Abstract
Endothelial barrier dysfunction is an important contributor to the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Even though approaches that target the prevention and repair of endothelial barrier dysfunction are clearly needed, our understanding of the molecular regulation of pulmonary microvascular endothelial permeability remains incomplete. Cultured pulmonary microvascular endothelial cells represent an attractive paradigm for the study of barrier function. Here, we describe a method for the harvest, identification and culture of human lung microvascular endothelial cells (HLMVEC). HLMVEC thus obtained, grow as a monolayer, exhibit contact inhibition and have the typical cobblestone appearance. They express endothelial proteins, such as von Willebrand Factor and endothelial nitric oxide synthase and take up acetylated LDL. Furthermore, HLMVEC respond predictably and with superior sensitivity to the barrier disruptive effects of Gram positive and Gram negative bacterial products, thrombin, vascular endothelial growth factor and microtubule disrupting agents. These HLMVEC present an in-house-derived alternative to commercially available human cells for the study of mechanisms contributing to ALI and ARDS.
INTRODUCTION
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) represent a continuum of progressive respiratory failure in the absence of left heart failure. ARDS patients represent a subset of ALI patients, distinguished by a greater severity. In ALI/ARDS, the integrity of the capillary barrier is compromised, leading to increased vascular permeability and alveolar flooding. Gram negative sepsis (indirect injury) is by far the most common cause of ALI (Hudson et al. 1995). Sepsis represents the systemic inflammatory response to infection3, (Jacobi 2002). Lungs are among the most frequently affected organs in severe sepsis leading to ALI and ARDS (Martin et al. 2003). The incidence of sepsis has increased by 8.7% from 1979–2000 (Martin et al. 2003) and mortality ranges from 30–50% (Rangel-Frausto et al. 1995; Angus et al. 2000; Annane et al. 2000). Clinical trials targeting inflammatory mediators have shown no survival benefit (Fisher et al. 1994; McCloskey et al. 1994; Abraham et al. 1998; Dhainaut et al. 1998; Fink 1998; Abraham et al. 2001) and other strategies have failed to reduce morbidity associated with severe sepsis except for the survival benefit with the use of recombinant activated protein C (Bernard et al. 2001).
Even though approaches that target the prevention and repair of endothelial barrier dysfunction are clearly needed, our understanding of the molecular regulation of pulmonary microvascular endothelial permeability remains incomplete. Cultured pulmonary microvascular endothelial cells represent an attractive paradigm for the study of barrier function. However animal-derived endothelial cells do not necessarily reflect the complex biology of human endothelial cells. Moreover, human endothelial cells, while available commercially, are expensive and, frequently of inconsistent quality. Here we present a method for the harvesting, identification and culture of human lung microvascular endothelial cells. We further provide data to suggest that these cells exhibit a strong baseline barrier function and respond predictable to common edemagenic agents.
MATERIALS AND METHODS
1. Materials
Tosyl activated Dynabeads and Prolong Gold were from Invitrogen; eNOS antibody was from Becton Dickinson; vWF antibody was from Sigma; goat anti-mouse IgG and Cy3 goat anti- rabbit IgG were from Jackson Laboratories. Fetal bovine serum (FBS) was from Hyclone. All other reagents were obtained from Sigma Chemical Co. (St. Louis, MO). Eight-well arrays were from Applied Biophysics (Albany, NY).
2. Harvest, culture and identification of human lung microvascular endothelial cells. (HLMVEC)
Peripheral lung specimens from patients that were undergoing lobectomy or pneumonectomy at the Medical College of Georgia Hospital were obtained. The overwhelming majority of these patients were undergoing curative surgery for lung cancer. All tissue was from anatomic resection specimens obtained away from the primary tumor, where tumor margins are not an issue. We did not use pulmonary wedge resection specimens because of the small amount of tissue that would be available. Patients with known underlying lung disease such as tuberculosis, pulmonary fibrosis or interstitial lung disease and patients with known systemic diseases such as HIV were not enrolled. All specimens were obtained from subjects 70 years of age or younger. We have observed that the age of the donor is a major determinant of the quality of the endothelial cell isolation. Specimens of varying size (5–15g) were placed on ice in a sterile 50ml tube containing culture medium and were transported to the laboratory for processing within one hour of resection. Isolation and culture of HLMVEC was performed by a modification of the methods of Hewett and Murray (Hewett & Murray 1993) and Burg et al. (Burg et al. 2002). Briefly, isolation of HLMVEC was performed as follows: sub-pleural lung tissue was cut into small fragments with scissors. After removal of debris and erythrocytes through a 40µm nylon net, the tissue collected in the net was treated with dispase (1 U/ml at 4°C for 18h). After filtration through a 100µm nylon net, the filtrate was treated in a volume of 15ml M199, 15% FBS, 1mg dispase/ml at 37°C for 1h, followed by a further 100µm net filtration. The cell clumps within the filtrate were repeatedly resuspended in M199 and filtered through a 40µm net, followed by centrifugation for 10min at 1000rpm and re-suspension in M199 with 20% FBS. The positive selection of HLMVEC was achieved by interacting the cell suspension with magnetic beads (Tosyl activated Dynabeads) coated with Ulex europaeus I, according to the method of Jackson et al (Jackson et al. 1990). Cells were cultured in M199 supplemented with 20% FBS, 100 Units/ml heparin, 150µg/ml ECGF, 1µg/ml hydrocortisone, 292mg/l L-glutamine, and 110mg/l sodium pyruvate. The cells thus collected were identified as HLMVEC by their 1) growth as a contact-inhibited monolayer; 2) exhibition of cobblestone-like appearance;3) uptake of 1,1_-dioctadecyl-1,3,3_,3_-tetramethyl-indocarbocyanine-acetylated low-density lipoprotein (Dil-Ac-LDL), 4) expression of endothelial nitric oxide synthase (eNOS or NOS3) and 5) expression of von Willebrand factor (vWF), as described below. Cells were sub-cultured 1:3 using standard techniques.
For purposes of identification, cells were grown on glass coverslips and incubated in Dil-Ac-LDL (10µg/ml medium) for 4h at 37°C. Cells were washed three times with PBS, fixed in 4% neutral buffered formalin for 15min, washed three times with PBS, and mounted with Prolong Gold on microscope slides. For immunostaining, cells were grown on coverslips, washed three times in PBS, fixed in acetone:methanol (1:1) at −20° C for 10min, washed three times in PBS and blocked for 1h in 1% BSA in PBS. Cells were then incubated overnight at 4° C in primary antibody at 1:1000 dilution in blocking buffer for eNOS and 1:200 dilution in blocking buffer for vWF. Cells were then washed three times in blocking buffer and then incubated in secondary antibody for 1h at room temperature: either 1:1000 Cy3 conjugated goat anti-mouse IgG antibody for eNOS or 1:1000 Cy3 goat anti- rabbit IgG antibody for vWF. Cells were washed three times in PBS and mounted with ProLong Gold on microscope slides. Dil-Ac-LDL uptake and eNOS and vWF expression were observed using an Axio Observer D1 microscope (Zeiss) with rhodamine filter.
3. Measurements of transendothelial resistance (TER) across endothelial monolayers
Cells were grown in special wells (0.8 cm2) at seeding density of 1.25×105 cells/cm2, as we have previously reported (Antonov et al. 2008; Chatterjee et al. 2008). Electrical resistance was continuously monitored during the course of treatments with an Applied Biophysics Electric Cell-Substrate Impedance Sensing System (Applied Biophysics, Albany, NY). The system can monitor 16 wells simultaneously and includes real time calculation and presentation of transendothelial resistance. At the bottom of each well there is a gold-film electrode. When cells cover the electrode, the impedance changes, because the cells block the current flow. The main contribution to impedance is due to narrow spaces beneath the cells and the intercellular junctions. As the cell alters its morphology or moves about, these passages vary causing changes in the electrical impedance. From changes in impedance, the barrier function of the cell can be determined (Tiruppathi et al. 1992).
4. Data analysis
All TER measurements were performed in triplicate or quadruplicate and each experiment was repeated at least three times. Values are presented as means ± SEM from one representative experiment. Differences among groups were examined by the two way ANOVA for repeated measures, followed by the Neuman-Keuls test, and were considered significant at P<0.05.
RESULTS
Identification of human lung microvascular endothelial cells
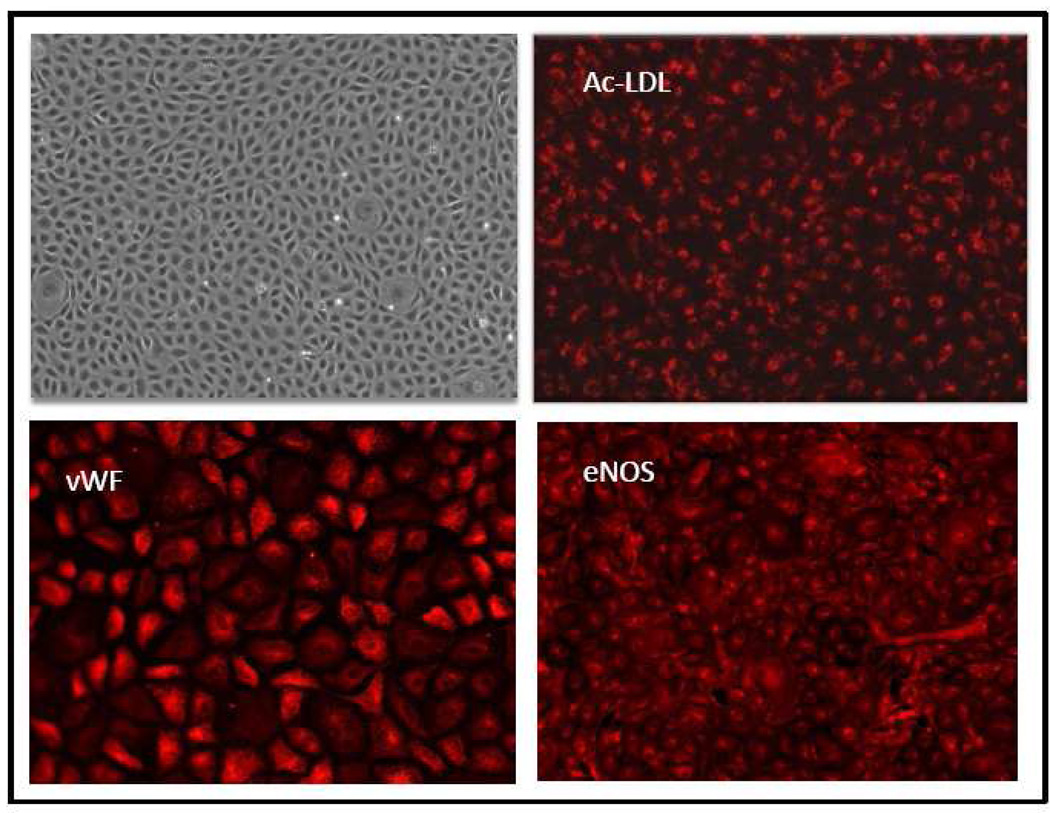
The harvesting procedures outlined in Methods consistently produced endothelial cells free of other cellular contamination. We call these cells microvascular because we utilized sub-pleural lung segments with no visible large vessels, as well as subjected the chopped tissue to two successive 100µm filtrations. Furthermore, these cells are endothelial cells for several reasons. As shown in Figure 1, left upper panel, they are of small size, form a contact-inhibited monolayer, exhibit the characteristic cobblestone appearance of endothelial cells and are highly homogeneous. These cells also possess properties and express proteins, which are characteristic of vascular endothelial cells. Thus, as shown in Figure 1, upper right panel, they take up acetylated LDL (Ac-LDL), while the two lower panels of Figure 1 demonstrate that these cells express widely two proteins constitutively found in endothelial cells: von Willebrand factor (vWF) and endothelial (type 3) nitric oxide synthase (eNOS). The HLMVEC depicted in Figure 1 are of passage 3; similar results were observed in HLMVEC at passages 4–10. We have not studied HLMVEC beyond passage 10.
FIGURE 1.
top left panel: phase contrast micrograph of HLMVEC (passage 3); the other three panels demonstrate well established characteristics of all endothelial cells, i.e., uptake of acetylated LDL (Ac-LDL: top right panel), expression of von Willebrand factor (vWF; bottom l;eft panel) and expression of endothelial (or type 3) nitric oxide synthase (eNOS; bottom right panel). See Materials and Methods for details.
Barrier properties of human lung microvascular endothelial cells
Twenty-four to forty-eight hours after being seeded on 8-well arrays, in preparation for measurements of transendothelial resistance (TER), HLMVEC exhibited a tight monolayer with excellent resistance (~1000MΩ). To demonstrate the barrier properties of the HLMVEC, we tested six substances known to increase pulmonary endothelial permeability (a Gram negative and two Gram positive bacterial products, two receptor-acting agents and one cytoskeleton disrupting agent).
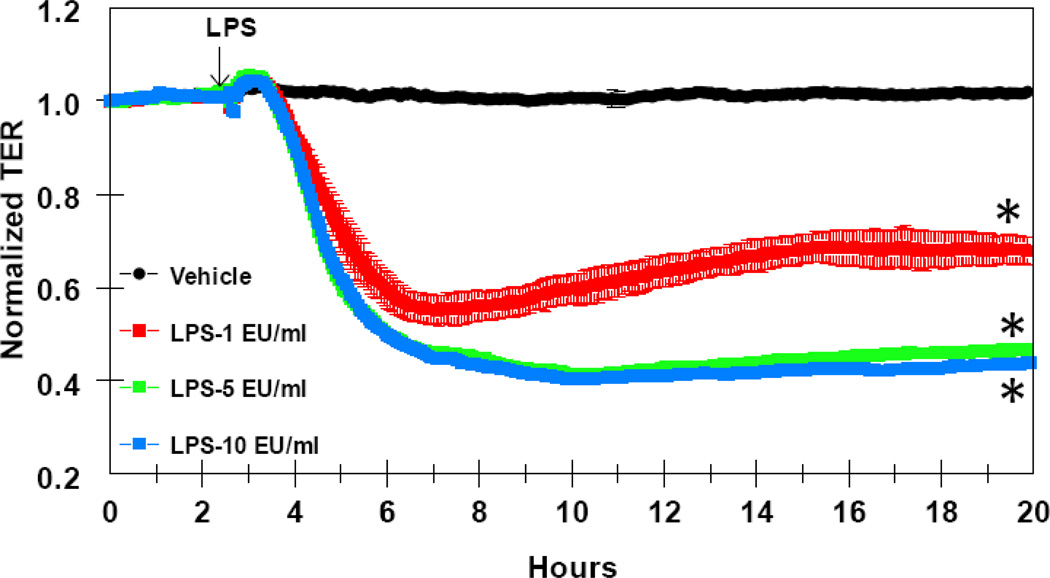
Figure 2 shows that lipopolysaccharide (LPS), a product of the wall of the Gram negative bacteria, E.coli, produced a time and concentration-dependent decrease in HLMVEC TER. LPS decreased TER at concentrations ranging from 1 to 10 EU (endotoxin units)/ml. At the lowest concentration tested, 1 EU/ml, LPS reduced TER as early as one hour after addition and reached a nadir of 45% decrease in TER by 3–4h.
FIGURE 2.
time and concentration-dependent decrease in HLMVEC transendothelial resistance (TER) by LPS. Eight-well arrays were inoculated with HLMVEC (100,000); 48h later, confluent monolayers were observed exhibiting resistance of ~900MΩ. LPS or vehicle were added (15µl) at the time indicated by the arrow and TER values were continuously recorded at 20sec intervals over the next 18h. Data shown are means ±SEM of four replicates. *:P<0.05 from Vehicle group.
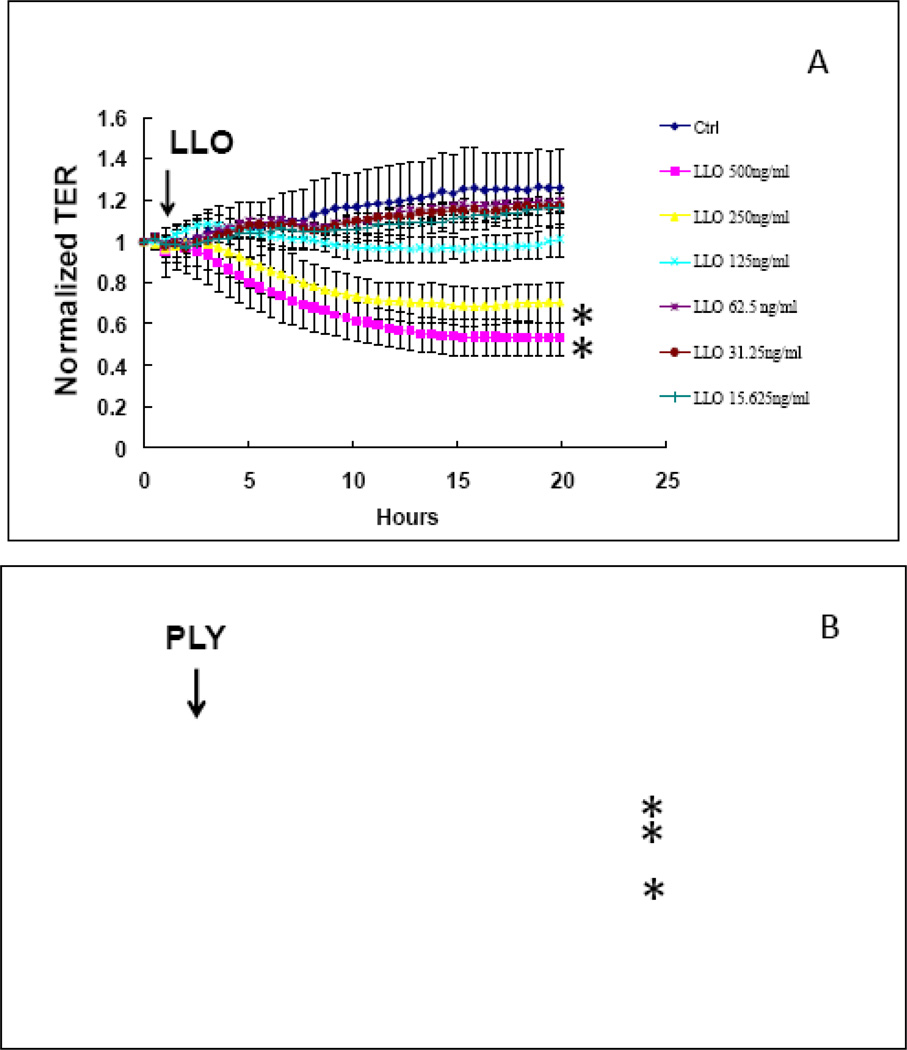
Figure 3 shows that the Gram positive bacterial products, listeriolysin (LLO; top panel) and pneumolysin (PLY; bottom panel) also decreased the TER of HLMVEC in a time- and concentration-dependent manner, but with different profiles. Whereas LLO produced a 20–40% inhibition in TER in concentrations of 250–500 ng/ml, PLY caused a 40–70% inhibition at much lower concentrations of 62.5–250 ng/ml; additionally, the effect of PLY was much quicker than that of LLO: the nadir of the PLY-induced decrease in TER occurred at about 40min, whereas for LLO it was at around 12–14 hours.
FIGURE 3.
time and concentration-dependent decrease in HLMVEC transendothelial resistance (TER) by the Gram positive bacteria products lysteriolysin (LLO; A) and pneumolysin (PLY; B). Eight-well arrays were inoculated with HLMVEC (100,000); 48h later, confluent monolayers were observed exhibiting resistance of ~900MΩ. LPS or vehicle were added (15µl) at the time indicated by the arrow and TER values were continuously recorded at 20sec intervals over the next 18h (A) or 150min (B). Data shown are means ±SEM of four replicates. *:P<0.05 from corresponding Vehicle group.
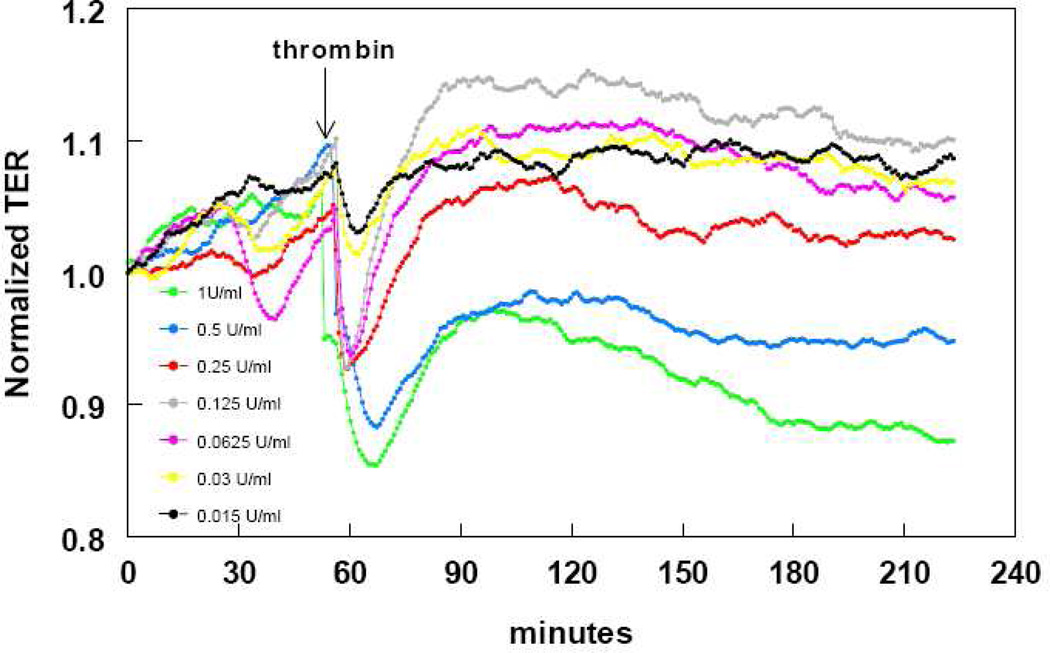
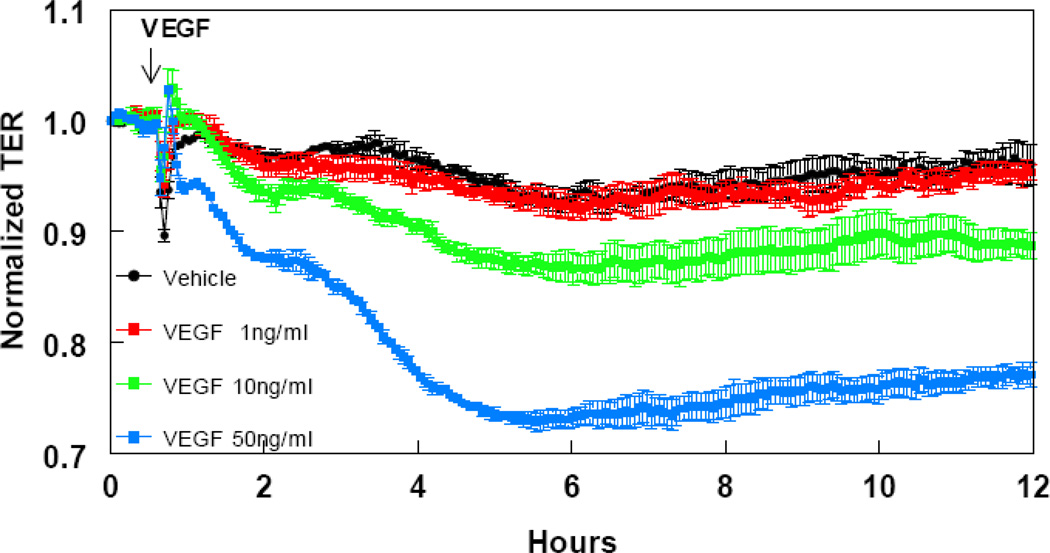
To investigate the response of HLMVEC to two well-characterized receptor-acting edemagenic agents, we studied time- and concentration- dependent responses of HLMVEC to changes in TER caused by thrombin (Figure 4) and VEGF (Figure 5). Both thrombin and VEGF induced a profound and both time- and concentration-dependent decrease in TER, as expected. Thrombin, at concentrations between 0.0625 and 1 U/ml, produced a rapid 5–15% decrease in TER, within a few minutes, that – at the two higher concentrations- partly recovered, but remained depressed for at least three hours. Conversely, VEGF, at concentrations between 10–50 ng/ml produced a slow, but sustained 10–30% decrease in TER, which remained depressed for at least 11 hours.
FIGURE 4.
time and concentration-dependent decrease in HLMVEC transendothelial resistance (TER) by thrombin. Eight-well arrays were inoculated with HLMVEC (100,000); 48h later, confluent monolayers were observed exhibiting resistance of ~900MΩ. Thrombin or vehicle was added (15µl) at the time indicated by the arrow and TER values were continuously recorded at 20sec intervals over the next 160min. Data shown are representative of three separate experiments with comparable results.
FIGURE 5.
time and concentration-dependent decrease in HLMVEC transendothelial resistance (TER) by vascular endothelial growth factor (VEGF). Eight-well arrays were inoculated with HLMVEC (100,000); 48h later, confluent monolayers were observed exhibiting resistance of ~900MΩ. VEGF or vehicle was added (15µl) at the time indicated by the arrow and TER values were continuously recorded at 20sec intervals over the next 11h. Data shown are means ±SEM of four replicates. *:P<0.05 from Vehicle group.
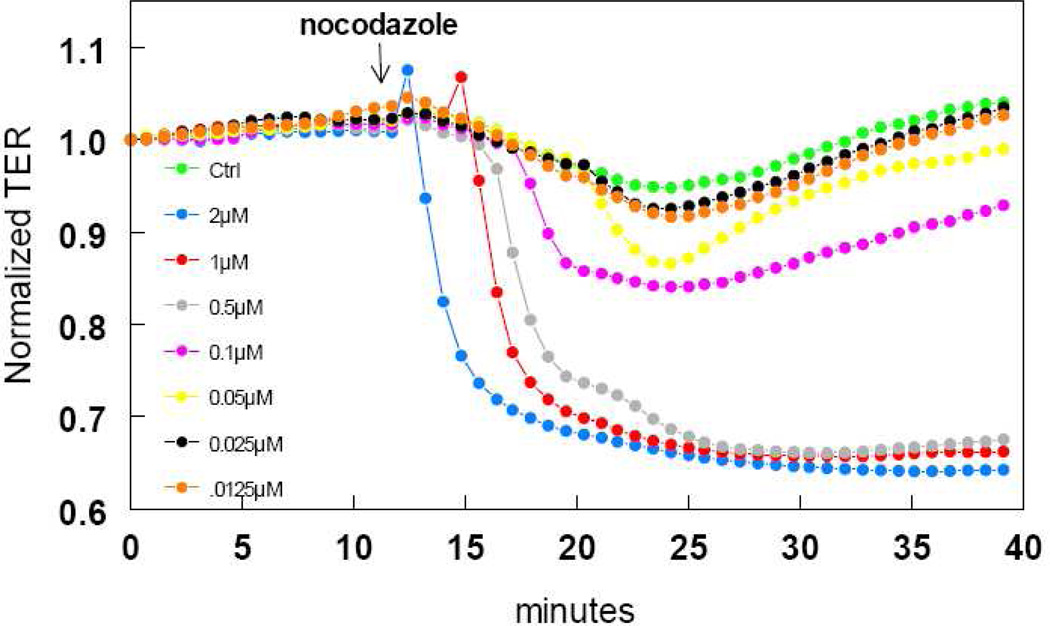
Microtubules are an important cytoskeletal regulator of paracellular endothelial permeability. We exposed HLMVEC to the microtubule depolymerizing agent, nocodazole (Figure 6). At concentrations between 0.05 and 2µM, nocodazole caused a quick, 5–35% decrease in TER that was sustained for at least 30min.
FIGURE 6.
time and concentration-dependent decrease in HLMVEC transendothelial resistance (TER) by the microtubule depolymerizing agent, nocodazole. Eight-well arrays were inoculated with HLMVEC (100,000); 48h later, confluent monolayers were observed exhibiting resistance of ~900MΩ. Nocodazole or vehicle was added (15µl) at the time indicated by the arrow and TER values were continuously recorded at 20sec intervals over the next 30min. Data shown are representative of three separate experiments with comparable results.
DISCUSSION
The maintenance of a barrier between blood and tissue is a very important function of vascular endothelium. This is especially true for pulmonary microvascular endothelium, because failure to maintain a healthy barrier may result in impaired gas exchange, reduced blood oxygenation, acid-base disturbances, acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS). Over the past three decades, cultured endothelial cells have been used frequently as a model for the study of mechanisms regulating endothelial barrier integrity. Most of these studies have utilized bovine and other animal endothelial cells or human umbilical vein endothelial cells, although recently a number of investigators have utilized commercially available human pulmonary arterial endothelial cells and a few of them, have employed human pulmonary microvascular endothelial cells. Because commercially available endothelial cells are both costly and of inconsistent quality, we developed a method for the routine and large scale in-house harvesting and culture of human lung microvascular endothelial cells (HLMVEC). This method produces consistently excellent quality HLMVEC, as shown under phase contrast microscopy (Figure 1). Importantly, they express proteins, characteristic of endothelial cells. Thus, HLMVEC strongly express both vWF and eNOS, two proteins that are nearly exclusively expressed by endothelial cells. vWF, a glycoprotein involved in hemostasis, is contained within the endothelial perinuclear Weibel-Palade bodies (Jaffe et al. 1974; Wagner et al. 1982). Outside of platelets, endothelial cells are the only cells expressing vWF; since the cells shown in Figure 1 are not platelets, their extensive expression of vWF strongly suggest that they are endothelial cells with undetectable non-endothelial contamination. Similarly, even though, both platelets and alveolar epithelial cells express endothelial NOS (Muruganandam & Mutus 1994; Vyas-Read et al. 2007), the simultaneous expression of both eNOS and vWF in our cells confirms their identity as endothelial cells.
With this approach, it is not possible –nor intended- to separate arterial from venous microvascular cells. It is reasonable to expect that these cultures contain a mixture of both. Similarly, we cannot be certain of the exact vessel size of origin of these cells, even though no specimen contained visible-size vessels.
To investigate the barrier function of HLMVEC, we tested a number of permeability-inducing agents. The Gram negative bacterial product, LPS, elicited a strong increase in HLMVEC permeability. This agrees with numerous literature reports. For example, in BPAEC, LPS decreases transendothelial electrical resistance through a mechanism sensitive to hsp90 inhibitors (Antonov et al. 2008), (Chatterjee et al. 2007). An important means of LPS-induced hyperpermeability involves the stimulation of endothelial contractile mechanisms and inhibition of Rho kinase effectively prevents endothelial contraction induced by LPS and reduces edema formation during septic inflammation (Essler et al. 2000; Tasaka et al. 2005). In general, Rho A is considered to be important for the increase of endothelial permeability in response to inflammatory stimuli, such as thrombin, tumor necrosis factor-[alpha], and LPS (Essler et al. 1998; van Nieuw Amerongen et al. 2000; Wojciak-Stothard & Ridley 2002; Baumer et al. 2008). LPS-induced lung edema is also blocked by sphingosine-1-phosphate, a known activator of Rac 1 (Peng et al. 2004), and by cAMP, which, in part, stabilizes endothelial barrier function also via activation of Rac 1 (Wojciak-Stothard et al. 2001; Birukova et al. 2007; Birukova et al. 2008). Endothelial barrier properties are known to be strictly dependent on the integrity of endothelial adherens and tight junctions (Bazzoni & Dejana 2004). LPS also increases paracellular permeability of human lung microvascular EC through tyrosine phosphorylation of VE-cadherin, p120 catenin, and [gamma]-catenin (Gong et al. 2008), as well as through inhibition of NADPH oxidase activity (Chen et al. 2008). In these studies, the overwhelming majority of human lung endothelial cells (arterial or microvascular) were from commercial sources (Chen et al. 2008; Gong et al. 2008; Tiruppathi et al. 2008). Our HLMVEC compare favorably and with increased sensitivity to those commercially available. Thus, Tiruppathi et al (Tiruppathi et al. 2008) reported that LPS concentrations as high as 4µg/ml (~12,000U/ml) did not alter the resting TER in commercially obtained HLMVEC, whereas at 1 U/ml, LPS produced a 45% decrease in TER in our own HLMVEC. Similarly, in our lab, the TER response of our HLMVEC to 1U/ml LPS is equivalent to the TER response of our in-house harvested bovine pulmonary arterial endothelial cells (BPAE) to 1000EU/ml (Chatterjee et al. 2008).
Our findings that both pneumolysin (PLY), the main virulence factor of Streptococcus pneumoniae and listeriolysin (LLO), the main virulence factor of Listeria monocytogenes, profoundly decrease TER in HLMVEC agree with published studies. The family of cholesterol-dependent pore-forming toxins, to which PLY and LLO belong, produce a rapid increase in intracellular Ca2+ and diacylglycerol levels (Repp H 2002) and have been implicated in severe pulmonary hyper-permeability (Ananthraman A 1983), (Witzenrath M 2006). The interaction of Listeria monocytogenes with endothelial cells represents a crucial step in the pathogenesis of listeriosis. Incubation of human umbilical vein endothelial cells (HUVEC) with wild-type L. monocytogenes provokes a strong, immediate NO synthesis, attributable to LLO and can be reproduced by purified LLO (Rose F 2001). In addition, incubation of HUVEC with LLO is a potent stimulus for sustained up-regulation of pro-inflammatory cytokines (IL-6, IL-8, and granulocyte-macrophage colony-stimulating factor) (Rose F 2001). The LLO-induced transmembrane Ca2+ flux in endothelial cells leads to the activation of phospholipase, generation of diacylglycerol, ceramide, and NF-κB, which may contribute to the pathogenic sequelae in severe listerial infection and sepsis. Recently, intravascular PLO dose-dependent increased pulmonary vascular resistance and lung microvascular permeability. In these studies, PLY was mainly detected in pulmonary arterial endothelial cells. PLY also increased permeability of HUVEC monolayers (Witzenrath M 2006). Furthermore, in neuroblastoma cells, PLY induced cholesterol- and Rho and Rac GTPase-dependent actin remodeling leading to the formation of actin stress fibers, filopodia, and lamellipodia (Iliev AI 2007). It is not clear why PLY exhibited a much faster time course in the decrease of TER in HLMVEC than LLO (1h vs. 12h). We have not yet investigated whether similar differences exist in the time course of calcium influx and stimulation of the previously described pro-inflammatory pathways between LLO and PLY in HLMVEC.
In agreement with literature reports, our HLMVEC responded to thrombin by decreasing TER in a concentration-dependent manner. Thrombin induces barrier dysfunction of pulmonary endothelial monolayer and this is associated with dramatic cytoskeletal reorganization, activation of actomyosin contraction, and gap formation (Birukova et al. 2004b). Thrombin-induced actin reorganization in BPAEC requires activation of both myosin light chain kinase (MLCK) and protein kinase C (PKC) (Zhao & Davis 1996). The thrombin-induced endothelial hyper-permeability occurs in conjunction with calcium mobilization as well as PKC activation (Lum et al. 1993), (Lum et al. 1992). Like LPS, thrombin‘s effect on endothelial cell cytoskeletal rearrangement and TER is inhibited by hsp90 inhibitors (Antonov et al. 2008). Also like LPS, the thrombin-induced barrier dysfunction requires RhoA (Vouret-Craviari et al. 1998; Hippenstiel et al. 2000; Woo & Kim 2002), as well as the induction of superoxide (Holland et al. 1998; Li et al. 2002; Pandian et al. 2005). These effects of thrombin are mediated via G-proteins, which couple the thrombin receptor to several key physiological responses.
VEGF increases permeability by at least two different pathways: one involving Raf-1, MEK, and ERK-1/2; and the other involving NOS. PKC, which increases permeability via increased NO production (Huang & Yuan 1997), is a mediator of VEGF-induced ERK-1/2 phosphorylation and hyperpermeability (Breslin et al. 2003). In vitro studies demonstrate that VEGF causes an increase in protein permeability across primary cultures of bovine macro- and microvascular lung endothelial cell monolayers and that this is associated with VE- and E-cadherin phosphorylation and the formation of actin stress fibers. Activation of the stress protein response prevents the VEGF-mediated changes in protein permeability across EC monolayers and reduces the phosphorylation of VE-and E-cadherin, as well as the formation of actin stress fibers (Godzich et al. 2006). The VEGF increase in endothelial cell permeability is prevented and reversed by hsp90 inhibitors (Antonov et al. 2008). The sensitivity of HLMVEC to VEGF was comparable to that of BPAEC: in both cell types 50ng/ml VEGF elicited ~30% decrease in TER. (Antonov et al. 2008)
The endothelial cytoskeleton plays a critical role in the regulation of endothelial barrier function (Dudek & Garcia 2001). Disassembly of microtubules by various agents, including nocodazole, results in a hyper-permeable endothelial monolayer (Verin et al. 2001; Birukova et al. 2004a; Birukova et al. 2005). We confirmed this observation in our HLMVEC. Nocodazole, produced a concentration-dependent decrease in HLMVEC TER, that was comparable to that previously observed in BPAE (Antonov et al. 2008).
In summary, we present a method for in-house harvesting, identification and culture of human lung microvascular endothelial cells and provide evidence that these cells exhibit predictable barrier functions. Altered pulmonary microvascular endothelial barrier function is a hallmark of ALI and ARDS, where mortality remains virtually unchanged in over 40 years. Whenever available, human tissue is a superior alternative to animal tissue for experimental studies; in addition in-house harvested human endothelial cells can provide a more economical and better quality alternative to those available commercially.
ACKNOWLEDGEMENTS
We thank Dr. Chakraborty for providing us with purified Listeriolysin and Pneumolysin. Supported by the American Heart Association, Southeast Affiliate and the National Institutes of Health, HL070214. This work was also supported by a Programmatic Development award (to SMB, JDC, DF, RL, and ADV) from the Cardiovascular Discovery Institute of the Medical College of Georgia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, Dal Nogare A, Nasraway S, Berman S, Cooney R, Levy H, Baughman R, Rumbak M, Light RB, Poole L, Allred R, Constant J, Pennington J, Porter S. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–933. [PubMed] [Google Scholar]
- Abraham E, Reinhart K, Svoboda P, Seibert A, Olthoff D, Dal Nogare A, Postier R, Hempelmann G, Butler T, Martin E, Zwingelstein C, Percell S, Shu V, Leighton A, Creasey AA. Assessment of the safety of recombinant tissue factor pathway inhibitor in patients with severe sepsis: a multicenter, randomized, placebo-controlled, single-blind, dose escalation study. Crit Care Med. 2001;29:2081–2089. doi: 10.1097/00003246-200111000-00007. [DOI] [PubMed] [Google Scholar]
- Ananthraman A IR, Magnussen CR. Pleural-pulmonary aspects of Listeria monocytogenes infection. Respiration. 1983;44:153–160. doi: 10.1159/000194542. [DOI] [PubMed] [Google Scholar]
- Angus DC, Birmingham MC, Balk RA, Scannon PJ, Collins D, Kruse JA, Graham DR, Dedhia HV, Homann S, MacIntyre N. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. E5 Study Investigators. Jama. 2000;283:1723–1730. doi: 10.1001/jama.283.13.1723. [DOI] [PubMed] [Google Scholar]
- Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. Jama. 2000;283:1038–1045. doi: 10.1001/jama.283.8.1038. [DOI] [PubMed] [Google Scholar]
- Antonov A, Snead C, Gorshkov B, Antonova GN, Verin AD, Catravas JD. Heat shock protein 90 inhibitors protect and restore pulmonary endothelial barrier function. Am J Respir Cell Mol Biol. 2008;39:551–559. doi: 10.1165/rcmb.2007-0324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumer Y, Drenckhahn D, Waschke J. cAMP induced Rac 1-mediated cytoskeletal reorganization in microvascular endothelium. Histochem Cell Biol. 2008;129:765–778. doi: 10.1007/s00418-008-0422-y. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, Verin AD. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J Cell Physiol. 2005;204:934–947. doi: 10.1002/jcp.20359. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Liu F, Garcia JG, Verin AD. Protein kinase A attenuates endothelial cell barrier dysfunction induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol. 2004a;287:L86–L93. doi: 10.1152/ajplung.00441.2003. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JG, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004b;67:64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol. 2008;215:715–724. doi: 10.1002/jcp.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313:2504–2520. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW, 2nd, Duran WN. VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am J Physiol Heart Circ Physiol. 2003;284:H92–H100. doi: 10.1152/ajpheart.00330.2002. [DOI] [PubMed] [Google Scholar]
- Burg J, Krump-Konvalinkova V, Bittinger F, Kirkpatrick CJ. GM-CSF expression by human lung microvascular endothelial cells: in vitro and in vivo findings. Am J Physiol Lung Cell Mol Physiol. 2002;283:L460–L467. doi: 10.1152/ajplung.00249.2001. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Dimitropoulou C, Drakopanayiotakis F, Antonova G, Snead C, Cannon J, Venema RC, Catravas JD. Heat shock protein 90 inhibitors prolong survival, attenuate inflammation, and reduce lung injury in murine sepsis. Am J Respir Crit Care Med. 2007;176:667–675. doi: 10.1164/rccm.200702-291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Snead C, Yetik-Anacak G, Antonova G, Zeng J, Catravas JD. Heat shock protein 90 inhibitors attenuate LPS-induced endothelial hyperpermeability. Am J Physiol Lung Cell Mol Physiol. 2008;294:L755–L763. doi: 10.1152/ajplung.00350.2007. [DOI] [PubMed] [Google Scholar]
- Chen W, Pendyala S, Natarajan V, Garcia JG, Jacobson JR. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am J Physiol Lung Cell Mol Physiol. 2008;295:L575–L583. doi: 10.1152/ajplung.00428.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhainaut JF, Tenaillon A, Hemmer M, Damas P, Le Tulzo Y, Radermacher P, Schaller MD, Sollet JP, Wolff M, Holzapfel L, Zeni F, Vedrinne JM, de Vathaire F, Gourlay ML, Guinot P, Mira JP. Confirmatory platelet-activating factor receptor antagonist trial in patients with severe gram-negative bacterial sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. BN 52021 Sepsis Investigator Group. Crit Care Med. 1998;26:1963–1971. doi: 10.1097/00003246-199812000-00021. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem. 1998;273:21867–21874. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- Essler M, Staddon JM, Weber PC, Aepfelbacher M. Cyclic AMP blocks bacterial lipopolysaccharide-induced myosin light chain phosphorylation in endothelial cells through inhibition of Rho/Rho kinase signaling. J Immunol. 2000;164:6543–6549. doi: 10.4049/jimmunol.164.12.6543. [DOI] [PubMed] [Google Scholar]
- Fink MP. Therapeutic options directed against platelet activating factor, eicosanoids and bradykinin in sepsis. J Antimicrob Chemother. 1998;41(Suppl A):81–94. doi: 10.1093/jac/41.suppl_1.81. [DOI] [PubMed] [Google Scholar]
- Fisher CJ, Jr, Opal SM, Lowry SF, Sadoff JC, LaBrecque JF, Donovan HC, Lookabaugh JL, Lemke J, Pribble JP, Stromatt SC, et al. Role of interleukin-1 and the therapeutic potential of interleukin-1 receptor antagonist in sepsis. Circ Shock. 1994;44:1–8. [PubMed] [Google Scholar]
- Godzich M, Hodnett M, Frank JA, Su G, Pespeni M, Angel A, Howard MB, Matthay MA, Pittet JF. Activation of the stress protein response prevents the development of pulmonary edema by inhibiting VEGF cell signaling in a model of lung ischemia-reperfusion injury in rats. Faseb J. 2006;20:1519–1521. doi: 10.1096/fj.05-4708fje. [DOI] [PubMed] [Google Scholar]
- Gong P, Angelini DJ, Yang S, Xia G, Cross AS, Mann D, Bannerman DD, Vogel SN, Goldblum SE. TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J Biol Chem. 2008;283:13437–13449. doi: 10.1074/jbc.M707986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett PW, Murray JC. Human microvessel endothelial cells: isolation, culture and characterization. In Vitro Cell Dev Biol Anim. 1993;29A:823–830. doi: 10.1007/BF02631356. [DOI] [PubMed] [Google Scholar]
- Hippenstiel S, Soeth S, Kellas B, Fuhrmann O, Seybold J, Krull M, Eichel-Streiber C, Goebeler M, Ludwig S, Suttorp N. Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood. 2000;95:3044–3051. [PubMed] [Google Scholar]
- Holland JA, Meyer JW, Chang MM, O'Donnell RW, Johnson DK, Ziegler LM. Thrombin stimulated reactive oxygen species production in cultured human endothelial cells. Endothelium. 1998;6:113–121. doi: 10.3109/10623329809072198. [DOI] [PubMed] [Google Scholar]
- Huang Q, Yuan Y. Interaction of PKC and NOS in signal transduction of microvascular hyperpermeability. Am J Physiol. 1997;273:H2442–H2451. doi: 10.1152/ajpheart.1997.273.5.H2442. [DOI] [PubMed] [Google Scholar]
- Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- Iliev AI DJ, Nau R, Mitchell TJ, Wouters FS. Cholesterol-dependent actin remodeling via RhoA and Rac1 activation by the Streptococcus pneumoniae toxin pneumolysin. Proc Natl Acad Sci U S A. 2007;104:2897–2902. doi: 10.1073/pnas.0608213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CJ, Garbett PK, Nissen B, Schrieber L. Binding of human endothelium to Ulex europaeus I-coated Dynabeads: application to the isolation of microvascular endothelium. J Cell Sci. 1990;96(Pt 2):257–262. doi: 10.1242/jcs.96.2.257. [DOI] [PubMed] [Google Scholar]
- Jacobi J. Pathophysiology of sepsis. Am J Health Syst Pharm. 2002;59(Suppl 1):S3–S8. doi: 10.1093/ajhp/59.suppl_1.S3. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Hoyer LW, Nachman RL. Synthesis of von Willebrand factor by cultured human endothelial cells. Proc Natl Acad Sci U S A. 1974;71:1906–1909. doi: 10.1073/pnas.71.5.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JM, Mullen AM, Yun S, Wientjes F, Brouns GY, Thrasher AJ, Shah AM. Essential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res. 2002;90:143–150. doi: 10.1161/hh0202.103615. [DOI] [PubMed] [Google Scholar]
- Lum H, Andersen TT, Siflinger-Birnboim A, Tiruppathi C, Goligorsky MS, Fenton JW, 2nd, Malik AB. Thrombin receptor peptide inhibits thrombin-induced increase in endothelial permeability by receptor desensitization. J Cell Biol. 1993;120:1491–1499. doi: 10.1083/jcb.120.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum H, Aschner JL, Phillips PG, Fletcher PW, Malik AB. Time course of thrombin-induced increase in endothelial permeability: relationship to Ca2+i and inositol polyphosphates. Am J Physiol. 1992;263:L219–L225. doi: 10.1152/ajplung.1992.263.2.L219. [DOI] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- McCloskey RV, Straube RC, Sanders C, Smith SM, Smith CR. Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double-blind, placebo-controlled trial. CHESS Trial Study Group. Ann Intern Med. 1994;121:1–5. doi: 10.7326/0003-4819-121-1-199407010-00001. [DOI] [PubMed] [Google Scholar]
- Muruganandam A, Mutus B. Isolation of nitric oxide synthase from human platelets. Biochim Biophys Acta. 1994;1200:1–6. doi: 10.1016/0304-4165(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Pandian RP, Kutala VK, Liaugminas A, Parinandi NL, Kuppusamy P. Lipopolysaccharide-induced alterations in oxygen consumption and radical generation in endothelial cells. Mol Cell Biochem. 2005;278:119–127. doi: 10.1007/s11010-005-6936-x. [DOI] [PubMed] [Google Scholar]
- Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. Jama. 1995;273:117–123. [PubMed] [Google Scholar]
- Repp H PZ, Koschinski A, Domann E, Darji A, Birringer J, Brockmeier D, Chakraborty T, Dreyer F. Listeriolysin of Listeria monocytogenes forms Ca2+- permeable pores leading to intracellular Ca2+ oscillations. Cell Microbiol. 2002;4:483–491. doi: 10.1046/j.1462-5822.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- Rose F ZS, Chakraborty T, Domann E, Machleidt T, Kronke M, Seeger W, Grimminger F, Sibelius U. Human endothelial cell activation and mediator release in response to Listeria monocytogenes virulence factors. Infect Immun. 2001;69:897–905. doi: 10.1128/IAI.69.2.897-905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaka S, Koh H, Yamada W, Shimizu M, Ogawa Y, Hasegawa N, Yamaguchi K, Ishii Y, Richer SE, Doerschuk CM, Ishizaka A. Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. Am J Respir Cell Mol Biol. 2005;32:504–510. doi: 10.1165/rcmb.2004-0009OC. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Malik AB, Del Vecchio PJ, Keese CR, Giaever I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci U S A. 1992;89:7919–7923. doi: 10.1073/pnas.89.17.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruppathi C, Shimizu J, Miyawaki-Shimizu K, Vogel SM, Bair AM, Minshall RD, Predescu D, Malik AB. Role of NF-kappaB-dependent caveolin-1. expression in the mechanism of increased endothelial permeability induced by lipopolysaccharide. J Biol Chem. 2008;283:4210–4218. doi: 10.1074/jbc.M703153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000;87:335–340. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- Verin AD, Birukova A, Wang P, Liu F, Becker P, Birukov K, Garcia JG. Microtubule disassembly increases endothelial cell barrier dysfunction: role of MLC phosphorylation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L565–L574. doi: 10.1152/ajplung.2001.281.3.L565. [DOI] [PubMed] [Google Scholar]
- Vouret-Craviari V, Boquet P, Pouyssegur J, Van Obberghen-Schilling E. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol Biol Cell. 1998;9:2639–2653. doi: 10.1091/mbc.9.9.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas-Read S, Shaul PW, Yuhanna IS, Willis BC. Nitric oxide attenuates epithelial-mesenchymal transition in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L212–L221. doi: 10.1152/ajplung.00475.2006. [DOI] [PubMed] [Google Scholar]
- Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982;95:355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzenrath M GB, Hocke AC, Schmeck B, Hippenstiel S, Berger K, Mitchell TJ, de los Toyos JR, Rosseau S, Suttorp N, Schütte H. Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit Care Med. 2006;34:1947–1954. doi: 10.1097/01.CCM.0000220496.48295.A9. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114:1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:187–199. doi: 10.1016/s1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]
- Woo CH, Kim JH. Rac GTPase activity is essential for lipopolysaccharide signaling to extracellular signal-regulated kinase and p38 MAP kinase activation in rat-2 fibroblasts. Mol Cells. 2002;13:470–475. [PubMed] [Google Scholar]
- Zhao Y, Davis HW. Thrombin-induced phosphorylation of the myristoylated alanine-rich C kinase substrate (MARCKS) protein in bovine pulmonary artery endothelial cells. J Cell Physiol. 1996;169:350–357. doi: 10.1002/(SICI)1097-4652(199611)169:2<350::AID-JCP14>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]