Abstract
This study demonstrates for the first time the ability to coat solid dispersions on microneedles as a means to deliver water-insoluble drugs through the skin. Polyethylene glycol (PEG) was selected as the hydrophilic matrix, and lidocaine base was selected as the model hydrophobic drug to create the solid dispersion. First, thermal characterization and viscosity measurements of the PEG-lidocaine mixture at different mass fractions were performed. The results show that lidocaine can remain stable at temperatures up to ~130 °C, and that viscosity of the PEG-lidocaine molten solution increases as the mass fraction of lidocaine decreases. Differential scanning calorimetry demonstrated that at lidocaine mass fraction less than or equal to 50%, lidocaine is well dispersed in the PEG-lidocaine mixture. Uniform coatings were obtained on microneedle surfaces. In vitro dissolution studies in porcine skin showed that microneedles coated with PEG-lidocaine dispersions resulted in significantly higher delivery of lidocaine in just 3 min compared to 1 h topical application of 0.15 g EMLA®, a commercial lidocaine-prilocaine cream. In conclusion, the molten coating process we introduce here offers a practical approach to coat water-insoluble drugs on microneedles for transdermal delivery.
Keywords: coating, dissolution, solid dispersion, solubility, transdermal drug delivery
1. INTRODUCTION
Coated microneedles have evolved in to a flexible platform for painless drug delivery. 1–3 A large variety of water-soluble or water-compatible compounds ranging from small molecules such as lidocaine hydrochloride and vitamin B to macromolecules such as peptides, proteins, oligonucleotides, and DNA to particles such as influenza virus and polymeric particles have been coated on microneedles. 4–11 Typically, microneedle surfaces are coated by dipping 4, 12 or spraying 13 them with an aqueous solution containing the drug and excipients. Water-insoluble drugs, which constitute about 40% of the top 200 drugs in the US, Great Britain, Spain and Japan 14 have not been incorporated into the scope of coated microneedles. A major obstacle that must be overcome to successfully design coated microneedles, which are practical for clinical use and can provide therapeutic doses of water-insoluble drugs is that the coating should dissolve rapidly, and the water-insoluble drug should exhibit enhanced aqueous solubility and bioavailability. Given that water-insoluble drugs cannot dissolve as promptly in the interstitial fluid of tissues as water-soluble drugs, the development of coated microneedle systems for delivery of water-insoluble drugs is challenging.
Poor dissolution of water-insoluble drugs in an aqueous environment is a common problem in the field of drug delivery and formulation, and different approaches are employed to address this issue. 15 Solid dispersion is one such technique, wherein the hydrophobic drug is homogeneously distributed at or near molecular level in a hydrophilic carrier matrix. In this arrangement the molecules of the hydrophilic carrier matrix can prevent the drug from acquiring a crystalline state, forcing it to adapt an amorphous or partially amorphous state. 16, 17 Since an amorphous phase exhibits better dissolution than its crystalline counterpart, a solid dispersion can exhibit high drug solubility. A solid dispersions’ improved aqueous drug dissolvability may also be attributed to other phenomena 17: (i) enhanced dissolution arising from high surface area created by fine drug crystals or even drug molecules released upon rapid dissolution of the carrier matrix in water, (ii) formation of a supersaturated drug solution upon dissolution in water, with the carrier-molecules acting as stabilizers preventing re-aggregation of the drug molecules, and, (iii) co-solvent effect of the carrier molecules, which can increase aqueous drug solubility.
Solid dispersions are prepared either by creating a molten mixture of the drug and carrier, which is then cooled, or by dissolving the drug and carrier in a suitable co-solvent and evaporating it. 18, 19 The solvent-based approach suffers from the limitation that since most solvents are toxic, all solvent must be completely removed to ensure biocompatibility and safety. Furthermore, even minute changes in evaporation conditions of the solvent can induce significant changes in the film microstructure and hence its dissolution behavior. 17 One study has indeed demonstrated the feasibility of coating solvent-based dispersions on to microneedles. 12 However, from a coated-microneedle perspective, use of organic solvents to formulate coating solutions can lead to significant manufacturing variability: the gradual evaporation of the typically-volatile organic solvent can steadily increase the concentration of the coating solution, which will over time lead to thicker films being coated on microneedles, 12 resulting in poor control over coated drug amount. On the other hand, a well-mixed molten solution of drug and carrier can potentially overcome these challenges, and can offer better reproducibility and control for creating solid dispersions on microneedles.
To help expand the domain of coated microneedles to include delivery of water-insoluble drugs, this study demonstrates for the first time, coating of solid dispersions onto microneedles using molten coating solutions. We selected lidocaine (base form) as the model lipophilic drug and polyethylene glycol (PEG) as the hydrophilic carrier matrix. Lidocaine (base form), is a local anesthetic, and is a poorly water soluble drug. 20, 21 Multiple approaches including non-ionic surfactants,22 microemulsions, 23 non-ionic surfactant vesicles, 24 and hot-melt extrusion films 25 have been employed to help increase its uptake across the skin and mucosa. Lidocaine can have applications as an anesthetic in venipuncture, and cutaneous and dental procedures. 26, 27 Thus, a microneedle patch coated with lidocaine with accelerated dissolution could have clinical application. We deposited solid dispersion coatings on microneedles, using a customized micro-precision coating device capable of controlling molten solution temperature. To facilitate development of optimized coatings, viscosity and thermal properties of the PEG-lidocaine mixture at different mass fractions were evaluated. Finally, in vitro dissolution and delivery efficiency of microneedles coated with PEG-lidocaine solid dispersions were compared to EMLA® (a commercial lidocaine-prilocaine cream), in a porcine skin model system in vitro. EMLA cream also contains lidocaine in its base form, and thus is a suitable formulation for comparison. It is important to note that lidocaine-base has a pK of about 7.9. 21 Thus, when lidocaine-base reaches the skin where pH is about 7.4, it can be converted to its ionized state, and can exhibit enhanced solubility. However, this phenomenon is expected to occur for both EMLA and solid dispersion coated-microneedles. Overall, this study provides a foundation to guide development of solid dispersion coatings on microneedles for delivery of lidocaine and other water-insoluble drugs.
2. MATERIALS AND METHODS
2.1 Microneedle fabrication and molten coating process
Planar 1D arrays with five microneedles per row were fabricated from 50 µm-thick stainless steel (304) sheets using a wet-etch process. Each microneedle measured 700-µm in length and 200-µm in width. Microneedles were coated using a micro-precision dip coating station developed in-house that comprises of an automated x-y-z linear computer-controlled stage onto which microneedles were mounted. The molten coating solution was housed in an orifice into which the microneedles were dipped. Two rubber heaters (Omega Engineering Inc., Stamford, CT) and a thermal sensor (Omega Engineering Inc., Stamford, CT), both attached to the orifice, were connected to a proportional-integral-derivative (PID) controller (Omega Engineering Inc., Stamford, CT). The PID controller was used to stabilize the temperature of the orifice. PEG (MW=3350, Fisher Scientific, manufacturer: Integra Chemical Company, Kent, WA) and lidocaine (Sigma-Aldrich, MO) were initially mixed in their powder states and then melted together on a hot stage heated to 120 °C. The molten mixture was further mixed by ultrasonication (Model 505, Fisher Scientific, Thermo Fisher Scientific Inc., Waltham, MA) with the power set at 25% with 4×5 s pulses with a 5 s gap. After ultrasonication, the molten mixture of PEG-lidocaine was loaded into the temperature controlled orifice (80 °C) of the coating system, ready for coating onto microneedles.
2.2 Thermal gravimetric analysis (TGA)
TGA was conducted with a Q50 TGA (TA Instrument. Inc, New Castle, DE, USA) under nitrogen atmosphere (flow rate: 10 ml/min). Pure lidocaine, pure PEG, and PEG-lidocaine solid dispersions of 5–10 mg were heated from 30 °C to 300 °C at a rate of 5 °C/min. The residual mass at each temperature was normalized to percentage by dividing with the initial sample mass.
2.3 Viscosity determination using rheometer
Viscosity measurements were performed on a shear rheometer (MCR 301, Anton Paar, USA). A parallel plate, 25 mm in diameter was used with temperature ramped from 70 °C to 100 °C, at 5 °C intervals. A gap of 1 mm was maintained during the course of the measurement. Dynamic frequency sweeps from ω = 0.01 to 100 s−1 were performed on each sample at each temperature to determine the constant viscosity region.
2.4 Thermal characterization using differential scanning calorimeter (DSC)
The miscibility of PEG and lidocaine in the solid dispersion was investigated by means of a differential scanning calorimeter (DSC Q20, TA instrument, New castle, DE). A small drop of PEG-lidocaine molten mixture (9–12 mg) was directly loaded into the aluminum pan after ultrasonication. The DSC was conducted after the sample cooled down to room temperature. An inert atmosphere was set up in the DSC with 50 ml/min of nitrogen gas. The sample was heated at a rate of 10 °C/min from 20 °C to 90 °C.
2.5 Imaging of PEG-lidocaine coatings on microneedles
Coated microneedles were characterized via brightfield microscopy by observing between crossed polarizers in an Olympus BX51 microscope with UMPlanFL 5x and 20x objectives. Images were recorded using a digital camera (Color Mosaic model 11.2, Diagnostic Instruments, Sterling Heights, MI) attached to the microscope. Scanning electron micrographs (SEMs) of coated microneedles were obtained using Hitachi S-4300 E/N (Hitachi High Technologies America, Inc., Dallas, TX).
2.6 Determination of lidocaine content in solid dispersion coatings and transdermal delivery efficiency
The amount of lidocaine in solid dispersions coated on microneedle arrays was obtained by dissolving the coatings and quantifying the lidocaine in the resulting solution with high-performance liquid chromatography (HPLC), based on a previously published method with minor modifications.5 Briefly, a freshly-coated microneedle (n=3 per PEG-lidocaine ratio) was added to 1000 µl 10% acetonitrile (Fisher scientific, Pittsburgh, PA) to dissolve the coating, and the resulting lidocaine concentration (C1 in µg/µl) was determined using a standard calibration curve correlating intensity to 10% acetonitrile solution containing known concentrations of lidocaine. The sample solution was injected into a HPLC system (Beckman Coulter, Brea, CA) equipped with a UV detector. A HPLC column (Discovery® BIO Wide Pore C18 HPLC Column, Sigma-Aldrich, St. Louis, MO) was used for separation. There were two mobile phases: A was 0.1% trifluoroacetic acid (TFA) (Fisher scientific, Pittsburgh, PA) water solution, and B was 100% acetonitrile with 0.1% TFA. Concentration gradients started from 5% B and went to 100% B. The flow rate was 0.2 ml/min, the gradient run time was 21 min while the total run time was 24 min, and the UV detector wavelength was 230 nm. The peak area and concentration of lidocaine (µg/ml) correlated linearly (R2=0.998).
To determine efficiency of delivery of solid dispersion coatings in skin, another set (n=5) of coated microneedle arrays (PEG:lidocaine :: 50:50) was inserted into porcine skin (Innovative research Inc., Novi, MI). Porcine skin from dorsal surface was received and stored at −80 °C until use. Prior to use, porcine skin was thawed, the underlying fat was removed to obtain skin tissue with total thickness of about 5 mm. Skin was first equilibrated by placing skin in a vertical Franz diffusion cell filled with saline in donor and acceptor chambers, and maintained at 37 °C for 1h. Next skin integrity was verified by measuring electrical impedance across donor and acceptor chambers using an LCR meter (Model LCR200, Extech Instruments, Nashua, NH). Only skin pieces with resistivity higher than 14 kohm.cm2 measured at 100 Hz were used for further evaluation. Skin pieces were removed from the Franz cell, gently blotted with a paper towel to remove excess solution from the top and bottom surfaces, and lidocaine-coated microneedles were inserted for 3 min and removed. The 3-min insertion time was selected based on a preliminary experiment wherein lidocaine-coated microneedles were held in saline solution for 1, 3 or 5 min, and based on microscopic evaluation the coating was found to be largely removed from the microneedles in 3 minutes. After removal of each array, a cotton swab pre-soaked in 10% acetonitrile (by dipping in a tube containing 500 µl 10% acetonitrile) was gently rubbed on the skin to collect lidocaine left on the skin surface. The swab was placed back into the tube containing 500 µl 10% acetonitrile, and the lidocaine concentration was measured (C2 in µg/µl). The amount of lidocaine left attached to the microneedle surface after insertions was similarly obtained by placing used microneedles in 1000 µl 10% acetonitrile to dissolve the residual material (C3 in µg/µl). The concentration of lidocaine was measured using HPLC as described above. The final delivery efficiency was calculated by obtaining the ratio of mass of lidocaine delivered to the lidocaine available on unused microneedles: [(C1*1000)-(C2*500)-(C3*1000)] / (C1*1000).
2.7 In vitro measurement of lidocaine diffusion in porcine skin
Six microneedle arrays freshly coated with PEG:lidocaine :: 50:50 molten dispersion were inserted into 1 cm×1 cm porcine skin for 3 min; then the microneedles were removed from the skin. Porcine skin was treated as described in section 2.6.The six microneedle arrays were inserted one after the other with each insertion taking less than 15 s. Microneedle arrays were removed in the order of insertion to ensure that each array stayed inserted for 3 minutes. Each inserted array was kept under gentle manual pressure to ensure that the next insertion did not affect the previously inserted arrays. The manual pressure was maintained during the entire 3 min duration to keep microneedles inserted in the skin. The skin was subsequently incubated at 37 °C for 1, 4, or 8 h in a humid chamber. The humid chamber was prepared by cutting a cap of an eppendorf tube and placing it with its flat surface upwards in a petridish containing water. A paper towel was placed in the petridish such that it also covered the cap, and the skin piece was placed on top of the moist paper towel (over the cap). The petridish was then covered with a lid. After incubation, the skin piece was embedded into OCT compound (Tissue-Tek, Fisher, Pittsburgh, PA) and frozen at −80 °C. EMLA cream, a commercially available topical formulation based on a eutectic mixture of lidocaine-base and prilocaine-base, served as the positive control. The recommended lidocaine dose, 0.15 g/cm2, was applied on the surface of the 1 cm×1 cm porcine skin and incubated for 1 h in the humid chamber. 28, 29 Clinically, EMLA cream is occluded to keep skin moisture content high, which enhances permeation of EMLA across the stratum corneum. However, in our experiment the humid chamber simulated the occlusion and we did not occlude EMLA with a plastic-wrap. After 1 h the cream was gently removed using a cotton swab, the porcine skin was frozen, sliced, and analyzed similar to the microneedle-inserted porcine skin.
For sectioning, the skin piece was placed in the cutting block ensuring that the stratum corneum faced the top, while the bottom of the skin was flush at the base. OCT was gently added so that it covered the stratum corneum by just a small amount to enable visualization of the skin through the top of the block. The skin piece was again gently pressed to ensure that the skin was lying flat, and the block was quickly frozen. Cryostat (LMD7000, Leica, Buffalo Grove, IL) was used to slice the frozen tissue into 100 µm-thick sections parallel to the stratum corneum. Since the OCT fully submerged the stratum corneum, the initial sections just contained OCT. The first section containing skin was discarded because for tissues treated with EMLA cream, superficial layers would contain lidocaine as remnants of EMLA cream, which would yield artificially-high lidcocaine amount. The next section was labelled as section at 0.1 mm depth. Sections at depths of 0.1, 0.3, 0.6, 0.9, 1.2, 1.5, 1.8, 2.1, 2.4, 2.7, 3.0, 3.3, 3.6, 3.9, 4.2, 4.5 and 4.8 mm were collected and individually dissolved in 100 µl 10% acetonitrile immediately after sectioning and incubated for 5 hours at 37 °C and 250 rpm shaking speed to extract lidocaine from the skin sections. After spinning for 5 min at 2000 g, the supernatant was collected and filtered using a centrifugal filtering device (Nanosep, Pall Corporation, Port Washington, NY). The filtered solution was injected into HPLC to measure the lidocaine content in each section.
3. RESULTS AND DISCUSSION
3.1 Design considerations for coating solid dispersions on microneedles
To coat a solid dispersion of lidocaine base, which is a water-insoluble compound, on microneedles, lidocaine must first be dispersed or solubilized in a water-soluble matrix compound. The water-soluble matrix compound is necessary so that its dissolution in the aqueous environment of the tissue can rapidly detach the coatings from the microneedle surface, thus minimizing wear-time of microneedles. Additionally, if the water-soluble matrix can enhance solubility of lidocaine base, the bioavailability of the water-insoluble drug can be enhanced. To meet these design criteria we selected PEG (MW 3350) to constitute the water-soluble polymer matrix. PEG is water-soluble and biocompatible. 17 PEG with a MW of 3350 has a melting point of approximately 60 °C, which is similar to that of lidocaine (~ 70 °C). Molten PEG (MW 3350) also possess viscosity in the range of 100–200 mPa.s, 17 which is similar to the viscosity of coating solutions typically employed for water-soluble molecules (47 mPa.s for 1% carboxymethylcellulose sodium salt solution 12); making it a suitable candidate to modulate the viscosity of the coating solution to help achieve uniform coverage of the microneedle surface with coatings. PEG 3350 is also relatively less hygroscopic compared to lower MW PEGs, which can help avoid problems associated with sticky coatings, and hydrolysis-based drug degradation in coatings during storage.
3.2 Thermal stability of lidocaine
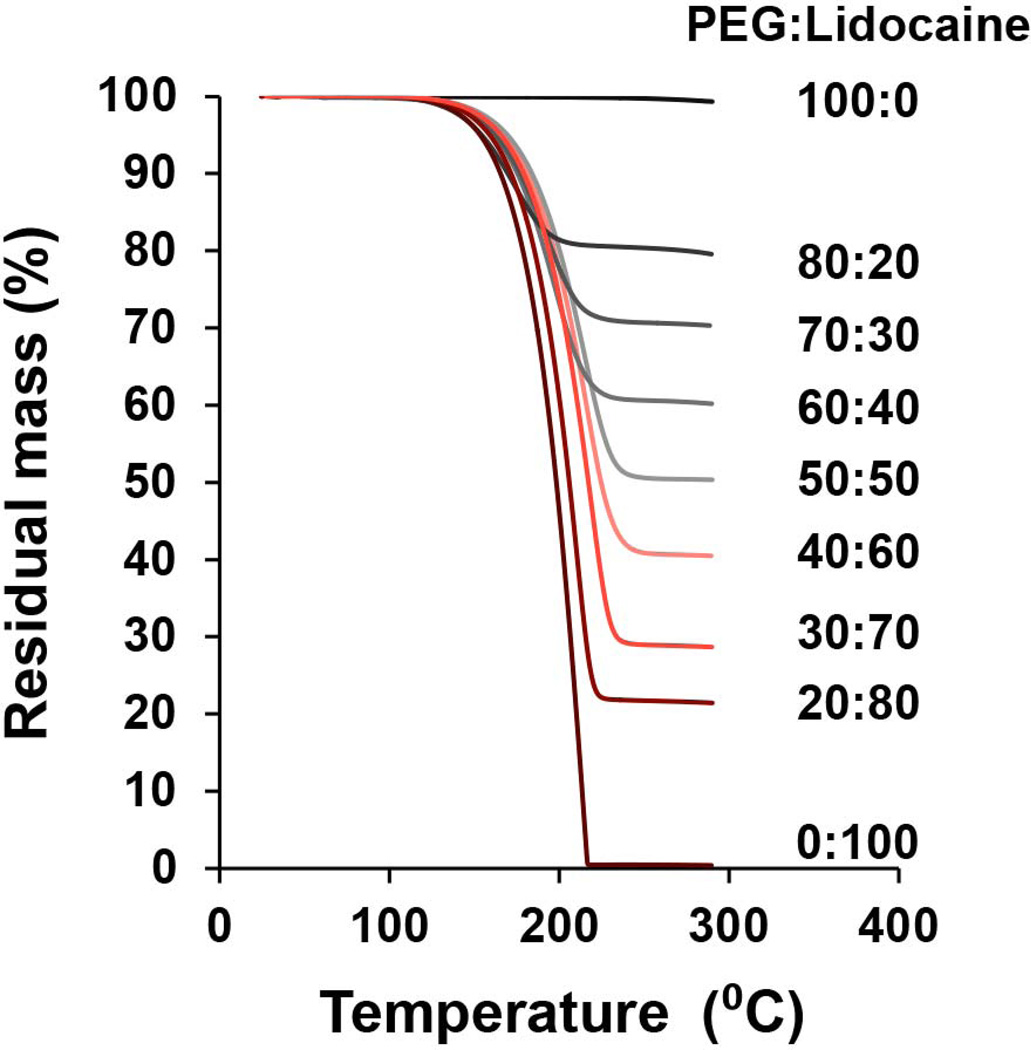
To coat a solid dispersion of PEG-lidocaine on to microneedles, a molten solution of PEG and lidocaine must be formed to dip-coat microneedles, and this solution must stay exposed to elevated temperature for extended durations to help perform coatings on multiple microneedle arrays. Thus, we first evaluated the thermal stability of both lidocaine and PEG to verify that the two compounds do not degrade and can remain stable at elevated temperatures that are needed to perform coatings. TGA analysis (Fig 1) shows that PEG has no obvious weight loss even upon heating to 300 °C. Lidocaine experiences significant weight loss after being heated to 130 °C, suggesting lidocaine vaporization. 20, 30 This indicates that the PEG-lidocaine solid dispersion-preparation process should not involve any heating process more than 130 °C. It is interesting to note that when different mass ratios of PEG and lidocaine are heated, the residual mass of the mixture approaches the initial mass fraction of PEG in the mixture. For example for a 50:50 PEG:lidocaine mixture, the residual mass fraction is approximately 50% after being heated to 300 °C. This confirms that even after mixing with PEG, lidocaine vaporization initiates at temperatures close to 130 °C. PEG, on the other hand remains stable up to 300 °C even in the mixture. Based on this data, we established the experimental protocol to create lidocaine and PEG melts at 120 °C to help form their solid dispersions.
Fig. 1. Thermogravimetric analysis (TGA) of polyethylene glycol (PEG) and lidocaine at different mass ratios.
3.2 Viscosity profile of PEG-lidocaine solid dispersion
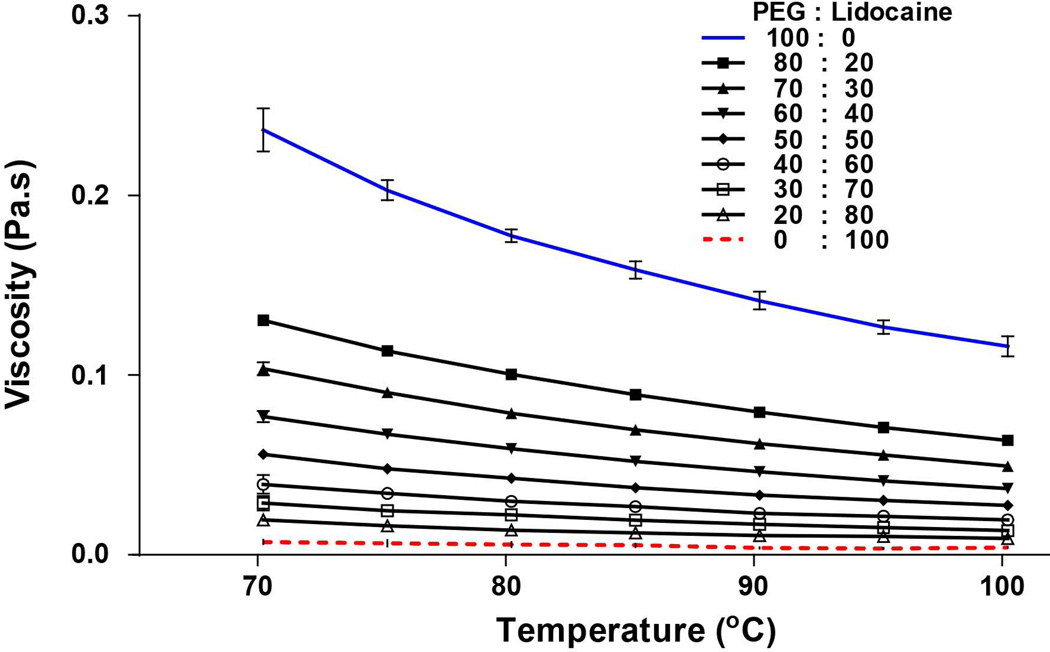
Once the material and surface morphology of the object being coated is fixed, the film thickness of the coating independently depends on three key parameters: the drag speed, viscosity of coating solution, and surface tension of coating solution. 31–33 The empirical Landau-Levich law describes how film thickness is related to these three parameters, at a macroscale coating process 34. The importance of coating solution viscosity in producing uniform films on microneedles has also been confirmed. It has been shown that a sufficiently-viscous coating solution is required to obtain films that uniformly cover the microneedle surface. 4, 12 The viscosity of a molten PEG solution can be affected by temperature and the amount of drug added. Accordingly we investigated the effect of temperature on viscosity of mixtures containing PEG-lidocaine in different proportions. As shown in Fig 2, the viscosity of 100% PEG was 0.2365±0.012 Pa.s at 70 °C, which decreased by 2 fold to 0.116±0.006 Pa.s upon reaching 100 °C. In contrast, 100% lidocaine has low viscosity (70 °C: 0.007195±0.001 Pa.s; 100 °C: 0.004±0.0003 Pa.s). Addition of lidocaine to PEG resulted in viscosity reduction of the melt solution. For example, addition of 20% lidocaine to PEG resulted in 1.8 fold decrease in the molten solution viscosity at 70 °C.
Fig. 2. Viscosity of molten polyethylene glycol (PEG) and lidocaine at different mass ratios as a function of temperature with two replicates.
Aqueous coating solutions that use 1% carboxymethylcellulose sodium salt as viscosity enhancer typically have a viscosity of about 0.047 Pa.s 12). As seen from Fig 2, upon addition of lidocaine to PEG, the viscosity of the molten solution ranges from about 0.020 to 0.120 Pa.s, suggesting that the molten mixture has adequate viscosity across a broad range of lidocaine composition (20–80% by mass) for dip coating microneedles. It is important to note that in aqueous dip coating solutions, the viscosity can only be modified typically by increasing or decreasing the amount of viscosity enhancer added to the solution, thus necessitating reformulation. On the other hand, for PEG-lidocaine molten formulations, the viscosity, and hence thickness of the coated film can be independently controlled by simply modulating the temperature of the molten coating solution, thus eliminating the need for reformulation.
3.3 Miscibility characterization of lidocaine in PEG by DSC
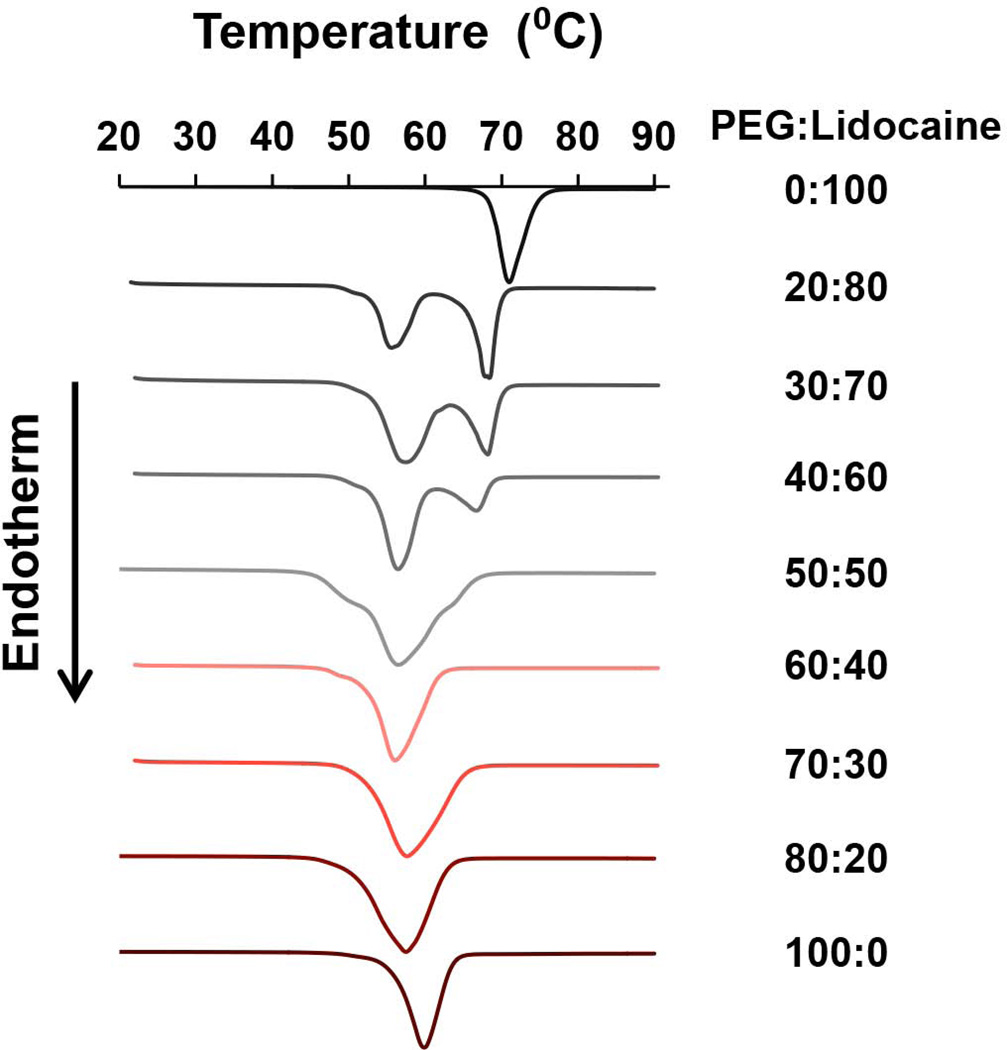
In solid dispersions, the proportion of drug in the carrier matrix has a major influence on how the drug molecules rearrange within the matrix. 17, 35 At high concentrations, the drug can form crystalline micro-domains leading to poor drug dissolution behavior; while at lower concentrations, the drug can facilitate molecular mixing of the drug in the carrier matrix to form an amorphous phase, thus improving dissolution and bioavailability. However, as drug fraction is reduced, it naturally reduces the overall drug content in the microneedle coatings too. Thus, it is important to assess miscibility of lidocaine in PEG, and to ascertain the maximum amount of lidocaine that can be incorporated in PEG without forming lidocaine crystalline micro-domains. We used DSC to analyze PEG-lidocaine miscibility characteristics, and the results are presented as normalized DSC curves (Fig 3). It can be seen that both 100% PEG and 100% lidocaine exhibit a single endothermic peak, which corresponds to their melting point (PEG: 59.8 °C; lidocaine: 68.1 °C). Based on the theory of crystallization, presence of melting point suggests certain crystalline state exists in the material. As seen in Fig 3, as the proportion of lidocaine decreases, its endothermic peak tends to disappear and gradually shifts to the melting peak of PEG. When mass fraction of lidocaine is 50% or lower in the binary system, there is only one endothermic peak. Further, the peak shifting, broadening and combining indicates that lidocaine is soluble in up to 50% PEG. The absence of a lidocaine melting peak at a mass fraction of less than 50% indicates that the drug is distributed in PEG at a molecular level or near molecular level. 36 In this condition, lidocaine remains in an amorphous state in the binary mixture and recrystallization is restrained. 37 Furthermore, PEG peaks of all solid dispersions were found to have shifted to lower values, around 56–57 °C, suggesting that there may have been a minor change in crystallinity of PEG due to the molecular interaction between PEG and lidocaine. 38, 39 Based on Fig 3, there is no obvious evidence of a eutectic point. This type of behavior has previously been reported for other drugs where PEG has been used as the carrier to create solid dispersions. 40, 41
Fig. 3. Differential scanning calorimetry (DSC) analysis of polyethylene glycol (PEG) and lidocaine solid dispersions.
3.4 Microscopy-based analysis of PEG/lidocaine coatings
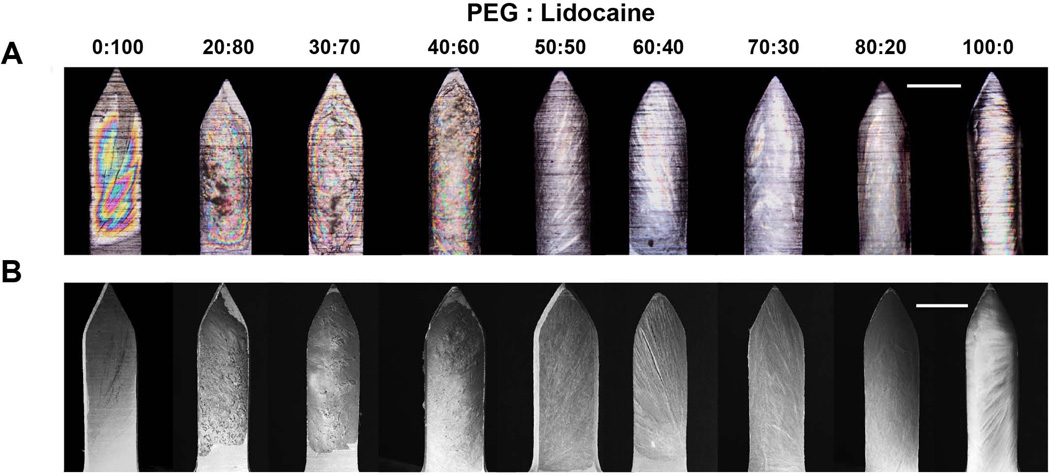
Successful formation of coatings also requires good wettability of the microneedle surface with the coating solution. While static contact angle is a good measure of wettability, the dynamic nature of the coating process makes it hard to directly relate static measurements to predict success of a coating process. 12, 42 Thus, we performed microneedle coatings with all the different solid dispersions to evaluate how PEG-lidocaine proportion affects coating uniformity. We decided to perform microneedle coatings by maintaining the molten solution at 80 °C, which is well below the vaporization temperature of lidocaine (~ 130 °C) identified via TGA, and still confers a suitable range of viscosity to the molten solutions (max: ~ 0.1 Pa.s). From polarized brightfield microscopy (Fig 4A) it can be seen that when lidocaine content is more than 80%, the molten mixture has less wettability, and can only partially coat microneedles. When lidocaine content is 100, 80, or 70%, there are circular rainbow patterns on the microneedles, suggesting that the film thickness is small, which creates interference patterns.43 Upon reduction of lidocaine content to 60% (40% PEG) the film thickness increases and the circular rainbow patterns begin to disappear, indicating formation of thicker films. When lidocaine content is less than or equal to 50%, the coatings appear smooth, homogeneous, and uniformly-cover the microneedle surface. Surface morphology of films was also observed using SEM (Fig 4B), which further confirms that thicker and uniform films are obtained when lidocaine content in the solid dispersion is 60% or lower.
Fig. 4. Microneedles coated with solid dispersions of polyethylene glycol (PEG) and lidocaine at different mass ratios.
(A) Polarized-light micrographs, (B) scanning electron micrographs. Scale bar in (A) and (B) represents 200 µm.
3.5 Analysis of lidocaine amount in the coatings
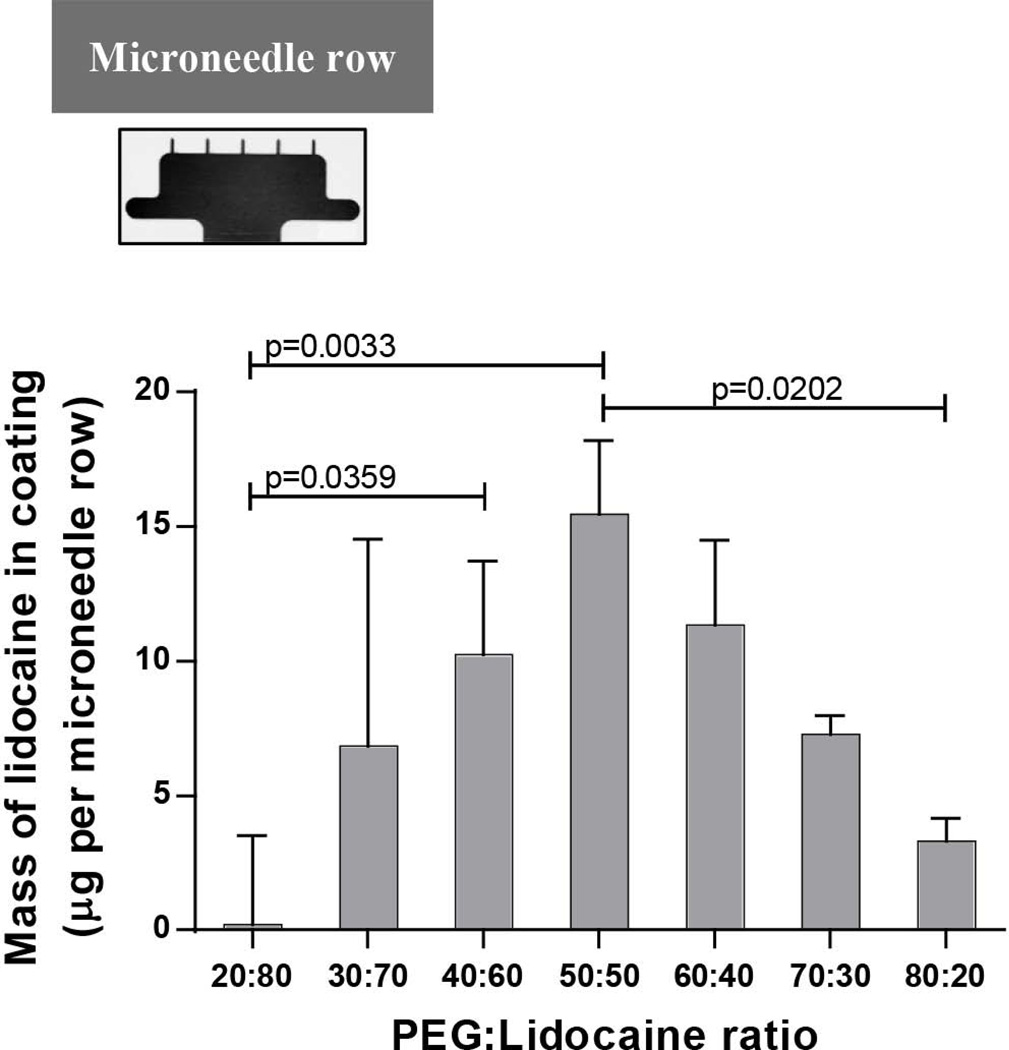
We next determined the mass of lidocaine that can be coated on microneedles and how it is affected by the fraction of lidocaine in the molten coating solution. As seen from Fig 5 when the lidocaine fraction in the molten solution is high (80%), very little lidocaine is coated on microneedles (0.203 ± 3.317 μg per array of five microneedles). This can be attributed to the low viscosity (0.0138 ± 0.00028 Pa.s) of the molten solution when the lidocaine fraction is high, which leads to formation of thin and non-uniform coatings. When the lidocaine fraction decreased to 70%, 6.8 ± 7.7 µg of lidocaine was found in coatings, but the variability was still high. Upon further decrease of the lidocaine fraction to 60%, the coating variation became smaller and the coating mass increased to 10.3 ± 3.5 μg. As the lidocaine fraction in the molten mixture further decreased to 50%, the lidocaine amount in the coatings reached a maximum of 15.5 ± 2.8 μg. Any further decrease in lidocaine fraction did not increase the lidocaine mass in coatings, but rather led to it’s decline. This unimodal effect can be explained by considering the opposing effects that reduction in lidocaine mass fraction in the PEG-lidocaine mixture produces. Upon decrease in lidocaine mass fraction, the viscosity of the coating solution increases, which causes thicker coatings to be formed on microneedles producing the initial rise in mass of lidocaine in coatings. However, as lidocaine mass fraction is reduced beyond a certain value, even though the coatings are thicker, the fraction of lidocaine in them is so small that the overall mass of lidocaine in the coatings starts to fall. It is important to note that the coating uniformity increased with higher PEG (lower lidocaine) content in the coating solution. Again, this is an effect of higher viscosity of the coating solution at higher PEG concentrations, which leads to better mass-uniformity on coatings The coefficient of variation in lidocaine-mass in coatings was 17.8% and 24.7% at 50% and 20% lidocaine content in the coating solution, respectively. We note that the coefficient of variation is relatively high, however this can be reduced by further carefully optimizing the coating conditions such as dipping and withdrawal speeds during dip-coating.
Fig. 5. Mass of lidocaine coated on microneedle arrays (inset) after dip-coating in molten solutions containing polyethylene glycol (PEG) and lidocaine at different mass ratios.
n=3 microneedle arrays per mass ratio.
3.6 Delivery efficiency of PEG-lidocaine coated microneedles
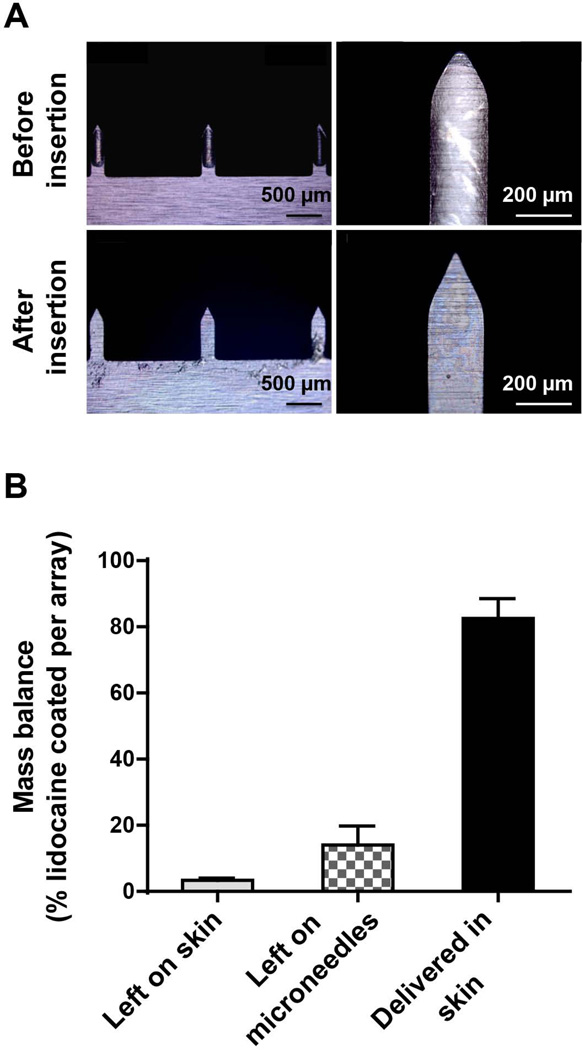
We next investigated the delivery efficiency of PEG-lidocaine solid dispersions coated on microneedles in porcine skin in vitro. To achieve maximum lidocaine dosage, we selected microneedles coated with PEG:lidocaine in a 50:50 ratio. After inserting coated microneedles in porcine skin for three minutes, most of the coating was found to be released from microneedle surfaces. This is qualitatively confirmed by the post-insertion image, which shows that most of the coating has been removed compared to unused microneedles (Fig 6A). Thus, even though lidocaine base is a water-insoluble drug, the coating film still dissolved in the tissue within three minutes, providing a low microneedle-wear time. This dissolution behavior can be explained by noting that PEG is water-soluble, and when lidocaine base at 50% weight fraction is added to PEG, it is sufficiently dispersed in the PEG matrix such that when PEG dissolves, lidocaine also gets detached from the microneedle surface. The near molecular dispersion of lidocaine in PEG can be inferred from the DSC isotherm for 50% lidocaine.
Fig. 6. Delivery efficiency of microneedles coated with a solid dispersion.
Solid dispersion coatings were formed on microneedles by dip-coating in a molten solution containing polyethylene glycol (PEG) and lidocaine at 50% mass ratio, and the coated microneedles were inserted in porcine skin in vitro for 3 min (A) Brightfield micrographs of coated microneedles before and after insertion providing visual evidence that most of the solid dispersion is detached from the microneedle surface, (B) mass balance quantifying lidocaine that is delivered into skin, left on skin and left on microneedle surface (n=3 microneedle arrays).
We next quantified the delivery efficiency of lidocaine from the PEG:lidocaine (50:50) coated microneedles. As shown in Fig 6B, lidocaine content in the coating left on microneedle after insertion was 10.2 ± 6.7%; the lidocaine left behind on the skin surface was 3.2 ± 1.2%; and the lidocaine delivered into tissue was 86.6 ± 7.4%. Previous research has shown that delivery efficiency of a coated microneedle whose film has been developed using aqueous dip-coating solutions is around 70% to 90% after 5 min of microneedle insertion in skin. 4, 44 Thus, delivery efficiency from PEG-lidocaine coated microneedles with a wear time of 3 min is higher than that observed from the hydrophilic form of lidocaine hydrochloride-coated microneedles, which demonstrate a delivery efficiency in the range of 28.3 ± 7.8% to 71.1 ± 1.4% with a 4 min wear time. 5 Thus, the solid dispersion-based coatings can help achieve similar delivery efficiency and wear-times for water-insoluble molecules as that of water-soluble molecules.
3.7 Analysis of lidocaine distribution in tissue after delivery
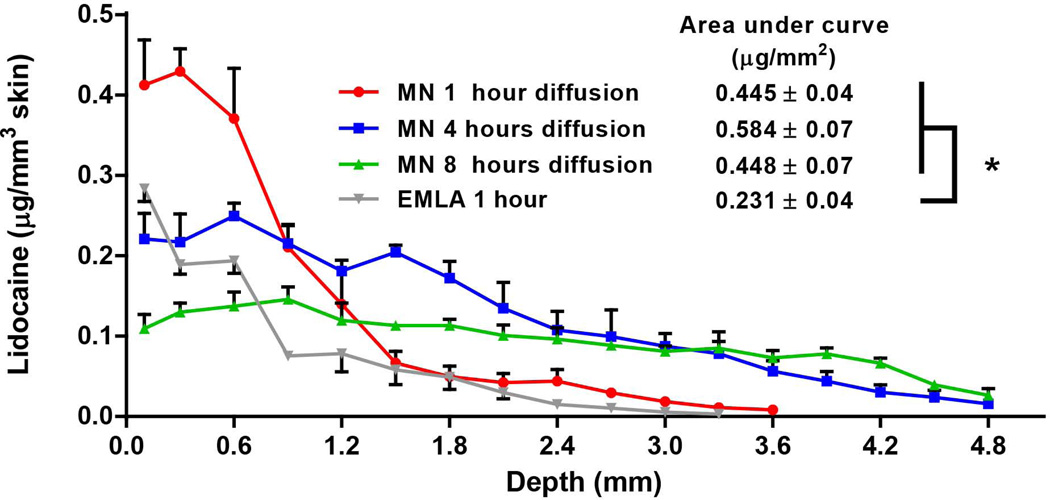
Having demonstrated rapid release of PEG-lidocaine from coated microneedles into skin, we next sought to assess whether lidocaine base, can achieve enhanced solubility due to the solid dispersion, and diffuse deeper into the tissue from the site of delivery. Accordingly, six arrays of microneedles coated with 50:50 PEG:lidocaine molten mixture, which could deliver a total of about 81 µg ([15.5 µg per array×6 arrays = 93 µg] × 87% delivery efficiency) lidocaine, were inserted into a piece of 1cm × 1cm porcine skin for 3 min. To achieve a uniform distribution of lidocaine in the tissue, the microneedle arrays were equally spaced in the 1cm2 surface area. Following incubation of microneedles in the skin for 3 min, microneedles were removed and the deposited lidocaine was allowed to diffuse within the skin. From Fig 7, it can be seen that lidocaine can diffuse up to a depth of 3.6 mm and 4.8 mm into the skin after 1 h and 4 h of diffusion, respectively. After 8 h, the lidocaine concentration curve extends even deeper and becomes more flat. These results suggest that lidocaine base delivered by microneedles as a solid dispersion, can diffuse in a fast manner within the skin. For comparison, 0.15 g EMLA cream, which is a commonly used topical anesthetic dosage in dentistry and other skin-based clinical procedures, 28, 45 was applied on a piece of similar porcine skin for 1 h. It is important to note though that EMLA contains lidocaine hydrochloride, which is a water-soluble form of lidocaine base. It can be seen from Fig 7, that lidocaine delivered by 0.15 g EMLA in an hour is lower than that delivered by microneedles delivering just 81 µg lidocaine. To further quantify the differences we computed the area under the curves (AUCs), which were 0.445±0.04, 0.584±0.07, and 0.448±0.7 µg/mm2, after 1h, 4h and 8h post microneedle insertion, respectively. These AUCs were not statistically different (p > 0.05). However, the AUC for EMLA cream was 0.231±0.04 µg/mm2, which was significantly lower than the AUCs from coated microneedle-based delivery of lidocaine (p < 0.05). Considering that AUCs are proportional to the amount of drug delivered, this result demonstrates that despite application of approximately 1850-fold lower lidocaine using microneedles, approximately twice the amount of lidocaine is delivered into the skin using coated microneedles with just 3 min of microneedle wear-time compared to 1 h application time needed for EMLA cream. Further, noting that even clinically, EMLA cream is applied for 1 h to achieve a therapeutic effect, 26 it can be inferred that coated microneedles can offer a significant reduction in onset and wear time. Thus, although lidocaine base is practically water-insoluble, it exhibited significant diffusion within skin, which is likely due to the enhanced solubility arising from the PEG:lidocaine solid dispersion.
Fig. 7. Diffusion of lidocaine in porcine skin in vitro.
Solid dispersion coatings were formed on microneedles by dip coating in a molten solution containing polyethylene glycol (PEG) and lidocaine at 50% mass ratio. Six arrays of microneedles were used to deliver 81 µg lidocaine into porcine skin measuring 1 cm×1 cm, in vitro, by inserting microneedles at equal spacings and leaving them inserted for 3 min. Upon removal of microneedles, the skin tissues were incubated in a humid chamber for 1, 4 or 8h (n=3 skin tissues per time point). For comparison, 0.15 g EMLA cream was topically applied on skin for 1 h. Skin samples were subsequently sectioned to quantify lidocaine that diffuses along the depth of the tissue.
From a previous study wherein lidocaine hydrochloride, the hydrophilic form of lidocaine, has been delivered using microneedles, it can be noted that lidocaine in the range of 80 - 220 µg delivered to 1 cm2 of rat skin could provide an anesthetic effect. 46 Although in this study we used linear arrays of microneedles with five microneedles per array, the coating apparatus can be easily adapted to coat 2D arrays of microneedles. A 2D array 1cm2 in size with 50 microneedles could be readily used to deliver approximately 150–200 µg lidocaine base, providing a convenient single-application dosage form.
As noted earlier, lidocaine has a pK of about 7.9, thus at a pH of about 7.4 inside the skin, greater than 60% of lidocaine base can be converted to ionized form, which could also partly explain the enhanced dissolution and diffusion of lidocaine. Thus, in future studies we will also focus on more lipophilic drug candidates to evaluate solid dispersion coatings on microneedles. However, because coated microneedle arrays are limited by how much drug can be coated on their surface, they are typically considered to be effective for delivery of drugs that require upto 1 mg dose. 12, 47 Thus it is important to select only potent lipophilic drugs for delivery by this approach. Additionally, we note that while in vitro studies were helpful in the development of solid dispersion coatings of lidocaine-base on microneedles, in vivo studies are ultimately necessary to fully evaluate effectiveness of lidocaine as an anesthetic compared to EMLA cream.
4. CONCLUSION
This study provides the first demonstration of coating microneedles with solid dispersions using molten coating solutions. Overall, the objective was to expand the application domain of microneedles from water-soluble drugs to water-insoluble drugs. Lidocaine base, which is a water-insoluble form of a commonly used topical anesthetic, was selected as the model drug. PEG served as the water-soluble polymer matrix to disperse lidocaine. Solid dispersions of PEG-lidocaine with different mass fractions of lidocaine were prepared and characterized to determine their viscosity, thermal stability and DSC profiles. PEG was found to be stable up to about 300 °C, while lidocaine was stable up to 130 °C. It was found that as lidocaine mass fraction increased, the viscosity of the molten solution decreased. At mass fraction less than or equal to 50%, lidocaine melting peak was not observed suggesting that at these concentrations lidocaine was well dispersed in the PEG matrix. Upon coating microneedles with different solid dispersion formulations, it was observed that mass of lidocaine in coatings increased as the lidocaine mass fraction increased in the molten mixture. A maxima in the mass of lidocaine coated on microneedles was observed when lidocaine was present in the molten coating solution at a mass fraction of 50%. Microneedle coatings obtained from 50:50 PEG:lidocaine molten solution could be released in porcine skin in vitro within three minutes with high efficiency. Investigation of in vitro diffusion profiles of lidocaine in porcine skin revealed that despite being practically water-insoluble, lidocaine base could readily diffuse up to 4.8 mm thickness of porcine skin. In comparison to topical application of 0.15 g of EMLA cream, microneedles coated with just 93 µg lidocaine and inserted into skin for only three min could still deliver approximately twice the amount of lidocaine into porcine skin, demonstrating not only low onset time but high delivery efficiency. Overall, this study provides the first demonstration of obtaining coatings of solid dispersions on microneedles using molten mixtures of water-insoluble drugs.
Acknowledgment
This work was supported in part by the National Institute of Health (1R03DE021667-01A1). HSG is a co-inventor on a patent related to microneedle coating technology. The patent application is still pending in the US patent office.
Footnotes
conflict of interest statement
This potential conflict of interest has been disclosed and is managed by Texas Tech University.
REFERENCES
- 1.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161(2):645–655. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 3.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24(7):585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117(2):227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Brown K, Siebenaler K, Determan A, Dohmeier D, Hansen K. Development of Lidocaine-Coated Microneedle Product for Rapid, Safe, and Prolonged Local Analgesic Action. Pharm Res. 2012;29(1):170–177. doi: 10.1007/s11095-011-0524-4. [DOI] [PubMed] [Google Scholar]
- 6.Peters EE, Ameri M, Wang X, Maa YF, Daddona PE. Erythropoietin-coated ZP-microneedle transdermal system: preclinical formulation, stability, and delivery. Pharm Res. 2012;29(6):1618–1626. doi: 10.1007/s11095-012-0674-z. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Q, Zarnitsyn VG, Ye L, Wen Z, Gao Y, Pan L, Skountzou I, Gill HS, Prausnitz MR, Yang C, Compans RW. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A. 2009;106(19):7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kommareddy S, Baudner BC, Bonificio A, Gallorini S, Palladino G, Determan AS, Dohmeier DM, Kroells KD, Sternjohn JR, Singh M, Dormitzer PR, Hansen KJ, O'Hagan DT. Influenza subunit vaccine coated microneedle patches elicit comparable immune responses to intramuscular injection in guinea pigs. Vaccine. 2013;31(34):3435–3441. doi: 10.1016/j.vaccine.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 9.Daddona PE, Matriano JA, Mandema J, Maa YF. Parathyroid hormone (1-34)-coated microneedle patch system: clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm Res. 2011;28(1):159–165. doi: 10.1007/s11095-010-0192-9. [DOI] [PubMed] [Google Scholar]
- 10.Gill HS, Soderholm J, Prausnitz MR, Sallberg M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 2010;17(6):811–814. doi: 10.1038/gt.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo Z, Ye T, Ma Y, Gill HS, Nitin N. Microprecision delivery of oligonucleotides in a 3D tissue model and its characterization using optical imaging. Mol Pharm. 2013;10(8):2868–2879. doi: 10.1021/mp300717f. [DOI] [PubMed] [Google Scholar]
- 12.Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24(7):1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Prow TW, Crichton ML, Jenkins DW, Roberts MS, Frazer IH, Fernando GJ, Kendall MA. Dry-coated microprojection array patches for targeted delivery of immunotherapeutics to the skin. J Control Release. 2009;139(3):212–220. doi: 10.1016/j.jconrel.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Takagi T, Ramachandran C, Bermejo M, Yamashita S, Yu LX, Amidon GL. A Provisional Biopharmaceutical Classification of the Top 200 Oral Drug Products in the United States, Great Britain, Spain, and Japan. Mol Pharm. 2006;3(6):631–643. doi: 10.1021/mp0600182. [DOI] [PubMed] [Google Scholar]
- 15.Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420(1):1–10. doi: 10.1016/j.ijpharm.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Kapsi SG, Ayres JW. Processing factors in development of solid solution formulation of itraconazole for enhancement of drug dissolution and bioavailability. Int J Pharm. 2001;229(1 – 2):193–203. doi: 10.1016/s0378-5173(01)00867-5. [DOI] [PubMed] [Google Scholar]
- 17.Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50(1):47–60. doi: 10.1016/s0939-6411(00)00076-x. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Lu M, Guo Z, Huang L, Feng X, Wu C. Improving the Chemical Stability of Amorphous Solid Dispersion with Cocrystal Technique by Hot Melt Extrusion. Pharm Res. 2012;29(3):806–817. doi: 10.1007/s11095-011-0605-4. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg AH, Gibaldi M, Kanig JL. Increasing dissolution rates and gastrointestinal absorption of drugs via solid solutions and eutectic mixtures III: Experimental evaluation of griseofulvin—succinic acid solid solution. J Pharm Sci. 1966;55(5):487–492. doi: 10.1002/jps.2600550610. [DOI] [PubMed] [Google Scholar]
- 20.Tatai A, Aigner Z, Erős I, Kata M. Preparation and investigation of mixtures containing lidocaine base and β-cyclodextrin. J Incl Phenom Macrocycl Chem. 2007;59(1 – 2):105–113. [Google Scholar]
- 21.Bergstrom CA, Luthman K, Artursson P. Accuracy of calculated pH-dependent aqueous drug solubility. Eur J Pharm Sci. 2004;22(5):387–398. doi: 10.1016/j.ejps.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Sarpotdar PP, Zatz JL. Evaluation of penetration enhancement of lidocaine by nonionic surfactants through hairless mouse skin in vitro. J Pharm Sci. 1986;75(2):176–181. doi: 10.1002/jps.2600750216. [DOI] [PubMed] [Google Scholar]
- 23.Kreilgaard M, Kemme MB, Burggraaf J, Schoemaker R, Cohen A. Influence of a Microemulsion Vehicle on Cutaneous Bioequivalence of a Lipophilic Model Drug Assessed by Microdialysis and Pharmacodynamics. Pharm Res. 2001;18(5):593–599. doi: 10.1023/a:1011068907416. [DOI] [PubMed] [Google Scholar]
- 24.vanHal DA, Jeremiasse E, deVringer T, Junginger HE, Bouwstra JA. Encapsulation of lidocaine base and hydrochloride into non-ionic surfactant vesicles (NSVs) and diffusion through human stratum corneum in vitro. Eur J Pharm Sci. 1996;4(3):147–157. [Google Scholar]
- 25.Repka MA, Gutta K, Prodduturi S, Munjal M, Stodghill SP. Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur J Pharm Biopharm. 2005;59(1):189–196. doi: 10.1016/j.ejpb.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Evers H, Von Dardel O, Juhlin L, Ohlsen L, Vinnars E. Dermal effects of compositions based on the eutectic mixture of lignocaine and prilocaine (EMLA) Studies in volunteers. Br J Anaesth. 1985;57(10):997–1005. doi: 10.1093/bja/57.10.997. [DOI] [PubMed] [Google Scholar]
- 27.Jeske AH. Xylocaine: 50 years of clinical service to dentistry. Tex Dent J. 1998;115(5):9–13. [PubMed] [Google Scholar]
- 28.Wahlgren C-F, Quiding H. Depth of cutaneous analgesia after application of a eutectic mixture of the local anesthetics lidocaine and prilocaine (EMLA cream) J Am Acad Dermatol. 2000;42(4):584–588. [PubMed] [Google Scholar]
- 29.Vanscheidt W, Sadjadi Z, Lillieborg S. EMLA anaesthetic cream for sharp leg ulcer debridement: a review of the clinical evidence for analgesic efficacy and tolerability. Eur J Dermatol. 2001;11(2):90–96. [PubMed] [Google Scholar]
- 30.Umeda Y, Fukami T, Furuishi T, Suzuki T, Makimura M, Tomono K. Molecular complex consisting of two typical external medicines: Intermolecular interaction between indomethacin and lidocaine. Chem Pharm Bull. 2007;55(5):832–836. doi: 10.1248/cpb.55.832. [DOI] [PubMed] [Google Scholar]
- 31.Strawbridge I, James PF. The factors affecting the thickness of sol-gel derived silica coatings prepared by dipping. J Non Cryst Solids. 1986;86(3):381–393. [Google Scholar]
- 32.Stokes RJ, Evans DF, Errico M. Liquid coating processes. In: Stokes RJ, Evans DF, editors. Fundamentals of Interfacial Engineering, ed. Wiley-VCH; 1997. pp. 399–456. [Google Scholar]
- 33.Scriven LE. Physics and applications of dip coating and spin coating. In: Brinker CJ, Clark DE, Ulrich DR, editors. Mat Res Soc Symp Proc, ed. Materials Research Society: 1988. pp. 717–729. [Google Scholar]
- 34.Brinker CJ, Frye GC, Hurd AJ, Ashley CS. Fundamentals of sol-gel dip coating. Thin Solid Film. 1991;201(1):97–108. [Google Scholar]
- 35.Yang D, Kulkarni R, Behme RJ, Kotiyan PN. Effect of the melt granulation technique on the dissolution characteristics of griseofulvin. Int J Pharm. 2007;329(1 – 2):72–80. doi: 10.1016/j.ijpharm.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 36.Biswal S, Sahoo J, Murthy P, Giradkar R, Avari J. Enhancement of dissolution rate of gliclazide using solid dispersions with polyethylene glycol 6000. AAPS PharmSciTech. 2008;9(2):563–570. doi: 10.1208/s12249-008-9079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joe JH, Lee WM, Park Y-J, Joe KH, Oh DH, Seo YG, Woo JS, Yong CS, Choi H-G. Effect of the solid-dispersion method on the solubility and crystalline property of tacrolimus. Int J Pharm. 2010;395(1 – 2):161–166. doi: 10.1016/j.ijpharm.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 38.Newa M, Bhandari K, Oh D, Kim Y, Sung J, Kim J, Woo J, Choi H, Yong C. Enhanced dissolution of ibuprofen using solid dispersion with poloxamer 407. Arch Pharm Res. 2008;31(11):1497–1507. doi: 10.1007/s12272-001-2136-8. [DOI] [PubMed] [Google Scholar]
- 39.Bizarria MTM, Giraldi ALFdM, de Carvalho CM, Velasco JI, d'Ávila MA, Mei LHI. Morphology and thermomechanical properties of recycled PET–organoclay nanocomposites. J Appl Polym Sci. 2007;104(3):1839–1844. [Google Scholar]
- 40.Damian F, Blaton N, Naesens L, Balzarini J, Kinget R, Augustijns P, Van den Mooter G. Physicochemical characterization of solid dispersions of the antiviral agent UC-781 with polyethylene glycol 6000 and Gelucire 44/14. Eur J Pharm Sci. 2000;10(4):311–322. doi: 10.1016/s0928-0987(00)00084-1. [DOI] [PubMed] [Google Scholar]
- 41.Yao W-W, Bai T-C, Sun J-P, Zhu C-W, Hu J, Zhang H-L. Thermodynamic properties for the system of silybin and poly (ethylene glycol) 6000. Thermochim Acta. 2005;437(1):17–20. [Google Scholar]
- 42.Blake TD, Ruschak KJ, editors. Wetting: static and dynamic contact lines. ed. London: Chapman & Hall; 1997. pp. 63–97. [Google Scholar]
- 43.Muller RH, Sand ML. Optimum angle of incidence for observing thin-film interference colors. Appl Opt. 1987;26(24):5211–5220. doi: 10.1364/AO.26.005211. [DOI] [PubMed] [Google Scholar]
- 44.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142(2):187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liossi C. Management of paediatric procedure-related cancer pain. Pain Rev. 1999;6(4):279–302. [Google Scholar]
- 46.Ito Y, Ohta J, Imada K, Akamatsu S, Tsuchida N, Inoue G, Inoue N, Takada K. Dissolving microneedles to obtain rapid local anesthetic effect of lidocaine at skin tissue. J Drug Target. 2013;21(8):770–775. doi: 10.3109/1061186X.2013.811510. [DOI] [PubMed] [Google Scholar]
- 47.Tuan-Mahmood T-M, McCrudden MTC, Torrisi BM, McAlister E, Garland MJ, Singh TRR, Donnelly RF. Microneedles for intradermal and transdermal drug delivery. Eur J Pharm Sci. 2013;50(5):623–637. doi: 10.1016/j.ejps.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]