Abstract
MicroRNAs (miRNA) are abundant classes of small, endogenous non-coding RNAs, which inhibit the expression of target gene via post-transcriptional regulation. In addition to an important functional role miRNAs play in carcinogenesis, emerging evidence has demonstrated the feasibility of miRNAs as robust cancer biomarkers. In particular, the recent discovery of miRNAs in the body fluids provides an attractive opportunity for the development of non-invasive biomarkers for the diagnosis, prognosis and predictive responses to cancer therapy. Colorectal cancer (CRC) is one of the most common cancers worldwide, and accumulating evidence provides a compelling case for the potential exploitation of miRNAs as CRC-biomarkers. This review summarizes the current state of literature in the field, and primarily focuses on the clinical relevance of miRNAs as potential biomarkers for CRC treatment, and discusses the forthcoming challenges to further advance this exciting field of “academic research” into “bedside clinical care” of patients suffering from CRC.
Keywords: miRNA, biomarker, colorectal cancer, adenoma, blood, feces, diagnosis, prediction, prognosis
Introduction
Colorectal cancer (CRC) is one of the most common and lethal malignancies worldwide[1]. Although the mortality rates have declined over the past decade, especially in developed countries as a result of improved screening and/or treatment options, CRC still remains the third leading cause of cancer-related death in the world, with an estimated incidence of 1.2 million new cases and 608,700 deaths estimated annually[2]. However, CRC is a potentially preventable disease, and early detection form of this disease is almost always curable. In view of these results, we clearly need to establish novel and more effective screening programs/strategies that would permit earlier detection of this disease, as well as develop safe and more effective therapies that can help reduce the significant global mortality rates associated with this malignancy. Several CRC screening tests, including fecal occult-blood testing and colonoscopy, have been available for years[3], Although colonoscopy is the current gold standard for early diagnosis of CRC, the invasive nature and the associated high costs have hampered its widespread application as an effective and compliant screening modality. Currently available noninvasive screening methods, such as fecal guaiac-based occult blood tests(FOBT) and fecal immunochemical occult blood tests (FIT), have resulted in somewhat decrease in mortality[7-9]. However, large-scale screening studies have noted the limitation of these tests due to the inherent low accuracy, particularly with regards to the detection of pre-neoplastic lesion, which should be the primary focus for any cancer-screening regimen[5,10,11]. In view of these caveats, there is an urgent need for the development of novel and highly specific non-invasive biomarkers that can help improve early detection of CRC. In contrast, patients with advanced disease frequently receive expensive cytotoxic chemotherapeutic drugs, or targeted monoclonal antibodies- but in both instances the overall benefits derived from these treatments are relatively modest, and leave much to be desired[12]. Hence, alternative strategies are needed to improve the patient outcomes and to identify patients that will benefit truly from treatment with cytotoxic chemotherapies and intensive post-treatment surveillance protocols. Taken together, there is a clear unmet need for developing novel biomarkers for early diagnosis, prognosis, and deciphering predictive responses to chemotherapy in CRC patients. Herein, we focus this article on the clinical utility of these molecular signatures in the context of current patient care with CRC.
MiRNA biogenesis and function
MicroRNAs (miRNAs, or miRs) are a class of small single stranded non coding RNAs (ncRNAs) that are ∼18-25 nucleotides in length, which were first discovered in 1993 as a developmental regulator of Caenorhabditis elegans[13,14]. MiRNA genes are transcribed by RNA polymerase II in the nucleus of a cell to produce primary miRNA (pri-miRNA), which consists of a 5′-cap, at least one ∼70-nucleotide hairpin structure and a 3′-poly(A) tail. After transcription, pri-miRNAs are processed to precursor miRNA (pre-miRNA) by Drosha and its cofactor DGCR8 by removing the 5′-cap, the 3′-poly(A) tail and sequences flanking the hairpin structure. Pre-miRNAs are transported to the cytoplasm by exportin 5, and further processed by the ribonuclease III enzyme Dicer, to produce a mature miRNA strand and its complementary miRNA* strand[15,16]. The resulting 18-25 nucleotide long mature miRNA is ultimately integrated into the RNA induced silencing complex (RISC), and negatively regulates the expression of hundreds of target messenger RNAs (mRNAs) by translation inhibition or mRNA degradation, through base-printing to partially complementary sites on the target mRNAs, usually residing within the 3′ untranslated regions[17,18]. Therefore, relatively minor variations of miRNA sequences could have important biological consequences for the cell via regulation of the large number of target mRNAs.
Aberrantly expressed MiRNAs in Colorectal Cancer
Accumulating data have shown that miRNAs are aberrantly expressed in virtually all human cancers, including CRC; in which, these ncRNAs may function either as tumor suppressors (tsmiRs) or as oncogenes (oncomiRs), depending on the downstream target genes or pathways they regulate. The expression status of individual miRNAs may also associate with a variety of clinicopathological and prognostic factors in cancer patients, highlighting the central role miRNAs play in CRC pathogenesis and its progression[19-23].
MiR-143 and miR-145 were the first reported miRNAs associated with CRC development. The expression of these miRNAs was down-regulated in precancerous tissues and neoplastic colorectal tissues compared with normal mucosa[20]. Subsequent functional studies demonstrated that knockdown of expression of these miRNAs induced growth inhibition in CRC cell lines suggesting that miR-143 and miR-145 act as tumor suppressors in this malignancy [24-27].
Mir-17-92a cluster was firstly identified as a critical genomic locus that linked miRNAs to cancer pathogenesis. This cluster, arising from 13q, encodes six miRNAs (miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a-1), and has been frequently implicated by the high incidence of amplification in various types of human cancers. Diosdado et al reported that the increased expression of miR-17-92a seen during the progression of colorectal adenoma to carcinoma is associated with the gain of miR-17-92a locus and increased c-myc transcriptional activity[28]. Moreover, recent evidence further highlights the oncogenic function of miR-17-92a cluster in CRC development[29,30].
The expanded use of high-throughput microarray-based miRNA profiling platforms clearly revolutionized the elucidation of miRNA expression pattern analysis and in unraveling their biological role in various diseases. The first systemic and comprehensive array-based analysis evaluated 389 miRNAs expression levels using 84 CRCs and matched normal colonic tissues. This study identified 37 differentially expressed miRNAs in CRC, including the overexpression of miR-20a, miR-21, miR-106a, miR-181b, and miR-203 in the tumor tissues[31]. Volina and colleagues also performed large scale miRNA profiling using various types of cancerous and normal tissues, and identified a miRNA signature for solid cancers. This study demonstrated that miRNAs, including miR-17-5p, miR-20a, miR-21, miR-24, miR-106, miR-155, miR-203, and miR-223, were dysregulated in colon cancer[19]. In a more recent meta-analysis conducted by Luo et al., the authors consolidated data from 20 tissue-based studies and revealed that up-regulation of miR-31 and down-regulation of miR-145 were most frequently reported miRNAs (both in 8 different studies) in CRC tissues vis-à-vis matched normals[32].
The list of aberrantly expressed miRNAs in CRC tissues is continuously growing, and currently more than several hundred studies have been published on this topic. Furthermore, in many of the publications, a given miRNA biomarker has been published only once, and has not been validated or substantiated by follow-up studies. Therefore, in Table 1, we chose to only list those miRNA biomarkers/studies, which have been validated in two independent articles, hence making the evidence presented in this table more credible and possibly clinically relevant.
Table 1. A comprehensive list of dysregulated miRNAs in primary colorectal cancer tissues.
| miRNA | Target gene | Chromosomal location | Reference |
|---|---|---|---|
| Up-regulated miRNA | |||
| miR-16 | BMI1, WIP1, CDK6 | 13q14.2/3q25.33 | [151,152] |
| miR-17 | PTEN, DLC1, ZBP1, p130 | 13q 31.3 | [47,84,153,154] |
| miR-18a | Dicer1, KRAS, PTEN, CTGF, ATM | 13q 31.3 | [155,156] |
| miR-20a | PTEN, RUNX1,E2F1,HIF1A | 13q 31.3 | [25,47,84,153,157] |
| miR-21 | PTEN, PDCD, RECK, JAG1, BCL2, TIMP3, DKK2 | 17q 23.1 | [31,49,84,158-160] |
| miR-29a | KLF4, DNMT3a, DNMT3b | 7q 32.3 | [153,161,162] |
| miR-31 | FIH1, RhoBTB1, RASA1 | 9q 21.3 | [49,153,163-165] |
| miR-32 | PTEN | 9q 31.3 | [19,166-168] |
| miR-33 | Pim1, p53, HMGA2 | 22q 13.2 | [19,168] |
| miR-92a | PTEN, P63, RECK, DUSP10, ITGA5 | 13q 31.3/Xq 26.2 | [31,47,84,153,169,170] |
| miR-95 | SNX1, SGPP1 | 4p16.1 | [31,47,153,162,171] |
| miR-96 | FOXO1, RECK, RAD51, REV1 | 7q 32.2 | [25,47,162,168,172,173] |
| miR-106a | PTEN, TIMP2, PDCD4, p130, RUNX3 | Xq 26.2 | [19,31,47,162] |
| miR-106b | APC, RBL1, RBL2, CASP8 | 7q 22.1 | [47,84,162] |
| miR-130b | PPARγ, ITGB1, PTEN | 22q 11.21 | [162,174-176] |
| miR-135a | APC, MCL1, c-MYC, HOXA10 | 3q 21.1 | [31,34] |
| miR-135b | APC, MTSS1, LATS2, β-TrCP, LZTS1 | 1q 32.1 | [25,34,47,173] |
| miR-155 | MLH1, MSH2, MSH6, APC, TP53, INP1 | 21q 21.3 | [177,178] |
| miR-181b | CBX7, CYLD, AC9 | 9q 33.3 | [31,84,162,179,180] |
| miR-182 | NDRG1, RECK, MTSS1, SMAD4 | 7q 32.2 | [153,168,181,182] |
| miR-183 | IDH2, PDCD4, Dkk3, SMAD4 | 7q 32.2 | [25,84,153,168,183] |
| miR-191 | TIMP3 | 3p 21.31 | [19,180,184] |
| miR-196b | HOXC8, HOXB7 | 7p 15.2 | [153,185] |
| miR-200c | ZEB1, ZEB2, BMI1 | 12p 13.31 | [177,180] |
| miR-203 | ZEB2, BMI1, SOCS3, VEGFA | 14q 32.33 | [31,84,162,186] |
| miR-223 | E2F1, FOXO1, EPB41L3 | Xq 12 | [31,47,84,186,187] |
| miR-224 | SMAD4, CXR4, PHLPP1, PHLPP2 | Xq 23 | [47,153,162,168,188] |
| miR-335 | RB1, ROCK1 | 7q 32.2 | [31,86,185] |
| miR-493 | IGF1R | 14q 32.2 | [153,173] |
| miR-584 | ROCK1 | 5q 32 | [168,173,187] |
| Down-regulated miRNA | |||
| let-7a | KRAS, NRAS | 9q22.32/11q24.1/22q13.31 | [186,187] |
| miR-1 | BDNF, MET, SLUG, CCND2, SDF1 | 18q 11.2/20q 13.33 | [31,50,162,168,173,187,189] |
| miR-7 | YY1, XRCC2 | 9q 21.32 | [190,191] |
| miR-9 | CXCR4, BRCA1, TLN1, ITGB1 | 1q22/5q14.3/15q26.1 | [168,192] |
| miR-30a | DTL, HIF2α | 6q 13 | [25,31,162,168] |
| miR-30b | SIX, KRAS,PIK3D, BCL21 | 8q 24.22 | [187,193,194] |
| miR-30c | BCL9, SNAI1 | 1q 34.2/6q 13 | [162,187] |
| miR-34b/c | BTG4, MET, CDK4, CDK6, RUNX2 | 11q 23.1/ | [31,195,196] |
| miR-101 | EZH2, COX2, TET2 | 9q 24.1 | [26,187,197] |
| miR-124a | SLUG, STAT3, EZH2, IL6R | 8p23.1/8q12.3/20q13.33 | [25,198,199] |
| miR-126 | CXCR4, IRS-1, VEGF | 9q34.3 | [200-204] |
| miR-129 | BCL2, CDK6, SOX4 | 7q32.1/11p11.2 | [25,109] |
| miR-133a | LASP1, RFFL, FSCN1 | 18q 11.2/20q 13.33 | [50,115,162,168,205] |
| miR-133b | CXCR4, MET | 6p12.2 | [25,189,206] |
| miR-137 | LSD1, FMNL2, CDC42, CDK6 | 1p 21.3 | [168,207] |
| miR-139 | IGF1R, NR5A2 | 11q 13.4 | [162,168,173,208] |
| miR-143 | KRAS, DNMT3A, TLR2, MACC1 | 5q 32 | [84,162,186,187,209] |
| miR-145 | MUC1, KLF4, Fascin1, SOC2, YES1 | 5q 32 | [25,84,162,173,187,210] |
| miR-148a | BCL2, MMP7, ROCK1 | 7p 15.2 | [122,187,211] |
| miR-192 | BCL2, ZEB2, VEGFA | 11q 13.1 | [31,186,187,212] |
| miR-195 | BCL2, IKKα, TAB3, E2F3 | 17p 13.1 | [162,173,187,213,214] |
| miR-212 | MnSOD | 17p 13.3 | [26,215] |
| miR-215 | RB1 | 1q 41 | [31,84,175,186,187,212,216] |
| miR-218 | BMI1, CDK6, DKK2 | 4p 15.31/5q 34 | [217,218] |
| miR-320a | Rac1, β-catenin, NRP1 | 8p 21.3 | [84,219,220] |
| miR-342 | DNMT1, ID4, BMP7 | 14q 32.2 | [31,187,221] |
| miR-363 | GATA6, SIPR1, PDPN | Xq 26.2 | [168,173] |
| miR-375 | PI3K, PDK1, AEG1, IGF1R, YAP | 2q 35 | [168,187,216,222,223] |
| miR-378 | IGF1R, MYC, VEGFA | 5q 32 | [168,173,187,216,224] |
| miR-497 | IGF1R, BCL2, RAF1 | 17p 13.1 | [113,162] |
Dysregulated miRNA expression in colorectal adenomatous polyps
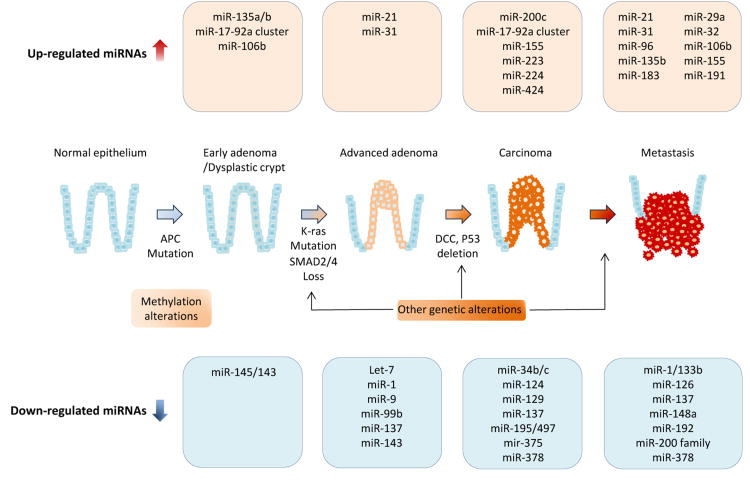
Colorectal adenomatous polyps, particularly advanced colorectal adenomas (colorectal polyps greater than 1 cm in diameter and/or villous component and/or severe dysplasia), are recognized as critical premalignant lesions for CRC development, and are the primary target lesions for CRC screening. Accumulating evidence indicates that a spectrum of dysregulated miRNAs is associated with tumorigenesis in colorectal adenomas. The following section summarizes some of the latest findings for miRNA expression alterations in colonic adenoma tissues, and a comprehensively tabulated list of such miRNAs is provided in Table 2.
Table 2. A comprehensive list of dysregulated miRNAs in colonic adenoma tissues.
| miRNA | Cases (n) | Controls (n) | Analysis Method | Reference |
|---|---|---|---|---|
| Up-regulated miRNA | ||||
| miR-21 | 18 | 18 | qPCR | [31] |
| miR-135a, -135b | 20 | 18 | qPCR | [34] |
| miR-21 | - | - | ISH | [159] |
| miR-17-92a cluster | 30 | 10 | qPCR | [28] |
| miR-21, -181b | 19 (SSA) | 20 | qPCR | [160] |
| miR-92a | - | - | copy number | [30] |
| miR-31, -135b | 41 | 55 | microarray | [33] |
| miR-1290 | - | - | ISH | [225] |
| Down-regulated miRNA | ||||
| miR-143, -145 | 65 | 65 | qPCR | [226] |
| miR-1,-9, -99b, -137 | 41 | 55 | microarray | [33] |
| miR-150 | - | - | qPCR | [227] |
SSA; sessile serrated adenoma, qPCR; quantitative polymerase chain reaction, ISH; in situ hybridization
MiR-21 is one of the most well-established oncogenic miRNAs, and it is frequently overexpressed in colorectal adenoma tissues compared with normal colonic mucosa. Schetter and colleagues demonstrated that the expression of miR-21 was significantly up-regulated in 18 adenoma tissues compared with the matched adjacent normal mucosa, while the expression of miR-21 in adenoma tissues was significantly lower than in CRC tissues[31]. Oberg and coworkers also performed comprehensive analysis using 41 adenoma and 55 normal tissue specimens, and found that miR-31 and miR-135b were overexpressed while miR-1, miR-9, miR-99b and miR-137 were downregulated in the adenoma tissues[33]. In accordance with this study, later studies confirmed that expression of miR-135a and miR-135b was upregulated in adenoma tissues in comparison with normal mucosa[34]. Increased expression or amplification of miR-92a in adenoma specimens have also been demonstrated in other independent studies[28,30]. MiR-21 has been shown to participate in the multistep process of colorectal tumorigenesis, by not only regulation of MAPK pathway, but also by associating with WNT/β-Catenin signaling by targeting PTEN, PDCD and DKK2[35-37]. MiR-135a/b and miR-17-92a cluster can also activate the WNT signaling pathway via suppression of APC or E2F1, respectively [34,38]. These evidences clearly highlight the functional role of a subgroup of miRNAs to be involved in the early phases of multi-step colorectal carcinogenesis via regulation of WNT/β-Catenin, and MAPK signal pathways.
Potential of MiRNAs as a disease biomarker
For any biomarker development endeavor, certain technical steps including sample collection and storage of clinical samples for extended periods of time (months or years) have been identified as potential sources of bias that can confound results[39-42]. In this context, miRNAs have a considerable potential for development as disease biomarkers compared to DNA and mRNAs that have been unsuccessfully exploited for decades. MiRNAs are remarkably small, can exist freely or in exosomal shells, are less vulnerable to RNase-mediated degradation, and can be easily extracted from a wide variety of biological and clinical materials, including archival formalin fixed paraffin embedded (FFPE) tissues and body fluids collected in clinical settings. In particular, RNase present in body fluids tends to degrade such biomolecules, particularly mRNAs, which posed the key impediment and success for developing mRNA-based biomarkers over the years. However, despite the presence of RNase activity in the blood, endogenous miRNAs tend to remain stable for long periods of time and can withstand several repeated freeze-thaw cycles[43-45]. Furthermore, accumulating evidence indicates that cancer cells secrete some miRNAs into systemic circulation[43,45]. This unique feature of miRNAs is one of the central reasons for the recent explosion of miRNA biomarker studies in the field of cancer research.
MiRNAs as non-invasive early detection biomarkers in CRC
Currently, large array of body fluids are being explored for their feasibility as non-invasive miRNA-based biomarkers for the early detection of various type of cancers. Considering that blood (serum/plasma) is one of the most easily accessible body fluids, it is most frequently used as a diagnostic material for the development of surrogate cancer biomarkers[46]. The first study of serum miRNA profiling in CRC reported 69 serum miRNAs that were differentially expressed in CRC patients, but not in healthy volunteers. Among these, 14 miRNAs were uniquely expressed in CRC patients and the authors suggested that these miRNAs may have high degree of CRC-specificity[44]. A follow-up study performed the first systematic and comprehensive analysis for miRNA biomarkers using plasma samples[47]. In the discovery phase of this study, miRNA profiling using tissue and plasma specimens from CRC and healthy volunteers detected 5 candidate miRNAs, and these markers were validated in a large set of plasma from 90 patients with CRC, 20 patients with gastric cancer, 20 patients with inflammatory bowel disease, and 50 healthy controls. The authors noted that the expression levels of both miR-17-3p and miR-92a were significantly over-expressed in plasma from CRC patients. MiR-92a expression status in plasma could discriminate patients with CRC from controls (sensitivity: 89%, specificity: 70%, AUC: 0.89). Intriguingly, the expression status of these over-expressed miRNAs was lowered following surgical removal of the tumor, indicating that the expression levels of these miRNAs in plasma was a reflection of underlying CRC. Furthermore, miR-92a expression was also capable of distinguishing CRC from other gastrointestinal diseases and tumors in this study, highlighting the specificity of miRNA biomarkers for each disease and suggested that plasma miR-92a expression levels may offer a valuable diagnostic biomarker for noninvasive screening of CRC[47]. The concept and results from this study contributed to significant progress in the CRC blood-based diagnostic marker research. Early diagnosis, including detection of adenomas, the precancerous lesions for CRC, is considered as a key concept for improving patient survival in CRC treatment. Huang and colleagues firstly conducted evaluation of miRNA expression analysis using 216 plasma specimens including specimens from 37 patients with colorectal adenomas[48]. This study successfully demonstrated that plasma miR-29a and 92a could be used to discriminate not only CRC, but also adenoma patients from healthy subjects. Hence the evaluation of plasma miR-29a and -92a status provided a strong rationale as novel non-invasive biomarkers for screening of CRC patients.
Recently, our group also discovered miR-21 as a potential biomarker for the early detection and prognosis in CRC patients using a large cohort of serum and matching tissue samples[49]. The study reported three key findings: First, miR-21 was identified as a novel secretory miRNA using supernatants from cultured CRC cell lines, and serum expression of this miR in CRC patients correlated positively with expression status in tumor tissues. Second, serum miR-21 expression levels could robustly identify not only patients with CRC (Sensitivity: 92%, Specificity: 81%, AUC: 0.92), but also patients with premalignant adenomatous polyps (Sensitivity: 81%, Specificity: 77%, AUC: 0.81). Third, serum expression status of miR-21 increased in a stage-dependent manner in CRC patients with stage I-IV disease, highlighting its potential use as a novel prognostic marker in CRC patients. Furthermore, we also demonstrated that serum miR-21 expression dropped significantly in post-operative serum samples from patients who underwent curative CRC surgery. Taken together, these data indicate that serum miR-21 is a promising biomarker that specifically reflects the tumor burden in the colon, and that serum miR-21 could be developed as a robust biomarker for early detection and prognosis in CRC patients. A subsequent study confirmed our findings and also reported the potential of plasma miR-21 as a diagnostic marker in CRC [50]. In this study during the discovery step, level of 380 miRNAs were determined using CRC tissues and corresponding normal mucosa from 30 patients, and the 4 most dysregulated miRNAs were then validated in tissue and plasma samples. This study also demonstrated that plasma miR-21 could be used to identify the patients with CRC from healthy volunteers with high sensitivity (90%) and specificity (90%). In line with these studies, another recent study using a large cohort of clinical specimens further reconfirmed that serum miR-21 could be a diagnostic biomarker for CRC patients [51].
In addition to blood, feces have also been widely used as potential substrate for developing non-invasive molecular screening tests for CRC patients. As mentioned earlier, considering the limitations of conventional stool-based screening tests including low sensitivity and specificity for detection of CRC and advanced adenoma, fecal DNA-based testing for CRC has been an area of active investigation since 1990s[52-56]. Although several studies have been reported fecal RNA-based assays[57-59], to date, no optimal method that offers superior detection accuracy compared to conventional screening tests (FOBT/FIT) have thus far been established. In contrast, exfoliated fecal colonocytes or tumor-secreted miRNAs are directly and continuously released from the tumors into intestinal lumen – providing a compelling rationale that a stool-based miRNA test might be better for CRC detection [60-62]. To date, several studies have attempted to identify the potential of fecal miRNAs as screening tools. Ahmed and colleagues evaluated miRNA expression status using fecal specimens from CRC, ulcerative colitis and healthy volunteers, and extended their study to establish a protocol for measuring miRNA levels using fecal specimens[60]. Using an approach to measure miRNA expression in fecal colonocytes, another laboratory extracted total RNA from fecal colonocytes isolated by immunomagnetic beads conjugated with epithelial cell adhesion molecule (EpCAM) monoclonal antibody, and evaluated expression status of 10 miRNAs from 197 CRC patients and 119 healthy volunteers[62]. This study showed that expression of miR-17-92a cluster and miR-135 was significantly higher in CRC patients than in healthy volunteers and suggested miRNA expression profile could be assessed in fecal colonocytes as a potential screening test in patients with CRC. Recently, the same group of investigators extended their research to further optimize their approach in the pursuit to develop a clinically viable assay[63]. In this study, the authors evaluated fecal miRNA expression from the samples extracted from the residual material collected in FOBT kits from CRC patients and healthy volunteers, and demonstrated that the combination of miR-106a status in feces with FOBT could reduce the rates of false negative results compared with FOBT alone to identify CRC patients.
Regarding the cell free miRNA in the feces, our research group was among the first to conduct a study analyzing fecal miRNA expression using a much simpler method[61]. We first confirmed the reproducibility of miRNA expression status using the specimens from fresh stool specimens and fecal samples collected in FOBT kits. Subsequently, we developed a one-step fecal miRNA extraction and amplification method (called direct miRNA analysis or DMA) and identified fecal miR-21 and miR-106a as potential candidates for fecal based CRC screening. In support of our data, another laboratory later also demonstrated the feasibility of using fecal miRNA expression as an early detection method of CRC[64]. The interesting feature of this latter study was evaluation of the miRNA status in feces using copy number dysregulation per ng of extracted stool RNA. This study revealed that combined analysis of stool miR-92a and miR-21 resulted in an acceptable sensitivity for the detection of CRC and precancerous advanced adenomas.
However, one of the major concerns for using miRNA expression analysis in body fluids, such as blood and stool, has been inconsistent and possible inadequate selection of internal controls for the normalization of miRNA expression across various published studies. Although many published studies have measured the commonly used reference gene, such as RNU6B, U6, 18S rRNA, and RNU43 as an internal control, these molecules are considered inadequate due to a non-excludable disease-specificity[65]. As a result, currently there is no established housekeeping miRNA gene for normalizing the expression of circulating miRNAs in body fluids [66,67]. Alternative normalization methods using Global means or Quantile normalization which could eliminate the need for endogenous controls are unrealistic, since such measures can only be achieved using large datasets. In these instances, the excessive cost constraints and requirements for large amounts of clinical specimens for such analysis, may limit the “practicality” of this approach. Considering the technical bias such as variability in serum RNA extraction and polymerase chain reaction amplification efficiencies, spike-in synthetics, non-human mature miRNA from Caenorhabditis elegans has been used recently for miRNA expression normalization in body fluids[68]. However, this method is not perfect, and can also be influenced by the sample quality. Further investigations for normalization of blood specimens are definitely needed, as we usher into the era on non-invasive diagnostics.
In addition, one of the key-limitations of fecal miRNA analysis is to overcome the complexity of fecal density and volume of sample needed for each assay. Since fecal conditions are more vulnerable to daily changes compared to serum/plasma, standardization of protocols for sample preparation are need to minimize the sample variability. In addition, candidates for the fecal miRNA test are roughly divided into three types: cell free miRNAs from fecal homogenates, exosomal miRNAs from fecal exosomes, and fecal colonocytes miRNAs. Taking into consideration these differences, further investigations and validation using standardized protocol in large cohort are also much required before such markers can be seriously considered for adaptation in the clinic for non-invasive CRC screening. Table 3 lists several non-invasive miRNA biomarkers with potential for clinical application of early detection of CRC.
Table 3. List of Diagnostic miRNA marker using blood or fecal specimen, which reported in Colonic adenoma and CRC.
| miRNA | Cases (n) | Controls (n) | Adenoma/CRC | Sensitivity (%) | Specificity (%) | AUC (95% CI) | Specimen Type | Dysregulation | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Blood-based biomarker | |||||||||
| miR-17-3p | 90 | 50 | CRC | 64 | 70 | 0.72 (0.63-0.80) | plasma | Up-regulated | [47] |
| miR-92a | 90 | 50 | CRC | 89 | 70 | 0.89 (0.83-0.94) | plasma | Up-regulated | [47] |
| miR-29a | 100 | 59 | CRC | 69 | 89.1 | 0.84 (0.79-0.90) | plasma | Up-regulated | [48] |
| 37 | 59 | Adenoma | 62.2 | 84.7 | 0.77 (0.67-0.87) | plasma | Up-regulated | ||
| miR-92a | 100 | 59 | CRC | 84 | 71.2 | 0.84 (0.78-0.90) | plasma | Up-regulated | [48] |
| 37 | 59 | Adenoma | 64.9 | 81.4 | 0.75 (0.64-0.86) | plasma | Up-regulated | ||
| miR-221 | 103 | 37 | CRC | 86 | 41 | 0.61 (0.49-0.72) | plasma | Up-regulated | [228] |
| miR-21 | 20 | 20 | CRC | 90 | 90 | 0.91 | plasma | Up-regulated | [50] |
| miR-601 | 90 | 58 | CRC | 69.2 | 72.4 | 0.75 (0.67-0.83) | plasma | Down-regulated | [229] |
| 43 | 58 | Adenoma | 72.1 | 51.7 | 0.64 (0.53-0.75) | plasma | Down-regulated | ||
| miR-760 | 90 | 58 | CRC | 80.0 | 72.4 | 0.79(0.71-0.86) | plasma | Down-regulated | [229] |
| 43 | 58 | Adenoma | 69.8 | 62.1 | 0.68 (0.58-0.79) | plasma | Down-regulated | ||
| miR-7, -93, -409-3p (panel) | 22 | 27 | CRC | 82 | 89 | 0.90(0.75-0.95) | plasma | - | [230] |
| miR-19a, -19b, -15b (panel) | 42 | 53 | CRC | 78.6 | 79.3 | 0.84 (0.76-0.92) | plasma | Up-regulated | [139] |
| miR-21 | 186 | 53 | CRC | 91.9 | 81.1 | 0.92 (0.87-0.96) | Serum | Up-regulated | [49] |
| 43 | 53 | Adenoma | 81.1 | 76.7 | 0.81(0.69-0.91) | Serum | Up-regulated | ||
| miR-21 | 200 | 80 | CRC | - | - | 0.8 | Serum | Up-regulated | [51] |
| 50 | 80 | Adenoma | - | - | 0.71 | Serum | Up-regulated | ||
| miR-92a | 200 | 80 | CRC | - | - | 0.77 | Serum | Up-regulated | [51] |
| 50 | 80 | Adenoma | - | - | 0.7 | Serum | Up-regulated | ||
| Feces-based biomarker | |||||||||
| miR-17-92 cluster | 197 | 119 | CRC | 69.5 | 81.5 | - | Fecal colonocyte | Up-regulated | [62] |
| miR-135 | 197 | 119 | CRC | 46.2 | 95 | - | Fecal colonocyte | Up-regulated | [62] |
| miR-21 | 19 | 10 | CRN (CRC+adenoma) | - | - | - | Feces | Up-regulated | [61] |
| miR-106a | 19 | 10 | CRN (CRC+adenoma) | - | - | - | Feces | Up-regulated | [61] |
| miR-144* | 35 | 40 | CRC | 74.3 | 87.2 | 0.83 | Feces | Up-regulated | [231] |
| miR-21 | 88 | 101 | CRC | 55.7 | 73.3 | 0.64 | Feces | Up-regulated | [64] |
| miR-92a | 88 | 101 | CRC | 71.6 | 73.3 | 0.78 | Feces | Up-regulated | [64] |
| miR-143 | 38 | 13 | CRC | - | - | - | Feces | Down-regulated | [232] |
| miR-145 | 38 | 13 | CRC | - | - | - | Feces | Down-regulated | [232] |
| miR-106a | 107 | 117 | CRC | 34.2 | 97.2 | - | Fecal colonocyte | Up-regulated | [63] |
MiRNA biomarkers for the identification of high-risk stage II patients
Accumulating evidence suggests that adjuvant chemotherapy following surgery significantly improves survival in stage III CRC patients, and is currently a well-accepted, standard treatment for these patients[69]. In contrast, in stage II CRC patients, surgical resection is highly effective; hence treating all stage II patients with adjuvant chemotherapy is still debatable[70-72]. This situation gets muddied further, since a significant proportion of Stage II CRC patients (25-30%) develop tumor recurrence and die because of disease progression. Currently, several clinical risk factors such as inadequately sampled lymph nodes, T4 lesions, intestinal obstruction, perforation, extramural venous invasion, or poorly differentiated histology are generally used to identify these high-risk subgroups in the clinic[71,73-76]. However these parameters are inadequate, highlighting the need for developing more accurate biomarkers for these high-risk populations of stage II CRC patients to improve their survival and spare the others from the toxicity of conventional chemotherapeutic drugs. The use of miRNAs as potential biomarkers using tissue samples in this area has been gradually expanding (Table 4). In one of the earliest studies, Schepeler and colleagues performed a systematic and comprehensive array-based miRNA profiling in stage II CRCs (including 37 microsatellite stable (MSS) and 12 microsatellite instability (MSI) tumor) using 315 miRNA probes[26]. These authors elegantly demonstrated that four miRNAs (miR-142-3p, miR-212, miR-151, and miR-144) could discriminate the microsatellite instability status of these patients with 84% accuracy (Sensitivity: 92%, Specificity: 81%). In addition, the expression level of miR-320 and miR-498 showed a significant correlation with recurrence free survival, and suggested that some of these miRNAs may have prognostic potential in CRC.
Table 4. List of prognostic markers responsible for poor outcome in stage II CRC patients.
| miRNA | N | Specimen Type | Dysregulation | Analysis Method | Reference |
|---|---|---|---|---|---|
| Tissue-based biomarker | |||||
| miR-320 | 37 (MSS) | Tissue | Down-regulated | qPCR | [26] |
| miR-498 | 37 (MSS) | Tissue | Down-regulated | qPCR | [26] |
| miR-21 | 42 | Tissue | Up-regulated | qPCR | [31,82] |
| miR-21 | 197 (Colon:130, Rectum:67) | Tissue (stroma) | Up-regulated | ISH | [233] |
| miR-29a | 59 | Tissue | Down-regulated | qPCR | [234] |
| miR-21 | 764 | Tissue | Up-regulated | ISH | [83] |
| miR-203 | 46 (African American) | Tissue | Up-regulated | qPCR | [235] |
| miR-20a, -21, -103a -106b, -143, -215 | 138 | Tissue | Up-regulated | Microarray, qPCR | [84] |
| miR-21 | 145 | Tissue | Up-regulated | qPCR | [158] |
| Blood-based biomarker | |||||
| miR-200c | 59 | Serum | Up-regulated | qPCR | [93] |
MSS; Microsatellite stable, qPCR; quantitative polymerase chain reaction, ISH; in situ hybridization
Currently, expression status of miR-21 in CRC tissues is the most well recognized miRNA biomarker for identifying high-risk stage II CRC patients. Schetter and coworkers identified miR-21 expression in primary tumor tissues as a prognostic marker in stage II CRC[31]. This group extended their study to evaluate the expression status of 23 inflammatory genes and discovered that combination of inflammatory risk score and miR-21 expression was a better predictor of prognosis than either individually[82]. The largest miR-21 cohort study in stage II CRC patients was reported from a research group in Denmark[83]. This population-based study conducted in situ hybridization using FFPE specimens from 520 stage II colon cancer patients, and showed that increased miR-21 expression levels in tumor tissues correlated significantly with poor recurrence-free cancer specific survival (HR=1.41, 95% CI:1.19-1.67). In another recent study, the investigators utilized 1849 miRNA probes and performed array-based profiling, in 40 paired stage II colorectal tumors and adjacent normal mucosa tissues. The study revealed 35 differentially expressed miRNAs between CRC vs. normal mucosa tissues. In the second phase of this study, expression analysis using quantitative polymerase chain reaction (qPCR) was performed to confirm differential expression of these miRNAs in 138 stage II patients. In this instance, six-miRNA-based classifiers (miR-20a-5p, miR-21-5p, miR-103a-3p, miR-106a-5p, miR-143-5p and miR-215) were used to discriminate high- and low-risk of disease progression in this group. In the final step, the performance of these classifiers were validated using independent cohort of samples (460 stage II patients), and concluded that these six-miRNAs emerged as promising prognostic and predictive tools for disease recurrence in patients with stage II CRC[84].
MiRNA biomarkers for the identification of distant metastasis in advanced CRCs
Distant metastasis is a major cause of death in patients with advanced CRC. Although approximately 70-80% of new CRC cases undergo potentially curative surgery, 40% of these patients develop metachronous metastatic disease[85]. Systemic chemotherapy plays a central role in treating such metastatic CRC (mCRC) patients, and the advances in chemotherapeutic treatments over the last few years have somewhat enabled management of mCRC, if the site of metastasis (especially lung, or liver) is restricted and accessible for local surgical treatment. In view of these clinical challenges, earlier detection of distant metastasis and selective criteria regarding which individuals would benefit the most from invasive treatments is essential for improving their long-term survival.
Although majority of published articles to date have focused on evaluation of miRNA expression in primary tumor specimens (Table 5), Vickers and colleagues were the first to investigate the differential miRNA expression between primary tissues and liver metastatic tissues[86]. This study evaluated 9 previously reported metastasis-associated miRNAs (miR-335, 206, -135a, -146a, -146b, -10b, -21, let-7a, let-7b) using 78 samples including 19 paired primary tumors with corresponding liver metastases. The authors demonstrated that a signature consisting of miR-21, -135a, -206, -335, and let-7a in primary tissues had a specificity of 87% and sensitivity of 76% for detecting the presence of metastasis in CRC. Similarly, another recent study performed miRNA-based profiling of 78 tissue specimens (23 normal colonic mucosa, 31 primary cancerous tissues, and 24 liver metastatic tissues) from 46 CRC patients[87]. It was shown that 3 miRNAs, miR-122, miR-146a and miR-210, were dysregulated in metastatic vs. primary cancer tissues.
Table 5. List of distant metastasis associated miRNAs in CRC.
| miRNA | N | Specimen Type | Dysregulated | Type of predictive metastasis | Reference |
|---|---|---|---|---|---|
| Tissue-based biomarker | |||||
| miR-21 | 156 | Tissues (primary) | Up-regulated in primary tissue | Hepatic metastasis | [178] |
| miR-125b*, - 139-3p, -150*, 1179 | - | Tissues (primary) | Up-regulated in primary tissue | Hepatic metastasis | [236] |
| let-7a, miR-21, -135a, -335 | 38 | Tissues (primary, metastasis) | Up regulated in metastatic tissue | Hepatic metastasis | [86] |
| miR-206 | 38 | Tissues (primary, metastasis) | Down regulated in metastatic tissue | Hepatic metastasis | [86] |
| miR-372 | 144 | Tissues (primary) | Up-regulated in primary tissue | Hepatic metastasis | [237] |
| miR-22 | 86 | Tissues (primary) | Down-regulated in primary tissue | Hepatic metastasis | [238] |
| miR-200c, -141 | 108 | Tissues (primary, metastasis) | Up-regulated in metastatic tissue | Hepatic metastasis | [92] |
| miR-96, -135b | 52 | Tissue (primary) | Up-regulated in primary tissue | Hepatic metastasis | [172] |
| miR-144 | 137 | Tissue (primary) | Down-regulated in primary tissue | Hepatic metastasis (Metachronous) | [239] |
| miR-199a-3p | 92 | Tissue (primary) Tissues (primary, | Up-regulated in primary tissue | Hepatic metastasis | [240] |
| miR-122 | 12 | metastasis) | Up regulated in metastatic tissue | Hepatic metastasis | [241] |
| miR-625 | 96 | Tissue (primary) | Down-regulated in primary tissue | Hepatic metastasis | [242] |
| miR-146, -201 | 55 | Tissues (primary, metastasis) | Up-/Down-regulated in metastatic tissue | Hepatic metastasis | [87] |
| miR-191 | 136 | Tissue (primary) | Up-regulated in primary tissue | Hepatic metastasis | [243] |
| miR-340 | 19 | Bone Marrow DTC | Down-regulated in Bone Marrow DTC | Hepatic metastasis | [244] |
| miR-214 | 99 | Tissue (primary) | Down-regulated in primary tissue | Hepatic metastasis | [245] |
| miR-185 | 50 | Tissues (primary) | Up-regulated in primary tissue | Distant metastasis | [246] |
| miR-133b | 50 | Tissues (primary) | Down-regulated in primary tissue | Distant metastasis | [246] |
| miR-9 | 25 | Tissues (primary) | Up-regulated in primary tissue | Distant metastasis | [247] |
| miR-139 | 35 | Tissue (primary) | Down-regulated in primary tissue | Distant metastasis | [86,208] |
| miR-103, 107 | 99 | Tissue (primary) | Up-regulated in primary tissue | Distant metastasis | [86,248] |
| miR-92a | 82 | Tissue (primary) | Up-regulated in primary tissue | Distant metastasis | [170,238] |
| miR-137 | 102 | Tissue (primary) | Down-regulated in primary tissue | Distant metastasis | [92,249] |
| miR-155 | - | Tissue (primary) | Up-regulated in primary tissue | Distant metastasis | [170,250] |
| miR-32 | 35 | Tissue (primary) | Up-regulated in primary tissue | Distant metastasis | [167,172] |
| miR-183 | 94 | Tissue (primary) | Up-regulated in primary tissue | Distant metastasis | [183,239] |
| miR-138 | 187 | Tissue (primary) | Down-regulated in primary tissue | Distant metastasis | [249,251] |
| miR-29a | 85 | Tissue (primary) | Up-regulated in primary tissue | Distant metastasis | [161,240] |
| miR-25 | 186 | Tissue (primary) | Up-regulated in primary tissue | Distant metastasis | [241,252] |
| miR-126 | 92 | Tissue (primary) | Down-regulated in primary tissue | Distant metastasis | [200,251] |
| Blood-based biomarker | |||||
| miR-21, 141 | 102 | Plasma | Up-regulated | Distant metastasis | [244,253] |
| miR-29a | 40 | Serum | Up-regulated | Hepatic metastasis | [200,254] |
| miR-21 | 186 | Serum | Up-regulated | Distant metastasis | [49,245] |
| miR-200c | 182 | Serum | Up-regulated | Distant metastasis | [93] |
| miR-155, -200c, -210 | 15 | Serum | Up-regulated | Distant metastasis/Local recurrence | [126,253] |
DTC; Disseminated tumor cell
One of the key molecular steps in the process of distant metastasis includes epithelial-to-mesenchymal transition (EMT) in primary tumor and mesenchymal-to-epithelial transition (MET) in metastasized location[88-91]. Our group recently demonstrated that miR-200c/141 cluster was up-regulated in metastasized tissue via EMT/MET process when we analyzed 54 paired primary CRC tissues and matched liver metastasis specimens[92]. Furthermore, we investigated the expression of miR-200c in serum specimens from these patients and showed that up-regulated miR-200c in metastatic tissues reflected the expression status in serum specimens[93]. High serum miR-200c expression was significantly correlated with lymph node metastasis and distant metastasis, and was an independent predictor of tumor recurrence and an independent prognostic maker for CRC. In view of these results, it is becoming more apparent that miRNAs play a crucial role in the metastatic process in CRC, and several miRNAs could be used as promising clinical tools for the identification of patients with micrometastasis who and will require intensive monitoring following surgery.
However, almost all of the studies published to date have focused on the predictive biomarkers for distant metastasis using resected primary tissues or preoperative serum/plasma samples, but there are no reports for the significance of monitoring biomarkers for metachronous metastasis using large cohort serum/plasma samples. In future, further investigations are required for a careful determination of the clinical significance of already published miRNAs as recurrence-monitoring biomarkers, such as CEA and CA19-9.
MiRNAs as predictive biomarkers for response to treatment
Predicting therapeutic response to chemotherapy in CRC patients
Unlike many other human cancers, the treatment options for advanced CRC patients have improved remarkably due to the development of several novel drugs over the last decade[94]. Traditional chemotherapeutic treatment for CRC has been based on 5-fluorouracil (5FU), with the more recent introduction of other cytotoxic agents, such as irinotecan and oxaliplatin. Furthermore, new monoclonal antibodies targeting the vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) have proven to be effective in combination with cytotoxic chemotherapy, or as single agents for the treatment of mCRC[95-97]. Notwithstanding considerable therapeutic advances, the prognosis for the patients with unresectable CRC still remains poor, with the median overall survival of only 18-21 months[98]. One of the best characterized major obstacles in treating CRC patients with unresectable disease CRC is the intrinsic or acquired resistance to chemotherapy. Understanding the molecular mechanisms of drug response through alterations in miRNA expression patterns could provide crucial additional information on patient's potential response, or the lack thereof, to a specific chemotherapeutic regimen prior to initiation of the therapy.
To date, growing evidence suggests that aberrant miRNA expression could play pivotal role in predicting resistance to chemotherapeutic drugs in CRC patients. Using a series of in vitro models for CRC, several studies have thus far demonstrated deregulation of several miRNAs to 5-fluorouracil resistance (miR-10b[99], miR-19b[100], miR-20a[101], miR-21[102-104], miR-23a[105],miR-31[106], miR-34[107,108], miR-129[109], miR-140[110], miR-145[111], miR-192/-215[112], miR-497[113]), irinotecan resistance (miR-21[104], miR-451[114]), as well as resistance to oxaliplatin (miR-20a[101], miR-21[104], miR-133a[115], miR-143[116], miR-153[117], miR-203[118], and miR-1915[119]). In Table 6, we summarized the published data related to the potential of predictive biomarker for chemosensitivity using clinical samples in CRC. In clinical settings, Nakajima et al. were the first to demonstrate the significance of miRNA dysregulation in relation to 5FU-based antimetabolite S-1 chemosensitivity, and identified that overexpression of let-7g and miR-181b in CRC tissues was significantly associated with response to S-1 based chemotherapy in CRC[120]. A recent study conducted more systematic and comprehensive analysis focused on response to chemotherapy by performing real time-PCR based profiling of 742 different miRNAs using the 26 cancer tissues with or without response to first-line capecitabine and oxaliplatin (XELOX)/5FU and oxaliplatin (FOLFOX) treatment[121]. In the screening phase of this study, high expression of miR-27b, miR-181b, and miR-625-3p was significantly associated with poor response to first-line treatment. In the second phase, an independent validation of these results was performed on the primary tumor tissues of 94 mCRC patients, and high expression of miR-625-3p was confirmed to be associated with poor response to XELOX/FOLFOX treatment. Our group also performed the screening of 21 candidate miRNAs, and revealed low-expression of miR-148a in primary tissues associated significantly with worse survival and poor therapeutic response to 5-FU and oxaliplatin based chemotherapy in advanced CRC patients[122].
Table 6. List of miRNA biomarker for resistance to chemotherapy in CRC.
| miRNA | N (Stage) | Specimen | Treatment | Neoadjuvant/Adjuvant/ Palliative | Dysregulation and response/prognosis | Reference |
|---|---|---|---|---|---|---|
| Tissue-based biomarker | ||||||
| let-7g, miR-181b | 46 | Tissue | S-1 +/- CDDP | Palliative | Up-regulated in poor response | [120] |
| miR-21 | 56(II/III) | Tissue (primary) | 5FU-based chemotherapy | Adjuvant | Up-regulated in poor prognosis | [31] |
| miR-150 | 352 (II/III) | Tissue (primary) | 5FU +/- LV/levamisole/CDDP | Adjuvant | Down-regulated in poor prognosis | [227] |
| miR-21 | 42 (II/III) | Tissue (primary) | FOLFOX4 | Neoadjuvant | Up-regulated in poor response | [255] |
| miR-126 | 89(IV) | Tissue (primary) | XELOX | Palliative | Down-regulated in poor response | [121] |
| miR-148a | 273 (II/III/IV) | Tissue (primary) | 5FU/5FU+oxaliplatin | Adjuvant/Palliative | Down-regulated in poor prognosis | [122] |
| miR-625-3p | 94 (IV) | Tissue (primary) | (XELOX or FOLFOX) | Palliative | Up-regulated in poor response/prognosis | [121] |
| miR-215 | 125(II/III) | Tissue (primary) | 5FU-based chemotherapy | Adjuvant | Down-regulated in poor prognosis | [256] |
| miR-21 | 301(II/III/IV) | Tissue (primary) | 5FU-based chemotherapy | Adjuvant/Palliative | Up-regulated in poor prognosis | [158] |
| miR-200b, -200c, -141, -429 | 127(I/II/III) | Tissue (primary) | Fluoropyrimidines | Adjuvant | Down-regulated in poor prognosis | [257] |
| miR-181a | 80(IV) | Tissue (primary) | anti-EGFR | Palliative | Down-regulated in poor prognosis | [123] |
| let-7c, miR-99a, -125b | 183(IV) | Tissue (primary) | anti-EGFR | Palliative | Down-regulated in poor prognosis | [124] |
| Blood-based biomarker | ||||||
| miR-106a, -130b, -484, -148, -27b, -326 | 150 (IV) | Plasma | 5FU+Oxaliplatin | Adjuvant | Up-regulated in poor response or prognosis | [125] |
| miR-20a, -130, -145,-216, -372 | 253 | Plasma | - | - | - | [258] |
| miR-19a | 72 (IV) | Serum | FOLFOX | Palliative | Up-regulated in poor response | [259] |
5FU;5-fluorouracil, CDDP; cisplatin, LV; leucovorin, FOLFOX; 5FU/LV/oxaliplatin, XELOX; capecitabine/oxaliplatin, EGFR; epidermal growth factor receptor
With regards to the predictive miRNA biomarkers for sensitivity to molecularly targeted therapies, a recent study demonstrated that decreased expression of miR-181a in micro-dissected tumor tissues associated with poor progression free survival (PFS) and overall survival (OS) in mCRC patients receiving EGFR targeted therapy[123]. Published around similar time, Cappuzzo and colleagues conducted a study by analyzing 2-different cohorts of mCRC patients treated with anti-EGFR based therapy and performed miRNA arrays for their discovery step. The authors identified dysregulation of a unique miRNA cluster (let-7c/miR-99c/miR-125b), which influenced the PFS and OS of patients with mCRC treated with cetuximab or panitumumab therapy[124].
Although almost all of miRNA biomarker studies for predicting response to chemotherapy are based on the analysis of archival “primary tumor tissues”, one of the major limitations of this tissue-based approach is heterogeneity, which characterizes most advanced cancers. Intra-tumoral heterogeneity might carry different expression profiles in primary tissues, and heterogeneity even exists between metastases within the same patient, such as inter-metastatic heterogeneity. A biopsy or tissue section from one part of a solitary tumor could not represent the overall molecular characteristics of harboring malignant disease. In addition, due to practical and ethical concerns, it is challenging to obtain repeated biopsies, especially metastatic tumors, during disease progression or prior to initiation of chemotherapy. Due to these issues, it has been difficult to characterize the molecular characteristics of growing tumor cells in patients. Therefore, evaluating miRNA expression from serum/plasma in patients with unresectable CRC may be potentially preferable for predicting response to chemotherapy.
Kjersem and colleagues conducted the first systematic and comprehensive array-based analysis by evaluating the expression of 742 miRNAs in plasma samples from 24 metastatic CRC patients (12 responders and 12 non-responders) before oxaliplatin-based chemotherapy[125]. In the validation step, the top differentially expressed 32 miRNAs between responders and non-responders were selected for further analysis using pre-treatment plasma samples from 150 CRC patients. High expression of miR-27b, miR-148a, and niR-326 in pre-treatment plasma was significantly correlated with response to oxaliplatin-based chemotherapy, and associated with poor progression-free survival. Likewsie, Chen et al. utilized a unique approach for dynamic monitoring of serum miRNA levels (miR-155 -200c, and -210) during a 3-year follow up period with adjuvant FOLFOX therapy with cetuximab in 15 patients with CRC patients, and suggested that re-elevation of serum miR-155 levels after surgery and chemotherapy might be a sign of chemoresistance in colon cancer[126]. Recently, as a non-invasive biomarker for response to chemotherapy, analysis of KRAS or BRAF mutations in cell free DNA in plasma/serum is gathering attention in metastatic CRC patients. In view of these results, it is becoming more apparent that miRNAs could be used as non-invasive biomarkers for response to chemotherapy in advanced CRC patients.
Predicting therapeutic response to chemo-radiotherapy or prognosis after treatment in rectal cancer
With regards to the treatment of advanced rectal cancer, the concept of combined modality therapy, which integrates surgery, chemotherapy, and radiotherapy, has been used for improving the odds for surgical operation and lowering local recurrence rates considerably over the last decades. In particular, preoperative chemo-radiotherapy (CRT) has become the preferred treatment modality for locally advanced rectal adenocarcinoma with a complete pathological response in up to 30% of patients[127,128]. However, at the same time, combined modality therapy including pelvic radiotherapy for rectal cancer can result in a number of acute and late toxicities. In addition, distant recurrence remains the major cause of mortality in patients with preoperative CRT followed by curative resection. Therefore, there is a clinical need to establish molecular biomarkers to predict response to pre-surgical chemo-radiation and/or to predict recurrence after surgery, which may spare the patients from the adverse toxicity and expense, who are poor candidates for such treatments[129,130].
Alteration of miRNA expression associated with clinical response to preoperative CRT and disease outcome in rectal cancer has also been explored in several studies (Table 7). In one of the first studies, an association of miRNA dysregulation and response to CRT in rectal cancer was demonstrated [131]. In this study, the investigators collected the tumor biopsy specimens from 35 patients with rectal cancer before CRT, and in parallel collected tumor biopsies from 31 patients two weeks after starting preoperative CRT. This study revealed that up-regulation of miR-125b and miR-137 after CRT was associated with worse response to CRT. These researchers later extended this study by doing a comprehensive analysis of differentially expressed miRNAs with or without response to CRT, using two sets of biopsy samples (10 responders and 10 non-responders to neoadjuvant CRT for rectal cancer[132]. The authors identified 8 significantly differentially expressed miRNAs in rectal tumors, and highlighted the potential use of miRNA expression status as a predictive biomarker for response to neoadjuvant CRT in rectal cancer. More recently, another study performed genome-wide miRNA profiling on 12 established CRC cell lines that were made resistant to CRT and confirmed the function of four miRNAs (let-7g, miR-132, miR-224, and miR-320a) as potential miRNA targets for resistance to CRT[133]. During the validation step, 128 pre-therapeutic biopsy specimens were used to analyze correlation between expression levels of these miRNAs and disease free survival (DFS). It was quite reassuring to observe that high expression of let-7g was significantly associated with better DFS in this well conducted study.
Table 7. List of miRNA biomarker for response to chemoradiotherapy in Rectal cancer.
| miRNA | N (Stage) | Neoadjuvant Treatment (Chemotherapy/Radiation) | Specimen Type | Dysregulation | Primary Endpoint | Reference |
|---|---|---|---|---|---|---|
| miR-125b, -137 | 35(II/III) | Capecitabine | Pre- and post-therapeutic Tissue | Up-regulated expression change after treatment in poor response | Response | [131] |
| 50.4Gy | ||||||
| miR-145 | 40 | 5FU | Pre- and post-therapeutic Tissue | Down-regulated of post-treatment tissue in poor response | Response | [260] |
| 50.4Gy | ||||||
| miR-215,-190, -29b-2 let-7e, miR-196b, -450a, -450b-5p, -99a | 20 (II/III) | 5FU or Capecitabine | Pre-therapeutic Tissue | Up-regulated in poor response Down-regulated in poor response | Response | [132] |
| 50.6 Gy | ||||||
| miR-1183, -483-5p, -622, -125a-3p, -1224-5p, -188-5p, -1471, -671-5p, -1909*, -630, -765 | 38(III) | Capecitabine + l-OHP | Pre-therapeutic Tissue | Up-regulated in poor response | Response | [261] |
| miR-1274b, -720 | 45 Gy | Down-regulated in poor response | ||||
| Let-7g | 128 (II-IV) | 5FU with/without l-OHP | Pre-therapeutic Tissue | Down-regulated in poor prognosis | prognosis (DFS) | [133] |
| 50.4 Gy | ||||||
| miR-16,-153, -519c, -561,-590 | 12 | - | Pre-therapeutic Tissue | - | Response | [262] |
5FU;5-fluorouracil, l-OHP; oxaliplatin, DFS: Disease Free Survival
One of the limitations of current studies exploring the potential relevance of miRNAs for predicting response to chemo- or chemo-radiation therapies is the lack of specificity for each treatment option. In order to realize the concept of “individualized/personalized treatment” of CRC patients in the near future, there is clearly an unmet need for the availability of drug-specific, regimen-specific, and treatment-specific biomarkers that can help predict the response to treatment specifically (such as 5FU, irinotecan, oxaliplatin, or radiation). Furthermore, validation using large cohort samples in well-designed prospective clinical studies is needed to confirm the significance of already discovered miRNAs as potential predictive biomarkers for therapeutic response in CRC.
Expert Comment and Five-year view: What's next step and where should we go?
At this moment, unequivocal body of literature exists suggesting a strong potential for the utilization of miRNAs as oncological biomarkers in various human cancers, particularly CRC. This is owing to several inherent advantages of using miRNAs as discussed previously, but most importantly, the stability of miRNAs in various clinical specimens remains at the pinnacle of attention for their development as clinically useful biomarkers. In particular, direct identification of circulating miRNAs would be expected as liquid biopsies that can provide information for diagnosis, prognosis and predictive responses to treatment in CRCs without the need for tumor-tissue biopsies. However, the paradigm of developing liquid biopsies remains very optimistic. In order to realize the fruits of efforts that have been put forth to date, we must collectively and collaboratively address several key issues, before any of these biomarkers can be translated for any meaningful clinical use.
One of the major issues we might need to overcome relates to the methodology for measuring miRNA expression levels. To date, as opposed to ‘absolute quantitation’, almost all published studies have evaluated miRNAs levels by qPCR using ‘relative quantitation’, approach by using cycle threshold (Ct) values. Therefore, the development of absolute quantitative method will be critical for inter-laboratory comparisons and standardization, before such assays can be faithfully used in different laboratories in clinical settings. Although several attempts have been made to establish the method of absolute quantification, especially using blood samples, a recent study elegantly demonstrated the absolute quantities of circulating miRNAs in classical Hodgkin lymphoma using copy number, which was calculated on the basis of known copy numbers of cel-miR-39 spiked-in in plasma[134]. This study showed a high correlation between relative and absolute quantification values for all evaluated miRNAs. This intriguing method allows the absolute value in plasma miRNA to be quantified by eliminating the technical bias of RNA extraction, and has the potential to be implemented in diagnostic laboratories. Furthermore, Droplet Digital PCR, a next generation qPCR method, provides absolute quantification of nucleic acids without the requirement of standard curve, and measures copy number changes of DNA and RNA targets with high sensitivity compared to qPCR methods [135,136]. Combining cel-miR-39 spiking approach and new PCR technology should, or might, help quantification of miRNA levels more close to absolute values, and may allow identification of low-abundance miRNA biomarkers in plasma.
Another major limitation of blood-based miRNA biomarkers for clinical use is the disease specificity. The majority of diagnostic miRNAs identified in CRC have also been recognized to be altered in other types of human cancers, and non-cancerous diseases. For example, miR-92a, which was the firstly reported miRNA as a plasma-based diagnostic marker in CRC, revealed the feasibility of exploiting plasma-based early detection biomarkers in breast cancer and coronary artery disease[137,138]. Recently, several investigators have made an attempt to address this limitation and provided some clues for overcoming this limitation. First major break-through came from studies focusing on the tumor-secreted miRNAs for diagnostic non-invasive biomarkers in CRC, in which the investigators made direct comparison between tumor tissue and blood expression status to clarify the interaction and origin of circulating miRNAs. Our research group demonstrated for the first time that the miR-21 up-regulation in CRC tissues corresponded with serum levels in large cohort samples, and showed that miR-21 was secreted from CRC cancer cells and confirmed this finding with positive correlation of miR-21 expression between tissue and serum[49]. Ng and colleagues also performed real-time-PCR based miRNA profiling using plasma, corresponding cancer and adjacent normal mucosal tissues from CRC patients, along with plasma from five healthy volunteers in their discovery step[47]. Such an approach aimed at evaluating tissue and matching blood specimens could clarify the correlation of expression status between different types of matched tissue specimens, and may further assist in addressing the issue of tumor specificity in future. Second major breakthrough came with advances in next generation sequencing technologies. Previous studies heavily relied upon the use of microarray-based technologies which often do not include the complete list of growing miRNAs, and hybridization-based non-quantitative techniques can yield data that may fail subsequent validation steps[47,139,140]. Advent of sequencing technologies, such as next-generation sequencing (NGS) for miRNA profiling in biomarker research, now allows the measurement of absolute abundance over dynamic range which was otherwise not possible using conventional microarray technology in the past. In addition, the use of NSG will permit identification of novel miRNAs, miRNA sequence changes, and miRNA variants such as isomiRs[141]. To date, isomiRs are explained to be derived from variable cleavage of Drosha and Dicer through pre-miRNA processing and they differ from each other only by a few nucleotides at the 5- and 3- ends[142-145]. However, more recent evidence suggests that these isomiRs have different physiological roles[146]. Furthermore, it has been suggested that the there is a tissue-dependent preference for isomiRs[143]. Collectively, these new emerging functional roles of isomiRs and the new profiling technologies will allow potential discovery of novel cancer-specific biomarkers. Final potential breakthrough comes from the concept of tumor-derived exosomes. Although previous studies suggested the feasibility of exosomal miRNAs as non-invasive biomarkers in oncology, circulating exosomes can also originate from other organs, and can compound lack of specificity of a given miRNA biomarker. However, Taylor and colleagues have recently proposed an elegant method for isolating circulating exosomes through modification of magnetic activated cell sorting procedure using anti-epithelial cell adhesion molecule (EpCAM), and revealed that the miRNA signature obtained from these tumor-derived (epithelia-derived) exosomes parallels that of the miRNA expression profiles of corresponding tumor tissues in ovarian cancer and lung cancer[147]. Such unique approaches for capturing exosomal miRNAs may be another pivotal step that will assist in enhancing the disease specificity of newly biomarkers in future.
Additional major limitations of miRNA biomarker studies for clinical use is individual variability in the expression of these molecules, due to various confounding factors associated with lifestyle factors. Although recent studies have suggested the potential for influencing miRNA expression profiles as a function of age, sex, and race, there is lack of robust data on the expression patterns of miRNAs as it relates to various life-style associated factors, such as dietary factors, smoking, and alcohol consumption – all of which may have profound effect in modu at ing miRNA expressio n, particularly in the body fluids[148-150]. To realize the actual clinical use of miRNA biomarkers in CRC treatment, further investigations are needed to clarify the effect of these factors on the miRNA expression patterns in body fluids.
Finally, MiRNA research has expanded remarkably over the past few years and accumulating evidence suggest the potential use of miRNA as oncological biomarkers in CRC. Although these results hold tremendous promise, majority of the studies have been based on the analysis of clinical tissues from retrospective patient cohorts and lack of external validation. Therefore, prospective, multicenter, large patient cohort studies are required to prove the clinical significance and promise miRNAs currently hold as diagnostic, prognostic and predictive biomarkers for CRC. Although none of us have a crystal ball to predict what an ideal “cancer assay” will look in the future, but it is logical and very likely that no single biomarker may be sufficient to capture the disease heterogeneity that underlies most CRCs. As a result, it is possible that for clinical purposes, we may end up using a panel of miRNAs rather than a single miRNA, or even combine different types of biomarkers or other available tests, such as CEA, and FOBT – all in an effort to enhance the sensitivity and specificity of these analytical approaches, and making an earnest effort to providing a true “personalized treatment” to every cancer patient in the future.
Key issues.
MicroRNAs (miRNAs) are a class of small, single stranded, noncoding RNAs (ncRNAs), and ever since their initial discovery in 1993, enough evidence has been gathered that implicates their role in cancer pathogenesis due to their ability to post-transcriptionally regulate the expression of various oncogenes and tumor suppressor genes.
Dysregulated expression of many miRNAs regulates the expression of hundreds of growth regulatory genes and pathways that are critical in the multistep model of colorectal carcinogenesis, and other human cancers.
Since the recognition that miRNA expression is stable, immune to RNase-mediated degradation, has allowed exploration of these molecular entities in a variety of biological fluids including archival tissues for biomarker studies.
Early diagnosis, including detection of premalignant adenomas, is considered as a key concept for improving patient survival in CRC treatment. Emerging evidence suggests promising potential of miRNAs (miR-21, miR-92a, miR-106a, etc.) as potential non-invasive biomarkers for non-invasive screening in blood (plasma/serum) and fecal specimens.
In stage II CRCs, surgical resection is highly effective; hence treating all patients with stage II CRC with adjuvant chemotherapy is still debatable. However a significant proportion of Stage II CRC patients develop recurrence and the identification of sensitive miRNA biomarkers in such high-risk populations is of key importance.
Early detection of distant metastasis and selective criteria regarding which individuals would benefit most from invasive treatments is essential for improving long term survival. Several lines of research suggests that miRNAs are intimately involved in the metastatic process in CRC and some of these miRNAs could be used as promising clinical tools for identifying patients with micro-metastasis who will require intensive monitoring after surgery.
Despite the progress of treatment options for advanced CRC during last decade, major obstacle is intrinsic and acquired resistance to chemotherapy and/or radiotherapy, necessitating the need to develop predictive biomarkers for response to such therapeutic treatments to facilitate personalized treatment of CRC patients. Several studies have identified alterations in miRNA expression (miR-21, miR-145, miR-148, etc.) which could provide crucial additional information on patient's sensitivity to chemotherapy before starting the therapy, and in supporting development of novel therapeutic strategies for enhancing the sensitivity to chemotherapeutic treatments.
Although miRNA biomarker studies have grown dramatically in numbers in the last few years, future prospective studies are essential in addressing limitations of retrospective trials, as we usher into the next era of miRNA biomarkers that are optimal for clinical practice.
Acknowledgments
Grant support: The present work was supported by grants R01 CA72851, CA181572 and CA184792 from the National Cancer Institute, National Institutes of Health, a pilot grant from the Charles A Sammons Cancer Center, and funds from the Baylor Research Institute.
Papers of particular interest, published recently, have been highlighted as: * of interest, or
** of considerable interest.
**23 Shetter AJ, et al. JAMA 2008; 299(4): 425-36.
This research group performed the first largest study analyzing miRNA profiles in colon cancer tissues using independent two cohorts, and demonstrated the systematic differences in miRNA expression patterns between colon tumors and paired non-tumor tissues.
**25 Ng EK, et al. Gut 2009; 58(10): 1375-81.
This study showed the first comprehensive analysis in the field of blood-based miRNA biomarkers in CRC., and suggested the feasibility of plasma miRNAs as diagnostic markers in CRC.
*58 Toiyama Y, et al. J Natl Cancer Inst 2013; 105(12): 849-59.
This study is the first miRNA biomarker study that used a large cohort of primary tissues and matching serum samples, and performed the direct comparison between tumor tissue and serum expression status to highlight the specificity of non-invasive detection of these non-coding RNAs in blood.
*71 Koga Y, et al. Cancer Prev Res (Phila) 2010; 3(11): 1435-42.
This was the first study for miRNA expression profiling using fecal colonocyte-based RNAs, and investigated the potential of miRNA expression analysis of exfoliated colonocytes as early detection biomarkers for CRC.
**89. Zhang, JX, et al. Lancet Oncol 2013; 14(13): 1295-306.
This study performed the most systematic and comprehensive study in the field of predictive biomarkers for the identification of high risk stage II population and concluded that a classifier based on six-miRNAs in primary tissues is an accurate prognostic and predictive approach in stage II colon cancer.
*152. Taylor DD, et al. Gynecol Oncol 2008; 110(1): 13-21.
This research group performed the isolation of tumor-derived (epithelia-derived) exosomes using anti-EpCAM and suggested the potential of miRNA profiling of circulating tumor-derived exosomes as a surrogate diagnostic markers for miRNA expression profiling in tissue biopsies.
Footnotes
Conflict of Interests: The authors have no conflict of interests to disclose.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Weizman AV, Nguyen GC. Colon cancer screening in 2010: an up-date. Minerva gastroenterologica e dietologica. 2010;56:181–188. [PubMed] [Google Scholar]
- 4.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. The American journal of gastroenterology. 2008;103:1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 5.Walsh JM, Terdiman JP. Colorectal cancer screening: clinical applications. JAMA : the journal of the American Medical Association. 2003;289:1297–1302. doi: 10.1001/jama.289.10.1297. [DOI] [PubMed] [Google Scholar]
- 6.Baxter NN, Warren JL, Barrett MJ, Stukel TA, Doria-Rose VP. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2664–2669. doi: 10.1200/JCO.2011.40.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine JS. Screening and surveillance for colorectal neoplasia: uncertainties of colonoscopic management. Polskie Archiwum Medycyny Wewnetrznej. 2008;118:302–306. [PubMed] [Google Scholar]
- 8.Dominic OG, McGarrity T, Dignan M, Lengerich EJ. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. The American journal of gastroenterology. 2009;104:2626–2627. doi: 10.1038/ajg.2009.419. author reply 2628-2629. [DOI] [PubMed] [Google Scholar]
- 9.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Annals of internal medicine. 2008;149:638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 10.Ahlquist DA, Sargent DJ, Loprinzi CL, et al. Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Annals of internal medicine. 2008;149:441–450. W481. doi: 10.7326/0003-4819-149-7-200810070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005;129:422–428. doi: 10.1016/j.gastro.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 12.Halama N, Herrmann C, Jaeger D, Herrmann T. Treatment with cetuximab, bevacizumab and irinotecan in heavily pretreated patients with metastasized colorectal cancer. Anticancer research. 2008;28:4111–4115. [PubMed] [Google Scholar]
- 13.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 14.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 15.Siomi H, Siomi MC. Posttranscriptional regulation of microRNA biogenesis in animals. Molecular cell. 2010;38:323–332. doi: 10.1016/j.molcel.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nature reviews Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 17.Garofalo M, Croce CM. microRNAs: Master regulators as potential therapeutics in cancer. Annual review of pharmacology and toxicology. 2011;51:25–43. doi: 10.1146/annurev-pharmtox-010510-100517. [DOI] [PubMed] [Google Scholar]
- 18.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michael MZ, SM OC, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Molecular cancer research : MCR. 2003;1:882–891. [PubMed] [Google Scholar]
- 21.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calin GA, Liu CG, Sevignani C, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11755–11760. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng EK, Tsang WP, Ng SS, et al. MicroRNA-143 targets DNA methyltransferases 3A in colorectal cancer. British journal of cancer. 2009;101:699–706. doi: 10.1038/sj.bjc.6605195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandres E, Cubedo E, Agirre X, et al. Identification by Real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Molecular cancer. 2006;5:29. doi: 10.1186/1476-4598-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schepeler T, Reinert JT, Ostenfeld MS, et al. Diagnostic and prognostic microRNAs in stage II colon cancer. Cancer research. 2008;68:6416–6424. doi: 10.1158/0008-5472.CAN-07-6110. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Guo X, Zhang H, et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28:1385–1392. doi: 10.1038/onc.2008.474. [DOI] [PubMed] [Google Scholar]
- 28.Diosdado B, van de Wiel MA, Terhaar Sive Droste JS, et al. MiR-17-92 cluster is associated with 13q gain and c-myc expression during colorectal adenoma to adenocarcinoma progression. British journal of cancer. 2009;101:707–714. doi: 10.1038/sj.bjc.6605037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Y, Zhang P, Wang F, et al. Elevated oncofoetal miR-17-5p expression regulates colorectal cancer progression by repressing its target gene P130. Nature communications. 2012;3:1291. doi: 10.1038/ncomms2276. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchida A, Ohno S, Wu W, et al. miR-92 is a key oncogenic component of the miR-17-92 cluster in colon cancer. Cancer science. 2011;102:2264–2271. doi: 10.1111/j.1349-7006.2011.02081.x. [DOI] [PubMed] [Google Scholar]
- 31.Schetter AJ, Leung SY, Sohn JJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA : the journal of the American Medical Association. 2008;299:425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo X, Burwinkel B, Tao S, Brenner H. MicroRNA signatures: novel biomarker for colorectal cancer? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:1272–1286. doi: 10.1158/1055-9965.EPI-11-0035. [DOI] [PubMed] [Google Scholar]
- 33.Oberg AL, French AJ, Sarver AL, et al. miRNA expression in colon polyps provides evidence for a multihit model of colon cancer. PloS one. 2011;6:e20465. doi: 10.1371/journal.pone.0020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagel R, le Sage C, Diosdado B, et al. Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer research. 2008;68:5795–5802. doi: 10.1158/0008-5472.CAN-08-0951. [DOI] [PubMed] [Google Scholar]
- 35.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talotta F, Cimmino A, Matarazzo MR, et al. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation. Oncogene. 2009;28:73–84. doi: 10.1038/onc.2008.370. [DOI] [PubMed] [Google Scholar]
- 37.Kawakita A, Yanamoto S, Yamada SI. MicroRNA-21 Promotes Oral Cancer Invasion via the Wnt/beta-Catenin Pathway by Targeting DKK2. Pathology oncology research : POR. 2013 doi: 10.1007/s12253-013-9689-y. [DOI] [PubMed] [Google Scholar]
- 38.Monzo M, Navarro A, Bandres E, et al. Overlapping expression of microRNAs in human embryonic colon and colorectal cancer. Cell research. 2008;18:823–833. doi: 10.1038/cr.2008.81. [DOI] [PubMed] [Google Scholar]
- 39.Olson J, Whitney DH, Durkee K, Shuber AP. DNA stabilization is critical for maximizing performance of fecal DNA-based colorectal cancer tests. Diagnostic molecular pathology : the American journal of surgical pathology, part B. 2005;14:183–191. doi: 10.1097/01.pas.0000176768.18423.7e. [DOI] [PubMed] [Google Scholar]
- 40.Rai AJ, Gelfand CA, Haywood BC, et al. HUPO Plasma Proteome Project specimen collection and handling: towards the standardization of parameters for plasma proteome samples. Proteomics. 2005;5:3262–3277. doi: 10.1002/pmic.200401245. [DOI] [PubMed] [Google Scholar]
- 41.Tuck MK, Chan DW, Chia D, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. Journal of proteome research. 2009;8:113–117. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou H, Harrington JJ, Klatt KK, Ahlquist DA. A sensitive method to quantify human long DNA in stool: relevance to colorectal cancer screening. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:1115–1119. doi: 10.1158/1055-9965.EPI-05-0992. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell research. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 45.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic acids research. 2011;39:7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brase JC, Wuttig D, Kuner R, Sultmann H. Serum microRNAs as non-invasive biomarkers for cancer. Molecular cancer. 2010;9:306. doi: 10.1186/1476-4598-9-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng EK, Chong WW, Jin H, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375–1381. doi: 10.1136/gut.2008.167817. [DOI] [PubMed] [Google Scholar]
- 48.Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. International journal of cancer Journal international du cancer. 2010;127:118–126. doi: 10.1002/ijc.25007. [DOI] [PubMed] [Google Scholar]
- 49.Toiyama Y, Takahashi M, Hur K, et al. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer. Journal of the National Cancer Institute. 2013;105:849–859. doi: 10.1093/jnci/djt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanaan Z, Rai SN, Eichenberger MR, et al. Plasma miR-21: a potential diagnostic marker of colorectal cancer. Annals of surgery. 2012;256:544–551. doi: 10.1097/SLA.0b013e318265bd6f. [DOI] [PubMed] [Google Scholar]
- 51.Liu GH, Zhou ZG, Chen R, et al. Serum miR-21 and miR-92a as biomarkers in the diagnosis and prognosis of colorectal cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34:2175–2181. doi: 10.1007/s13277-013-0753-8. [DOI] [PubMed] [Google Scholar]
- 52.Dong SM, Traverso G, Johnson C, et al. Detecting colorectal cancer in stool with the use of multiple genetic targets. Journal of the National Cancer Institute. 2001;93:858–865. doi: 10.1093/jnci/93.11.858. [DOI] [PubMed] [Google Scholar]
- 53.Ahlquist DA, Skoletsky JE, Boynton KA, et al. Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology. 2000;119:1219–1227. doi: 10.1053/gast.2000.19580. [DOI] [PubMed] [Google Scholar]
- 54.Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME Colorectal Cancer Study G. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. The New England journal of medicine. 2004;351:2704–2714. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 55.Traverso G, Shuber A, Levin B, et al. Detection of APC mutations in fecal DNA from patients with colorectal tumors. The New England journal of medicine. 2002;346:311–320. doi: 10.1056/NEJMoa012294. [DOI] [PubMed] [Google Scholar]
- 56.Nagasaka T, Tanaka N, Cullings HM, et al. Analysis of fecal DNA methylation to detect gastrointestinal neoplasia. Journal of the National Cancer Institute. 2009;101:1244–1258. doi: 10.1093/jnci/djp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanaoka S, Yoshida K, Miura N, Sugimura H, Kajimura M. Potential usefulness of detecting cyclooxygenase 2 messenger RNA in feces for colorectal cancer screening. Gastroenterology. 2004;127:422–427. doi: 10.1053/j.gastro.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 58.Leung WK, To KF, Man EP, et al. Detection of hypermethylated DNA or cyclooxygenase-2 messenger RNA in fecal samples of patients with colorectal cancer or polyps. The American journal of gastroenterology. 2007;102:1070–1076. doi: 10.1111/j.1572-0241.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- 59.Takai T, Kanaoka S, Yoshida K, et al. Fecal cyclooxygenase 2 plus matrix metalloproteinase 7 mRNA assays as a marker for colorectal cancer screening. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:1888–1893. doi: 10.1158/1055-9965.EPI-08-0937. [DOI] [PubMed] [Google Scholar]
- 60.Ahmed FE, Jeffries CD, Vos PW, et al. Diagnostic microRNA markers for screening sporadic human colon cancer and active ulcerative colitis in stool and tissue. Cancer genomics & proteomics. 2009;6:281–295. [PubMed] [Google Scholar]
- 61.Link A, Balaguer F, Shen Y, et al. Fecal MicroRNAs as novel biomarkers for colon cancer screening. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:1766–1774. doi: 10.1158/1055-9965.EPI-10-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koga Y, Yasunaga M, Takahashi A, et al. MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer prevention research. 2010;3:1435–1442. doi: 10.1158/1940-6207.CAPR-10-0036. [DOI] [PubMed] [Google Scholar]
- 63.Koga Y, Yamazaki N, Yamamoto Y, et al. Fecal miR-106a is a useful marker for colorectal cancer patients with false-negative results in immunochemical fecal occult blood test. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22:1844–1852. doi: 10.1158/1055-9965.EPI-13-0512. [DOI] [PubMed] [Google Scholar]
- 64.Wu CW, Ng SS, Dong YJ, et al. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut. 2012;61:739–745. doi: 10.1136/gut.2011.239236. [DOI] [PubMed] [Google Scholar]
- 65.Baraniskin A, Nopel-Dunnebacke S, Ahrens M, et al. Circulating U2 small nuclear RNA fragments as a novel diagnostic biomarker for pancreatic and colorectal adenocarcinoma. International journal of cancer Journal international du cancer. 2013;132:E48–57. doi: 10.1002/ijc.27791. [DOI] [PubMed] [Google Scholar]
- 66.Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nature reviews Clinical oncology. 2011;8:467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zen K, Zhang CY. Circulating microRNAs: a novel class of biomarkers to diagnose and monitor human cancers. Medicinal research reviews. 2012;32:326–348. doi: 10.1002/med.20215. [DOI] [PubMed] [Google Scholar]
- 68.Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR) Methods. 2010;50:298–301. doi: 10.1016/j.ymeth.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA : the journal of the American Medical Association. 1990;264:1444–1450. [PubMed] [Google Scholar]
- 70.Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. The Cochrane database of systematic reviews. 2008:CD005390. doi: 10.1002/14651858.CD005390.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benson AB, 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 72.Quasar Collaborative G, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 73.Morris EJ, Maughan NJ, Forman D, Quirke P. Who to treat with adjuvant therapy in Dukes B/stage II colorectal cancer? The need for high quality pathology. Gut. 2007;56:1419–1425. doi: 10.1136/gut.2006.116830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. Journal of the National Cancer Institute. 2001;93:583–596. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 75.Akiyoshi T, Kobunai T, Watanabe T. Recent approaches to identifying biomarkers for high-risk stage II colon cancer. Surgery today. 2012;42:1037–1045. doi: 10.1007/s00595-012-0324-4. [DOI] [PubMed] [Google Scholar]
- 76.Labianca R, Nordlinger B, Beretta GD, Brouquet A, Cervantes A Group E.G.W. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010;21(Suppl 5):v70–77. doi: 10.1093/annonc/mdq168. [DOI] [PubMed] [Google Scholar]
- 77.Agesen TH, Sveen A, Merok MA, et al. ColoGuideEx: a robust gene classifier specific for stage II colorectal cancer prognosis. Gut. 2012;61:1560–1567. doi: 10.1136/gutjnl-2011-301179. [DOI] [PubMed] [Google Scholar]
- 78.Okugawa Y, Miki C, Toiyama Y, Koike Y, Inoue Y, Kusunoki M. Serum level of soluble vascular cell adhesion molecule 1 is a valuable prognostic marker in colorectal carcinoma. Diseases of the colon and rectum. 2009;52:1330–1336. doi: 10.1007/DCR.0b013e3181a0d144. [DOI] [PubMed] [Google Scholar]
- 79.Okugawa Y, Miki C, Toiyama Y, et al. Loss of tumoral expression of soluble IL-6 receptor is associated with disease progression in colorectal cancer. British journal of cancer. 2010;103:787–795. doi: 10.1038/sj.bjc.6605827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Toiyama Y, Miki C, Inoue Y, Okugawa Y, Tanaka K, Kusunoki M. Serum hepatocyte growth factor as a prognostic marker for stage II or III colorectal cancer patients. International journal of cancer Journal international du cancer. 2009;125:1657–1662. doi: 10.1002/ijc.24554. [DOI] [PubMed] [Google Scholar]
- 81.Toiyama Y, Miki C, Inoue Y, et al. Soluble intercellular adhesion molecule-1 as a prognostic marker for stage II colorectal cancer patients. Annals of surgical oncology. 2008;15:1617–1624. doi: 10.1245/s10434-008-9874-5. [DOI] [PubMed] [Google Scholar]
- 82.Schetter AJ, Nguyen GH, Bowman ED, et al. Association of inflammation-related and microRNA gene expression with cancer-specific mortality of colon adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5878–5887. doi: 10.1158/1078-0432.CCR-09-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kjaer-Frifeldt S, Hansen TF, Nielsen BS, et al. The prognostic importance of miR-21 in stage II colon cancer: a population-based study. British journal of cancer. 2012;107:1169–1174. doi: 10.1038/bjc.2012.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang JX, Song W, Chen ZH, et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. The lancet oncology. 2013;14:1295–1306. doi: 10.1016/S1470-2045(13)70491-1. [DOI] [PubMed] [Google Scholar]
- 85.Lombardi L, Morelli F, Cinieri S, et al. Adjuvant colon cancer chemotherapy: where we are and where we'll go. Cancer treatment reviews. 2010;36(Suppl 3):S34–41. doi: 10.1016/S0305-7372(10)70018-9. [DOI] [PubMed] [Google Scholar]
- 86.Vickers MM, Bar J, Gorn-Hondermann I, et al. Stage-dependent differential expression of microRNAs in colorectal cancer: potential role as markers of metastatic disease. Clinical & experimental metastasis. 2012;29:123–132. doi: 10.1007/s10585-011-9435-3. [DOI] [PubMed] [Google Scholar]
- 87.Pizzini S, Bisognin A, Mandruzzato S, et al. Impact of microRNAs on regulatory networks and pathways in human colorectal carcinogenesis and development of metastasis. BMC genomics. 2013;14:589. doi: 10.1186/1471-2164-14-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Developmental cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 89.Jou J, Diehl AM. Epithelial-mesenchymal transitions and hepatocarcinogenesis. The Journal of clinical investigation. 2010;120:1031–1034. doi: 10.1172/JCI42615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yates CC, Shepard CR, Stolz DB, Wells A. Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. British journal of cancer. 2007;96:1246–1252. doi: 10.1038/sj.bjc.6603700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature reviews Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 92.Hur K, Toiyama Y, Takahashi M, et al. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut. 2013;62:1315–1326. doi: 10.1136/gutjnl-2011-301846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Toiyama Y, Hur K, Tanaka K, et al. Serum miR-200c Is a Novel Prognostic and Metastasis-Predictive Biomarker in Patients With Colorectal Cancer. Annals of surgery. 2014;259:735–743. doi: 10.1097/SLA.0b013e3182a6909d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1254–1261. doi: 10.1200/JCO.2009.24.6116. [DOI] [PubMed] [Google Scholar]
- 95.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. The New England journal of medicine. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 96.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 97.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. The New England journal of medicine. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 98.Poston GJ, Figueras J, Giuliante F, et al. Urgent need for a new staging system in advanced colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:4828–4833. doi: 10.1200/JCO.2008.17.6453. [DOI] [PubMed] [Google Scholar]
- 99.Nishida N, Yamashita S, Mimori K, et al. MicroRNA-10b is a prognostic indicator in colorectal cancer and confers resistance to the chemotherapeutic agent 5-fluorouracil in colorectal cancer cells. Annals of surgical oncology. 2012;19:3065–3071. doi: 10.1245/s10434-012-2246-1. [DOI] [PubMed] [Google Scholar]
- 100.Kurokawa K, Tanahashi T, Iima T, et al. Role of miR-19b and its target mRNAs in 5-fluorouracil resistance in colon cancer cells. Journal of gastroenterology. 2012;47:883–895. doi: 10.1007/s00535-012-0547-6. [DOI] [PubMed] [Google Scholar]
- 101.Chai H, Liu M, Tian R, Li X, Tang H. miR-20a targets BNIP2 and contributes chemotherapeutic resistance in colorectal adenocarcinoma SW480 and SW620 cell lines. Acta biochimica et biophysica Sinica. 2011;43:217–225. doi: 10.1093/abbs/gmq125. [DOI] [PubMed] [Google Scholar]
- 102.Valeri N, Gasparini P, Braconi C, et al. MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2) Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21098–21103. doi: 10.1073/pnas.1015541107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deng J, Lei W, Fu JC, Zhang L, Li JH, Xiong JP. Targeting miR-21 enhances the sensitivity of human colon cancer HT-29 cells to chemoradiotherapy in vitro. Biochemical and biophysical research communications. 2014;443:789–795. doi: 10.1016/j.bbrc.2013.11.064. [DOI] [PubMed] [Google Scholar]
- 104.Faltejskova P, Besse A, Sevcikova S, et al. Clinical correlations of miR-21 expression in colorectal cancer patients and effects of its inhibition on DLD1 colon cancer cells. International journal of colorectal disease. 2012;27:1401–1408. doi: 10.1007/s00384-012-1461-3. [DOI] [PubMed] [Google Scholar]
- 105.Shang J, Yang F, Wang Y, et al. MicroRNA-23a antisense enhances 5-fluorouracil chemosensitivity through APAF-1/caspase-9 apoptotic pathway in colorectal cancer cells. Journal of cellular biochemistry. 2014;115:772–784. doi: 10.1002/jcb.24721. [DOI] [PubMed] [Google Scholar]
- 106.Wang CJ, Stratmann J, Zhou ZG, Sun XF. Suppression of microRNA-31 increases sensitivity to 5-FU at an early stage, and affects cell migration and invasion in HCT-116 colon cancer cells. BMC cancer. 2010;10:616. doi: 10.1186/1471-2407-10-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siemens H, Jackstadt R, Kaller M, Hermeking H. Repression of c-Kit by p53 is mediated by miR-34 and is associated with reduced chemoresistance, migration and stemness. Oncotarget. 2013;4:1399–1415. doi: 10.18632/oncotarget.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Akao Y, Noguchi S, Iio A, Kojima K, Takagi T, Naoe T. Dysregulation of microRNA-34a expression causes drug-resistance to 5-FU in human colon cancer DLD-1 cells. Cancer letters. 2011;300:197–204. doi: 10.1016/j.canlet.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 109.Karaayvaz M, Zhai H, Ju J. miR-129 promotes apoptosis and enhances chemosensitivity to 5-fluorouracil in colorectal cancer. Cell death & disease. 2013;4:e659. doi: 10.1038/cddis.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Song B, Wang Y, Xi Y, et al. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene. 2009;28:4065–4074. doi: 10.1038/onc.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang J, Guo H, Zhang H, et al. Putative tumor suppressor miR-145 inhibits colon cancer cell growth by targeting oncogene Friend leukemia virus integration 1 gene. Cancer. 2011;117:86–95. doi: 10.1002/cncr.25522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boni V, Bitarte N, Cristobal I, et al. miR-192/miR-215 influence 5-fluorouracil resistance through cell cycle-mediated mechanisms complementary to its post-transcriptional thymidilate synthase regulation. Molecular cancer therapeutics. 2010;9:2265–2275. doi: 10.1158/1535-7163.MCT-10-0061. [DOI] [PubMed] [Google Scholar]
- 113.Guo ST, Jiang CC, Wang GP, et al. MicroRNA-497 targets insulin-like growth factor 1 receptor and has a tumour suppressive role in human colorectal cancer. Oncogene. 2013;32:1910–1920. doi: 10.1038/onc.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bitarte N, Bandres E, Boni V, et al. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem cells. 2011;29:1661–1671. doi: 10.1002/stem.741. [DOI] [PubMed] [Google Scholar]
- 115.Dong Y, Zhao J, Wu CW, et al. Tumor suppressor functions of miR-133a in colorectal cancer. Molecular cancer research : MCR. 2013;11:1051–1060. doi: 10.1158/1541-7786.MCR-13-0061. [DOI] [PubMed] [Google Scholar]
- 116.Qian X, Yu J, Yin Y, et al. MicroRNA-143 inhibits tumor growth and angiogenesis and sensitizes chemosensitivity to oxaliplatin in colorectal cancers. Cell cycle. 2013;12:1385–1394. doi: 10.4161/cc.24477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang L, Pickard K, Jenei V, et al. miR-153 supports colorectal cancer progression via pleiotropic effects that enhance invasion and chemotherapeutic resistance. Cancer research. 2013;73:6435–6447. doi: 10.1158/0008-5472.CAN-12-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou Y, Wan G, Spizzo R, et al. miR-203 induces oxaliplatin resistance in colorectal cancer cells by negatively regulating ATM kinase. Molecular oncology. 2014;8:83–92. doi: 10.1016/j.molonc.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu K, Liang X, Cui D, Wu Y, Shi W, Liu J. miR-1915 inhibits Bcl-2 to modulate multidrug resistance by increasing drug-sensitivity in human colorectal carcinoma cells. Molecular carcinogenesis. 2013;52:70–78. doi: 10.1002/mc.21832. [DOI] [PubMed] [Google Scholar]
- 120.Nakajima G, Hayashi K, Xi Y, et al. Non-coding MicroRNAs hsa-let-7g and hsa-miR-181b are Associated with Chemoresponse to S-1 in Colon Cancer. Cancer genomics & proteomics. 2006;3:317–324. [PMC free article] [PubMed] [Google Scholar]
- 121.Rasmussen MH, Jensen NF, Tarpgaard LS, et al. High expression of microRNA-625-3p is associated with poor response to first-line oxaliplatin based treatment of metastatic colorectal cancer. Molecular oncology. 2013;7:637–646. doi: 10.1016/j.molonc.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Takahashi M, Cuatrecasas M, Balaguer F, et al. The clinical significance of MiR-148a as a predictive biomarker in patients with advanced colorectal cancer. PloS one. 2012;7:e46684. doi: 10.1371/journal.pone.0046684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pichler M, Winter E, Ress AL, et al. miR-181a is associated with poor clinical outcome in patients with colorectal cancer treated with EGFR inhibitor. Journal of clinical pathology. 2014;67:198–203. doi: 10.1136/jclinpath-2013-201904. [DOI] [PubMed] [Google Scholar]
- 124.Cappuzzo F, Sacconi A, Landi L, et al. MicroRNA signature in metastatic colorectal cancer patients treated with anti-EGFR monoclonal antibodies. Clinical colorectal cancer. 2014;13:37–45. e34. doi: 10.1016/j.clcc.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 125.Kjersem JB, Ikdahl T, Lingjaerde OC, Guren T, Tveit KM, Kure EH. Plasma microRNAs predicting clinical outcome in metastatic colorectal cancer patients receiving first-line oxaliplatin-based treatment. Molecular oncology. 2014;8:59–67. doi: 10.1016/j.molonc.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen J, Wang W, Zhang Y, Chen Y, Hu T. Predicting distant metastasis and chemoresistance using plasma miRNAs. Medical oncology. 2014;31:799. doi: 10.1007/s12032-013-0799-x. [DOI] [PubMed] [Google Scholar]
- 127.Valentini V, Coco C, Picciocchi A, et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. International journal of radiation oncology, biology, physics. 2002;53:664–674. doi: 10.1016/s0360-3016(02)02764-5. [DOI] [PubMed] [Google Scholar]
- 128.Reerink O, Karrenbeld A, Plukker JT, et al. Molecular prognostic factors in locally irresectable rectal cancer treated preoperatively by chemo-radiotherapy. Anticancer research. 2004;24:1217–1221. [PubMed] [Google Scholar]
- 129.Heriot AG, Tekkis PP, Fazio VW, Neary P, Lavery IC. Adjuvant radiotherapy is associated with increased sexual dysfunction in male patients undergoing resection for rectal cancer: a predictive model. Annals of surgery. 2005;242:502–510. doi: 10.1097/01.sla.0000183608.24549.68. discussion 510-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Birgisson H, Pahlman L, Gunnarsson U, Glimelius B Swedish Rectal Cancer Trial G. Adverse effects of preoperative radiation therapy for rectal cancer: long-term follow-up of the Swedish Rectal Cancer Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:8697–8705. doi: 10.1200/JCO.2005.02.9017. [DOI] [PubMed] [Google Scholar]
- 131.Svoboda M, Izakovicova Holla L, Sefr R, et al. Micro-RNAs miR125b and miR137 are frequently upregulated in response to capecitabine chemoradiotherapy of rectal cancer. International journal of oncology. 2008;33:541–547. [PubMed] [Google Scholar]
- 132.Svoboda M, Sana J, Fabian P, et al. MicroRNA expression profile associated with response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer patients. Radiation oncology. 2012;7:195. doi: 10.1186/1748-717X-7-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Salendo J, Spitzner M, Kramer F, et al. Identification of a microRNA expression signature for chemoradiosensitivity of colorectal cancer cells, involving miRNAs-320a, -224, -132 and let7g. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2013;108:451–457. doi: 10.1016/j.radonc.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 134.Jones K, Nourse JP, Keane C, Bhatnagar A, Gandhi MK. Plasma microRNA are disease response biomarkers in classical Hodgkin lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:253–264. doi: 10.1158/1078-0432.CCR-13-1024. [DOI] [PubMed] [Google Scholar]
- 135.Day E, Dear PH, McCaughan F. Digital PCR strategies in the development and analysis of molecular biomarkers for personalized medicine. Methods. 2013;59:101–107. doi: 10.1016/j.ymeth.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 136.Vogelstein B, Kinzler KW. Digital PCR. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9236–9241. doi: 10.1073/pnas.96.16.9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chan M, Liaw CS, Ji SM, et al. Identification of circulating microRNA signatures for breast cancer detection. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:4477–4487. doi: 10.1158/1078-0432.CCR-12-3401. [DOI] [PubMed] [Google Scholar]
- 138.Ren J, Zhang J, Xu N, et al. Signature of circulating microRNAs as potential biomarkers in vulnerable coronary artery disease. PloS one. 2013;8:e80738. doi: 10.1371/journal.pone.0080738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Giraldez MD, Lozano JJ, Ramirez G, et al. Circulating microRNAs as biomarkers of colorectal cancer: results from a genome-wide profiling and validation study. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:681–688. e683. doi: 10.1016/j.cgh.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 140.Hofsli E, Sjursen W, Prestvik WS, et al. Identification of serum microRNA profiles in colon cancer. British journal of cancer. 2013;108:1712–1719. doi: 10.1038/bjc.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kuchenbauer F, Morin RD, Argiropoulos B, et al. In-depth characterization of the microRNA transcriptome in a leukemia progression model. Genome research. 2008;18:1787–1797. doi: 10.1101/gr.077578.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guo L, Yang Q, Lu J, et al. A comprehensive survey of miRNA repertoire and 3′ addition events in the placentas of patients with pre-eclampsia from high-throughput sequencing. PloS one. 2011;6:e21072. doi: 10.1371/journal.pone.0021072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Landgraf P, Rusu M, Sheridan R, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee LW, Zhang S, Etheridge A, et al. Complexity of the microRNA repertoire revealed by next-generation sequencing. Rna. 2010;16:2170–2180. doi: 10.1261/rna.2225110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morin RD, O'Connor MD, Griffith M, et al. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome research. 2008;18:610–621. doi: 10.1101/gr.7179508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Llorens F, Banez-Coronel M, Pantano L, et al. A highly expressed miR-101 isomiR is a functional silencing small RNA. BMC genomics. 2013;14:104. doi: 10.1186/1471-2164-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecologic oncology. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 148.Takahashi K, Yokota S, Tatsumi N, Fukami T, Yokoi T, Nakajima M. Cigarette smoking substantially alters plasma microRNA profiles in healthy subjects. Toxicology and applied pharmacology. 2013;272:154–160. doi: 10.1016/j.taap.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 149.Bala S, Petrasek J, Mundkur S, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Witwer KW. XenomiRs and miRNA homeostasis in health and disease: evidence that diet and dietary miRNAs directly and indirectly influence circulating miRNA profiles. RNA biology. 2012;9:1147–1154. doi: 10.4161/rna.21619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 152.Zhang X, Wan G, Mlotshwa S, et al. Oncogenic Wip1 phosphatase is inhibited by miR-16 in the DNA damage signaling pathway. Cancer research. 2010;70:7176–7186. doi: 10.1158/0008-5472.CAN-10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Motoyama K, Inoue H, Takatsuno Y, et al. Over- and under-expressed microRNAs in human colorectal cancer. International journal of oncology. 2009;34:1069–1075. doi: 10.3892/ijo_00000233. [DOI] [PubMed] [Google Scholar]
- 154.Kanaan Z, Rai SN, Eichenberger MR, et al. Differential microRNA expression tracks neoplastic progression in inflammatory bowel disease-associated colorectal cancer. Human mutation. 2012;33:551–560. doi: 10.1002/humu.22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wu CW, Dong YJ, Liang QY, et al. MicroRNA-18a attenuates DNA damage repair through suppressing the expression of ataxia telangiectasia mutated in colorectal cancer. PloS one. 2013;8:e57036. doi: 10.1371/journal.pone.0057036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tsang WP, Kwok TT. The miR-18a* microRNA functions as a potential tumor suppressor by targeting on K-Ras. Carcinogenesis. 2009;30:953–959. doi: 10.1093/carcin/bgp094. [DOI] [PubMed] [Google Scholar]
- 157.Valladares-Ayerbes M, Blanco M, Haz M, et al. Prognostic impact of disseminated tumor cells and microRNA-17-92 cluster deregulation in gastrointestinal cancer. International journal of oncology. 2011;39:1253–1264. doi: 10.3892/ijo.2011.1112. [DOI] [PubMed] [Google Scholar]
- 158.Oue N, Anami K, Schetter AJ, et al. High miR-21 expression from FFPE tissues is associated with poor survival and response to adjuvant chemotherapy in colon cancer. International journal of cancer Journal international du cancer. 2014;134:1926–1934. doi: 10.1002/ijc.28522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yamamichi N, Shimomura R, Inada K, et al. Locked nucleic acid in situ hybridization analysis of miR-21 expression during colorectal cancer development. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:4009–4016. doi: 10.1158/1078-0432.CCR-08-3257. [DOI] [PubMed] [Google Scholar]
- 160.Schmitz KJ, Hey S, Schinwald A, et al. Differential expression of microRNA 181b and microRNA 21 in hyperplastic polyps and sessile serrated adenomas of the colon. Virchows Archiv : an international journal of pathology. 2009;455:49–54. doi: 10.1007/s00428-009-0804-0. [DOI] [PubMed] [Google Scholar]
- 161.Tang W, Zhu Y, Gao J, et al. MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. British journal of cancer. 2014;110:450–458. doi: 10.1038/bjc.2013.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Arndt GM, Dossey L, Cullen LM, et al. Characterization of global microRNA expression reveals oncogenic potential of miR-145 in metastatic colorectal cancer. BMC cancer. 2009;9:374. doi: 10.1186/1471-2407-9-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu RS, Wu XD, Zhang SQ, et al. The tumor suppressor gene RhoBTB1 is a novel target of miR-31 in human colon cancer. International journal of oncology. 2013;42:676–682. doi: 10.3892/ijo.2012.1746. [DOI] [PubMed] [Google Scholar]
- 164.Chen T, Yao LQ, Shi Q. MicroRNA-31 contributes to colorectal cancer development by targeting factor inhibiting HIF-1alpha (FIH-1) Cancer biology & therapy. 2014;15 doi: 10.4161/cbt.28017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sun D, Yu F, Ma Y, et al. MicroRNA-31 activates the RAS pathway and functions as an oncogenic MicroRNA in human colorectal cancer by repressing RAS p21 GTPase activating protein 1 (RASA1) The Journal of biological chemistry. 2013;288:9508–9518. doi: 10.1074/jbc.M112.367763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu W, Yang J, Feng X, et al. MicroRNA-32 (miR-32) regulates phosphatase and tensin homologue (PTEN) expression and promotes growth, migration, and invasion in colorectal carcinoma cells. Molecular cancer. 2013;12:30. doi: 10.1186/1476-4598-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu W, Yang P, Feng X, et al. The relationship between and clinical significance of MicroRNA-32 and phosphatase and tensin homologue expression in colorectal cancer. Genes, chromosomes & cancer. 2013;52:1133–1140. doi: 10.1002/gcc.22108. [DOI] [PubMed] [Google Scholar]
- 168.Sarver AL, French AJ, Borralho PM, et al. Human colon cancer profiles show differential microRNA expression depending on mismatch repair status and are characteristic of undifferentiated proliferative states. BMC cancer. 2009;9:401. doi: 10.1186/1471-2407-9-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang G, Zhou H, Xiao H, Liu Z, Tian H, Zhou T. MicroRNA-92a functions as an oncogene in colorectal cancer by targeting PTEN. Digestive diseases and sciences. 2014;59:98–107. doi: 10.1007/s10620-013-2858-8. [DOI] [PubMed] [Google Scholar]
- 170.Zhou T, Zhang G, Liu Z, Xia S, Tian H. Overexpression of miR-92a correlates with tumor metastasis and poor prognosis in patients with colorectal cancer. International journal of colorectal disease. 2013;28:19–24. doi: 10.1007/s00384-012-1528-1. [DOI] [PubMed] [Google Scholar]
- 171.Huang Z, Huang S, Wang Q, et al. MicroRNA-95 promotes cell proliferation and targets sorting Nexin 1 in human colorectal carcinoma. Cancer research. 2011;71:2582–2589. doi: 10.1158/0008-5472.CAN-10-3032. [DOI] [PubMed] [Google Scholar]
- 172.Xu XM, Qian JC, Deng ZL, et al. Expression of miR-21, miR-31, miR-96 and miR-135b is correlated with the clinical parameters of colorectal cancer. Oncology letters. 2012;4:339–345. doi: 10.3892/ol.2012.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hamfjord J, Stangeland AM, Hughes T, et al. Differential expression of miRNAs in colorectal cancer: comparison of paired tumor tissue and adjacent normal mucosa using high-throughput sequencing. PloS one. 2012;7:e34150. doi: 10.1371/journal.pone.0034150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhao Y, Miao G, Li Y, et al. Microrna 130b suppresses migration and invasion of colorectal cancer cells through downregulation of integrin beta1. PloS one. 2014;9:e87938. doi: 10.1371/journal.pone.0087938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Slattery ML, Wolff E, Hoffman MD, Pellatt DF, Milash B, Wolff RK. MicroRNAs and colon and rectal cancer: differential expression by tumor location and subtype. Genes, chromosomes & cancer. 2011;50:196–206. doi: 10.1002/gcc.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Colangelo T, Fucci A, Votino C, et al. MicroRNA-130b promotes tumor development and is associated with poor prognosis in colorectal cancer. Neoplasia. 2013;15:1218–1231. doi: 10.1593/neo.13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang M, Zhang P, Li Y, et al. The quantitative analysis by stem-loop real-time PCR revealed the microRNA-34a, microRNA-155 and microRNA-200c overexpression in human colorectal cancer. Medical oncology. 2012;29:3113–3118. doi: 10.1007/s12032-012-0241-9. [DOI] [PubMed] [Google Scholar]
- 178.Shibuya H, Iinuma H, Shimada R, Horiuchi A, Watanabe T. Clinicopathological and prognostic value of microRNA-21 and microRNA-155 in colorectal cancer. Oncology. 2010;79:313–320. doi: 10.1159/000323283. [DOI] [PubMed] [Google Scholar]
- 179.Xi Y, Nakajima G, Gavin E, et al. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. Rna. 2007;13:1668–1674. doi: 10.1261/rna.642907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xi Y, Formentini A, Chien M, et al. Prognostic Values of microRNAs in Colorectal Cancer. Biomarker insights. 2006;2:113–121. [PMC free article] [PubMed] [Google Scholar]
- 181.Liu H, Du L, Wen Z, et al. Up-regulation of miR-182 expression in colorectal cancer tissues and its prognostic value. International journal of colorectal disease. 2013;28:697–703. doi: 10.1007/s00384-013-1674-0. [DOI] [PubMed] [Google Scholar]
- 182.Rapti SM, Kontos CK, Papadopoulos IN, Scorilas A. Enhanced miR-182 transcription is a predictor of poor overall survival in colorectal adenocarcinoma patients. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2014 doi: 10.1515/cclm-2013-0950. [DOI] [PubMed] [Google Scholar]
- 183.Zhou T, Zhang GJ, Zhou H, Xiao HX, Li Y. Overexpression of microRNA-183 in human colorectal cancer and its clinical significance. European journal of gastroenterology & hepatology. 2014;26:229–233. doi: 10.1097/MEG.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 184.Lanza G, Ferracin M, Gafa R, et al. mRNA/microRNA gene expression profile in microsatellite unstable colorectal cancer. Molecular cancer. 2007;6:54. doi: 10.1186/1476-4598-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen XM, Gao HJ. Initial study of microRNA expression profiles of colonic cancer without lymph node metastasis. Journal of digestive diseases. 2010;11:50–54. doi: 10.1111/j.1751-2980.2009.00413.x. [DOI] [PubMed] [Google Scholar]
- 186.Earle JS, Luthra R, Romans A, et al. Association of microRNA expression with microsatellite instability status in colorectal adenocarcinoma. The Journal of molecular diagnostics : JMD. 2010;12:433–440. doi: 10.2353/jmoldx.2010.090154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gaedcke J, Grade M, Camps J, et al. The rectal cancer microRNAome--microRNA expression in rectal cancer and matched normal mucosa. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4919–4930. doi: 10.1158/1078-0432.CCR-12-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang GJ, Zhou H, Xiao HX, Li Y, Zhou T. Up-regulation of miR-224 promotes cancer cell proliferation and invasion and predicts relapse of colorectal cancer. Cancer cell international. 2013;13:104. doi: 10.1186/1475-2867-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Balaguer F, Moreira L, Lozano JJ, et al. Colorectal cancers with microsatellite instability display unique miRNA profiles. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:6239–6249. doi: 10.1158/1078-0432.CCR-11-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang N, Li X, Wu CW, et al. microRNA-7 is a novel inhibitor of YY1 contributing to colorectal tumorigenesis. Oncogene. 2013;32:5078–5088. doi: 10.1038/onc.2012.526. [DOI] [PubMed] [Google Scholar]
- 191.Xu K, Chen Z, Qin C, Song X. miR-7 inhibits colorectal cancer cell proliferation and induces apoptosis by targeting XRCC2. Onco Targets and therapy. 2014;7:325–332. doi: 10.2147/OTT.S59364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cekaite L, Rantala JK, Bruun J, et al. MiR-9, -31, and -182 deregulation promote proliferation and tumor cell survival in colon cancer. Neoplasia. 2012;14:868–879. doi: 10.1593/neo.121094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhao H, Xu Z, Qin H, Gao Z, Gao L. MiR-30b regulates migration and invasion of human colorectal cancer via SIX1. The Biochemical journal. 2014 doi: 10.1042/BJ20131535. [DOI] [PubMed] [Google Scholar]
- 194.Liao WT, Ye YP, Zhang NJ, et al. MicroRNA-30b functions as a tumour suppressor in human colorectal cancer by targeting KRAS, PIK3CD and BCL2. The Journal of pathology. 2014;232:415–427. doi: 10.1002/path.4309. [DOI] [PubMed] [Google Scholar]
- 195.Toyota M, Suzuki H, Sasaki Y, et al. Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer research. 2008;68:4123–4132. doi: 10.1158/0008-5472.CAN-08-0325. [DOI] [PubMed] [Google Scholar]
- 196.Roy S, Levi E, Majumdar AP, Sarkar FH. Expression of miR-34 is lost in colon cancer which can be re-expressed by a novel agent CDF. Journal of hematology & oncology. 2012;5:58. doi: 10.1186/1756-8722-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Strillacci A, Valerii MC, Sansone P, et al. Loss of miR-101 expression promotes Wnt/beta-catenin signalling pathway activation and malignancy in colon cancer cells. The Journal of pathology. 2013;229:379–389. doi: 10.1002/path.4097. [DOI] [PubMed] [Google Scholar]
- 198.Wang MJ, Li Y, Wang R, et al. Downregulation of microRNA-124 is an independent prognostic factor in patients with colorectal cancer. International journal of colorectal disease. 2013;28:183–189. doi: 10.1007/s00384-012-1550-3. [DOI] [PubMed] [Google Scholar]
- 199.Zhang J, Lu Y, Yue X, et al. MiR-124 suppresses growth of human colorectal cancer by inhibiting STAT3. PloS one. 2013;8:e70300. doi: 10.1371/journal.pone.0070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu Y, Zhou Y, Feng X, et al. Low expression of MicroRNA-126 is associated with poor prognosis in colorectal cancer. Genes, chromosomes & cancer. 2014;53:358–365. doi: 10.1002/gcc.22146. [DOI] [PubMed] [Google Scholar]
- 201.Zhou Y, Feng X, Liu YL, et al. Down-regulation of miR-126 is associated with colorectal cancer cells proliferation, migration and invasion by targeting IRS-1 via the AKT and ERK1/2 signaling pathways. PloS one. 2013;8:e81203. doi: 10.1371/journal.pone.0081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang Y, Wang X, Xu B, et al. Epigenetic silencing of miR-126 contributes to tumor invasion and angiogenesis in colorectal cancer. Oncology reports. 2013;30:1976–1984. doi: 10.3892/or.2013.2633. [DOI] [PubMed] [Google Scholar]
- 203.Li XM, Wang AM, Zhang J, Yi H. Down-regulation of miR-126 expression in colorectal cancer and its clinical significance. Medical oncology. 2011;28:1054–1057. doi: 10.1007/s12032-010-9637-6. [DOI] [PubMed] [Google Scholar]
- 204.Liu Y, Zhou Y, Feng X, et al. MicroRNA-126 functions as a tumor suppressor in colorectal cancer cells by targeting CXCR4 via the AKT and ERK1/2 signaling pathways. International journal of oncology. 2014;44:203–210. doi: 10.3892/ijo.2013.2168. [DOI] [PubMed] [Google Scholar]
- 205.Wang H, An H, Wang B, et al. miR-133a represses tumour growth and metastasis in colorectal cancer by targeting LIM and SH3 protein 1 and inhibiting the MAPK pathway. European journal of cancer. 2013;49:3924–3935. doi: 10.1016/j.ejca.2013.07.149. [DOI] [PubMed] [Google Scholar]
- 206.Duan FT, Qian F, Fang K, Lin KY, Wang WT, Chen YQ. miR-133b, a muscle-specific microRNA, is a novel prognostic marker that participates in the progression of human colorectal cancer via regulation of CXCR4 expression. Molecular cancer. 2013;12:164. doi: 10.1186/1476-4598-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Balaguer F, Link A, Lozano JJ, et al. Epigenetic silencing of miR-137 is an early event in colorectal carcinogenesis. Cancer research. 2010;70:6609–6618. doi: 10.1158/0008-5472.CAN-10-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shen K, Liang Q, Xu K, et al. MiR-139 inhibits invasion and metastasis of colorectal cancer by targeting the type I insulin-like growth factor receptor. Biochemical pharmacology. 2012;84:320–330. doi: 10.1016/j.bcp.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 209.Guo H, Chen Y, Hu X, Qian G, Ge S, Zhang J. The regulation of Toll-like receptor 2 by miR-143 suppresses the invasion and migration of a subset of human colorectal carcinoma cells. Molecular cancer. 2013;12:77. doi: 10.1186/1476-4598-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Feng Y, Zhu J, Ou C. MicroRNA-145 inhibits tumour growth and metastasis in colorectal cancer by targeting fascin-1. British journal of cancer. 2014 doi: 10.1038/bjc.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 211.Chen Y, Song Y, Wang Z, et al. Altered expression of MiR-148a and MiR-152 in gastrointestinal cancers and its clinical significance. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2010;14:1170–1179. doi: 10.1007/s11605-010-1202-2. [DOI] [PubMed] [Google Scholar]
- 212.Karaayvaz M, Pal T, Song B, et al. Prognostic significance of miR-215 in colon cancer. Clinical colorectal cancer. 2011;10:340–347. doi: 10.1016/j.clcc.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang X, Wang J, Ma H, Zhang J, Zhou X. Downregulation of miR-195 correlates with lymph node metastasis and poor prognosis in colorectal cancer. Medical oncology. 2012;29:919–927. doi: 10.1007/s12032-011-9880-5. [DOI] [PubMed] [Google Scholar]
- 214.Liu L, Chen L, Xu Y, Li R, Du X. microRNA-195 promotes apoptosis and suppresses tumorigenicity of human colorectal cancer cells. Biochemical and biophysical research communications. 2010;400:236–240. doi: 10.1016/j.bbrc.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 215.Meng X, Wu J, Pan C, et al. Genetic and epigenetic down-regulation of microRNA-212 promotes colorectal tumor metastasis via dysregulation of MnSOD. Gastroenterology. 2013;145:426–436. e421–426. doi: 10.1053/j.gastro.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 216.Faltejskova P, Svoboda M, Srutova K, et al. Identification and functional screening of microRNAs highly deregulated in colorectal cancer. Journal of cellular and molecular medicine. 2012;16:2655–2666. doi: 10.1111/j.1582-4934.2012.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yu H, Gao G, Jiang L, et al. Decreased expression of miR-218 is associated with poor prognosis in patients with colorectal cancer. International journal of clinical and experimental pathology. 2013;6:2904–2911. [PMC free article] [PubMed] [Google Scholar]
- 218.He X, Dong Y, Wu CW, et al. MicroRNA-218 inhibits cell cycle progression and promotes apoptosis in colon cancer by downregulating BMI1 polycomb ring finger oncogene. Molecular medicine. 2012;18:1491–1498. doi: 10.2119/molmed.2012.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhao H, Dong T, Zhou H. miR-320a suppresses colorectal cancer progression by targeting Rac1. Carcinogenesis. 2013 doi: 10.1093/carcin/bgt378. [DOI] [PubMed] [Google Scholar]
- 220.Sun JY, Huang Y, Li JP, et al. MicroRNA-320a suppresses human colon cancer cell proliferation by directly targeting beta-catenin. Biochemical and biophysical research communications. 2012;420:787–792. doi: 10.1016/j.bbrc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 221.Wang H, Wu J, Meng X, et al. MicroRNA-342 inhibits colorectal cancer cell proliferation and invasion by directly targeting DNA methyltransferase 1. Carcinogenesis. 2011;32:1033–1042. doi: 10.1093/carcin/bgr081. [DOI] [PubMed] [Google Scholar]
- 222.Wang Y, Tang Q, Li M, Jiang S, Wang X. MicroRNA-375 inhibits colorectal cancer growth by targeting PIK3CA. Biochemical and biophysical research communications. 2014;444:199–204. doi: 10.1016/j.bbrc.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 223.Dai X, Chiang Y, Wang Z, et al. Expression levels of microRNA-375 in colorectal carcinoma. Molecular medicine reports. 2012;5:1299–1304. doi: 10.3892/mmr.2012.815. [DOI] [PubMed] [Google Scholar]
- 224.Zhang GJ, Zhou H, Xiao HX, Li Y, Zhou T. MiR-378 is an independent prognostic factor and inhibits cell growth and invasion in colorectal cancer. BMC cancer. 2014;14:109. doi: 10.1186/1471-2407-14-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wu J, Ji X, Zhu L, et al. Up-regulation of microRNA-1290 impairs cytokinesis and affects the reprogramming of colon cancer cells. Cancer letters. 2013;329:155–163. doi: 10.1016/j.canlet.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 226.Akao Y, Nakagawa Y, Hirata I, et al. Role of anti-oncomirs miR-143 and -145 in human colorectal tumors. Cancer gene therapy. 2010;17:398–408. doi: 10.1038/cgt.2009.88. [DOI] [PubMed] [Google Scholar]
- 227.Ma Y, Zhang P, Wang F, et al. miR-150 as a potential biomarker associated with prognosis and therapeutic outcome in colorectal cancer. Gut. 2012;61:1447–1453. doi: 10.1136/gutjnl-2011-301122. [DOI] [PubMed] [Google Scholar]
- 228.Pu XX, Huang GL, Guo HQ, et al. Circulating miR-221 directly amplified from plasma is a potential diagnostic and prognostic marker of colorectal cancer and is correlated with p53 expression. Journal of gastroenterology and hepatology. 2010;25:1674–1680. doi: 10.1111/j.1440-1746.2010.06417.x. [DOI] [PubMed] [Google Scholar]
- 229.Wang Q, Huang Z, Ni S, et al. Plasma miR-601 and miR-760 are novel biomarkers for the early detection of colorectal cancer. PloS one. 2012;7:e44398. doi: 10.1371/journal.pone.0044398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wang S, Xiang J, Li Z. A plasma microRNA panel for early detection of colorectal cancer. International journal of cancer Journal international du cancer. 2013 [Google Scholar]
- 231.Kalimutho M, Del Vecchio Blanco G, Di Cecilia S, et al. Differential expression of miR-144* as a novel fecal-based diagnostic marker for colorectal cancer. Journal of gastroenterology. 2011;46:1391–1402. doi: 10.1007/s00535-011-0456-0. [DOI] [PubMed] [Google Scholar]
- 232.Li JM, Zhao RH, Li ST, et al. Down-regulation of fecal miR-143 and miR-145 as potential markers for colorectal cancer. Saudi medical journal. 2012;33:24–29. [PubMed] [Google Scholar]
- 233.Nielsen BS, Jorgensen S, Fog JU, et al. High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clinical & experimental metastasis. 2011;28:27–38. doi: 10.1007/s10585-010-9355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Weissmann-Brenner A, Kushnir M, Lithwick Yanai G, et al. Tumor microRNA-29a expression and the risk of recurrence in stage II colon cancer. International journal of oncology. 2012;40:2097–2103. doi: 10.3892/ijo.2012.1403. [DOI] [PubMed] [Google Scholar]
- 235.Bovell LC, Shanmugam C, Putcha BD, et al. The prognostic value of microRNAs varies with patient race/ethnicity and stage of colorectal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:3955–3965. doi: 10.1158/1078-0432.CCR-12-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lin M, Chen W, Huang J, et al. MicroRNA expression profiles in human colorectal cancers with liver metastases. Oncology reports. 2011;25:739–747. doi: 10.3892/or.2010.1112. [DOI] [PubMed] [Google Scholar]
- 237.Yamashita S, Yamamoto H, Mimori K, et al. MicroRNA-372 is associated with poor prognosis in colorectal cancer. Oncology. 2012;82:205–212. doi: 10.1159/000336809. [DOI] [PubMed] [Google Scholar]
- 238.Zhang G, Xia S, Tian H, Liu Z, Zhou T. Clinical significance of miR-22 expression in patients with colorectal cancer. Medical oncology. 2012;29:3108–3112. doi: 10.1007/s12032-012-0233-9. [DOI] [PubMed] [Google Scholar]
- 239.Iwaya T, Yokobori T, Nishida N, et al. Downregulation of miR-144 is associated with colorectal cancer progression via activation of mTOR signaling pathway. Carcinogenesis. 2012;33:2391–2397. doi: 10.1093/carcin/bgs288. [DOI] [PubMed] [Google Scholar]
- 240.Wan D, He S, Xie B, et al. Aberrant expression of miR-199a-3p and its clinical significance in colorectal cancers. Medical oncology. 2013;30:378. doi: 10.1007/s12032-012-0378-6. [DOI] [PubMed] [Google Scholar]
- 241.Iino I, Kikuchi H, Miyazaki S, et al. Effect of miR-122 and its target gene cationic amino acid transporter 1 on colorectal liver metastasis. Cancer science. 2013;104:624–630. doi: 10.1111/cas.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lou X, Qi X, Zhang Y, Long H, Yang J. Decreased expression of microRNA-625 is associated with tumor metastasis and poor prognosis in patients with colorectal cancer. Journal of surgical oncology. 2013;108:230–235. doi: 10.1002/jso.23380. [DOI] [PubMed] [Google Scholar]
- 243.Qin S, Zhu Y, Ai F, et al. MicroRNA-191 correlates with poor prognosis of colorectal carcinoma and plays multiple roles by targeting tissue inhibitor of metalloprotease 3. Neoplasma. 2014;61:27–34. [PubMed] [Google Scholar]
- 244.Takeyama H, Yamamoto H, Yamashita S. Decreased miR-340 expression in bone marrow is associated with liver metastasis of colorectal cancer. Molecular cancer therapeutics. 2014 doi: 10.1158/1535-7163.MCT-13-0571. [DOI] [PubMed] [Google Scholar]
- 245.Chen DL, Wang ZQ, Zeng ZL, et al. Identification of miR-214 as a negative regulator of colorectal cancer liver metastasis via regulation of FGFR1 expression. Hepatology. 2014 doi: 10.1002/hep.27118. [DOI] [PubMed] [Google Scholar]
- 246.Akcakaya P, Ekelund S, Kolosenko I, et al. miR-185 and miR-133b deregulation is associated with overall survival and metastasis in colorectal cancer. International journal of oncology. 2011;39:311–318. doi: 10.3892/ijo.2011.1043. [DOI] [PubMed] [Google Scholar]
- 247.Zhu L, Chen H, Zhou D, et al. MicroRNA-9 up-regulation is involved in colorectal cancer metastasis via promoting cell motility. Medical oncology. 2012;29:1037–1043. doi: 10.1007/s12032-011-9975-z. [DOI] [PubMed] [Google Scholar]
- 248.Chen HY, Lin YM, Chung HC, et al. miR-103/107 promote metastasis of colorectal cancer by targeting the metastasis suppressors DAPK and KLF4. Cancer research. 2012;72:3631–3641. doi: 10.1158/0008-5472.CAN-12-0667. [DOI] [PubMed] [Google Scholar]
- 249.Chen DL, Wang DS, Wu WJ, et al. Overexpression of paxillin induced by miR-137 suppression promotes tumor progression and metastasis in colorectal cancer. Carcinogenesis. 2013;34:803–811. doi: 10.1093/carcin/bgs400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhang GJ, Xiao HX, Tian HP, Liu ZL, Xia SS, Zhou T. Upregulation of microRNA-155 promotes the migration and invasion of colorectal cancer cells through the regulation of claudin-1 expression. International journal of molecular medicine. 2013;31:1375–1380. doi: 10.3892/ijmm.2013.1348. [DOI] [PubMed] [Google Scholar]
- 251.Long L, Huang G, Zhu H, Guo Y, Liu Y, Huo J. Down-regulation of miR-138 promotes colorectal cancer metastasis via directly targeting TWIST2. Journal of translational medicine. 2013;11:275. doi: 10.1186/1479-5876-11-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Li X, Yang C, Wang X, Zhang J, Zhang R, Liu R. The expression of miR-25 is increased in colorectal cancer and is associated with patient prognosis. Medical oncology. 2014;31:781. doi: 10.1007/s12032-013-0781-7. [DOI] [PubMed] [Google Scholar]
- 253.Cheng H, Zhang L, Cogdell DE, et al. Circulating plasma MiR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PloS one. 2011;6:e17745. doi: 10.1371/journal.pone.0017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wang LG, Gu J. Serum microRNA-29a is a promising novel marker for early detection of colorectal liver metastasis. Cancer epidemiology. 2012;36:e61–67. doi: 10.1016/j.canep.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 255.Liu K, Li G, Fan C, Zhou X, Wu B, Li J. Increased expression of microRNA-21and its association with chemotherapeutic response in human colorectal cancer. The Journal of international medical research. 2011;39:2288–2295. doi: 10.1177/147323001103900626. [DOI] [PubMed] [Google Scholar]
- 256.Li S, Gao J, Gu J, Yuan J, Hua D, Shen L. MicroRNA-215 inhibits relapse of colorectal cancer patients following radical surgery. Medical oncology. 2013;30:549. doi: 10.1007/s12032-013-0549-0. [DOI] [PubMed] [Google Scholar]
- 257.Diaz T, Tejero R, Moreno I. Role of miR-200 family members in survival of colorectal cancer patients treated with fluoropyrimidines. Journal of surgical oncology. 2014 doi: 10.1002/jso.23572. [DOI] [PubMed] [Google Scholar]
- 258.Zhang J, Zhang K, Bi M, Jiao X, Zhang D, Dong Q. Circulating microRNA expressions in colorectal cancer as predictors of response to chemotherapy. Anti-cancer drugs. 2014;25:346–352. doi: 10.1097/CAD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 259.Chen Q, Xia HW, Ge XJ, Zhang YC, Tang QL, Bi F. Serum miR-19a predicts resistance to FOLFOX chemotherapy in advanced colorectal cancer cases. Asian Pacific journal of cancer prevention : APJCP. 2013;14:7421–7426. doi: 10.7314/apjcp.2013.14.12.7421. [DOI] [PubMed] [Google Scholar]
- 260.Drebber U, Lay M, Wedemeyer I, et al. Altered levels of the onco-microRNA 21 and the tumor-supressor microRNAs 143 and 145 in advanced rectal cancer indicate successful neoadjuvant chemoradiotherapy. International journal of oncology. 2011;39:409–415. doi: 10.3892/ijo.2011.1036. [DOI] [PubMed] [Google Scholar]
- 261.Della Vittoria Scarpati G, Falcetta F, Carlomagno C, et al. A specific miRNA signature correlates with complete pathological response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. International journal of radiation oncology, biology, physics. 2012;83:1113–1119. doi: 10.1016/j.ijrobp.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 262.Kheirelseid EA, Miller N, Chang KH, et al. miRNA expressions in rectal cancer as predictors of response to neoadjuvant chemoradiation therapy. International journal of colorectal disease. 2013;28:247–260. doi: 10.1007/s00384-012-1549-9. [DOI] [PubMed] [Google Scholar]