Abstract
Background
Pulmonary rehabilitation (PR) is able to improve dyspnea, endurance capacity, and health-related quality of life in chronic obstructive pulmonary disease (COPD) patients, but it is rarely used in China. This study aimed to assess the effectiveness and safety of PR after exacerbation of COPD.
Material/Methods
Patients admitted to hospital due to an exacerbation of COPD were randomized to receive either PR or routine care (control group). The PR program was performed from the second day of admission until discharge. The pre-post changes in 6-minute walk distance (6MWD), self-reported quality of life (QOL) assessed by CAT score and CRQ-SAS score, and activity of daily life assessed by ADL-D score were determined. The perceived end-effort dyspnea (Borg scale) was measured throughout the study.
Results
A total of 101 patients were enrolled, of whom 7 withdrew after randomization, and 94 completed this study. There were 66 patients in the PR group and 28 in the control group. The 6MWD, resting SpO2, and exercise Borg dyspnea score were significantly improved in the PR group. In addition, the PR group had greater improvement in the total CRQ-SAS score and had a lower CAT score. Significant improvements were also found in the ADL-D and BODE index in the PR group. No adverse events were recorded during exercise.
Conclusions
Our study provides evidence that it is safe and feasible to apply an early PR in patients with acute exacerbation of COPD.
Keywords: Disease Progression; Pulmonary Disease, Chronic Obstructive; Rehabilitation Centers
Background
Chronic obstructive pulmonary disease (COPD) is characterized by exertional dyspnea, wheezing, and sputum production, and often decreased daily life activities [1]. Acute exacerbations of COPD (AECOPD) are defined as changes in sputum volume, color, or consistency, accompanied by an increase in dyspnea. Exacerbations usually result in decreases in lung function, exercise tolerance, and quality of life (QOL) [2]. It has been shown that, even with optimal medical treatment during hospitalization, patients at discharge need considerable time to recover to baseline levels of physical function and health status [2]. Up to 25% of patients after an acute exacerbation may not fully recover to baseline peak flow at 3 months [3]. The recovery period of health status is very long, but there is no further exacerbation [4]. Frequent exacerbators have a reduced response to treatment of AECOPD in terms of inflammatory indices and quality of life [5]. Pulmonary rehabilitation (PR) plays an important role in the management of COPD [6]. PR includes respiratory system interventions (e.g., smoking cessation and medications), psychological support (e.g., patients’ education, and psychological and social support), and physical exercise, and there is evidence showing that PR is able to improve exercise capacity and QOL [7–9]. Most studies on PR have been done in stable COPD patients, and trials with small sample size also suggest that PR is feasible and effective in patients with exacerbations of COPD [10–13]. Therefore, an early PR has become a component of recovery programs in the management of acute COPD. However, PR is rarely applied in China. The main reasons might be that physicians need more knowledge on PR, and concern about whether PR is safe after exacerbations of COPD, especially in patients with severe COPD [14,15]. Therefore, this study was conducted to assess the effectiveness and safety of PR after exacerbation of COPD. The feasibility and safety of an early PR was evaluated in AECOPD inpatients, and the effects of PR on the exercise capacity and QOL were determined.
Material and Methods
Subjects
This was a prospective, randomized, open trial conducted in inpatients admitted due to AECOPD. These patients received standard medical treatments and were then randomized to receive either routine care (control group) or PR (PR group) from the second day of admission until discharge.
We recruited 101 consecutive inpatients with AECOPD between December 2011 and November 2013 from Tongji Hospital, School of Medicine of Tongji University. All the patients had a diagnosis of COPD prior to admission. The diagnosis and determination of COPD severity were conducted according to the Global Initiative for Chronic Obstructive Lung Disease guidelines. An exacerbation of COPD was defined as the worsening of respiratory symptoms beyond normal day-to-day variation and leading to a change in medication [16]. Patients were eligible if they reported a limitation in daily activities due to dyspnea on exertion, as categorized using the Modified Medical Research Council (mMRC) dyspnea grade >0. When patients had any disease not associated with COPD (e.g., uncontrolled heart failure, sever lower limb arthritis, and symptomatic peripheral vascular disease, which may affect the outcome of dyspnea or exercise tolerance) they were excluded from this study. Other exclusion criteria included severe orthopedic or neurological disorders limiting exercise performance, unstable cardiac disease, and inability to understand or complete questionnaires. This study was registered in the Chinese Clinical Trial Registry (ChiCTR-TRC-13003068) and approved by the Human Research Ethics Board of Affiliated Tongji Hospital of Tongji University in Shanghai, China (2011-161). All participants signed written informed consent before participating in the study.
Baseline measurements
Baseline measurements were age, gender, body mass index (BMI), smoking status, use of long-term oxygen therapy (LTOT), and pulmonary function. The outcome measures in this study were dyspnea and functional status, functional exercise capacity, limitation in daily activities, and health status. This information was collected at baseline and on the day of discharge.
Spirometry, static lung volumes, and lung diffusion capacity for carbon monoxide were also measured in accordance with a standard protocol [17,18]. Arterial blood gas analysis was done at rest, or in the presence of oxygen supplementation for subjects receiving LTOT.
Dyspnea and functional status were evaluated using the mMRC dyspnea grade [19]. Subjects were asked to point to the Borg scale, and rate the severity of perceived breathlessness.
The 6-minute walk test was performed according to published guidelines along a 30-meter corridor [20]. The test was performed twice on consecutive days and the longest distance [6-minute walk distance (6MWD)] was used for analysis. Oxygen saturation was monitored continuously throughout the test, and the test was terminated if oxygen saturation fell to below 80%. Pre-exercise and the lowest oxygen saturation were recorded during the test. Heart rate (HR) was monitored continuously throughout the test (Polar, Polar Electro, Oy, Finland). In the 6-minute walk test, the same flow rate of supplemental oxygen was used as was prescribed for their normal daily activities in subjects receiving LTOT. All follow-up exercise tests were performed in subjects using the same flow rate of supplemental oxygen as applied in the exercise test at baseline.
Limitations in daily life activities were assessed using an Activity of Daily Living Dyspnea scale (ADL-D scale), which was a validated scale for COPD patients [21]. Paul et al. reported that the CAT correlated well with CRQ-SAS domains and SGRQ scores in health status measures in pulmonary rehabilitation [22]. Thus, QOL was measured using the Chronic Respiratory Questionnaire-Self Administered Standardized (CRQ-SAS) scale [23] and COPD assessment test (CAT) [24].
Pulmonary rehabilitation program
Briefly, each PR session included exercise training, relaxation, and breathing retraining and education [25]. Symptom-limited exercise training was used. Patients were trained at between 3 and 5 on the Borg breathlessness score (moderate to severe). Resting oxygen saturation, HR, and blood pressure were measured and recorded before, during, and at the end of PR. Patients received 30-minute exercise twice-daily. Exercise training included stretches, endurance, and strength training. Lower-limb endurance training was performed using a treadmill with the initial workload prescribed at 60% of the peak work rate achieved in the 6-minute walk test at baseline. Walking was controlled within 5–10 min and progressively increased, within symptom tolerance, to 20 min of continuous walking. Upper-limb endurance training comprised repetitive bilateral shoulder flexion and abduction using a light weight and was synchronized with expiration for 2 min. Strength training was accomplished using free weights or the subject’s own body weight. One set of 10 repetitions was initially prescribed and then increased to 3 sets when the subject could perform the exercises without any difficulty. Arterial oxygen saturation was monitored, and supplemental oxygen was given to maintain arterial oxygen saturation above 85%. Breathing retraining consisted of relaxation with breathing control, pursed-lip breathing, and pacing during exercise training and daily life activities. The pursed-lip breathing was to assist subjects to control their breathing by reducing respiratory frequency. All subjects received the same instructions.
The education component was provided by a physiotherapist at each class and consisted of the benefits and importance of daily exercise, pacing and energy-conservation techniques to manage daily-life activities, and self-management strategies for coping with an exacerbation of COPD.
Statistical analysis
Data are presented as mean ± standard error. A value of P<0.05 was considered statistically significant. Student’s unpaired t-test was used for comparisons of age, BMI, Brinkman Index, and days of PR, and lung function after determination of normal distribution. Student’s paired t-test was used for comparisons of data before and after 6-MWD, Borg, mMRC score, CAT score, CRQ-SAS score, ADL-D score, and BODE score.
Results
Characteristics of patients and baseline measurements
A total of 101 patients were enrolled into this study, of whom 94 completed this study and 7 withdraw after randomizations. The characteristics of 94 patients in 2 groups before PR are presented in Table 1. They had moderate-to-severe COPD, and most were males and smokers. There were no significant differences in age, BMI, Brinkman index, and FEV1% predicted at baseline between the 2 groups.
Table 1.
Baseline characteristics of patients completing this study.
| PR group | Control group | P | |
|---|---|---|---|
| Patients, No. | 66 | 28 | |
| Age, yr | 69.2±1.53 | 73.9±1.84 | 0.017 |
| Sex, M/F, No. | 60/6 | 23/5 | |
| BMI, kg/m2 | 22.3±0.80 | 23.4±0.76 | 0.147 |
| Brinkman index | 851±85.03 | 783±107.65 | 0.313 |
| Current smoker | 2 | 4 | |
| PR duration, days | 9.1±0.85 | 10.1±1.33 | 0.212 |
| FEV1, % Pred. | 38±3.04 | 39±5.07 | 0.481 |
| FEV1, L | 0.86±0.13 | 0.90±0.15 | 0.101 |
| GOLD stage, No. | |||
| I | 0 | 0 | |
| II | 0 | 1 | |
| III | 28 | 15 | |
| IV | 38 | 13 | |
Exercise capacity
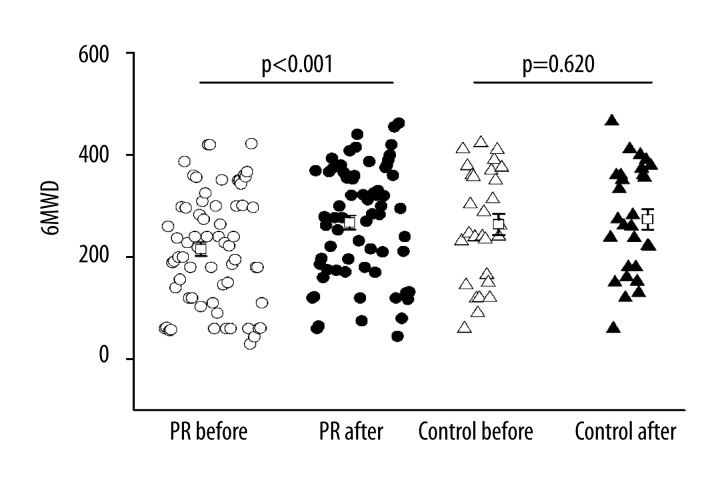
No adverse events were recorded during exercise training. The 6MWD was increased by 49.0m in PR group as compared to that at baseline (242.0±15.03 m vs. 291.0±14.61m, P<0.001). In contrast, the 6MWD was increased by 9.9 m (263.9±20.75 m vs. 273.7±20.03 m) in the control group as compared to that at baseline, showing no significant difference (P=0.620, Figure 1). There was a significant improvement in resting SpO2 at rest in the PR group as compared to baseline. There was a significant improvement in the dyspnea at rest as measured by Borg dyspnea score in both the PR and control groups as compared to that at baseline. However, a significant improvement was observed in dyspnea at exercises (P=0.008) only in the PR group, but not in the control group (P=0.108). These findings suggested that there was a significant improvement in the exercise capacity after PR (Table 2).
Figure 1.
Effects of PR on 6MWD. Open circle: PR group before PR, solid circle: PR group after PR, open triangle: control group before PR, solid triangle: control group after usual care.
Table 2.
Effects of PR on dyspnea and exercise capacity during 6-minute walk test.
| PR group | Control group | |||||
|---|---|---|---|---|---|---|
| Before | After | P | Before | After | P | |
| Resting SpO2 (%) | 95.8±0.66 | 97.1±0.24 | 0.001 | 96.7±0.50 | 96.3±0.42 | 0.183 |
| Exercise SpO2 (%) | 90.7±1.58 | 91.5±1.21 | 0.329 | 90.9±1.50 | 92.3±1.11 | 0.500 |
| Resting Borg dyspnea score | 0.4±0.13 | 0.1±0.05 | 0.013 | 0.5±0.15 | 0.3±0.15 | 0.033 |
| Exercise Borg dyspnea score | 3.5±2.50 | 1.8±0.32 | 0.008 | 3.1±0.38 | 2.2±0.41 | 0.108 |
Effects of exercise on QOL and daily life activities
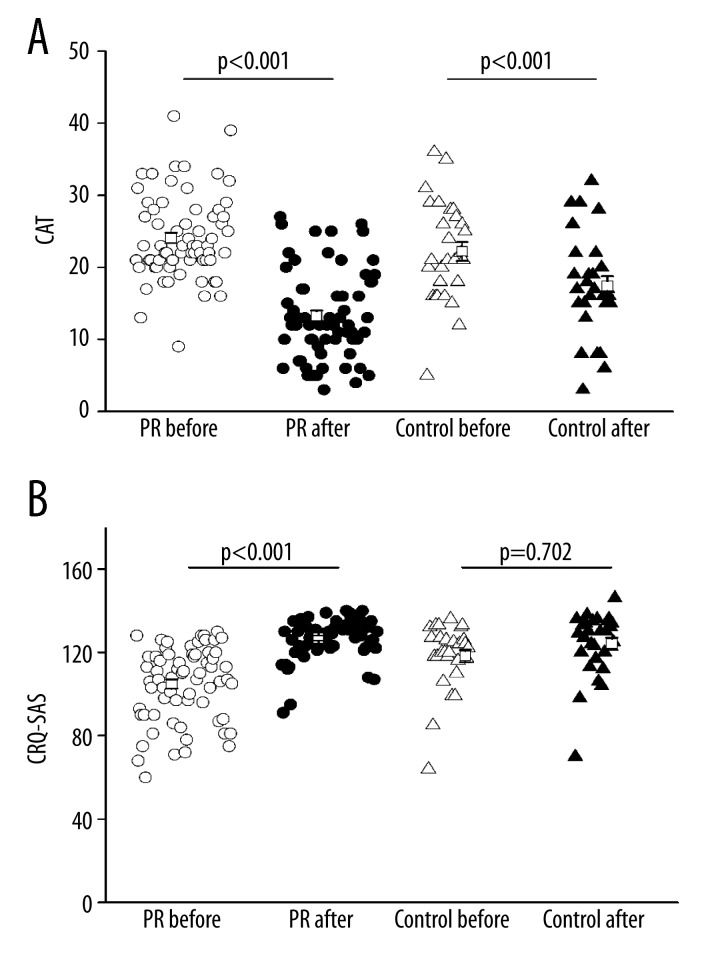
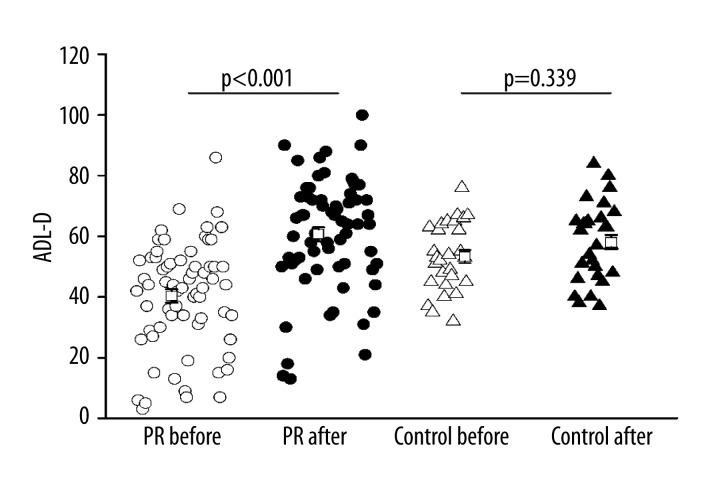
There was a significant improvement in CAT score (the lower the score, the better the QOL was) in both the PR group and the control group. There was a significant improvement in CRQ-SAS score in the PR group from baseline to post-PR (P<0.001) and a slight decline in the control group (Figure 2). The ADL-D score was improved as indicated by an increase in total score from 40.4±2.19 to 60.7±2.31 in the PR group, and a significant increase was observed after PR. ADL-D score was also improved as indicated by an increase in total score form 53.3±2.19 to 58.07±2.43 in the control group, but there was no significant difference (Figure 3).
Figure 2.
Effects of PR on QOL, assessed by CAT score (A) and CRQ-SAS score (B). Open circle: PR group before PR, solid circle: PR group after PR, open triangle: control group before PR, solid triangle: control group after routine care.
Figure 3.
Effects of PR on daily life activities, assessed by ADL-D score. Open circle: PR group before PR, solid circle: PR group after PR, open triangle: control group before PR, solid triangle: control group after routine care.
mMRC and BODE index
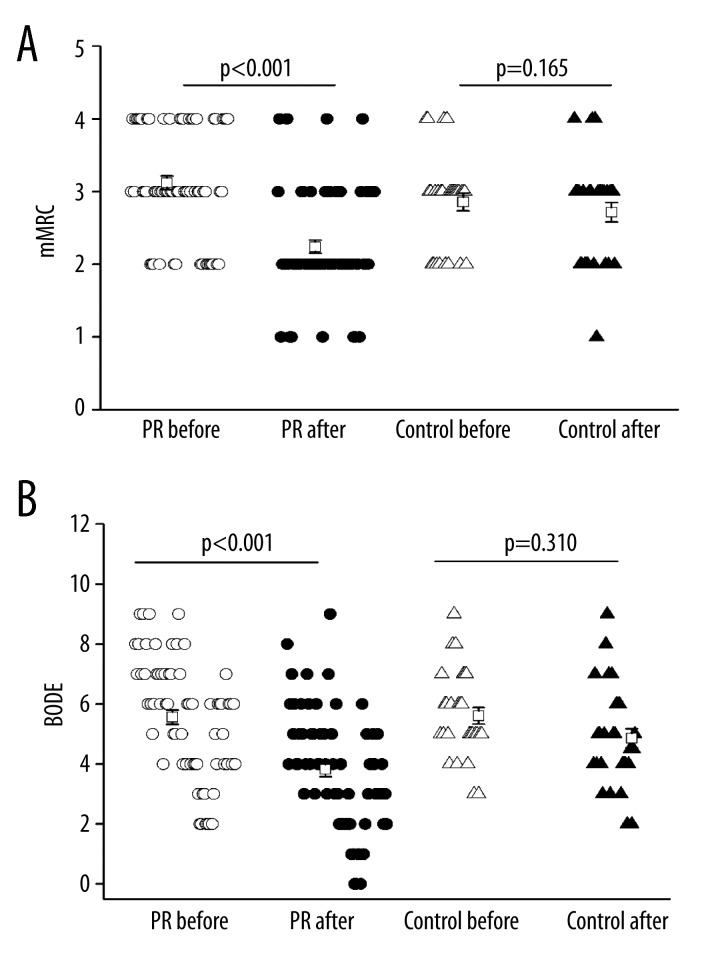
Dyspnea is a symptom associated with exercise performance and QOL. One of the major goals of COPD treatment is to attenuate dyspnea. To measure the severity of dyspnea during exercise and daily life activities, the mMRC dyspnea scale was used. Results showed that the mMRC was improved from 3.1±0.93 at baseline to 2.2±0.09 after PR in the PR group, showing a significant difference. The mMRC was improved from 2.9±0.65 at baseline to 2.7±0.71 in the control group without marked difference (Figure 4A).
Figure 4.
Effects of PR on mMRC (A) and BODE index (B). Open circle: PR group before PR, solid circle: PR group after PR, open triangle: control group before PR, solid triangle: control group after routine care.
The BODE index is a multi-dimensional grading system determined on the basis of BMI, FEV1%, mMRC dyspnea score, and 6MWD. The BODE index has been validated in COPD patients, and is responsive to PR [26]. Our study indicated that the BODE index was improved from 5.6±0.24 at baseline to 3.8±0.24 after PR in the PR group, showing a significant difference. The BODE index was improved from 5.6±0.27 at baseline to 4.9±0.32 in the control group without significant difference (Figure 4B).
Discussion
Our study provides preliminary evidence that it is safe and feasible to perform PR in inpatients with AECOPD. Of 94 patients completing this trial, none experienced a serious adverse event, nor had an increase in hospital stay. In a large population of severely impaired COPD patients with high exacerbation rates, a significant reduction in exacerbation and hospitalization frequency was observed after participation in a comprehensive pulmonary rehabilitation program [27]. In a previous study, 14 of 126 patients with stable COPD experienced a serious adverse event during PR in a community setting [28]. The relaxation training (e.g., stretches and strength training before exercise training, breathing training, and symptom-limited exercise training) may help these patients to finish PR [29].
Our results showed that the PR group had significant improvements in 6MWD, resting SpO2, and exercise Borg dyspnea score, indicating that PR is beneficial in improving the exercise capacity of patients with AECOPD. In addition, greater improvement in total CRQ-SAS score and lower CAT score were also observed in the PR group, indicating that PR is helpful to improve the QOL of patients with AECOPD.
Several factors have contributed to the improvements identified in this study. PR was applied with symptom-limited exercise training, which is a low-intensity exercise. Moderate-to-high-intensity exercise may be inappropriate for acutely ill patients with a compromised respiratory function. In other studies, low-intensity exercise has been found to benefit patients experiencing AECOPD [11,30]. A second contribution to the improvements was that the “respiratory conditioning” of PR was a procedure for improving the flexibility of the chest with the correction of posture and the stretching and mobilization of the rib cage. Our PR incorporated physical therapist-assisted manual stretching of the respiratory muscles to reduce dyspnea before starting exercise training. Respiratory conditioning was reported to decrease the chest wall stiffness by stretching the respiratory muscles of the chest wall during breathing, and contributed to expanding expiratory flow and reducing hyperinflation of the lungs at rest [31,32]. In addition, by decreasing chest wall stiffness and increasing its flexibility, the reduced hyperinflation of the lung at rest may generate greater mobility of the diaphragm. Consequently, this effect improves the exercise capacity. In this context, respiratory conditioning in our PR ameliorated symptoms, and raised exercise capacity. Moreover, physical therapist-assistant manual compression and relaxation of the thoracic cage of our respiratory conditioning created a strong interaction between patient and physical therapist, thereby decreasing the anxiety about exercise training and reducing the breathlessness, which is another factor playing a role in the improvement of daily life activities and QOL. Our PR may ensure that all the patients including the severe COPD patients can finish their exercise sessions. A third contributing factor to the improvements was the breath training of PR. Our PR included pursed-lips breathing and diaphragmatic breathing, aiming to alter the respiratory muscle recruitment, improve respiratory muscle performance, and reduce dyspnea [33,34], which have been reported to improve the 6MWD [33].
Our study has several limitations: the sample size was relatively small; the majority of patients were males, reflecting the gender difference in disease morbidity; and we did not assess social background. In addition, the mechanism by which PR achieves its effectiveness was not explored. Therefore, these factors should be taken into account in future studies.
PR is recommended by the international guidelines as a part of COPD management. PR is becoming more popular for patients. An awareness of the clinical sequelae of acute exacerbation of chronic obstructive pulmonary disease enables approaches, such as early post-exacerbation rehabilitation to mitigate its negative effects [35]. However, it is still not adequately used in China [36], and the major barriers are the low referral rate in the primary-care center and difficulty in accessing suitable programs. Studies from Shanghai, China reported that PR was rarely used in local hospitals. The main reason was that respiratory physicians have only limited knowledge about PR, which leads to a poor attendance of COPD patients to PR. Therefore, research on the availability of suitable and safe PR programs and interrelated issues of referral and access is needed [14,15].
Conclusions
The present study provides evidence that it is safe and feasible to implement an early PR for inpatients with AECOPD, and clinicians should promote PR after AECOPD no matter how severe AECOPD is.
Acknowledgments
We thank all the patients and staff members at each rehabilitation center. We also thank the technical assistance and helpful discussion from Prof Hideaki Senjyu (Department of Rehabilitation Medicine, Nagasaki University Hospital, Nagasaki, Japan). We also thank Mr. Kozo Fujiwara (Rehabilitation Department of Kurashiki Daiichi Hospital, Fukuoka, Japan) for his assistance.
Footnotes
Source of support: This study was supported by grants from National Natural Science Foundation of China (81200044) and Shanghai Pujiang Program (12PJ1407800) and Research Fund for the Doctoral Program of Higher Education of China (20120072120070) to M.H.
References
- 1.Wesseling G, Vrijhoef HJ. Acute exacerbations of COPD: recommendations for integrated care. Expert Rev Respir Med. 2008;2:489–94. doi: 10.1586/17476348.2.4.489. [DOI] [PubMed] [Google Scholar]
- 2.Clini EM, Crisafulli E, Costi S, et al. Effects of early inpatient rehabilitation after acute exacerbation of COPD. Respir Med. 2009;103:1526–31. doi: 10.1016/j.rmed.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Seemungal TA, Donaldson GC, Bhowmik A, et al. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–13. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 4.Spencer S, Jones PW. Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax. 2003;58:589–93. doi: 10.1136/thorax.58.7.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang C, Yao W. Time course of inflammation resolution in patients with frequent exacerbations of chronic obstructive pulmonary disease. Med Sci Monit. 2015;21:311–20. doi: 10.12659/MSM.889828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casaburi R, ZuWallack R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:1329–35. doi: 10.1056/NEJMct0804632. [DOI] [PubMed] [Google Scholar]
- 7.Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 8.Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131:4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 9.Pitta F, Troosters T, Probst VS, et al. Are patients with COPD more active after pulmonary rehabilitation? Chest. 2008;134:273–80. doi: 10.1378/chest.07-2655. [DOI] [PubMed] [Google Scholar]
- 10.Puhan M, Scharplatz M, Troosters T, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009:CD005305. doi: 10.1002/14651858.CD005305.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Troosters T, Probst VS, Crul T, et al. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:1072–77. doi: 10.1164/rccm.200908-1203OC. [DOI] [PubMed] [Google Scholar]
- 12.Eaton T, Young P, Fergusson W, et al. Does early pulmonary rehabilitation reduce acute health-care utilization in COPD patients admitted with an exacerbation? A randomized controlled study. Respirology. 2009;14:230–38. doi: 10.1111/j.1440-1843.2008.01418.x. [DOI] [PubMed] [Google Scholar]
- 13.Clini E, Roversi P, Crisafulli E. Early rehabilitation: much better than nothing. Am J Respir Crit Care Med. 2010;181:1016–17. doi: 10.1164/rccm.201001-0054ED. [DOI] [PubMed] [Google Scholar]
- 14.Chen YH, Yao WZ, Kang J, et al. Attitudes and actions of chronic obstructive pulmonary disease patients on treatment: a national multi-center investigative study. Zhonghua Jie He He Hu Xi Za Zhi. 2010;33:750–53. [in Chinese] [PubMed] [Google Scholar]
- 15.Chen XD, Jin XD, Li M, et al. Knowledge Level about Pulmonary Rehabilitation: A Questionnaire Analysis in Respiratory Physicians in Shanghai. Chinese J Respir Crit Care Med. 2012;11:375–77. [Google Scholar]
- 16.The Global Initiative for Chronic Obstructive Lung Disease. [Accessed May 30, 2014]. Global Strategy for the Diagnosis, Management, and Prevention of COPD (2014 Update) [editorial] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 18.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–35. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 19.Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–86. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test [editorial] Am J Respir Crit Care Med. 2002;166:111–17. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 21.Yoza Y, Ariyoshi K, Honda S, et al. Development of an activity of daily living scale for patients with COPD: the Activity of Daily Living Dyspnoea scale. Respirology. 2009;14:429–35. doi: 10.1111/j.1440-1843.2009.01479.x. [DOI] [PubMed] [Google Scholar]
- 22.Jones PW, Harding G, Wiklund I, et al. Tests of the responsiveness of the COPD assessment test following acute exacerbation and pulmonary rehabilitation. Chest. 2012;142:134–40. doi: 10.1378/chest.11-0309. [DOI] [PubMed] [Google Scholar]
- 23.Schunemann HJ, Goldstein R, Mador MJ, et al. A randomised trial to evaluate the self-administered standardised chronic respiratory questionnaire. Eur Respir J. 2005;25:31–40. doi: 10.1183/09031936.04.00029704. [DOI] [PubMed] [Google Scholar]
- 24.Gupta N, Pinto LM, Morogan A, Bourbeau J. The COPD assessment test: a systematic review. Eur Respir J. 2014;44(4):873–84. doi: 10.1183/09031936.00025214. [DOI] [PubMed] [Google Scholar]
- 25.Tydeman DE, Chandler AR, Graveling BM, et al. An investigation into the effects of exercise tolerance training on patients with chronic airways obstruction. Physiotherapy. 1984;70:261–64. [PubMed] [Google Scholar]
- 26.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 27.van Ranst D, Stoop WA, Meijer JW, et al. Reduction of exacerbation frequency in patients with COPD after participation in a comprehensive pulmonary rehabilitation program. Int J Chron Obstruct Pulmon Dis. 2014;9:1059–67. doi: 10.2147/COPD.S69574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maltais F, Bourbeau J, Shapiro S, et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2008;149:869–78. doi: 10.7326/0003-4819-149-12-200812160-00006. [DOI] [PubMed] [Google Scholar]
- 29.Kozu R, Senjyu H, Jenkins SC, et al. Differences in response to pulmonary rehabilitation in idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. Respiration. 2011;81:196–205. doi: 10.1159/000315475. [DOI] [PubMed] [Google Scholar]
- 30.Tang CY, Blackstock FC, Clarence M, Taylor NF. Early rehabilitation exercise program for inpatients during an acute exacerbation of chronic obstructive pulmonary disease: a randomized controlled trial. J Cardiopulm Rehabil Prev. 2012;32:163–69. doi: 10.1097/HCR.0b013e318252f0b2. [DOI] [PubMed] [Google Scholar]
- 31.Minoguchi H, Shibuya M, Miyagawa T, et al. Cross-over comparison between respiratory muscle stretch gymnastics and inspiratory muscle training. Intern Med. 2002;41:805–12. doi: 10.2169/internalmedicine.41.805. [DOI] [PubMed] [Google Scholar]
- 32.Yamada M, Kakizaki F, Sibuya M, et al. Clinical effects of four weeks of respiratory muscle stretch gymnastics in patients with chronic obstructive pulmonary disease. Nihon Kyobu Shikkan Gakkai Zasshi. 1996;34:646–52. [in Japanese] [PubMed] [Google Scholar]
- 33.Holland AE, Hill CJ, Jones AY, McDonald CF. Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;10:CD008250. doi: 10.1002/14651858.CD008250.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguti WP, Claudino RC, Neto AP, et al. Diaphragmatic Breathing Training Program Improves Abdominal Motion During Natural Breathing in Patients With Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial. Archives Of Physical Medicine And Rehabilitation. 2012;93:571–77. doi: 10.1016/j.apmr.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein R, Brooks D. Pulmonary rehabilitation at the time of the COPD exacerbation. Clin Chest Med. 2014;35:391–98. doi: 10.1016/j.ccm.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Johnston K, Grimmer-Somers K. Pulmonary rehabilitation: overwhelming evidence but lost in translation? Physiother Can. 2010;62:368–73. doi: 10.3138/physio.62.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]