Abstract
The perinucleolar compartment (PNC) is a nuclear substructure associated with, but structurally distinct from, the nucleolus. The PNC contains several RNA processing proteins and several RNA pol III transcripts, which form novel complexes. As determined by cell culture experiments and human tumor samples, the PNC forms exclusively in cancer cells and the percentage of cancer cells in a population that have one or more PNCs directly correlates with the malignancy of that population of cells. Therefore, the PNC is being developed as a prognostic marker for several malignancies. PNC elimination in cancer cells has proven to be a useful as screening method to discover probe compounds used to elucidate PNC biology and to discover compounds with the potential to be developed as minimally toxic anti-cancer drugs.
Keywords: Nuclear architecture, PNC, RNPs, Chromatin, Cancer
1 Introduction
1.1 Structure of the PNC
The perinucleolar compartment (PNC) is a nonmembrane bound nuclear substructure associated with, but structurally distinct from, the nucleolus. The PNC is irregularly shaped with dimensions ranging from 0.25 to 4 microns. Electron microscopy studies have revealed the structure of the PNC consists of several 80–180 nm electron dense strands that form a reticulated meshwork on the surface of the nucleolus [1, 2]]. The PNC persists through interphase with limited movement, disassembles in pro-metaphase, and reassembles in late telophase along with the biogenesis of nucleoli [1]. The PNC is generally heritable from mother to daughter cells, which can be observed in newly divided daughter cells, which often display PNCs that are mirror images (Fig. 1). PNC is a dynamic structure through which some of the components shuttle in and out rapidly [[2], our unpublished data].
Fig. 1.
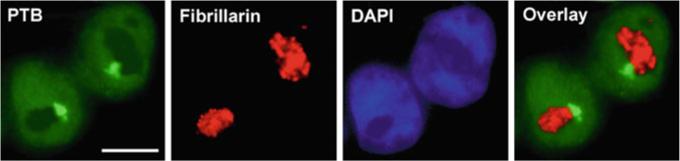
The numbers and the position of the PNC in relation to the nucleolus are heritable to daughter cells during cell division as shown by immunostaining. Labeling of PTB in two newly divided daughter cells marks the PNCs (bright spots at the nucleolar periphery) and nucleoplasm. Fibrillarin marks the nucleoli and DAPI marks the nucleoplasm. Overlay of the PTB and fibrillarin signals shows the association of the PNC with the nucleolus. Scale bar = 10 μm
1.2 Molecular Components of the PNC
Although the molecular composition of the structure is not fully elucidated, the PNC is known to be enriched with several RNAs and RNA binding proteins. The PNC was initially described during the characterization of the polypyrimidine tract-binding protein (PTB) [3], which is a multi-functional RNA binding protein involved in pre-mRNA splicing, stability, and translational regulation. Although PTB shuttles between the nucleus and the cytoplasm, the protein is enriched primarily in the nucleoplasm and is highly concentrated in the PNC when the structure is present (Fig. 1). Since the discovery of PTB localization to the PNC, several other RNA binding proteins have also been found to be concentrated in the PNC. They include CUG-BP [4], KSRP [5], Raver1 [6], Raver2 [7], Rod1 [our unpublished data], and nucleolin [our unpublished data]. The PTB-associated splicing factor (PSF) [8] and the apobec editosome [9] may also localize to the PNC. All these proteins are implicated primarily in the processing of pol II RNAs, with the exception of nucleolin. However, not all pre-mRNA processing factors are enriched in the PNC nor are pol II transcription factors [our unpublished data]. Since PNC is physically associated with the nucleolus and can be co-purified with nucleoli [our unpublished data], we examined the localization of several nucleolar proteins to determine if they are in the PNC. Surprisingly, many nucleolar proteins are not enriched in the PNC. These proteins include UBF, pol I subunits, SL1 components, RRN3, fibrillarin, and B23 [our unpublished data], all of which are either involved in pol I transcription or pre-rRNA processing.
In addition to proteins, a set of small non-coding pol III RNAs have also been found enriched in the PNC. They include MRP RNA [10], RNAse P RNA [10], hY RNAs (hY1, 2, and 5) [10], Alu RNA [11], and SRP (7SL) RNA [11]. MRP and RNAse P RNAs are highly conserved components of two different site specific endoribonucleases that are involved in pre-rRNA processing [12]. hY is an abundant RNA species that associates with the Ro protein, whose function remains unclear. SRP RNA is a transcript component of the signal recognition particle which functions in trafficking nascent proteins containing the endoplasmic reticulum (ER) signal and the associated ribosome to the ER [13]. Alu is a relatively newly evolved RNA that is derived from 7SL RNA and also associates with the signal recognition particle [14]. However, in situ hybridization to other pol III RNAs including U6 [[10], our unpublished data], tRNA [our unpublished data], 7SK, and hY4 [10] RNAs did not show localization of these RNAs in the PNC.
Therefore, the PNC is concentrated with RNA binding proteins that have been primarily implicated in the metabolism of pol II RNAs and is also enriched with pol III synthesized small RNAs (Table 1). Our recent observations demonstrate novel interactions among the PNC-associated RNA and the proteins [15]. The enrichment of the RNA and proteins in the PNC does not appear to be simply due to overexpression of these components in PNC containing cells, since overexpression of the protein components (PTB or CUG-BP) alone or in concert with PNC-associated RNAs (such as MRP RNA and RNase P RNA) does not induce the formation of the PNCs in primary or immortalized cells [our unpublished data]. The complete molecular composition of the PNC has not been fully resolved and the interactions among the known PNC-associated components are just beginning to be explored. This chapter will discuss the possible functions of the PNC in RNA metabolism based on published and our unpublished data, the association of the PNC with the malignant phenotype, and the potential utilization of the PNC as a prognostic and drug discovery cancer marker.
Table 1.
RNA and protein components of the PNC
| RNAs | Proteins |
|---|---|
| MRP | PTB |
| CUG-BP | |
| Alu | Raver1 |
| Raver2 | |
| Rnase P | Rod1 |
| KSRP | |
| hY (1, 2, and 5) | nucleolin |
| PSF | |
| SRP (7SL) | apobec |
Some of these components have previously been linked to cancer and metastasis. Alu RNAs have been shown to be over expressed in cancers but no functional link has been established [15]. PTB is often up regulated in cancer cells [[16], our unpublished data], may support malignant transformation [our unpublished data], and possibly promote metastasis in some cell types [[17], our unpublished data]. Nucleolin can also be unregulated in cancer cells [18] and is involved in the production of MMP-9 [19], which promotes metastatic behavior. However, how these components interact in the PNC and the function of the PNC in malignancy remains unclear
2 The PNC and RNA Metabolism
2.1 The PNC is Likely Involved in RNA Processing
Although the precise function of the PNC is unknown, several lines of evidence have suggested that it might be involved in RNA metabolism. First, RNase treatment disassembles the PNC in unfixed permeabilized cells while DNase has no effect [1]. Second, pulse labeling with Br-U shows enrichment of these nucleotides in the PNC after only 5 min, indicating that the PNC is enriched with newly transcribed RNAs [2]. The third piece of evidence is the fact that all known protein components of the PNC are RNA binding proteins involved in RNA processing. Finally, the RNA-binding capacity of PTB is required for its localization to the PNC and PTB is essential for maintaining the integrity of the PNC [1]. All these findings support a role for PNC in RNA metabolism.
2.2 PNC is Enriched with Pol III Transcripts, but not Pol I or Pol II RNAs
To determine the origin of newly synthesized PNC-associated RNAs, we have performed experiments that selectively inhibit pol I, II, or III transcriptional activity. The initial experiments using a low dose of actinomycin D (0.4 lg/mL), which selectively inhibits pol I transcription [20] caused elimination the PNC [2]. However, further experiments with cycloheximide, which also selectively inhibits pol I transcription (5 h at 100 ug/ml) [21], did not induce PNC disassembly nor did it affect the labeled nucleotide enrichment in the PNC. Thus, the affect of actinomycin D on the PNC is likely due to an “off target” affect of the drug, perhaps such as DNA intercalation, rather than pol I transcription inhibition. This is further supported by the finding that neither 28S and 18S RNAs nor pre-rRNA processing factors, such as fibrillarin, are detected in the PNC through in situ hybridization and immunolabeling [[1, 10], our unpublished data]. Therefore, the source of these newly synthesized PNC-associated RNAs is unlikely rRNA. It is also unlikely that the primary source of PNC-localized RNA is from pol II as the PNC is not sensitive to pol II transcription inhibition by α-amanatin [2], nor is BrU incorporation into the PNC [our unpublished data]. In addition, in situ hybridization to several pre-mRNAs did not show any enrichment in the PNC [5], our unpublished data]; however, this does not exclude the possibility that some pol II transcripts might localize to the PNC.
The structural integrity of the PNC is dependent upon continuous pol III transcription. Injection of tagetin, a specific pol III inhibitor, into HeLa cells results in a disassembly of the PNC within 2 h [11]. This finding suggests that the majority of PNC-associated RNAs are of pol III origins and these RNAs are essential components of the PNC. Furthermore, over expression of one PNC-associated RNA, MRP RNA, from a pol II promoter followed by injection of tagetin partially blocks the tagetin-induced PNC disassembly [11], demonstrating that it is the RNA rather than activity of the polymerase that is important for the integrity of the PNC. In addition, inhibition of pol III transcription disassembles the PNC within a short period of time but does not disrupt the localization of MRP RNA to its functional RNP within the nucleolus [11]. These findings, along with the observation that none of the genes of known PNC-associated RNAs localize to the PNC [[10], our unpublished data], demonstrates that the PNC rapidly recruits a subset of newly synthesized pol III transcripts.
2.3 Novel RNP Associates with the PNC
Reciprocal precipitation experiments were used to analyze the in vivo interactions to determine whether the pol III RNAs in the PNC interact with the RNA binding proteins enriched in the PNC. MRP RNA pulled-down by a specific oligo or immunoprecipitation of PTB or CUGBP by specific antibodies show a reciprocal co-precipitation between the RNA and the RNA binding proteins [15]. Glycerol gradient analyses show that this complex is large and sediments at a different fraction from known MRP RNA containing complexes, the MRP RNP ribozyme, and hTERT. Tethering PNC components to a LacO locus recruits other PNC components, further confirming the in vivo interactions. High-resolution localization analyses demonstrate that MRP RNA, CUGBP, and PTB colocalize at the PNC as a reticulated network [15]. These findings indicate that the pol III RNA containing complexes in the PNC represent non-canonical interactions that may have novel roles in their metabolism and in the PNC.
2.4 Potential Functions of the PNC
What might be the functional significance of the enrichment of newly synthesized RNA and RNA binding proteins in the PNC? Several possibilities can be envisioned. Since PNC is not the site of transcription for the known PNC-associated RNAs, one obvious possibility is that the PNC may serve as an assembly site for the RNPs themselves. However, localization studies of the subunits of MRP RNase, RNase P, and Ro RNP, whose RNA components are associated with the PNC, show that neither the subunits nor functional RNPs localized to the PNC [[10], our unpublished data], indicating that the PNC is not likely involved in the canonical RNP assembly. These findings together with the fact that the PNC is highly enriched with newly synthesized RNA suggest that the RNAs are concentrated in the PNC prior to being assembled into mature or functional RNPs that are subsequently distributed to the nucleolus or other cellular destinations. Since live cell analyses showed that PTB shuttles in and out of PNCs rapidly [1], the association of these RNAs with the PNC is likely to be dynamic rather than long-term storage or simple aggregation. All these findings lead to a working model that the PNC is primarily involved in the metabolism of a subset of newly synthesized pol III RNA transcripts in transformed cells.
There are several possibilities for the specific role for PNC in the metabolism of these RNAs: (1) the PNC could be the site of RNA processing; (2) the RNAs could be sequestered in the PNC prior to being assembled into functional complexes, which may serve as a regulatory mechanism; (3) the PNC may act as the site of assembly for RNA export complexes for the cytoplasmic translocation of some of the PNC-associated RNAs; or (4) the PNC could also be the site for degradation of excess RNAs.
The PNC might be involved in the processing of the pol III PNC-associated transcripts; however, the post-transcriptional processing of pol III RNAs is poorly understood, with the exception of tRNAs. La, an RNA binding protein, is the only protein shown to mediate processing of newly made pol III transcripts [22]. To determine whether PNC is involved in La-mediated processes, the localization of La was evaluated through immunofluorescence and expression of tagged La with GFP and T7 individually. La was not enriched in the PNC when compared to the concentration of pol III RNAs in the structure [our unpublished data]. This finding suggests that the PNC is unlikely to be part of La-mediated pol III RNA processing, but there is increasing evidence to support that the PNC contains novel RNA–protein complexes [our unpublished data] that may be possibly involved in pol III RNA processing. Localization, RNA pull-down, and immunoprecipitation experiments reveal that the pol III RNAs and proteins primarily implicated in pol II transcription, including PTB and CUG-BP are co-enriched in the PNC and interact with each other in vivo. In addition, dynamic studies in live cells show that these RNA binding proteins have much slower dynamics in the PNC as compared to their counterparts in the nucleoplasm [our unpublished data], which suggests altered function for these proteins when in the PNC. Further, understanding of the novel RNP complexes associated with the PNC could help elucidate the function of PNC and novel mechanisms of post-transcriptional metabolism of a subset of pol III transcripts.
The PNC may also serve as a regulatory depot that sequesters a subset of pol III RNAs and regulates their functional availability. During transformation, there is an up-regulation of pol III transcription [23]. As a large number of pol III transcripts are functional RNAs (for example, the RNA components of RNase MRP, RNase P, snRNPs, and tRNA) the level of these RNAs could significantly impact a broad spectrum of cellular activities. The up-regulation of these RNAs during transformation may indeed reflect the increase demand for these RNAs throughout the process. It is possible that the PNC participates in regulating the post-transcriptional availability of these RNAs in transformed cells.
In addition, it is also possible that the PNC acts as the assembly site of RNA export complexes for the cytoplasmic translocation of some of the PNC-associated RNAs. The RNase MRP complex is known to be involved in mitochondrial DNA replication [24] and Ro RNPs are predominantly localized to the cytoplasm [25] although their function remains to be clarified. Treatment of cells with leptomycin B or ratjadone, which block the XPO1 mediated nuclear export of rRNA, some mRNAs, and several RNPs, effectively disassembles the PNC [our unpublished data]. Since inhibition of pol II transcription dose not eliminate PNCs [2], the disassembly of PNCs by leptomycin B and ratjadone is unlikely due to the loss of protein synthesis from the block of mRNA export. Therefore, the requirement of XPO1 activity for PNC suggests that the PNC could play a role XPO1 mediated export.
Finally, the PNC could also be the site for degradation of excess RNAs. The over expression of pol III transcription may trigger the RNA degradation machinery to eliminate the excess amount the RNA. Evidence supporting a possible role of PNC in this function are the following: two components of the PNC, CUG-BP, [26] and KSRP [27], are known to promote the degradation of RNAs in addition to their roles in RNA processing. Second, preliminary proteomic analyses suggest the presence of several other classes of RNA degradation proteins in the PNC [our unpublished data]. To distinguish what role the PNC actually plays in RNA metabolism, parallel studies using different techniques are underway to identify the molecular complexes that are associated with the PNC and to characterize their functional interactions.
3 The PNC and Malignant Transformation
3.1 PNC Selectively Forms in Metastatic Solid Tumor Cells
3.1.1 In Vitro Studies
During the initial characterization of the PNC, it was observed that PNC prevalence (% non-mitotic and non-apoptotic cells with one or more PNC) was invariably low (< 5 %) in normal cell lines while heterogeneous, but much higher (15–95 %) in transformed and cancerous cell lines [1]. Examination of many more (> 50) primary cells, normal cell lines, and cancer cell lines has demonstrated the same trend. The PNC can form not only in carcinomas, but also in sarcomas and blastomas; however, the PNC prevalence is invariably low in hematological malignancies. The PNC is not prevalent in primary cells or normal cell lines derived from stromal, endothelial, haematopoetic, or embryonic stem cell origins [36]. These findings suggest that PNCs selectively form in cells from solid tumors.
3.1.2 In Vivo Studies
The increased PNC prevalence in transformed and cancerous cells prompted examination of the PNC in vivo. Breast cancer has been used as a model system since the disease progression and molecular basis of the disease have been extensively characterized. Histological samples of normal breast tissue, primary tumors, affected lymph nodes, and distant metastasis were immunolabeled with SH54, an antibody to PTB, via microwave antigen retrieval protocol. For each sample, > 500 cells in the most active area (with the highest histological grading) were scored for PNC prevalence. The results showed that PNC prevalence is 0 % in normal breast tissue, but increases in the primary tumor along with the progression of the disease from benign, to ductal carcinoma in situ, to affected lymph nodes, and finally reaches near 100 % in distant metastasis. The PNC prevalence also increases in a step wise fashion from the primary tumor, to the cancerous lymph node lesions, to the distant metastasis. In addition, high PNC prevalence in primary tumors positively correlates with disease relapse and inversely correlates with disease free and overall survival in a retrospective 17 year follow-up study. A multivariate examination of the data from this study showed that PNC prevalence provides additional prognostic information for stage I, node negative patients [28]. More recently, we found similar correlations between PNC prevalence and the disease progression in colon and ovarian cancers [37]. Together with the findings that PNC forms in solid tumor cell lines derived from a wide range of tissue types, PNC prevalence has the potential to be a useful pan-cancer prognostic marker, making it the first marker of its kind.
3.2 The PNC and Metastatic Behavior
Results from the breast cancer study [28] showed that PNC prevalence increased with the progression of the disease, ultimately reaching near 100 % in distant metastasis, suggesting that PNC containing cells have a metastatic advantage over those lacking PNCs. High PNC prevalence correlates with increased risk of relapse and decreased overall survival in breast cancer patients, further supporting that the PNC marks the malignant (metastatic) breast cancer cells.
To empirically determine if PNC marks metastatic cells, the PC-3 series of cell lines, which have varying levels of metastatic capacities as selected for in mouse models, were examined for PNC prevalence. The PC-3 cell line was created from a human prostate carcinoma [29]. The PC-3 M cell line is a metastatic variant of PC-3 created by injecting PC-3 cells into a nude mouse, allowing the cells to metastasize, and then resecting the metastatic cells for culturing [30]. The PNC prevalence in PC-3 cells is 4 % and significantly increases to 85 % in the meta-static variant PC-3M [36]. The PC-3 M cell line was further enriched for meta-static cells by injecting them into the prostate of a nude mouse, allowing lymph node metastasis, resecting the metastases and reinjecting the metastatic cells into the prostate of another nude mouse. This process was iterated four times to obtain the highly metastatic variant PC-3M LN4 [31], which has a PNC prevalence of 98 % and abnormally large PNCs [our unpublished data]. Conversely, PC-3 M cells were used to create a cell line enriched with nonmetastatic cells by resecting the primary prostate tumors, reinjecting into the prostate of another nude mouse, and iterating this process four times [31]. This cell line, PC-3 M Pro4, has a PNC prevalence of 71 %, most of which are atypically small and nearly undetectable [36]. When the PNC prevalence is adjusted to the percentage of cells with PNCs greater than 2.2 μm, it correlates very closely with the metastatic behavior of these cells. These observations in cells of the same origin, but of varying metastatic capacities, further confirm that the presence of typical PNCs reflects the metastatic capability of cancer cells. To evaluate the association of PNC prevalence with metastatic behavior in an alternative system, PNC prevalence was examined in cell lines over expressing the breast cancer related metastatic suppressor protein (BRMS). BRMS is a chromatin remodeling protein that suppresses the ability of cells to proliferate at distant sites [32]. Stable over expression in two breast cancer cell lines, MB-MDA-231 and MB-MDA-435, caused a great decrease in metastasis formation compared to the parental cells when injected into a nude mouse [32]. The PNC prevalence in the BRMS overexpressing cell lines is significantly lower than in the parental cells [36] further confirming the association of the PNC with metastatic cells.
3.3 PNC is not a Marker of Differentiation or Growth Rate
Although metastasis is a trait specific to cancer cells, some characteristics of cancer cells can be shared by normal cells, including rapid proliferation, high glycolytic rate, and undifferentiation. To determine whether the PNC prevalence also correlates with traits that are shared by normal cells, the PNC prevalence was examined in several in vitro experimental systems. The proliferation of MCF-10A (normal breast epithelium) cells is over 5 times more rapid than the proliferation of MCF-7 (breast carcinoma) cells. However, the PNC prevalence of the MCF-10A cells is about 7 times lower than the MCF-7 cells [36], which dissociates PNC prevalence from proliferation. To further examine the relationship of PNC prevalence and proliferation, HeLa cells were treated under normal serum or serum starved conditions. The serum starved cells proliferated much slower than the cells grown under normal conditions, but PNC prevalence remains the same under both conditions. Thus, the PNC does not indicate rapid proliferation. In addition, peripheral blood mononuclear cells (PBMCs) were treated with the antigen phytohemagglutinin (PHA), which stimulates metabolism and proliferation of these cells. PBMCs have 0 % PNC, as they are primary cells, and their PNC prevalence remains at 0 % even after 72 h treatment with PHA demonstrating that normal cells cannot form PNC simply by increasing proliferation rate [our [36]], which further dissociates the PNC from proliferation. To address whether the PNC is associated with a high rate of glycolysis, HeLa cells were grown in a medium lacking glucose and pyruvate for 24 h, which significantly inhibits growth when compared to cells grown in normal glucose conditions. PNC prevalence remains the same in cells grown under both conditions, demonstrating that PNC does not correlate with a high rate of glycolysis [our unpublished data] and further demonstrating that the PNC does not associate with proliferation rate. Cancer cells are generally less differentiated than normal somatic cells and an undifferentiated state is also characteristic of normal progenitor cells. To determine whether PNC formation reflects an undifferentiated state, the PNC prevalence was examined in human embryonic stem cells and the results show a 0 % PNC prevalence. In addition, a blastoma cell line (NIE-115) and teratoma cell line (F9) that can each be differentiated by specific chemical treatment [[34, 35] respectively] showed no change in PNC prevalence after induced differentiation, demonstrating that PNC is not a marker of an undifferentiated state [our unpublished data]. These observations together show that PNC formation does not associate with cellular proliferation rate, glycolytic rate, or the differentiation state, all of which are characteristics shared by normal and cancer cells. Therefore, PNC prevalence selectively associates with the metastatic capability of cancer cells.
3.4 Why does the PNC Form in Transformed Cells?
The unique association of the PNC with metastatic capable cancer cells from solid tumors suggests that it forms due to cellular conditions specific to these cells. Two possible mechanisms for PNC formation, which are not mutually exclusive, can be speculated. (1) PNC formation could partially be due to increased need for metabolism or regulation of PNC-associated components in cancer cells. Pol III transcription is significantly increased during transformation [23], which may lead to an excess of RNP RNAs, allowing increased interaction with the protein components of the PNC, leading to the nucleation of the PNC. While the interactions between the protein and RNA components of the PNC have not yet been fully characterized, initial studies suggest that several of the protein and RNA components are in the same complex in vivo as determined by immunoprecipitation and RNA pull-down experiments [[10], our unpublished data]. (2) Another possibility is that yet to be identified factors in the PNC are altered at the level of expression or function specifically in solid tumor cells, which nucleate the RNAs and RNA binding proteins in the PNC. Formation of the PNC is likely a late event during malignant transformation (Fig. 2), possibly as a result of the heterogeneous multi-step process of malignant transformation. These steps can include: increased pol III transcription, altered molecular complex expression levels or functions, and possibly alterations in expression or function of unidentified PNC-nucleation factors. While PNC is most likely a result of an advanced cellular transformation state, the PNC may also functionally promote or maintain malignant phenotype through regulating the molecular complexes associated with the PNC. Studies are currently underway to identify these molecular complexes, their functions in the PNC, and how they impact malignant phenotype.
Fig. 2.

The PNC forms at a late stage in the multi-step process of malignant transformation and is indicative of metastatic cells
4 Potential Utilization of the PNC
4.1 Prognostic Marker for Solid Tumors
Our findings in breast, ovary, and colon cancers, as well as cancer cell lines from multiple tissue origins suggest that PNC is an ideal candidate to be a pan-cancer marker for solid tumors. The PNC is a multi-component complex structure whose presence may reflect the malignant behavior of cancer cells more comprehensively than molecular markers, since malignancy is induced by complex and heterogeneous mechanisms among, and even with in, tumors. In addition, PNC is selectively associated with metastasis without obvious links to proliferation, glycolysis, or differentiation state. Such specific association with malignant behavior makes the PNC a unique tumor marker that may more selectively represent the malignant characteristics of cancer cells than other markers. Therefore, PNC prevalence can be an ideal tumor marker that increases the accuracy of disease prognosis. There is still a great need for selective and specific tumor markers to help make appropriate treatment decisions for many cancer patients. For example, stage I, node negative breast cancer patients often undergo adjuvant chemotherapy, which causes severe side effects and long-term health problems. Yet, the majority of the patients will not have relapses even without the chemotherapy. The lack of accurate prognostic markers that distinguish high risk from low risk patients causes a large proportion of the patients to be over-treated. PNC prevalence provides additional prognostic information for this group of patients than the existing markers [28], demonstrating the potential for PNC prevalence to be developed into a useful prognostic marker. Currently, histological PNC prevalence scoring in tissue samples is being refined to make it a reproducible and reliable marker.
4.2 Anti-cancer Drug Discovery Marker
As the PNC marks metastatic cells in vitro and in vivo, PNC elimination could serve as a surrogate marker that indicates changes in cancer cell behavior toward a more benign phenotype (Fig. 2). This hypothesis rationalizes the use of PNC elimination as a drug discovery marker with the goal of discovering broadly efficacious and selective anti-cancer compounds. Since the PNC is unique to cancer cells and marks metastatic cells, it is reasonable to expect discoveries of compounds that inhibit metastatic behavior of cancer cells while minimally affecting normal cells. The findings from our lab that clinically used cytotoxic cancer drugs and experimental cancer drugs are enriched with PNC reducing compounds while random small molecule libraries are not [35], preliminarily validating that PNC prevalence reduction is a sound screening strategy that should be pursued further [35]. Compounds that eliminate the PNC not only have potential to be developed into novel drugs [38], but can also be used as chemical biology tools to help understand the biology driving PNC formation, maintenance, and its function in malignant cells. Further elucidation of PNC function will lead to a better understanding of novel biology underlying all solid tumor cells and potentially provide novel targeted pan-cancer treatment strategies.
Acknowledgments
We thank Drs. Scott Cameron, Alexander Pertsemlidis, and Zain Paroo for useful comments on this manuscript. We apologize to our colleagues whose outstanding contributions could not be cited due to the space constraint of this minireview. Q.L. is a W.A. “Tex” Moncrief Jr. Scholar in Medical Research and a Damon Runyon Scholar, supported by the Damon Runyon Cancer Research Foundation (DRS-43). The work is also supported by a Welch foundation grant (I-1608) and a National Institute of Health grant (GM078163) awarded to Q.L.
Contributor Information
John T. Norton, Department of Cell and Molecular Biology, Northwestern University Feinberg School of Medicine, 303 E. Chicago Ave, Ward Building 11-240, Chicago, IL 60614, USA
Sui Huang, Department of Cell and Molecular Biology, Northwestern University, Chicago, IL, USA.
References
- 1.Huang S, Deerinck TJ, Ellisman MH, Spector DL. The dynamic organization of the perinucleolar compartment in the cell nucleus. J Cell Biol. 1997;137(5):965–974. doi: 10.1083/jcb.137.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang S, Deerinck TJ, Ellisman MH, Spector DL. The perinucleolar compartment and transcription. J Cell Biol. 1998;143(1):35–47. doi: 10.1083/jcb.143.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghetti A, Piñol-Roma S, Michael WM, Morandi C, Dreyfuss G. hnRNP I, the polypyrimidine tract-binding protein: distinct nuclear localization and association with hnRNAs. Nucleic Acids Res. 1992;20(14):3671–3678. doi: 10.1093/nar/20.14.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timchenko LT, Miller JW, Timchenko NA, DeVore DR, Datar KV, Lin L, et al. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucleic Acids Res. 1996;24:4407–4414. doi: 10.1093/nar/24.22.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall MP, Huang S, Black DL. Differentiation-induced colocalization of the KH-type splicing regulatory protein with polypyrimidine tract binding protein and the c-src premRNA. Mol Biol Cell. 2004;15(2):774–786. doi: 10.1091/mbc.E03-09-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hüttelmaier S, Illenberger S, Grosheva I, Rüdiger M, Singer RH, Jockusch BM. Raver1, a dual compartment protein, is a ligand for PTB/hnRNPI and microfilament attachment proteins. J Cell Biol. 2001;155(5):775–786. doi: 10.1083/jcb.200105044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleinhenz M, Fabienke S, Swiniarski N, Wittenmayer J, Kirsch B, Jockusch H, et al. Raver2, a new member of the hnRNP family. FEBS Lett. 2005;579(20):4254–4258. doi: 10.1016/j.febslet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Shav-Tal Y, Lee BC, Bar-Haim S, Schori H, Zipori D. Reorganization of nuclear factors during myeloid differentiation. J Cell Biochem. 2001;81:379–392. doi: 10.1002/1097-4644(20010601)81:3<379::aid-jcb1052>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Lau PP, Chan L. Involvement of a chaperone regulator, Bcl2-associated athanogene-4, in apolipoprotein B mRNA editing. J Biol Chem. 2003;278(52):52988–52996. doi: 10.1074/jbc.M310153200. [DOI] [PubMed] [Google Scholar]
- 10.Matera AG, Frey MR, Margelot K, Wolin SL. A perinucleolar compartment contains several RNA polymerase III transcripts as well as the polypyrimidine tract-binding protein, hnRNP I. J Cell Biol. 1995;129(5):1181–1193. doi: 10.1083/jcb.129.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Politz JC, Pederson T, Huang S. RNA polymerase III transcripts and the PTB protein are essential for the integrity of the perinucleolar compartment. Mol Biol Cell. 2003;14:2425–2435. doi: 10.1091/mbc.E02-12-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollock C, Daily K, Nguyen VT, Wang C, Lewandowska M, Bensaude O, Huang S. Characterization of MRP RNA-protein interactions within the perinucleolar compartment. Mol Biol Cell. 2011;22(6):858–867. doi: 10.1091/mbc.E10-09-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch HG, Moser M, Müller M. Signal recognition particle-dependent protein targeting, universal to all kingdoms of life. Rev Physiol Biochem Pharmacol. 2003;146:55–94. doi: 10.1007/s10254-002-0002-9. [DOI] [PubMed] [Google Scholar]
- 14.Häsler J, Strub K. Alu elements as regulators of gene expression. Nucleic Acids Res. 2006;34(19):5491–5497. doi: 10.1093/nar/gkl706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fähling M, Steege A, Perlewitz A, Nafz B, Mrowka R, Persson PB, Thiele BJ. Role of nucleolin in posttranscriptional control of MMP-9 expression. Biochim Biophys Acta. 2005;1731(1):32–40. doi: 10.1016/j.bbaexp.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Perry RP. Selective effects of actinomycin D on the intracellular distribution of RNA synthesis in tissue culture cells. Exp Cell Res. 1963;29:400–406. [Google Scholar]
- 17.Higashi K, Matsuhisa T, Kitao A, Sakamoto Y. Selective suppression of nucleolar RNA metabolism in the absence of protein synthesis. Biochim Biophys Acta. 1968;166:388–393. doi: 10.1016/0005-2787(68)90226-8. [DOI] [PubMed] [Google Scholar]
- 18.Wolin SL, Cedervall T. The La protein. Annu Rev Biochem. 2002;71:375–403. doi: 10.1146/annurev.biochem.71.090501.150003. [DOI] [PubMed] [Google Scholar]
- 19.White RJ. RNA polymerase III transcription and cancer. Oncogene. 2004;23:3208–3216. doi: 10.1038/sj.onc.1207547. [DOI] [PubMed] [Google Scholar]
- 20.Lee DY, Clayton DA. Initiation of mitochondrial DNA replication by transcription and R-loop processing. Biol Chem. 1998;273:30614–30621. doi: 10.1074/jbc.273.46.30614. [DOI] [PubMed] [Google Scholar]
- 21.Pruijn GJ, Simons FH, van Venrooij WJ. Intracellular localization and nucleocytoplasmic transport of Ro RNP components. Eur J Cell Biol. 1997;74:123–132. [PubMed] [Google Scholar]
- 22.Paillard L, Legagneux V, Beverley OH. A functional deadenylation assay identifies human CUG-BP as a deadenylation factor. Biol Cell. 2003;95:107–113. doi: 10.1016/s0248-4900(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 23.Norton JT, Wang C, Gjidoda A, Henry RW, Huang S. Perinucleolar compartment is directly associated with DNA. J Biol Chem. 2009;284:4090–4101. doi: 10.1074/jbc.M807255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gherzi R, Lee KY, Briata P, Wegmüller D, Moroni C, Karin M, Chen CY. A KH domain RNA binding protein, KSRP, promotes ARE-directed mRNA turnover by recruiting the degradation machinery. Mol Cell. 2004;14:571–583. doi: 10.1016/j.molcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Norton JT, Pollock CB, Wang C, Schink JC, Kim JJ, Huang S. Perinucleolar compartment prevalence is a phenotypic pan-cancer marker of malignancy. Cancer. 2008;113:861–869. doi: 10.1002/cncr.23632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamath RV, Thor AD, Wang C, Edgerton SM, Slusarczyk A, Leary DJ, et al. Perinucleolar compartment prevalence has an independent prognostic value for breast cancer. Cancer Res. 2005;65(1):246–253. [PubMed] [Google Scholar]
- 27.Kaighn ME, Lechner JF, Narayan KS, Jones LW. Prostate carcinoma: tissue culture cell lines. Natl Cancer Inst Monogr. 1978;49:17–21. [PubMed] [Google Scholar]
- 28.Kozlowski JM, Fidler IJ, Campbell D, Xu ZL, Kaighn ME, Hart IR. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984;44:3522–3529. [PubMed] [Google Scholar]
- 29.Pettaway CA, Pathak S, Greene G, Ramirez E, Wilson MR, Killion JJ, et al. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res. 1996;2:1627–1636. [PubMed] [Google Scholar]
- 30.Oh JE, Karlmark KR, Shin JH, Pollak A, Freilinger A, Hengstschlager M, et al. Differentiation of neuroblastoma cell line N1E - 115 involves several signaling cascades. Neurochem Res. 2005;30(3):333–348. doi: 10.1007/s11064-005-2607-2. [DOI] [PubMed] [Google Scholar]
- 31.Strickland S, Smith KK, Marotti KR. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- 32.Slusarczyk A, Kamath R, Wang C, Anchel D, Pollock C, Lewandowska MA, Fitzpatrick T, Bazett-Jones DP, Huang S. Structure and Function of the Perinucleolar Compartment in Cancer Cells. Cold Spring Harb Symp Quant Biol. 2010;75:599–605. doi: 10.1101/sqb.2010.75.026. Epub 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang RB, Wang HY, Lu HY, Xiong J, Li HH, Qiu XH, Liu HQ. Increased level of polymerase III transcribed Alu RNA in hepatocellular carcinoma tissue. Mol Carcinog. 2005;42(2):93–96. doi: 10.1002/mc.20057. [DOI] [PubMed] [Google Scholar]
- 34.McCutcheon IE, Hentschel SJ, Fuller GN, Jin W, Cote GJ. Expression of the splicing regulator polypyrimidine tract-binding protein in normal and neoplastic brain. Neuro Oncol. 2004;6(1):9–14. doi: 10.1215/S1152851703000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He X, Pool M, Darcy KM, Lim SB, Auersperg N, Coon JS, Beck WT. Knockdown of polypyrimidine tract-binding protein suppresses ovarian tumor cell growth and invasiveness in vitro. Oncogene. 2007;26(34):4961–4968. doi: 10.1038/sj.onc.1210307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storck S, Shukla M, Dimitrov S, Bouvet P. Functions of the histone chaperone nucleolin in diseases. Subcell Biochem. 2007;41:125–144. doi: 10.1007/1-4020-5466-1_7. [DOI] [PubMed] [Google Scholar]
- 37.Samant RS, Seraj MJ, Saunders MM, Sakamaki TS, Shevde LA, Harms JF, et al. Analysis of mechanisms underlying BRMS1 suppression of metastasis. Clin Exp Metastasis. 2000;18(8):683–693. doi: 10.1023/a:1013124725690. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Norton JT, Witshi MA, Xu Q, Lou G, hen Wang C, Appella DH, Chen Z, Huang S. Methoxyethlyamino-numonafide is an efficacious and minimally toxic amonafide derivative in murine models of human cancer. Neoplasia. 2011;13(5):453–460. doi: 10.1593/neo.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Eenennaam H, Jarrous N, van Venrooij WJ, Pruijn GJ. Architecture and function of the human endonucleases RNase P and RNase MRP. IUBMB Life. 2000;49:265–272. doi: 10.1080/15216540050033113. [DOI] [PubMed] [Google Scholar]


