetic innervation of the spleen: V. Acute drug-induced depletion of lymphocytes in the target fields of innervation results in redistribution of noradrenergic fibers but maintenance of compartmentation. J Neurosci Res. 1987;18:64–69. 130–131. doi: 10.1002/jnr.490180111. [DOI] [PubMed] [Google Scholar]
- 44.Catania A, Lonati C, Sordi A, Gatti S. Detrimental consequences of brain injury on peripheral cells. Brain Behav Immun. 2009;23:877–884. doi: 10.1016/j.bbi.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Caulfield MP, Birdsall NJ International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 46.Chapleau MW, Sabharwal R. Methods of assessing vagus nerve activity and reflexes. Heart Fail Rev. 2011;16:109–127. doi: 10.1007/s10741-010-9174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen XH, Itoh M, Sun W, Miki T, Takeuchi Y. Localization of sympathetic and parasympathetic neurons innervating pancreas and spleen in the cat. J Auton Nerv Syst. 1996;59:12–16. doi: 10.1016/0165-1838(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 48.Ching S, Zhang H, Belevych N, He L, Lai W, Pu XA, Jaeger LB, Chen Q, Quan N. Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J Neurosci. 2007;27:10476–10486. doi: 10.1523/JNEUROSCI.3357-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cirmanova V, Bayer M, Starka L, Zajickova K. The effect of leptin on bone: an evolving concept of action. Physiol Res. 2008;57 (Suppl 1):S143–151. doi: 10.33549/physiolres.931499. [DOI] [PubMed] [Google Scholar]
- 50.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 51.Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol. 2009;310:21–29. doi: 10.1016/j.mce.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conolly ME, Greenacre JK. The beta-adrenoceptor of the human lymphocyte and human lung parenchyma. Br J Pharmacol. 1977;59:17–23. doi: 10.1111/j.1476-5381.1977.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cornall RJ, Goodnow CC. B cell antigen receptor signalling in the balance of tolerance and immunity. Novartis Found Symp. 1998;215:21–30. doi: 10.1002/9780470515525.ch3. discussion 30–40. [DOI] [PubMed] [Google Scholar]
- 54.Crandall CG, Etzel RA, Farr DB. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol. 1999;277:H2348–2352. doi: 10.1152/ajpheart.1999.277.6.h2348. [DOI] [PubMed] [Google Scholar]
- 55.Cremaschi GA, Fisher P, Boege F. Beta-adrenoceptor distribution in murine lymphoid cell lines. Immunopharmacology. 1991;22:195–206. doi: 10.1016/0162-3109(91)90044-y. [DOI] [PubMed] [Google Scholar]
- 56.Cunningham ET, Jr, Wada E, Carter DB, Tracey DE, Battey JF, De Souza EB. In situ histochemical localization of type I interleukin-1 receptor messenger RNA in the central nervous system, pituitary, and adrenal gland of the mouse. J Neurosci. 1992;12:1101–1114. doi: 10.1523/JNEUROSCI.12-03-01101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 58.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 59.Dampney RA, Horiuchi J, Tagawa T, Fontes MA, Potts PD, Polson JW. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand. 2003;177:209–218. doi: 10.1046/j.1365-201X.2003.01070.x. [DOI] [PubMed] [Google Scholar]
- 60.Davisson RL, Zimmerman MC. Angiotensin II, oxidant signaling, and hypertension: down to a T? Hypertension. 2010;55:228–230. doi: 10.1161/HYPERTENSIONAHA.109.144477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 62.del Rey A, Wolff C, Wildmann J, Randolf A, Hahnel A, Besedovsky HO, Straub RH. Disrupted brain-immune system-joint communication during experimental arthritis. Arthritis Rheum. 2008;58:3090–3099. doi: 10.1002/art.23869. [DOI] [PubMed] [Google Scholar]
- 63.DeRubertis FR, Zenser TV, Adler WH, Hudson T. Role of cyclic adenosine 3′,5′-monophosphate in lymphocyte mitogenesis. J Immunol. 1974;113:151–161. [PubMed] [Google Scholar]
- 64.Desai MY, Watanabe MA, Laddu AA, Hauptman PJ. Pharmacologic modulation of parasympathetic activity in heart failure. Heart Fail Rev. 2011;16:179–193. doi: 10.1007/s10741-010-9195-1. [DOI] [PubMed] [Google Scholar]
- 65.Diamantstein T, Ulmer A. The antagonistic action of cyclic GMP and cyclic AMP on proliferation of B and T lymphocytes. Immunology. 1975;28:113–119. [PMC free article] [PubMed] [Google Scholar]
- 66.DiBona GF, Sawin LL. Reflex regulation of renal nerve activity in cardiac failure. Am J Physiol. 1994;266:R27–39. doi: 10.1152/ajpregu.1994.266.1.R27. [DOI] [PubMed] [Google Scholar]
- 67.DiBona GF, Sawin LL, Jones SY. Characteristics of renal sympathetic nerve activity in sodium-retaining disorders. Am J Physiol. 1996;271:R295–302. doi: 10.1152/ajpregu.1996.271.1.R295. [DOI] [PubMed] [Google Scholar]
- 68.Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, Prass K, Meisel A. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- 69.Dubois F, Foley J. Experimental studies on the vagus and spinal accessory nerves in the cat. The Anatomical Record. 1936;64:285–307. [Google Scholar]
- 70.Ek M, Kurosawa M, Lundeberg T, Ericsson A. Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins. J Neurosci. 1998;18:9471–9479. doi: 10.1523/JNEUROSCI.18-22-09471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 72.Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci. 1997;17:7166–7179. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ericsson A, Liu C, Hart RP, Sawchenko PE. Type 1 interleukin-1 receptor in the rat brain: distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J Comp Neurol. 1995;361:681–698. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- 75.Estes G, Solomon SS, Norton WL. Inhibition of lymphocyte stimulation by cyclic and non-cyclic nucleotides. J Immunol. 1971;107:1489–1492. [PubMed] [Google Scholar]
- 76.Fabry Z, Fitzsimmons KM, Herlein JA, Moninger TO, Dobbs MB, Hart MN. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J Neuroimmunol. 1993;47:23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- 77.Farrar WL, Kilian PL, Ruff MR, Hill JM, Pert CB. Visualization and characterization of interleukin 1 receptors in brain. J Immunol. 1987;139:459–463. [PubMed] [Google Scholar]
- 78.Felten DL, Ackerman KD, Wiegand SJ, Felten SY. Noradrenergic sympathetic innervation of the spleen: I. Nerve fibers associate with lymphocytes and macrophages in specific compartments of the splenic white pulp. J Neurosci Res. 1987;18:28–36. 118–121. doi: 10.1002/jnr.490180107. [DOI] [PubMed] [Google Scholar]
- 79.Felten DL, Felten SY. Sympathetic noradrenergic innervation of immune organs. Brain Behav Immun. 1988;2:293–300. doi: 10.1016/0889-1591(88)90031-1. [DOI] [PubMed] [Google Scholar]
- 80.Felten DL, Felten SY, Bellinger DL, Madden KS. Fundamental aspects of neural-immune signaling. Psychother Psychosom. 1993;60:46–56. doi: 10.1159/000288679. [DOI] [PubMed] [Google Scholar]
- 81.Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135:755s–765s. [PubMed] [Google Scholar]
- 82.Felten DL, Livnat S, Felten SY, Carlson SL, Bellinger DL, Yeh P. Sympathetic innervation of lymph nodes in mice. Brain Res Bull. 1984;13:693–699. doi: 10.1016/0361-9230(84)90230-2. [DOI] [PubMed] [Google Scholar]
- 83.Felten SY, Felten DL, Bellinger DL, Carlson SL, Ackerman KD, Madden KS, Olschowka JA, Livnat S. Noradrenergic sympathetic innervation of lymphoid organs. Prog Allergy. 1988;43:14–36. [PubMed] [Google Scholar]
- 84.Felten SY, Olschowka J. Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp. J Neurosci Res. 1987;18:37–48. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- 85.Feng QP, Carlsson S, Thoren P, Hedner T. Characteristics of renal sympathetic nerve activity in experimental congestive heart failure in the rat. Acta Physiol Scand. 1994;150:259–266. doi: 10.1111/j.1748-1716.1994.tb09685.x. [DOI] [PubMed] [Google Scholar]
- 86.Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology. 2009;89:370–376. doi: 10.1159/000211202. [DOI] [PubMed] [Google Scholar]
- 87.Ferguson DW, Berg WJ, Roach PJ, Oren RM, Mark AL. Effects of heart failure on baroreflex control of sympathetic neural activity. Am J Cardiol. 1992;69:523–531. doi: 10.1016/0002-9149(92)90998-e. [DOI] [PubMed] [Google Scholar]
- 88.Ferguson DW, Berg WJ, Sanders JS. Clinical and hemodynamic correlates of sympathetic nerve activity in normal humans and patients with heart failure: evidence from direct microneurographic recordings. J Am Coll Cardiol. 1990;16:1125–1134. doi: 10.1016/0735-1097(90)90544-y. [DOI] [PubMed] [Google Scholar]
- 89.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 90.Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol. 2004;286:H2264–2271. doi: 10.1152/ajpheart.01072.2003. [DOI] [PubMed] [Google Scholar]
- 91.Fu LW, Longhurst JC. Interleukin-1beta sensitizes abdominal visceral afferents of cats to ischaemia and histamine. J Physiol. 1999;521(Pt 1):249–260. doi: 10.1111/j.1469-7793.1999.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fuchs BA, Albright JW, Albright JF. Beta-adrenergic receptors on murine lymphocytes: density varies with cell maturity and lymphocyte subtype and is decreased after antigen administration. Cell Immunol. 1988;114:231–245. doi: 10.1016/0008-8749(88)90318-8. [DOI] [PubMed] [Google Scholar]
- 93.Fujii T, Kawashima K. Calcium signaling and c-Fos gene expression via M3 muscarinic acetylcholine receptors in human T- and B-cells. Jpn J Pharmacol. 2000;84:124–132. doi: 10.1254/jjp.84.124. [DOI] [PubMed] [Google Scholar]
- 94.Furuyashiki T, Narumiya S. Stress responses: the contribution of prostaglandin E(2) and its receptors. Nat Rev Endocrinol. 2011;7:163–175. doi: 10.1038/nrendo.2010.194. [DOI] [PubMed] [Google Scholar]
- 95.Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Front Neuroendocrinol. 2012;33:116–125. doi: 10.1016/j.yfrne.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ganta CK, Blecha F, Ganta RR, Helwig BG, Parimi S, Lu N, Fels RJ, Musch TI, Kenney MJ. Hyperthermia-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Physiol Genomics. 2004;19:175–183. doi: 10.1152/physiolgenomics.00109.2004. [DOI] [PubMed] [Google Scholar]
- 97.Ganta CK, Lu N, Helwig BG, Blecha F, Ganta RR, Zheng L, Ross CR, Musch TI, Fels RJ, Kenney MJ. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol. 2005;289:H1683–1691. doi: 10.1152/ajpheart.00125.2005. [DOI] [PubMed] [Google Scholar]
- 98.Gaykema RP, Goehler LE, Hansen MK, Maier SF, Watkins LR. Subdiaphragmatic vagotomy blocks interleukin-1beta-induced fever but does not reduce IL-1beta levels in the circulation. Auton Neurosci. 2000;85:72–77. doi: 10.1016/s1566-0702(00)00222-8. [DOI] [PubMed] [Google Scholar]
- 99.Gayle D, Ilyin SE, Plata-Salaman CR. Feeding status and bacterial LPS-induced cytokine and neuropeptide gene expression in hypothalamus. Am J Physiol. 1999;277:R1188–1195. doi: 10.1152/ajpregu.1999.277.4.R1188. [DOI] [PubMed] [Google Scholar]
- 100.Gilbey MP. Sympathetic rhythms and nervous integration. Clin Exp Pharmacol Physiol. 2007;34:356–361. doi: 10.1111/j.1440-1681.2007.04587.x. [DOI] [PubMed] [Google Scholar]
- 101.Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, Watkins LR. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci. 1999;19:2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 103.Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, Watkins LR. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 104.Goldbach JM, Roth J, Zeisberger E. Fever suppression by subdiaphragmatic vagotomy in guinea pigs depends on the route of pyrogen administration. Am J Physiol. 1997;272:R675–681. doi: 10.1152/ajpregu.1997.272.2.R675. [DOI] [PubMed] [Google Scholar]
- 105.Grisanti LA, Woster AP, Dahlman J, Sauter ER, Combs CK, Porter JE. alpha1-adrenergic receptors positively regulate Toll-like receptor cytokine production from human monocytes and macrophages. J Pharmacol Exp Ther. 2011;338:648–657. doi: 10.1124/jpet.110.178012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gross PM, Weindl A. Peering through the windows of the brain. J Cereb Blood Flow Metab. 1987;7:663–672. doi: 10.1038/jcbfm.1987.120. [DOI] [PubMed] [Google Scholar]
- 107.Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev. 2005;29:493–500. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 108.Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, Marini H, Squadrito G, Minutoli L, Bertolini A, Marini R, Adamo EB, Venuti FS, Squadrito F. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–1194. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 109.Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, Francis J. TNF-alpha blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol. 2007;293:H599–609. doi: 10.1152/ajpheart.00286.2007. [DOI] [PubMed] [Google Scholar]
- 110.Guggilam A, Patel KP, Haque M, Ebenezer PJ, Kapusta DR, Francis J. Cytokine blockade attenuates sympathoexcitation in heart failure: cross-talk between nNOS, AT-1R and cytokines in the hypothalamic paraventricular nucleus. Eur J Heart Fail. 2008;10:625–634. doi: 10.1016/j.ejheart.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haensel A, Mills PJ, Nelesen RA, Ziegler MG, Dimsdale JE. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology. 2008;33:1305–1312. doi: 10.1016/j.psyneuen.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporos Int. 2008;19:905–912. doi: 10.1007/s00198-007-0487-9. [DOI] [PubMed] [Google Scholar]
- 113.Hansen MK, Daniels S, Goehler LE, Gaykema RP, Maier SF, Watkins LR. Subdiaphragmatic vagotomy does not block intraperitoneal lipopolysaccharide-induced fever. Auton Neurosci. 2000;85:83–87. doi: 10.1016/S1566-0702(00)00224-1. [DOI] [PubMed] [Google Scholar]
- 114.Hansen MK, Krueger JM. Subdiaphragmatic vagotomy blocks the sleep- and fever-promoting effects of interleukin-1beta. Am J Physiol. 1997;273:R1246–1253. doi: 10.1152/ajpregu.1997.273.4.R1246. [DOI] [PubMed] [Google Scholar]
- 115.Hansen MK, O’Connor KA, Goehler LE, Watkins LR, Maier SF. The contribution of the vagus nerve in interleukin-1beta-induced fever is dependent on dose. Am J Physiol Regul Integr Comp Physiol. 2001;280:R929–934. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- 116.Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986;73:615–621. doi: 10.1161/01.cir.73.4.615. [DOI] [PubMed] [Google Scholar]
- 117.Hasko G, Elenkov IJ, Vizi ES. Presynaptic receptors involved in the modulation of release of noradrenaline from the sympathetic nerve terminals of the rat thymus. Immunol Lett. 1995;47:133–137. doi: 10.1016/0165-2478(95)00085-j. [DOI] [PubMed] [Google Scholar]
- 118.Heiffer MH, Mundy RL, Mehlman B. Effect of lethal doses of bacterial endotoxin (E. coli) on sympathetic neurohormones in the rabbit. Am J Physiol. 1960;198:1307–1311. doi: 10.1152/ajplegacy.1960.198.6.1307. [DOI] [PubMed] [Google Scholar]
- 119.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Helwig BG, Craig RA, Fels RJ, Blecha F, Kenney MJ. Central nervous system administration of interleukin-6 produces splenic sympathoexcitation. Auton Neurosci. 2008;141:104–111. doi: 10.1016/j.autneu.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Helwig BG, Musch TI, Craig RA, Kenney MJ. Increased interleukin-6 receptor expression in the paraventricular nucleus of rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1165–1173. doi: 10.1152/ajpregu.00507.2006. [DOI] [PubMed] [Google Scholar]
- 122.Henricks PA, Van Esch B, Van Oosterhout AJ, Nijkamp FP. Specific and non-specific effects of beta-adrenoceptor agonists on guinea pig alveolar macrophage function. Eur J Pharmacol. 1988;152:321–330. doi: 10.1016/0014-2999(88)90727-3. [DOI] [PubMed] [Google Scholar]
- 123.Higgins TJ, David JR. Effect of isoproterernol and aminiphylline on cyclic AMP levels of guinea pig macrophages. Cell Immunol. 1976;27:1–10. doi: 10.1016/0008-8749(76)90147-7. [DOI] [PubMed] [Google Scholar]
- 124.Hjemdahl P, Larsson K, Johansson MC, Zetterlund A, Eklund A. Beta-adrenoceptors in human alveolar macrophages isolated by elutriation. Br J Clin Pharmacol. 1990;30:673–682. doi: 10.1111/j.1365-2125.1990.tb03835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hosking KG, Fels RJ, Kenney MJ. Inhibition of RVLM synaptic activation at peak hyperthermia reduces visceral sympathetic nerve discharge. Auton Neurosci. 2009;150:104–110. doi: 10.1016/j.autneu.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang BS, Yuan B, Leenen FH. Chronic blockade of brain “ouabain” prevents sympathetic hyper-reactivity and impairment of acute baroreflex resetting in rats with congestive heart failure. Can J Physiol Pharmacol. 2000;78:45–53. doi: 10.1139/cjpp-78-1-45. [DOI] [PubMed] [Google Scholar]
- 127.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med. 2011;269:45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ichijo T, Katafuchi T, Hori T. Central interleukin-1 beta enhances splenic sympathetic nerve activity in rats. Brain Res Bull. 1994;34:547–553. doi: 10.1016/0361-9230(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 130.Ikegami K. Modulation of adenosine 3′,5′-monophosphate contents of rat peritoneal macrophages mediated by beta2-adrenergic receptors. Biochem Pharmacol. 1977;26:1813–1816. doi: 10.1016/0006-2952(77)90351-3. [DOI] [PubMed] [Google Scholar]
- 131.Jetschmann JU, Benschop RJ, Jacobs R, Kemper A, Oberbeck R, Schmidt RE, Schedlowski M. Expression and in-vivo modulation of alpha- and beta-adrenoceptors on human natural killer (CD16+) cells. J Neuroimmunol. 1997;74:159–164. doi: 10.1016/s0165-5728(96)00221-4. [DOI] [PubMed] [Google Scholar]
- 132.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 133.Kanemi O, Zhang X, Sakamoto Y, Ebina M, Nagatomi R. Acute stress reduces intraparenchymal lung natural killer cells via beta-adrenergic stimulation. Clin Exp Immunol. 2005;139:25–34. doi: 10.1111/j.1365-2249.2005.02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kang YM, He RL, Yang LM, Qin DN, Guggilam A, Elks C, Yan N, Guo Z, Francis J. Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res. 2009;83:737–746. doi: 10.1093/cvr/cvp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009;82:503–512. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H227–236. doi: 10.1152/ajpheart.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kannan H, Tanaka Y, Kunitake T, Ueta Y, Hayashida Y, Yamashita H. Activation of sympathetic outflow by recombinant human interleukin-1 beta in conscious rats. Am J Physiol. 1996;270:R479–485. doi: 10.1152/ajpregu.1996.270.2.R479. [DOI] [PubMed] [Google Scholar]
- 138.Kasprowicz DJ, Kohm AP, Berton MT, Chruscinski AJ, Sharpe A, Sanders VM. Stimulation of the B cell receptor, CD86 (B7-2), and the beta 2-adrenergic receptor intrinsically modulates the level of IgG1 and IgE produced per B cell. J Immunol. 2000;165:680–690. doi: 10.4049/jimmunol.165.2.680. [DOI] [PubMed] [Google Scholar]
- 139.Katafuchi T, Duan S, Take S, Yoshimura M. Cytokine-induced suppression of medial preoptic neurons: mechanisms and neuroimmunomodulatory effects. Ann N Y Acad Sci. 2009;1153:76–81. doi: 10.1111/j.1749-6632.2008.03963.x. [DOI] [PubMed] [Google Scholar]
- 140.Katafuchi T, Ichijo T, Take S, Hori T. Hypothalamic modulation of splenic natural killer cell activity in rats. J Physiol. 1993;471:209–221. doi: 10.1113/jphysiol.1993.sp019898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Katafuchi T, Take S, Hori T. Roles of sympathetic nervous system in the suppression of cytotoxicity of splenic natural killer cells in the rat. J Physiol. 1993;465:343–357. doi: 10.1113/jphysiol.1993.sp019680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 143.Kawashima K, Fujii T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front Biosci. 2004;9:2063–2085. doi: 10.2741/1390. [DOI] [PubMed] [Google Scholar]
- 144.Kawashima K, Fujii T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 2003;74:675–696. doi: 10.1016/j.lfs.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 145.Kees MG, Pongratz G, Kees F, Scholmerich J, Straub RH. Via beta-adrenoceptors, stimulation of extrasplenic sympathetic nerve fibers inhibits lipopolysaccharide-induced TNF secretion in perfused rat spleen. J Neuroimmunol. 2003;145:77–85. doi: 10.1016/j.jneuroim.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 146.Kelleher DJ, Pessin JE, Ruoho AE, Johnson GL. Phorbol ester induces desensitization of adenylate cyclase and phosphorylation of the beta-adrenergic receptor in turkey erythrocytes. Proc Natl Acad Sci U S A. 1984;81:4316–4320. doi: 10.1073/pnas.81.14.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kenney MJ. Animal aging and regulation of sympathetic nerve discharge. J Appl Physiol (1985) 2010;109:951–958. doi: 10.1152/japplphysiol.00506.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kenney MJ. Frequency characteristics of sympathetic nerve discharge in anesthetized rats. Am J Physiol. 1994;267:R830–840. doi: 10.1152/ajpregu.1994.267.3.R830. [DOI] [PubMed] [Google Scholar]
- 149.Kenney MJ, Barney CC, Hirai T, Gisolfi CV. Sympathetic nerve responses to hyperthermia in the anesthetized rat. J Appl Physiol (1985) 1995;78:881–889. doi: 10.1152/jappl.1995.78.3.881. [DOI] [PubMed] [Google Scholar]
- 150.Kenney MJ, Blecha F, Fels RJ, Morgan DA. Altered frequency responses of sympathetic nerve discharge bursts after IL-1beta and mild hypothermia. J Appl Physiol (1985) 2002;93:280–288. doi: 10.1152/japplphysiol.01250.2001. [DOI] [PubMed] [Google Scholar]
- 151.Kenney MJ, Blecha F, Morgan DA, Fels RJ. Interleukin-1 beta alters brown adipose tissue but not renal sympathetic nerve responses to hypothermia. Am J Physiol Heart Circ Physiol. 2001;281:H2441–2445. doi: 10.1152/ajpheart.2001.281.6.H2441. [DOI] [PubMed] [Google Scholar]
- 152.Kenney MJ, Blecha F, Wang Y, McMurphy R, Fels RJ. Sympathoexcitation to intravenous interleukin-1beta is dependent on forebrain neural circuits. Am J Physiol Heart Circ Physiol. 2002;283:H501–505. doi: 10.1152/ajpheart.00181.2002. [DOI] [PubMed] [Google Scholar]
- 153.Kenney MJ, Claassen DE, Bishop MR, Fels RJ. Regulation of the sympathetic nerve discharge bursting pattern during heat stress. Am J Physiol. 1998;275:R1992–2001. doi: 10.1152/ajpregu.1998.275.6.R1992. [DOI] [PubMed] [Google Scholar]
- 154.Kenney MJ, Fels RJ. Forebrain and brain stem neural circuits contribute to altered sympathetic responses to heating in senescent rats. J Appl Physiol (1985) 2003;95:1986–1993. doi: 10.1152/japplphysiol.00438.2003. [DOI] [PubMed] [Google Scholar]
- 155.Kenney MJ, Fels RJ. Sympathetic nerve regulation to heating is altered in senescent rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R513–520. doi: 10.1152/ajpregu.00683.2001. [DOI] [PubMed] [Google Scholar]
- 156.Kenney MJ, Musch TI, Weiss ML. Renal sympathetic nerve regulation to heating is altered in rats with heart failure. Am J Physiol Heart Circ Physiol. 2001;280:H2868–2875. doi: 10.1152/ajpheart.2001.280.6.H2868. [DOI] [PubMed] [Google Scholar]
- 157.Kenney MJ, Pickar JG, Weiss ML, Saindon CS, Fels RJ. Effects of midbrain and spinal cord transections on sympathetic nerve responses to heating. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1329–1338. doi: 10.1152/ajpregu.2000.278.5.R1329. [DOI] [PubMed] [Google Scholar]
- 158.Kenney MJ, Weiss ML, Haywood JR. The paraventricular nucleus: an important component of the central neurocircuitry regulating sympathetic nerve outflow. Acta Physiol Scand. 2003;177:7–15. doi: 10.1046/j.1365-201X.2003.01042.x. [DOI] [PubMed] [Google Scholar]
- 159.Khan MM, Sansoni P, Silverman ED, Engleman EG, Melmon KL. Beta-adrenergic receptors on human suppressor, helper, and cytolytic lymphocytes. Biochem Pharmacol. 1986;35:1137–1142. doi: 10.1016/0006-2952(86)90150-4. [DOI] [PubMed] [Google Scholar]
- 160.Kobilka B. Adrenergic receptors as models for G protein-coupled receptors. Annu Rev Neurosci. 1992;15:87–114. doi: 10.1146/annurev.ne.15.030192.000511. [DOI] [PubMed] [Google Scholar]
- 161.Kohm AP, Sanders VM. Norepinephrine: a messenger from the brain to the immune system. Immunol Today. 2000;21:539–542. doi: 10.1016/s0167-5699(00)01747-3. [DOI] [PubMed] [Google Scholar]
- 162.Kohm AP, Sanders VM. Suppression of antigen-specific Th2 cell-dependent IgM and IgG1 production following norepinephrine depletion in vivo. J Immunol. 1999;162:5299–5308. [PubMed] [Google Scholar]
- 163.Komaki G, Arimura A, Koves K. Effect of intravenous injection of IL-1 beta on PGE2 levels in several brain areas as determined by microdialysis. Am J Physiol. 1992;262:E246–251. doi: 10.1152/ajpendo.1992.262.2.E246. [DOI] [PubMed] [Google Scholar]
- 164.Krawietz W, Werdan K, Schober M, Erdmann E, Rindfleisch GE, Hannig K. Different numbers of beta-receptors in human lymphocyte subpopulations. Biochem Pharmacol. 1982;31:133–136. doi: 10.1016/0006-2952(82)90252-0. [DOI] [PubMed] [Google Scholar]
- 165.Kregel KC, Stauss H, Unger T. Modulation of autonomic nervous system adjustments to heat stress by central ANG II receptor antagonism. Am J Physiol. 1994;266:R1985–1991. doi: 10.1152/ajpregu.1994.266.6.R1985. [DOI] [PubMed] [Google Scholar]
- 166.Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B. Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J Comp Neurol. 2013;521:3389–3405. doi: 10.1002/cne.23355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lankar D, Briken V, Adler K, Weiser P, Cassard S, Blank U, Viguier M, Bonnerot C. Syk tyrosine kinase and B cell antigen receptor (BCR) immunoglobulin-alpha subunit determine BCR-mediated major histocompatibility complex class II-restricted antigen presentation. J Exp Med. 1998;188:819–831. doi: 10.1084/jem.188.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Larsson J, Scadden D. Nervous activity in a stem cell niche. Cell. 2006;124:253–255. doi: 10.1016/j.cell.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 169.Lavis VR, Strada SJ, Ross CP, Hersh EM, Thompson WJ. Comparison of the responses of freshly isolated and cultured human monocytes and P388D1 cells to agents affecting cyclic AMP metabolism. J Lab Clin Med. 1980;96:551–561. [PubMed] [Google Scholar]
- 170.Ledbetter JA, Parsons M, Martin PJ, Hansen JA, Rabinovitch PS, June CH. Antibody binding to CD5 (Tp67) and Tp44 T cell surface molecules: effects on cyclic nucleotides, cytoplasmic free calcium, and cAMP-mediated suppression. J Immunol. 1986;137:3299–3305. [PubMed] [Google Scholar]
- 171.Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation. 1986;73:913–919. doi: 10.1161/01.cir.73.5.913. [DOI] [PubMed] [Google Scholar]
- 172.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–124. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 173.Liggett SB. Identification and characterization of a homogeneous population of beta 2-adrenergic receptors on human alveolar macrophages. Am Rev Respir Dis. 1989;139:552–555. doi: 10.1164/ajrccm/139.2.552. [DOI] [PubMed] [Google Scholar]
- 174.Lindstrom J. Nicotinic acetylcholine receptors in health and disease. Mol Neurobiol. 1997;15:193–222. doi: 10.1007/BF02740634. [DOI] [PubMed] [Google Scholar]
- 175.Lips KS, Konig P, Schatzle K, Pfeil U, Krasteva G, Spies M, Haberberger RV, Grando SA, Kummer W. Coexpression and spatial association of nicotinic acetylcholine receptor subunits alpha7 and alpha10 in rat sympathetic neurons. J Mol Neurosci. 2006;30:15–16. doi: 10.1385/JMN:30:1:15. [DOI] [PubMed] [Google Scholar]
- 176.Livnat S, Felten SY, Carlson SL, Bellinger DL, Felten DL. Involvement of peripheral and central catecholamine systems in neural-immune interactions. J Neuroimmunol. 1985;10:5–30. doi: 10.1016/0165-5728(85)90031-1. [DOI] [PubMed] [Google Scholar]
- 177.Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–283. 276–283. doi: 10.1161/HYPERTENSIONAHA.109.142646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lob HE, Schultz D, Marvar PJ, Davisson RL, Harrison DG. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension. 2013;61:382–387. doi: 10.1161/HYPERTENSIONAHA.111.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Loewy AD. Central Autonomic pathways. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. pp. 88–103. [Google Scholar]
- 180.Loveland BE, Jarrott B, McKenzie IF. The detection of beta-adrenoceptors on murine lymphocytes. Int J Immunopharmacol. 1981;3:45–55. doi: 10.1016/0192-0561(81)90044-8. [DOI] [PubMed] [Google Scholar]
- 181.Lu N, Wang Y, Blecha F, Fels RJ, Hoch HP, Kenney MJ. Central interleukin-1beta antibody increases renal and splenic sympathetic nerve discharge. Am J Physiol Heart Circ Physiol. 2003;284:H1536–1541. doi: 10.1152/ajpheart.00891.2002. [DOI] [PubMed] [Google Scholar]
- 182.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 183.Mac Grory B, O’Connor ET, O’Halloran KD, Jones JF. The effect of pro-inflammatory cytokines on the discharge rate of vagal nerve paraganglia in the rat. Respir Physiol Neurobiol. 2010;171:122–127. doi: 10.1016/j.resp.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.MacNeil BJ, Jansen AH, Greenberg AH, Nance DM. Effect of acute adrenalectomy on sympathetic responses to peripheral lipopolysaccharide or central PGE(2) Am J Physiol Regul Integr Comp Physiol. 2000;278:R1321–1328. doi: 10.1152/ajpregu.2000.278.5.R1321. [DOI] [PubMed] [Google Scholar]
- 185.MacNeil BJ, Jansen AH, Janz LJ, Greenberg AH, Nance DM. Peripheral endotoxin increases splenic sympathetic nerve activity via central prostaglandin synthesis. Am J Physiol. 1997;273:R609–614. doi: 10.1152/ajpregu.1997.273.2.R609. [DOI] [PubMed] [Google Scholar]
- 186.Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2004;286:R320–325. doi: 10.1152/ajpregu.00515.2003. [DOI] [PubMed] [Google Scholar]
- 187.Madden KS, Ackerman KD, Livnat S, Felten SY, Felten DL. Neonatal sympathetic denervation alters development of natural killer (NK) cell activity in F344 rats. Brain Behav Immun. 1993;7:344–351. doi: 10.1006/brbi.1993.1034. [DOI] [PubMed] [Google Scholar]
- 188.Madden KS, Felten SY, Felten DL, Hardy CA, Livnat S. Sympathetic nervous system modulation of the immune system. II. Induction of lymphocyte proliferation and migration in vivo by chemical sympathectomy. J Neuroimmunol. 1994;49:67–75. doi: 10.1016/0165-5728(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 189.Madden KS, Felten SY, Felten DL, Sundaresan PR, Livnat S. Sympathetic neural modulation of the immune system. I. Depression of T cell immunity in vivo and vitro following chemical sympathectomy. Brain Behav Immun. 1989;3:72–89. doi: 10.1016/0889-1591(89)90007-x. [DOI] [PubMed] [Google Scholar]
- 190.Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol. 1995;35:417–448. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- 191.Maiorov DN, Wilton ER, Badoer E, Petrie D, Head GA, Malpas SC. Sympathetic response to stimulation of the pontine A5 region in conscious rabbits. Brain Res. 1999;815:227–236. doi: 10.1016/s0006-8993(98)01150-0. [DOI] [PubMed] [Google Scholar]
- 192.Makman MH. Properties of adenylate cyclase of lymphoid cells. Proc Natl Acad Sci U S A. 1971;68:885–889. doi: 10.1073/pnas.68.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Maness LM, Kastin AJ, Banks WA. Relative contributions of a CVO and the microvascular bed to delivery of blood-borne IL-1alpha to the brain. Am J Physiol. 1998;275:E207–212. doi: 10.1152/ajpendo.1998.275.2.E207. [DOI] [PubMed] [Google Scholar]
- 194.Marquette C, Linard C, Galonnier M, Van Uye A, Mathieu J, Gourmelon P, Clarencon D. IL-1beta, TNFalpha and IL-6 induction in the rat brain after partial-body irradiation: role of vagal afferents. Int J Radiat Biol. 2003;79:777–785. doi: 10.1080/09553000310001610998. [DOI] [PubMed] [Google Scholar]
- 195.Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62:1214–1222. doi: 10.1136/gutjnl-2012-302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Meinkoth JL, Alberts AS, Went W, Fantozzi D, Taylor SS, Hagiwara M, Montminy M, Feramisco JR. Signal transduction through the cAMP-dependent protein kinase. Mol Cell Biochem. 1993;127–128:179–186. doi: 10.1007/BF01076769. [DOI] [PubMed] [Google Scholar]
- 197.Meurs H, van den Bogaard W, Kauffman HF, Bruynzeel PL. Characterization of (−)-[3H]dihydroalprenolol binding to intact and broken cell preparations of human peripheral blood lymphocytes. Eur J Pharmacol. 1982;85:185–194. doi: 10.1016/0014-2999(82)90464-2. [DOI] [PubMed] [Google Scholar]
- 198.Miles K, Atweh S, Otten G, Arnason BG, Chelmicka-Schorr E. Beta-adrenergic receptors on splenic lymphocytes from axotomized mice. Int J Immunopharmacol. 1984;6:171–177. doi: 10.1016/0192-0561(84)90014-6. [DOI] [PubMed] [Google Scholar]
- 199.Miles K, Chelmicka-Schorr E, Atweh S, Otten G, Arnason BG. Sympathetic ablation alters lymphocyte membrane properties. J Immunol. 1985;135:797s–801s. [PubMed] [Google Scholar]
- 200.Morrison SF. 2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: Central neural pathways for thermoregulatory cold defense. J Appl Physiol (1985) 2011;110:1137–1149. doi: 10.1152/japplphysiol.01227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol. 2001;281:R683–698. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- 202.Morrison SF. Raphe pallidus neurons mediate prostaglandin E2-evoked increases in brown adipose tissue thermogenesis. Neuroscience. 2003;121:17–24. doi: 10.1016/s0306-4522(03)00363-4. [DOI] [PubMed] [Google Scholar]
- 203.Muthu K, Iyer S, He LK, Szilagyi A, Gamelli RL, Shankar R, Jones SB. Murine hematopoietic stem cells and progenitors express adrenergic receptors. J Neuroimmunol. 2007;186:27–36. doi: 10.1016/j.jneuroim.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nakada MT, Stadel JM, Poksay KS, Crooke ST. Glucocorticoid regulation of beta-adrenergic receptors in 3T3-L1 preadipocytes. Mol Pharmacol. 1987;31:377–384. [PubMed] [Google Scholar]
- 205.Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1207–1228. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- 206.Nakamura Y, Nakamura K, Morrison SF. Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience. 2009;161:614–620. doi: 10.1016/j.neuroscience.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nance DM, Burns J. Innervation of the spleen in the rat: evidence for absence of afferent innervation. Brain Behav Immun. 1989;3:281–290. doi: 10.1016/0889-1591(89)90028-7. [DOI] [PubMed] [Google Scholar]
- 208.Nance DM, Hopkins DA, Bieger D. Re-investigation of the innervation of the thymus gland in mice and rats. Brain Behav Immun. 1987;1:134–147. doi: 10.1016/0889-1591(87)90016-x. [DOI] [PubMed] [Google Scholar]
- 209.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 210.Niijima A, Hori T, Aou S, Oomura Y. The effects of interleukin-1 beta on the activity of adrenal, splenic and renal sympathetic nerves in the rat. J Auton Nerv Syst. 1991;36:183–192. doi: 10.1016/0165-1838(91)90042-2. [DOI] [PubMed] [Google Scholar]
- 211.Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst. 1997;63:61–67. doi: 10.1016/s0165-1838(96)00134-8. [DOI] [PubMed] [Google Scholar]
- 212.O’Leary DM, Murphy A, Pickering M, Jones JF. Arterial chemoreceptors in the superior laryngeal nerve of the rat. Respir Physiol Neurobiol. 2004;141:137–144. doi: 10.1016/j.resp.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 213.Ohashi K, Saigusa T. Sympathetic nervous responses during cytokine-induced fever in conscious rabbits. Pflugers Arch. 1997;433:691–698. doi: 10.1007/s004240050333. [DOI] [PubMed] [Google Scholar]
- 214.Oka T. Prostaglandin E2 as a mediator of fever: the role of prostaglandin E (EP) receptors. Front Biosci. 2004;9:3046–3057. doi: 10.2741/1458. [DOI] [PubMed] [Google Scholar]
- 215.Oke SL, Tracey KJ. From CNI-1493 to the immunological homunculus: physiology of the inflammatory reflex. J Leukoc Biol. 2008;83:512–517. doi: 10.1189/jlb.0607363. [DOI] [PubMed] [Google Scholar]
- 216.Ootsuka Y, Blessing WW, Steiner AA, Romanovsky AA. Fever response to intravenous prostaglandin E2 is mediated by the brain but does not require afferent vagal signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1294–1303. doi: 10.1152/ajpregu.00709.2007. [DOI] [PubMed] [Google Scholar]
- 217.Palsson J, Ricksten SE, Delle M, Lundin S. Changes in renal sympathetic nerve activity during experimental septic and endotoxin shock in conscious rats. Circ Shock. 1988;24:133–141. [PubMed] [Google Scholar]
- 218.Pan W, Kastin AJ. TNFalpha transport across the blood-brain barrier is abolished in receptor knockout mice. Exp Neurol. 2002;174:193–200. doi: 10.1006/exnr.2002.7871. [DOI] [PubMed] [Google Scholar]
- 219.Pardini BJ, Jones SB, Filkins JP. Cardiac and splenic norepinephrine turnovers in endotoxic rats. Am J Physiol. 1983;245:H276–283. doi: 10.1152/ajpheart.1983.245.2.H276. [DOI] [PubMed] [Google Scholar]
- 220.Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, Al-Abed Y, Tracey KJ. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci U S A. 2006;103:5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, Chavan S, Al-Abed Y, Tracey KJ. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23:41–45. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- 223.Perez SD, Kozic B, Molinaro CA, Thyagarajan S, Ghamsary M, Lubahn CL, Lorton D, Bellinger DL. Chronically lowering sympathetic activity protects sympathetic nerves in spleens from aging F344 rats. J Neuroimmunol. 2012;247:38–51. doi: 10.1016/j.jneuroim.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Peri F, Piazza M, Calabrese V, Damore G, Cighetti R. Exploring the LPS/TLR4 signal pathway with small molecules. Biochem Soc Trans. 2010;38:1390–1395. doi: 10.1042/BST0381390. [DOI] [PubMed] [Google Scholar]
- 225.Pernow J, Schwieler J, Kahan T, Hjemdahl P, Oberle J, Wallin BG, Lundberg JM. Influence of sympathetic discharge pattern on norepinephrine and neuropeptide Y release. Am J Physiol. 1989;257:H866–872. doi: 10.1152/ajpheart.1989.257.3.H866. [DOI] [PubMed] [Google Scholar]
- 226.Peruzzo B, Pastor FE, Blazquez JL, Schobitz K, Pelaez B, Amat P, Rodriguez EM. A second look at the barriers of the medial basal hypothalamus. Exp Brain Res. 2000;132:10–26. doi: 10.1007/s002219900289. [DOI] [PubMed] [Google Scholar]
- 227.Pochet R, Delespesse G. beta-Adrenoreceptors display different efficiency on lymphocyte subpopulations. Biochem Pharmacol. 1983;32:1651–1655. doi: 10.1016/0006-2952(83)90344-1. [DOI] [PubMed] [Google Scholar]
- 228.Pochet R, Delespesse G, Gausset PW, Collet H. Distribution of beta-adrenergic receptors on human lymphocyte subpopulations. Clin Exp Immunol. 1979;38:578–584. [PMC free article] [PubMed] [Google Scholar]
- 229.Quan N. Immune-to-brain signaling: how important are the blood-brain barrier-independent pathways? Mol Neurobiol. 2008;37:142–152. doi: 10.1007/s12035-008-8026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 231.Radojcic T, Baird S, Darko D, Smith D, Bulloch K. Changes in beta-adrenergic receptor distribution on immunocytes during differentiation: an analysis of T cells and macrophages. J Neurosci Res. 1991;30:328–335. doi: 10.1002/jnr.490300208. [DOI] [PubMed] [Google Scholar]
- 232.Ramer-Quinn DS, Baker RA, Sanders VM. Activated T helper 1 and T helper 2 cells differentially express the beta-2-adrenergic receptor: a mechanism for selective modulation of T helper 1 cell cytokine production. J Immunol. 1997;159:4857–4867. [PubMed] [Google Scholar]
- 233.Reardon C, Duncan GS, Brustle A, Brenner D, Tusche MW, Olofsson PS, Rosas-Ballina M, Tracey KJ, Mak TW. Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proc Natl Acad Sci U S A. 2013;110:1410–1415. doi: 10.1073/pnas.1221655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Redwine L, Snow S, Mills P, Irwin M. Acute psychological stress: effects on chemotaxis and cellular adhesion molecule expression. Psychosom Med. 2003;65:598–603. doi: 10.1097/01.psy.0000079377.86193.a8. [DOI] [PubMed] [Google Scholar]
- 235.Remold-O’Donnell E. Stimulation and desensitization of macrophage adenylate cyclase by prostaglandins and catecholamines. J Biol Chem. 1974;249:361–321. [PubMed] [Google Scholar]
- 236.Rinner I, Kawashima K, Schauenstein K. Rat lymphocytes produce and secrete acetylcholine in dependence of differentiation and activation. J Neuroimmunol. 1998;81:31–37. doi: 10.1016/s0165-5728(97)00155-0. [DOI] [PubMed] [Google Scholar]
- 237.Rivest S, Lacroix S, Vallieres L, Nadeau S, Zhang J, Laflamme N. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med. 2000;223:22–38. doi: 10.1046/j.1525-1373.2000.22304.x. [DOI] [PubMed] [Google Scholar]
- 238.Romanovsky AA. The inflammatory reflex: the current model should be revised. Exp Physiol. 2012;97:1178–1179. doi: 10.1113/expphysiol.2011.064071. [DOI] [PubMed] [Google Scholar]
- 239.Romanovsky AA, Steiner AA, Matsumura K. Cells that trigger fever. Cell Cycle. 2006;5:2195–2197. doi: 10.4161/cc.5.19.3321. [DOI] [PubMed] [Google Scholar]
- 240.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rosas-Ballina M, Tracey KJ. The neurology of the immune system: neural reflexes regulate immunity. Neuron. 2009;64:28–32. doi: 10.1016/j.neuron.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rosati C, Hannaert P, Dausse JP, Braquet P, Garay R. Stimulation of beta-adrenoceptors inhibits calcium-dependent potassium-channels in mouse macrophages. J Cell Physiol. 1986;129:310–314. doi: 10.1002/jcp.1041290307. [DOI] [PubMed] [Google Scholar]
- 244.Roth J, De Souza GE. Fever induction pathways: evidence from responses to systemic or local cytokine formation. Braz J Med Biol Res. 2001;34:301–314. doi: 10.1590/s0100-879x2001000300003. [DOI] [PubMed] [Google Scholar]
- 245.Ruhl A, Hurst S, Collins SM. Synergism between interleukins 1 beta and 6 on noradrenergic nerves in rat myenteric plexus. Gastroenterology. 1994;107:993–1001. doi: 10.1016/0016-5085(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 246.Saigusa T. Participation of interleukin-1 and tumor necrosis factor in the responses of the sympathetic nervous system during lipopolysaccharide-induced fever. Pflugers Arch. 1990;416:225–229. doi: 10.1007/BF00392057. [DOI] [PubMed] [Google Scholar]
- 247.Saindon CS, Blecha F, Musch TI, Morgan DA, Fels RJ, Kenney MJ. Effect of cervical vagotomy on sympathetic nerve responses to peripheral interleukin-1beta. Auton Neurosci. 2001;87:243–248. doi: 10.1016/s1566-0702(00)00280-0. [DOI] [PubMed] [Google Scholar]
- 248.Sanders VM. The role of adrenoceptor-mediated signals in the modulation of lymphocyte function. Adv Neuroimmunol. 1995;5:283–298. doi: 10.1016/0960-5428(95)00019-x. [DOI] [PubMed] [Google Scholar]
- 249.Sanders VM. The role of norepinephrine and beta-2-adrenergic receptor stimulation in the modulation of Th1, Th2, and B lymphocyte function. Adv Exp Med Biol. 1998;437:269–278. doi: 10.1007/978-1-4615-5347-2_30. [DOI] [PubMed] [Google Scholar]
- 250.Sanders VM, Baker RA, Ramer-Quinn DS, Kasprowicz DJ, Fuchs BA, Street NE. Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J Immunol. 1997;158:4200–4210. [PubMed] [Google Scholar]
- 251.Sanders VM, Munson AE. Beta adrenoceptor mediation of the enhancing effect of norepinephrine on the murine primary antibody response in vitro. J Pharmacol Exp Ther. 1984;230:183–192. [PubMed] [Google Scholar]
- 252.Sanders VM, Munson AE. Norepinephrine and the antibody response. Pharmacol Rev. 1985;37:229–248. [PubMed] [Google Scholar]
- 253.Sanders VM, Munson AE. Role of alpha adrenoceptor activation in modulating the murine primary antibody response in vitro. J Pharmacol Exp Ther. 1985;232:395–400. [PubMed] [Google Scholar]
- 254.Sanders VM, Powell-Oliver FE. Beta 2-adrenoceptor stimulation increases the number of antigen-specific precursor B lymphocytes that differentiate into IgM-secreting cells without affecting burst size. J Immunol. 1992;148:1822–1828. [PubMed] [Google Scholar]
- 255.Sanders VM, Straub RH. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain Behav Immun. 2002;16:290–332. doi: 10.1006/brbi.2001.0639. [DOI] [PubMed] [Google Scholar]
- 256.Saper CB, Romanovsky AA, Scammell TE. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat Neurosci. 2012;15:1088–1095. doi: 10.1038/nn.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sayk F, Vietheer A, Schaaf B, Wellhoener P, Weitz G, Lehnert H, Dodt C. Endotoxemia causes central downregulation of sympathetic vasomotor tone in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2008;295:R891–898. doi: 10.1152/ajpregu.90444.2008. [DOI] [PubMed] [Google Scholar]
- 258.Schiltz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J Neurosci. 2002;22:5606–5618. doi: 10.1523/JNEUROSCI.22-13-05606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Schmidt P, Holsboer F, Spengler D. Beta(2)-adrenergic receptors potentiate glucocorticoid receptor transactivation via G protein beta gamma-subunits and the phosphoinositide 3-kinase pathway. Mol Endocrinol. 2001;15:553–564. doi: 10.1210/mend.15.4.0613. [DOI] [PubMed] [Google Scholar]
- 260.Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schabitz WR. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Schneider A, Kuhn HG, Schabitz WR. A role for G-CSF (granulocyte-colony stimulating factor) in the central nervous system. Cell Cycle. 2005;4:1753–1757. doi: 10.4161/cc.4.12.2213. [DOI] [PubMed] [Google Scholar]
- 262.Schobitz B, de Kloet ER, Sutanto W, Holsboer F. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Eur J Neurosci. 1993;5:1426–1435. doi: 10.1111/j.1460-9568.1993.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 263.Schocken DD, Roth GS. Reduced beta-adrenergic receptor concentrations in ageing man. Nature. 1977;267:856–858. doi: 10.1038/267856a0. [DOI] [PubMed] [Google Scholar]
- 264.Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- 265.Serrats J, Schiltz JC, Garcia-Bueno B, van Rooijen N, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160:3251–3258. [PubMed] [Google Scholar]
- 267.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shi Z, Gan XB, Fan ZD, Zhang F, Zhou YB, Gao XY, De W, Zhu GQ. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf) 2011;203:289–297. doi: 10.1111/j.1748-1716.2011.02313.x. [DOI] [PubMed] [Google Scholar]
- 269.Siso S, Jeffrey M, Gonzalez L. Sensory circumventricular organs in health and disease. Acta Neuropathol. 2010;120:689–705. doi: 10.1007/s00401-010-0743-5. [DOI] [PubMed] [Google Scholar]
- 270.Skok MV, Voitenko LP, Voitenko SV, Lykhmus EY, Kalashnik EN, Litvin TI, Tzartos SJ, Skok VI. Alpha subunit composition of nicotinic acetylcholine receptors in the rat autonomic ganglia neurons as determined with subunit-specific anti-alpha(181–192) peptide antibodies. Neuroscience. 1999;93:1427–1436. doi: 10.1016/s0306-4522(99)00160-8. [DOI] [PubMed] [Google Scholar]
- 271.Smith JW, Steiner AL, Parker CW. Human lymphocytic metabolism. Effects of cyclic and noncyclic nucleotides on stimulation by phytohemagglutinin. J Clin Invest. 1971;50:442–448. doi: 10.1172/JCI106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Soares JB, Pimentel-Nunes P, Roncon-Albuquerque R, Leite-Moreira A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int. 2010;4:659–672. doi: 10.1007/s12072-010-9219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Solaroglu I, Cahill J, Jadhav V, Zhang JH. A novel neuroprotectant granulocyte-colony stimulating factor. Stroke. 2006;37:1123–1128. doi: 10.1161/01.STR.0000208205.26253.96. [DOI] [PubMed] [Google Scholar]
- 274.Solaroglu I, Jadhav V, Zhang JH. Neuroprotective effect of granulocyte-colony stimulating factor. Front Biosci. 2007;12:712–724. doi: 10.2741/2095. [DOI] [PubMed] [Google Scholar]
- 275.Spengler RN, Allen RM, Remick DG, Strieter RM, Kunkel SL. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J Immunol. 1990;145:1430–1434. [PubMed] [Google Scholar]
- 276.Steiner AA, Chakravarty S, Rudaya AY, Herkenham M, Romanovsky AA. Bacterial lipopolysaccharide fever is initiated via Toll-like receptor 4 on hematopoietic cells. Blood. 2006;107:4000–4002. doi: 10.1182/blood-2005-11-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Straub RH. Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol Sci. 2004;25:640–646. doi: 10.1016/j.tips.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 279.Straub RH, Herfarth H, Falk W, Andus T, Scholmerich J. Uncoupling of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis in inflammatory bowel disease? J Neuroimmunol. 2002;126:116–125. doi: 10.1016/s0165-5728(02)00047-4. [DOI] [PubMed] [Google Scholar]
- 280.Straub RH, Westermann J, Scholmerich J, Falk W. Dialogue between the CNS and the immune system in lymphoid organs. Immunol Today. 1998;19:409–413. doi: 10.1016/s0167-5699(98)01297-3. [DOI] [PubMed] [Google Scholar]
- 281.Straub RH, Wiest R, Strauch UG, Harle P, Scholmerich J. The role of the sympathetic nervous system in intestinal inflammation. Gut. 2006;55:1640–1649. doi: 10.1136/gut.2006.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sun MK. Central neural organization and control of sympathetic nervous system in mammals. Prog Neurobiol. 1995;47:157–233. doi: 10.1016/0301-0082(95)00026-8. [DOI] [PubMed] [Google Scholar]
- 283.Sun MK. Pharmacology of reticulospinal vasomotor neurons in cardiovascular regulation. Pharmacol Rev. 1996;48:465–494. [PubMed] [Google Scholar]
- 284.Take S, Mori T, Katafuchi T, Hori T. Central interferon-alpha inhibits natural killer cytotoxicity through sympathetic innervation. Am J Physiol. 1993;265:R453–459. doi: 10.1152/ajpregu.1993.265.2.R453. [DOI] [PubMed] [Google Scholar]
- 285.Tanaka M, McKinley MJ, McAllen RM. Roles of two preoptic cell groups in tonic and febrile control of rat tail sympathetic fibers. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1248–1257. doi: 10.1152/ajpregu.91010.2008. [DOI] [PubMed] [Google Scholar]
- 286.Taylor JA, Lipsitz LA. Heart rate variability standards. Circulation. 1997;95:280–281. [PubMed] [Google Scholar]
- 287.Terao A, Oikawa M, Saito M. Tissue-specific increase in norepinephrine turnover by central interleukin-1, but not by interleukin-6, in rats. Am J Physiol. 1994;266:R400–404. doi: 10.1152/ajpregu.1994.266.2.R400. [DOI] [PubMed] [Google Scholar]
- 288.Thames MD, Kinugawa T, Smith ML, Dibner-Dunlap ME. Abnormalities of baroreflex control in heart failure. J Am Coll Cardiol. 1993;22:56A–60A. doi: 10.1016/0735-1097(93)90464-c. [DOI] [PubMed] [Google Scholar]
- 289.Thayer JF, Sternberg EM. Neural aspects of immunomodulation: focus on the vagus nerve. Brain Behav Immun. 2010;24:1223–1228. doi: 10.1016/j.bbi.2010.07.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.The F, Cailotto C, van der Vliet J, de Jonge WJ, Bennink RJ, Buijs RM, Boeckxstaens GE. Central activation of the cholinergic anti-inflammatory pathway reduces surgical inflammation in experimental post-operative ileus. Br J Pharmacol. 2011;163:1007–1016. doi: 10.1111/j.1476-5381.2011.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Thyagarajan S, Madden KS, Boehm GW, Stevens SY, Felten DL, Bellinger DL. L-Deprenyl reverses age-associated decline in splenic norepinephrine, interleukin-2 and interferon-gamma production in old female F344 rats. Neuroimmunomodulation. 2013;20:72–78. doi: 10.1159/000345043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.ThyagaRajan S, Madden KS, Stevens SY, Felten DL. Restoration of splenic noradrenergic nerve fibers and immune reactivity in old F344 rats: a comparison between L-deprenyl and L-desmethyldeprenyl. Int J Immunopharmacol. 2000;22:523–536. doi: 10.1016/s0192-0561(00)00016-3. [DOI] [PubMed] [Google Scholar]
- 293.Tobey JC, Fry HK, Mizejewski CS, Fink GD, Weaver LC. Differential sympathetic responses initiated by angiotensin and sodium chloride. Am J Physiol. 1983;245:R60–68. doi: 10.1152/ajpregu.1983.245.1.R60. [DOI] [PubMed] [Google Scholar]
- 294.Tonges L, Schlachetzki JC, Weishaupt JH, Bahr M. Hematopoietic cytokines--on the verge of conquering neurology. Curr Mol Med. 2007;7:157–170. doi: 10.2174/156652407780059186. [DOI] [PubMed] [Google Scholar]
- 295.Tonhajzerova I, Mokra D, Visnovcova Z. Vagal function indexed by respiratory sinus arrhythmia and cholinergic anti-inflammatory pathway. Respir Physiol Neurobiol. 2013;187:78–81. doi: 10.1016/j.resp.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 296.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 297.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Trotter RN, Stornetta RL, Guyenet PG, Roberts MR. Transneuronal mapping of the CNS network controlling sympathetic outflow to the rat thymus. Auton Neurosci. 2007;131:9–20. doi: 10.1016/j.autneu.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 300.Vallieres L, Lacroix S, Rivest S. Influence of interleukin-6 on neural activity and transcription of the gene encoding corticotrophin-releasing factor in the rat brain: an effect depending upon the route of administration. Eur J Neurosci. 1997;9:1461–1472. doi: 10.1111/j.1460-9568.1997.tb01500.x. [DOI] [PubMed] [Google Scholar]
- 301.Vallieres L, Rivest S. Regulation of the genes encoding interleukin-6, its receptor, and gp130 in the rat brain in response to the immune activator lipopolysaccharide and the proinflammatory cytokine interleukin-1beta. J Neurochem. 1997;69:1668–1683. doi: 10.1046/j.1471-4159.1997.69041668.x. [DOI] [PubMed] [Google Scholar]
- 302.van de Borne P, Montano N, Pagani M, Oren R, Somers VK. Absence of low-frequency variability of sympathetic nerve activity in severe heart failure. Circulation. 1997;95:1449–1454. doi: 10.1161/01.cir.95.6.1449. [DOI] [PubMed] [Google Scholar]
- 303.Van Der Zanden EP, Boeckxstaens GE, de Jonge WJ. The vagus nerve as a modulator of intestinal inflammation. Neurogastroenterol Motil. 2009;21:6–17. doi: 10.1111/j.1365-2982.2008.01252.x. [DOI] [PubMed] [Google Scholar]
- 304.van Esch B, Henricks PA, van Oosterhout AJ, Nijkamp FP. Guinea pig alveolar macrophages possess beta-adrenergic receptors. Agents Actions. 1989;26:123–124. doi: 10.1007/BF02126582. [DOI] [PubMed] [Google Scholar]
- 305.Van Oosterhout AJ, Nijkamp FP. Anterior hypothalamic lesions prevent the endotoxin-induced reduction of beta-adrenoceptor number in guinea pig lung. Brain Res. 1984;302:277–280. doi: 10.1016/0006-8993(84)90240-3. [DOI] [PubMed] [Google Scholar]
- 306.Van Tits LJ, Michel MC, Grosse-Wilde H, Happel M, Eigler FW, Soliman A, Brodde OE. Catecholamines increase lymphocyte beta 2-adrenergic receptors via a beta 2-adrenergic, spleen-dependent process. Am J Physiol. 1990;258:E191–202. doi: 10.1152/ajpendo.1990.258.1.E191. [DOI] [PubMed] [Google Scholar]
- 307.Vayssettes-Courchay C, Bouysset F, Verbeuren TJ. Sympathetic activation and tachycardia in lipopolysaccharide treated rats are temporally correlated and unrelated to the baroreflex. Auton Neurosci. 2005;120:35–45. doi: 10.1016/j.autneu.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 308.Verghese MW, Snyderman R. Hormonal activation of adenylate cyclase in macrophage membranes is regulated by guanine nucleotides. J Immunol. 1983;130:869–873. [PubMed] [Google Scholar]
- 309.Vischer TL. The differential effect of cyclic AMP on lymphocyte stimulation by T- or B-cell mitogens. Immunology. 1976;30:735–739. [PMC free article] [PubMed] [Google Scholar]
- 310.Vizi ES, Sershen H, Balla A, Mike A, Windisch K, Juranyi Z, Lajtha A. Neurochemical evidence of heterogeneity of presynaptic and somatodendritic nicotinic acetylcholine receptors. Ann N Y Acad Sci. 1995;757:84–99. doi: 10.1111/j.1749-6632.1995.tb17466.x. [DOI] [PubMed] [Google Scholar]
- 311.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 312.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 313.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 314.Wang W, Chen JS, Zucker IH. Carotid sinus baroreceptor sensitivity in experimental heart failure. Circulation. 1990;81:1959–1966. doi: 10.1161/01.cir.81.6.1959. [DOI] [PubMed] [Google Scholar]
- 315.Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci. 1995;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- 316.Weinstein Y, Segal S, Melmon KL. Specific mitogenic activity of 8-Br-guanosine 3′,5′-monophosphate (Br-cyclic GMP) on B lymphocytes. J Immunol. 1975;115:112–117. [PubMed] [Google Scholar]
- 317.Welscher HD, Cruchaud A. The influence of various particles and 3′, 5′ cyclic adenosine monophosphate on release of lysosomal enzymes by mouse macrophages. J Reticuloendothel Soc. 1976;20:405–420. [PubMed] [Google Scholar]
- 318.Westly HJ, Kelley KW. Down-regulation of glucocorticoid and beta-adrenergic receptors on lectin-stimulated splenocytes. Proc Soc Exp Biol Med. 1987;185:211–218. doi: 10.3181/00379727-185-42537. [DOI] [PubMed] [Google Scholar]
- 319.Wiest R, Moleda L, Zietz B, Hellerbrand C, Scholmerich J, Straub R. Uncoupling of sympathetic nervous system and hypothalamic-pituitary-adrenal axis in cirrhosis. J Gastroenterol Hepatol. 2008;23:1901–1908. doi: 10.1111/j.1440-1746.2008.05456.x. [DOI] [PubMed] [Google Scholar]
- 320.Williams JM, Felten DL. Sympathetic innervation of murine thymus and spleen: a comparative histofluorescence study. Anat Rec. 1981;199:531–542. doi: 10.1002/ar.1091990409. [DOI] [PubMed] [Google Scholar]
- 321.Williams JM, Peterson RG, Shea PA, Schmedtje JF, Bauer DC, Felten DL. Sympathetic innervation of murine thymus and spleen: evidence for a functional link between the nervous and immune systems. Brain Res Bull. 1981;6:83–94. doi: 10.1016/s0361-9230(81)80072-x. [DOI] [PubMed] [Google Scholar]
- 322.Williams LT, Snyderman R, Lefkowitz RJ. Identification of beta-adrenergic receptors in human lymphocytes by (−) (3H) alprenolol binding. J Clin Invest. 1976;57:149–155. doi: 10.1172/JCI108254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Xiao J, Huang HW, Peng YP, Bao JY, Huang Y, Qiu YH. Modulation of natural killer cell function by alpha-adrenoreceptor-coupled signalling. Neuro Endocrinol Lett. 2010;31:635–644. [PubMed] [Google Scholar]
- 324.Ye S, Mozayeni P, Gamburd M, Zhong H, Campese VM. Interleukin-1beta and neurogenic control of blood pressure in normal rats and rats with chronic renal failure. Am J Physiol Heart Circ Physiol. 2000;279:H2786–2796. doi: 10.1152/ajpheart.2000.279.6.H2786. [DOI] [PubMed] [Google Scholar]
- 325.Yokotani K, Nishihara M, Murakami Y, Hasegawa T, Okuma Y, Osumi Y. Elevation of plasma noradrenaline levels in urethane-anaesthetized rats by activation of central prostanoid EP3 receptors. Br J Pharmacol. 1995;115:672–676. doi: 10.1111/j.1476-5381.1995.tb14985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci. 2009;29:11954–11964. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest. 2012;122:3960–3964. doi: 10.1172/JCI64583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Yu Y, Kang YM, Zhang ZH, Wei SG, Chu Y, Weiss RM, Felder RB. Increased cyclooxygenase-2 expression in hypothalamic paraventricular nucleus in rats with heart failure: role of nuclear factor kappaB. Hypertension. 2007;49:511–518. doi: 10.1161/01.HYP.0000257356.20527.c5. [DOI] [PubMed] [Google Scholar]
- 329.Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 2010;55:652–659. doi: 10.1161/HYPERTENSIONAHA.109.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Yujiri T, Tagami K, Tanimura A, Tanizawa Y. Alteration of adrenergic signals during peripheral blood stem cell mobilization induced by granulocyte colony-stimulating factor. Leuk Res. 2008;32:195–197. doi: 10.1016/j.leukres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 331.Zhang ZH, Wei SG, Francis J, Felder RB. Cardiovascular and renal sympathetic activation by blood-borne TNF-alpha in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol. 2003;284:R916–927. doi: 10.1152/ajpregu.00406.2002. [DOI] [PubMed] [Google Scholar]
- 332.Zhang ZH, Yu Y, Wei SG, Felder RB. Centrally administered lipopolysaccharide elicits sympathetic excitation via NAD(P)H oxidase-dependent mitogen-activated protein kinase signaling. J Hypertens. 2010;28:806–816. doi: 10.1097/HJH.0b013e3283358b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zhang ZH, Yu Y, Wei SG, Nakamura Y, Nakamura K, Felder RB. EP(3) receptors mediate PGE(2)-induced hypothalamic paraventricular nucleus excitation and sympathetic activation. Am J Physiol Heart Circ Physiol. 2011;301:H1559–1569. doi: 10.1152/ajpheart.00262.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Zhu GQ, Patel KP, Zucker IH, Wang W. Microinjection of ANG II into paraventricular nucleus enhances cardiac sympathetic afferent reflex in rats. Am J Physiol Heart Circ Physiol. 2002;282:H2039–2045. doi: 10.1152/ajpheart.00854.2001. [DOI] [PubMed] [Google Scholar]
- 335.Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Prog Biophys Mol Biol. 2004;84:125–149. doi: 10.1016/j.pbiomolbio.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 336.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 337.Zucker IH. Baro- and Cardiac Reflex Abnormalities in Chronic Heart Failure. In: Zucker IH, editor. Reflex Control of Circulation. Boca Raton: 1991. pp. 849–874. [Google Scholar]