Abstract
Adiponectin is an adipocyte-specific adipocytokine with anti-atherogenic and anti-diabetic properties. Here, we investigated whether adiponectin regulates angiogenic processes in vitro and in vivo. Adiponectin stimulated the differentiation of human umbilical vein endothelium cells (HUVECs) into capillary-like structures in vitro and functioned as a chemoattractant in migration assays. Adiponectin promoted the phosphorylation of AMP-activated protein kinase (AMPK), protein kinase Akt/protein kinase B, and endothelial nitric oxide synthesis (eNOS) in HUVECs. Transduction with either dominant-negative AMPK or dominant-negative Akt abolished adiponectin-induced eNOS phosphorylation as well as adiponectin-stimulated HUVEC migration and differentiation. Dominant-negative AMPK also inhibited adiponectin-induced Akt phosphorylation, suggesting that AMPK is upstream of Akt. Dominant-negative Akt or the phosphatidylinositol 3-kinase inhibitor LY294002 blocked adiponectin-stimulated Akt and eNOS phosphorylation, migration, and differentiation without altering AMPK phosphorylation. Finally, adiponectin stimulated blood vessel growth in vivo in mouse Matrigel plug implantation and rabbit corneal models of angiogenesis. These data indicate that adiponectin can function to stimulate the new blood vessel growth by promoting cross-talk between AMP-activated protein kinase and Akt signaling within endothelial cells.
Adipose tissue secretes various bioactive substances, referred to as adipocytokines, whose dysregulation directly contributes to obesity-related diseases (1–4). Adiponectin/ACRP30 is an adipocytokine that is abundantly present in plasma (5, 6) but is down-regulated in association with obesity-linked diseases including coronary artery diseases (7, 8) and type 2 diabetes (9). Adiponectin is normally present in plasma at 30 µg/ml, but levels below 4 µg/ml are associated with coronary artery disease (7–9). Adiponectin exists as a trimer, hexamer, high molecular weight form, and small proteolytic cleavage product in both human and mouse plasma (10–12). However, the biological activities of these different forms are poorly understood, and it is possible that they are specific for different receptors and cell types.
Adiponectin inhibits monocyte adhesion to endothelial cells (7), macrophage transformation to foam cells (13), and vascular smooth muscle cell proliferation (14) in vitro. In vivo, forced adiponectin expression reduces atherosclerotic lesions in a mouse model of atherosclerosis (15), whereas adiponectin-deficient mice exhibit excessive vascular remodeling response to acute injury (16) and diet-induced insulin resistance (17). Therefore, adiponectin acts as a biologically relevant modulator of vascular remodeling with anti-atherogenic and anti-diabetic properties.
Vascular endothelial cells are in direct contact with plasma and they play pivotal roles in angiogenesis and maintaining whole body homeostasis (18, 19). Dysregulated angiogenesis is a characteristic of obesity-related disorders including atherosclerosis, diabetes, and hypertension (20). However, the interaction between adiponectin and angiogenesis has not been elucidated.
A number of recent studies have explored the intracellular signaling pathways within endothelial cells that regulate angiogenesis. Several endothelial cell stimuli including vascular endothelial growth factor (VEGF),1 angiopoietin-1, and statins promote vascular homeostasis and angiogenesis through their ability to activate Akt signaling (21). Akt signaling promotes endothelial cell survival (22–24). Akt also phosphorylates endothelial nitric-oxide synthase (eNOS), resulting in NO production and the regulation of vasomotor responses (25–27). Akt signaling also regulates VEGF-stimulated endothelial cell migration and differentiation (21, 28). AMP-activated protein kinase (AMPK) is a stress-activated protein kinase that participates in the regulation of energy and metabolic homeostasis (29, 30). With regard to the vascular endothelium, AMPK signaling is required for VEGF-stimulated endothelial cell NO production, migration, and differentiation under conditions of hypoxia (31). Therefore, AMPK signaling may contribute to the regulation of angiogenesis in ischemic tissues. Recent evidence indicates that adiponectin stimulates the phosphorylation and activation of AMPK in skeletal muscle (32, 33), liver (33), and adipocytes (34), leading to the regulation of glucose metabolism. AMPK signaling is also reported to mediate adiponectin-stimulated NO production in endothelial cells through its ability to phosphorylate eNOS (35).
In the present study, we investigated whether adiponectin modulates the angiogenic process. We examined the ability of adiponectin to induce endothelial cell migration, promote tube formation, and activate AMPK and Akt signaling pathways in endothelial cells. We also tested the effect of adiponectin on blood vessel growth using mouse Matrigel plug and rabbit corneal assays. Our observations indicate that adiponectin promotes angiogenesis via activation of AMPK- and phosphatidylinositol 3-kinase (PI3-kinase)-Akt-dependent pathways in endothelial cells.
EXPERIMENTAL PROCEDURES
Materials
Phospho-AMPK (Thr-172), pan-α-AMPK, and phospho-Akt (Ser-473), phospho-eNOS (Ser-1177), phospho-p42/44 extracellular signal-regulated kinase (ERK) (Thr 202/Tyr 204), ERK, and Akt antibodies were purchased from Cell Signaling Technology (Beverly, MA). c-Myc tag antibody was purchased from Upstate Biotechnology (Lake Placid, New York). eNOS antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Tubulin antibody was purchased from Oncogene (Cambridge, MA). Recombinant human VEGF was purchased from Sigma.
Recombinant Proteins
Mouse adiponectin (amid acids 15–247) was cloned into the bacterial expression vector pTrcHisB (Amersham Biosciences). The histidine-tagged proteins were purified using nickel ionagarose column, mono Q column, and for removal of lipopolysaccharide, Detoxi-Gel Affinity Pak column (Pierce).
Cell Culture, Adenoviral Infection, and Western Blot Analysis
Human umbilical vein endothelium cells (HUVECs) were cultured in endothelial cell growth medium-2. Before each experiment cells were placed in endothelial cell basal medium-2 with 0.5% fetal bovine serum for 16 h for serum starvation. Experiments were performed by the addition of the indicated amount of mouse recombinant adiponectin, VEGF, or vehicle for the indicated lengths of time. In some experiments HUVECs were infected with adenoviral constructs encoding dominant-negative AMPKα2 (31), dominant-negative Akt1 (22), or green fluorescence protein at a multiplicity of infection of 50 for 24 h. In some experiments HUVECs were pretreated with LY294002 (10 µm) or vehicle for 1 h before stimulation with adiponectin. Cell lysates were resolved by SDS-PAGE. The membranes were immunoblotted with the indicated antibodies at a 1:1000 dilution followed by the secondary antibody conjugated with horseradish peroxidase at a 1:5000 dilution. ECL-PLUS Western blotting detection kit (Amersham Biosciences) was used for detection.
Migration Assay
Migration activity was measured using a modified Boyden chamber assay. Serum-starved cells were trypsinized and resuspended in endothelial cell growth medium-2 with 0.5% fetal bovine serum. Cell suspension (250 µl, 2.0 × 104 cells/well) were added to the transwell fibronectin-coated insert (6.4-mm diameter, 3.0-µm pore size, BD Biosciences). Then 750 µl of endothelial cell growth medium-2 with 0.5% fetal bovine serum supplemented with adiponectin (30 µg/ml), VEGF (20 ng/ml), or bovine serum albumin (BSA) (30 µg/ml) was added to lower chamber and incubated for 4 h. Migrated cells on the lower surface of the membrane were fixed and stained with Giemsa stain solution, and eight random microscopic fields per well were quantified. All assays were performed in triplicate.
Tube Formation Assay
The formation of vascular-like structures by HUVECs on growth factor-reduced Matrigel (BD Biosciences) was performed as previously described (31). Twenty-four-well culture plates were coated with Matrigel according to the manufacturer’s instructions. Serum-starved HUVECs were seeded on coated plates at 5 × 104 cells/well in endothelial cell growth medium-2 with 0.5% fetal bovine serum containing the indicated concentrations of adiponectin, VEGF (20 ng/ml), or BSA (30 µg/ml) and incubated at 37 °C for 18 h. Tube formation was observed using an inverted phase contrast microscope (Nikon, Tokyo, Japan). Images were captured with a video graphic system (DEI-750 CE Digital Output Camera, Optronics, Goleta, CA). The degree of tube formation was quantified by measuring the length of tubes in three randomly chosen fields from each well using the angiogenic activity quantification program (Kurabo, Osaka, Japan). Each experiment was repeated for three times.
Mouse Angiogenesis Assay
The formation of new vessels in vivo was evaluated by Matrigel plug assay as described previously (31). For these experiments, 400 µl of Matrigel containing adiponectin (100 µg/ml) or vehicle was injected subcutaneously into the abdomen of C57BL mice. Mice were sacrificed 14 days after the injection. The Matrigel plugs with adjacent subcutaneous tissues were carefully recovered by en bloc resection, fixed in 4% paraformaldehyde, dehydrated with 30% sucrose, and embedded in OCT compound (GTI Microsystems, Tempe, AZ) in liquid nitrogen. Immunohistostaining for CD31 (PECAM-1; BD Biosciences) was performed on adjacent frozen sections. Primary antibody was used at a 1:50 dilution followed by incubation of secondary antibody (horseradish peroxidase-conjugated anti-rat IgG at a 1:100 dilution). The AEC substrate pack (Biogenex, San Ramon, CA) was used for detection. CD31-positive capillaries were counted in 4 randomly chosen low power (×100) microscopic fields.
Rabbit Corneal Angiogenesis Assay
Rabbit corneal assay was performed with minor modification as previously described (36). Male New Zealand White rabbits weighing 3.0–3.9 kg were used. Two pockets, about 2 × 3 mm in size and 5 mm apart, were surgically prepared in the cornea extending toward a point 2 mm from the limbus. Hydron pellets, which contain an indicated amount of adiponectin, VEGF (100 ng), or phosphate-buffered saline that enables its slow release (37), were implanted into the pocket. On day 7 after surgery, eyes were photographed, and cornea neovascularization was examined in a single blind manner. The angiogenic activity was evaluated on the basis of the number and growth rate of newly formed capillaries. An angiogenic score was calculated (vessel density × distance from limbus) (36). A density value of 1 corresponded to 0–25 vessels per cornea, 2 for 25–30 vessels, 3 for 50–75 vessels, 4 for 75–100 vessels, and 5 for >100 vessels.
Statistical Analysis
Data are presented as mean ± S.E. Differences were analyzed by Student’s unpaired t test. A level of p < 0.05 was accepted as statistically significant.
RESULTS
Adiponectin Accelerates Vascular Structure Formation in Vitro
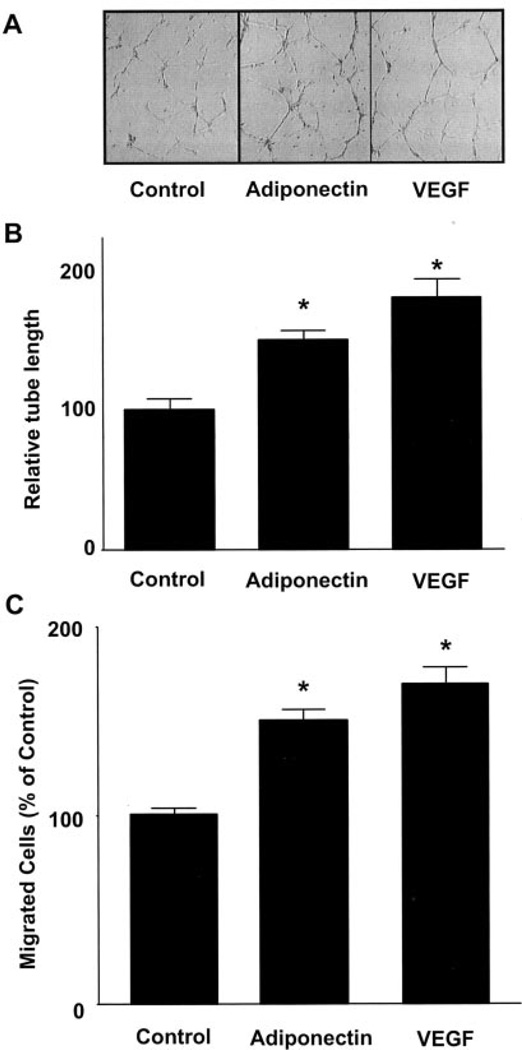
We first examined whether adiponectin affected endothelial cell differentiation into capillary-like structure when HUVECs were plated on a Matrigel matrix. Treatment with a physiological concentration of adiponectin promoted the formation of capillary-like tubes (Fig. 1A). Quantitative analyses revealed a trend toward increased tube length in the VEGF-treated cultures relative to adiponectin, but this was not statistically significant (Fig. 1B). To test whether adiponectin modulated endothelial migration, a modified Boyden chamber assay was performed. Adiponectin significantly stimulated HUVEC migration, as did VEGF (Fig. 1C). Quantitative analyses revealed a trend toward greater migration with VEGF compared with adiponectin, but this was not statistically significant. Adiponectin also induced endothelial migration in a cell-wounding assay.2 These results suggest that adiponectin promotes pro-angiogenic cellular responses in endothelial cells.
Fig. 1. Adiponectin promotes endothelial cell migration and differentiation into tube-like structures.
Tube formation assays were performed (A and B). HUVECs were seeded on Matrigel-coated culture dishes in the presence of adiponectin (30 µg/ml), VEGF (20 ng/ml), or BSA (30 µg/ml) (Control). A, representative cultures are shown. B, quantitative analysis of tube formation. C, a modified Boyden chamber assay was performed using HUVECs. HUVECs were treated with adiponectin (30 µg/ml), VEGF (20 ng/ml), or BSA (30 µg/ml) (Control). Results are shown as mean ± S.E. Results are expressed relative to control. *, p < 0.01 versus control.
Adiponectin Induces the Phosphorylation of AMPK, Akt, and eNOS
Endothelial AMPK signaling is associated with the regulation of angiogenesis under certain conditions (31). Therefore, to test whether adiponectin induces AMPK signaling in endothelial cells, cultured HUVECs were incubated with adiponectin, and AMPK phosphorylation at Thr-172 of the α subunit was assessed by Western blot analyses. Treatment of HUVECs with adiponectin enhanced the phosphorylation of AMPK in a time-dependent manner with maximal AMPK phosphorylation occurring at 15 min (Fig. 2A). Akt plays important roles in the angiogenic response to several growth factors and cytokines (21). Therefore, the effect of adiponectin on the activating phosphorylation of Akt at Ser-473 was investigated. Adiponectin treatment led to a time-dependent increase in Akt phosphorylation (Fig. 2A). In contrast to these signaling protein kinases, adiponectin treatment had no effect on the phosphorylation of extracellular signal-regulated kinase at Thr-202/Tyr-204 (Fig. 2A). Both AMPK and Akt can phosphorylate eNOS at Ser-1179 (25, 26, 38, 39). Therefore, eNOS phosphorylation was examined in these cultures. Adiponectin stimulation promoted a time-dependent increase in eNOS phosphorylation at Ser-1179 but had no effect on eNOS protein levels (Fig. 2A).
Fig. 2. Adiponectin-stimulated signaling in endothelial cells.
A, time-dependent changes in the phosphorylation (p-) of AMPK, Akt, eNOS, and extracellular signal-regulated kinase (ERK) after adiponectin treatment (30 µg/ml). B, role of AMPK in the regulation of adiponectin-induced protein phosphorylation. HUVECs were transduced with an adenoviral vector expressing dominant-negative AMPK tagged with c-Myc (dn-AMPK) or an adenoviral vector expressing green fluorescence protein (Control) 24 h before serum-starvation. After 16-h serum starvation, cells were treated with adiponectin (30 µg/ml) for the indicated lengths of time. C, role of Akt in the regulation of adiponectin-induced protein phosphorylation. HUVECs were transduced with an adenoviral vector expressing dominant-negative Akt (dn-Akt) or an adenoviral vector expressing green fluorescence protein (Control) 24 h before serum starvation. After 16 h of serum starvation, cells were treated with adiponectin (30 µg/ml) for the indicated lengths of time. Representative blots are shown.
The regulation of eNOS by mitogen-stimulated phosphorylation is complicated by the possibility of AMPK-Akt cross-talk (31, 40). To examine the relative contribution of AMPK and Akt to the regulation of adiponectin-induced phosphorylation of eNOS, HUVECs were transduced either with an adenoviral vector expressing a c-Myc-tagged dominant-negative mutant of AMPK (ad-dnAMPK) or dominant-negative Akt (ad-dnAkt). Transduction with ad-dnAMPK suppressed adiponectin-induced AMPK and eNOS phosphorylation (Fig. 2B). Transduction with addnAMPK also blocked adiponectin-induced phosphorylation of Akt, suggesting signaling cross-talk between these two protein kinases (Fig. 2B). Of note, transduction with ad-dnAkt suppressed the adiponectin-induced phosphorylation of eNOS without altering that of AMPK (Fig. 2C). These data indicate that Akt is a downstream kinase of AMPK and that Akt mediates eNOS phosphorylation under these conditions.
AMPK and Akt Signaling Are Required for Adiponectin-stimulated Migration and Differentiation
To test whether AMPK and Akt signaling participate in adiponectin-stimulated endothelial differentiation and migration, HUVECs were infected with ad-dnAMPK or ad-dnAkt and evaluated in tube formation and Boyden chamber assays, respectively. Transduction with either ad-dnAMPK or ad-dnAkt suppressed adiponectin-induced endothelial tube structure formation to basal levels (Fig. 3, A and B). In contrast, VEGF-stimulated differentiation was blocked by transduction with ad-dnAkt but not by transduction with ad-dnAMPK (Fig. 3B). Transduction with ad-dnAMPK and ad-dnAkt had no effect on non-stimulated, basal tube formation (Fig. 3B). Adiponectin-stimulated endothelial migration was also significantly suppressed by transduction with either ad-dnAMPK or ad-dnAkt (Fig. 3C). In contrast, transduction with ad-dnAkt blocked VEGF-stimulated migration, whereas transduction with ad-dnAMPK had no effect (Fig. 3C). Transduction with ad-dnAMPK and ad-dnAkt had no effect on the basal migration rate (Fig. 3C). These results indicate that both AMPK and Akt signals are required for adiponectin-induced endothelial migration and differentiation, whereas only Akt signaling participates in these endothelial cell responses to VEGF.
Fig. 3. Contribution of AMPK and Akt to adiponectin-induced angiogenic cellular responses.
HUVECs were transduced with an adenoviral vector expressing dn-AMPK (gray), dn-Akt (open) or green fluorescence protein (Control, solid) 24 h before the change to low-serum media. After 16 h of serum starvation, in vitro Matrigel (A and B) or modified Boyden chamber assays (C) were performed. Cells were treated with adiponectin (30 µg/ml) or BSA (30 µg/ml) (Vehicle). A, representative cultures displaying tube formation are shown. B, quantitative analysis of tube lengths. C, modified Boyden chamber assay was performed with adiponectin or VEGF as chemoattractant. Results are shown as the mean ± S.E. Results are expressed relative to control. *, p < 0.01 versus each control.
Role of PI3-kinase Signaling in Adiponectin-induced Angiogenic Response
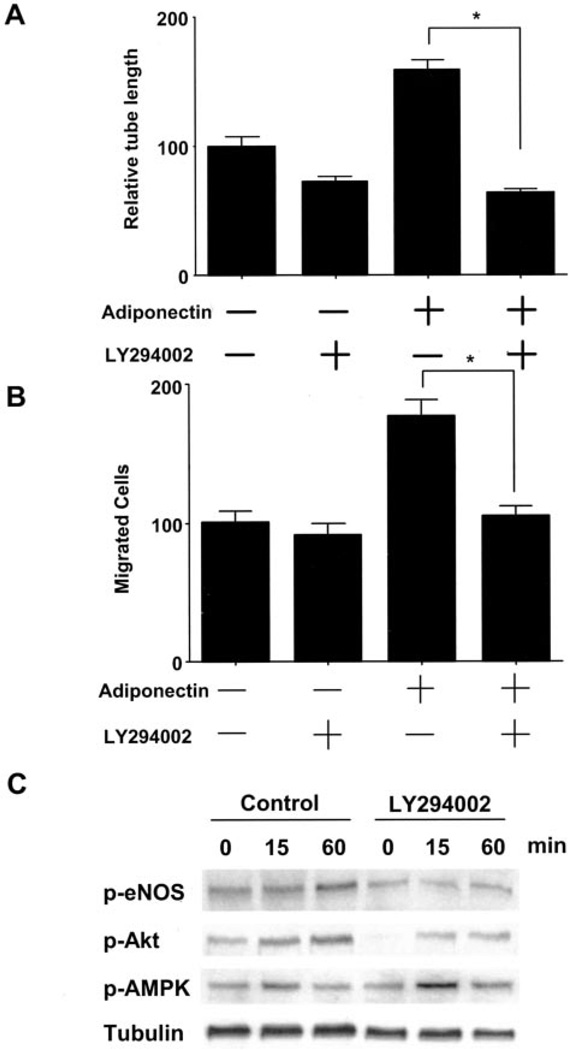
Akt is activated by many growth factors and cytokines in a PI3-kinase-dependent manner (21). To investigate whether PI3-kinase signaling is involved in the adiponectin- induced angiogenic response, HUVECs were incubated with PI3-kinase inhibitor, LY294002, in the absence or presence of adiponectin. Brief treatment with LY294002 abolished adiponectin-stimulated tube formation and migration (Fig. 4, A and B). Adiponectin-stimulated phosphorylation of Akt and eNOS was blocked by treatment with LY294002, whereas LY294002 treatment had no effect on AMPK phosphorylation (Fig. 4C). These data indicate that PI3-kinase is critical for adiponectin-induced angiogenic cell responses and that PI3-kinase functions upstream from the Akt-eNOS regulatory axis in adiponectin-stimulated endothelial cells.
Fig. 4. PI3-kinase signaling is involved in adiponectin-induced angiogenic pathway.
A, quantitative analysis of tube formation is shown. HUVECs were treated with adiponectin (30 µg/ml) or BSA (30 µg/ml) in the presence of LY294002 (10 µm) or vehicle at the time of seeding. B, a modified Boyden chamber assay was performed using adiponectin as the chemoattractant. HUVECs were pretreated with LY294002 (10 µm) or vehicle for 1 h and then incubated with adiponectin (30 µg/ml) or BSA (30 µg/ml) for 4 h. C, effects of LY294002 on adiponectin-stimulated protein phosphorylation (p-). Representative blots are shown. HUVECs were pretreated with LY294002 (10 µm) or vehicle for 1 h and then incubated with adiponectin (30 µg/ml) or BSA (30 µg/ml) for the indicated lengths of time. Results are presented as the mean ± S.E. For A and B, results are expressed relative to control. *, p < 0.01.
Adiponectin Promotes Vessel Growth in Vivo
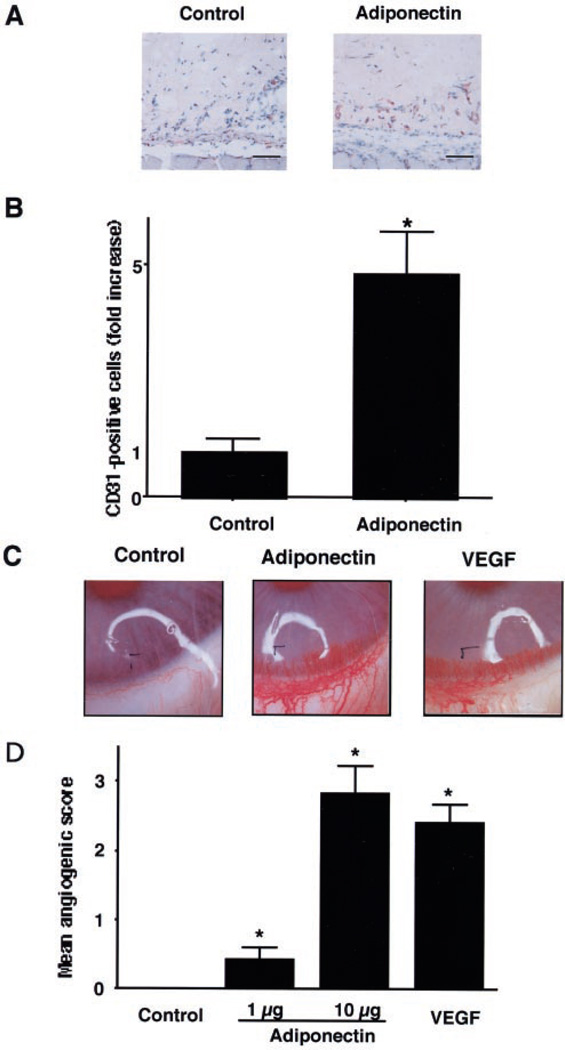
To examine the effect of adiponectin on angiogenesis, mouse Matrigel plug and rabbit corneal assays were performed. In the Matrigel plug assay, endothelial cell infiltration of the plugs was assessed by immunohistochemical analysis of CD31-positive cells (Fig. 5A). Quantitative analyses of histological sections revealed that plugs containing adiponectin displayed a significantly higher density of CD31-positive cells compared with controls (Fig. 5B). In addition, neovascularization in corneal implants containing adiponectin was markedly accelerated compared with controls (Fig. 5, C and D). The stimulatory effect of adiponectin was comparable with that of VEGF in this model (Fig. 5, C and D). These data show that adiponectin can promote neovascularization in vivo.
Fig. 5. Adiponectin promotes angiogenesis in vivo.
An in vivo Matrigel plug assay was performed to evaluate the effect of adiponectin on angiogenesis (A and B). Matrigel plugs containing adiponectin (100 µg/ml, n = 3) or phosphate-buffered saline (Control, n = 3) were injected subcutaneously into mice. A, plugs were stained with the endothelial cell marker CD31. Bar, 100 µm. B, the frequency of CD31-positive cells in five low power fields was determined for each Matrigel plug. Data were presented as fold increase of CD31-positive cells relative to the control. A rabbit cornea assay was performed (C and D). Pellets containing adiponectin (1 and 10 µg, n = 8), VEGF (100 ng, n = 8), or phosphate-buffered saline (Control, n = 8) were implanted in the cornea. C, photographs of rabbit eyes are shown (Control, adiponectin 10 µg; VEGF, 100 ng). D, an angiogenic score was calculated (vessel density × distance from limbus). Results are shown as the mean ± S.E. *, p < 0.01 versus control.
DISCUSSION
This study identifies the promotion of blood vessel growth as a new role for the adipocytokine adiponectin. Proangiogenic activity was demonstrated in two well established models of angiogenesis, the mouse Matrigel plug and rabbit corneal assays. The ability of adiponectin to stimulate angiogenesis is likely due, at least in part, to its ability to promote endothelial cell migration and stimulate the differentiation of these cells into capillary-like structures.
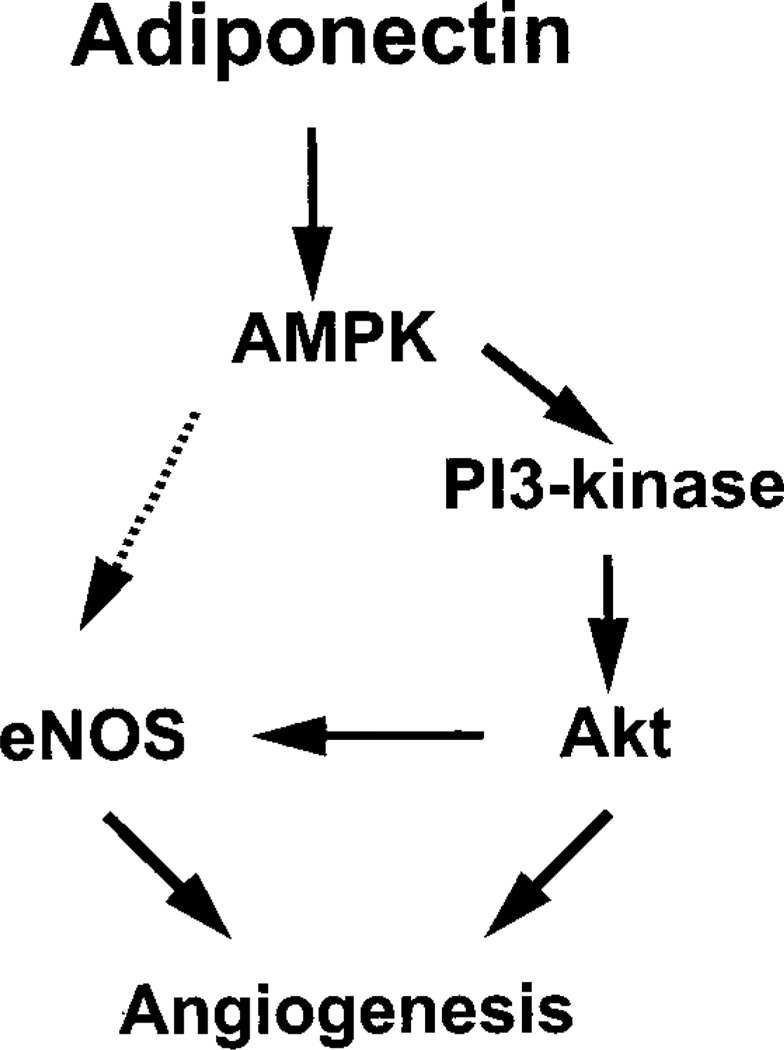
Adiponectin functions as an AMPK activator in multiple cell types (32–35, 41). Recently, we reported that endothelial AMPK signaling is essential for angiogenesis under conditions of hypoxia but dispensable in normoxic cells. Here it is shown that AMPK activation by adiponectin can activate angiogenic cellular responses in normoxic endothelial cells. Furthermore, it is shown that cross-talk between AMPK and Akt protein kinases is required for several cellular responses downstream of adiponectin including the activating phosphorylation of eNOS at Ser-1179. Several recent reports demonstrate the importance of AMPK-Akt cross-talk (31, 40). Both Akt and AMPK are reported to directly phosphorylate eNOS (25, 26, 38, 39). Our study found that transduction with either addnAMPK or ad-dnAkt effectively blocked adiponectin-induced eNOS phosphorylation. Both of these reagents also suppressed adiponectin-stimulated endothelial cell migration and differentiation. Furthermore, inhibition of AMPK signaling suppressed adiponectin-induced Akt phosphorylation, suggesting that Akt functions downstream of AMPK in adiponectin-stimulated endothelial cells (Fig. 6). Importantly, the PI3-kinase inhibitor LY294002 or dominant-negative Akt blocked adiponectin-stimulated cell migration, differentiation, and Akt and eNOS phosphorylation without altering the phosphorylation status of AMPK. These data suggest that the pro-angiogenic effects of adiponectin-stimulated AMPK activity are due in large part to an activation of Akt signaling under these conditions. Although we cannot exclude the possibility that AMPK directly phosphorylates eNOS, the data are most consistent with a model that comprises an adiponectin-AMPK-PI3-kinase-Akt-eNOS signaling axis under the conditions of our assays (Fig. 6).
Fig. 6. Proposed scheme for adiponectin-stimulated signaling in endothelial cells.
Adiponectin activates AMPK, which in turn promotes Akt activation, eNOS phosphorylation, and angiogenesis. PI3-kinase is essential for adiponectin-mediated activation of Akt. Both AMPK and Akt can directly phosphorylate eNOS. However, inhibition of Akt or PI3-kinase was found to suppress adiponectin-stimulated eNOS phosphorylation without interfering with AMPK activation. Therefore, the data are most consistent with an AMPK-PI3-kinase-Akt-eNOS-signaling axis.
The hypothesis that AMPK functions upstream of Akt signaling is consistent with data obtained from studies in other systems. For example, it has been shown that the AMPK stimulator 5-aminoimidazole-4-carboxamide riboside enhances insulin-stimulated activation of IRS-1-associated PI3-kinase in C2C12 myocytes (42). Furthermore, adiponectin-deficient mice exhibit severe diet-induced insulin resistance that coincides with a reduction of muscle IRS-1-associated PI3-kinase activity (17). Conversely, adiponectin stimulates IRS-1-associated PI3-kinase activity in C2C12 myocytes (17), and adiponectin treatment increases insulin-stimulated Akt phosphorylation in the skeletal muscle of lipoatrophic mice (43).
Plasma adiponectin levels are low in patients with type 2 diabetes (9). Low levels of adiponectin expression have also been observed in the visceral fat of diabetic fa/fa Zucker rats in comparison with lean rats (44). Collateral vessel development is impaired in diabetic patients including those with myocardial and limb ischemia (45, 46), and there is an impaired angiogenic response after ischemic injury in nonobese diabetic mice and obese diabetic fa/fa Zucker rats (47, 48). Adiponectin has a protective action on the vasculature in addition to its ability to promote signaling in insulin-responsive tissues (49). Both of these activities probably involve the ability of adiponectin to promote Akt signaling, an important regulator of angiogenesis and endothelial cell homeostasis (21). Therefore, low adiponectin levels may contribute to endothelial cell dysfunction and impaired collateral growth in diabetic states. Taken together, these data suggest that exogenous supplementation of adiponectin could have utility for therapeutic angiogenesis in patients who suffer from the vascular complications of diabetes.
Footnotes
This work was supported by National Institutes of Health Grants AR40197, HD23681, AG17241, and AG15052 (to K. W.) and by the Japanese Ministry of Education and the Japan Society for Promotion of Science-Research for the Future Program.
Supported by a grant from the Uehara Memorial Foundation.
The abbreviations used are: VEGF, vascular endothelial growth factor; AMPK, AMP-activated kinase; eNOS, endothelial nitric-oxide synthase; PI3-kinase, phosphatidylinositol 3-kinase; HUVEC, human umbilical vein endothelial cell; BSA, bovine serum albumin; dn, dominant negative; ad, adenovirus.
N. Ouchi and K. Walsh, unpublished data.
REFERENCES
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS, Shargill NS, Spiegelman BM. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 3.Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, Tokunaga K, Matsuzawa Y. Nat. Med. 1996;2:800–803. doi: 10.1038/nm0796-800. [DOI] [PubMed] [Google Scholar]
- 4.Trayhurn P, Beattie JH. Proc. Nutr. Soc. 2001;60:329–339. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- 5.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. Biochem. Biophys. Res. Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 6.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Biochem. Biophys. Res. Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 7.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 8.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Arterioscler. Thromb. Vasc. Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 9.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Arterioscler. Thromb. Vasc. Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 10.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. J. Biol. Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 11.Kishida K, Nagaretani H, Kondo H, Kobayashi H, Tanaka S, Maeda N, Nagasawa A, Hibuse T, Ohashi K, Kumada M, Nishizawa H, Okamoto Y, Ouchi N, Maeda K, Kihara S, Funahashi T, Matsuzawa Y. Biochem. Biophys. Res. Commun. 2003;306:286–292. doi: 10.1016/s0006-291x(03)00940-9. [DOI] [PubMed] [Google Scholar]
- 12.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 14.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, Nakamura T, Shimomura I, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Circulation. 2002;105:2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, Kishida K, Komuro R, Ouchi N, Kihara S, Nagai R, Funahashi T, Matsuzawa Y. J. Biol. Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 17.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Nat. Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Alitalo K. Nat. Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 19.Vita JA, Loscalzo J. Circulation. 2002;106:164–166. doi: 10.1161/01.cir.0000023452.26135.34. [DOI] [PubMed] [Google Scholar]
- 20.Carmeliet P. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 21.Shiojima I, Walsh K. Circ. Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 22.Fujio Y, Walsh K. J. Biol. Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim I, Kim HG, So J-N, Kim JH, Kwak HJ, Koh GY. Circ. Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 24.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. Nat. Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AZ. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 27.Luo Z, Fujio Y, Kureishi Y, Rudic RD, Daumerie G, Fulton D, Sessa WC, Walsh K. J. Clin. Invest. 2000;106:493–499. doi: 10.1172/JCI9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morales-Ruiz M, Fulton G, Sowa G, Languino LR, Fujio Y, Walsh K, Sessa WC. Circ. Res. 2000;86:892–896. doi: 10.1161/01.res.86.8.892. [DOI] [PubMed] [Google Scholar]
- 29.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. Mol. Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 30.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. J. Biol. Chem. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- 31.Nagata D, Mogi M, Walsh K. J. Biol. Chem. 2003;278:31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 32.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CcC, Itani SI, Lodish HF, Ruderman NB. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Diabetes. 2003;52:1355–1363. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. J. Biol. Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 36.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. J. Clin. Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips GD, Stone AM, Jones BD, Schultz JC, Whitehead RA, Knighton DR. In Vivo (Athens) 1994;8:961–965. [PubMed] [Google Scholar]
- 38.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 39.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. J. Biol. Chem. 2003;278:31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 40.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. J. Biol. Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 42.Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. J. Biol. Chem. 2001;276:46912–46916. doi: 10.1074/jbc.C100483200. [DOI] [PubMed] [Google Scholar]
- 43.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 44.Milan G, Granzotto M, Scarda A, Calcagno A, Pagano C, Federspil G, Vettor R. Obes. Res. 2002;10:1095–1103. doi: 10.1038/oby.2002.149. [DOI] [PubMed] [Google Scholar]
- 45.Waltenberger J. Cardiovasc. Res. 2001;49:554–560. doi: 10.1016/s0008-6363(00)00228-5. [DOI] [PubMed] [Google Scholar]
- 46.Schaper W, Buschmann I. Circulation. 1999;99:2224–2226. doi: 10.1161/01.cir.99.17.2224. [DOI] [PubMed] [Google Scholar]
- 47.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner JM. Am. J. Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janiak P, Lainee P, Grataloup Y, Luyt CE, Bidouard JP, Michel JB, O’Connor SE, Herbert JM. Cardiovasc. Res. 2002;56:293–302. doi: 10.1016/s0008-6363(02)00538-2. [DOI] [PubMed] [Google Scholar]
- 49.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Curr. Opin. Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]