Abstract
Purpose
This study was conducted to assess the effect of Two-week L-carnitine supplementation on known markers of oxidative stress and muscle damage following acute bouts of exercise in active healthy young men.
Methods
Twenty-one active healthy men volunteered for this study. Participants were randomized in a double-blind placebo-controlled fashion into two groups: L-carnitine (C group; n=10) and placebo group (P group; n=11). They arrived at the laboratory after overnight fasting. A baseline blood sample was taken. Afterwards, subjects consumed either L-carnitine (2 capsules containing totally 2000 mg L-carnitine) or placebo (2 capsules containing totally 2000 mg lactose) daily for 14 days. On the day of the test, participants attended the athletics arena after overnight fasting. Then, participants were asked to run 14 km on the track at their highest ability. Blood samples were taken immediately, 2, and 24 hours after exercise. Plasma total antioxidant capacity (TAC), malondialdehyde (MDA) as thiobarbituric acid-reactive substance (TBARS) as a marker of lipid peroxidation, creatine kinase (CK) and lactate dehydrogenase (LDH) as markers of muscle damage were measured.
Results
TAC increased significantly 14 days after supplementation and 24h after exercise in C group compared with P group (P<0.05). Serum MDA-TBARS, CK, and LDH were significantly lower 24h after exercise in C group compared with P group (P<0.05).
Conclusion
These results suggest that two-week daily oral supplementation of L-carnitine has alleviating effects on lipid peroxidation and muscle damage markers following an acute bout of exercise in active healthy young men.
Keywords: Lipid Peroxidation, Antioxidants, Thiobarbituric Acid-Reactive Substance, Creatine Kinase, Oxidative Stress
Introduction
The shift in balance between oxidant/antioxidant in favor of oxidants is termed oxidative stress [1]. Physical exercise may increase accumulation of free radicals and induce oxidative stress [2]. During strenuous exercise, there is a dramatic increase in oxygen uptake in the skeletal muscle [3], and antioxidant defense system may temporarily become overwhelmed by insufficiency of endogenous antioxidants. Therefore, supplementation with antioxidants may be one intervention to reduce oxidative stress [4]. Antioxidant is defined as any mechanism, structure and/or substance that delays, prevents or removes oxidative modifications on a target molecule [5, 6].
L-carnitine (L-3-hydroxytrimethylaminobutanoate) is a naturally occurring compound that can be synthesized in mammals from the essential amino acids lysine and methionine or ingested through diet [7]. L-carnitine is required for mitochondrial long-chain fatty acid oxidation [8]. L-carnitine is also reported to possess antioxidant properties [9]. The antioxidant benefits have been implicated in several forms of toxicity [10,11]. To the best of our knowledge, there is a paucity of investigation about the effect of L-carnitine as an antioxidant on exercise-induced oxidative stress and muscle damage [12]. Although there are lots of studies about the effects of L-carnitine supplementation on fatty acid oxidation [7,13,14], and a plenty of works about its influence on some diseases [10,11,15], we did not find any work on the effect of L-carnitine on exercise-induced oxidative stress as an antioxidant. In recent years, there has been strong interest in the effect of antioxidant supplementations such as vitamin C and E on exercise-induced oxidative stress. In our previous works, we observed the significant effects of vitamin C [16,17] and Methylesulfonylmethane (MSM) supplementation[18] as antioxidants on exercise-induced oxidative stress. Because L-carnitine has been such a prevalent ergogenic substance among athletes, we decided to evaluate antioxidant properties of this supplement during heavy endurance-like exercise.
Therefore, the purpose of this study was to investigate the effect of 2-week L-carnitine supplementation on exercise-induced oxidative stress and muscle damage in young active healthy men.
METHODS AND SUBJECTS
Participants
Twenty-one active healthy young men volunteered for this study. They were not eligible to participate to the study if they: a) had a history of medical or surgical procedures that might significantly affect the study outcome, including cardiovascular disease or metabolic, renal, hepatic, or musculoskeletal disorders; b) were smokers or used medication that might have significantly affected the study outcome; c) used any nutritional supplements (i.e. creatine, protein drinks, amino acids, vitamins) in the 8 weeks before the beginning of the study; d) had enrolled in another trial or ingested another experimental product in the 30 days before screening and enrollment and e) having VO2max between 40-50 ml/kg/min. All subjects were informed verbally and in writing about the nature and demands of study, and subsequently completed a health history questionnaire and gave their written informed consent. The protocol of the study was approved by the university ethics committee in accordance with the Helsinki Declaration.
Preliminary measurements
The Balke 15-min run test was used to estimate VO2max [19]. Participants warmed up for 10-min and then ran around the track for 15-min, and the distance covered was recorded. The participants must have been encouraged to push themselves as hard as they could. The formula used to calculate VO2max is:
((Total distance covered ÷ 15) − 133) × 0.172) + 33.3
This test was performed almost two weeks before the main trial.
Experimental Design
Participants were randomized in a double blind study placebo-controlled fashion into two groups, L-carnitine (C) (n=10) and placebo (P) (n=11) (Table 1). At first, the participants were 22 persons. They were randomly divided into two groups on the basis of their VO2max (They were matched based on their VO2max). One of the participants of C group left the study because of personal problems. Subjects arrived at the laboratory after overnight fasting. A baseline blood sample was taken. Afterwards, subjects consumed either L-carnitine (2 capsules containing totally 2000 mg L-carnitine) or placebo (2 capsules containing totally 2000 mg lactose) daily for 14 days. On the day of the test, participants attended the athletics arena after overnight fasting.
Table 1.
Subject characteristics
| Variable | Carnitine (n=10) Mean (SE) | Placebo (n=11) Mean (SE) |
|---|---|---|
| Age (years) | 22.2 (1.1) | 22.0 (1.0) |
| Height (cm) | 180.0 (2.2) | 180.2 (1.9) |
| Weight (kg) | 72.3 (2.50) | 74.6 (2.1) |
| Body mass index (kg.m-2) | 22.4 (0.8) | 23.0 (0.6) |
| VO2max (ml.kg-1.min-1) | 43.5 (0.9) | 43.0 (1.0) |
SE: standard error; VO2max: maximal oxygen consumption
After a second blood sampling, participants had a 10-min warm-up involving running at almost 50% VO2max for 5-min and stretching for 5-min. Then participants were asked to run 14 km on the track at their maximum ability and highest record. They were allowed to consume water ad libitum throughout the trial. There was a general practitioner and an ambulance at the arena to control potential cardio respiratory problems and other health-related problems during trial. Blood samples were taken immediately, 2, and 24 hours after exercise.
Diet Control
Since total antioxidant capacity was measured at baseline, the subjects were requested to go on their normal diet during supplementation and test. Furthermore, a small list of accessible seasonal fruits and other foods with high antioxidant power was provided to subjects and they were requested to avoid extra use of these types of foods.
Blood sampling and analysis
Approximately 5 ml of blood was withdrawn at each time point. Two milliliters of blood was placed in heparinized tubes and centrifuged at 3000 rpm for 10-min at 4°C. Plasma was transferred to microtubes and stored at -20°C for subsequent analysis. The rest of the blood was allowed to clot and centrifuged at 5000 rpm for 10-min. Serum was removed and aliquoted in 0.2 ml volumes and stored at -20°C until analysis. Serum creatine kinase (CK) and lactate dehydrogenase (LDH) were measured using commercial available kits (Darmankave, Iran). Total antioxidant capacity (TAC) was analyzed by Varga et al method [20]. Plasma lipid peroxidation was estimated using the measurement malondialdehyde (MDA) as thiobarbituric acid-reactive substance (TBARS) level according to the method of Okhawa et al [21].
Statistical analysis
All data are presented as mean (± standard error), and statistical significance was set at the P<0.05 level. Subject characteristics and dietary data were compared under two groups using independent samples t-test (Table 1). The data, which contained multiple time points during the main trial, were analyzed using mixed-model repeated-measures ANOVA. Mauchly’s test was consulted and Greenhouse-Geisser correction was applied if the assumption of sphericity was violated. If a significant p value was identified for the main effect of time (time of sample), multiple pair wise comparisons were made using bonferroni confidence interval adjustment. Moreover, the dependent variable data in multiple time points between two groups were compared using independent samples t-test.
RESULTS
Participants’ characteristics
The characteristics of participants, including age, height, BMI and preliminary VO2max, are summarized in Table 1. There were no significant differences between physical characteristics of both groups (P<0.05).
Antioxidant Capacity
Baseline resting serum TAC was not different between groups after 2-weeks supplementation compared with baseline and P group (P<0.05). TAC significantly increased immediately, and 2h after exercise in both groups (P<0.05). TAC was significantly higher in C group compared with P group 24h after exercise (P= 0.02) (Fig. 1).
Fig. 1.
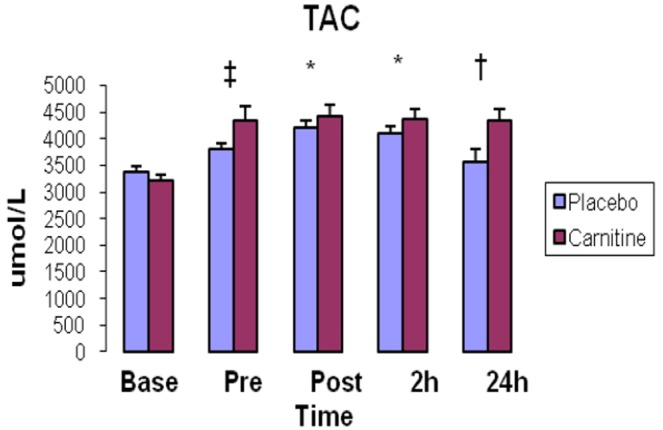
Total antioxidant capacity (TAC) concentrations in plasma. Values represent means (± Standard error).
* shows significant increase in comparison with pre-exercise (P<0.05). ‡ demonstrates significant increase in carnitine group compared with placebo group and baseline (P<0.05). † demonstrates significant increase in carnitine group compared with placebo group (P<0.05).
Lipid Peroxidation
Baseline resting serum TBARS was not different between groups (P>0.05). TBARS significantly increased just immediately after exercise in both groups (P<0.05). TBARS was significantly lower in C group compared with P group 24h after exercise (P= 0.003) (Fig. 2).
Fig. 2.
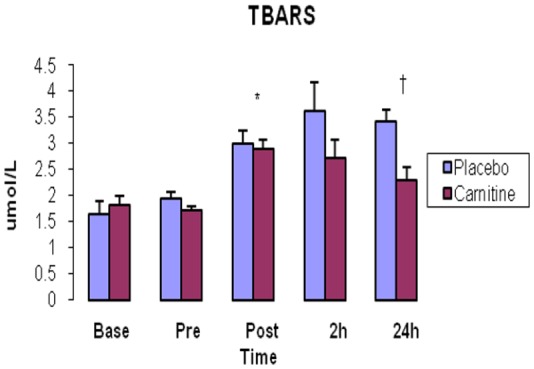
Thiobarbituric acid-reactive substance (TBARS) concentrations in serum. Values represent means (± standard error).
* shows significant increase in comparison with pre-exercise (P<0.05). † demonstrates significant enhancement in carnitine group compared with placebo group (P<0.05).
Muscle Damage Indices
Baseline resting serum CK and LDH were not different between groups (P>0.05). CK significantly increased immediately, 2, and 24h after exercise in both groups (P<0.05). CK was significantly higher in P group compared with C group 24h after exercise (P=0.03) (Fig. 3).
Fig. 3.
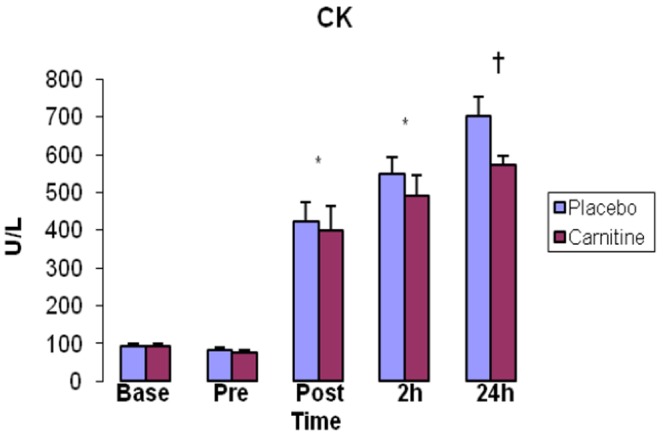
Creatine kinase (CK) activity in serum. Values represent means (± standard error).
* shows significant increase in comparison with pre-exercise (P<0.05). † demonstrates significant increase in carnitine group compared with placebo group (P<0.05).
LDH significantly increased just immediately after exercise in both groups (P<0.05). LDH were significantly higher in P group compared with C group 2 and 24h after exercise (P=0.01 and P=0.003, respectively) (Fig. 4).
Fig. 4.
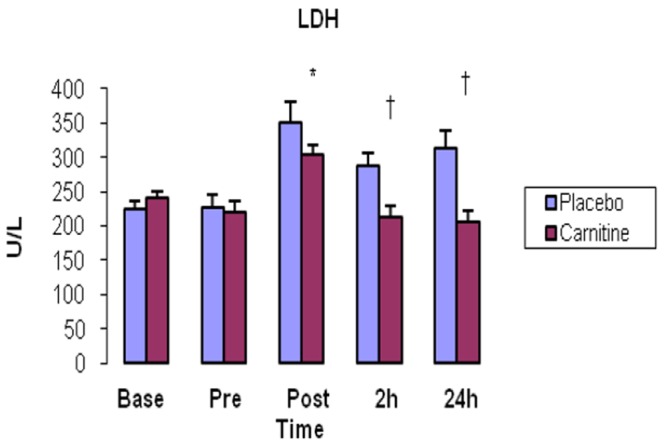
Lactate dehydrogenase (LDH) activity in serum. Values represent means (± standard error).
* shows significant increase in comparison with pre-exercise (P<0.05). † demonstrates significant increase in carnitine group compared with placebo group (P<0.05).
DISCUSSION
The main purpose of this study was to investigate whether L-carnitine can influence exercise-induced oxidative stress and muscle damage in young healthy men. There was no significant difference between the two groups’ running records (P group: 73±8.2 versus C group: 71±6.8 min) possibly because they had been matched on the basis of their VO2max.
In the present study, similar to our previous study [18], 14Km running has been able to induce oxidative stress after exercise. It can be seen in Fig. 2 that MDA- TBARS concentration as a marker of lipid peroxidation significantly increased after exercise in both groups demonstrating 14 km continuous running in our subjects has been able to begin oxidative stress. VO2max of our subjects were not so high (40-50 ml/kg/min) in this study, indicating possibly why 14 km running has been able to initiate exercise-induced oxidative stress.
Furthermore, in our previous works, we had considered supplementation with some antioxidants such as vitamin C and MSM in similar supplementation patterns and had been able to attenuate exercise-induced oxidative stress and muscle damage markers[16-18]. L-carnitine is reported to possess antioxidant properties. In the present study, it seems 2-week L-carnitine supplementation has been able to increase antioxidant capacity in C group compared with P group (Fig. 1). Furthermore, TAC was significantly higher in C group than P group 24h after exercise showing the possible effect of L-carnitine supplementation on exercise–induced oxidative stress.
Assessment of MDA-TBARS changes further supports this hypothesis. MDA-TBARS as a marker of lipid peroxidation significantly increased immediately and 24h afterwards in P group and just immediately after exercise in C group (Fig. 2). MDA-TBARS concentration is significantly lower in C group compared with P group 24h after exercise demonstrating possible influence of antioxidant properties of L-carnitine. It seems 2-week administration of L-carnitine as an antioxidant has been able to blunt lipid peroxidation via enhancement of antioxidant capacity.
The other finding of our study was promotion of CK and LDH as indirect markers of muscle damage. CK and LDH significantly increased immediately after, as well as 2 and 24h after exercise in both groups, (Fig. 3 and 4) indicating the intensity and duration of exercise was enough to cause muscle damage in our participants. In C group, LDH was significantly higher than P group 2h after exercise. Furthermore, Both CK and LDH were lower in C group than P group 24h after exercise showing the possible effect of L-carnitine supplementation. According to Figure 2, 3 and 4, the pattern of alterations in MDA-TBARS is almost similar in CK and LDH. There is significant reduction in all of these markers 24h after exercise in C group compared to P group. The potential reason of this phenomenon is possibly decrease of membrane permeability and escape of constituents such as CK and LDH via inhibition of lipid peroxidation possibly stemming from antioxidant supplementation in our study [22, 23].
CONCLUSION
The present study demonstrates that an acute bout of exercise can induce oxidative stress in active healthy young men. Two-week daily oral supplementation with L-carnitine has some alleviating effects on lipid peroxidation and muscle damage indices in favour of increased antioxidant capacity. Nevertheless, the exact mechanism of L-carnitine in attenuating the markers of oxidative stress is not well established and further exploration is needed.
Acknowledgments
We would like to thank the subjects that participated in this study as well as all exercise physiology laboratory assistants of Ardabil branch, Islamic Azad University who assisted with data collection and analysis.
Footnotes
Conflict of interests: None
References
- 1.Birben E, Sahiner UM, Sackesen C, et al. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prigol M, Luchese C, Nogueira CW. Antioxidant effect of diphenyl diselenide on oxidative stress caused by acute physical exercise in skeletal muscle and lungs of mice. Cell Biochem Funct. 2009;27:216–22. doi: 10.1002/cbf.1559. [DOI] [PubMed] [Google Scholar]
- 3.Morillas-Ruiza JM, Villegas JA, Garci AB, et al. Effects of polyphenolic antioxidants on exercise-induced oxidative stress. Clin Nutr. 2006;25:444–53. doi: 10.1016/j.clnu.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Vincent HK, Bourguignon CM, Vincent K, et al. Antioxidant supplementation lowers exercise-induced oxidative stress in young overweight adults. Obesity (Silver Spring) 2006;14:2224–35. doi: 10.1038/oby.2006.261. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B, Gutteridge J, Vijayarani MP, et al. Free Radicals in Biology and Medicine. New York: Oxford University Press; 2007. [Google Scholar]
- 6.Pamplona R, Costantini D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am J Physiol Regul Integr Comp Physiol. 2011;301:843–63. doi: 10.1152/ajpregu.00034.2011. [DOI] [PubMed] [Google Scholar]
- 7.Kraemer WJ, Volek JS, Spiering BA, Vingren JL, et al. L-carnitine supplementation: a new paradigm for its role in exercise. Monatshefte für Chemie. 2005;136:1383–90. [Google Scholar]
- 8.Bremer J. Carnitine-metabolism and functions. Physiol Rev. 1983;63:1420–80. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- 9.Izgüt-Uysal VN, Agaç A, Derin N. Effect of carnitine on stress-induced lipid peroxidation in rat gastric mucosa. J Gastroenterol. 2001;36:231–6. doi: 10.1007/s005350170108. [DOI] [PubMed] [Google Scholar]
- 10.Demirdag K, Bahcecioglu IH, Ozercan IH, et al. Role of L-carnitine in the prevention of acute liver damage induced by carbon tetrachloride in rats. J Gastroenterol Hepatol. 2004;19:333–8. doi: 10.1111/j.1440-1746.2003.03291.x. [DOI] [PubMed] [Google Scholar]
- 11.Kraemer WJ, Volek JS, Spiering BA, Vingren JL, et al. L-carnitine supplementation: a new paradigm for its role in exercise. Monatshefte für Chemie. 2005;136:1383–90. [Google Scholar]
- 12.Cao Y, QU HJ, Li P, et al. Single dose administration of L-carnitine improves antioxidant activities in healthy subjects. Tohoku J Exp Med. 2011;224:209–13 . doi: 10.1620/tjem.224.209. [DOI] [PubMed] [Google Scholar]
- 13.Wall BT, Stephens FB, Constantin-Teodosiu D, et al. Chronic oral ingestion of L-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans. J Physiol. 2011;589(Pt 4):963–73. doi: 10.1113/jphysiol.2010.201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brass EP. Supplemental carnitine and exercise. Am J Clin Nutr. 2000;72(2 Suppl):618S–23S. doi: 10.1093/ajcn/72.2.618S. [DOI] [PubMed] [Google Scholar]
- 15.Schaevitz LR, Nicolai R, Lopez CM, et al. Acetyl-L-carnitine improves behavior and dendritic morphology in a mouse model of Rett syndrome. PLoS One. 2012;7:e51586. doi: 10.1371/journal.pone.0051586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barmaki S, Bohlooli S, Khoshkhahesh F, Nakhostin-Roohi B. Effect of methylsulfonylmethane supplementation on exercise - Induced muscle damage and total antioxidant capacity. J Sports Med Phys Fitness. 2012;52:170–4. [PubMed] [Google Scholar]
- 17.Nakhostin-Roohi B, Babaei P, Rahmani-Nia F, Bohlooli S. Effect of vitamin C supplementation on lipid peroxidation, muscle damage and inflammation after 30-min exercise at 75%VO2max. J Sports Med Phys Fitness. 2008;48:217–4. [PubMed] [Google Scholar]
- 18.Nakhostin-Roohi B, Barmaki S, Khoshkhahesh F, Bohlooli S. Effect of chronic supplementation with methylsulfonylmethane on oxidative stress following acute exercise in untrained healthy men. J Pharm Pharmacol. 2011;63:1290. doi: 10.1111/j.2042-7158.2011.01314.x. [DOI] [PubMed] [Google Scholar]
- 19.Balke B. Rep Civ Aeromed Res Inst US. Oklahoma City: Federal Aviation Agency; 1963. A simple field test for the assessment of physical fitness; pp. 63–18. [PubMed] [Google Scholar]
- 20.Varga SZ, Matkovics B, Sasvqri M, Salgr L. Comparative study of plasma antioxidant status in normal and pathological cases. Curr Topics Biophys. 1998;22(suppl):219–24. [Google Scholar]
- 21.Okhawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbitoric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fiber injury. Sports Med. 1991;12:184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- 23.Jackson M. Oxygen radical production and muscle damage during running exercise. Marconnet P, Saltin B, Komi P, Poortmans J, editors. Med Sci Sport. 1996:121–33. [Google Scholar]


