Abstract
This report examined the effect of methylphenidate on social communication and self-regulation in children with Pervasive Developmental Disorders and hyperactivity in a secondary analysis of RUPP Autism Network data. Participants were 33 children (29 boys) between the ages of 5 and 13 years who participated in a four-week crossover trial of placebo and increasing doses of methylphenidate given in random order each for one week. Observational measures of certain aspects of children's social communication, self-regulation, and affective behavior were obtained each week. A significant positive effect of methylphenidate was seen on children's use of joint attention initiations, response to bids for joint attention, self-regulation, and regulated affective state. The results go beyond the recent literature and suggest that methylphenidate may have positive effects on social behaviors in children with PDD and hyperactivity.
Keywords: Methylphenidate, Pervasive Developmental Disorders, Hyperactivity
Children with pervasive developmental disorders (PDD) exhibit deficits in social interaction, language, and also show restrictive interests or stereotyped behaviors. Some 40-50% of children with PDD also display high levels of symptoms consistent with Attention Deficit Hyperactivity Disorder (ADHD), including hyperactivity, distractibility, and impulsiveness (Hazell, 2007; Lecavalier, 2006; Posey et al., 2007). Psychostimulant medications such as methylphenidate (MPH) are considered standard treatments for these symptoms in typically-developing children with ADHD. Until recently, only a limited number of controlled psychopharmacological studies of MPH have been conducted with children with PDD (Aman & Langworthy, 2000; Handen, Johnson, & Lubetsky, 2000; Quintana, et al., 1995) with findings confirming small to medium positive effect sizes of MPH on clinical measures of hyperactivity in children with PDD (RUPP Autism Network, 2005; Posey, et al., 2007). The purpose of this analysis was to examine the effect of MPH on certain aspects of social-communication and self-regulation behaviors in children with PDD.
Earlier psychopharmacological studies of the effect of MPH on children with PDD, mainly uncontrolled case studies, suggested that this medication may not be well-suited for children with PDD, due to a lack of significant response to treatment, as well as an increased frequency of adverse events, including negative effects on social behaviors and significant rates of intolerable side effects. (Aman, 1996; Hazell, 2007; Stigler, Desomond, Posey, Wiegand, & McDougle, 2004). Results from more rigorous but small controlled studies have still yielded ambiguous clinical implications (Quintana et al., 1995; Handen, et al., 2000). The largest study to date was the trial undertaken by the Research Units on Pediatric Psychopharmacology (RUPP) Autism Network. This study included 72 children with PDD and hyperactivity between the ages of 5 and 14 years (RUPP Autism Network, 2005). Thirty-five of the 72 children were identified as responders to MPH, based on hyperactivity subscale scores on the Aberrant Behavior Checklist as well as the Clinical Global Impression-Improvement scale. In follow-up analyses of their original study, RUPP researchers (Posey et al., 2007) examined the effects of MPH on secondary clinical outcome measures. MPH was superior to placebo on parent and teacher ratings of DSM-IV ADHD and oppositional defiant disorder (ODD) symptoms using the Swanson, Nolan, and Pelham Questionnaire (SNAP-IV). There was no difference between drug and placebo on repetitive behaviors as measured on the Yale-Brown Obsessive Compulsive Scale (CYBOCS) for PDD (Scahill, et al., 2006). In addition, there was a statistically-significant increase in parent rated “Social Withdrawal” subscale of the Aberrant Behavior Checklist on the high dose, as well as a trend for increases in ratings of “irritability.” Together, the extant research on MPH, though still limited in scope, suggested small to medium effects of MPH on the clinical symptoms of ADHD.
Less information exists on whether this medication has broader effects on children's everyday social behaviors. These behaviors are of particular interest because they reflect core features of PDD. Delayed development of social-communication skills is one such core deficit associated with PDD (Kasari, Freeman, & Paparella, 2006; Mundy, Sigman, & Kasari, 1990). Social communication skills, which typically develop in the first year of life, include the ability to engage in joint or shared attention (e.g., pointing, showing and coordinating looks between objects and people). Such skills are important developmental milestones in early childhood and have significant consequences for later social and language competence (Bakeman & Adamson, 1984; Van Hecke, et al., 2007). Behavioral interventions have shown to be effective for improving these skills in young children with autism over treatment periods as short as six weeks (Kasari et al., 2006; Yoder & Stone, 2006), and treatments that improve joint attention skills have been shown to significantly improve children's expressive language trajectories (Kasari, Freeman, Paparella, & Jahromi, in press). Given the importance of improving joint attention skills for the subsequent language and social outcomes of children with PDD, we explored the effect of MPH treatment on social-communication skills.
Self-regulation, another element of children's social behaviors, refers to the ability to engage in strategies to cope with or modulate heightened affect, or to act according to social standards (Kopp, 1982; Kochanska, Coy, & Murray, 2001; Thompson, 1994). Subsumed under the larger construct of self-regulation is the capacity for emotion regulation and compliance. Emotion regulation entails control over one's emotional expressions, for example in situations involving frustration or stress (Thompson, 1994). Children with difficulties in emotional regulation often show signs of aggression, angry outbursts, and negative peer interactions (Eisenberg, et al., 1995). Compliance to adult requests or commands, another form of self-regulation, is important for the development of positive social competence and excessive noncompliance is often associated with explosive, disruptive behavior, and risk of injury (Kuczynski & Kochanska, 1990). Although self-regulation has not traditionally been identified as a core issue in the study of children with PDD, it is regarded as a critical dimension of adaptive functioning in social settings for children with PDD (Wetherby, Prizant, & Schuler, 2000). Moreover, there is a growing consensus that these children have difficulty regulating their emotional reactions and behaviors, as is evidenced by lengthy tantrums and trouble returning to a calm or regulated affective state (Loveland, 2005; RUPP Autism Network, 2002). Data are extremely limited on the impact of medications on core symptoms of PDD. Using the Aberrant Behavior Checklist, one double-blind trial did report a significant decrease in parent ratings of stereotypies and repetitive behaviors with risperidone associated with greater reductions than placebo treatment (RUPP Autism Network, 2002). No studies to our knowledge have attempted more in-depth procedures to assess medication effects on social behaviors as we report herein.
The goals of the present study were to explore the efficacy of MPH on social-communication and self-regulation in children with PDD and hyperactivity as measured with blinded ratings of a structured video recordings collected in a multisite trial. Given that most MPH trials in children with PDD rely heavily on parent reports, this study is unique in two aspects: it uses observational measures, which may be more objective than other change indices; it examines the impact of MPH on social interaction and self-regulation. In this secondary analysis of RUPP Autism Network data (RUPP Autism Network, 2005), we tested two hypotheses: whether MPH would be superior to placebo on both social-communication and on self-regulation in children with PDD accompanied by hyperactivity.
Method
Sample
The study was conducted under approved IRB protocols at each site. Details concerning the study participants, informed consent, and treatment design were reported previously (RUPP Autism Network, 2005). Briefly, 72 subjects with PDD (autistic disorder, Asperger's disorder, or PDD-NOS) based on DSM-IV criteria supported by results on the Autism Diagnostic Interview-Revised (ADI-R) were enrolled in the original trial; 6 subjects were excluded due to inability to tolerate MPH, leaving 59 boys and 7 girls. Study subjects also had moderate to severe hyperactivity, according to SNAP-IV and CGI-Severity ratings. Subjects were excluded for any comorbid neuropsychiatric disorders that might require alternative clinical management (e.g., major depressive disorder, bipolar disorder, Rett's disorder, childhood disintegrative disorder, tic disorder). The present study reports on a subset of 33 children (29 boys and 4 girls) with PDD who had data on the observational measure. Fewer participants were included in individual analyses due to missing observational data (technical difficulty, video camera malfunction, missed visits, or an uncooperative child). An additional inclusion criterion for the observational study was a mental age of < 9 years as the social behavioral constructs and measures used in the present study would not be developmentally appropriate for older children. Thus, children had a mean age of 6.93 years (SD = 1.83; Range = 5 – 13 years), which was slightly lower than the mean age of 7.5 years in the original sample. Of the children from the original sample who were not included in the present study, 12 had a mental age above our inclusion criteria, 2 terminated early due to side effects, 1 terminated early due to the parent declining participation, 1 had incomplete data due to the child begin uncooperative, 1 had incomplete data due to the caregiver begin unavailable, 2 had technical difficulties with videos, and 14 did not schedule videotaping.
Twenty-three children were Caucasian, 7 African American, 2 Asian, and 1 Hispanic.
Procedure
Prior to randomization, participants entered a 1-week test-dose phase in which they received placebo for 1 day followed by increasing doses of methylphenidate (low, medium, high dose), each given for two days. Dosage was based on body weight, at approximately .125, .25, and .50 mg/kg per dose, twice daily with an additional half-dose given in the afternoon. Subjects tolerating the drug during the test-dose phase underwent a 4-week, randomized, double-blind, crossover trial with one week each of placebo, low, medium, or high dose methylphenidate. Best dose was ascertained by a blinded comparison of parent and teacher ratings as well as adverse effects for each dose during the crossover trial (RUPP Autism Network, 2005). Each week, children participated in a scripted, semi-structured social-communication task and parent-child interaction which were videotaped and subsequently coded at UCLA by observers who were blind to the child's treatment condition. Research staff at each site had received instruction and training on the approach to the interaction task.
Social Communication Measure
A brief social communication measure, the Joint Attention Measure from the EScs (JAMES), was administered each week to capture the initiation of joint attention, response to the experimenter's bids for joint attention, and the child's spontaneous requesting behaviors. Derived from the Early Social Communication Scales (Mundy et al., 2003), the JAMES assessment is a shortened version of the ESCS, involving only those items that differentiated children with autism from children with nonspecific developmental delays and typically developing children in previous research (Mundy, Sigman, Ungerer, & Sherman, 1986). In the JAMES, the experimenter and child sat facing each other at a table, and the experimenter engaged in a semi-structured interaction with the child using a variety of toys. Based on the child's level of functioning, one of two modules of the JAMES assessment was used. The toys for Module 1 (for lower functioning children) included wind-up toys, balloons, books, glasses, pictures on the walls, bubbles, and a silly hat. Materials for Module 2 (for higher functioning children) consisted of a racing car and Mr. Potato Head in addition to the books, pictures on the walls, and a silly hat. The procedure was videotaped and subsequently coded. All coding was completed blind to treatment condition.
Coding of Social Communication Behaviors
Two observers, trained to reliability, individually coded the JAMES procedure tapes. The variables of interest were: frequency of joint attention initiations (coordinated looks, points to share, shows, and verbal joint attention initiations), frequency of joint attention responding behaviors (following an invitation by the experimenter), and frequency of requesting behaviors (gives, reaches, points to request, and verbal requests). Fifteen percent of the videotapes were dually coded by two observers to assess reliability (ICC = .89, .91, and .99 for initiating, responding, and requesting behaviors respectively).
Caregiver-Child Interaction
Each week, the child and a primary caregiver engaged in a semi-structured caregiver-child interaction. To begin, the caregiver and child were escorted into a small room containing a few storage boxes and toys. The interaction consisted of a Competing Demands task (Diener & Mangelsdorf, 1999; Diener, Mangelsdorf, McHale, & Frosch, 2002), during which the parent completed questionnaires and acted busy and unavailable for interaction while the child played alone with toys, and a Clean-Up task, during which the parent asked the child to clean up the toys while the parent remained in the room. At the start of the caregiver-child interaction, the caregiver was instructed to play with the child for a few minutes until the experimenter returned with forms for the caregiver to complete. The Competing Demands task began once the experimenter provided the caregiver with a set of forms to complete and left the room. Caregivers were instructed to complete the forms and to refrain from interacting with the child while the child played with the toys provided. Parents were instructed to respond to their child's bids for attention in a natural tone indicating that they were very busy filling out forms and to otherwise not respond to their child's behaviors. After approximately 3 minutes, the experimenter returned to the room and quietly instructed the caregiver to ask the child to clean up the toys. The Clean-Up task lasted for approximately 3 minutes. The six minutes of child behaviors during the parent-child interactions (3 minutes in each condition) were subsequently coded as present or absent from videotapes in 10-second intervals.
Coding of Competing Demands Task
Seven self-regulation behaviors were coded as present or absent in each 10-second interval during the Competing Demands task. Fifteen percent of Competing Demands sessions were double-coded by two independent observers to assess reliability. The coded self-regulation behaviors (and corresponding ICC α reliability) were: (a) Engaging Parent, .84, (b) Social Referencing, .90, (c) Distraction, .89, (d) Self-Soothing, .66, (e) Directed Fussing, .66, (f) Passive Disengagement,.72, and (g) Leave-Taking, .66. See Table 1 for a description of behavioral codes. These categories were not mutually-exclusive; therefore, more than one behavior could be coded in a given 10-second interval. The child's predominant affective state within each 10-second interval (high positive, low positive, neutral, low negative, and high negative) was also coded during the task (ICC α = .89). Based on previous research on children's behaviors indicative of constructively coping with negativity and less reliance on the parent (Eisenberg, et al., 1995), a self-regulation composite variable was created for all subsequent analyses. The composite measure combined distraction (focused attention) and self-soothing in the context of neutral affect, minus bids for parent's attention (engaging and referencing parent), leave-taking, and passive disengagement behaviors.
Table 1.
Child Behaviors Coded in Caregiver-Child Interaction
| Self-Regulation Behaviors Coded in Competing Demands Task | |
|---|---|
| Engaging parent | Attempts to engage the parent in interaction or make bids for attention from the parent |
| Social referencing | Looking at the parent's face |
| Distraction | Prolonged or intense attention to, or manipulation of, an object or toy in the room |
| Self-soothing | Self-manipulative behaviors such as thumb-sucking, fingering clothing, or twirling hair |
| Directed fussing | Child expresses distress vocalizations clearly directed at the parent in an attempt to change the parent's behavior |
| Passive disengagement | Child withdraws passively, or sits without focus on any particular object |
| Leave taking | Attempts by the child to leave the room by banging or opening the door, or verbally indicating desire to leave |
|
Compliance/Defiance Behaviors Coded in Clean-up Task | |
| Compliance | Immediate obedience to the parent's initial request or directive |
| Passive noncompliance | Child ignores or does not acknowledge the request without overtly refusing, defying, or showing negativity |
| Direct defiance | Noncompliance by overt refusal, with angry, defiant, or negative affect; includes temper tantrums, whining |
| Simple refusal | Matter-of-fact verbal refusal without negative affect |
| Negotiation | Child proposes bargains, alternate solutions or compromises, or asks for or offers explanations or excuses |
| Unengaged | Child is completely unengaged from the interaction |
Coding of Behaviors in Clean-up Task
The following child behaviors were coded as present or absent in each 10-second interval of the Clean-up Task. Fifteen percent of Clean-up sessions were double-coded by two independent observers to assess reliability. The coded self-regulation behaviors (and corresponding ICC α) were: (a) Compliance, .88, (b) Passive Non- Compliance, .76, (c) Simple Refusal, 1.0, (d) Negotiation, .79, (e) Unengaged, .80, and (f) Direct Defiance. Due to the lack of variance in the Direct Defiance measure, the reliability measurement was not a valid assessment of agreement (percent agreement for this variable was 98.7%). See Table 1 for descriptions of coded behaviors. As these categories were mutually exclusive, only one behavior was coded in a given interval. As with the Competing Demands task, the child's predominant affective state within each 10-second interval was also coded during this task (ICC α = .74). The compliance composite variable for subsequent analyses reflected greater compliance with less direct defiance, passive noncompliance, and simple refusal.
RESULTS
Repeated-measures Analyses of Variance (RM-ANOVA) were conducted using SAS Proc GLM to assess differences in observed child outcome behaviors as a function of MPH dosage. Effect sizes (Cohen's ds) were calculated for all significant mean difference effects (MPH dose mean – placebo mean/pooled SD). To control for the effect of children's mental age on social behavior, mental age (Slosson or Leiter-R) was included as a covariate in all analyses assessing MPH effects (Slosson, 1983; Roid & Miller, 1995). Participants had a mean mental age of 43.91 months (SD = 19.72, Range = 20 – 84). Table 2 provides descriptive data for all study variables.
Table 2.
Descriptive Data for Study Variables
| Low Dose MPH | Medium Dose MPH | High Dose MPH | Best Dose MPH | Placebo | Dosage Effectsb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | ||
| Social Communication | |||||||||||
| Joint Attention Initiations | 23.29 | 16.62 | 20.85 | 13.01 | 19.27 | 14.23 | 25.09 | 15.55 | 18.59 | 12.03 | B>P; p < .05; d = .49 |
| L>P; p < .05; d = .34 | |||||||||||
| M v. P; p = .59; ns | |||||||||||
| H v. P; p = 1.0; ns | |||||||||||
| Joint Attention Responses | 2.48 | 1.45 | 1.69 | 1.42 | 1.93 | 1.48 | 2.24 | 1.69 | 1.90 | 1.71 | B v. P; p = .31; ns |
| L>P; p<.01; d = .28 | |||||||||||
| M v. P; p = .39; ns | |||||||||||
| H v. P; p = .90; ns | |||||||||||
| Joint Attention Requesting | 4.04 | 2.25 | 3.81 | 2.65 | 3.69 | 3.27 | 4.13 | 2.58 | 3.67 | 2.40 | B v. P; p = .75; ns |
| L v. P; p = .62; ns | |||||||||||
| M v. P; p = .55; ns | |||||||||||
| H v. P; p = .80; ns | |||||||||||
| Competing Demands Task | |||||||||||
| Self-Regulation Behaviors | 19.77 | 10.89 | 16.21 | 9.03 | 15.80 | 12.65 | 16.47 | 13.91 | 12.47 | 11.29 | B>P; p = .09; d = .29 |
| L>P; p= .09; d = .62 | |||||||||||
| M>P; p< .01; d = .36 | |||||||||||
| H v. P; p = .11; ns | |||||||||||
| Regulated Affective Statea | 12.91 | 4.98 | 12.96 | 3.85 | 11.67 | 5.53 | 12.47 | 4.99 | 9.57 | 6.72 | B>P; p=.09; d = .61 |
| L v. P; p=.07, d = .66 | |||||||||||
| M>P; p< .05; d = .63 | |||||||||||
| H>P; p< .05; d = .34 | |||||||||||
| Clean-Up Task | |||||||||||
| Compliance Behaviors | 3.67 | 4.87 | 3.67 | 5.64 | 4.06 | 4.55 | 3.94 | 4.88 | 3.47 | 5.41 | B v. P; p = .61; ns |
| L v. P; p = .46; ns | |||||||||||
| M v. P; p = .79; ns | |||||||||||
| H v. P; p = .23; ns | |||||||||||
| Regulated Affective Statea | 8.10 | 5.18 | 6.88 | 4.86 | 6.65 | 6.03 | 7.42 | 5.05 | 7.33 | 4.86 | B v. P; p = .79; ns |
| L v. P; p = .69; ns | |||||||||||
| M v. P; p = .91; ns | |||||||||||
| H v. P; p = .32; ns | |||||||||||
Note.
Greater neutral and less negative affect
P = placebo; B = best dose; L = low dose; M = medium dose; H = high dose. Findings reflect dosage effects after controlling for child's mental age.
Prior to conducting the primary study analyses, we conducted a comparison between the subset of participants included in this study to the full sample of 66 children reported in RUPP Autism Network (RUPP Autism Network, 2005) on the Hyperactivity subscale of the Aberrant Behavior Checklist (ABC; Aman, Singh, Stewart, & Field, 1985a, 1985b); and on mental age. Results of one-way ANOVAs revealed that there was no significant difference between the participants in the present study and the full sample in either the parent-rated or teacher-rated ABC hyperactivity subscale (p = .84 and .24, respectively). The present sample was younger (6.92 vs. 7.5 years) and had a significantly lower mental age (M = 43.91 months, SD = 19.72) than did the full sample (M = 63.28 months, SD = 38.74), F (1, 91) = 7.01, p < .05, as was expected due to the lower mental age cutoff for the present sample.
Effect of MPH on Social-Communication Behaviors
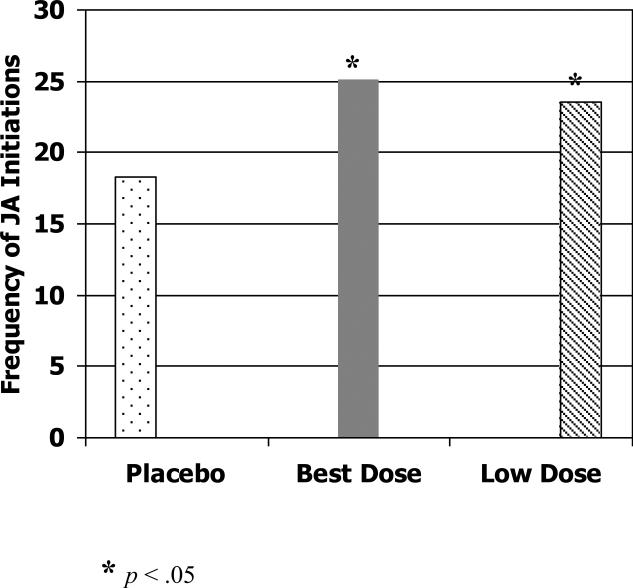
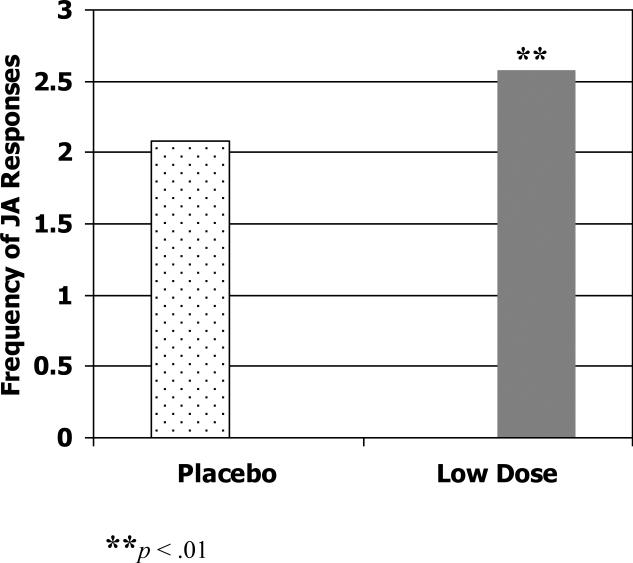
First we examined the effect of methylphenidate on child-initiated joint attention. Children showed significantly more joint attention behaviors when receiving their best dose of methylphenidate compared to placebo, F (1, 29) = 6.68, p < .05 (Cohen's d = .49). The best dose was determined by a blinded comparison of parent and teacher ratings, as well as adverse effects for each dose during the crossover trial (RUPP Autism Network, 2005). See Figure 1. When children's joint attention initiations on each of the three doses of methylphenidate was compared to that on placebo, the low dose showed significant improvement over placebo, F (1, 29) = 5.76, p < .05 (Cohen's d = .34), but no significant differences were found between placebo and either the medium or high doses. On the response to experimenter-initiated joint attention, children showed significantly more joint attention responses on the low dose of methylphenidate than on placebo, F (1, 31) = 10.80, p < .01 (Cohen's d = .28). See Figure 2.
Figure 1.
Frequency of total joint attention initiations in JAMES task at placebo, best dose and low dose.
Figure 2.
Frequency of total responses to joint attention in JAMES task at placebo and low dose.
On child requesting behaviors, there was no difference between any dose of methylphenidate and placebo (effect sizes ranging from .04 to .18; ps > .05).
Effect of MPH on Self-Regulation Behaviors in Competing Demands Task
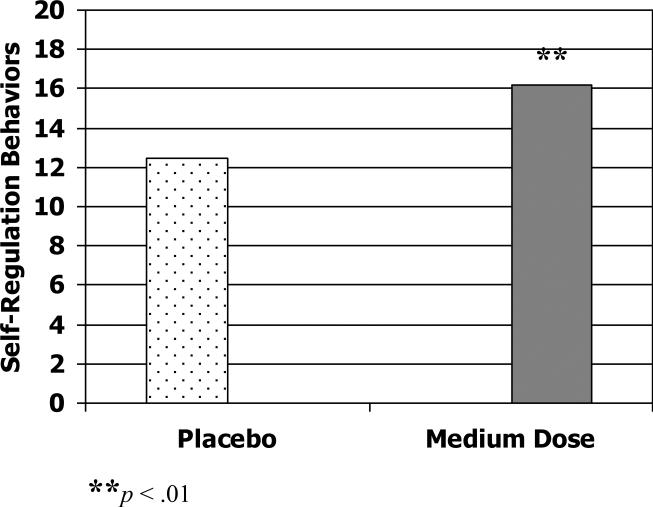
On self-regulation behaviors during the Competing Demands task, the best dose of methylphenidate was associated with marginal, but non significant, improvement compared to placebo, F (1, 21) = 3.47, p = .09 (Cohen's d = .29). There was, however, a significant effect of the medication on self-regulation behaviors when comparing varying doses to placebo. Children showed greater self-regulation in the Competing Demands task when on a low dose of methylphenidate, F (1, 24) = 4.71, p < .05 (Cohen's d = .66), although this effect was partially moderated by children's mental age (p = .09, Cohen's d = .62 after controlling for mental age); medium dose also was associated with significantly greater self-regulation behaviors, F (1, 25) = 9.99, p < .01 (Cohen's d = .36). See Figure 3. No improvement in self-regulation behaviors was found at the high dose level over placebo.
Figure 3.
Self-regulation in the Competing Demands task on placebo versus low and medium dose of methylphenidate.
Effect of MPH on Compliance in Clean-Up Task
There were no significant differences in compliance on any of the three dose levels of methylphenidate compared to placebo, or between placebo and the best dose of methylphenidate (effect sizes ranging from .01 to .26; ps > .05).
Effect of MPH on Children's Affective State
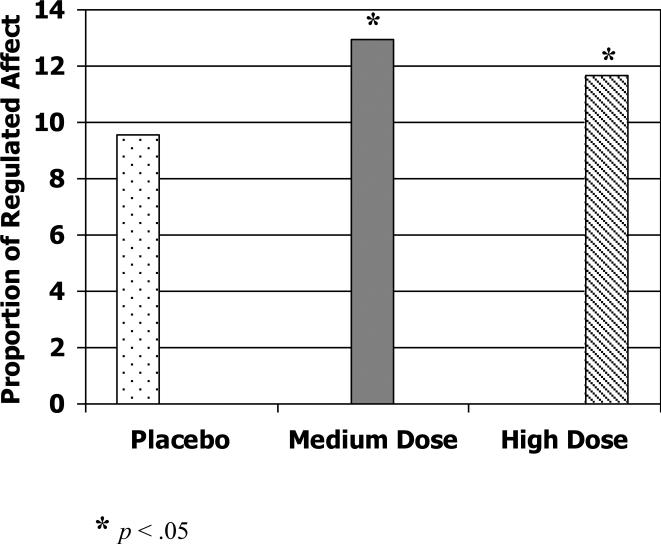
Finally, we examined the impact of methylphenidate on affective state during the Competing Demands task and the Clean Up task. For this analysis, the counts of neutral affect and negative affect were combined (neutral – negative) to create a continuous measure reflecting greater neutral and less negative affect, and compared across each drug condition. On the Competing Demands task, there was a marginal effect of the best dose of methylphenidate over placebo on greater neutral affect, F (1, 21) = 3.70, p = .09 (Cohen's d = .61) and of the low dose, F (1, 21) = 3.88, p = .07 (Cohen's d = .66). There was, however, a statistically significant increase in neutral affect for the medium dose, F (1, 25) = 7.51, p < .05 (Cohen's d = .63) and the high dose of MPH compared to placebo, F (1, 27) = 6.73, p < .05 (Cohen's d = .34) (See Figure 4). Analyses of affective behavior in the Clean-up task revealed no significant impact of MPH at any dose (effect sizes ranging from .05 to .14; ps > .05).
Figure 4.
Proportion of time in which children displayed a regulated affective state (greater neutral affect and less negative affect) in the Competing Demands task on placebo versus medium and high dose of methylphenidate.
Discussion
The intent of the present study was to examine the effects of methylphenidate on key domains of social functioning, social communication and self-regulation behaviors, in young school age children with PDDs. The study is novel in its focus on social endpoint changes associated with drug treatment. Although modest in sample size, the multi-site study included rigorous characterization of subjects, three dose levels of MPH, multiple-informants to judge medication response, use of well validated semi-structured parent-child interaction with valid and blinded coding procedures for the observational data.
Research on social communication and self-regulation behaviors indicates that both processes are a function of the neural attentional system (Rothbart, Posner, & Boylan, 1990). Executive attention regulation (attention control and inhibitory control) underlies joint attention development by enabling the child to focus his or her attention on an object to which another person is referring, and to actively deploy attention between the object and his social partner (Mundy & Acra, 2006; Van Hecke, et al., 2007). Individual differences in attentional control have also been shown to be related to the child's ability to regulate emotional expressions and behaviors, possibly by enabling the child to disengage or distract his or her attention from a source of distress and to orient toward an alternative stimulus (Howse, Calkins, Anastopoulos, Keane, & Shelton, 2003; Jahromi & Stifter, in press; Morales, Mundy, Crowson, Neal, & Delgado, 2005). Together, this research points to children's attention as an underlying construct affecting social-communication and self-regulation behaviors, suggesting a possible positive effect of psychostimulant medications on such behaviors.
The results of the present study suggest that a relatively brief interactive observational task can be incorporated as a repeated measure in a randomized, multisite, clinical treatment trial and yield interpretable data which are sensitive to treatment effects. Second, MPH treatment was associated with improvement in a variety of domains of social functioning that appeared to parallel improvements in previously reported ADHD symptoms. Third, the improvements in joint attention initiations and emotion regulation suggest that MPH may benefit core features of PDD, an effect not previously reported to our knowledge. On the other hand, improvements in attention may account for the observed improvements on these observational tasks. It is unknown if such improvements in social functioning would be observed in children with PDD without hyperactivity.
On joint attention behaviors, drug effects were evident on child- initiations, with an overall MPH effect estimated in the moderate range (Cohen's d = 0.49). No clear dose-response relationship was observed, although the lower dose appeared to show the largest impact on these behaviors. A smaller effect was found for experimenter-initiated joint attention responses (Cohen's d = 0.28). This may reflect the truncated sampling of the JAMES, which allowed for coding of this behavior in only two tasks (posters and book). Thus, there were fewer opportunities and little room for variability in these behaviors. Joint attention is considered an essential component of language development. Because the higher dose conditions have been associated with greater parent ratings of “irritability” and “social withdrawal” (Posey et al., 2007) this may have interfered with observing more robust effects on social communication measures associated with higher doses. Future work should assess whether these effects predict children's later language development, and whether examining responding behaviors in contexts allowing for greater number of observed behaviors, such as during a parent-child interaction, may reveal broader joint attention effects of MPH.
Some what surprisingly, MPH had no effect on compliance with parental requests. It should be noted, however, that baseline ratings of oppositional and defiant behavior on the eight-item ODD SNAP-IV scale (mean 8.83 ± 5.19) were actually low. It may be that the low baseline ratings created a floor effect, and/or that the treatment period of only one week was not long enough to see considerable changes in the children's compliance behaviors. However, studies of typically developing children with ADHD have documented improvements in compliance in response to acute MPH administration (Barkley, 1998). Furthermore, as a significant change in child affective behavior was also not found in the Clean-up task, it may be that parents’ behaviors in this context (e.g., helping children clean up the toys) resulted in lower child negativity and less need for compliance. It may be, therefore, that participants did not show improvements in their affective states because this tasks was less demanding. The construct of compliance is less directly related to attentional processes as it requires more than simply overcoming negativity but also engaging in specific behaviors to meet another person's expectations (e.g., cleaning up the toys). Perhaps a modification of our probe in the experimental task to more strongly challenge the subject would have revealed MPH effects.
Positive effects of MPH on subjects’ affective state were also significant. In particular, more neutral and regulated affect in the Competing Demands task, which can elicit negative affect, may indicate a clinically significant benefit of MPH for some children which could reduce strain in some contexts. Effect size estimates were from small to moderate (0.34 – 0.61), and higher doses were more likely to be associated with more regulated affect. A related, but separate, measure of children's responses to the Competing Demands task was that of self-regulation behaviors. Whereas children's affective state reflected their displays of emotional reactivity, our measure of self-regulation captured the behavioral coping strategies that children deployed in this context. Effect sizes again ranged from small to moderate (0.36 - .62), and low and medium doses of methylphenidate were associated with greater use of self-regulation strategies.
Overall, the pattern of MPH reflected improvement or enhancement across several measures of social-communication and affective behavior. Similar to our analyses of ADHD behaviors using parent- and teacher-rated change (Posey, et al., 2007), a clear dose-response relationship was not evident at the group level. Additional analyses could examine the intra-individual profile of response on both disruptive and social behaviors, and the possible relationships between improvements in different domains. Pearson et al. (2004) analyzed the cognitive and behavioral improvements in children with intellectual disability and ADHD, and found considerable independence of optimal dose effects comparing cognitive versus behavioral domains. In this study, the greatest clinical effect was seen with the medium dose, but optimal dose for social behavior tended to vary. It may be the case that with developmentally disabled children, there may be greater disparity in dose-response across different domains for optimal change.
Limitations
This study was a pilot project to examine the effect of MPH on certain aspects of social-communication and self-regulation behaviors in children with PDD. The study also offered the opportunity to explore the feasibility and sensitivity of established observational measures to detect medication effects on social behaviors. The study has several limitations. The one week time period for each treatment condition was brief; hence the durability of apparent benefits remains undetermined. In addition, our sample was a smaller subset of the larger trial, due to technical difficulties with video equipment, drop outs, and additional inclusion criteria. The reduced sample size increases the risk of both Type I and Type II error. It should be noted, however, that the present study's sample did not differ significantly from that in the larger trial on parent or teacher scores from the ABC hyperactivity subscale, thus can be considered reflective of the larger group with respect to this primary outcome measure and inclusion criteria. Future, larger investigations of social behaviors could control for inattention and hyperactivity to determine the degree to which medication effects are specific to the social behaviors. Another important consideration is the expense of such observational measures. Future studies may provide further support for the unique contribution of observation measures that justify the additional expense.
In summary, social communication deficits involving joint attention and self regulation are fundamental problems in children with PDD. They are also a critical focus for intervention as they are relevant to broader impairments in language development and social functioning. Although specific psychosocial treatments are increasingly targeting these skills as goals for intervention, there is a need to explore the possible benefits of medication on these behaviors. It may be that drug therapies could promote development in joint attention and self- regulation, either alone or in combination with other treatments. The positive effects seen of MPH on social behaviors in a sample of children with PDD should encourage additional research incorporating measures of social communication in future clinical trials, and suggests that other perhaps more targeted medications could yield enhanced social functioning in individuals with PDD.
Clinical Implications
When confronted with hyperactivity as a prominent co-occurring feature in children with PDD, clinicians can feel more confident that a trial of MPH will not exacerbate the core social and communication problems and may actually help them some. Whether MPH helps these core problems enough to justify targeting them for treatment would require further research. Until more data are available, we would not recommend stimulants for treatment of core features of PDD in the absence of hyperactivity. The response of patients to any medication has to be individually evaluated. For example, in the trial from which this sample was drawn, 18% of the participants had to stop treatment because of intolerable side effects, while others benefited significantly.
Acknowledgements
This work was supported by National Institute of Mental Health Contracts N01 MH-70070 (principal investigator: Dr. McCracken), N01 MH-70009 (principal investigator: Dr. Scahill), N01 MH-70001 (principal investigator: Dr. McDougle), and N01 MH 80011 (principal investigator: Dr. Aman); by National Institutes of Health Division of Research Resources General Clinical Research Center Grants M01 RR-00750 (to Indiana University), M01 RR-00052 (to Johns Hopkins University), M01 RR-00034 (to Ohio University), and M01 RR-06022 (to Yale University); by National Institute of Mental Health Grants MH-01805 (to Dr. McCracken) and MH-68627 (to Dr. Posey); by funding from the Korczak Foundation (to Dr. Scahill); and by NIMH Postdoctoral Training Grant 5 T32 MH18372 to Dr. Jahromi. The RUPP Autism Network comprises the following investigators, listed by role and study site. Ohio State University: principal investigator Michael G. Aman, Ph.D., co-investigators L. Eugene Arnold, M.Ed., M.D., Yaser Ramadan, M.D., Andrea N. Witwer, M.A., Ronald Lindsay, M.D., and Patricia Nash, M.D.; University of California at Los Angeles: principal investigator James T. McCracken, M.D., co-investigators Bhavik Shah, M.D., James McGough, M.D., Pegeen Cronin, Ph.D., and Lisa Lee, B.A.; Indiana University: principal investigator Christopher J. McDougle, M.D., co-investigators David J. Posey, M.D., Naomi Swiezy, Ph.D., and Arlene Kohn, B.A.; Yale University: principal investigator Lawrence Scahill, M.S.N., Ph.D., co-investigators Andres Martin, M.D., Kathleen Koenig, M.S.N., Fred Volkmar, M.D., Deirdre H. Carroll, M.S.N., and Allison Lancor, B.S.; Kennedy Krieger Institute: principal investigator Elaine Tierney, M.D., co-investigators Jaswinder Ghuman, M.D., Nilda Gonzalez, M.D., and Marco Grados, M.D.; National Institute of Mental Health: principal investigator Benedetto Vitiello, M.D., co-investigator Louise Ritz, M.B.A. The Network also includes statisticians Shirley Z. Chuang, M.S., and Mark Davies, M.P.H., of Columbia University, and data managers James Robinson, M.Ed., and Don McMahon, M.S., of the Nathan Kline Institute. Affiliations. Dr. Aman has affiliations with Bristol-Myers Squibb Co., Forest Research Institute, Johnson & Johnson, Neuropharm, and Supernus. Dr. Arnold has affiliations with Shire, Neuropharm, Lilly, Novartis, Organon, Janssen, and McNeil. Dr. Ghuman has affiliations with Bristol-Myers Squibb Co. Dr McCracken has affliations with Bristol Myers Squibb, Eli Lilly, McNeil Pediatrics, Janssen Pharmaceutica, Pfizer, Shire, UCB, Wyeth, and Novartis. Dr. McDougle has affiliations with Bristol-Myers Squibb Co., Eli Lilly and Co., Forest Research Institute, Janssen Pharmaceutica, and McNeil Pediatrics. Dr. Scahill has affiliations with Janssen Pharmceutica, Bristol-Myers Squibb, Neuropharm and Supernus. Dr. Posey has affiliations with Bristol-Myers Squibb, Eli Lilly, Forest, and Shire. The remaining authors have no affiliations.
References
- Aman MG, Singh NN, Stewart AW, Field CJ. The Aberrant Behavior Checklist: A behavior rating scale of the assessment of treatment effects. American Journal of Mental Deficiency. 1985a;89:485–491. [PubMed] [Google Scholar]
- Aman MG, Singh NN, Stewart AW, Field CJ. Psychometric characteristics of the Aberrant Behavior Checklist. American Journal of Mental Deficiency. 1985b;49:492–502. [PubMed] [Google Scholar]
- Aman MG. Stimulant drugs in the developmental disabilities revisited. Journal of Developmental and Physical Disabilities. 1996;8:347–365. [Google Scholar]
- Aman MG, Langworthy KS. Pharmacotherapy for hyperactivity in children with autism and other pervasive developmental disorders. Journal of Autism and Developmental Disorders. 2000;30:451–459. doi: 10.1023/a:1005559725475. [DOI] [PubMed] [Google Scholar]
- Bakeman R, Adamson LB. Coordinating attention to people and objects in mother-infant and peer-infant interaction. Child Development. 1984;55:1278–1289. [PubMed] [Google Scholar]
- Barkley RA. The effects of methylphenidate on interactions of preschool ADHD children with their mothers. Journal of American Academy of Child and Adolescent Psychiatry. 1988;27:336–341. doi: 10.1097/00004583-198805000-00012. [DOI] [PubMed] [Google Scholar]
- Diener ML, Mangelsdorf SC. Behavioral strategies for emotion regulation in toddlers: Associations with maternal involvement and emotional expressions. Infant Behavior and Development. 1999;22:569–583. [Google Scholar]
- Diener ML, Mangelsdorf SC, McHale JL, Frosch CA. Infants’ behavioral strategies for emotion regulation with fathers and mothers: Associations with emotional expressions and attachment quality. Infancy. 2002;3:153–174. doi: 10.1207/S15327078IN0302_3. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes R, Murphy B, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children’s social functioning: A longitudinal study. Child Development. 1995;66:1360–1384. [PubMed] [Google Scholar]
- Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. Journal of Autism and Developmental Disorders. 2000;30:245–255. doi: 10.1023/a:1005548619694. [DOI] [PubMed] [Google Scholar]
- Hazell P. Drug therapy for attention-deficit/hyperactivity disorder-like symptoms in autistic disorder. Journal of Pediatric and Child Health. 2007;43:19–24. doi: 10.1111/j.1440-1754.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- Howse RB, Calkins SD, Anastopoulos AD, Keane SP, Shelton TL. Regulatory contributors to children’s kindergarten achievement. Early Education & Development. 2003;14:101–119. [Google Scholar]
- Jahromi LB, Stifter CA. Individual differences in preschoolers’ self-regulation and theory of mind. Merrill-Palmer Quarterly. 2008;54:125–150. [Google Scholar]
- Kasari C, Freeman S, Paparella T. Joint attention and play in young children with autism: A randomized controlled intervention study. Journal of Child Psychology and Psychiatry. 2006;47:611–620. doi: 10.1111/j.1469-7610.2005.01567.x. [DOI] [PubMed] [Google Scholar]
- Kasari C, Freeman S, Paparella T, Jahromi LB. Predicting language outcome in autism from controlled comparison of joint attention and play interventions. Journal of Clinical and Consulting Psychology. 2008;76:125–137. doi: 10.1037/0022-006X.76.1.125. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Coy KC, Murray K. The development of self-regulation in the first four years of life. Child Development. 2001;72:1091–1111. doi: 10.1111/1467-8624.00336. [DOI] [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18:199–214. [Google Scholar]
- Kuczynski L, Kochanska G. Development of children’s noncompliance strategies from toddlerhood to age 5. Developmental Psychology. 1990;26:398–408. [Google Scholar]
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: Relative prevalence, effects of subject characteristics, and empirical classification. Journal of Autism and Developmental Disorders. 2006;36:1101–1114. doi: 10.1007/s10803-006-0147-5. [DOI] [PubMed] [Google Scholar]
- Loveland KA. Social-emotional impairment and self-regulation in autism spectrum disorder. In: Nadel J, Muir D, editors. Emotional development: Recent research advance. Oxford University Press; New York: 2005. pp. 365–382. [Google Scholar]
- Morales M, Mundy P, Crowson MM, Neal AR, Delgado CEF. Individual differences in infant attention skills, joint attention, and emotion regulation behaviour. International Journal of Behavior and Development. 2005;29:259–263. [Google Scholar]
- Mundy P, Delgado C, Block J, Venezia M, Hogan A, Seibert J. A manual for the abridged Early Social Communication Scales (ESCS) University of Miami; Coral Gables. FL: 2003. [Google Scholar]
- Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. Journal of Autism and Developmental Disorders. 1990;20:115–128. doi: 10.1007/BF02206861. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: The contribution of non-verbal communication measures. Journal of Child Psychology and Psychiatry. 1986;27:657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Mundy P, Acra F. Joint attention, social engagement and the development of social competence. In: Marshall P, Fox N, editors. The development of social engagement neurobiological perspectives. Oxford University Press; New York: 2006. pp. 81–117. [Google Scholar]
- Pearson DA, Lane DM, Santos CW, Casat CD, Jerger SW, Loveland KA, et al. Effects of methylphenidate treatment in children with mental retardation and ADHD: Individual variation in medication response. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:686–698. doi: 10.1097/01.chi.0000120024.14101.96. [DOI] [PubMed] [Google Scholar]
- Posey DJ, Aman MG, McCracken JT, Scahill L, Tierney E, Arnold LE, et al. Positive Effects of Methylphenidate on Inattention and Hyperactivity in Pervasive Developmental Disorders: An Analysis of Secondary Measures. Biological Psychiatry. 2007;61:538–544. doi: 10.1016/j.biopsych.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Quintana H, Birmaher B, Stedge D, Lennon S, Freed J, Bridge J, et al. Use of methylphenidate in treatment of children with autistic disorder. Journal of Autism and Developmental Disorders. 1995;25:283–295. doi: 10.1007/BF02179289. [DOI] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology Autism Network Risperidone in children with autism and serious behavioral problems. New England Journal of Medicine. 2002;347:314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology (RUPP) Autism Network Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Archives of General Psychiatry. 2005;62:1266–1274. doi: 10.1001/archpsyc.62.11.1266. [DOI] [PubMed] [Google Scholar]
- Roid GH, Miller LJ. Leiter International Performance Scale−Revised. Stoelting; Wood Dale, IL: 1995. [Google Scholar]
- Rothbart M, Posner M, Boylan A. Regulatory mechanisms in infant development. In J. Enns (Ed.), The development of attention: Research and theory. Elsevier; Holland: 1990. [Google Scholar]
- Scahill L, McDougle CJ, Williams SK, Dimitropoulos A, Aman MG, McCracken JT, et al. Children’s Yale-Brown obsessive compulsive scale modified for pervasive developmental disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1114–1123. doi: 10.1097/01.chi.0000220854.79144.e7. [DOI] [PubMed] [Google Scholar]
- Slosson RL. Slosson Intelligence Test. Slosson Educational Publications; East Aurora, NY: 1983. [Google Scholar]
- Stigler KA, Desomond LA, Posey DJ, Wiegand RE, McDougle CJ. A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. Journal of Child and Adolescent Psychopharmacology. 2004;14:49–56. doi: 10.1089/104454604773840481. [DOI] [PubMed] [Google Scholar]
- Thompson R. Emotion regulation: A theme in search of definition. Monographs of the Society for Research in Child Development. 1994;59:25–52. 2-3, Serial No. 240. [PubMed] [Google Scholar]
- Van Hecke AV, Mundy P, Acra CF, Block JJ, Delgado CEF, Parlade MV, et al. Infant joint attention, temperament, and social competence in preschool children. Child Development. 2007;78:53–69. doi: 10.1111/j.1467-8624.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby AM, Prizant BM, Schuler AL. Understanding the nature of communication and language impairments. In: Wetherby AM, Prizant BM, editors. Autism spectrum disorders: A transactional developmental perspective. Paul H. Brookes; Baltimore, MD: 2000. pp. 109–141. [Google Scholar]
- Yoder P, Stone W. Randomized Comparison of Two Communication Interventions for Preschoolers with Autism Spectrum Disorders. Journal of Consulting and Clinical Psychology. 2006;74:426–435. doi: 10.1037/0022-006X.74.3.426. [DOI] [PubMed] [Google Scholar]