Abstract
Increased catalytic activity of cystathionine β-synthase (CBS) was recently shown to mediate vasodilation of the cerebral microcirculation, which is initiated within minutes after the onset of acute hypoxia. To test whether chronic hypoxia was a stimulus for increased CBS expression, U87-MG human glioblastoma and PC12 rat pheochromocytoma cells were exposed to 1% or 20% O2 for 24 to 72 h. CBS mRNA and protein expression were increased in hypoxic cells. Hypoxic induction of CBS expression was abrogated in cells transfected with vector encoding short hairpin RNA targeting hypoxia-inducible factor (HIF) 1α or 2α. Exposure of rats to hypobaric hypoxia (0.35 atm) for 3 d induced increased Cbs mRNA, protein, and catalytic activity in the cerebral cortex and cerebellum, which was blocked by administration of the HIF inhibitor digoxin. HIF binding sites, located 0.8 and 1.2 kb 5′ to the transcription start site of the human CBS and rat Cbs genes, respectively, were identified by chromatin immunoprecipitation assays. A 49-bp human sequence, which encompassed an inverted repeat of the core HIF binding site, functioned as a hypoxia response element in luciferase reporter transcription assays. Thus, HIFs mediate tissue-specific CBS expression, which may augment cerebral vasodilation as an adaptive response to chronic hypoxia.
Keywords: hydrogen sulfide, oxygen, cerebral circulation
Introduction
The brain represents 2% of body weight but is responsible for 20% of total body oxygen consumption because the considerable energy requirements of neuronal activity are met primarily by mitochondrial respiration [1]. Measurement of tissue partial pressure of O2 (PTO2) in the rat cerebral cortex has yielded mean values from 14 to 27 mm Hg with considerable spatial and temporal variation [2-4]. When the ambient O2 concentration was reduced from 21% to 10%, the mean cerebral PTO2 decreased to a mean of 7 to 13 mm Hg [5]. A homeostatic balance between O2 supply and demand is maintained by autoregulation of the cerebral microcirculation, in which reduced local O2 availability triggers vasodilation, thereby increasing local blood flow and O2 delivery. Cerebral blood flow is regulated by three gaseous signaling molecules: nitric oxide (NO) and hydrogen sulfide (H2S) induce vasodilation, whereas carbon monoxide (CO) induces vasoconstriction [6]. Cystathionine β-synthase (CBS), which is expressed by glia and astrocytes in the cerebral cortex and cerebellum, is a heme protein that catalyzes the conversion of cysteine into pyruvate and H2S [7-10]. The catalytic activity of CBS is negatively regulated by binding of CO to its heme moiety [1]. Heme oxygenase (HO) catalyzes the conversion of heme and O2 to biliverdin and CO; under conditions of reduced O2 availability in the brain, CO levels decline, leading to increased CBS activity and vasodilation [11]. Immunohistochemistry revealed that the end feet of CBS-expressing astrocytes directly abut cerebral blood vessels, which is consistent with their role in paracrine regulation of vascular tone [11].
Acute hypoxia (exposure lasting seconds to minutes) induces vasodilation of the cerebral microcirculation, which is due in part to direct, but transient, changes in the catalytic activity of HO and CBS [11]. In contrast, responses to chronic hypoxia (exposure lasting from hours to days) involve changes in gene expression, leading to altered protein levels. The principal regulators of gene expression in response to chronic hypoxia are the hypoxia-inducible factors (HIFs), which consist of an O2-regulated HIF-1α or HIF-2α subunit that heterodimerizes with the constitutively expressed HIF-1β subunit to form the active transcription factors HIF-1 and HIF-2, respectively [12]. HIF-1α levels increase exponentially at O2 concentrations less than 6%, both in cultured cells [13] and isolated perfused and ventilated lung preparations [14]. HIFs bind to the consensus DNA sequence 5′-RCGTG-3′ (R = A or G) and over 1200 HIF target genes have been identified, but most are expressed in a tissue-specific manner such that, in any given cell type, a battery of several hundred genes is induced by hypoxia [15]. In this study, we tested the hypothesis that chronic hypoxia induces increased CBS expression in the brain that is mediated by HIF-dependent transcriptional activation.
Materials and Methods
Cell Culture
U87-MG cells were cultured in DMEM with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) at 37°C in a humidified 5% CO2, 95% air incubator. PC12 cells were cultured in DMEM with 5% FBS, 10% horse serum, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) at 37°C in a humidified 10% CO2, 90% air incubator. Before hypoxic exposure, the media was replaced with fresh media containing 25 mM HEPES, then the cells were placed in a modulator incubator chamber (Billups-Rothenberg) that was flushed with a gas mixture consisting of 1% O2, 5% CO2, with balance N2, and incubated at 37°C. The HIF inhibitors digoxin [16] and acriflavine [17] were purchased from Sigma; cells were pre-treated with inhibitors for 1 h before hypoxic exposure. Control cells were treated with vehicle (0.1% [v/v] DMSO).
Immunoblot Assays
Cell and tissue lysates were prepared with modified RIPA buffer (25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% IGEPAL CA-630, 1% sodium deoxycholate, 0.1% SDS) supplemented with a protease inhibitor cocktail (Roche). The antibodies against the following proteins were used for immunoblot assays: human HIF-1α (BD Biosciences), rat HIF-1α (Novus Biologicals), human and rat HIF-2α (Novus Biologicals), human and rat CBS (Abnova), human and rat Actin (Santa Cruz).
Lentivirus Production
Recombinant lentivirus was produced by transfection of HEK293T cells with the following plasmids: pMD.G, pCMV-ΔR8.91, and recombinant pLKO.1 expression vector encoding one of the following short hairpin RNAs (shRNAs): shNT, non-targeting control shRNA; shHIF-1α, shRNA targeting shHIF-1α# or shHIF-2α shRNA targeting shHIF-2α. The following shRNAs were previously described: shHIF-1α#1 and shHIF-2α#1 [18]; and shHIF-2α#3 [19]; shNT and shHIF-1α#9 were purchased from Sigma. Lentiviral supernatants were harvested, passed through a 0.45-μm filter, concentrated by centrifugation through an Amicon filter with a 100 kDa size exclusion, and resuspended in fresh media.
Luciferase Reporter Assays
Oligonucleotides were inserted into pGL2-Promoter (Promega), which encodes firefly luciferase downstream of an SV40 basal promoter. Oligonucleotides were either wild type or contained the following mutations: MUT1, GCGTG→GAAAG; MUT2, CTGTG→GGCAT; MUT3, CACGT→CTTTT. U87-MG or PC12 cells were transfected with firefly luciferase reporter plasmid and control reporter plasmid pSV-Renilla, which encodes Renilla luciferase, with or without pcDNA3.1-HIF-1α or HIF-2α expression vector, or vector encoding shRNA targeting HIF-1α or HIF-2α. Twenty four hours after transfection, cells were exposed under the 20% or 1% O2 for 24 h. Luciferase reporter activities were measured with the Dual-Luciferase Assay System (Promega).
Reverse Transcription and Real-time PCR (RT-qPCR)
Total RNA was isolated by TRIZOL (Invitrogen), treated with DNase I (Ambion), and cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad). Real- time PCR was performed as described using SYBR Green (Fermentas) [20]. The hypoxia-induced change in target mRNA expression was calculated based on the Δ(ΔCt) method: ΔCt = Cttarget- Ct18SrRNA for U87-MG and PC12 cells; ΔCt = Cttarget- CtRpl13a for rat brain samples; Δ(ΔCt) = Ctnon-hypoxic – Cthypoxic. Primer sequences are as follows: 18S rRNA, CGG CGA CGA CCC ATT CGA AC and GAA TCG AAC CCT GAT TCC CCG TC; CBS, CCA CAT CAC CAC ACT GCC and GCC GAA CTT CTTCCC AAT CT; SLC2A1, CGG GCC AAG AGT GTG CTA AA and TGA CGA TAC CGG AGC CAA TG; CTH, TCT ACC TGC GTG CTT TAG and AAG AAC ACC GAA GAT ATA ACC; MPST, GAA CAT CCC CTT CAC AGA and TTC TTC TCC TGG AAC AGA T; Rpl13a, GAT GAA CAC CAA CCC GTC TC and ATC CCA TCC AAC ACC TTG AG; Cbs, AGA CAC CGA CTG GTT TCC AC and TAG GGA GCT GAG CGT TAG GA; Vegf, GGA GGA TGT CCT CAC TTG GA and CAA ACA GAC TTC GGC CTC TC; and Slc2a1, GCC TGA GAC CAG TTG AAA GC and CAG TGC ACA GGA GAC CTG AA.
Chromatin Immunoprecipitation (ChIP) Assays
ChIP assays were performed as described [19] with ChIP assay kit (Millipore). U87-MG and PC12 cells were exposed to 20% or 1% O2 for 24 or 4 h, respectively. Cells were fixed with 1% formaldehyde for 25 min and quenched with 125 mM glycine. Immunoprecipitation was performed with the following antibodies: anti-human HIF-1α (Santa Cruz); anti-rat HIF-1α (Novus Biologicals); anti-HIF-2α (Novus Biologicals); and anti-HIF-1β (Novus Biologicals). Real-time PCR was performed with SYBR Green (Fermentas) using the following primers: CBS, TGC CAT TTC TCG TCT GGT and CTG TCC AGT CTT TAT AAG AAG AGA G; Cbs, GTC AGG CTC CTG CCG TTC A and ACA GCC TGG CCA AGT GTG TC.
Exposure of Rats to Hypobaric Hypoxia
Experiments were approved by the Institutional Animal Care and Use Committee of the University of Chicago and were performed using male Sprague-Dawley rats (200-250 g). Rats were exposed to hypobaric hypoxia (0.35 atm, 7.35% O2, simulating an altitude of 8,500 m) or normoxia (1 atm, 21% O2) continuously without interruption for 3 d, during which they received daily intraperitoneal (IP) injections of digoxin (1 mg/kg/d) or vehicle (0.1% [v/v] DMSO), were not restrained, and fed ad libitum. The rats were then anesthetized with urethane (1.2 g/kg IP) and the cerebral cortex and cerebellum were harvested and stored at -80°C for subsequent analysis.
Measurement of CBS Activity
Brain tissue was lysed in 3 volumes of 0.05 M Tris-HCl (pH 7.4) buffer containing 10 mM EDTA and 20% (v/v) glycerol. The tissue extract was pretreated with 0.2 mM DL-propargylglycine to inhibit cystathionine γ-lyase (CTH) activity. To determine CBS activity, an assay based on detection of H2S was used. The reaction was performed in sealed tubes containing center wells to trap the gaseous H2S generated during the reaction as described previously [21]. The reaction mixture (100 mM potassium phosphate buffer (pH 7.4), 2 mM L-cysteine, 0.2 mM pyridoxal 5′ -phosphate, 0.5 mM Ado-Met and 2-10 μg protein equivalent of extract) and the center well containing H2S trapping solution (0.2 ml 1% zinc acetate and a 2 × 2.5 cm piece of filter paper) were placed in a sealed tube and incubated at 37°C for up to 4 h. The reaction was stopped by addition of 0.5 ml of 50% trichloroacetic acid. To the center wells containing trapped H2S, 50 μl of 20 mM N,N-dimethyl-p-phenylenediamine sulfate in 7.2 M HCl and 50 μl of 30 mM FeCl3 in 1.2 M HCl were added. The absorbance of the resulting solution was measured at 670 nm. The concentration of H2S was determined using Na2S as standard.
Vascular Smooth Muscle Cell Cultures
Male Wistar rats (150-200 g) were anesthetized with sodium pentobarbital, the heart and lungs were removed, and the thoracic aorta and intralobar pulmonary arteries (200-600 μm outer diameter) were isolated and cleaned of connective tissue in ice-cold HEPES-buffered saline solution (HBSS) containing (in mM): 130 NaCl, 5 KCl, 1.2 MgCl2, 10 HEPES and 10 glucose, with pH adjusted to 7.2 with 5 M NaOH. The arteries were opened, the endothelium removed and the cleaned tissue allowed to recover for 30 min in cold HBSS followed by 20 min in reduced-Ca2+ HBSS (20 μM CaCl2) at room temperature. Following recovery, the tissue was incubated at 37°C (20 min for pulmonary arteries or 40 min for aorta) in reduced-Ca2+ HBSS containing: type I collagenase (1750 U/ml), papain (9.5 U/ml), bovine serum albumin (2 mg/ml) and dithiothreitol (1 mM). Single smooth muscle cells were dispersed in Ca2+-free HBSS and cultured in Smooth Muscle Growth Media (Lonza) supplemented with 1% penicillin/streptomycin until 80% confluent. Cells were placed in low serum (0.3%) Smooth Muscle Basal Media for 24 h before beginning experiments. >90% of cells expressed heavy chain myosin by immunofluorescence (Abcam).
Vascular Endothelial Cell Cultures
Human lung microvascular and aortic endothelial cells, from 3 different donors each, were obtained from Lonza at passage 3-4. Cells were cultured in Endothelial Basal Medium (EBM-2, Lonza) with growth supplements (EGM-2 Bullet Kit, Lonza) at 37° in a humidified atmosphere of 5% CO2/95% air. For lung microvascular endothelial cells, the media was supplemented with an additional 5% serum. Cells were used for experiments at passage 7; immunofluorescence demonstrated expression of von Willebrand factor (Abcam) but not smooth muscle-specific α-actin (Sigma).
Results
CBS Expression is Induced in Hypoxic Human Glioblastoma Cells
Because CBS is expressed in glial cells and astrocytes in the brain, glial-derived U87-MG cells were exposed to 20% or 1% O2 for 24 h and mRNA levels were quantified by RT-qPCR. CBS mRNA expression was significantly induced by hypoxia, similar to the established HIF target gene product, SLC2A1 mRNA (Figure 1A). In contrast, expression of mRNAs encoding the other two enzymes that generate H2S, CTH and 3-mercaptopyruvate sulfurtransferase (MPST), was not induced in hypoxic U87-MG cells. CBS protein levels increased in U87-MG cells exposed to 1% O2 for 72 h (Figure 1B). Hypoxia did not induce CBS mRNA expression in cultured human aortic or lung microvascular endothelial cells, whereas the expression of SLC2A1 mRNA was induced in both endothelial cell types (Figure 1C).
Figure 1. Expression of CBS in U87-MG cells under hypoxic conditions.
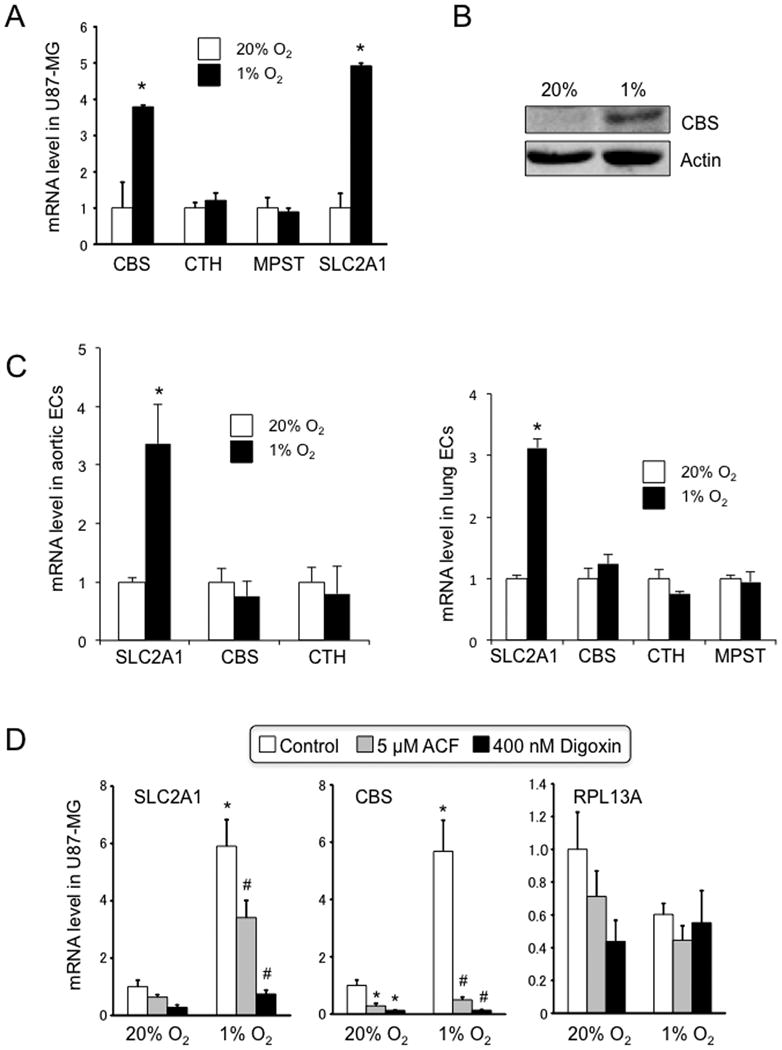
(A) Cells were exposed to 20% or 1% O2 for 24 h and total RNA was isolated. Reverse transcription and real-time quantitative PCR (RT-qPCR) were performed to determine levels of mRNA encoding cystathionine β-synthase (CBS), cystathionine γ-lyase (CTH), and 3-mercaptopyruvate sulfurtransferase (MPST). Levels of SLC2A1 mRNA, which is encoded by a known HIF target gene, were also determined as a positive control. Data shown are mean ± S.E.M., n = 3; *p < 0.05 vs cells exposed to 20% O2. (B) immunoblot assays were performed to analyze CBS and actin protein levels. U87-MG cells were exposed to 20% or 1% O2 for 72 h and whole cell lysates were prepared. Asterisks show nonspecific band and arrow indicates CBS. (C) Primary cultures of human aortic (left panel) and lung microvascular (right panel) endothelial cells (ECs) were exposed to 20% or 1% O2 for 24 h and qRT-PCR assays were performed to quantify levels of the indicated mRNAs, normalized to the levels at 20% O2 (mean ± S.E.M., n = 3). MPST mRNA was not detected in aortic ECs. (D) U87-MG cells were exposed to 20% or 1% O2 for 24 h in the presence of vehicle (0.1% DMSO), 5 μM acriflavine (ACF), or 400 nM digoxin. Total RNA was isolated and RT-qPCR assays were performed using primers specific for SLC2A1, CBS, and RPL13A mRNA. Data shown are mean ± S.E.M., n = 3; *p < 0.05 vs vehicle-treated cells exposed to 20% O2; #p < 0.05 vs vehicle-treated cells exposed to 1% O2.
To investigate whether HIF was required for hypoxia-induced CBS expression, U87-MG cells were treated with digoxin or acriflavine, which inhibit HIF accumulation and dimerization, respectively [16, 17]. Both inhibitors blocked the induction of CBS mRNA expression in cells exposed to 1% O2, which was similar to their effect on SLC2A1 mRNA levels, whereas the expression of RPL13A mRNA, which is not HIF regulated, was unaffected by drug treatment under hypoxic conditions (Figure 1D). The finding that two chemically unrelated HIF inhibitors blocked the induction of CBS in response to hypoxia provides strong evidence that HIF activity is required for the change in gene expression.
To complement the pharmacological approach to HIF loss-of-function, U87-MG subclones were generated that stably expressed shRNAs that efficiently inhibited the expression of HIF-1α or HIF-2α protein, as shown by immunoblot assays (Figure 2A). Both of the shRNAs inhibited the expression of CBS and SLC2A1 mRNA (Figure 2B) and CBS protein (Figure 2C) in hypoxic U87-MG cells, demonstrating that both HIF-1 and HIF-2 contribute to hypoxia-induced CBS expression in these cells. The results were confirmed with an independent set of shRNAs targeting different sequences in HIF-1α or HIF-2α (Figures 2D-F). Taken together, the data presented in Figures 1 and 2 indicate that hypoxia induces HIF-dependent CBS expression in a cell type-specific manner.
Figure 2. Expression of CBS in U87-MG cells with HIF loss of function.
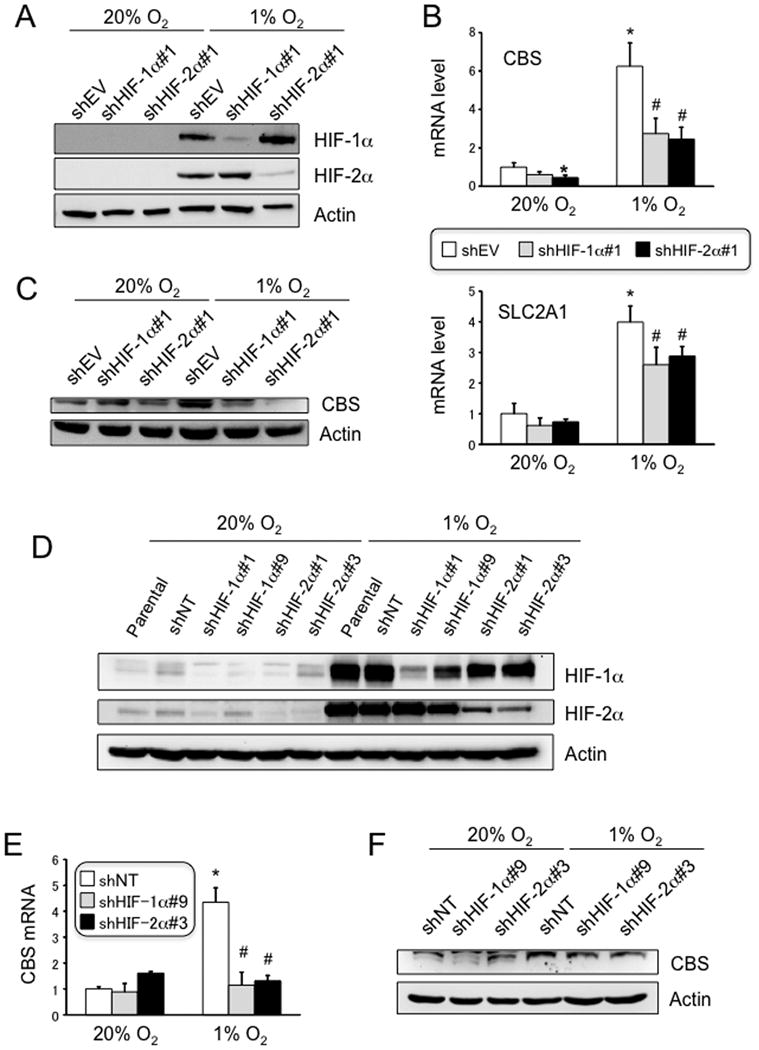
(A) U87-MG subclones, which were stably transfected with vector encoding short hairpin RNA (shRNA) targeting HIF-1α (shHIF-1α) or HIF-2α (shHIF-2α), or with empty vector (shEV), were exposed to 20% or 1% O2 for 4 h and whole cell lysates were prepared for immunoblot assays. (B) U87-MG subclones were exposed to 20% or 1% O2 for 24 h, followed by RT-qPCR assays of CBS and SLC2A1 mRNA expression (mean ± S.E.M., n = 3); *p < 0.05 vs shEV cells exposed to 20% O2; #p < 0.05 vs shEV cells exposed to 1% O2. (C) U87-MG subclones were exposed to 20% or 1% O2 for 72 h, followed by immunoblot assays. Arrow indicates CBS. Independent U87-MG subclones stably transfected with shRNAs that target different sequences in HIF-1α (shHIF-1α#9) or HIF-2α (shHIF-2α#3) or were transfected with a non-targeting control shRNA (shNT) were analyzed for expression of HIF proteins (D), CBS mRNA (E), and CBS protein (F) as described above.
Identification of a Hypoxia Response Element (HRE) in the Human CBS Gene
In order to determine the molecular mechanism by which HIFs induce CBS expression, the human CBS gene sequence was analyzed for matches to the HIF consensus binding site sequence, 5′-RCGTG-3′. Approximately 0.8 kb 5′ to the transcription start site, an inverted repeat of HIF sites, separated by 8 bp, was identified that was similar to previously characterized HREs in the human LDHA [22] and mouse Slc2a1 [23] genes (Figure 3A). ChIP assays demonstrated that binding of HIF-1α, HIF-1β and to a lesser extent, HIF-2α, to the -0.8-kb site was induced when U87-MG cells were exposed hypoxia (Figure 3B).
Figure 3. Identification of a hypoxia response element (HRE) in the human CBS gene.
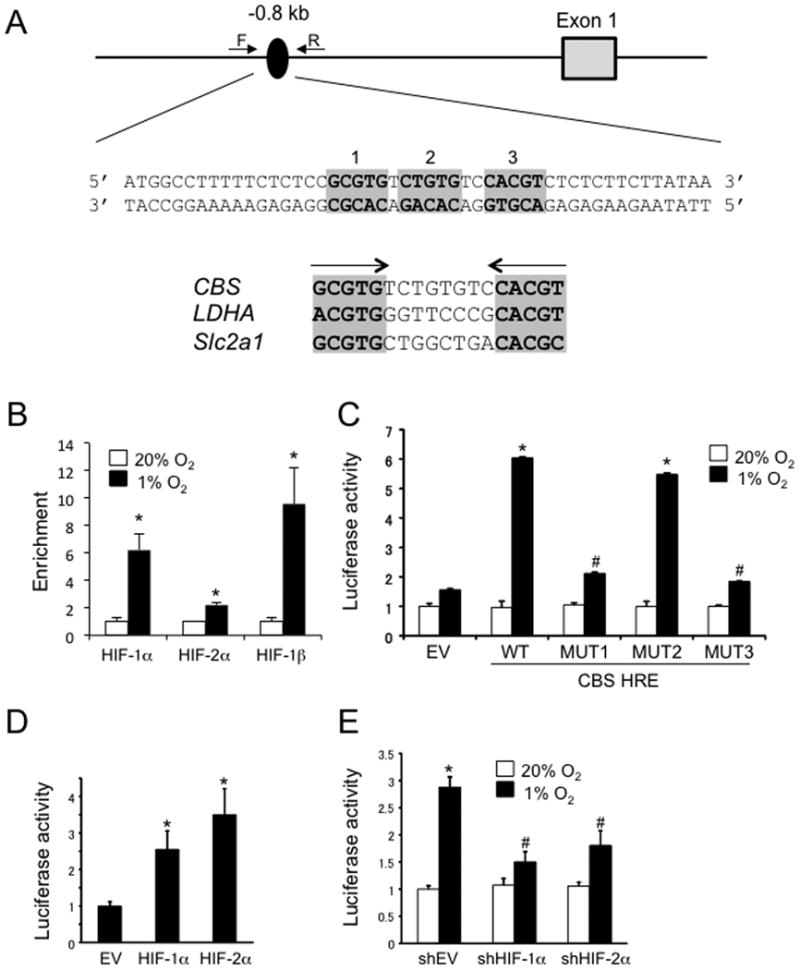
(A) Candidate HIF binding sites (oval, with nucleotide sequence shown below), located 0.8 kb 5′ to the transcription start site of the CBS gene, were interrogated by ChIP using the indicated primers. The HIF binding sites are organized as an inverted repeat separated by 8 bp, similar to the organization of previously identified hypoxia response elements (HREs) from the LDHA and Slc2a1 genes. (B) ChIP assay of U87-MG cells exposed to 20% or 1% O2 for 24 h, using antibodies against HIF-1α HIF-2α and HIF-1β Enrichment of each sequence in the immunoprecipitate relative to the starting lysate was determined by qPCR (mean ± S.E.M., n = 3); *p < 0.05 vs cells exposed to 20% O2. (C) U87-MG cells were transiently co-transfected with pSV-Renilla and recombinant pGL2-Promoter containing the wild-type CBS gene sequence (WT) or a sequence with mutation of site 1 (MUT1), site 2 (MUT2), or site 3 (MUT3). Transfected cells were exposed to 20% or 1% O2 for 24 h, and the ratio of firefly: Renilla luciferase activity was determined (mean ± S.E.M., n = 3); *p < 0.05 vs cells transfected with WT reporter and exposed to 20% O2; #p < 0.05 vs cells transfected with WT reporter and exposed to 1% O2. (D) U87-MG cells were co-transfected with pSV-Renilla, pGL2-Promoter-WT, and a vector encoding HIF-1α or HIF-2α, or empty vector (EV). Transfected cells were exposed to 1% O2 for 24 h, and the ratio of firefly:Renilla luciferase activity was determined (mean ± S.E.M., n = 3); *p < 0.05 vs cells transfected with EV. (E) U87-MG cells were co-transfected with pSV-Renilla, pGL2-Promoter-WT, and vector encoding shHIF-1α or shHIF-2α, or empty vector (shEV). Transfected cells were exposed to 20% or 1% O2 for 24 h, and the ratio of firefly:Renilla luciferase activity was determined (mean ± S.E.M., n = 3); *p < 0.05 vs cells transfected with shEV and exposed to 20% O2; #p < 0.05 vs cells transfected with shEV and exposed to 1% O2.
To demonstrate functional activity in a transcriptional assay, a 49-bp sequence encompassing the inverted repeat (Figure 3A) was inserted into reporter plasmid pGL2-Promoter, which encodes firefly luciferase under the control of a basal SV40 promoter, and co-transfected into U87-MG cells with pSV-Renilla, which encodes Renilla luciferase under the control of the same SV40 promoter. The ratio of firefly:Renilla luciferase activity in transfected cells exposed to 20% or 1% O2 was determined. The ratio of firefly:Renilla luciferase activity was similar under non-hypoxic and hypoxic conditions when the pGL2-Promoter empty vector (EV) was transfected; in contrast, when recombinant pGL2-Promoter containing the wild type (WT) 49-bp CBS gene sequence was transfected, the ratio of firefly:Renilla luciferase activity was increased by more than 5-fold under hypoxic conditions (Figure 3C). Mutation of either HIF binding site (labeled site 1 and site 3 in Figure 3A) resulted in a significant loss of hypoxia-induced firefly luciferase activity (MUT1 and MUT3 in Figure 3C), whereas mutation of a sequence between the two HIF binding sites (site 2 in Figure 3A) had no significant effect on luciferease activity (MUT2 in Figure 3C).
To demonstrate that the hypoxia-induced reporter activity was HIF dependent, U87-MG cells were transiently co-transfected with firefly (WT) and Renilla luciferase reporters and either an expression vector encoding HIF-1α or HIF-2α, or an empty vector (EV). Overexpression of either HIF-1α or HIF-2α was sufficient to specifically increase firefly luciferase activity under hypoxic conditions (Figure 3D). Finally, U87-MG cells were transiently co-transfected with firefly and Renilla reporters and an expression vector encoding shHIF-1α or shHIF-2α, or an empty vector (EV). Knockdown of either HIF-1α or HIF-2α significantly impaired the induction of firefly luciferase activity in hypoxic cells (Figure 3E). From the data presented in Figure 3, we conclude that HIF binding to an HRE located 0.8 kb 5′ to the transcription start site is necessary and sufficient for transcriptional activation of the human CBS gene.
Exposure of Rats to Hypoxia Induces Cbs Expression in the Brain
Cbs is expressed by astrocytes in rat brain, leading to the production of H2S, which mediates cerebral vasodilatation in response to acute hypoxia [11]. To investigate whether chronic hypoxia induces HIF-dependent expression of Cbs in the brain, rats were placed in a hypobaric chamber (0.35 atm) or maintained under control conditions (1 atm) for 3 d after which the cortex and cerebellum were harvested. Cbs mRNA levels were increased in cortex (Figure 4A) and cerebellum (data not shown) of rats subjected to hypobaric hypoxia, as were levels of Vegf mRNA, another HIF target gene, whereas Slc2a1 mRNA expression was not induced in rat cortex. Treatment of the rats with digoxin (1 mg/kg/d IP) during the hypobaric exposure blunted the induction of Cbs and Vegf mRNA in the cerebral cortex (Figure 4A) and cerebellum (data not shown). CBS protein levels also increased in the cerebral cortex (Figures 4B-C) and cerebellum (data not shown) of hypoxic rats and this induction was blunted by concurrent digoxin treatment. As observed for the mRNA and protein expression, Cbs enzymatic activity was also significantly increased in the cerebral cortex of rats exposed to hypobaric hypoxia and this induction was blunted by digoxin treatment (Figure 4D).
Figure 4. Expression of Cbs in rat cerebral cortex.
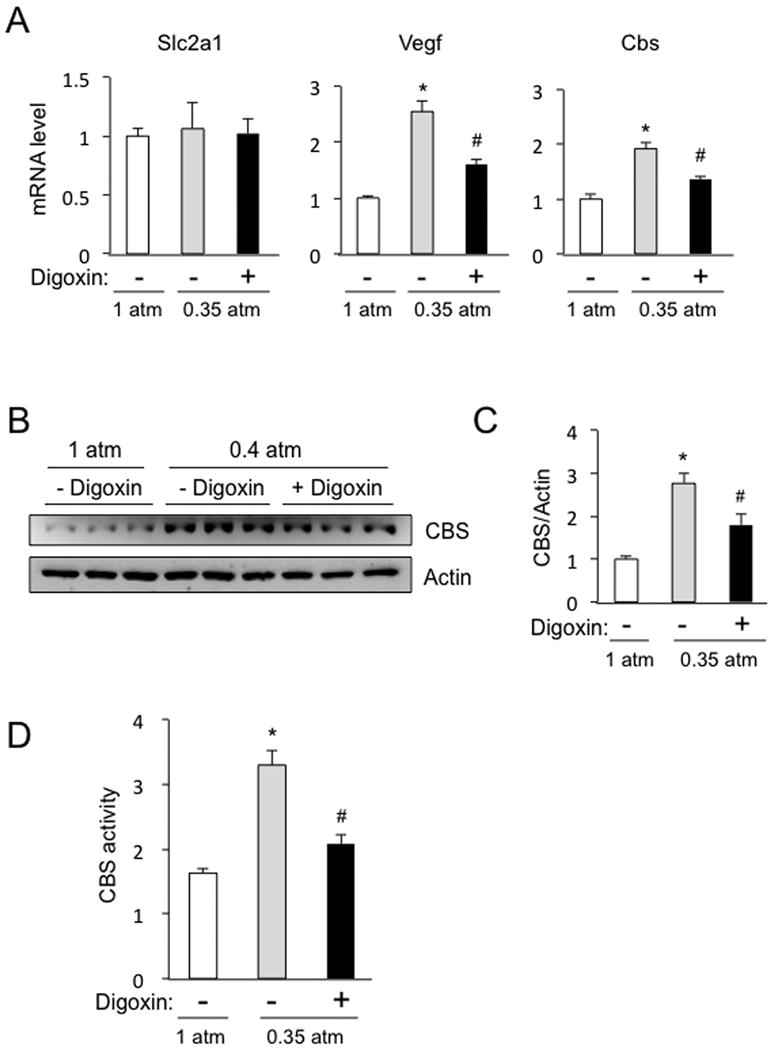
Rats were exposed to normobaric (1 atm) or hypobaric (0.35 atm) conditions for 3 d, during which time they were treated with digoxin (+) or vehicle (-), and then the cerebral cortex was harvested for analysis. (A) RT-qPCR assays were performed to quantify Slc2a1, Vegf, and Cbs mRNA levels. (B) immunoblot assays were performed to analyze Cbs and actin protein levels. (C) immunoblot band intensity was determined by densitometry (mean ± S.E.M., n = 3); *p < 0.05 vs 1 atm; #p < 0.05 vs vehicle. (D) Cbs enzymatic activity was measured (nmol H2S/h/mg protein; mean ± S.E.M., n = 3); *p < 0.05 vs 1 atm; #p < 0.05 vs vehicle.
Identification of an HRE in the Rat Cbs Gene
Inspection of the rat Cbs gene sequence revealed the presence of candidate HIF binding sites ∼1.2 kb 5′ to the transcription start site, consisting of two sites arranged as an inverted repeat separated by 7 bp followed by a third site located 22 bp downstream (Figure 5A). PC12 pheochromocytoma cells are a rat cell line that has been utilized extensively to study HIF-mediated transcriptional responses to hypoxia [24]. Exposure of PC12 cells to hypoxia induced Cbs mRNA expression (Figure 5B). ChIP assays in PC12 cells, using primers flanking the putative HIF binding sites in the 5′-flanking region of the Cbs gene (Figure 5A), revealed hypoxia-induced binding of HIF-1α and HIF-1β but not HIF-2α (Figure 5C). A 64-bp sequence encompassing the HIF binding sites (Figure 5A) was inserted into pGL2-Promoter (Cbs-HRE) and transiently co-transfected into PC12 cells with pSV-Renilla. Compared to co-transfection of pGL2-Promoter empty vector (EV), firefly luciferase activity was significantly induced in hypoxic PC12 cells co-transfected with Cbs-HRE (Figure 5D). In contrast to the hypoxia-induced expression of Cbs mRNA in PC12 cells, hypoxia did not induce increased expression of Cbs (or Cth or Mpst) mRNA in rat aortic or pulmonary arterial smooth muscle cells, whereas expression of Slc2a1 mRNA was induced in both vascular smooth muscle cell types (Figure 5E). Taken together, the data presented in Figure 5 demonstrate that exposure of PC12 cells to hypoxia induces cell type-specific Cbs gene expression that is associated with the binding of HIF-1 to an HRE located in the 5′-flanking region of the Cbs gene.
Figure 5. Identification of an HRE in the rat Cbs gene.
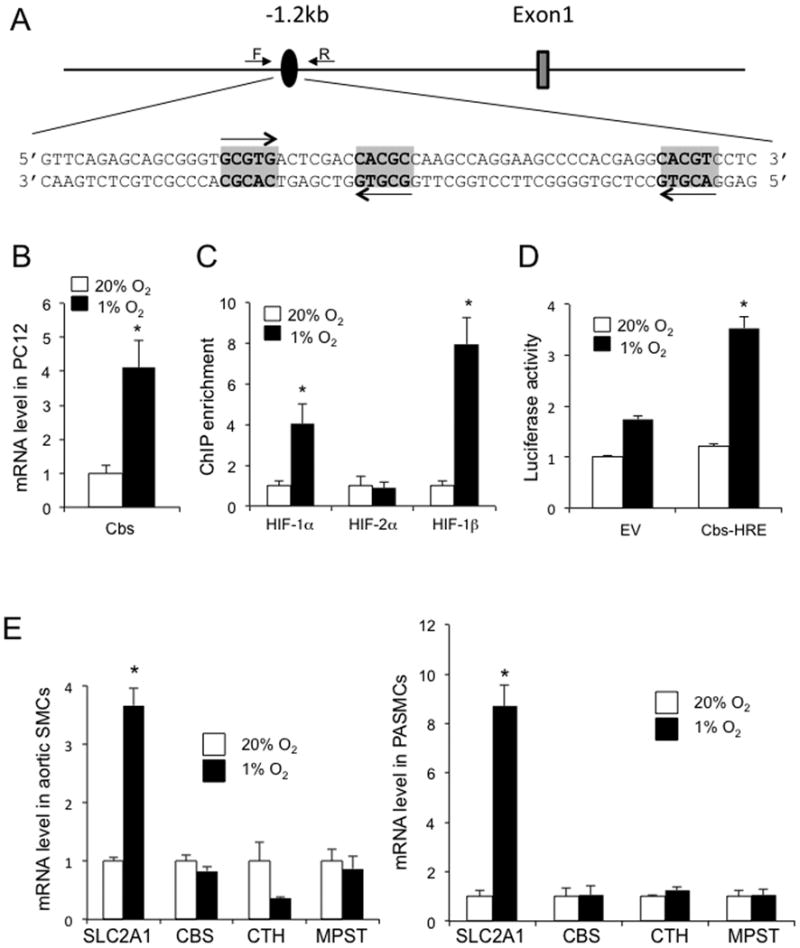
(A) Candidate HIF binding sites (oval, with nucleotide sequence shown below), located 1.2 kb 5′ to the transcription start site of the Cbs gene, were interrogated by ChIP using the indicated primers. (B) CBS mRNA levels were quantified by RT-qPCR in rat PC12 cells exposed to 20% or 1% O2 for 24 h (mean ± S.E.M., n = 3); *p < 0.05 vs 20% O2. (C) ChIP assay of PC12 cells exposed to 20% or 1% O2 for 4 h using antibodies against HIF-1α HIF-2α or HIF-1β. (D) PC12 cells were transiently co-transfected with pSV-Renilla and pGL2-Promoter (EV) or recombinant pGL2-Promoter containing the Cbs gene sequence shown in panel A (Cbs-HRE). Transfected cells were exposed to 20% or 1% O2 for 24 h, and the ratio of firefly:Renilla luciferase activity was determined (mean ± S.E.M., n = 3); *p < 0.05 vs cells transfected with EV. (E) Rat aortic (left panel) and pulmonary arterial (PA; right panel) smooth muscle cells (SMCs) were exposed to 20% or 1% O2 for 24 h and qRT-PCR assays were performed to quantify levels of the indicated mRNAs, normalized to the levels at 20% O2 (mean ± S.E.M., n = 3).
Discussion
In this study, we demonstrate that HIFs mediate hypoxia-induced CBS expression in U87-MG glioblastoma cells, PC12 pheochromocytoma cells, and the cerebral cortex and cerebellum of rats exposed to hypobaric conditions that simulated an altitude of 8,500 m. Our results complement a recent report that in response to acute hypoxia, H2S produced by CBS mediates cerebral vasodilation, which is required to maintain ATP levels in the brain [11]. In addition, H2S may have direct protective effects on neurons, either by scavenging reactive oxygen species or by activating KATP channels [25-28]. The results of our study suggest that under conditions of sustained (chronic) hypoxia, H2S production may be further augmented by a HIF-dependent increase in CBS mRNA and protein expression. Additional studies will be required to demonstrate that chronic hypoxia induces augmented cerebral blood flow in a HIF- and CBS-dependent manner.
Chronic exposure to high altitude is associated with increased cerebral blood flow, which has been attributed to hypoxia-induced vasodilation leading to increased oxygenation [29-31]. Our results suggest that HIF-dependent CBS expression may contribute to this adaptive response. Previous studies have shown that HIFs mediate transactivation of the EPO [32] and VEGF [33] genes in response to chronic hypoxia, leading to increased blood O2-carrying capacity and angiogenesis, respectively, which also contribute to improved tissue oxygenation [34]. Thus, HIFs function as master regulators by activating transcription of multiple genes that play critical roles in mediating adaptation to high altitude hypoxia [9].
The majority of HIF target genes are induced by hypoxia in a cell type-restricted manner. Recent studies indicate that in cell types in which target gene expression is activated by HIF-1, the HIF binding sites are located in DNase-hypersensitive chromatin domains [35]. We found that CBS expression was induced by hypoxia in glioblastoma and pheochromocytoma cell lines, as well as rat cerebral cortex and cerebellum, but not in vascular endothelial or smooth muscle cells (whereas the opposite was true for SLC2A1 expression). Further studies are required to determine whether hypoxia-induced CBS expression occurs in other cell types, in which it may contribute to the regulation of blood flow or other adaptive responses to hypoxia. We did not observe hypoxia-induced expression of either CTH or MPST mRNA in the cell types analyzed in this study. However, it is intriguing that CTH is required for oxygen sensing in the carotid body [21], suggesting that expression of this H2S-generating enzyme may also be induced by hypoxia in cell type-restricted manner.
Finally, several recent studies have reported that H2S modulates HIF transcriptional activity [36-39]. These results suggest the possibility that complex feedback regulation between CBS and HIF may be critical to balance cerebral blood flow (oxygen supply) and oxygen demand.
Summary statement.
Hypoxia induced expression of cystathionine β-synthase in U87-MG cells, PC12 cells, and rat brain, which was blocked by inhibition of hypoxia-inducible factors (HIFs). Chromatin immunoprecipitation assays demonstrated that HIFs bound directly to the CBS gene in response to hypoxia.
Acknowledgments
We thank Karen Padgett of Novus Biologicals for generously providing antibodies against HIF-1α, HIF-2α, and HIF-1β.
Funding: This work was supported in part by the Japan Science and Technology Agency (JST) Exploratory Research for Advanced Technology (ERATO-ICORP) Suematsu Gas Biology Project and by funds from the Johns Hopkins Institute for Cell Engineering. G.L.S. is the C. Michael Armstrong Professor at the Johns Hopkins University School of Medicine.
Abbreviations
- CBS
cystathionine β-synthase
- HIF
hypoxia-inducible factor
- H2S
hydrogen sulfide
- NO
nitric oxide
- CO
carbon monoxide
- CTH
cystathionine γ-lyase
- MPST
3-mercaptopyruvate sulfurtransferase
Footnotes
Author Contribution: Naoharu Takano, Nanduri Prabhakar, and Gregg Semenza designed the study and wrote the paper; Naoharu Takano, Ying-Jie Peng, Ganesh Kumar, Weibo Luo, Hongxia Hu, and Larissa Shimoda performed experiments; and Makoto Suetmatsu advised on experimental procedures and manuscript revision.
References
- 1.Surmeier DJ, Guzman JN, Sanchez J, Schumacker PT. Physiological phenotype and vulnerability in Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2:a009290. doi: 10.1101/cshperspect.a009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lubbers DW. The meaning of the tissue oxygen distribution curve and its measurement by means of Pt electrodes. Prog Resp Res. 1969;3:112–123. [Google Scholar]
- 3.Kozniewska E, Weller L, Hoper J, Harrison DK, Kessler M. Cerebrocortical microcirculation in different stages of hypoxic hypoxia. J Cereb Blood Flow Metab. 1987;7:464–470. doi: 10.1038/jcbfm.1987.89. [DOI] [PubMed] [Google Scholar]
- 4.Nwaigwe CI, Roche MA, Grinberg O, Dunn JF. Effect of hyperventilation on brain tissue oxygenation and cerebrovenous PO2 in rats. Brain Res. 2000;868:150–156. doi: 10.1016/s0006-8993(00)02321-0. [DOI] [PubMed] [Google Scholar]
- 5.Dunn JF, Khan MN, Hou HG, Merlis J, Abajian MA, Demidenko E, Grinberg OY, Swartz HM. Cerebral oxygenation in awake rats during acclimation and deacclimation to hypoxia: an in vivo electron paramagnetic resonance study. High Alt Med Biol. 2011;12:71–77. doi: 10.1089/ham.2010.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hishiki T, Yamamoto T, Morikawa T, Kubo A, Kajimura M, Suematsu M. Carbon monoxide: impact on remethylation/transsulfuration metabolism and its pathophysiologic implications. J Mol Med (Berl) 2012;90:245–254. doi: 10.1007/s00109-012-0875-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Predmore BL, Lefer DJ, Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal. 2012;17:119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 9.Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010;12:1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- 10.Kajimura M, Fukuda R, Bateman RM, Yamamoto T, Suematsu M. Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid Redox Signal. 2010;13:157–192. doi: 10.1089/ars.2009.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morikawa T, Kajimura M, Nakamura T, Hishiki T, Nakanishi T, Yukutake Y, Nagahata Y, Ishikawa M, Hattori K, Takenouchi T, Takahashi T, Ishii I, Matsubara K, Kabe Y, Uchiyama S, Nagata E, Gadalla MM, Snyder SH, Suematsu M. Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc Natl Acad Sci USA. 2012;109:1293–1298. doi: 10.1073/pnas.1119658109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 14.Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor 1 in the lung. Am J Physiol. 1998;275:L818–L826. doi: 10.1152/ajplung.1998.275.4.L818. [DOI] [PubMed] [Google Scholar]
- 15.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, et al. Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci USA. 2009;106:17910–17915. doi: 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Zhang H, Wong CC, Wei H, Gilkes DM, Korangath P, Chaturvedi P, Schito L, Chen J, Krishnamachary B, Winnard PT, Jr, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31:1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 21.Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA. 2010;107:10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 23.Ebert BL, Firth JD, Ratcliffe PJ. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct cis-acting sequences. J Biol Chem. 1995;270:29083–29089. doi: 10.1074/jbc.270.49.29083. [DOI] [PubMed] [Google Scholar]
- 24.Yuan G, Peng YJ, Reddy VD, Makarenko VV, Nanduri J, Khan SA, Garcia JA, Kumar GK, Semenza GL, Prabhakar NR. Mutual antagonism between hypoxia-inducible factors 1α and 2α regulates oxygen sensing and cardio-respiratory homeostasis. Proc Natl Acad Sci USA. 2013;110:E1788–E1796. doi: 10.1073/pnas.1305961110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 26.Lu M, Hu LF, Hu G, Bian JS. Hydrogen sulfide protects astrocytes against H2O2-induced neural injury via enhancing glutamate uptake. Free Radic Biol Med. 2008;45:1705–1713. doi: 10.1016/j.freeradbiomed.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Luo Y, Liu X, Zheng Q, Wan X, Ouyang S, Yin Y, Sui X, Liu J, Yang X. Hydrogen sulfide prevents hypoxia-induced apoptosis via inhibition of an H2O2-activated calcium signaling pathway in mouse hippocampal neurons. Biochem Biophys Res Commun. 2012;425:473–477. doi: 10.1016/j.bbrc.2012.07.131. [DOI] [PubMed] [Google Scholar]
- 28.Tay AS, Hu LF, Lu M, Wong PT, Bian JS. Hydrogen sulfide protects neurons against hypoxic injury via stimulation of ATP-sensitive potassium channel/protein kinase C/extracellular signal-regulated kinase/heat shock protein 90 pathway. Neuroscience. 2010;167:277–286. doi: 10.1016/j.neuroscience.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Dunn JF, Khan MN, Hou HG, Merlis J, Abajian MA, Demidenko E, Grinberg OY, Swartz HM. Cerebral oxygenation in awake rats during acclimation and deacclimation to hypoxia: an in vivo electron paramagnetic resonance study. High Alt Med Biol. 2011;12:71–77. doi: 10.1089/ham.2010.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas SJ, Burgess KR, Thomas KN, Donnelly J, Peebles KC, Lucas RA, Fan JL, Cotter JD, Basnyat R, Ainslie PN. Alterations in cerebral blood flow and cerebrovascular reactivity during 14 days at 5050 m. J Physiol. 2011;589:741–753. doi: 10.1113/jphysiol.2010.192534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villien M, Bouzat P, Rupp T, Robach P, Lamalle L, Tropres I, Esteve F, Krainik A, Levy P, Warnking JM, et al. Changes in cerebral blood flow and vasoreactivity to CO2 measured by arterial spin labeling after 6 days at 4350 m. Neuroimage. 2013;72:272–279. doi: 10.1016/j.neuroimage.2013.01.066. [DOI] [PubMed] [Google Scholar]
- 32.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu K, Lamanna JC. Chronic hypoxia and the cerebral circulation. J Appl Physiol. 2006;100:725–730. doi: 10.1152/japplphysiol.00940.2005. 1985. [DOI] [PubMed] [Google Scholar]
- 35.Schodel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011;117:e207–e217. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budde MW, Roth MB. Hydrogen sulfide increases hypoxia-inducible factor-1 activity independently of von Hippel-Lindau tumor suppressor-1 in C. elegans. Mol Biol Cell. 2010;21:212–217. doi: 10.1091/mbc.E09-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Pan L, Zhuo Y, Gong Q, Rose P, Zhu Y. Hypoxia-inducible factor-1α is involved in the pro-angiogenic effect of hydrogen sulfide under hypoxic stress. Biol Pharm Bull. 2010;33:1550–1554. doi: 10.1248/bpb.33.1550. [DOI] [PubMed] [Google Scholar]
- 38.Kai S, Tanaka T, Daijo H, Harada H, Kishimoto S, Suzuki K, Takabuchi S, Takenaga K, Fukuda K, Hirota K. Hydrogen sulfide inhibits hypoxia- but not anoxia-induced hypoxia-inducible factor 1 activation in a von Hippel-Lindau- and mitochondria-dependent manner. Antioxid Redox Signal. 2012;16:203–216. doi: 10.1089/ars.2011.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu B, Teng H, Yang G, Wu L, Wang R. Hydrogen sulfide inhibits the translational expression of hypoxia-inducible factor-1α. Br J Pharmacol. 2012;167:1492–1505. doi: 10.1111/j.1476-5381.2012.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


