Abstract
Modest antidepressant response rates of mood disorders (MD) encourage benzodiazepine (BZD) co-medication with debatable benefit. Adult hippocampal neurogenesis may underlie antidepressant responses, but diazepam co-administration impairs murine neuron maturation and survival in response to fluoxetine. We counted neural progenitor cells (NPCs), mitotic cells, and mature granule neurons postmortem in dentate gyrus (DG) from subjects with: untreated DSM-IV MD (n=17); antidepressant-treated MD (MD*ADT, n=10); benzodiazepine-antidepressant-treated MD (MD*ADT*BZD, n=7); no psychopathology or treatment (controls, n=18).
MD*ADT*BZD had fewer granule neurons vs. MD*ADT in anterior DG and vs. controls in mid DG, and did not differ from untreated-MD in any DG subregion. MD*ADT had more granule neurons than untreated-MD in anterior and mid DG and comparable granule neuron number to controls in all dentate subregions. Untreated-MD had fewer granule neurons than controls in anterior and mid DG, and did not differ from any other group in posterior DG. MD*ADT*BZD had fewer NPCs vs. MD*ADT in mid DG. MD*ADT had more NPCs vs. untreated-MD and controls in anterior and mid DG. MD*ADT*BZD and MD*ADT had more mitotic cells in anterior DG vs. controls and untreated-MD. There were no between-group differences in mid DG in mitotic cells or in posterior DG for any cell type.
Our results in mid-dentate, and to some degree anterior dentate, gyrus are consistent with murine findings that benzodiazepines counteract antidepressant-induced increases in neurogenesis by interfering with progenitor proliferation. We also confirmed, in this expanded sample, our previous finding of granule neuron deficit in untreated MD.
Keywords: Neurogenesis, nestin, Ki-67, NeuN, stereology
INTRODUCTION
To manage the anxiety component of mood disorders (MD), a benzodiazepine (BZD) is often co-administered with antidepressant medication (Bandelow et al., 2008). Selective serotonin reuptake inhibitors (SSRIs) may increase anxiety in MD during the initial treatment of MD and, as an alternative to lowering SSRI dosage, adjunctive BZD use may be recommended (Edwards and Anderson, 1999; Bandelow et al., 2008). Recently, concern has arisen that such BZD administration may worsen depression, cause transient cognitive or motor impairment, and potentially lead to abuse or dependence (Lader, 2011; Dell'osso and Lader, 2013). Additional studies have also suggested longer-term cognitive impairment with prolonged BZD use (Klein et al., 2009; Wu et al., 2009).
New neurons develop from neural progenitor cells (NPCs) in adult human dentate gyrus (DG), and mature into functional granule neurons (Eriksson et al., 1998) that contribute to hippocampal-dependent functions, such as learning and memory (Saxe et al., 2006) and pattern discrimination (Clelland et al., 2009; Aimone et al., 2011). Increased neurogenesis and cell survival improves pattern discrimination (Sahay et al., 2011). Neurogenesis mediates recovery from stress via environmental enrichment (Schloesser et al., 2010) and antidepressant effects on chronic unpredictable stress, novelty suppressed feeding (Surget et al., 2008), and contextual discrimination (Tronel et al., 2012). Nevertheless, adult neurogenesis is not necessary for all antidepressant effects responses in animal models, suggesting the existence of neurogenesis-dependent and -independent mechanisms of antidepressant action, at least in rodent depression models (David et al., 2009).
Antidepressant treatment in major depression is associated with more mitotic cells, NPCs (Boldrini et al., 2009; Boldrini et al., 2012) and mature granule neurons (Boldrini et al., 2013) in human DG. This antidepressant effect would mitigate the defective neurogenesis that is hypothesized to contribute to the pathogenesis of major depression (Kempermann and Kronenberg, 2003). In support of this hypothesis, we find fewer mature granule neurons in hippocampal DG of untreated subjects with major depression compared with controls, as well as a decrease in DG granule neurons associated with earlier onset of major depression (Boldrini et al., 2013). In addition, we found that more lifetime major depressive episodes are correlated with smaller DG volume (Boldrini et al., 2013), consistent with magnetic resonance (MR) volume findings in vivo (Sheline et al., 2003; McKinnon et al., 2009).
In rodents, co-administration of diazepam and fluoxetine inhibits the neurogenesis effect of fluoxetine and the suppression of anxious and depressive behavior (Wu and Castren, 2009; Sun et al., 2013). Although specific underlying mechanisms of BZD function remain unknown, tonic and phasic gamma-amino-butyric acid (GABA) activation regulates the synaptic integration of newborn neurons in murine DG (Ge et al., 2006). Additionally, a balance of glutamatergic and GABAergic transmission closely regulates adult hippocampal neurogenesis (Sun et al., 2009). BZDs, which act as GABAA receptor agonists (Rudolph et al., 1999) may impact hippocampal neurogenesis by enhancing GABAergic signaling and causing an imbalance in neuronal activity.
The effect of BZDs on the relationship between adult hippocampal neurogenesis and antidepressant use has not been studied in the brain of depressed patients. In this study we assessed the relationship of BZD to antidepressant co-treatment by quantifying neural progenitor cell, mitotic cell, and mature granule neuron number in human DG of subjects with mood disorders. We hypothesized that fewer mature granule neurons, NPCs, and mitotic cells would be observed in subjects with MD co-treated with BZD and antidepressants, compared with those treated with antidepressants alone. Other comparison groups were untreated MD and non-psychiatric controls.
METHOD
Brain Collection
IRB approval was obtained for all research conducted. Postmortem tissue was acquired from the Macedonian/New York State Psychiatric Institute brain collection. We dissected the hippocampus from two-cm thick coronal blocks of the right hemisphere that were frozen in dichlorodifluoromethane (−30°C) and stored at −80°C at the time of autopsy. Samples of selected brain areas were formalin-fixed for neuropathology screening and brain pH determination. Toxicology tests were performed on cerebellar tissue, blood and other body fluids.
Clinical Measures
Subjects were diagnosed using a psychological autopsy and the SCID I or SCID NP (Non-Patient edition) and II (Lobbestael et al., 2011), using a method validated for DSM axis I and II diagnoses (Kelly and Mann, 1996). History of lifetime mood disorders, developmental history and recent medication history were obtained. Other instruments and assessments included the Global Assessment Scale (Endicott et al., 1976). Cause of death and time to autopsy, and freezer storage time were noted.
Subjects
Four groups of subjects were studied: benzodiazepine-antidepressant-treated MDs (MD*ADT*BZD; N=7), MDs treated with antidepressants only (MD*ADT; N=10), untreated MDs (N=17) and controls without psychiatric disease or treatment (N=18). The percentage of bipolar and major depressive disorder subjects was not different between groups (Table 1). Subjects were included in treated groups if they received drug prescriptions in the last three months of life and tested positive for such drugs (brain or blood toxicology) at autopsy. Groups were matched for sex and postmortem interval (PMI) because of the influence of estrogen on neurogenesis (Saravia et al., 2007) and the possible effect of PMI on antigen potency. Males and females were equally distributed in the different subject groups (Chi-Square=3.456; df=3; p=.327). There was no difference between groups in terms of PMI (p=.903). Age differed between groups (F=3.2230; df=3,48; p=.030): MD*ADT were younger than MD*ADT*BZD (p=.036) and untreated-MDs (p=.030). No differences were found between other groups. Therefore, age was included as a covariate in analyses of group effect.
Table 1.
Demographic and clinical characteristics of subjects
| Group (n) | Age (years) (Mean ± SEM) |
PMI (hours) (Mean ± SEM) |
Brain pH (Mean ± SEM) |
Sex (M:F) |
Suicide (n) |
DSM Axis I Diagnosis |
Blood and Brain Toxicology |
GAS (Mean ± SEM) |
N. Episodes of MDE (Mean ± SEM) |
Age of First MDE (Mean ± SEM) |
Medication Prescribed During the last 3 Months of Life |
Smoking Tobacco |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
C (n=18) |
46 ± 4.1 | 14.7 ± 1.2 | 6.42 ± 0.08 | 11:7 | 0 | None (18) | None (9) Anesthetics (4) Carbon Monoxide (1) |
84.3 ± 2.51 | - | - | None (18) | Yes (7) No (10) |
|
MD (n=17) |
51 ± 4.6 | 14.7 ± 1.8 | 6.08 ± 0.07 | 11:6 | 13 |
Primary: MDD (16) BPD (1) Comorbidity: OCD (1) GD (1) AA (1) |
None (14) Analgesic (1) |
54.4 ± 5.88 | 1.66 ± 0.32 | 44.6 ± 5.09 | None (16) Nitroglycerine (1) Atenolol (1) Albuterol (1) |
Yes (6) No (8) |
|
MD*ADT (n=10) |
34 ± 2.6 | 15.8 ± 2.2 | 6.39 ± 0.12 | 3:7 | 8 |
Primary: MDD (7) BPD (3) Comorbidity: CA (1) BA (2) |
Anesthetics (1) Antidepressants (Bupropion Fluoxetine Nortriptyline Paroxetine Sertraline) (10) Lithium (2) Opioids (1) Solvents (1) |
35.3 ± 7.24 | 3 ± 0.96 | 25.3 ± 2.69 | Amitriptyline (1) Benztropine Mesylate (1) Bupropion (1) Fluoxetine (3) Fluphenazine (1) Lithium (2) Nortriptyline (2) Paroxetine (1) Sertraline (2) Trazodone (3) Valproic Acid (1) |
Yes (3) No (7) |
|
MD*ADT*BZD (n=7) |
57 ± 6.8 | 16.9 ± 4.3 | 6.57 ± 0.18 | 4:3 | 4 |
Primary: MDD (4) BPD (3) |
Analgesic (2) Antidepressants (7) Antipsychotics (1) Barbiturates (2) BZD (7) Fluoxetine (1) Mianserin (1) Opioids (2) Sertraline (2) |
11.2 ± 7.14 | 1 | 68.7 ± 7.66 | Sertraline (1) Clomipramine (1) |
Yes (1) No (3) |
AA = Alcohol Abuse; BA = Bulimia/ Anorexia; BPD = Bipolar Disorder; BZD = Benzodiazepines; C = controls without psychopathology or treatment; CA = Cannabis Abuse; GAS = Global Assessment Scale; GD = Gambling Disorder; MDD = Major Depressive Disorder; MD = Untreated subjects with mood disorder; MD*ADT = Subjects with mood disorder treated with antidepressant only; MD*ADT*BZD = Subjects with mood disorder treated with antidepressants and benzodiazepines; OCD = Obsessive Compulsive Disorder; SEM = standard error of the mean.
Hippocampus Preparation
The whole right hippocampus was dissected from consecutive frozen coronal blocks, then fixed in 4% paraformaldehyde phosphate buffer saline at 4°C and cryoprotected in 30% sucrose. Sections were cut (at 50µm) using a freezing microtome (Microm HM440E) and stored in 40-well boxes at −20°C in cryoprotectant (30% ethylene glycol in 0.1M phosphate buffer). While sectioning, reference slides at 1-mm intervals were stained with Cresyl violet, and were later used to align sections processed for immunohistochemistry along the anterior-posterior axis of the hippocampal formation.
Immunohistochemistry and Stereology
Sections were processed to identify mature granule neurons (anti-Neuronal Nuclear antigen [NeuN] mouse monoclonal antibody, 1:100,000; Chemicon, Temecula, CA), NPCs (anti-nestin mouse monoclonal antibody, 1:8000, Chemicon) and mitotic cells (anti-Ki-67 (Scholzen and Gerdes, 2000) mouse monoclonal antibody, 1:200, Novocastra Clone-MM1, Newcastle Upon Tyne, UK). Immunohistochemistry and stereology were performed as previously described (Boldrini et al., 2009; Boldrini et al., 2012; Boldrini et al., 2013). The choice of nestin as a marker to detect NPCs was determined by the fact that nestin (NEural STem cell proteIN) is a class VI intermediate filament protein expressed during development, until around postnatal day 11 in rat cortex and gradually replaced by intermediate filament proteins specific for mature cells, such as glial fibrillary acidic protein (GFAP) in glial cells and other types of neurofilament in neurons (Kalman and Ajtai, 2001). In transgenic adult mice expressing green fluorescent protein under the control of regulatory regions of the nestin gene, nestin-positive cells eventually express markers of neuroblasts: polysialated neuronal cell adhesion molecule, doublecortin and the transcription factor NeuroD (Yamaguchi et al., 2000). In culture, nestin-immunoreactive cell spheres differentiate into neurons and glia (Itoh et al., 2006). Moreover, nestin-positive type II NPCs, but not GFAP-positive type I NPCs, are targeted by antidepressants (Encinas et al., 2006) (15). Therefore, nestin seems an ideal marker to examine NPCs and their response to antidepressants in the adult brain. However, nestin re-expression in reactive astrocytes is induced by cerebral ischemia (Duggal et al., 1997), traumatic brain injury (Sahin et al., 1999), de-afferentation (Brook et al., 1999), and neurotoxicity (Yoo et al., 2005), but reactive astrocytes can be distinguished by morphology and because they express both nestin and GFAP (Yoo et al., 2005). Also a marker of neovascularization, nestin labels capillaries and newly formed vessels after ischemia and is not expressed in absence of vascular response (Mokry et al., 2008; Salehi et al., 2008).
Statistical Analysis
Regression analysis tested correlations between cell numbers and continuous variables. Age was used as covariate in a MANCOVA analysis to assess the effect of group on cell numbers. We used ANOVA with Tukey post-hoc test for between-group (MD*ADT*BZD, MD*ADT, untreated MDs and non-psychiatric controls) comparisons of continuous dependent variables. Mitotic cell number was compared using a non-parametric test (Mann-Whitney). For the comparison of qualitative variables, a Chi2-test was employed. We set p<.05 for significance level. All statistics were analyzed using SPSS (18.0.3; Apache Software Foundation) and data were expressed as mean ± SEM.
RESULTS
Between-Groups Comparisons for DG Granule Neurons, NPCs and Mitotic Cells
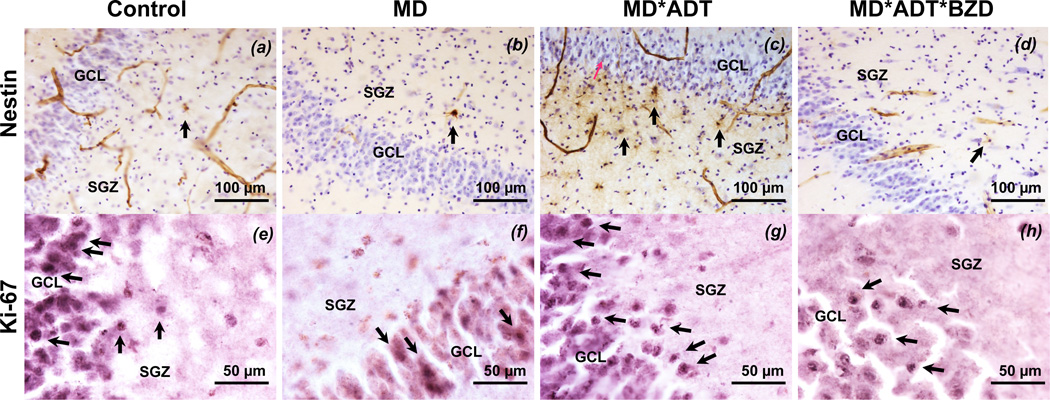
There was a group effect on mature granule neuron, NPC, and mitotic cell numbers in anterior and mid DG, using a MANCOVA with group as the independent variable and age as covariate. No group differences were found in posterior DG for any cell type. For representative images of hippocampal tissue from each group showing NPCs and mitotic cells, see Figure 1.
Figure 1. Nestin-immunoreactive (IR) neural progenitor cells (NPCs) and capillaries, and Ki-67-IR mitotic cells in the dentate gyrus (DG) from representative subjects from each group.
(a, e) Control subject without psychopathology or treatment (control); (b, f) untreated subject with mood disorder (MD); (c, g) subject with mood disorder treated with antidepressants only (MD*ADT); and (d, h) subject with mood disorder treated with antidepressants and benzodiazepines (MD*ADT*BZD), average age is 48 years, two males and two females. The subgranular zone (SGZ) and granule cell layer (GCL) are indicated. (a–d) Nestin-immunoreactive (IR) cells and capillaries appear in brown (diaminobenzidine). Cells are stained for Nissl using Cresyl Violet. (e–h) Ki-67-IR cells appear in black (nickel-diaminobenzidine). Cell cytoplasm is stained with eosin. The control (a, e) and MD*ADT (c, g) show more nestin-IR and cells (arrows) and capillaries (in brown) and more Ki-67-IR cells (arrows) compared with the untreated MD (b, f). The MD*ADT*BZD (d, h) shows as few nestin-IR and cells (arrows) and capillaries (in brown) as the control (b, f) but more Ki-67-IR cells (arrows) compared with the untreated MD (b, f). In the untreated MD (f), Ki-67-IR cells do not show the classical SGZ localization, thus most probably they are not replicating NPCs.
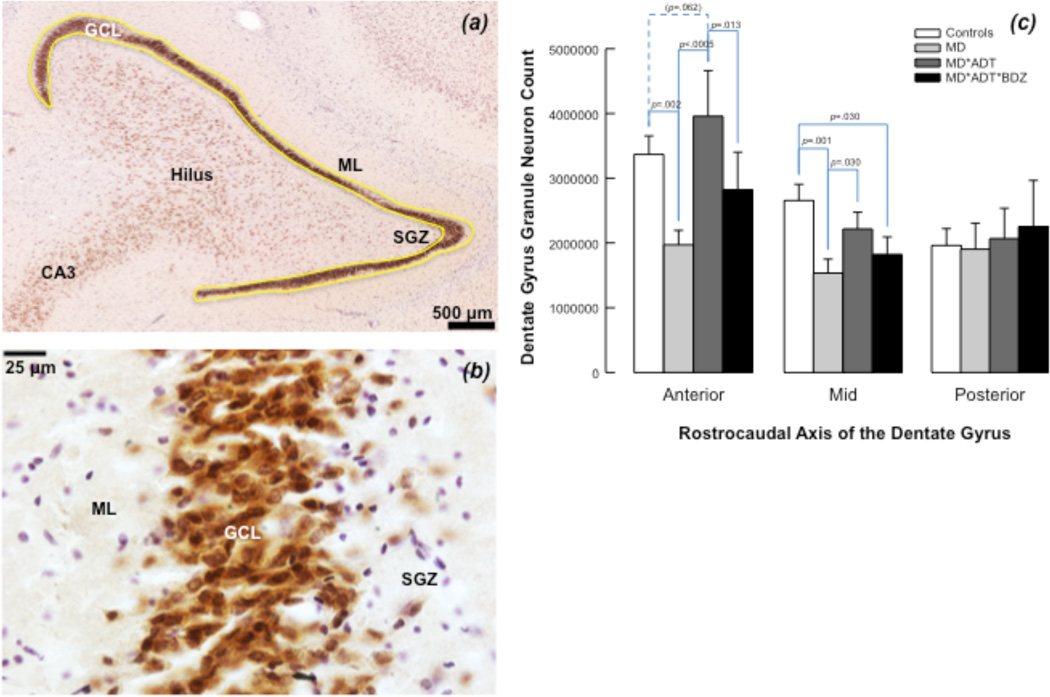
Mature granule neuron (NeuN-immunoreactive) number differed between groups in the anterior (F=6.846; df=3; p=.001) and mid (F=4.475; df=3,40; p=.008) DG. MD*ADT*BZD had fewer granule neurons compared with MD*ADT in anterior DG, and with controls in mid DG; MD*ADT had more granule neurons than untreated-MD in anterior and mid DG, and comparable granule neuron number to controls in all DG regions. Untreated-MD had fewer granule neurons than controls in anterior and mid DG, but did not differ from any other group in posterior DG; no between-groups differences in granule neuron number were found in posterior DG (Figure 2).
Figure 2. Mature granule neurons in the human hippocampus.
(a) Neuronal nuclear antigen (NeuN)-immunoreactive neurons (in brown) are found in the granule cell layer (GCL), hilus, and cornu ammonis (CA) regions. The molecular layer (ML) and subgranular zone (SGZ) of the dentate gyrus are indicated. The yellow outline defines the region of interest for cell counting with stereology. (b) NeuN-positive granule cells are packed within the GCL. Non-neuronal cells are stained for Nissl with Cresyl violet. (c) NeuN-positive granule neuron number in the dentate gyrus (DG). Antidepressant-treated subjects with mood disorders (MD*ADT) had more granule neurons compared with untreated subjects (MD) and subjects treated with antidepressants and benzodiazepines (MD*ADT*BZD) in the anterior DG and more granule neurons than untreated MD in mid DG. Granule neuron number in MD*ADT*BZD did not differ from untreated MD in any DG subregion. Untreated MD subjects had fewer granule neurons than subjects with no psychopathology or treatment (Controls) in anterior and mid DG.
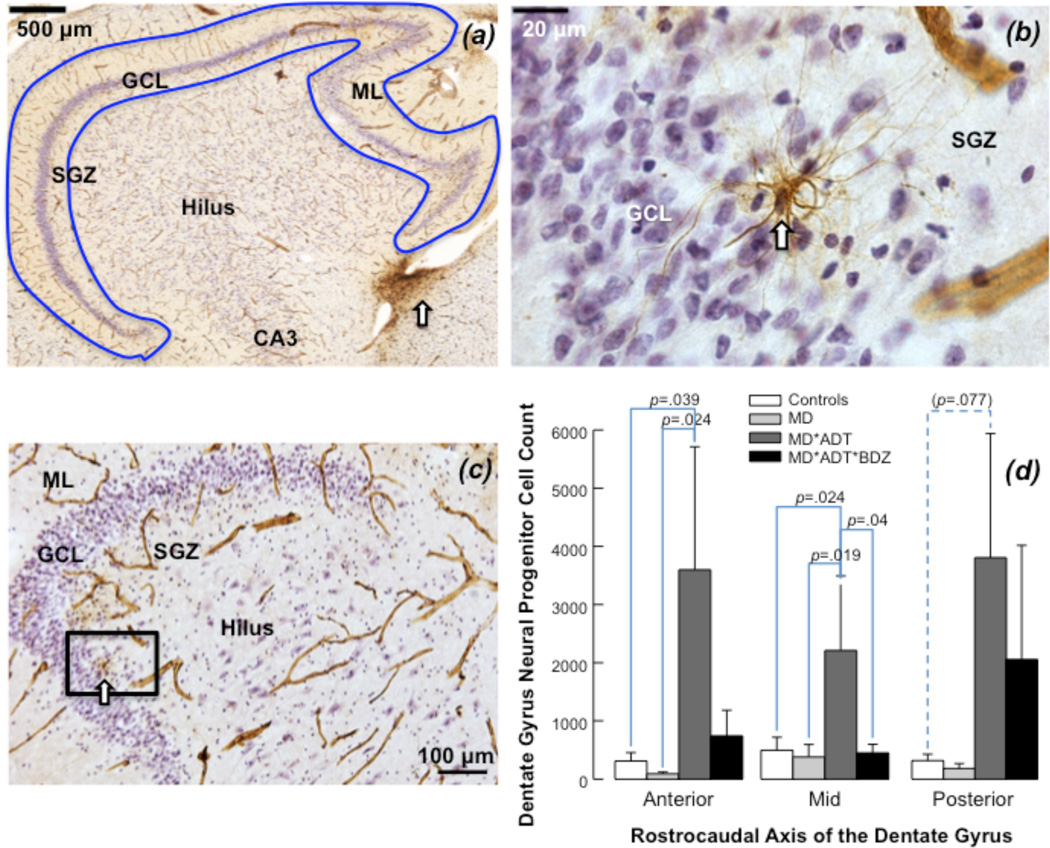
NPC (nestin-immunoreactive) number differed between groups in anterior (F=4.949; df=3; p=.014) and mid DG (F=12.965; df=3; p<.001), but not in posterior DG. MD*ADT*BZD had fewer NPCs compared with MD*ADT in mid DG. MD*ADT had more NPCs compared with controls and untreated MD in anterior and mid DG. Post-hoc test for multiple comparisons did not show significance differences in NPC number between untreated-MD (U-MD) and controls (C) in anterior (U-MD= 84±24; C= 583±180), mid (U-MD= 323±196; C= 649±221) or posterior (U-MD= 171±65; C= 1253±60) DG. No between-group differences in NPC number were found in posterior DG (Figure 3). The nestin-immunoreactive cells counted in the present study were found in the SGZ of the human DG and displayed the morphology of amplifying (type 2) NPCs, as previously shown (Boldrini et al., 2012). Nestin-immunoreactive cells did not have vertical processes crossing the granule cell layer and ending in elaborate arbors in the molecular layer, a characteristic of quiescent (type 1) NPCs that double label with nestin and GFAP (Figure 4). Nestin is seen also in capillaries (Figures 3, 4), as previously shown (Boldrini et al., 2012).
Figure 3. Neural progenitor cells in the human hippocampus.
(a) Nestin-immunoreactive neural progenitor cells (NPCs) and capillaries (in brown) were primarily found within the subgranular zone (SGZ) and in the subventricular zone (white arrow). Granule cells in the granule cell layer (GCL) and glia were stained for Nissl using Cresyl Violet. Capillaries are visible also in the hilus and Cornu Ammonis (CA) regions. The molecular layer (ML) is indicated. The blue outline defines the region of interest for cell counting with stereology. (b) NPCs are seen in the SGZ (white arrow). Capillaries are visible throughout the section. (c) Multipolar Nestin-immunoreactive NPC (enlargement of rectangle in b) shows dendrite processes extending into the GCL and touching the capillary located in the SGZ. Nestin-positive blood vessels display their characteristic appearance. (d) Nestin-immunoreactive NPC number in the dentate gyrus (DG). Antidepressant-treated subjects with mood disorders (MD*ADT) had more NPCs in the anterior and mid DG compared with untreated MD subjects (MD) and subjects with no psychopathology or treatment (Controls); subjects treated with antidepressants and benzodiazepines (MD*ADT*BZD) had fewer NPCs in mid DG compared with MD*ADT and similar number of NPCs as untreated MD in every DG subregion. Untreated MD did not show fewer NPCs than Controls in any DG subregion.
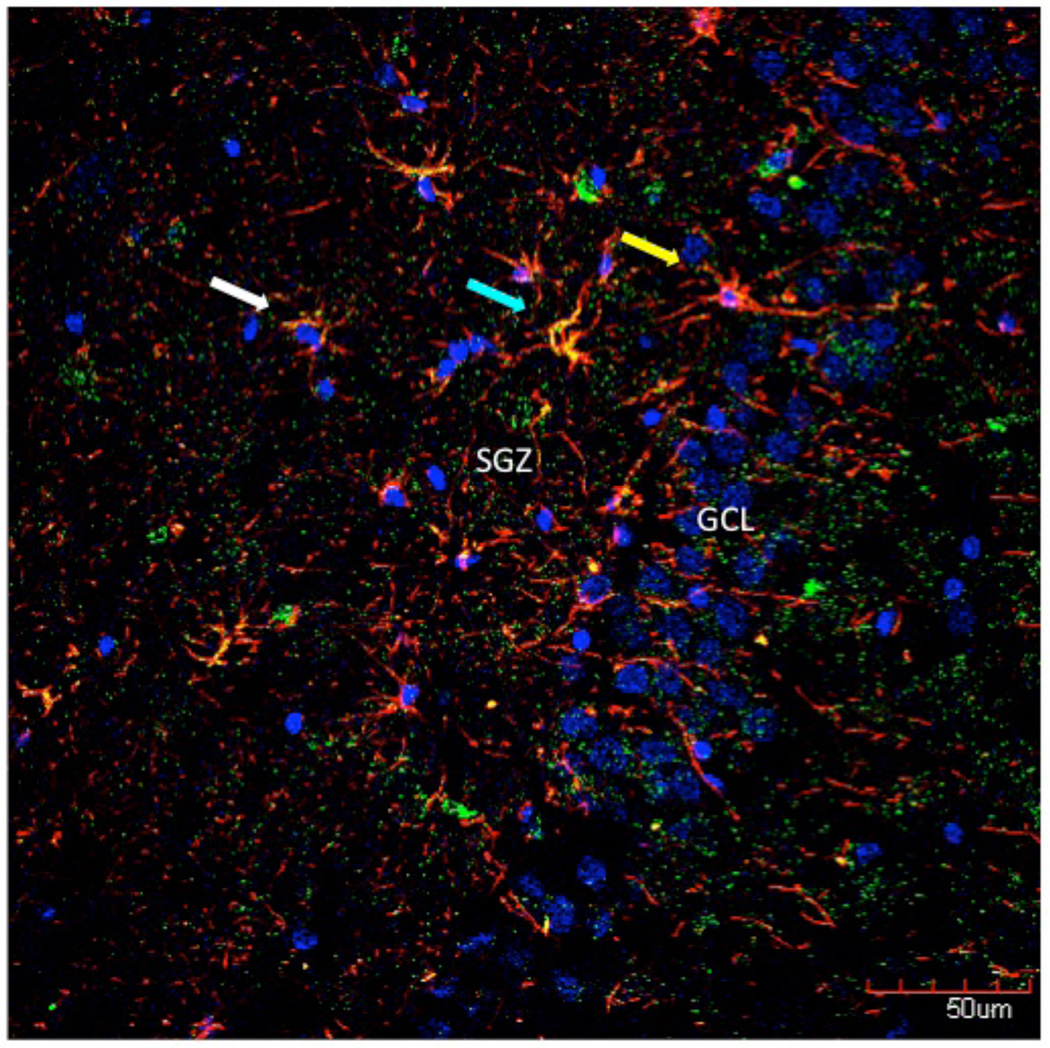
Figure 4. Stem cells and neural progenitor cells in the human dentate gyrus.
Stem cells, labeled with antibody against glial fibrillary acid protein (GFAP, in red) were seen in the human dentate gyrus (yellow arrow). They show the typical apical dendrite crossing the granule cell layer (GCL) typical of stem cells, or type 1 progenitors. Neural progenitor cells, or type 2 progenitors, double labeled with nestin (green) and GFAP (in orange, cyan arrow) and were found in the subgranular zone (SGZ); they are the cells that we find proliferating in the human brain in adulthood. GFAP labeling (in red) was also found in astrocytes (white arrow), as expected. Cell nuclei were stained with DAPI (in blue).
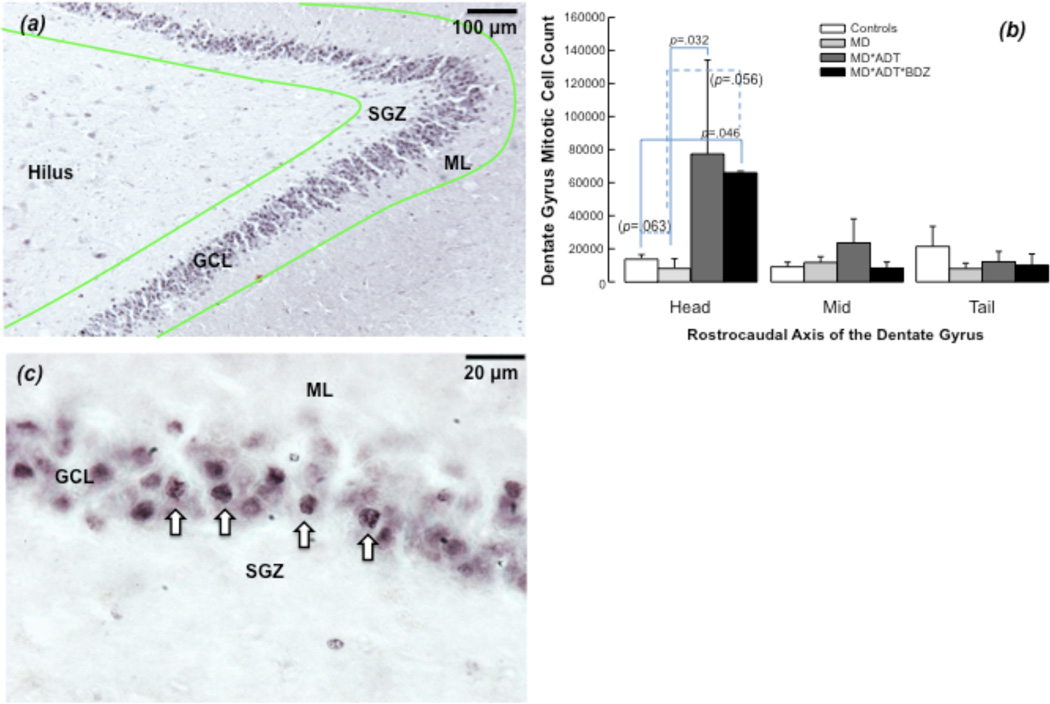
Mitotic cell (Ki-67-immunoreactive) number differed between groups only in anterior DG (F=32.31; df=3; p=.003). MD*ADT*BZD had more mitotic cells compared with controls and comparable numbers to MD*ADT in anterior DG; MD*ADT had more mitotic cells than untreated MD in anterior DG; untreated-MD showed a trend for fewer mitotic cells compared with controls in anterior DG. No between-group differences in mitotic cell number were found in mid or posterior DG (Figure 5).
Figure 5. Mitotic cells in the human hippocampus.
(a) Mitotic cells visualization with monoclonal antibody against the nuclear proliferation antigen Ki-67. This antigen is present in the nuclei of cells in the G1, S, and G2 phases of the cell division cycle as well as in mitosis, while quiescent or resting cells in the G0 phase do not express Ki-67 (Scholzen and Gerdes, 2000). The green outline defines the region of interest for cell counting with stereology. The granule cell layer (GCL), subgranular zone (SGZ), molecular layer (ML) and hilus are indicated. (b) Ki-67 immunoreactive cells with stained nuclei (in black) are found in the inner portion of the GCL of the dentate gyrus and in the SGZ. (c) Ki-67-immunoreactive mitotic cell number in the dentate gyrus (DG). Non-parametric statistical analysis shows that antidepressant-treated subjects with mood disorders (MD*ADT) had more mitotic cells compared with untreated subjects (MD) in the anterior DG. Subjects treated with antidepressants and benzodiazepines (MD*ADT*BZD) had more mitotic cells compared with subjects with no psychopathology or treatment (Controls) in the anterior DG. Mitotic cell number in MD*ADT*BZD did not differ from untreated MD*ADT. Untreated MD showed a trend to fewer mitotic cells than Controls in anterior DG. No between-groups differences in mitotic cell number were found in mid and posterior DG.
Clinical Comparison of Depression Groups
Age at first major depressive episode was earlier in MD*ADT compared with MD*ADT*BZD (p=.002) and untreated-MD (p=.029). Number of major depressive episodes did not differ between groups. Global Assessment Scale (GAS) score was worse in MD*ADT*BZD compared with untreated-MD (p=.030), but did not differ between MD*ADT*BZD and MD*ADT.
Potential Confounding Variables in Subject Groups
There was no group differences in brain pH (p=.752), brain weight (p=.757), or body mass index (BMI, p=.440). There was no relationship between cell numbers and PMI, brain pH, brain weight, or BMI. Males and females were equally distributed in the different groups (see methods). There was no sex difference in number of NPC, mitotic cells, and granule neurons in any group. Age differed between groups and was used as a covariate in the statistical analysis to test between-group differences (see methods).
DISCUSSION
This first human brain study of the effect of augmenting antidepressant treatment with adjunctive BZDs on neurogenesis in the dentate gyrus in mood disorders finds evidence consistent with mouse studies showing BZDs inhibit the antidepressant enhancement of neurogenesis. MD treated with BZDs and antidepressants had fewer NPCs and mature granule neurons in DG than MD treated with antidepressant alone. Both MD groups had comparable mitotic cell number.
Mature Granule Neurons
In the anterior DG, MD co-treated with antidepressants and BZDs had granule neuron number comparable to untreated-MD and fewer granule neurons than MD treated with antidepressants. Long-term BZD use has been linked to sustained cognitive and psychomotor impairment (Lader, 2011; Dell'osso and Lader, 2013). Our results suggest longer-term impairment may be related to fewer mature granule neurons in subjects co-treated with BZD.
Untreated MD had fewer granule neurons compared with non-psychiatric controls in anterior and mid DG. We had previously reported fewer granule neurons in anterior and mid DG in untreated major depressive disorder, in a partially overlapping, smaller sample (Boldrini et al., 2013). This study includes subjects with bipolar disorder. The percentage of bipolar and major depressive disorder (MDD) subjects did not differ between treatment groups. Our subsample sizes were too small to determine whether MD subtype influences cell numbers.
MD treated with antidepressants alone had more mature granule neurons in anterior and mid DG compared with untreated MD. In a previous study (Boldrini et al., 2013), we reported that SSRI-treated subjects with MDD had more granule neurons than untreated MDD subjects only in mid DG. Our previous study sample differed from this sample because it did not exclude from the SSRI-treated MDD group subjects that had received BZD treatment. The present study also included in the antidepressant-treated group subjects treated with tricyclic antidepressants and subjects treated with lithium, but without BDZ treatment. The number of subjects investigated was insufficient to allow analysis of potentially different effects of SSRIs, tricyclics, and lithium. The absence of subjects with BZD treatment likely strengthened the results in terms of detecting the overall effect of antidepressants.
Neural Progenitor Cells
Based on morphology and antigenicity, the nestin-immunoreactive cells we counted are amplifying NPCs. MD co-treated with antidepressants and BZD did not differ from untreated-MD in NPC number and had fewer NPCs in mid DG compared with MDs treated with antidepressants alone. This picture is consistent with BZDs inhibiting early stage neuronal development. Another possibility is that subjects treated with BZD and antidepressants may have had more severe depression such as higher levels of anxiety compared to subjects treated with antidepressants alone. Such clinical characteristics may be a consequence or a cause of diminished NPC and mature neuron number. However, age of onset of major depression, number of episodes of depression, and GAS score, do not suggest that the BZD-antidepressant co-treated MD group was more severely ill than those treated with antidepressants only. Therefore severity of illness is unlikely to explain the findings.
Untreated MD did not have fewer NPCs compared with non-psychiatric controls in anterior and mid DG. This is in agreement with our findings in smaller samples of untreated major depression (Boldrini et al., 2009; Boldrini et al., 2012). Ablation of neurogenesis in mice precipitates behavioral despair and anhedonia without exposing mice to any behavioral stress (Snyder et al., 2011). Of note, we found the mean NPC number in anterior DG of MD was one seventh that of controls, but the difference was not statistically significant due to the low number of NPCs in both groups and the variance in counts. Alternatively, since stress does induce a form of depression in mice (Santarelli et al., 2003; Surget et al., 2008), an NPC deficit in mood disorders may be present only in a phenotype that involves stress exposure.
Antidepressant-alone treated MD had higher NPC number in the mid-DG compared with controls and untreated MD. Our results are consistent with rodent (Malberg et al., 2000), non-human primate (Perera et al., 2011), and our previous human studies (Boldrini et al., 2009; Boldrini et al., 2012), which suggest that antidepressants increase amplifying or type 2 NPCs (Encinas et al., 2006).
Mitotic Cells
MD subjects co-treated with antidepressants and BZD did not differ from antidepressants-alone treated MD in mitotic cell number, and both treatment groups had more mitotic cells compared with untreated MD and controls in anterior DG. Thus. We do not observe an effect of BZD co-treatment on mitotic cells stained with Ki-67, but note that these cells include glial and neuronal lineages. Moreover, the effects of BZDs on mitotic cells in rodents are complex, depending on duration of exposure. Short-term exposure increases proliferation, whereas long-term exposure reduces overall neurogenesis (Zhao et al., 2012).
Consistent with our previous findings (Boldrini et al., 2009), in this larger sample, although the mean number of mitotic cells in untreated MD subjects is about a half that in controls, the difference did not reach statistical significance.
Effects of GABA-Mediated Signaling on Hippocampal Neurogenesis
A BZD effect on neurogenesis may be mediated by their allosteric effect on the GABA receptor. Type-2 progenitors in adult hippocampus express GABAA receptors that can be activated by synaptic stimuli and stimulate differentiation (Deisseroth and Malenka, 2005; Wang et al., 2005). Antiepileptic drugs, such as the barbiturate phenobarbital, which also act on the GABAergic system by binding to GABAA receptors (Rudolph et al., 1999), decrease neurogenesis by about 60% in the DG (Chen et al., 2009) and have cognitive effects that persist after drug cessation (Chen et al., 2009). Alcohol, which, like BZDs, modulates GABAA receptors to induce sedation (Laukkanen et al., 2013), decreases DG neurogenesis by up to 50% in adult rats after long-term exposure (Jang et al., 2002; Nixon and Crews, 2002; Rice et al., 2004). The reduction in hippocampal neurogenesis and the depression-like responses induced by chronic alcohol self-administration in mice are reversible by fluoxetine (Stevenson et al., 2009). Whether treatment with BDZ alone would be associated with an apparent detrimental effect on hippocampal neurogenesis or granule cell number relative to untreated depression should be investigated in future studies.
Increased GABA activation via GABAA receptor agonists, such as BZD, appears to cause neuronal apoptosis in the developing brain of rodents (Chen et al., 2009). We found that mature granule neuron were fewer in subjects co-treated with BZD compared with subjects treated with antidepressant alone, and in BZD co-treated MD were comparable to untreated MD. Based on rodent studies, our findings may be partly the result of increased apoptosis.
Clinical Relevance of Hippocampal Neuroplasticity
We found that cell number changes associated with MD and with treatment occur selectively in the anterior and mid DG and are not detected in posterior DG. The posterior primate hippocampus, and its homologue in the rodent, the dorsal hippocampus, are mainly implicated in memory (Risold and Swanson, 1996; Bannerman et al., 2004), whereas the anterior hippocampus in primates projects to prefrontal cortex and amygdala (Thierry et al., 2000), and participates in emotional regulation (Nettles et al., 2000; Sahay and Hen, 2007) and pattern discrimination (Clelland et al., 2009; Aimone et al., 2011). Our results are consistent with a functional relationship between anterior and mid DG NPCs and granule neuron number and indicate that mood disorders or treatment effects may impact emotional responses and pattern discrimination via the anterior hippocampus.
We found fewer mature DG neurons correlated with worse global clinical functioning, earlier onset of major depressive disorder, and more lifetime major depressive episodes, indicating the potential clinical relevance of DG cell viability in major depression (Boldrini et al., 2013). Adult neurogenesis, like developmental neurogenesis, in non-human primates is about five times less than in mice (Kohler et al., 2011). Human levels of neurogenesis decline modestly during aging (Spalding et al., 2013), but our MD treated and untreated groups did not differ in age, thus age does not explain our findings.
The proportion of subjects who died by suicide was not different between the MD groups, and so suicide as a cause of death did not explain our treatment group findings. Of note, clinician-rated severity of depression is weakly related to risk for suicidal behavior (Mann et al., 1999) and therefore, suicide is not considered a useful measure of depression severity. The pathophysiology of suicide, as opposed to depression, is related to the diathesis for suicidal behavior (Mann et al., 1999). What is puzzling is why patients with robust increase in neurogenesis still die by suicide? Perhaps, neurogenesis alone is insufficient protection from suicide, and other mechanisms and pathways lead to suicide. Alternatively, an intermediate level of improvement in depression and neurogenesis, while suicidal ideation persists, may provide an increase in drive and effectiveness to overcome the helplessness and inertia of depression and act on suicidal thoughts.
Study Limitations
The sample included major depressive disorder and bipolar disorder. It is not known whether the two disorders differ in hippocampal DG morphometry. The mood disorder-treated group included subjects treated with lithium, SSRIs, and tricyclic antidepressants. Larger sample sizes would allow for testing the effect of BZD co-treatment with different classes of antidepressants and lithium. Subjects co-treated with BZD may be anxious compared with MD not prescribed BZD. The relationship between anxiety and neurogenesis remains unclear, and excessive anxiety might inhibit neurogenesis instead of the BZD used to treat it and thereby could explain our results.
Animal studies and an expanded human study may begin to further address these questions which are of critical importance to doctors and patients trying to decide whether to use a benzodiazepine for treatment of anxiety symptoms in major depression.
ACKNOWLEDGEMENTS
Supported by MH83862, MH94888, MH64168, MH40210, the American Foundation for Suicide Prevention and the Diane Goldberg Foundation.
Dr. Andrew J. Dwork received loans and gifts of equipment and software from Olympus and Visiopharm for research unrelated to this study. Dr. René Hen receives compensation as a consultant for Roche and Lundbeck. Dr. J. John Mann received past unrelated grants from GlaxoSmithKline and Novartis.
Footnotes
STATEMENT OF INTEREST
Maura Boldrini, Tanya H Butt, Adrienne N. Santiago, Andrew Dwork, Gorazd Rosoklija, Hadassah Tamir, and Victoria Arango declare that, except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional services and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
REFERENCES
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders - first revision. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2008;9:248–312. doi: 10.1080/15622970802465807. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neuroscience and biobehavioral reviews. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John MJ, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Hen R, Underwood M, Rosoklija G, Dwork A, Mann JVA. Hippocampal Angiogenesis and Progenitor Cell Proliferation Are Increased with Antidepressant Use in Major Depression. Biological psychiatry. 2012;75:562–571. doi: 10.1016/j.biopsych.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, Arango V, John Mann J. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook GA, Perez-Bouza A, Noth J, Nacimiento W. Astrocytes re-express nestin in deafferented target territories of the adult rat hippocampus. Neuroreport. 1999;10:1007–1011. doi: 10.1097/00001756-199904060-00021. [DOI] [PubMed] [Google Scholar]
- Chen J, Cai F, Cao J, Zhang X, Li S. Long-term antiepileptic drug administration during early life inhibits hippocampal neurogenesis in the developing brain. Journal of neuroscience research. 2009;87:2898–2907. doi: 10.1002/jnr.22125. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Malenka RC. GABA excitation in the adult brain: a mechanism for excitation-neurogenesis coupling. Neuron. 2005;47:775–777. doi: 10.1016/j.neuron.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Dell'osso B, Lader M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. European psychiatry : the journal of the Association of European Psychiatrists. 2013;28:7–20. doi: 10.1016/j.eurpsy.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Duggal N, Schmidt-Kastner R, Hakim AM. Nestin expression in reactive astrocytes following focal cerebral ischemia in rats. Brain research. 1997;768:1–9. doi: 10.1016/s0006-8993(97)00588-x. [DOI] [PubMed] [Google Scholar]
- Edwards JG, Anderson I. Systematic review and guide to selection of selective serotonin reuptake inhibitors. Drugs. 1999;57:507–533. doi: 10.2165/00003495-199957040-00005. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Satou T, Nishida S, Hashimoto S, Ito H. Cultured rat astrocytes give rise to neural stem cells. Neurochem Res. 2006;31:1381–1387. doi: 10.1007/s11064-006-9186-8. [DOI] [PubMed] [Google Scholar]
- Jang MH, Shin MC, Kim EH, Kim CJ. Acute alcohol intoxication decreases cell proliferation and nitric oxide synthase expression in dentate gyrus of rats. Toxicol Lett. 2002;133:255–262. doi: 10.1016/s0378-4274(02)00129-7. [DOI] [PubMed] [Google Scholar]
- Kalman M, Ajtai BM. A comparison of intermediate filament markers for presumptive astroglia in the developing rat neocortex: immunostaining against nestin reveals more detail, than GFAP or vimentin. Int J Dev Neurosci. 2001;19:101–108. doi: 10.1016/s0736-5748(00)00058-7. [DOI] [PubMed] [Google Scholar]
- Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: a comparison with clinician ante-mortem diagnosis. Acta Psychiatr Scand. 1996;94:337–343. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kronenberg G. Depressed new neurons--adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biological psychiatry. 2003;54:499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- Klein DN, Arnow BA, Barkin JL, Dowling F, Kocsis JH, Leon AC, Manber R, Rothbaum BO, Trivedi MH, Wisniewski SR. Early adversity in chronic depression: clinical correlates and response to pharmacotherapy. Depress Anxiety. 2009;26:701–710. doi: 10.1002/da.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler SJ, Williams NI, Stanton GB, Cameron JL, Greenough WT. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10326–10331. doi: 10.1073/pnas.1017099108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lader M. Benzodiazepines revisited--will we ever learn? Addiction. 2011;106:2086–2109. doi: 10.1111/j.1360-0443.2011.03563.x. [DOI] [PubMed] [Google Scholar]
- Laukkanen V, Storvik M, Hakkinen M, Akamine Y, Tupala E, Virkkunen M, Tiihonen J. Decreased GABA(A) benzodiazepine binding site densities in postmortem brains of Cloninger type 1 and 2 alcoholics. Alcohol. 2013 doi: 10.1016/j.alcohol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Lobbestael J, Leurgans M, Arntz A. Inter-Rater Reliability of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) and Axis II Disorders (SCID II) Clin Psychol Psychot. 2011;18:75–79. doi: 10.1002/cpp.693. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. American Journal of Psychiatry. 1999;156:181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- Mokry J, Ehrmann J, Karbanova J, Cizkova D, Soukup T, Suchanek J, Filip S, Kolar Z. Expression of intermediate filament nestin in blood vessels of neural and non-neural tissues. Acta Medica (Hradec Kralove) 2008;51:173–179. doi: 10.14712/18059694.2017.20. [DOI] [PubMed] [Google Scholar]
- Nettles KW, Pesold C, Goldman MB. Influence of the ventral hippocampal formation on plasma vasopressin, hypothalamic-pituitary-adrenal axis, and behavioral responses to novel acoustic stress. Brain research. 2000;858:181–190. doi: 10.1016/s0006-8993(99)02281-7. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N, Lange C, Higley JD, Rosoklija G, Hen R, Sackeim HA, Coplan JD. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One. 2011;6:e17600. doi: 10.1371/journal.pone.0017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AC, Bullock MR, Shelton KL. Chronic ethanol consumption transiently reduces adult neural progenitor cell proliferation. Brain Res. 2004;1011:94–98. doi: 10.1016/j.brainres.2004.01.091. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Structural evidence for functional domains in the rat hippocampus. Science. 1996;272:1484–1486. doi: 10.1126/science.272.5267.1484. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy J-M, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin KS, Mahmood A, Li Y, Yavuz E, Chopp M. Expression of nestin after traumatic brain injury in rat brain. Brain research. 1999;840:153–157. doi: 10.1016/s0006-8993(99)01757-6. [DOI] [PubMed] [Google Scholar]
- Salehi F, Kovacs K, Cusimano MD, Horvath E, Bell CD, Rotondo F, Scheithauer BW. Immunohistochemical expression of nestin in adenohypophysial vessels during development of pituitary infarction. J Neurosurg. 2008;108:118–123. doi: 10.3171/JNS/2008/108/01/0118. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of Hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Saravia F, Beauquis J, Pietranera L, De Nicola AF. Neuroprotective effects of estradiol in hippocampal neurons and glia of middle age mice. Psychoneuroendocrinology. 2007;32:480–492. doi: 10.1016/j.psyneuen.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Molecular psychiatry. 2010;15:1152–1163. doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. Journal of cellular physiology. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. American Journal of Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JR, Schroeder JP, Nixon K, Besheer J, Crews FT, Hodge CW. Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1209–1222. doi: 10.1038/npp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Halabisky B, Zhou Y, Palop JJ, Yu G, Mucke L, Gan L. Imbalance between GABAergic and Glutamatergic Transmission Impairs Adult Neurogenesis in an Animal Model of Alzheimer's Disease. Cell Stem Cell. 2009;5:624–633. doi: 10.1016/j.stem.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Evans J, Russell B, Kydd R, Connor B. A benzodiazepine impairs the neurogenic and behavioural effects of fluoxetine in a rodent model of chronic stress. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biological psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Degenetais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tronel S, Belnoue L, Grosjean N, Revest JM, Piazza PV, Koehl M, Abrous DN. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2012;22:292–298. doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- Wang LP, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Molecular and cellular neurosciences. 2005;29:181–189. doi: 10.1016/j.mcn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Wu CS, Wang SC, Chang IS, Lin KM. The association between dementia and long-term use of benzodiazepine in the elderly: nested case-control study using claims data. Am J Geriatr Psychiatry. 2009;17:614–620. doi: 10.1097/JGP.0b013e3181a65210. [DOI] [PubMed] [Google Scholar]
- Wu X, Castren E. Co-treatment with diazepam prevents the effects of fluoxetine on the proliferation and survival of hippocampal dentate granule cells. Biol Psychiatry. 2009;66:5–8. doi: 10.1016/j.biopsych.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport. 2000;11:1991–1996. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
- Yoo YM, Lee U, Kim YJ. Apoptosis and nestin expression in the cortex and cultured astrocytes following 6-OHDA administration. Neurosci Lett. 2005;382:88–92. doi: 10.1016/j.neulet.2005.02.070. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang Z, Dai J, Chen L, Huang Y, Zhan Z. Beneficial effects of benzodiazepine diazepam on chronic stress-induced impairment of hippocampal structural plasticity and depression-like behavior in mice. Behavioural brain research. 2012;228:339–350. doi: 10.1016/j.bbr.2011.12.013. [DOI] [PubMed] [Google Scholar]