Abstract
In the wheat (Triticum aestivum L.) cultivar ‘Zenkoujikomugi’, a single nucleotide polymorphism (SNP) in the promoter of MOTHER OF FT AND TFL1 on chromosome 3A (MFT-3A) causes an increase in the level of gene expression, resulting in strong grain dormancy. We used a DNA marker to detect the ‘Zenkoujikomugi’-type (Zen-type) SNP and examined the genotype of MFT-3A in Japanese wheat varieties, and we found that 169 of 324 varieties carry the Zen-type SNP. In Japanese commercial varieties, the frequency of the Zen-type SNP was remarkably high in the southern part of Japan, but low in the northern part. To examine the relationship between MFT-3A genotype and grain dormancy, we performed a germination assay in three wheat-growing seasons. On average, the varieties carrying the Zen-type SNP showed stronger grain dormancy than the varieties carrying the non-Zen-type SNP. Among commercial cultivars, ‘Iwainodaichi’ (Kyushu), ‘Junreikomugi’ (Kinki-Chugoku-Shikoku), ‘Kinuhime’ (Kanto-Tokai), ‘Nebarigoshi’ (Tohoku-Hokuriku), and ‘Kitamoe’ (Hokkaido) showed the strongest grain dormancy in each geographical group, and all these varieties, except for ‘Kitamoe’, were found to carry the Zen-type SNP. In recent years, the number of varieties carrying the Zen-type SNP has increased in the Tohoku-Hokuriku region, but not in the Hokkaido region.
Keywords: Triticum aestivum L., grain dormancy, pre-harvest sprouting, MFT, genealogical pedigree
Introduction
Germination of wheat grains on the mother plant before harvest is called pre-harvest sprouting (PHS). PHS decreases the quality of the end product, coupled with lower grain yield, and causes economic losses (Bewley and Black 1994). Therefore, PHS tolerance has been an important breeding objective in wheat-breeding programs. The most suitable way to improve PHS tolerance is to select varieties with higher grain dormancy at harvest. However, grain dormancy is strongly influenced by environmental conditions, such as temperature and humidity (rainfall and aridity), during grain development (King 1993, Lunn et al. 2002, Mares et al. 2009). Thus, unstable evaluation of grain dormancy makes it difficult to select PHS-tolerant varieties in the field.
The wheat acreage in Japan is about 210,200 ha and its production reached nearly 811,700 tons in 2012, while the wheat consumption per year is 7,167,000 tons. Annual production of wheat covers only 11% of Japanese consumption. One of the factors that limit wheat production in Japan is the low quality due to PHS. Wheat is mainly cultivated in the Hokkaido, Kanto-Tokai, and Kyushu regions. In southern parts of Japan, including the Kanto-Tokai and Kyushu regions, the harvest maturity of wheat coincides with the beginning of the monsoon season. Hokkaido, which is the most productive region in Japan, has a cool humid summer every few years. These weather conditions easily lead to sprouting if rain comes before harvest. Although the newly bred commercial varieties in the Hokkaido region were improved for PHS tolerance (Yanagisawa et al. 2005), varieties with still higher PHS tolerance are needed. In Hokkaido, wheat production in 2009 (400,100 t) was 26% lower than that in 2008 (541,500 t) because of the occurrence of PHS under conditions of continuous rain and cool weather just before the harvest (Nishio et al. 2011).
The history of wheat breeding in Japan has been described in detail by Hoshino and Seko (1996). Since 1934, through the Japanese wheat-breeding programs, many Japanese and introduced foreign varieties have been subjected to germination assays and/or sprouting tests to find the source of PHS tolerance (Akihama 1936, Fukunaga et al. 1987, Oda and Seko 1992). Japanese varieties, especially the varieties that originated or bred in southern parts of Japan, are generally more dormant than foreign varieties. Hoshino et al. (1989) reported that ‘Zenkoujikomugi’ has the highest dormancy in the genealogical pedigree of Japanese wheat varieties that they used. Although many breeders have tried to establish a new variety with stronger grain dormancy than ‘Zenkoujikomugi’, no such variety has been developed from crosses between ‘Zenkoujikomugi’ and other varieties with strong grain dormancy. It is presumed that the key gene regulating grain dormancy is identical in ‘Zenkoujikomugi’ and the varieties used by breeders. The varieties developed in southern parts of Japan might have potentially acquired PHS tolerance during the long history of wheat cultivation under the moist conditions; however, the crucial factor that affects PHS tolerance has not been identified.
Many analyses of quantitative trait loci (QTLs) have been conducted for PHS tolerance and grain dormancy in wheat, and a number of QTLs have been found on wheat chromosomes (Anderson et al. 1993, Flintham et al. 2002, Groos et al. 2002, Kato et al. 2001, Kulwal et al. 2012, Mori et al. 2005, Munkvold et al. 2009, Osa et al. 2003, Torada et al. 2008). A major QTL, QPhs.ocs-3A.1, was mapped to the terminal region of the short arm of chromosome 3A by using recombinant inbred lines derived from a cross between the highly dormant variety ‘Zenkoujikomugi’ and the less dormant variety ‘Chinese Spring’ (Mori et al. 2005). In recent years, a wheat homologue of MOTHER OF FT AND TFL1 (MFT) underlying QPhs.ocs-3A.1 was identified, and a cleaved amplified polymorphic sequence (CAPS) marker was developed to detect a single nucleotide polymorphism (SNP) in the promoter of MFT on chromosome 3A (MFT-3A) (Nakamura et al. 2011). They showed that a nucleotide substitution in MFT-3A of ‘Zenkoujikomugi’ increases the transcriptional level of MFT, resulting in strong grain dormancy. Although the ‘Zenkoujikomugi’-type (Zentype) SNP in the promoter of MFT-3A is thought to play an important role in the control of grain dormancy in wheat, it remains unknown whether the Zen-type SNP is common in other Japanese wheat varieties.
The objective of our study was to identify varieties carrying the Zen-type SNP in the promoter of MFT-3A and to lay the foundation for marker-assisted breeding aimed at improving PHS tolerance in wheat. We performed CAPS marker analysis to identify the genotype of MFT-3A in Japanese wheat varieties. The level of grain dormancy in each variety was evaluated using germination assays on freshly harvested grains, and the relationship between MFT-3A genotype and grain dormancy was discussed. The genealogical pedigree of newly developed varieties with strong grain dormancy was also discussed.
Materials and Methods
Plant materials
A total of 324 wheat varieties (Supplemental Table 1), including 183 commercial varieties released in Japan (174 Norin varieties and 9 varieties without Norin number), 62 Japanese landraces, 39 breeding lines, and 40 foreign varieties introduced for breeding, were used in the present study. Norin varieties have been registered with the Ministry of Agriculture, Forestry and Fisheries, and have also been assigned a name in addition to the Norin number, starting from Norin 76 (‘Yuyakekomugi’). The geographical origins are summarized in Supplemental Table 1.
CAPS marker analysis
For DNA extraction, all wheat genotypes were grown in a growth chamber maintained at 20°C with a natural photoperiod. Genomic DNA was extracted from 2-week-old seedlings by using a modified CTAB method (Murray and Thompson 1980). Wheat varieties were genotyped by using a CAPS marker for the SNP in the promoter region of MFT-3A, according to the method described by Nakamura et al. (2011). PCR was performed with a gene-specific primer pair (CS3A06Proseq-F3: 5′-GTAGCGGGTGAAATCTGCAT-3′ and CS3A06Proseq-R5: 5′-GGGACGTACGAGGGTGTAGA-3′) that was designed to amplify the region around the SNP in the promoter sequence of MFT-3A and Ex Taq DNA polymerase (Takara Bio Inc., Shiga, Japan), according to the manufacturer’s instructions. The PCR conditions were as follows: 30 s denaturation at 98°C, followed by 40 cycles of 10 s at 98°C, 30 s at 60°C, and 1 min at 72°C. The amplified fragments of about 800 bp were treated with ClaI restriction enzyme (Takara Bio Inc.), and the fragment size was analyzed using polyacrylamide gel electrophoresis. The amplified fragments with the Zen-type SNP cannot be cut by ClaI because of nucleotide substitution. The amplified fragments with the ‘Chinese spring’-type SNP were cut by ClaI, producing two fragments of approximately 400 bp. To achieve the required separation efficiency, a high-efficiency genome scanning system (Kawasaki and Murakami 2000) was used.
Evaluation of grain dormancy
A total of 267 varieties (160 commercial varieties, 29 Japanese landraces, 39 breeding lines, and 39 foreign varieties introduced for breeding) were sown and grown in a field at NARO Agricultural Research Center (36°01′ N, 140°06′ E) in the Kanto region and subjected to a germination assay for 2004/2005, 2005/2006, and 2006/2007 wheat-growing seasons. Each experimental plot consisted of a single 1.0-m-long row, and the planting distance was 70 cm between rows and 8.5 cm between plants. For germination assay, spikes were harvested from the field at physiological maturity, as estimated by the loss of green color from the glumes, and were dried at room temperature for 10 days. Dried spikes were gently threshed, and the collected grains were stored at 4°C before use. Thirty whole grains were placed in a petri dish (9 cm diameter) containing two Whatman No. 2 filter papers (8.5 cm diameter) and 6 ml of water, with three replicates. Dishes were placed in a growth cabinet at 20°C. Germinated grains (germination was defined as pericarp rupture over the embryo) were counted and removed from the dishes every 24 h for 7 days. Germination was represented by the index GI. GI was calculated with the maximum weight given to the grains that germinated first and less weight to those that germinated later.
where n1, n2, … n7 is the number of grains germinated on the first, second, and subsequent days until the seventh day, respectively. The average and standard error (SE) were calculated. The GI values of the varieties carrying the Zen-type SNP and non-Zen-type SNP were compared using a t-test.
To evaluate the grain dormancy of ‘Kitahonami’, the commercial varieties bred in the Hokkaido region (‘Horoshirikomugi’, ‘Takunekomugi’, ‘Chihokukomugi’, ‘Hokushin’, ‘Kitamoe’, and ‘Kitahonami’) were sown in a field at NARO Agricultural Research Center and subjected to a germination assay for 2006/2007 and 2007/2008 wheat-growing seasons. These six varieties were sown separately from the varieties in the large-scale evaluation of grain dormancy for the 2006/2007 wheat-growing season. Germination was represented by GI.
Results and Discussion
MFT-3A genotyping in Japanese wheat varieties
The genotype of MFT-3A in Japanese wheat varieties was examined using PCR with the CAPS marker developed by Nakamura et al. (2011). A total of 324 wheat varieties, consisting of commercial varieties, Japanese landraces, breeding lines, and foreign varieties introduced for breeding, were subjected to the analysis. The DNA fragment of the expected size (about 800 bp) was amplified in 320 varieties. We could not amplify the DNA fragment from two commercial varieties (‘Horoshirikomugi’ and ‘Chihokukomugi’) and two breeding lines (‘Kitakei 320’ and ‘Kitakei 497’) with this CAPS marker (Supplemental Table 1). These varieties were developed in the Hokkaido region. Detailed analysis of the MFT-3A sequence of ‘Chihokukomugi’ revealed that ‘Chihokukomugi’ has an insertion in MFT-3A (Nakamura et al. unpublished data). Further investigation of MFT-3A genotype in wheat varieties will soon be reported. Sequence variation of MFT in wheat has also been reported by Lei et al. (2013) and Liu et al. (2013).
On the basis of the fragment pattern after ClaI digestion, 320 varieties were classified into two types: one carrying the Zen-type SNP (cannot be cut by ClaI) and the other carrying the non-Zen-type SNP (can be cut by ClaI). In our experiments, 110 of 181 commercial varieties, 34 of 62 Japanese landraces, 23 of 37 breeding lines, and 2 of 40 foreign varieties were found to carry the Zen-type SNP in the promoter of MFT-3A (Supplemental Table 1). Two foreign varieties carrying the Zen-type SNP were ‘Joshuu’ and ‘Shikan’, both originated in China.
Geographic distribution of the Zen-type SNP in Japanese commercial varieties
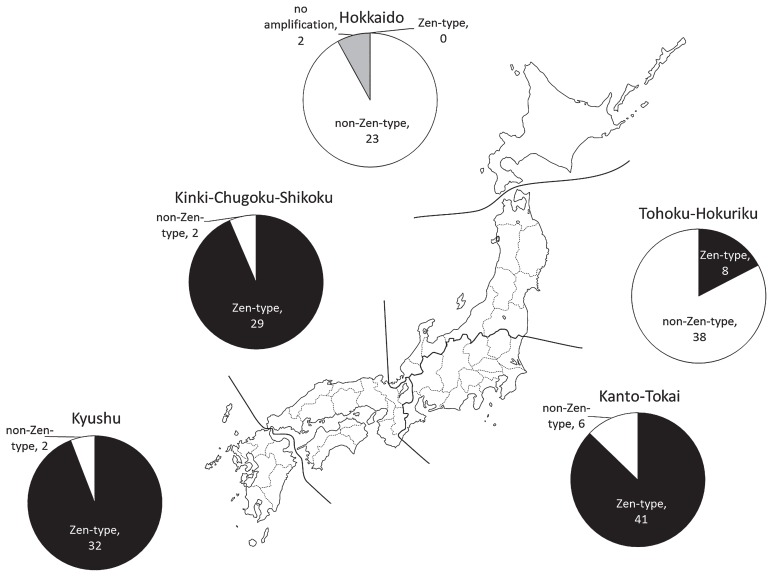
The frequency of the Zen-type SNP was different among the geographical groups of 183 commercial varieties (Fig. 1). The varieties carrying the Zen-type SNP were mainly developed in the southern parts of Japan. In the Kyushu, Kinki-Chugoku-Shikoku, and Kanto-Tokai regions, 32 of 34 varieties (94.1%), 29 of 31 varieties (93.5%), and 41 of 47 varieties (87.2%), respectively, were found to carry the Zen-type SNP. In contrast, few varieties carrying the Zen-type SNP were developed in the northern parts of Japan. In the Tohoku-Hokuriku region, only 8 of 46 varieties (17.4%) were found to carry the Zen-type SNP. In the Hokkaido region, none of the 25 commercial varieties carried the Zen-type SNP.
Fig. 1.
Genotype distribution of the Zen-type SNP and non-Zen-type SNP in Japanese commercial varieties. Solid and open parts of the circular chart indicate the proportions of wheat varieties carrying the Zen-type SNP and non-Zen-type SNP, respectively. Gray parts of the circular chart indicate the proportion of wheat varieties with no amplification with the CAPS marker. Number of varieties is indicated.
During wheat harvest in Japan, rain and high humidity are frequent. In such conditions, a variety carrying the Zen-type SNP may be preferred because of its potentially strong grain dormancy. Although strong grain dormancy prevents PHS at harvest, it hinders rapid and uniform germination at the beginning of the next growing season. In the southern parts of Japan, dormancy decay during dry storage may be sufficient to minimize the non-uniform germination at sowing.
Relationship between MFT-3A genotype and grain dormancy in Japanese wheat varieties
Grain dormancy in Japanese wheat varieties was evaluated by GI calculated from the data obtained from germination assays for three wheat-growing seasons (Supplemental Table 1). A total of 267 varieties were subjected to the germination assay, and three varieties (‘Asakazekomugi’, ‘Fukuhokomugi’, and ‘Furutsudaruma’) were not grown in 2004/2005 wheat growing season. The average GI values of ‘Igachikugo Oregon’, ‘Aki 9’, ‘Junreikomugi’, ‘Kinuhime’, ‘Chugoku 81’, and ‘Shinrikikomugi’ were lower than that of ‘Zenkoujikomugi’. The GI values and the origin of these varieties are summarized in Table 1. All these varieties carry the Zen-type SNP and were developed and/or originated in the southern part of Japan.
Table 1.
Varieties of wheat with lower average GI values than ‘Zenkoujikomugi’. All the varieties carried the Zen-type SNP in the promoter of MFT-3A
| Name | Origin | Germination Index | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 2004/2005 | 2005/2006 | 2006/2007 | Average | SE | ||
| Igachikugo Oregon | Kanto-Tokai | 9.7 | 10.7 | 16.2 | 12.2 | 2.0 |
| Aki 9 | Kyushu | 25.2 | 16.0 | 3.6 | 14.9 | 6.3 |
| Junreikomugi | Kinki-Chugoku-Shikoku | 11.0 | 23.8 | 21.2 | 18.7 | 3.9 |
| Kinuhime | Kanto-Tokai | 31.9 | 16.4 | 12.6 | 20.3 | 5.9 |
| Chugoku 81 | Kinki-Chugoku-Shikoku | 11.4 | 33.8 | 16.0 | 20.4 | 6.8 |
| Shinrikikomugi | Kinki-Chugoku-Shikoku | 32.4 | 12.1 | 25.2 | 23.3 | 5.9 |
| Zenkoujikomugi | Kanto-Tokai | 5.7 | 36.2 | 29.3 | 23.7 | 9.2 |
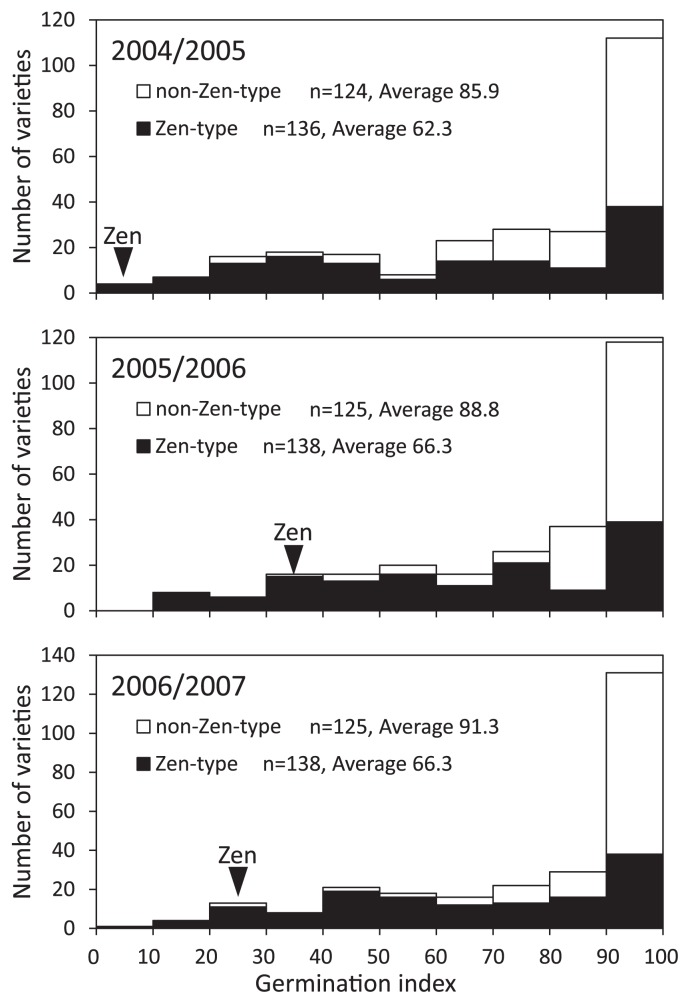
Frequency distribution of the GI values for the varieties carrying the Zen-type SNP and non-Zen-type SNP is shown in Fig. 2. Four varieties with no amplification with the CAPS marker were excluded. The average GI values of the varieties carrying the Zen-type SNP and non-Zen-type SNP were 62.3 and 85.9, respectively, for 2004/2005 wheat-growing season. Similar genotype difference was observed in other growing seasons. The average GI values of the varieties carrying the Zen-type SNP were 66.3 (2005/2006) and 66.3 (2006/2007), and the average GI values of the varieties carrying the non-Zen-type SNP were 88.8 (2005/2006) and 91.3 (2006/2007). The correlation between MFT-3A genotype and grain dormancy was significant in three seasons (P < 0.001). The GI values of the varieties carrying Zen-type SNP were widely spread. A number of QTLs related to the PHS tolerance and/or dormancy have been reported in wheat (reviewed in Gao et al. 2013, Kulwal et al. 2010). Other genes related to the regulation of PHS tolerance and/or dormancy are supposed to have an effect on grain dormancy in each variety.
Fig. 2.
Frequency distribution of Japanese wheat varieties for GI grown in 2004/2005, 2005/2006, and 2006/2007 growing seasons. Sample size (n) and the average GI values for the varieties carrying the Zen-type SNP (solid columns) and non-Zen-type SNP (open columns) are shown. A black arrowhead indicates the GI value for ‘Zenkoujikomugi’.
Genealogical pedigree of the varieties with strong grain dormancy in Japan
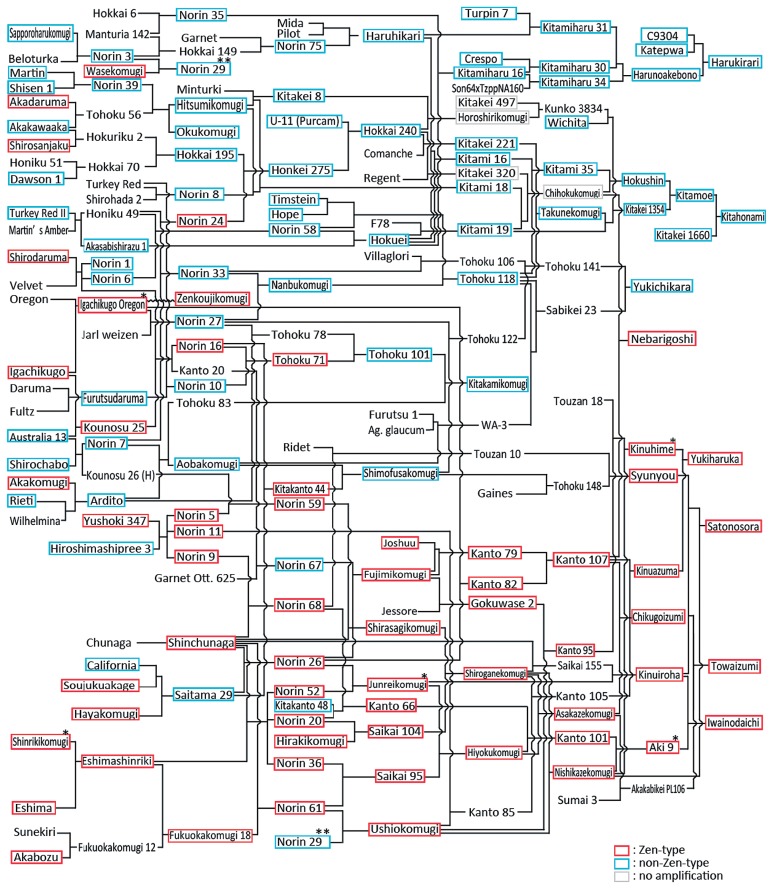
Among commercial varieties, ‘Iwainodaichi’ (Kyushu), ‘Junreikomugi’ (Kinki-Chugoku-Shikoku), ‘Kinuhime’ (Kanto-Tokai), ‘Nebarigoshi’ (Tohoku-Hokuriku), and ‘Kitamoe’ (Hokkaido) showed the lowest average GI value in each geographical group (Supplemental Table 1). MFT-3A genotypes in the pedigree of these varieties are summarized in Fig. 3. ‘Igachikugo Oregon’, which showed the lowest average GI value, is a parent of the variety ‘Zenkoujikomugi’. ‘Zenkoujikomugi’ was derived from ‘Igachikugo Oregon’ by mutation breeding using gamma rays (Fig. 3). ‘Aki 9’ showed strong grain dormancy and is a cross parent of ‘Iwainodaichi’ developed in the Kyushu region (Fig. 3).
Fig. 3.
MFT-3A genotype in the pedigree of varieties with strong grain dormancy. Red and blue frames indicate the varieties carrying the Zen-type SNP and non-Zen-type SNP, respectively. Gray frames indicate varieties with no amplification with the CAPS marker. A single asterisk indicates varieties with lower average GI values than ‘Zenkoujikomugi’. ‘Norin 29’ (double asterisk) is shown separately in the top and bottom sections of this figure. A wavy line indicates mutation breeding.
The varieties carrying the Zen-type SNP were mainly developed in the Kyushu, Kinki-Chugoku-Shikoku, and Kanto-Tokai regions (Fig. 1, Supplemental Table 1). As shown in Fig. 3, the old varieties carrying the Zen-type SNP, such as ‘Yushoki 347’, ‘Eshimashinriki’, and ‘Shinchunaga’, were used as cross parents of the old Norin varieties in these regions. In particular, ‘Shinchunaga’ was used as a cross parent of 22 Norin varieties, of which 20 varieties (‘Norin 20’, ‘Norin 26’, ‘Norin 30’, ‘Norin 32’, ‘Norin 34’, ‘Norin 36’, ‘Norin 43’, ‘Norin 47’, ‘Norin 48’, ‘Norin 49’, ‘Norin 50’, ‘Norin 52’, ‘Norin 57’, ‘Norin 60’, ‘Norin 61’, ‘Norin 64’, ‘Norin 68’, ‘Norin 73’, ‘Shirasagikomugi’, and ‘Mikunikomugi’) were found to carry the Zen-type SNP. Thus, the varieties carrying the Zen-type SNP were distributed in the southern parts of Japan.
In the Tohoku-Hokuriku region, the trend in the MFT-3A genotype of commercial varieties has changed significantly. Only 8 of the 46 commercial varieties were found to carry the Zen-type SNP (Fig. 1). Among them, 5 of the 8 commercial varieties carrying the Zen-type SNP have been developed after 2000 (‘Nebarigoshi’, ‘Haruibuki’, ‘Mochihime’, ‘Ginganochikara’, and ‘Yukiharuka’). In recent years, a variety developed in the southern parts of Japan has been used as a cross parent in this region. For example, ‘Nebarigoshi’ is selected from double haploid lines of the cross of ‘Kanto 107’/‘Chihokukomugi’ (Fig. 3). ‘Kanto 107’ carrying the Zen-type SNP is a breeding line developed in the Kanto-Tokai region and shows a moderately strong dormancy (Supplemental Table 1). ‘Yukiharuka’ is developed form the cross between ‘Kinuhime’ and ‘Kinuazuma’, both of which carry the Zen-type SNP and were developed in the Kanto-Tokai region (Fig. 3). The newly developed varieties carrying the Zen-type SNP may, in part, be useful to improve the PHS tolerance of an upcoming variety in the Tohoku-Hokuriku region.
At the time when we started evaluating grain dormancy in Japanese wheat varieties, ‘Kitahonami’, which is now becoming a leading variety in Japan, had not been developed. To evaluate the grain dormancy of ‘Kitahonami’, the commercial varieties developed in the Hokkaido region were subjected to a germination assay for two wheat-growing seasons. As shown in Table 2, the GI value of ‘Kitahonami’ was the lowest in each season. In comparison with ‘Kitamoe’, the dormancy level was greatly improved in ‘Kitahonami’. None of the varieties in the Hokkaido region carried the Zen-type SNP, and the strong dormancy in ‘Kitahonami’ may be derived from other gene(s) that regulate grain dormancy. To examine the effect of the Zen-type SNP on grain dormancy in the Hokkaido region, we are preparing a new variety carrying the Zen-type SNP in a ‘Kitahonami’ background. Our future studies will clarify whether the Zen-type SNP in the promoter of MFT-3A improves the grain dormancy in varieties developed in the Hokkaido region.
Table 2.
Evaluation of grain dormancy in commercial varieties developed in the Hokkaido region
| Name | MFT-3A Genotype | Germination Index | |
|---|---|---|---|
|
| |||
| 2006/2007 | 2007/2008 | ||
| Horoshirikomugi | no amplification | 73.5 | 86.9 |
| Takunekomugi | non-Zen-type | 82.9 | 57.6 |
| Chihokukomugi | no amplification | 92.5 | 82.9 |
| Hokushin | non-Zen-type | 85.7 | 69.8 |
| Kitamoe | non-Zen-type | 66.0 | 45.0 |
| Kitahonami | non-Zen-type | 11.4 | 11.9 |
Acknowledgements
We acknowledge Dr. S. Taya for his helpful comments. We thank M. Nakamura, K. Yokoi, K. Suzuki, C. Amemiya, M. Someya, A. Yamase, and Y. Maki at NARO Institute of Crop Science for their technical assistance; the members of the field cultivation team at NARO Agricultural Research Center for cultivation of the experimental material; and Dr. H. Hanai for his helpful comments and ongoing support. This work was supported by Grants-in-Aid from NARO.
Literature Cited
- Akihama, K. (1936) Varietal differences in seed dormancy of Wheat. Agr. and Hort. 11: 2937–2942. [Google Scholar]
- Anderson, J.A., Sorrells, M.E. and Tanksley, S.D. (1993) RFLP analysis of genomic regions associated with resistance to preharvest sprouting in wheat. Crop Sci. 33: 453–459. [Google Scholar]
- Bewley, J.D. and Black, M. (1994) Seeds: Physiology of development and germination. Second edition (New York: Plenum Press; ). [Google Scholar]
- Flintham, J., Adlam, R., Bassoi, M., Holdsworth, M. and Gale, M. (2002) Mapping genes for resistance to sprouting damage in wheat. Euphytica 126: 39–45. [Google Scholar]
- Fukunaga, K., Hoshino, T., Matsukura, U., Taira, H. and Oda, S. (1987) Differences in preharvest sprouting and alpha-amylase activity among wheat cultivars. In: Mares, D.J. (ed.) 4th Int. Symp. on Pre-Harvest Sprouting in Cereals, Westview Press, Boulder, Co. USA, pp. 116–122. [Google Scholar]
- Gao, X., Hu, C.H., Li, H.Z., Yao, Y.J., Meng, M., Dong, J., Zhao, W.C., Chen, Q.J. and Li, X.Y. (2013) Factors affecting pre-harvest sprouting resistance in wheat (Triticum aestivum L.): a review. J. Animal Plant Sci. 23: 556–565. [Google Scholar]
- Groos, C., Gay, G., Perretant, M.-R., Gervais, L., Bernard, M., Dedryver, F. and Charmet, G. (2002) Study of the relationship between preharvest sprouting and grain color by quantitative trait loci analysis in a white×red grain bread-wheat cross. Theor. Appl. Genet. 104: 39–47. [DOI] [PubMed] [Google Scholar]
- Hoshino, T., Tomooka, N., Fukunaga, K. and Seko, H. (1989) Testing method of pre-harvest sprouting and genealogical pedigree of pre-harvest sprouting resistant cultivars in wheat. Japan. J. Breed. 39: 365–372. [Google Scholar]
- Hoshino, T. and Seko, H. (1996) History of wheat breeding for a half century in Japan. Euphytica 89: 215–221. [Google Scholar]
- Kato, K., Nakamura, W., Tabiki, T., Miura, H. and Sawada, S. (2001) Detection of loci controlling seed dormancy on group 4 chromosomes of wheat and comparative mapping with rice and barley genomes. Theor. Appl. Genet. 102: 980–985. [Google Scholar]
- Kawasaki, S. and Murakami, Y. (2000) Genome analysis of Lotus japonicas. J. Plant Res. 113: 497–506. [Google Scholar]
- King, R.W. (1993) Manipulation of grain dormancy in wheat. J. Exp. Bot. 44: 1059–1066. [Google Scholar]
- Kulwal, P., Ishikawa, G., Benscher, D., Feng, Z., Yu, L.-X., Jadhav, A., Mehetre, S. and Sorrells, M.E. (2012) Association mapping for pre-harvest sprouting resistance in white winter wheat. Theor. Appl. Genet. 125: 793–805. [DOI] [PubMed] [Google Scholar]
- Kulwal, P.L., Mir, R.R., Kumar, S. and Gupta, P.K. (2010) QTL analysis and molecular breeding for seed dormancy and pre-harvest sprouting tlerance in bread wheat. J. Plant Biol. 37: 59–74 [Google Scholar]
- Lei, L., Zhu, X., Wang, S., Zhu, M., Carver, B.F. and Yan, L. (2013) TaMFT-A1 is associated with seed germination sensitive to temperature in winter wheat. PLoS ONE 8:e73330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S., Sehgal, S.K., Li, J., Lin, M., Trick, H.N., Yu, J., Gill, B.S. and Bai, G. (2013) Cloning and characterization of a critical regulator for preharvest sprouting in wheat. Genetics 195: 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn, G.D., Kettlewell, P.S., Major, B.J. and Scott, R.K. (2002) Variation in dormancy duration of the U.K. wheat cultivar Hornet due to environmental conditions during grain development. Euphytica 126: 89–97. [Google Scholar]
- Mares, D., Rathjen, J., Mrva, K. and Cheong, J. (2009) Genetic and environmental control of dormancy in white-grained wheat (Triticum aestivum L.). Euphytica 168: 311–318. [Google Scholar]
- Mori, M., Uchino, N., Chono, M., Kato, K. and Miura, H. (2005) Mapping QTLs for grain dormancy on wheat 3A and group 4 chromosomes, and their combined effect. Theor. Appl. Genet. 110: 1315–1323. [DOI] [PubMed] [Google Scholar]
- Munkvold, J.D., Tanaka, J., Benscher, D. and Sorrells, M.E. (2009) Mapping quantitative trait loci for preharvest sprouting resistance in white wheat. Theor. Appl. Genet. 119: 1223–1235. [DOI] [PubMed] [Google Scholar]
- Murray, M.G. and Thompson, W.F. (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8: 4321–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, S., Abe, F., Kawahigashi, H., Nakazono, K., Tagiri, A., Matsumoto, T., Utsugi, S., Ogawa, T., Handa, H., Ishida, H.et al. (2011) A wheat homolog of MOTHER OF FT AND TFL1 acts in the regulation of germination. Plant Cell 23: 3215–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio, Z., Ito, M., Tabkiki, T., Nagasawa, K. and Yamauchi, H. (2011) Analysis of pre-harvest sprouting damages of winter wheat in Hokkaido by continuous low temperature and rainy weather in July 2009. Miscellaneous publication of the NARO Hokkaido Agricultural Research Center 69: 17–23. [Google Scholar]
- Oda, S. and Seko, H. (1992) Evaluation of pre-harvest sprouting resistance in wheat using germination tests conducted at two temperatures. In: Waker-Simmons, M.K. and Ried, J.L. (eds.) 6th Int. Symp. on Pre-Harvest Sprouting in Cereals, American Association of Cereal Chemists, St. Paul, MN, USA, pp. 69–75. [Google Scholar]
- Osa, M., Kato, K., Mori, M., Shindo, C., Torada, A. and Miura, H. (2003) Mapping QTLs for seed dormancy and the Vp1 homologue on chromosome 3A in wheat. Theor. Appl. Genet. 106: 1491–1496. [DOI] [PubMed] [Google Scholar]
- Torada, A., Ikeguchi, S. and Koike, M. (2005) Mapping and validation of PCR-based markers associated with a major QTL for seed dormancy in wheat. Euphytica 143: 251–255. [Google Scholar]
- Yanagisawa, A., Nishimura, T., Amano, Y., Torada, A. and Shibata, S. (2005) Development of winter wheat with excellent resistance to pre-harvest sprouting and rain damage. Euphytica 143: 313–318. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.