Abstract
Tie1 is a receptor tyrosine kinase with broad expression in embryonic endothelium. Reduction of Tie1 levels in mouse embryos with a hypomorphic Tie1 allele resulted in abnormal lymphatic patterning and architecture, decreased lymphatic draining efficiency, and ultimately, embryonic demise. Here we report that Tie1 is present uniformly throughout the lymphatics and from late embryonic/early postnatal stages, becomes more restricted to lymphatic valve regions. To investigate later events of lymphatic development, we employed Cre-loxP recombination utilizing a floxed Tie1 allele and an Nfatc1Cre line, to provide loxP excision predominantly in lymphatic endothelium and developing valves. Interestingly, unlike the early prenatal defects previously described by ubiquitous endothelial deletion, excision of Tie1 with Nfatc1Cre resulted in abnormal lymphatic defects in postnatal mice and was characterized by agenesis of lymphatic valves and a deficiency of collecting lymphatic vessels. Attenuation of Tie1 signaling in lymphatic endothelium prevented initiation of lymphatic valve specification by Prox1 high expression lymphatic endothelial cells that is associated with the onset of turbulent flow in the lymphatic circulation. Our findings reveal a fundamental role for Tie signaling during lymphatic vessel remodeling and valve morphogenesis and implicate it as a candidate gene involved in primary lymphedema.
Keywords: Tie1, lymphatic valve, lymphovenous valve, lymphatic vessel remodeling, conditional knockout, Prox1
Introduction
The lymphatic vasculature is essential for the maintenance of normal tissue fluid homeostasis and for support of the immune response. It consists of a complex network of capillaries and collecting vessels. The lymphatic capillaries have discontinuous basement membrane and loose intercellular junctions, lack pericyte coverage, and are therefore highly permeable to large macromolecules. In contrast, larger collecting lymphatic vessels are surrounded by a substantial basement membrane and by smooth muscle cells (SMC)/mural cells, which helps to pump the lymph forward while the luminal valves in these vessels prevent lymph backflow (Oliver and Detmar 2002). These structural features allow efficient fluid uptake of protein-rich lymph from tissue interstitium by capillaries and transport of lymph back to the blood vascular system by collecting vessels (Tammela and Alitalo, 2010). Lymphatic vessel hypoplasia or defective lymphatic valves can impair the ability of the lymphatic vasculature to collect and transport fluids and lead to lymphedema (Alitalo, 2011; Schute-Merker et al., 2011).
The mammalian lymphatic system has been shown to originate from embryonic veins. Lymphatic vessel development starts at embryonic day (E) 9.5 in mice. After formation of the primary lymph sacs, primitive lymphatic capillary networks assemble by a process of centrifugal sprouting from the lymph sacs, and then subsequently these networks combine and remodel into a hierarchal network of capillaries and collecting lymphatic vessels (Koltowska et al., 2013; Tammela and Alitalo, 2010). Maturation of collecting vessels is accompanied by the downregulation of lymphatic marker molecules such as Lyve1, the acquisition of partial smooth muscle cell coverage, and the formation of intraluminal valves (Bazigou et al., 2009; Sabine et al., 2012).
Formation of intraluminal lymphatic valves is one of the hallmarks of the collecting lymphatic vessel characteristic of later stages of lymphatic development. Lymphatic valve formation in mice is initiated during late embryonic development (around E16.5). It has recently been shown that mechanical stimulus of turbulent hemodynamics caused by the onset of lymph flow establishes the location, often at branch points of lymphatic vessels, where valves develop by upregulating the expression of Prox1 and Foxc2 (Sabine et al., 2012). Foxc2 cooperates with Prox1 to control Connexin37 (Cx37) expression and to activate Nfatc1/Calcineurin signaling (Sabine et al., 2012). Other molecular regulators such as EphrinB2 (Makinen et al., 2005), integrin 9 and its extracellular matrix (ECM) ligand FN-EIIIA (Bazigou et al., 2009), EMILIN1/α9β1 Integrin (Danussi et al., 2013), Connexin37 and Connexin43 (Kanady et al., 2011), Bmp9 (Levet et al., 2013), and Semaphorin3a and their receptors Neuropilin1/plexin A1 (Bouvrée et al., 2012; Jurisic et al., 2012) have been reported to be required for either lymphatic valve formation, elongation of lymphatic valve leaflets, or valve maturation. Despite this knowledge, the signaling mechanisms regulating lymphatic remodeling and maturation, and early stages of lymphatic-valve development are still not well understood. Importantly, although FOXC2 is induced by oscillatory shear stress in vitro (Sabine, et al., 2012), surprisingly Prox1 is not. Thus further analysis of lymphatic endothelial cell mediators of altered hemodynamic shear stress should provide important insights on the molecular mechanisms required for the initiation of lymphatic valve formation.
Tie1 is an orphan endothelial receptor tyrosine kinase sharing a high degree of homology with Tie2, the receptor for the angiopoietins (Peters et al., 2004; Yancopoulos et al., 2000). It is expressed throughout both the blood and lymphatic vasculature endothelium from early embryonic stages to the adult (Partanen et al., 1992; Dumont et al., 1995; Qu et al., 2010) and has previously been shown to be involved in the regulation of growth and integrity of lymphatic capillaries (D'Amico et al., 2010; Qu, et al., 2010). Accordingly, Tie1-deficient embryos have abnormal lymphatic patterning and architecture, leading to a lymphedema associated with inefficient lymph drainage and increased leakage and embryonic demise. In addition, our laboratory has recently shown that Tie 1 is at least partially responsible for mechanotransduction of turbulent flow required for initiation and maintenance of atherosclerotic plaque formation at the branch points of the systemic vasculature in the adult animal (Woo, et al., 2011). Here, we report that Tie1 is expressed in developing collecting lymphatics and becomes progressively enriched at lymphatic valves. We subsequently demonstrate that Tie1 is a crucial regulator of lymphatic remodeling and valve morphogenesis by conditional deletion of Tie1 predominantly in the lymphatic valvular endothelium. Early post-natal animals exhibit major lymphatic defects, including a failure to remodel their primary lymphatic capillary plexus into a hierarchical vessel network with a definitive collecting vessels, and lack of luminal valve formation. Furthermore, we demonstrate that Tie1 is necessary for assembling the critical patterned high expression of Prox1 and Foxc2 in prospective valve-forming cells, which is required for the initiation of valve morphogenesis. This suggests that as previously demonstrated in the adult systemic circulation, Tie1 may serve as a component of the mechanotransduction machinery required to mediate the altered hemodynamics essential for lymphatic valve ontogeny.
Materials and methods
Mouse models
Generation of Tie1+/lz (Puri et al., 1995) Tie1fl/fl (Qu et al., 2002), and Nfatc1Cre mice (Wu et al., 2012) has been described previously. R26R reporter (R26fslz) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All strains were maintained on a mixed 129 and C57/BL6 background. The animals were handled in accordance with institutional guidelines with the approval of the Institutional Animal Care and Use Committee of Vanderbilt University School of Medicine.
Antibodies and whole-mount immunohistochemistry
Mesenteries, hearts, diaphragm, head and back skin (from embryos), and ventral skin (from neonates) were dissected, fixed in paraformaldehyde (PFA) in phosphate-buffered saline (PBS, pH 7.4) overnight at 4°C and then washed with PBS. Whole-mount immunostaining was performed as described previously (Qu et al., 2002). Briefly, the samples were washed 3 × 10 min with PBST (0.1% Tween-20 in PBS) following by 2 washes with PBTD (1% dimethyl sulfoxide in PBST), blocked for minimum of 2 hours with blocking solution (10% serum in PBTD) and then incubated with primary antibodies diluted in blocking solution overnight at 4°C. At day 2, the samples were washed at least 4 hours with PBTD and then incubated with secondary antibodies diluted in blocking solution overnight at 4°C. At day 3, the samples were washed 2-3 hours with PBTD. The following primary antibodies were used: rat anti-mouse CD31 (Pharmingen, monoclonal MEC13.3), goat anti-mouse Vegfr3 (R&D Systems, AF743), rabbit anti-Lyve1 (Abcam, ab14917), and Cy3 conjugated anti-α-Smooth Muscle Actin (SMA) (Sigma, C-6198), mouse anti-human Nfatc1 (Santa Cruz Biotechnology, clone 7A6), rabbit anti-Prox1 (Abcam, ab37128), goat anti–human Prox1 (R&D Systems, AF2727), rat anti–mouse CD105 (endoglin) (eBioscience, 14-1051), goat anti-mouse integrin-α9 (R&D Systems, AF3827), rabbit anti-human laminin α5 (Ringelmann et al., 1999). For fluorescence staining, Alexa Fluor 488, 594, and 647 fluorochrome-conjugated secondary antibodies (Invitrogen) were used for signal detection. Images were acquired with a Leica TCS SP2 confocal system (Leica Microsystems). To visualize anti-Vegfr3 staining with light microscopy, biotinylated rabbit anti-goat IgG (Vector Laboratories, BA-5000) secondary antibodies were used in horseradish peroxidase stainings with the Vectastain kit (Vector Elite PK-6100) and DAB kit (Vector Laboratories, SK-4100).
Staining for β-galactosidase activity
Whole-mount X-gal staining was performed as previously described (Qu et al., 2002). Briefly, whole embryos or intestine with mesenteries were harvested in PBS, fixed in 4% PFA in PBS, and then washed three times for 15 min with a detergent rinse (2 mM MgCl2, 0.01% sodium deoxycholate, and 0.02% Nonidet P-40, in PBS). The staining was developed in staining solution (2 mM MgCl2, 0.01% sodium deoxycholate, and 0.02% Nonidet P-40, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and X-gal 1 mg/ml, in PBS) at 37 °C overnight. After staining, the samples were used for histological analysis or imaged using a Leica M205 FA stereomicroscope and a Leica DFC310 FX digital camera (Leica Microsystems).
Quantitative RT-PCR
RNA from mesenteries of Tie1 conditional knockout (Tie1-cko) and their control littermates at E18.5 and P1 was isolated using TRIZOL Reagent (Invitrogen) with additional DNase treatment (Promega). cDNA was then generated using the SuperScript III Reverse Transcriptase kit (Invitrogen). Quantitative PCR was performed on the CFX96 Touch™ thermal cycler (Bio-Rad) using iQ™ SYBR Green Supermix (Bio-Rad). All assays were repeated at least twice, and all samples were run in triplicate. Analysis of gene expression was carried out using the comparative Ct (ΔΔCt) method as described by the manufacturer. Relative quantification of gene expression was normalized to 18S mRNA expression level. Sequences of the PCR primers used are listed in supplemental Table 1.
Quantification of lymphatic valves
Mesenteric valves were identified as areas of strongly positive Prox1 staining and counted from duodenum to ileum. The number of valves at each developmental stage was quantified using four mesenteric vessels in 4 mice/genotype.
Presentation of data and statistical analysis
Macroscopic pictures and microscopic images are representative of three or more independent experiments. Microscopic image processing and analysis were performed using Photoshop (Adobe) software. Data are expressed as the means ± s.d. Statistical analysis was conducted using the 2-tailed Student's t test. A P value < 0.05 was considered significant.
Results
Tie1 expression is accentuated in developing and mature lymphatic valves
We (Qu, et al., 2010) and others (D'Amico et al., 2010) have previously documented that Tie1 was expressed in the lymphatic endothelial cell (LEC) progenitors and all lymphatic vessels, and that structural capillary defects were associated with inefficient lymph drainage and lymphedema observed in hypomorphic mutant Tie1 embryos. This prompted us to examine if Tie1 might be involved in the formation, maturation, and function of the luminal valves and collecting-vessels. Utilizing the Tie 1 LacZ knockin/ knockout reporter line (Puri et al., 1995), we confirmed Tie1 expression in developing collecting lymphatic vessels and developing lymphatic valves by whole mount X-gal staining of mesenteries of heterozygous Tie1+/lz mice. Formation of lymphatic valves is initiated around E16.0 (Bazigou et al., 2013; Sabine et al., 2012). At E16.5, Tie1 is clearly expressed in developing collecting lymphatics although its expression level in lymphatics is lower than in either the arteries or veins (Fig. 1A). Furthermore, regions of accentuated Tie1 expression are detected throughout the collecting vessel. From E17.5, X-gal (Tie1) continues to stain intensely in the arteries, and veins, but is clearly enriched in the regions of lymphatic vessel constriction where developing lymphatic valves will form (Fig. 1B). Tie1 expression level in valvular endothelium persists during subsequent maturation and is more robust in valvular endothelium than in lymphangion cells (the segment between valves). At postnatal day 1 (P1) (Fig. 1C) and P7 (Fig. 1D), Tie1 is highly expressed in the leaflets of mature valves and this expression profile of Tie1 in mature lymphatic valves persists throughout adulthood (Fig. 1E). Therefore, Tie1 is expressed in developing collecting lymphatics and becomes progressively enriched at lymphatic valves. This expression data suggests that Tie1 might have a role in the initiation of lymphatic valve leaflet development and maintenance of the mature valve.
Figure 1. Expression of Tie1 in developing and mature lymphatic valves.
Whole-mount X-gal staining of mesenteric lymphatic vessels from Tie1lz/+ mice at the indicated ages (E16.5–young adult) showing progressively enrichment of Tie1 (X-gal) at lymphatic valves. At E16.5 (A) Tie1 expression in detected developing collecting lymphatics with accentuated expression in the region of the developing valves (Arrows). As valve development progresses (B-E), Tie1 expression becomes primarily restricted to the lymphatic valves (arrows). a, arteries; v, veins. Scale bars, 100 μm.
Nfatc1Cre mediates loxP excision in lymphatic progenitors and endothelium of the developing lymphatic valves
To specifically determine the role of Tie1 in development of collecting lymphatics, we utilized an Nfatc1Cre line as Nfatc1 expression has previously been shown to be accentuated in the endothelium of the developing lymphatic valves and essential for normal lymphatic valve development (Kulkarni et al., 2009; Norrmen et al., 2009). In this line, a nuclear localized Cre-Recombinase is “knocked in” to the 3’ UTR of Nfatc1 preceded by an IRES (internal bacterial ribosomal entry site) such that Cre recombinase is expressed under control of endogenous Nfatc1 regulatory domains without disrupting endogenous expression (Wu et al., 2012). To characterize lymphatic expression of Cre, we analyzed Cre activity by lacZ staining of the transgenic mice bred with R26R reporter mice. As seen in Fig. 2A,B, in addition to our previous reports on endogenous Nfatc1 expression pattern, which is restricted to the endocardium of developing hearts (Ranger et al., 1998; Wu et al., 2012), we detected clear Cre activity in a subset of endothelium within the lymph sacs and superficial lymphatic vessels of the jugular region at E12.5. Eosin stained transverse section through the jugular region of the whole-mount X-gal staining embryo reveals that Cre is highly active in the endothelium of the developing jugular lymphatic sacs but not the endothelium of the cardinal veins (Fig. 2C,D). In addition, whole-mount X-gal staining of postnatal mesenteries of Nfatc1Cre;R26fslz mice revealed robust expression in the early postnatal and mature (Fig. 2E,F) lymphatic valves but not in arteries or veins. Thus, Nfatc1Cre mediates loxP excision in lymphatic endothelium with persistent activation in the leaflets of mature luminal valves.
Figure 2. Nfatc1Cre mediates loxP excision in lymphatic progenitors and valve endothelium.
(A) Whole-mount X-gal staining of E12.5 Nfatc1Cre;R26fslz embryo. (B) A higher magnification view of boxed area in (A) showing X-gal positive vessels in the jugular region consistent with lymph sacs and superficial lymphatic vessels (arrows). (C) Transverse section through the jugular region of (A). (D) Boxed area in (C) is enlarged. Nfatc1 driven Cre is active in the endothelium of the developing jugular lymphatic sacs (jls) but not the endothelium of cardinal veins (cv). Whole-mount X-gal staining of postnatal mesenteries of Nfatc1Cre;R26fslz mice at P4 (E) and 3 weeks of age (F) reveals that the β-gal activity Cre is clearly present in leaflets of mature lymphatic valves (arrows) with minimal Cre mediated deletion in arteries (a) or veins (v). b, blood vessel. lv, lymphatic vessels. Scale bars: (A) 500μm; (D-F) 100μm.
Agenesis of lymphatic valves in Tie1-cko mice
Conditional deletion of Tie1 in lymphatic endothelium circumvents early embryonic lethality seen in germ line deletion (Puri et al., 1995; Sato et al., 1995) or hypomorphic Tie1 embryos (Qu et al., 2010). Nfatc1Cre;Tie1fl/fl mice (designated as Tie1-cko mice) embryos were indistinguishable from wild-type mice in utero and were born at normal frequencies. However, all newborn Tie1-cko mice developed chylous ascites shortly after their initial feeding (Fig. 3B), which was obvious by P1. The accumulation of chyle was also commonly found in the hyper-dilated lymphatic capillaries of intestine wall from Tie1-cko pups, but not in their normal littermates (data not shown). Tie1+/fl, Tie1fl/fl, and Nfatc1Cre;Tie1+/fl pups were all normal and showed no evidence of lymphatic defects. Chylous ascites is characteristic of defective lymphatic function, resulting from poor uptake and transport of chyle produced by the intestine following feeding and the leakage of lymphatic fluid into the peritoneal cavity, where it can cause an inflammatory response and lead to death (Gupta et al., 2007). Consistent with generalized lymphatic dysfunction, Tie1-cko mice demonstrate abnormal lymphatic pattern in internal organs similar to Tie1 hypomorphic mutant embryos including hyper-dilated lymphatic capillaries in the intestine wall and disorganized regressing or developmental retarded lymphatic vessels at the surface of the heart and the diaphragm (supplementary Fig. S1). Closer examination of the chyle-filled mesenteric lymphatic vessels revealed that the mesenteric collecting vessels in control neonates had characteristic constrictions reflecting functional valves (Fig. 3C). In contrast, no such constrictions corresponding to presumptive valve territories (even at sites of branching and bifurcations) were visible, indicating lack of valve formation (Fig. 3E). The presumptive collecting vessels in Tie1-cko pups were morphologically tortuous and thinner with blind-ended outcroppings, indicating regression and leakage. In addition, from P3 onward, mesenteric presumptive collecting lymphatics and superficial intestinal lymphatics in Tie1-cko mice began to be filled with blood (Fig. 3D). Once this phenotype developed, demise of the Tie1-cko pups was inevitable, occurring within one week of age.
Figure 3. Chylous ascites and abnormal structure of mesenteric lymphatic vessels in Tie1-cko neonates.
(A and B) A newborn Tie1-cko pup (B) compared to a Tie1+/f littermate (A) at P2 displaying chylous ascites (arrow) in the peritoneal cavity and also frequently found in the pleural cavity (upper arrowhead). Representative macroscopic views of mesenteric vessels in Tie1+/f (C) and Tie1-cko (E) pups at P2. The lymphatic vessels are filled with white chyle and constrictions along the vessels reflect the presence of valves (arrows in C) in control mice, but there are no such constrictions in Tie1-cko pups, even at sites of lymphatic vessel branching (arrows in E) where valve often form. Blind-ended outcroppings of presumptive collecting vessels in Tie1-cko mice suggest leakage and regression of the developing lymphatic vessels. (D) Blood-filled mesenteric presumptive collecting lymphatics (arrows) as well as bloody superficial intestinal lymphatics (arrowheads) were observed in Tie1-cko specimen at P3. Representative neonates are shown from 25 littermate control and 18 Tie1-cko animals examined from 9 independent litters. b, blood vessel. Scale bars: 500μm.
To confirm the observations of the defective lymphatic valve phenotype in Tie1-cko mice, we visualized the lymphatic vessels by co-immunofluorescence staining with the pan-endothelial marker CD31, the lymphatic markers (Prox1 and Vegfr3), and the markers for lymphatic valve cells (integrin-α9 and laminin-α5) (Bazigou et al., 2009). Expression of Prox1 or Vegfr3 is elevated in valve LECs, and CD31 staining also highlights the valve leaflets. As seen in Fig. 4, valves were abundant in the mesenteric collecting lymphatics of control neonates, and could be visualized easily in control embryos by wholemount co-immunostaining for Prox1 (Fig. 4 A, C) or Vegfr3 (Fig. 4 E, G) and CD31. However, lymphatic valves were completely absent at the corresponding sites of Tie1-cko presumptive collecting vessels (Fig. 4B, D, F, H). Interestingly, in comparison to control pups, Vegfr3 expression in the presumptive collecting vessels of Tie1-cko pups (Fig. 4H) was not downregulated in the lymphangion (segments between two consecutive valves) and not upregulated at the presumptive valve territories as observed in the controls. Similarly, expression of integrin-α9 (Fig. 4I-L) and laminin-α5 (Fig. 4M-P) revealed that characteristic mature valves are present in neonatal control mesenteries but completely missing in Tie1-cko mesenteric samples.
Figure 4. A deficiency of lymphatic valves in Tie1-cko mice.
Dual whole mount immunofluorescence of mesenteries from Tie1-cko pups and their Tie1+/f littermates at P2 for CD31 (green) and Prox1 (red, A-D), Vegfr3 (red, E-H), Integrin α9 (red, I-L), and Laminin α5 (M-P). Arrows indicate lymphatic valves as demoted by elevated expression of Prox1, Vegfr3, integrin-α9, or Laminin α5 co-localized with CD31 in control lymphatic collecting vessels. Similar lymphatic valves were completely absent at the corresponding sites (arrowheads) of Tie1-cko presumptive collecting vessels. Representative images are shown from 5 control and 6 Tie1-cko pups examined from 3 independent litters in each group. b, blood vessel. Scale bars, 100 μm.
Attenuation of Tie1 in lymphatic endothelium prevents the initiation of valve morphogenesis
In mice, valves originate around E16 by specification of valve-forming cells (Bazigou et al., 2009; Sabine et al., 2012). These cells express high levels of Prox1 and Foxc2. Thus, the earliest sign of valve formation is a localized upregulation of transcription factors Prox1 and Foxc2, which is accompanied by decreased expression of Lyve1. In developing collecting vessels of wild type mice, only immature lymphatic valves are observed up to E17.5. By E18.5, lymphatic valves at all four different stages could be found in the collecting vessels. To determine the onset of the defective valve phenotype and the mechanism underlying absence of valves in Tie1-cko mice, we performed wholemount co-immunostaining of mesenteric and dermal lymphatics at E17.0, E17.5, and E18.5 for CD31 and Prox1. At E17.0 and E17.5, normal developing lymphatic valves at early stages (stage 1-3 according to Bazigou et al., 2009; Sabine et al., 2012) were often observed in mesenteric or dermal developing collecting vessels of control embryos (Fig. 5A, C, F, and supplementary Fig. S2A, C), but only a few isolated Prox1high LEC clusters were occasionally detected in the presumptive collecting vessels of Tie1-cko embryos (B, D, G and supplementary Fig. S2B), indicating initiation of lymphatic valve specification was abnormal in the Tie1-cko embryos. The number of constrictions that contained Prox1high LEC clusters in Tie1-cko mesenteries at E17.5 was dramatically reduced when compared to the control littermates (18.1 ± 2.5 vs 5.4 ± 0.8, n = 4; P < 0.001; Fig. 5M). At E18.5, even less Prox1high LEC clusters were observed in the mesenteric (supplementary Fig. S2F, G and Fig. 5E) or dermal (data not shown) developing collecting vessels of Tie1-cko embryos. The total number of valves (stage 2-3 according to Sabine et al., 2012) in Tie1-cko mesenteries at E18.5 was dramatically decreased (22.1 ± 3.2 vs 2.6 ± 0.6, n = 4; P < 0.001; Fig. 5E). No any fully mature lymphatic valves were detected in all Tie1-cko tissues tested.
Figure 5. Attenuation of Tie1 in lymphatic endothelium prevents initiation of valve morphogenesis.
Whole mount dual immunofluorescence of mesenteric (A-D) or back skin (F, G) vessels from Tie1-cko pups and their Tie1+/f littermate controls at E17.0 with Prox1 (red) and CD31 (green, not shown in skin samples). Normal developing lymphatic valves (arrows) at early stages were often observed in mesenteric or dermal developing collecting vessels of control embryo (A, C, F), but only a few isolated Prox1high LEC clusters (arrowheads) were occasionally detected in the presumptive collecting vessels of Tie1-cko embryos (B, D, G). Representative images are shown from 5 control and 6 Tie1-cko pups examined from 3 independent litters in each group. b, blood vessel. Scale bars, 100 μm. (E) Quantification of the luminal valves (in Tie1-cko mice, counted as the number of constrictions that contained clusters of cells expressing high levels of Prox1) in control and Tie1-cko embryo mesenteric lymphatic vessels at E17.5 and E18.5. Quantitative data are represented as mean and s.d. from 4 control and 4 Tie1-cko embryos examined from 3 independent litters (P < 0.01). (H) RT-qPCR analysis of control and Tie1-cko mesenteries at E18.5. Expression of 18S was used to normalize the samples. The results are represented as mRNA fold changes measured in Tie1-cko vs control. Data are the mean ± s.d. from 4 independent experiments performed in duplicate. *, P < 0.05.
To identify the potential change of several key molecular regulators probably associated with lymphatic remodeling and valve formation, we analyzed their expression in mesenteries by quantitative real-time RT-PCR at E18.5 and P1. At E18.5, consistent with the above immunofluorescence staining, expression level of Prox1 and Foxc2 in Tie1-cko samples was reduced by 18.0% and 26.3%, respectively when compared with control embryos; while expression levels of CD31, Lyve1, Vegfr3, Nfatc1, Tie2, and Ang2 had no significant change (Fig. 5H). In Tie1-cko neonates (P1), expression level of Prox1 and Foxc2 was further reduced by 33.0% and 45.5%, respectively (supplementary Fig. S2I). Reduced expression level of Lyve1 and Vegfr3 by 15.3% and 17.5% is in agreement with the observation of regression of certain lymphatics in Tie1-cko mesenteries (data not shown and Qu et al., 2010). It should be noted that CD31 and Tie2 are normally expressed in both blood and lymphatic vessels, they have higher expression in blood vessels than in lymphatics. Taken together, Tie1 is required for the initiation of lymphatic valve development characterized by high expression of Prox1 and Foxc2.
Lymphovenous valve formation and lymphatic-blood vessel separation are normal in Tie1-cko embryos
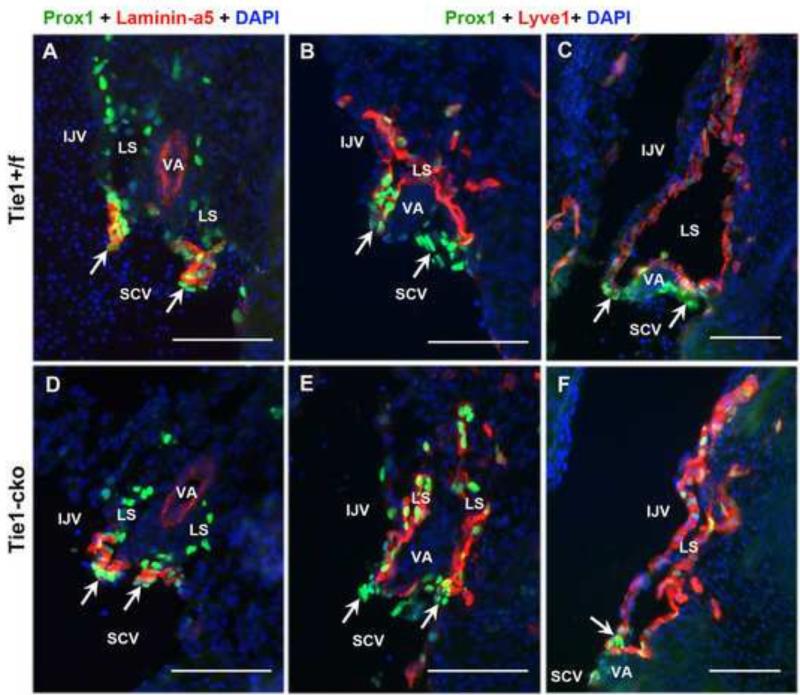
Since blood-filled mesenteric lymphatics and bloody superficial intestinal lymphatics were often observed in Tie1-cko mice from P3, this phenotype could be attributed to defects in lymphovenous valve formation and/or lymphatic-blood vessel separation in Tie1-cko embryos. Lymphovenous valves, which located at the junctions of the lymphatic ducts with the venous circulation, allow fluid to drain into the veins and prevent blood from entering into the lymphatic circulation (Srinivasan and Oliver, 2011). Failure to separate the lymphatic sacs from the veins leads to prolonged bleeding into the lymph sacs, tortuous blood-filled cutaneous lymphatic vessels and eventually embryonic death (Abtahian et al., 2003). As described by Srinivasan and Oliver (2011), and Turner et al. (2014), the lymphovenous valves form through the fusion of the lymph sacs with the veins in a region containing a subpopulation of Prox1+ Foxc2+ venous ECs within the internal jugular (IJV) and subclavian (SCV) veins. As seen in Fig. 6, sequential coronal cryosections at the junction of the IJV and SCV veins were co-stained with Prox1 (green) and Laminin-α5 (red), or Lyve1 (red), showing the normal morphology of the lymphovenous valves in both control (Fig. 6A–C) and Tie1-cko (Fig. 6D–F) embryos at E13.5 and at E15.5 (supplementary Fig. S3). Consistent to the previous reports (Srinivasan and Oliver, 2011; Turner et al., 2014), on each of the left and right sides of both the control and Tie1-cko embryos, two valves connecting the lymph sacs (labeled with Lyve1) with the surrounding veins were identified. At this location the lymph sac is split into two portions by the vertebral artery. One of these valves is located more dorsomedially than does the other one. The valves have high expression of the valve-specific markers Prox1 and Laminin-α5. Furthermore, separation (with Lyve1 positive endothelial cells) of the lymph sac from the IJV is also normal in both control (C) and Tie1-cko (F) embryos. We detected no blood cells within the lymph sacs in both control and Tie1-cko mice. Thus, the phenotype of blood-filled mesenteric lymphatics in Tie1-cko neonates is not attributed to defects in lymphovenous valve formation or lymphatic-blood vessel separation.
Figure 6. Normal lymphovenous valve formation and lymphatic-blood vessel separation in Tie1-cko embryos.
Sequential coronal cryosections at the junction of the internal jugular (IJV) and subclavian (SCV) veins co-stained with Prox1 (green) and Laminin-α5 (red), or Lyve1 (red), showing the normal morphology of the left lymphovenous valves with high expression of Prox1 and Laminin-α5 in a control (A–C) and a Tie1-cko (D–F) embryo at E13.5. The head is orientated to the top and the heart towards the bottom of the figures. Lyve1 labels lymph sacs (LS), while Prox1 and Laminin-α5 (which is also positive in the wall of arteries) are valve specific markers. In both control and Tie1-cko mice, the first valve leaflets open into the IJV (upper arrows), while the second valve drains into the SCV/IJV junction (lower arrows). In addition, separation (with Lyve1 positive endothelial cells) of the LS from the IJV is normal in both control (C) and Tie1-cko (F) embryos. Note the lack of blood cells within the LS in both control and Tie1-cko mice. Representative images are shown from 5 control and 5 Tie1-cko pups examined from 3 independent litters in each group. VA, the vertebral artery. Scale bars: 100 μm.
Attenuation of Tie1 in lymphatic endothelium prevents formation of collecting lymphatic vessels
Because Tie1-cko mice exhibit a deficit of intraluminal lymphatic valves, we sought to determine whether there was also a deficiency of collecting lymphatic vessels development. Wholemount immunofluorescence staining of neonatal skin (P1) for Vegfr3 and Lyve1 revealed a highly branched network of lymphatic vessels in both Tie1-cko mice and their control littermates (Fig. 7A). In control embryos, lymphatic capillaries expressed high levels of both Vegfr3 as well as Lyve1. Collecting lymphatic vessels were identified as vessels in which Lyve1 was downregulated but expression of Vegfr3 remained high, particularly in regions of the lymphatic valves. Thus both initial lymphatics with high expression of Lyve1 and Lyve1-downregulated/Vegfr3-positive collecting lymphatic vessels are present in the control skin as previously described (Dellinger, et al., 2008). In contrast, all lymphatic vessels throughout the immature network of Tie1-cko skin expressed the initial lymphatic marker Lyve1, and downregulation of Lyve1 characteristic of collecting lymphatic vessel development did not occur.
Figure 7. A deficit of Lyve1 down-regulated collecting lymphatic vessels in Tie1-cko mice.
(A) P1 ventral skin of Tie1+/f pups and their Tie1-cko littermates was whole-mount co-stained for Vegfr3 (green) and Lyve1 (red). Both initial lymphatics (arrowheads) with high expression of Lyve1 and Lyve1-down-regulated-Vegfr3-positive collecting lymphatic vessels (arrow) are present in the control skin. In contrast, all lymphatic vessels throughout the immature network of Tie1-cko skin express the initial lymphatic marker Lyve1. (B) E18.5 mesenteries of Tie1+/f pups (G-I) and their Tie1-cko littermates (J-L) were whole-mount co-stained for CD31 (green) and Lyve1 (red). Mesenteric lymphatic vessels of Tie1+/f mice exhibit a mature collecting vessel phenotype, have very low expression of Lyve1, and contain valves (arrow). In contrast, the comparable mesenteric lymphatic vessels of Tie1-cko mice are immature, have uniformly high expression of Lyve1 (arrowheads), and lack valves. A nonlymphatic Lyve1 immunoreactivity is also observed in isolated cells outside vessels, which probably correspond to macrophages. Representative images are shown from at least 5 control and 5 Tie1-cko pups examined from 3 independent litters in each group at each age. b, blood vessel. Scale bars, 100 μm.
To determine whether lymphatic vessels in other regions of the body of Tie1-cko mice fail to exhibit a collecting vessel phenotype, we examined the mesenteries of Tie1-cko mice and their control littermates at E18.5 by whole-mount immunofluorescence (Fig. 7B). Similar to observation in neonatal skin, mesenteric lymphatic vessels of Tie1+/f mice exhibit a mature collecting vessel phenotype, have low expression of Lyve1 and relatively low level of Vegfr3 in the lymphangion, and contain valves. In contrast, the comparable mesenteric lymphatic vessels of Tie1-cko mice are immature, have uniformly high expression of Lyve1 and Vegfr3, and lack valves (Fig. 7B). Similarly, at E17.5, the lymphatic capillaries both in Tie1-cko mice and in control littermates continue to express high levels of Lyve1. However, in control embryos a relatively low level of Lyve1 in observed in developing collecting vessels while all the lymphatics in Tie1-cko embryos exhibit a uniformly high expression of Lyve1 (data not shown). These data show that the failure of Tie1-cko lymphatic vessels to exhibit an abnormal collecting vessel phenotype is not restricted to the skin. Collectively, our data show that loss of Tie1 leads to a failure in the maturation of the primary lymphatic plexus whole bodily into functional collecting lymphatic vessels.
SMCs associate with initial lymphatic vessels of Tie1-cko mice
A sparse covering by mural cells is another characteristic feature of collecting lymphatic vessels. Normally immature collecting vessels lack pericytes/SMCs, and only at later stages they acquire sparse coverage, but the valve areas are usually devoid of these cells (Oliver and Alitalo, 2005). To determine the potential effect of Tie1 attenuation on the association of SMCs with dermal lymphatic vessels, we performed wholemount immunofluorescence staining of skin for SMA and Lyve1. Dermal lymphatic capillaries of the control mice at E18.5, P1 and P3 lacked SMC coverage. In contrast, SMCs were associated with about 30% of initial lymphatic vessels in Tie1-cko mice at P3 (Fig. 8A), while this ectopic SMC coverage occurred at a lower frequency (~8%) at P1 (supplementary Fig. S4A-D). At E18.5, this defect was only occasionally observed in certain severe dilated dermal lymphatic capillaries (supplementary Fig. S4E-H). Similarly, in the mesenteries of control mice at E18.5 and P3, initial lymphatic vessels lacked SMC coverage while mural cells were only associated with Lyve1 downregulated collecting lymphatic vessels. In contrast, SMCs were uniformly associated with almost all lymphatic vessels in Tie1-cko mice (Fig. 8B and supplementary Fig. S4I-L).
Figure 8. Ectopic SMCs and increased endoglin expression in lymphatics of Tie1-cko mice.
(A) P3 ventral skin of Tie1+/f pups and their Tie1-cko littermates were whole-mount co-stained for SMA (red) and Lyve1 (green) showing that SMA positive cells are associated with Lyve1 positive initial lymphatic vessels (arrow) in Tie1-cko pups but not in control mice. (B) E18.5 mesenteries of Tie1+/f pups and their Tie1-cko littermates were whole-mount co-stained for SMA (red) and Vegfr3 (green). SMA positive cells are only associated with Lyve1-down-regulated collecting lymphatic vessels in control, but with both collecting lymphatic vessels (arrowheads) and lymphatic capillaries (arrows) in the mutants. (C) E18.5 mesenteries were whole-mount co-stained for Vegfr3 (red) and endoglin (green) showing increased lymphatic endothelial endoglin expression in Tie1-cko embryos. Note very low level of endoglin expression at sites of lymphatic valves (arrowheads) in the control but uniformly high level at the comparable sites of Tie1-cko embryos. Representative images are shown from at least 4 control and 5 Tie1-cko pups examined from 3 independent litters in each group at each age. Arrows indicate lymphatic capillaries. b, blood vessel. Scale bars, 100 μm.
Endoglin, a co-receptor for members of the transforming growth factor (TGF) superfamily, has been implicated in the recruitment of SMCs to blood vessels (Li, et al., 1999). As seen in Fig. 8C, the mesenteric lymphatic vessels in Tie1-cko mice expressed higher levels of endoglin than in control embryos at E18.5. Consistent with immunofluorescence staining, our qPCR revealed that endoglin expression level in Tie1-cko mesenteries at E18.5 and at P1 is higher than that in control samples by 25% and 22%, respectively (Fig. 5H and supplementary Fig. S2I).
Discussion
Previously we (Qu et al., 2010) and others (D'Amico et al., 2010) demonstrated that mice hypomorphic for Tie1 exhibit dilated and disorganized lymphatic vessels with impaired lymphatic drainage capacity which resulted in embryonic demise. In the current study, we document, for the first time, that while Tie1 is ubiquitously expressed at the onset of lymphatic development, there is accentuated expression in LECs at the onset of lymphatic valve development, and expression in the lymphatic system is almost exclusively restricted to the endothelium of mature lymphatic valves. Furthermore, utilizing a Cre deletor mouse line controlled by Nfatc1 regulatory elements (Wu, et. al., 2012), a transcription factor whose activity has been shown to be essential for lymphatic valve formation (Normen, et al., 2009), we demonstrate that Tie1 is necessary for lymphatic valve morphogenesis, as well as vessel remodeling and maturation.
In preparation of this manuscript, a report by Shen et al. (2014) also described that perturbation of Tie1 is results in abnormal lymphatic valve development and collecting vessel remodeling further supporting the results reported here. The mouse model used in their study was homozygous mutated allele, Tie1ΔICD/ΔICD, where the putative kinase domain of Tie1 was ubiquitously deleted throughout the vasculature. In addition to defects in lymphatic valve and collecting vessel remodeling similar to those observed in our present study, the investigators documented earlier defects in formation of the primary lymphatic network, which we did not observe. This is likely at least partially the result of our use of the Nfatc1-Cre line to inactivate Tie1 in cells expressing Nfatc1, which may be more specific for lymphatic valve cells. The phenotype observed by Shen and colleagues was similar to that seen in the hypomorphic mouse model used in a previous report by our group (Qu et al., 2010) and that of D'Amico, et al., 2010. In addition, we observed abnormal SMC coverage of lymphatic vasculature (Fig. 8B and Sup;. Fig. S4) similar to that observed in the Ang2 null mutants (Dellinger et al, 2008:Gale et al., 2002)), but this was not appreciated in the Tie1ΔICD/ΔICD mutants. The reason for this discrepancy is uncertain.
A role for Tie1 in mechanotransduction of lymphatic flow required for valve formation
Despite the clinical importance of lymphatic valves, the molecular mechanisms and cellular events required for the formation of luminal valves are poorly understood and have only recently begun to be characterized. Based on the analysis of mouse mesenteric lymphatics (Bazigou et al., 2009; Kazenwadel et al., 2012; Koltowska et al., 2013; Norrmén et al., 2009; Sabine et al., 2012), four different stages of valve development have been recently defined: (1) valve initiation (formation of discrete clusters of valve-forming cells), (2) condensation (formation of a circumferential valve territory, alteration in cell shape and molecular identity), (3) elongation (valve leaflet formation from deposition of ECM and ring-like constriction), and (4) maturation (leaflet elongation and sinus formation). The initiation of lymphatic valve development in the mouse embryos can be first recognized at E16, when Prox1, Foxc2 and Gata2 become visible at high levels within discrete clusters of cells in the initial lymphatic plexus. These cells create a clear boundary with the neighboring lymphangion cells that express reduced levels of these markers. Compared to controls, in mesenteric or dermal developing collecting vessels of Tie1-cko embryos by E18.5, not only no any mature lymphatic valves were detected, but also Prox1high LEC clusters were dramatically reduced. Thus Tie1 appears to be required for lymphatic valve morphogenesis. Specifically, Tie1 is required for initiation of lymphatic valve specification.
Recent studies (Bazigou et al., 2009; Sabine et al., 2012) support the hypothesis that the cooperation between lymphatic endothelial transcription factors Foxc2 and Prox1, and mechanical forces is essential for determining where valves ultimately form: (1) Lymphatic valves often form at sites of lymphatic vessel branching and bifurcations, where lymph flow may be disturbed; (2) The initiation of valve formation in the mouse embryo coincides with the onset of lymph flow and occurs concomitantly with remodeling of a primary lymphatic vascular plexus into functional collecting vessels; (3) Oscillatory but not laminar flow introduced to LECs in vitro induces expression of two crucial downstream regulators (connexin 37 and calcineurin/Nfatc signaling) of valve morphogenesis in a Foxc2- and Prox1-dependent manner, similar to the upregulation that precedes valve induction in vivo; (4) Lymphatic vessels cultured ex vivo, in the absence of fluid flow, lose the patterned expression of Prox1 and Foxc2 and fail to assemble valves properly. However, to date, the molecular pathways responsible for transducing the mechanosensory signals that initiate lymphatic valve morphogenesis remain elusive. Tie1 has recently been identified as an essential component of the endothelial mechanosensory complex (Woo and Baldwin, 2011), and as we demonstrated here, it is highly expressed in lymphatic-valve-forming cells and mature lymphatic valves (Fig. 1). Moreover, Tie1 expression in the adult correlates distinctively to vascular regions exposed to disturbed flow in both physiological and pathological conditions (Woo et al., 2011). Experiments in vivo and in vitro revealed that Tie1 is up-regulated and activated upon exposure of cells to disturbed flow, but down-regulated by brief application of laminar flow (Chen-Konak et al., 2003; Porat et al., 2004, Woo, et al., 2011), suggesting that Tie1 mediated signaling may be involved in regulating flow-mediated responses, and thus early stages of lymphatic valve formation. In supporting of this hypothesis, attenuation of Tie1 abolishes Prox1 and Foxc2 upregulation in prospective valve-forming cells of embryonic mesenteric and dermal lymphaics.
Tie1 is required for lymphatic collecting vessel development
Tie1-cko embryos also demonstrated remodeling defects including the failure to form mature collecting vessels. Lymphatic vessel remodeling commences at E15.5 and continues after birth in mice. The lymphatic vasculature develops first as a primary capillary plexus, and the subsequent remodeling involves sprouting of new lymphatic capillaries from the initial plexus. In the absence of Tie1, the early development of the dermal lymphatic vessels occurred normally, but presumptive collecting vessels are not able to remodel and to down-regulate the expression of lymphatic capillary markers Lyve-1, and Vegfr3. In addition, some dilated lymphatic capillaries particularly in skin of Tie1-cko mice acquire ectopic mural cell coverage. Normally lymphatic capillaries devoid of pericytes and SMCs, while a sparse coverage of SMCs is acquired to lymphatic vessels only after valve formation and concomitant remodeling of a primitive vascular plexus to mature collecting vessels (Oliver and Srinivasan, 2008). In line with the abnormal recruitment of SMCs, presumptive collecting vessels in Tie1-cko mice demonstrated significantly increased expression of endoglin, a co-receptor for members of TGF-β superfamily, which has been implicated in the recruitment of SMCs to blood vessels (Li, et al., 1999). Interestingly, increased expression of endoglin has been reported in Pik3r1 null mice (Mouta-Bellu et al., 2009), and as discussed below, PI3K is a downstream target of the Tie1/Tie2 signaling pathway. Recent studies using endoglin transgenic mice indicated that endoglin overexpression in vascular precursor cells promoted vascular smooth muscle cell investment of major vessels (Mancini et al., 2007).
Blood-filled mesenteric lymphatics and bloody superficial intestinal lymphatics were often observed in Tie1-cko mice from 3 days of age. We confirmed that these bloody vessels were lymphatics rather than blood vessels by whole-mount Lyve1 staining (Fig. 3D and data not shown). This phenotype shares similarity with Cx37−/−Cx43−/− double knockout mice (Kanady et al., 2011), which also demonstrated lymphatic valve defects. However, in that model blood in the lymphatic system was observed much earlier (from late embryo stage E18.5). A similar observation of blood filled lymphatics was observed in the slp-76 null mice and was attributed to nonseparation of the venous and lymphatic circulations (Abtahian et al., 2003). As described by Srinivasan and Oliver (2011) and Turner et al. (2014), the lymphatic vessels communicate with the blood vessels only at the junction of the jugular and subclavian veins and the embryonic lymphovenous valves control this vital communication from E13.5. They are formed by the intercalation of LECs with a subpopulation of venous ECs at the junction of the jugular and subclavian veins. We observed normal lymphovenous valve formation and lymphatic-blood vessel separation in Tie1-cko embryos. In addition, we saw no evidence of aberrant connections between lymphatic vessels and blood vessels of Tie1-cko neonates. Blood vessels of the mesenteries in the Tie1-cko mice looked normal (data not shown) and tissue hemorrhage was not a source of red blood cells in the lymphatics. Thus, a nonseparation phenotype in Tie1-cko mice was unlikely to be the etiology of retrograde flow of venous blood to entering the lymphatic vasculature but is likely a compound effect of lymphatic valve deficiencies and subsequent heart failure in postnatal animals.
Tie1 cko mice display phenotypic similarity to Ang2 null mice
The Tie1-deficient mice in this study share many features with the Ang2 null mice that have been shown to have abnormal lymphatic vessel architecture, defective remodeling, ectopic SMC coverage, and valve agenesis (Dellinger et al., 2008; Gale et al., 2002). Recently, Ang2 had been reported to be indispensable before and during lymphatic valve morphogenesis (Zheng et al., 2014). Ang2 is a known ligand for Tie2, another endothelial receptor tyrosine kinase which often serves as an antagonist of Tie2 activity during angiogenesis but as an agonist during lymphangiogenesis (Gale et al., 2002). The similarity in defects observed between the Ang2 null mice and Tie1-cko mice raises the possibility of interactions between Tie1 and Tie2 signaling during lymphatic valve development. Tie1 shares a high degree of homology and is able to form heterodimers with Tie2 (Marron et al., 2000; Peters et al., 2004; Yancopoulos et al., 2000). The Tie receptors are co-expressed in both blood vascular and lymphatic ECs. In fact, to date no EC population has been described to only express Tie1 or Tie2. Increasing, evidences suggest that Tie1 can play a role as an inhibitory coreceptor for Tie2 since Tie1 and Tie2 appear to associate on the cell surface and Tie1 also attenuates Ang1 activation or Ang2 inhibition of Tie2 activation (Marron et al., 2007; Seegar et al., 2010). Therefore, Tie1 might modulate Ang2 function via the Tie2 receptor in the developing valvular LECs in vivo as has documented by in vitro studies utilizing mature lymphatic endothelial cells (Song et al., 2012). The absolute requirement for Tie1 and Tie2 during angiogenic remodeling and vessel maintenance suggests that a well-balanced Tie2/Tie1 ratio is critical to the development of new vessels and the regression of existing vessels (Augustin et al., 2009). Interestingly, Tie2 is highly expressed in venous valves, starting from the earliest developmental stages (Bazigou et. al, 2011) and experiments using cultured endothelial monolayers in vitro also revealed that Tie2 was upregulated and activated upon exposure of cells to shear stress (Chlench, et al., 2007; Lee, et al., 2003). Thus, it is tempting to speculate that Tie2, in concert with Tie1 serves as a shear stress mediator involved in initiating lymphatic valve formation. In support of this possibility, Tie1 deletion in the systemic vasculature in regions of disturbed flow results in an increase in Tie2 activation without increase in Tie2 expression (Woo, et al., 2011).
Tie1 is capable of activating both PI3K and Akt to promote endothelial survival (Kontos et al., 2002; Yuan, et al., 2007) and signaling through PI3K and Akt is essential for Ang1/Tie2-induced endothelial survival, sprouting, migration and capillary tube formation (Fukuhara et al. 2008; Saharinen et al. 2008). It is interesting to note that both PI3K and Akt are required for valve morphogenesis. Pik3r1 null mice lacking the regulatory isoforms of the class Ia PI3K, p85α/p55α/p50α, died by P1 and showed severe lymphatic defects including defective lymphatic vessel remodeling, and lack of valves (Mouta-Bellum, et al., 2009). The newborn mice with a point mutation abrogating the interaction between p110α (a catalytic subunit of PI3K) and Ras were also reported to display chylous ascites and defective lymphatic development (Gupta et al., 2007). In addition, lack of Akt1 leads to more specific defects in the formation of lymphatic valves in the superficial collecting vessels of the skin (Zhou et al., 2010). The phenotype observed in the Tie1-cko mice described here are consistent with perturbation in the PI3K and Akt pathways resulting from either Tie1 deletion alone and/or loss of Tie1 attenuation of Tie2 activation. Thus, we propose that Tie signaling is an important component of the mechanosensors of shear stress in endothelial cells contributing to initiate the Prox1high Foxc2 high LEC clusters and establish the valve territory via the activation of calcineurin/Nfatc1, thus triggering subsequent steps of lymphatic valve development: valve leaflet formation, maturation and sinus formation (Fig.9). In the disruption of Tie1/Tie2 signaling (as Tie1/PI3K/Akt mutants), lymphatic valve-forming cells fail to form Prox1high Foxc2 high LEC clusters and assemble the ring-like valve territory, resulting lack of valves.
Figure 9. Proposed mechanism of Tie1 pathway function in lymphatic valve formation.
The Tie signaling is an important component of the mechanosensors of shear stress in lymphatic valve initiation. Lymph reversing/disturbed flow (dashed arrows) activates the Tie signaling which inducing high-expression of Prox1 and Foxc2 at the sites of future valves to form discrete clusters of valve-forming cells. Shear stress mediators Tie1/Tie2 also contribute to maintain these Prox1high Foxc2 high LEC clusters and establish the valve territory via the activation of calcineurin/Nfatc1, thus triggering subsequent steps of lymphatic valve development: valve leaflet formation, maturation and sinus formation (Modified from Sabine et al. (2012)). Once lymphatic valve is formed, flow becomes mostly unidirectional (solid arrow). With disruption of Tie1/Tie2 signaling (as Ang2/Tie1/PI3K/Akt mutants), lymphatic valve-forming cells fail to form Prox1high Foxc2 high LEC clusters and assemble the ring-like valve territory, resulting lack of valves and unidirectional blood flow is never established in the lymphatic circulation. The key regulators at each stage are indicated.
Conclusions
Our findings identify Tie1 as a critical regulator of lymphatic vessel remodeling and maturation as well as valve morphogenesis. Our study also sheds light on early steps of lymphatic valve morphogenesis, and suggests that Tie signaling is an important component for mechanosensing of shear stress in lymphatic endothelial cells maintaining the critical and patterned high expression of Prox1 and Foxc2 in prospective valve-forming cells. Future studies, including the functional knockdown of Tie2 in primary lymphatic endothelial cells during development as well as in the postanatal period, should help to identify the direct target genes of Ang-Tie system and enhance understanding of the mechanisms regulating lymphatic remodeling and maturation, and the formation of luminal valves. This work should help in elucidating basic mechanisms underlying genetic defects in lymphatic development as well inform potential therapies to accelerate post-surgical lymphatic repair.
Supplementary Material
Highlights.
Tie1 is expressed in developing and mature lymphatic valves.
The Nfatc1-Cre line provides loxP excision in lymphatic developing valves.
Tie1 is required for the initiation of valve morphogenesis.
Tie1 deletion resulted in lack of lymphatic valves and collecting vessel defects.
Tie1 is dispensable for lymphovenous valve formation.
Acknowledgements
We thank professor Lydia Sorokin (Institute of Physiological Chemistry and Pathobiochemistry, University of Muenster, Germany) for generously sharing their laminin α5 antibody with us. We thank M. Puri for Tie1+/lz mice.
Sources of Funding
This work was supported by National Institutes of Health grants R01 HL118386 (S.B.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
X.Q. and S.B. designed research; X.Q. performed research; X.Q. and S.B. analyzed data, and co-authored the manuscript. B.Z. developed the Nfatc1-Cre mouse line and assisted with editing the manuscript.
Competing interests statement
The authors declare no competing financial interests.
References
- Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K. The lymphatic vasculature in disease. Nat. Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Bazigou E, Makinen T. Flow control in our vessels: vascular valves make sure there is no way back. Cell. Mol. Life Sci. 2013;70:1055–1066. doi: 10.1007/s00018-012-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Lyons OT, Smith A, Venn GE, Cope C, Brown NA, Makinen T. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J. Clin. Invest. 2011;121:2984–2992. doi: 10.1172/JCI58050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, Adams R, Muro AF, Sheppard D, Makinen T. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev. Cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvrée K, Brunet I, Del Toro R, Gordon E, Prahst C, Cristofaro B, Mathivet T, Xu Y, Soueid J, Fortuna V, et al. Semaphorin3A, Neuropilin-1, and PlexinA1 are required for lymphatic valve formation. Circ. Res. 2012;111:437–445. doi: 10.1161/CIRCRESAHA.112.269316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Konak L, Guetta-Shubin Y, Yahav H, Shay-Salit A, Zilberman M, Binah O, Resnick N. Transcriptional and post-translation regulation of the Tie1 receptor by fluid shear stress changes in vascular endothelial cells. FASEB J. 2003;17:2121–2123. doi: 10.1096/fj.02-1151fje. [DOI] [PubMed] [Google Scholar]
- Chlench S, Mecha Disassa N, Hohberg M, Hoffmann C, Pohlkamp T, Beyer G, Bongrazio M, Da Silva-Azevedo L, Baum O, Pries AR, Zakrzewicz A. Regulation of Foxo-1 and the angiopoietin-2/Tie2 system by shear stress. FEBS Lett. 2007;581:673–680. doi: 10.1016/j.febslet.2007.01.028. [DOI] [PubMed] [Google Scholar]
- D'Amico G, Korhonen EA, Waltari M, Saharinen P, Laakkonen P, Alitalo K. Loss of endothelial Tie1 receptor impairs lymphatic vessel development-brief report. Arterioscler. Thromb. Vasc. Biol. 2010;30:207–209. doi: 10.1161/ATVBAHA.109.196618. [DOI] [PubMed] [Google Scholar]
- Danussi C, Del Bel Belluz L, Pivetta E, Modica TM, Muro A, Wassermann B, Doliana R, Sabatelli P, Colombatti A, Spessotto P. EMILIN1/[g302]9[g533]1 Integrin Interaction Is Crucial in Lymphatic Valve Formation and Maintenance. Mol. Cell. Biol. 2013;33:4381–4394. doi: 10.1128/MCB.00872-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger M, Hunter R, Bernas M, Gale N, Yancopoulos G, Erickson R, Witte M. Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice. Dev. Biol. 2008;319:309–320. doi: 10.1016/j.ydbio.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont DJ, Fong GH, Puri MC, Gradwohl G, Alitalo K, Breitman ML. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev. Dyn. 1995;203:80–92. doi: 10.1002/aja.1002030109. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat. Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev. Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- Gupta S, Ramjaun AR, Haiko P, Wang Y, Warne PH, Nicke B, Nye E, Stamp G, Alitalo K, Downward J. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- Jurisic G, Maby-El Hajjami H, Karaman S, Ochsenbein AM, Alitalo A, Siddiqui SS, Ochoa Pereira C, Petrova TV, Detmar M. An unexpected role of semaphorin3a-neuropilin-1 signaling in lymphatic vessel maturation and valve formation. Circ. Res. 2012;111:426–436. doi: 10.1161/CIRCRESAHA.112.269399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanady JD, Dellinger MT, Munger SJ, Witte MH, Simon AM. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev. Biol. 2011;354:253–266. doi: 10.1016/j.ydbio.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazenwadel J, Secker GA, Liu YJ, Rosenfeld JA, Wildin RS, Cuellar-Rodriguez J, Hsu AP, Dyack S, Fernandez CV, Chong CE, et al. Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood. 2012;119:1283–1291. doi: 10.1182/blood-2011-08-374363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltowska K, Betterman KL, Harvey NL, Hogan BM. Getting out and about: the emergence and morphogenesis of the vertebrate lymphatic vasculature. Development. 2013;140:1857–1870. doi: 10.1242/dev.089565. [DOI] [PubMed] [Google Scholar]
- Kontos CD, Cha EH, York JD, Peters KG. The endothelial receptor tyrosine kinase Tie1 activates phosphatidylinositol 3-kinase and Akt to inhibit apoptosis. Mol. Cell Biol. 2002;22:1704–1713. doi: 10.1128/MCB.22.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RM, Greenberg JM, Akeson AL. NFATc1 regulates lymphatic endothelial development. Mech. Dev. 2009;126:350–365. doi: 10.1016/j.mod.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Koh GY. Shear stress activates Tie2 receptor tyrosine kinase in human endothelial cells. Biochem. Biophys. Res. Commun. 2003;304:399–404. doi: 10.1016/s0006-291x(03)00592-8. [DOI] [PubMed] [Google Scholar]
- Levet S, Ciais D, Merdzhanova G, Mallet C, Zimmers TA, Lee SJ, Navarro FP, Texier I, Feige JJ, Bailly S, Vittet D. Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation. Blood. 2013;122:598–607. doi: 10.1182/blood-2012-12-472142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini ML, Verdi JM, Conley BA, Nicola T, Spicer DB, Oxburgh LH, Vary CP. Endoglin is required for myogenic differentiation potential of neural crest stem cells. Dev. Biol. 2007;308:520–533. doi: 10.1016/j.ydbio.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marron MB, Hughes DP, Edge MD, Forder CL, Brindle NP-J. Evidence for heterotypic interaction between the receptor tyrosine kinases Tie-1 and Tie-2. J. Biol. Chem. 2000;275:39741–39746. doi: 10.1074/jbc.M007189200. [DOI] [PubMed] [Google Scholar]
- Marron MB, Singh H, Tahir TA, Kavumkal J, Kim H-Z, Koh GY, Brindle NP. Regulated proteolytic processing of Tie1 modulates ligand responsiveness of the receptor tyrosine kinase Tie2. J. Biol. Chem. 2007;282:30509–30517. doi: 10.1074/jbc.M702535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouta-Bellum C, Kirov A, Miceli-Libby L, Mancini ML, Petrova TV, Liaw L, Prudovsky I, Thorpe PE, Miura N, Cantley LC, et al. Organ-specific lymphangiectasia, arrested lymphatic sprouting, and maturation defects resulting from gene-targeting of the PI3 K regulatory isoforms p85alpha, p55alpha, and p50alpha. Dev. Dyn. 2009;238:2670–2679. doi: 10.1002/dvdy.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmén C, Ivanov KI, Cheng J, Zangger N, Delorenzi M, Jaquet M, Miura N, Puolakkainen P, Horsley V, Hu J, et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J. Cell Biol. 2009;185:439–457. doi: 10.1083/jcb.200901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Alitalo K. The lymphatic vasculature: recent progress and paradigms. Annu. Rev. Cell Biol. 2005;21:457–483. doi: 10.1146/annurev.cellbio.21.012704.132338. [DOI] [PubMed] [Google Scholar]
- Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- Oliver G, Srinivasan RS. Lymphatic vasculature development: current concepts. Ann. N. Y. Acad. Sci. 2008;1131:75–81. doi: 10.1196/annals.1413.006. [DOI] [PubMed] [Google Scholar]
- Partanen J, Armstrong E, Makela TP, Korhonen J, Sandberg M, Renkonen R, Knuutila S, Huebner K, Alitalo K. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol. Cell. Biol. 1992;12:1698–1707. doi: 10.1128/mcb.12.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KG, Kontos CD, Lin PC, Wong AL, Rao P, Huang L, Dewhirst MW, Sankar S. Functional significance of Tie2 signaling in the adult vasculature. Recent Prog. Horm. Res. 2004;59:51–71. doi: 10.1210/rp.59.1.51. [DOI] [PubMed] [Google Scholar]
- Porat RM, Grunewald M, Globerman A, Itin A, Barshtein G, Alhonen L, Alitalo K, Keshet E. Specific induction of tie1 promoterby disturbed flow in atherosclerosis-prone vascularniches and flow-obstructing pathologies. Circ. Res. 2004;94:394–401. doi: 10.1161/01.RES.0000111803.92923.D6. [DOI] [PubMed] [Google Scholar]
- Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995;14:5884–5891. doi: 10.1002/j.1460-2075.1995.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Tompkins K, Batts LE, Puri M, Baldwin S. Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice. Development. 2010;137:1285–1295. doi: 10.1242/dev.043380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger AM, Grusby MJ, Hodge MR, Gravallese EM, de la Brousse FC, Hoey T, Mickanin C, Baldwin HS, Glimcher LH. The transcription factor NF-ATc is essential for cardiac valve formation. Nature. 1998;392:186–90. doi: 10.1038/32426. [DOI] [PubMed] [Google Scholar]
- Ringelmann B, Roder C, Hallmann R, Maley M, Davies M, Grounds M, Sorokin L. Expression of laminin alpha1, alpha2, alpha4, and alpha5 chains, fibronectin, and tenascin-C in skeletal muscle of dystrophic 129ReJ dy/dy mice. Exp. Cell Res. 1999;246:165–182. doi: 10.1006/excr.1998.4244. [DOI] [PubMed] [Google Scholar]
- Sabine A, Agalarov Y, Maby-El Hajjami H, Jaquet M, Hägerling R, Pollmann C, Bebber D, Pfenniger A, Miura N, Dormond O, et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev. Cell. 2012;22:430–445. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat. Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell Biol. 2011;193:607–618. doi: 10.1083/jcb.201012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegar TC, Eller B, Tzvetkova-Robev D, Kolev MV, Henderson SC, Nikolov DB, Barton WA. Tie1–Tie2 interactions mediate functional differences between angiopoietin ligands. Mol. Cell. 2010;37:643–655. doi: 10.1016/j.molcel.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Shang Z, Wang B, Zhang L, Zhou F, Li T, Chu M, Jiang H, Wang Y, Qiao T, et al. Genetic dissection of tie pathway in mouse lymphatic maturation and valve development. Arterioscler. Thromb. Vasc. Biol. 2014;34:1221–1230. doi: 10.1161/ATVBAHA.113.302923. [DOI] [PubMed] [Google Scholar]
- Song SH, Kim KL, Lee KA, Suh W. Tie regulates the Tie2 agonistic role of angiopoietin-2 in human lymphatic endothelial cells. Bichem. Biophys. Res. Commun. 2012;419:281–286. doi: 10.1016/j.bbrc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Srinivasan RS, Oliver G. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev. 2011;25:2187–2197. doi: 10.1101/gad.16974811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Turner CJ, Badu-Nkansah K, Crowley D, van der Flier A, Hynes RO. Integrin-[g302]5[g533]1 is not required for mural cell functions during development of blood vessels but is required for lymphatic-blood vessel separation and lymphovenous valve formation. Dev. Biol. 2014;392:381–392. doi: 10.1016/j.ydbio.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo KV, Baldwin HS. Role of Tie1 in shear stress and atherosclerosis. Trends Cardiovasc. Med. 2011;21:118–123. doi: 10.1016/j.tcm.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo KV, Qu X, Babaev VR, Linton MF, Guzman RJ, Fazio S, Baldwin HS. Tie1 attenuation reduces murine atherosclerosis in a dose-dependent and shear stress-specific manner. J. Clin. Invest. 2011;121:1624–1635. doi: 10.1172/JCI42040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, Moreno-Rodriguez RA, Markwald RR, O'Rourke BP, Sharp DJ, et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012;151:1083–1096. doi: 10.1016/j.cell.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Yuan HT, Venkatesha S, Chan B, Deutsch U, Mammoto T, Sukhatme VP, Woolf AS, Karumanchi SA. Activation of the orphan endothelial receptor Tie1 modifies Tie2-mediated intracellular signaling and cell survival. FASEB J. 2007;21:3171–3183. doi: 10.1096/fj.07-8487com. [DOI] [PubMed] [Google Scholar]
- Zheng W, Nurmi H, Appak S, Sabine A, Bovay E, Korhonen EA, Orsenigo F, Lohela M, D'Amico G, Holopainen T, et al. Angiopoietin 2 regulates the transformation and integrity of lymphatic endothelial cell junctions. Genes Dev. 2014;28:1592–1603. doi: 10.1101/gad.237677.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Chang Z, Zhang L, Hong YK, Shen B, Wang B, Zhang F, Lu G, Tvorogov D, Alitalo K, et al. Akt/protein kinase B is required for lymphatic network formation, remodeling, and valve development. Am. J. Pathol. 2010;177:2124–2133. doi: 10.2353/ajpath.2010.091301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.