Abstract
We evaluated the efficacy of a 6-month clinic and home-based behavioral intervention (Learning about Activity and Understanding Nutrition for Child Health; LAUNCH) to reduce obesity in preschool children ≥95th BMI percentile compared to enhanced standard of care (Pediatrician Counseling; PC). LAUNCH was a family-based behavioral intervention that taught parents to use child behavior management strategies to increase healthy eating and activity for their children and themselves. PC presented the same diet and activity recommendations, but was delivered in a one-time PC session. Eighteen children aged 2–5 years (mean 4.71 ± 1.01) with an average BMI percentile of 98 (±1.60) and an overweight parent were randomized to LAUNCH or PC. Assessments were conducted at baseline, 6 months (end of LAUNCH treatment) and 12 months (6 months following LAUNCH treatment). LAUNCH showed a significantly greater decrease on the primary outcomes of child at month 6 (post-treatment) BMI z (−0.59 ± 0.17), BMI percentile (−2.4 ± 1.0), and weight gain (−2.7 kg ± 1.2) than PC and this difference was maintained at follow-up (month 12). LAUNCH parents also had a significantly greater weight loss (−5.5 kg ± 0.9) at month 6 and 12 (−8.0 kg ± 3.5) than PC parents. Based on the data from this small sample, an intensive intervention that includes child behavior management strategies to improve healthy eating and activity appears more promising in reducing preschool obesity than a low intensity intervention that is typical of treatment that could be delivered in primary care.
INTRODUCTION
Obesity (≥95th percentile of BMI) in children aged 2–5 years has risen steadily from 5% in 1971–1980 to 10.4% by 2000 (1) and to 13.9% by 2003–2004 (2). Obese preschool children are at greater risk for developing high blood pressure (3), asthma at age 7 years (4), and behavior problems upon entrance to kindergarten (5) than children <95th percentile. Longitudinal data also demonstrate that children ≥95th percentile BMI at any time during the preschool years are two to four times more likely to be overweight (≥85th percentile BMI) at age 12 than children who were <95th percentile BMI (6).
Given the high percentage of obese preschoolers, the potential negative health implications, and the likelihood of remaining overweight at later ages, effective interventions for those preschoolers already obese are urgently needed. We developed a 6-month behavioral intervention for preschool obesity (Learning about Activity and Understanding Nutrition for Child Health; LAUNCH) (7) based on social cognitive theory (8) and modeled on successful behavioral family-based interventions for school-age children (9), but specifically targeting behaviors unique to the preschool years (e.g., food neophobia and tantruming for food (10)). We found that three of the five preschoolers who completed treatment demonstrated a decrease in BMI z-score ranging from −0.18 to −0.99 and maintained this decrease at 12 months (7).
The purpose of this study was to conduct a pilot randomized clinical trial of LAUNCH compared to an enhance standard of care condition (Pediatrician Counseling; PC). We hypothesized that LAUNCH would show greater effectiveness on the primary outcomes of reducing child BMI z-score and parent weight and on secondary outcomes of child caloric intake and changes in the home food environment compared to PC at 6 (end of LAUNCH treatment) and 12 months (6 months following the end of LAUNCH). We included measures of parenting, feeding, and mealtimes to assess whether treatment had unintended negative consequences (11).
METHODS AND PROCEDURES
Design
This study was a pilot randomized controlled trial conducted at Cincinnati Children’s Hospital Medical Center (CCHMC) from February 2008 to September 2009. The protocol was approved by the institutional review board and parents provided informed consent for participation. Assessments were conducted at baseline, month 6 (post-treatment for LAUNCH), and month 12 (6 months post-treatment for LAUNCH).
Study participants
Participants were recruited from a large Midwestern pediatric practice. Inclusion criteria were (i) child age between 2 and 5 years; (ii) child ≥95th percentile BMI (12), but not more than 100% above the mean BMI; (iii) at least one parent with a BMI ≥25; and (iv) medical clearance from the child’s pediatrician. Exclusion criteria were (i) non-English speaking; (ii) living >50 miles from the medical center; (iii) a disability or illness that would interfere with at least moderate physical activity; (iv) medical condition/medication associated with weight gain; or (v) currently enrolled in another weight-control program.
Recruitment
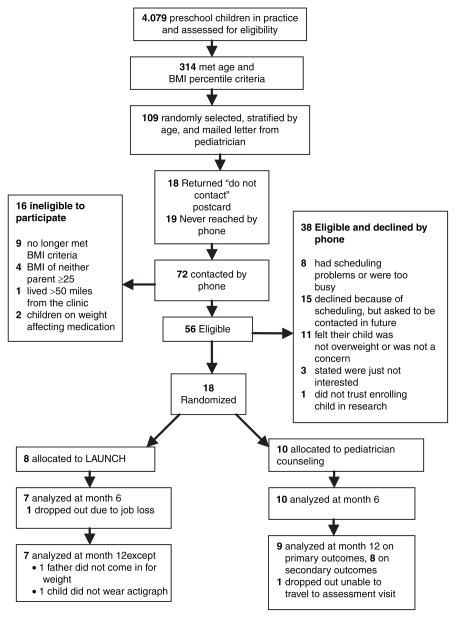
A systematic chart review of preschool aged children was conducted. Figure 1 shows recruitment and retention. Of the 314 children identified as being ≥95th percentile BMI at their last well-child visit, 109 were randomly selected to receive a letter from the child’s pediatrician that introduced the study and included a “Do Not Contact” postcard for families to decline further screening follow-up. Baseline assessments were scheduled with eligible, interested families. Randomization was conducted using a random numbers table and was concealed until all baseline assessments were completed.
Figure 1.
CONSORT flow diagram of participants to LAUNCH and pediatrician counseling at all time points. (LAUNCH, Learning about Activity and Understanding Nutrition for Child Health).
Interventions
Enhanced standard of care
Pediatricial Counseling. PC was designed to deliver dietary and physical activity recommendations outlined by the American Academy of Pediatrics (13). Following a scripted manual a board-certified pediatrician met each family individually for one 45-min visit to review the child’s growth chart and to explain BMI, BMI percentiles, and the child’s current BMI percentile. The following recommendations were made in accordance with the Stage 1 Intervention: “Prevention Plus” for obese preschool children (14); (i) ≤2 h/day of screen time; (ii) 60 min/day of active play; (iii) eliminating soda and limiting juice to 4 oz./day); (iv) providing ≥5 servings/day of fruits and vegetables; (v) limiting eating out; and (vi) appropriate portion sizes for preschoolers. Each family was given 1-page healthy food and activity brochure created by the Collaboration for Healthy Ohio (http://www.healthyohioprogram.org/healthylife/nutri2/nutrikids2/ounce.aspx).
Clinic and home-based behavioral intervention
LAUNCH was designed to produce small decreases or stabilize the rate of children’s weight gain, consistent with current recommendations for treatment of preschool obesity (14,15). The 6-month intervention consisted of two phases. Phase 1 (Intensive Intervention) was 12 weekly sessions that alternated between group-based clinic sessions (parent and child concurrent groups) and individual home visits. Phase 2 (Maintenance) was 12 weeks of every other week sessions, alternating between group sessions in clinic and home sessions.
Parent-group clinic sessions (90 min each) addressed three components: dietary education, physical activity and parenting skills. Dietary education covered the same topics as described for the PC, but recommendations were embedded in separate sessions targeting snack and beverages (Session 2), breakfast/lunch (Session 4), and dinner (Session 6). Parents kept 7-day diet diaries on their and their child’s intake during weeks 1–12. Calorie goals were set to gradually achieve an energy intake in the range of 1,000–1,200 per day depending on the child’s age, and at a caloric intake commensurate with a weight below a BMI of 25 for parents. Although food choices and barriers continued to be addressed, the primary focus of Sessions 8, 10, and 12 was on decreasing screen time to <2 h/day and increasing physical activity to 60 min of active play/day. Children and parents were provided with pedometers and given goals of 5,000 and 10,000 steps per day, respectively. Pedometer and diet diary data were used as feedback tools.
Throughout treatment, parents were taught to use child behavior management skills (16) to implement dietary and activity changes including: (i) praise and attention to increase healthy eating and physical activity; (ii) ignoring and time-out to manage tantrums; (iii) contingency management; and (iv) modeling. They were also taught stimulus control strategies, such as setting up the food environment to encourage healthy eating by eliminating high calorie/low nutrient foods and having fruits and vegetables in the home. To ensure children were exposed to new vegetables repeatedly, parents were given a 14-day supply of a vegetables at each of six clinic sessions and instructed on how to apply behavioral strategies to effectively conduct a daily taste with their child following the protocol described by Wardle et al. (17). Parents and children were weighed at each clinic visit with the goal of keeping the child’s weight stable. The parent group was conducted by a licensed clinical psychologist following a written manual.
Children were seen concurrently in a group format. They received nutrition education through games and art activities, tried new foods during a structured meal, and completed 15 min of moderate to vigorous activity. Child groups were conducted by pediatric psychology postdoctoral fellows and a research coordinator.
In-home sessions (60–90 min each) were designed to support generalization of the clinic-taught skills to the home environment and were conducted by psychology postdoctoral fellows. For example, the home therapist observed the taste-test and provided feedback and/or modeling of skills, such as praise or conducting time-out for tantrums (depending on child behavior). They also conducted home “clean outs” with parents where high-calorie/low-nutrient foods were identified and a plan was made to eliminate them from the home. Therapists assisted parents with setting up a safe place in the home for active play.
Phase 2 (Maintenance) sessions focused on helping families continue to make or maintain changes in eating and activity by identifying barriers and problem-solving with the families on using strategies taught during phase 1 to address these barriers. To prepare families for end of treatment diet diary recording was reduced to 3 days/week (2 weekdays, 1 weekend) and pedometers were worn but no longer recorded.
Measures
All measures except demographic, Barriers to Treatment Scale, and treatment satisfaction were obtained at baseline, 6 and 12 months. Demographics were self-reported by parents.
Primary outcomes
Child and parent weight and height were measured in triplicate following standard anthropometric procedures (18) by trained personnel from the General Clinical Research Center who were unaware of the child’s treatment condition at baseline, 6, and 12 months. Children’s BMI z-score and BMI percentile for sex and age were calculated using the Centers for Disease Control and Prevention (12) growth curves. Adult BMI was calculated as kg/m2.
Secondary outcomes
Children’s dietary intake was assessed by a registered dietitian from the General Clinical Research Center unaware of the child’s treatment assignment via three scheduled 24-h recalls (2 weekdays and 1 weekend) with the child’s parent over a 2-week period using the multiple-pass method (19). This method has been validated against doubly labeled water and deemed accurate for estimates of energy intake at the group level for young children aged 3–4 years (20) and 4–7 years (21). Average caloric intake per day was calculated using the Minnesota Nutrient Data Systems software, version 5.0 (22).
Home food environment was assessed based on the presence/absence of predefined categories of fresh fruits and vegetables, unhealthy (e.g., potato chips), and healthy foods (e.g., skim milk) based on criteria adapted from the traffic light diet (23). An independent coder established inter-rater reliability on 37% of randomly selected home assessments. Alpha coefficients on the presence of high calorie foods (0.76), high calorie beverages (0.82), and on fruits and vegetables (0.98) suggested adequate to excellent inter-rater reliability (24).
Children’s physical activity was measured by the MTI actigraph that has been validated and calibrated for use with preschool children (25). Actigraphs were worn for 7 days during all waking hours, with re-wearing requested if worn for less than 5 valid days (26). A valid day was a day in which the 60% of the total awake time with valid hours (27) with a valid hour defined as one with less than 10-min of consecutive zero counts. Across all time points, there was 86% compliance in meeting these criteria. Fifteen-second epochs were programmed for data collection (28). Average minutes of moderate and vigorous activity per day were calculated for each assessment period (25).
Exploratory outcomes
Parenting Styles and Dimensions (PDS)
Parenting Styles and Dimensions (29) is 53-item questionnaire assessing three parenting styles (authoritarian, authoritative, and permissive) included to explore hypothesized links between parenting styles, parental feeding practices, and child nutritional intake (30). Parents rated each item on a 5-point Likert-type scale (“never” to “always”).
About Your Child’s Eating—Revised (AYCE-R)
About Your Child’s Eating— Revised (31) is a 25-item questionnaire assessing caregiver beliefs and concerns about children’s eating and family mealtime interactions. We used two of the three subscales, Positive Mealtime Interaction and Resistance to Eating. Parents rated items on a 5-point Likert-type scale (“never” to “nearly all the time”).
Child Feeding Questionnaire (CFQ)
Child Feeding Questionnaire (32) is a 31-item questionnaire assessing parental child feeding attitudes and practices. We used two subscales, Restriction and Pressure to Eat, that have been linked with child eating and weight status (30). Parents rated items on a 5-point Likert-type scale (“disagree” to “agree”).
The PedsQL Generic Core Scales
(33) parent proxy form is a 23-item questionnaire assessing health-related quality. We used the Total Score and the Physical Functioning, Emotional Functioning, and Social Functioning subscales only. Parents rated items on a 5-point scale (“never a problem” to “almost always a problem”).
Perception of treatment and satisfaction
Parent Motivation Inventory (PMI)
Parent Motivation Inventory (34) is a 25-item questionnaire measuring parent motivation for treatment including: (i) desire for child change, (ii) readiness to change, and (iii) perceived ability to change was adapted for obesity treatment. Parents rated items on a 5-point Likert-type scale (“strongly disagree” to “strongly agree”).
Barrier to Treatment Participation Scale (BTPS)
Barrier to Treatment Participation Scale (35) is a 58-item questionnaire assessing barriers to participation in outpatient treatment that was administered at month 6 to assess: (i) stressors and obstacles that compete with treatment, (ii) treatment demands and issues, and (iii) perceived relevance of treatment.
Treatment Satisfaction Questionnaire
We asked parents to provide ratings of their satisfaction with treatment content and their ability to make the recommended changes using a 5-point Likert scale (“extremely unsatisfied” to “extremely satisfied”) upon treatment completion.
Data analysis
Independent samples t-tests were conducted on change from baseline to month 6 to test for pre- to post-treatment changes, and change from baseline to month 12 to test for maintenance of treatment effects 6 months following treatment for each outcome of interest using intent-to-treat analysis. All hypothesis tests used a two-tailed level of significance of 0.05. Given the pilot nature of the work no adjustments were made for multiple tests.
Effect sizes were calculated by using Cohen’s d (36). The stability of the results for the primary outcomes to missing data were examined through imputing missing change scores based on the regression of the change scores on the baseline scores. These stability analyses yielded essentially identical results to the reported analyses, which are based on complete cases at each time point.
RESULTS
Study population
Of the 18 families enrolled, 8 were randomized to LAUNCH and 10 to PC (32% recruitment rate; Figure 1). Treatment groups did not differ significantly on any demographic variables at baseline (Tables 1 and 2), except children’s Physical Functioning (PedsQL).
Table 1.
Baseline characteristics of children in LAUNCH and pediatrician counseling
| LAUNCH (N = 8) mean (s.d.) | Pediatrician counseling (N = 10) mean (s.d.) | |
|---|---|---|
| Child age (years) | 4.4 (0.92) | 3.9 (1.1) |
| Female (%) | 2 (25) | 4 (40) |
| White (%) | 6 (75%) | 9 (90%) |
| Hispanic (%) | 2 (25%) | 1 (10%) |
| BMI percentile for age and sex | 99 (0.9) | 97.7 (2.5) |
| Physical functioning on peds QL | 76.6 (15.9) | 89.4 (10.0)* |
LAUNCH, Learning about Activity and Understanding Nutrition for Child Health.
P = 0.05.
Table 2.
Baseline characteristics of families in LAUNCH and Pediatrician Counseling (PC)
| LAUNCH (N = 8) | PC (N = 10) | |
|---|---|---|
| Overweight parent targeted: mothers (%) | 7 (87.5) | 9 (90) |
| Mother’s age (mean years ± s.d.) | 36 (3.61) | 35 (4.24) |
| Mother’s education | ||
| Junior high school (%) | 1 (12.5) | 0 |
| High school/GED (%) | 0 | 1 (10) |
| Partial college/specialized training (%) | 2 (25) | 3 (30) |
| 4-Year university (%) | 5 (62.5) | 2 (20) |
| Graduate school (%) | 0 | 4 (40) |
| Father’s age (mean years ± s.d.) | 37 (3.43) | 39 (5.26) |
| Father’s education (years)* | ||
| High school/GED (%) | 0 | 1 (11) |
| Partial college/specialized training (%) | 5 (62.5) | 3 (33) |
| 4-Year university (%) | 2 (25) | 4 (44) |
| Graduate school (%) | 1 (12.5) | 1 (11) |
| Income before taxes (%) | ||
| $0–49,999 | 0 | 2 (20) |
| $50,000–74,999 | 2 (25) | 0 |
| $75,000–99,999 | 3 (38) | 5 (50) |
| $100,000–124,999 | 2 (25) | 3 (30) |
| $125,000–149,999 | 1 (13) | 0 |
| Hollingshead classification | 3.6 (0.7) | 4.2 (0.4) |
LAUNCH, Learning about Activity and Understanding Nutrition for Child Health.
One family in the control condition did not complete paternal education.
Primary outcomes: BMI z and weight
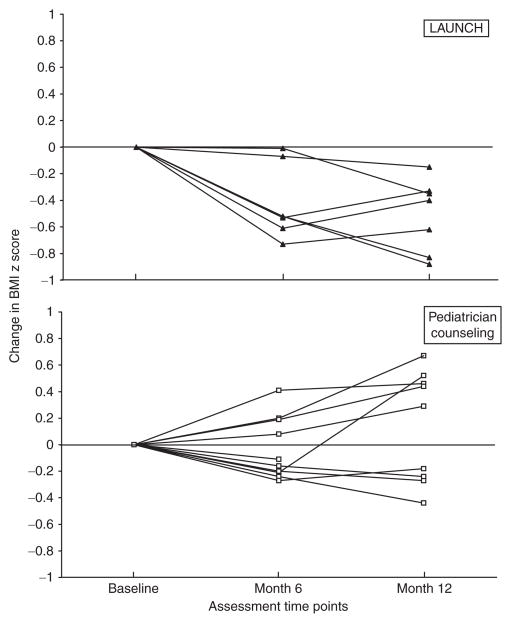
Children in LAUNCH had a significantly greater decrease in BMI z, BMI percentile, and gained significantly less weight than children in PC at month 6. These differences remained significant at month 12 (Table 3). Six of seven children who completed LAUNCH decreased their BMI z-score from baseline to month 6 and maintained a decrease at month 12. In contrast, 5 of the 10 children in PC increased their BMI z-across this same time period (Figure 2). None of the children in either group achieved a BMI percentile <85th. However, by month 12, 5 children in LAUNCH decreased their BMI percentile and 2 remained the same, while none of the children in PC decreased their BMI percentile. At month 12, 5 children in PC were ≥99th percentile (severely obese (37)), while only 1 child in LAUNCH was ≥99th percentile. LAUNCH parents achieved a statistically significant greater weight loss and lower BMI than parents in PC parents from baseline to month 6 and this difference was maintained at month 12.
Table 3.
Change on weight outcomes for children and parents in LAUNCH and pediatrician counseling (PC)
| Weight outcomes | Change, mean ± s.d.
|
Treatment effect, mean ± s.e. (CI) | Effect size (Cohen’s d) | Between group P | |
|---|---|---|---|---|---|
| LAUNCH | PC | ||||
| Child | |||||
| BMI z | |||||
| 6 months | −0.49 (0.36) | 0.10 (0.32) | −0.59 (0.17) [−0.94, −0.24] | 1.7 | 0.003 |
| 12 months | −0.37 (0.41) | 0.40 (0.49) | −0.77 (0.23) [−1.26, −0.27] | 1.7 | 0.005 |
| BMI percentile | |||||
| 6 months | −2.1 (1.9) | 0.3 (2.0) | −2.4 (1.0) [−4.4, −0.3] | 1.2 | 0.030 |
| 12 months | −1.1 (1.9) | 1.6 (2.7) | −2.7 (1.2) [−5.3, −0.2] | 1.1 | 0.040 |
| Weight (kg) | |||||
| 6 months | −0.9 (2.3) | 1.8 (0.9) | −2.7 (0.8) [−4.4, −1.0] | 1.7 | 0.004 |
| 12 months | 0.6 (3.5) | 4.8 (1.5) | −4.3 (1.3) [−7.0, −1.5] | 1.7 | 0.005 |
| Parent | |||||
| Weight (kg) | |||||
| 6 months | −7.8 (5.0) | −2.3 (5.1) | −5.5 (2.5) [−10.8, −0.1] | 1.1 | 0.046 |
| 12 months | −8.3 (3.9) | −0.4 (7.9) | −8.0 (3.5) [−15.5, −0.4] | 1.2 | 0.040 |
| BMI | |||||
| 6 months | −2.8 (1.7) | −0.8 (1.8) | −2.0 (0.9) [−3.9, −0.1] | 1.1 | 0.038 |
| 12 months | −3.1 (1.3) | −0.0 (2.8) | −3.0 (1.2) [−4.4, −1.7] | 1.3 | 0.029 |
LAUNCH, Learning about Activity and Understanding Nutrition for Child Health.
Figure 2.
Change in BMI z-score for all children in LAUNCH and pediatrician counseling from baseline to month 6 and month 12 assessments. LAUNCH, Learning about Activity and Understanding Nutrition for Child Health.
Secondary outcomes
Caloric intake and home food environment
LAUNCH child participants achieved a significantly greater decrease in daily caloric intake than PC from baseline to month 6 and maintained this difference at month 12 (Table 4). LAUNCH participants also showed significantly greater decreases in the number of high calorie foods and beverages and an increase in the number of fruits and vegetable in the home at month 6 than PC participants. At month 12, only decrease in high calorie foods for LAUNCH families remained significant.
Table 4.
Change in caloric intake and home food environment for children in LAUNCH and Pediatrician Counseling (PC)
| Outcomes | Change, mean ± s.d.
|
Treatment effect, mean ± s.e. (CI) | Effect size (Cohen’s d) | Between group P | |
|---|---|---|---|---|---|
| LAUNCH | PC | ||||
| Caloric intake (avg/day) | |||||
| 6 months | −218 (354) | 322 (280) | −540 (158) [−880, −200] | 1.7 | 0.004 |
| 12 months | −214 (327) | 311 (172) | −525 (132) [−811, −240] | 2.1 | 0.002 |
| In home High calorie foods | |||||
| 6 months | −1.8 (0.8) | 0.2 (1.3) | −2.1 (1.3) [−3.3, −0.8] | 1.9 | 0.003 |
| 12 months | −0.6 (1.6) | 1.5 (1.2) | −1.5 (1.2) [0.5, 2.5] | 1.5 | 0.010 |
| Fruits and vegetables | |||||
| 6 months | 2.2 (2.9) | −0.5 (2.2) | 2.7 (1.3) [−2.2, 1.2] | 1.1 | 0.050 |
| 12 months | 00 (2.0) | −0.4 (3.3) | 0.4 (1.5) [−2.8, 3.5] | 0.1 | 0.80 |
| High calorie beverages | |||||
| 6 months | −0.6 (0.9) | 0.5 (0.8) | −1.1 (0.4) [−2.3, 0.2] | 1.2 | 0.025 |
| 12 months | −0.5 (1.0) | 0.5 (1.2) | −1.0 (0.6) [−2.3, 0.2] | 0.9. | 0.09 |
Significant P values are denoted in boldface.
LAUNCH, Learning about Activity and Understanding Nutrition for Child Health.
Child activity
Treatment groups did not differ significantly on changes in level of vigorous or moderate physical activity from baseline to month 6 or 12 (all P > 0.05). Children in both groups engaged in an average of approximately 20 min of vigorous activity and 59–75 min of moderate activity per day at all time points.
Exploratory outcomes
There were no statistically significant differences in parenting styles on the PDS, with authoritative style endorsed as the predominate approach by both groups at all time points. There were no statistically significant differences on the Restriction or Pressure to Eat subscales of the Child Feeding Questionnaire. Scores on the Restriction subscale remained stable for both groups across all time points (approximately 4). Scores on the Pressure to Eat subscale decreased from 2.4 (LAUNCH) and 2.1 (PC) at baseline to 1.5 and 1.8 at month 6 and 12, respectively, for both groups. All parents reported Positive Mealtime (approximately 4) that was consistently above the level of Resistance to Eating (approximately 2) on the About Your Child’s Eating—Revised across all time points, suggesting mealtimes were generally positive for both groups. However, parent Aversion to Mealtime decreased significantly for LAUNCH (−0.6 ± 0.6) from 1.8 at baseline to 1.3 at month 12 compared to PC who showed an increase on this scale (0.1 ± 0.4) from 1.6 to 1.7, t(13) = 2.66, P = 0.02, Cohen’s d = 1.4.
On quality of life, LAUNCH parents reported significantly lower physical functioning for their child at baseline (76.6 ± 15.9) compared to PC parents (89.4 ± 10). However, LAUNCH showed a statistically significant greater improvement on physical functioning from baseline to month 6 (9.5 ± 13), t(14) = 2.24, P = 0.042 (Cohen’s d = 1.1), and to month 12 (13.8 ± 8.6), t(13) = 4.46, P = 0.001 (Cohen’s d = 2.3), compared to children in PC, whose score decreased slightly at month 6 (−1.7 ± 6.5) and 12 (−2.7 ± 5.6).
Parent perception and satisfaction with treatment
Change in parent motivation for change, readiness to change, or perceived ability to make changes did not differ significantly between groups from baseline to month 6. However, parents in LAUNCH reported a significantly greater increase (4.0 ± 5.6) in their desire for child change than parents in PC (−3.9 ± 3.6), t(14) =3.43, P = 0.004. Groups were also not statistically different on parent perceptions of treatment demands (11 for both) or relevance of treatment (11.3 vs. 10.6, respectively). However, LAUNCH parents reported significantly greater stressors and obstacles (33 ± 8.2) compared to parents in PC (25.6 ± 4.7), t(14) = 2.29, P = 0.038, Cohen’s d = 1.2.
Parents in LAUNCH and PC were highly satisfied with treatment and did not differ significantly on their satisfaction ratings for information on nutritional (4.86 ± .38 and 4.30 ± 1.25, respectively) or physical activity (4.71 ± .49 and 4.00 ± 1.25, respectively), or in their satisfaction with ability to make recommended changes (4.26 ± .49 and 4.20 ± 1.23, respectively), all P > 0.05.
DISCUSSION
LAUNCH demonstrated significantly greater decreases in BMI z-scores and BMI percentile in obese preschool children than PC. The trajectory of weight gain achieved by children receiving LAUNCH of 0.21 kg/2 cm of linear growth over 12 months is consistent with current treatment recommendations for preschoolers (<1 kg/2 cm of linear growth (15) or maintenance of weight (13) during linear growth so that a BMI percentile of <85th can be achieved). In contrast, children receiving PC gained an average of 1.2 kg/2 cm of linear growth during this same time period, a rate of weight gain approaching the 1.8 kg/2 cm found to be predictive of remaining overweight in elementary school (38). The results of the PC intervention indicate that low intensity intervention, which is the current standard of care in pediatric practice with young children (14), does not result in the kind of change needed by these children.
The clinical significance of these outcomes is apparent when comparing BMI percentile change, which decreased for five of seven children in LAUNCH, but remained the same (n = 3) or increased (n = 4) for all seven children in PC. At month 12, only one child in LAUNCH was at the 99th percentile BMI (little change from baseline of 99.9th percentile). In contrast, five children in PC were >99th BMI percentile. Two children increased into this percentile and three maintained this percentile, representing trajectories mirroring the concern that the heaviest children are getting heavier (37).
The health implications of being ≥99th vs. the 95th–96.9th are only now being discovered but appear significant, as Freedman and colleagues (39) found that children ≥99th percentile are significantly more likely to have ≥3 cardiovascular risk factors (33%), to remain obese (100%) and severely obese (88%) as adults compared to children at the 95th percentile. Therefore, while none of the children in this study achieved a BMI percentile <85th, being below the 99th may have cardiovascular health benefits and reduce the risk of being severely obese in adulthood.
The secondary outcomes suggest that the lower weight gain in LAUNCH participants was associated with changes in diet (caloric intake and home food environment). These dietary changes were made without any detrimental effects on parenting style, parent feeding practices, or mealtimes. In fact, parents in LAUNCH reported a significant decrease in parent aversion to mealtimes. Moderate and vigorous physical activity did not change, perhaps because children in both groups were at the recommended level for preschoolers (40) at baseline.
We believe there are several factors unique to the preschool age group that makes this an especially difficult population to treat and warrants the intensity of the LAUNCH intervention, particularly home visitation. Parents often do not recognize that their preschooler is obese (41), especially if the child resembles others in the family (42). Even if parents do recognize weight as a problem, they may feel they are depriving their child when not serving common high calorie foods and are frustrated when a child rejects healthy alternative foods (42). These feelings may interfere with parental limit-setting around food and the inability to persevere when faced with tantrums or child food refusal.
LAUNCH addresses these issues through a combination of a group clinic sessions and individual home visits that encourage learning via didactics, peer sharing, and in vivo practice of behavioral parenting skills with guidance and support of a home therapist. Anecdotally, parents reported the home component to be essential to following recommendations. We estimate that the cost of providing this intervention would be $1,276 based upon estimates that 10 group therapy sessions at $75.00 each would be $750.00 and 8 home visits estimated at $65.80 each. The cost of home visits were estimated using the Bureau of Labor Statistics (43) of the 75th percentile hourly rate for a social worker ($29.25) plus 50% overhead ($14.63) for an hourly rate of $43.87, for an average charge of $65.80 per 90-min home visit. This seems a reasonable cost given the high cost of treating the health conditions associated with obesity. Moreover, while this intervention is approximately $1,100 more than the cost of a detailed office visit with a pediatrician (approximately $150), LAUNCH was more effective in reducing obesity than the one-time visit. That parents in both groups reported equal satisfaction with the treatment indicates both were credible treatments. It was reassuring that despite the greater time commitment LAUNCH parents reported similar burden to the one-time counseling. LAUNCH parents did report more stressors, but this is not surprising as they were directly addressing difficult child behaviors like tantrums and food refusal.
These results are encouraging, but limitations of our study must be acknowledged. Our recruitment rate of 34% limits these findings to families who accept that their child’s weight is problematic. Because of the multifaceted nature of LAUNCH, it is not possible to determine the contribution of any one component to the weight outcomes. However, while we believe that information on healthy diet and activity is a necessary component of any obesity intervention, we hypothesize that teaching parents child behavior management strategies is critical to parents being able to implement the recommended diet and activity changes. In LAUNCH, we also hypothesize that the in vivo practice of these skills, tailored to the behaviors exhibited by the individual child that was possible during home visits, was also a critical component to the success. This hypothesis would need to be examined in future studies in which LAUNCH is compared to clinic only treatment. Such a study would also address the limitation of differential contact of participants with health care providers between the two treatments in the current study.
In summary, this pilot randomized trial demonstrated preliminary efficacy for improved weight outcomes for obese preschoolers using a behavioral, family intervention that includes both clinic and home visits compared to one-time education session at a well-child visit. The results of this trial will need to be replicated with a larger sample size, but LAUNCH appears to have much promise to reduce obesity in preschool children.
Acknowledgments
This study was supported by grant D24 DK 059492 from the National Institutes of Health (L.J.S.) and USPHS grant UL1RR026314 from the National Center for Research Resources of the NIH.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Williams CL, Strobino B, Bollella M, Brotanek J. Body size and cardiovascular risk factors in a preschool population. Prev Cardiol. 2004;7:116–121. doi: 10.1111/j.1520-037x.2004.03224.x. [DOI] [PubMed] [Google Scholar]
- 4.Mannino DM, Mott J, Ferdinands JM, et al. Boys with high body masses have an increased risk of developing asthma: findings from the National Longitudinal Survey of Youth (NLSY) Int J Obes (Lond) 2006;30:6–13. doi: 10.1038/sj.ijo.0803145. [DOI] [PubMed] [Google Scholar]
- 5.Datar A, Sturm R, Magnabosco JL. Childhood overweight and academic performance: national study of kindergartners and first-graders. Obes Res. 2004;12:58–68. doi: 10.1038/oby.2004.9. [DOI] [PubMed] [Google Scholar]
- 6.Nader PR, O’Brien M, Houts R, et al. National Institute of Child Health and Human Development Early Child Care Research Network. Identifying risk for obesity in early childhood. Pediatrics. 2006;118:e594–e601. doi: 10.1542/peds.2005-2801. [DOI] [PubMed] [Google Scholar]
- 7.Boles RE, Scharf C, Stark LJ. Developing a Treatment Program for Obesity in Preschool Age Children: Preliminary Data. Child Health Care. 2010;39:34. doi: 10.1080/02739610903455137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 9.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26:381–391. doi: 10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: a prospective study from birth to 9. 5 years. J Pediatr. 2004;145:20–25. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Faith MS, Kerns J. Infant and child feeding practices and childhood overweight: the role of restriction. Matern Child Nutr. 2005;1:164–168. doi: 10.1111/j.1740-8709.2005.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC Growth Charts: United States Advance Data from Vital and Health Statistics. 314. Hyattsville, MD: National Center for Health Statistics; 2000. [PubMed] [Google Scholar]
- 13.Spear BA, Barlow SE, Ervin C, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120 (Suppl 4):S254–S288. doi: 10.1542/peds.2007-2329F. [DOI] [PubMed] [Google Scholar]
- 14.Barlow SE Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120 (Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 15.Daniels SR, Arnett DK, Eckel RH, et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111:1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 16.Forehand RL, McMahon RJ. Helping the Noncompliant Child: A Clinician’s Guide to Parent Training. New York: Guilford Press; 1981. [Google Scholar]
- 17.Wardle J, Cooke LJ, Gibson EL, et al. Increasing children’s acceptance of vegetables; a randomized trial of parent-led exposure. Appetite. 2003;40:155–162. doi: 10.1016/s0195-6663(02)00135-6. [DOI] [PubMed] [Google Scholar]
- 18.Cameron N. The methods of auxological anthropometry. In: Falkner F, Tanner J, editors. Human Growth. New York: Plenum Press; 1986. pp. 3–43. [Google Scholar]
- 19.Guenther PM, DeMaio TJ, Ingwersen LA, Berline M, editors. The multiple-pass approach for the 24-hour recall in the Continuing Survey of Food Intakes by Individuals (CSFII) 1994–1996; International Conference on Dietary Assessment Methods; Boston, MA. 1995. [Google Scholar]
- 20.Reilly JJ, Armstrong J, Dorosty AR, et al. Avon Longitudinal Study of Parents and Children Study Team. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc. 1996;96:1140–1144. doi: 10.1016/S0002-8223(96)00293-3. [DOI] [PubMed] [Google Scholar]
- 22.Nutrition Data Systems (NDS) 5.0. Minneapolis: Nutrition Data Systems Nutrition Coordinating Center, University of Minnesota, Division of Epidemiology; 2004. [Google Scholar]
- 23.Epstein LH, Squires S. The Stop-Light Diet for Children. Boston: Little Brown; 1988. [Google Scholar]
- 24.Nunnally J, Bernstein I. Psychometric Theory. 2. New York: McGraw-Hill; 1978. [Google Scholar]
- 25.Pate RR, Almeida MJ, McIver KL, Pfeiffer KA, Dowda M. Validation and calibration of an accelerometer in preschool children. Obesity (Silver Spring) 2006;14:2000–2006. doi: 10.1038/oby.2006.234. [DOI] [PubMed] [Google Scholar]
- 26.Trost SG, Pate RR, Freedson PS, Sallis JF, Taylor WC. Using objective physical activity measures with youth: how many days of monitoring are needed? Med Sci Sports Exerc. 2000;32:426–431. doi: 10.1097/00005768-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 27.Mâsse LC, Fuemmeler BF, Anderson CB, et al. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc. 2005;37:S544–S554. doi: 10.1249/01.mss.0000185674.09066.8a. [DOI] [PubMed] [Google Scholar]
- 28.Trost SG, Sirard JR, Dowda M, Pfeiffer KA, Pate RR. Physical activity in overweight and nonoverweight preschool children. Int J Obes Relat Metab Disord. 2003;27:834–839. doi: 10.1038/sj.ijo.0802311. [DOI] [PubMed] [Google Scholar]
- 29.Robinson CC, Mandelco BL, Olsen SF, Hart CH. Authoritative, authoritarian, and permissive parenting practices: Development of a new measure. Psychological Reports. 1995;77:819–830. [Google Scholar]
- 30.Faith MS. Development and modification of child food preferences and eating patterns: behavior genetics strategies. Int J Obes (Lond) 2005;29:549–556. doi: 10.1038/sj.ijo.0802981. [DOI] [PubMed] [Google Scholar]
- 31.Davies WH, Nuzzo LK, Zeller MH, editors. Mealtime behaviors in families with chronically ill youth and controls. Washington, D.C: American Psychological Association; 2000. [Google Scholar]
- 32.Birch LL, Fisher JO, Grimm-Thomas K, et al. Confirmatory factor analysis of the Child Feeding Questionnaire: a measure of parental attitudes, beliefs and practices about child feeding and obesity proneness. Appetite. 2001;36:201–210. doi: 10.1006/appe.2001.0398. [DOI] [PubMed] [Google Scholar]
- 33.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Nock MK, Photos V. Parent motivation to participate in treatment: Assessment and prediction of subsequent participation. J Child Fam Studies. 2006;15:345–358. [Google Scholar]
- 35.Kazdin AE, Holland L, Crowley M, Breton S. Barriers to Treatment Participation Scale: evaluation and validation in the context of child outpatient treatment. J Child Psychol Psychiatry. 1997;38:1051–1062. doi: 10.1111/j.1469-7610.1997.tb01621.x. [DOI] [PubMed] [Google Scholar]
- 36.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 37.Skelton JA, Cook SR, Auinger P, Klein JD, Barlow SE. Prevalence and trends of severe obesity among US children and adolescents. Acad Pediatr. 2009;9:322–329. doi: 10.1016/j.acap.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams CL, Strobino BA, Bollella M, Brotanek J. Cardiovascular risk reduction in preschool children: the “Healthy Start” project. J Am Coll Nutr. 2004;23:117–123. doi: 10.1080/07315724.2004.10719351. [DOI] [PubMed] [Google Scholar]
- 39.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150:12–17. e2. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 40.National Association for Sport and Physical Education. Active Start: A Statement of Physical Activity Guidelines for Children Birth to Five Years. Reston, VA: American Alliance for Health, Physical Education, Recreation and Dance; 2002. [Google Scholar]
- 41.Jain A, Sherman SN, Chamberlin LA, et al. Why don’t low-income mothers worry about their preschoolers being overweight? Pediatrics. 2001;107:1138–1146. doi: 10.1542/peds.107.5.1138. [DOI] [PubMed] [Google Scholar]
- 42.Pagnini DL, Wilkenfeld RL, King LA, Booth ML, Booth SL. Mothers of pre-school children talk about childhood overweight and obesity: The Weight Of Opinion Study. J Paediatr Child Health. 2007;43:806–810. doi: 10.1111/j.1440-1754.2007.01199.x. [DOI] [PubMed] [Google Scholar]
- 43.Bureau of Labor Statistics United States Department of Labor. Occupational Employment and Wages, May 2008: 21–1029 Social Workers, All Other. 2009 http://www.bls.gov/oes/current/oes211029.htm. updated 4 May 2009; cited 12 March 2010.