Abstract
Background
Coronary artery calcium (CAC) is a well-established predictor of clinical outcomes for population screening. Limited evidence is available as to its predictive value in symptomatic patients without obstructive coronary artery disease (CAD). The aim of the current study was to assess the prognostic value of CAC scores among symptomatic patients with nonobstructive CAD.
Methods
From the COronary Computed Tomographic Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter (CONFIRM) registry, 7,200 symptomatic patients with nonobstructive CAD (<50% coronary stenosis) on coronary-computed tomographic angiography were prospectively enrolled and followed for a median of 2.1 years. Patients were categorized as without (0% stenosis) or with (>0% but <50% coronary stenosis) a luminal stenosis. CAC scores were calculated using the Agatston method. Univariable and multivariable Cox proportional hazard models were employed to estimate all-cause mortality and/or myocardial infarction (MI). Four-year death and death or MI rates were 1.9% and 3.3%.
Results
Of the 4,380 patients with no luminal stenosis, 86% had CAC scores of <10 while those with a luminal stenosis had more prevalent and extensive CAC with 31.9% having a CAC score of ≥100. Among patients with no luminal stenosis, CAC was not predictive of all-cause mortality (P = .44). However, among patients with a luminal stenosis, 4-year mortality rates ranged from 0.8% to 9.8% for CAC scores of 0 to ≥400 (P < .0001). The mortality hazard was 6.0 (P = .004) and 13.3 (P < .0001) for patients with a CAC score of 100–399 and ≥400. In patients with a luminal stenosis, CAC remained independently predictive in all-cause mortality (P < .0001) and death or MI (P < .0001) in multivariable models containing CAD risk factors and presenting symptoms.
Conclusions
CAC allows for the identification of those at an increased hazard for death or MI in symptomatic patients with nonobstructive disease. From the CONFIRM registry, the extent of CAC was an independent estimator of long-term prognosis among symptomatic patients with luminal stenosis and may further define risk and guide preventive strategies in patients with nonobstructive CAD.
Keywords: Coronary calcification, prognosis, nonobstructive coronary artery disease
INTRODUCTION
The presence and extent of coronary artery calcium (CAC) is well-established predictor of clinical outcomes in asymptomatic individuals.1–4 Several large population registries and clinical trials have been published which confirm a role for CAC scanning in the detection of risk among intermediate Framingham risk, asymptomatic patients.1–4 A consistent message within this evidence is that the rate of cardiovascular events increases proportionally with the extent of CAC and is predictive across diverse populations.4–8
However, a modicum of evidence has been put forth on the prognostic value of CAC in symptomatic patients.9–12 Among patients evaluated with suspected cardiac symptoms, management is largely based on defining the extent and severity of obstructive coronary artery disease (CAD).13 A large proportion of patients undergoing a diagnostic evaluation will not have any significant obstructive lesions (i.e., ≥50%)14,15 and additional tools may prove useful to further risk stratify this subset of patients. It remains unclear whether the extent to which patients without obstructive CAD have an identifiable burden of atherosclerosis that elevates their long-term risk. Recent evidence is supportive that coronary-computed tomographic angiography (CCTA) may prove useful to define atherosclerotic plaque in those without obstructive CAD.14 Specifically, the quantification of CAC is a reliable and easily quantifiable measure of the burden of atherosclerotic plaque extent.16 Preliminary work in relatively small patient series suggests that patients without obstructive CAD have an elevated mortality risk.7,14,17 The aim of the current study was to assess the independent contribution of CAC extent among symptomatic patients without obstructive CAD from a multinational registry of patients referred for CCTA.
METHODS
Eligibility Criteria
The design of the COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter (CONFIRM) registry is described in detail elsewhere.11 As a prospective, observational, multicenter registry, adults ≥18 years were prospectively enrolled at each of the 12 centers between 2005 and 2009 (Capital Cardiology Associates, Albany, NY; Cedars Sinai Medical Center, Los Angeles, CA; Harbor UCLA Medical Center, Los Angeles, CA; Henry Ford Medical Center, Detroit, MI; Tennessee Heart and Vascular Institute, Hendersonville, TN; William Beaumont Hospital, Royal Oak, MI; Walter Reed Army Medical Center, Washington, DC; Ottawa Heart Institute, ON, Canada; University of Munich, Munich, Germany; University Hospital of Parma, Parma, Italy; University Hospital, Zurich, Switzerland; and Yonsei Medical Center, Seoul, Korea). Enrolled patients included prospective referrals of those undergoing ≥64 detector row CCTA. All centers had institutional review board approval for patient enrollment including follow-up procedures. For this analysis, only symptomatic patients presenting for evaluation of chest pain or the anginal equivalent of dyspnea were eligible for the current analysis. Patients with a prior diagnosis of CAD defined as documented myocardial infarction (MI) or coronary revascularization were excluded. Asymptomatic patients, such as those referred for cardiovascular risk assessment, pre-operative evaluation, electrophysiologic indication, or congenital heart disease evaluation, were also excluded. As the focus of this report was to identify patient subsets with nonobstructive CAD who may be at higher risk, patients defined as having a stenosis of ≥50% were excluded in this series.
Among 27,125 consecutive patients referred for CCTA to the participating centers, a total of 9,938 were symptomatic and without a prior CAD diagnosis. From this subset, a total of 8,098 had <50% stenosis; with 7,200 having a CAC score calculated. Of the 7,200 patients, a total of 4,380 had no luminal stenosis (defined as 0% stenosis in all coronary arteries) and 2,820 had luminal stenosis (>0% but <50% stenosis).
Pretest Clinical History Data
Prior to CCTA, demographic data and a focused history of cardiovascular risk factors was obtained from each patient. Hypertension, diabetes mellitus, and dyslipidemia were defined based on a prior diagnosis or medical treatment use. Current cigarette smokers or those who quit smoking within 3 months of testing were established to have a positive smoking history. Significant family history of CAD was defined as that occurring in a prior relative<65 years of age in women and <55 years of age in men. Current risk factor modifying and anti-ischemic therapy drug use was also documented.
CCTA Image Acquisition and Interpretation Procedures
We employed standardized protocols, defined by the Society of Cardiovascular Computed Tomography, for image acquisition.18 Details of the CCTA procedures employed in the CONFIRM registry were detailed in prior manuscripts.8,14 All participating sites employed standardized anatomic segmental analysis for the CCTA interpretation. For this analysis, each of the 16 segments was coded for 0% stenosis and stenosis defined as >0% but <50%. Patients with nonobstructive CAD were categorized as those with no luminal stenosis (defined as 0% stenosis in all coronary arteries) and with luminal stenosis (>0% but <50% stenosis). Additionally, all patients had CAC scoring performed using the methods of Agatston et al.16 CAC score subsets were defined as 0, 1–10, 11–99, 100–399, and ≥400, respectively.6,19,20 The segment involvement score was calculated as the number of segments with a luminal stenosis; from a total of 16 segments.21 The number of vessels with a luminal stenosis was used to define extent of mild CAD.
Follow-Up Methods
The methods for follow-up have been previously described.8,14 Participants were followed for the primary endpoint of all-cause mortality and the secondary endpoint of death or MI. For participating US centers, the national death index was queried to ascertain vital status for all enrollees. The secondary endpoint of acute MI was ascertained from the patient’s medical records (i.e., primary or secondary discharge diagnosis) or verbal confirmation from the patient’s primary care physician. In non-US sites, all patients, their relatives, or primary care physicians were interviewed to ascertain vital status and index hospitalization for acute MI. During follow-up, a total of 48 patients (0.7%) were lost during follow-up. The median duration of follow-up for surviving patients was 2.1 years (interquartile range: 1.5–3.2 years).
Statistical Analysis
We initially performed a series of categorical analyses that compared CAC subsets with the patient’s presenting symptoms and risk factors. A plot of the cumulative prevalence of CAC scores was drawn for patients with and without a luminal stenosis. Additional analyses examined CCTA parameters including mild proximal lesions and the number of vessels with a luminal stenosis by CAC subsets. The categorical analyses were performed using a χ2 log rank statistic. Univariable and multivariable (covariate adjustment included age, cardiac risk factors, and presenting symptoms) logistic regression models were used to estimate the odds ratio (and 95% confidence intervals [CIs]) of detectable CAC, a CAC score ≥100, and a CAC score ≥400 in patients with as compared to those without a luminal stenosis.
The primary aim of the current manuscript was to define the independent predictive value of CAC scoring in Cox proportional hazard models estimating all-cause mortality and secondarily in a model estimating death or MI. Univariable and multivariable Cox proportional hazards models were calculated to estimate clinical outcomes while controlling for confounding through risk-adjusting patient symptoms and cardiac risk factors. A hazard ratio and 95% CI was calculated for each variable within the Cox models. Covariates included in the multivariable models were age and other cardiac risk factors (hypertension, diabetes, and smoking), and presenting symptoms. From the multivariable model, estimated mortality rates were calculated. The proportional hazards assumption was met for all survival analyses. Model overfitting procedures were considered by limiting the number of variables in a model to 1/10 clinical outcomes. For the anatomic variables, collinearity was considered by limiting highly correlated variables with a spearman’s rho r ≤ 0.80. Cox survival curves were plotted by CAC subsets through 4 years of follow-up. The number at risk during each year of follow-up was calculated. The estimated or predictive mortality rate was calculated from the Cox models. General linear models were used to compare CAC subsets by age subsets; from this model, an adjusted r2 or explanatory variance estimate was also calculated. A life table analysis was employed to estimate changes in mortality rates by CAC subsets at 6 months, 1 year, 2 years, 3 years, and 4 years, respectively, of follow-up. A receiver operating characteristic (ROC) analysis was performed to assess the ability of CAC and the segment involvement score to classify mortality. From the ROC analysis, an area under the curve (AUC) and the asymptotic 95% CI was calculated.
Finally, the proportion of excess risk was calculated using the equation: [HRU − HRA]/[HRU − 1] where HR is the hazard ratio and HRU is an unadjusted HR and HRA is a risk-adjusted HR.22,23 For this calculation, HR were continued until the third decimal place.
RESULTS
Clinical Characteristics of the Study Cohort
Table 1 provides clinical characteristics of the study cohort with nonobstructive CAD by their CAC scores. In general, patients with more extensive CAC were older, less often female, and had a greater prevalence of cardiac risk factors. Patients with higher CAC scores more often had luminal stenosis in the proximal segments of the right, left anterior descending, and circumflex coronary arteries (all P < .0001 when compared to lower CAC scores). Additionally, nearly one-fourth of patients with a CAC score ≥400 had a luminal stenosis in the left main coronary artery.
Table 1.
Clinical and CCTA descriptors of 7,200 symptomatic patients by CAC score
| CAC 0 (n = 4,047) (%) | CAC 1–10 (n = 524) (%) | CAC 11–100 (n = 1,300) (%) | CAC 101–399 (n = 823) (%) | CAC ≥ 400 (n = 384) (%) | P value | |
|---|---|---|---|---|---|---|
| Age (in deciles) | <.0001 | |||||
| <40 | 13.7 | 4.8 | 3.2 | 5.3 | 7.0 | |
| 40–49 | 24.9 | 20.0 | 14.5 | 12.9 | 11.0 | |
| 50–59 | 32.8 | 28.4 | 29.7 | 24.6 | 19.1 | |
| 60–69 | 21.6 | 31.1 | 33.6 | 30.7 | 29.8 | |
| 70–79 | 6.2 | 13.2 | 15.9 | 20.7 | 24.0 | |
| ≥80 | 0.8 | 2.5 | 3.1 | 5.8 | 9.2 | |
| Female gender | 57.5 | 47.8 | 48.9 | 49.1 | 44.1 | <.0001 |
| Risk factors | ||||||
| Hypertension | 44.9 | 50.1 | 56.9 | 53.9 | 58.5 | <.0001 |
| Diabetes | 9.5 | 13.4 | 15.7 | 15.3 | 19.0 | <.0001 |
| Dyslipidemia | 51.6 | 59.4 | 59.8 | 57.4 | 54.5 | <.0001 |
| Family history | 27.1 | 28.8 | 32.3 | 31.3 | 34.4 | <.0001 |
| Smoker | 16.0 | 20.7 | 14.7 | 15.0 | 17.3 | .62 |
| Obesity | 20.7 | 28.9 | 29.1 | 31.1 | 31.4 | <.0001 |
| Chest pain | <.0001 | |||||
| Dyspnea | 7.8 | 11.4 | 11.7 | 15.2 | 17.0 | |
| Non-cardiac | 11.1 | 8.3 | 10.4 | 10.1 | 6.9 | |
| Atypical | 65.8 | 65.0 | 61.9 | 60.0 | 63.6 | |
| Typical | 15.3 | 15.3 | 15.9 | 14.7 | 12.5 | |
| Mild stenosis on CCTA | ||||||
| Left main | 1.8 | 10.6 | 13.6 | 20.6 | 27.7 | <.0001 |
| Proximal LAD | 7.9 | 34.9 | 50.3 | 58.3 | 62.8 | <.0001 |
| Proximal RCA | 3.1 | 11.8 | 19.0 | 30.5 | 45.5 | <.0001 |
| Proximal left Cx | 1.9 | 8.2 | 14.2 | 23.6 | 43.8 | <.0001 |
| Medication use | ||||||
| Aspirin | 32.2 | 43.9 | 52.7 | 52.7 | 61.7 | <.0001 |
| Statins | 23.3 | 38.2 | 43.0 | 52.1 | 48.5 | <.0001 |
| Hyperglycemics | 4.0 | 5.9 | 8.1 | 8.1 | 9.4 | <.0001 |
| Insulin | 0.5 | 0.8 | 1.3 | 1.9 | 3.1 | <.0001 |
| ACE inhibitor | 8.6 | 17.1 | 17.0 | 18.6 | 23.4 | <.0001 |
| Beta-blockers | 22.6 | 30.7 | 31.9 | 32.7 | 44.9 | <.0001 |
| CCD | 16.9 | 23.9 | 26.6 | 25.4 | 26.0 | <.0001 |
| Nitrates | 13.0 | 12.8 | 15.6 | 13.5 | 21.7 | .004 |
Available data for medications: aspirin (n = 5,294), statins (n = 4,891), hyperglycemics (n = 5,056), insulin (n = 5,064), ACE inhibitor (n = 5,295), beta-blockers (n = 5,295), CCB (n = 5,081), nitrates (n = 5,050).
LAD, Left anterior descending; RCA right coronary artery; Cx, circumflex; ACE, angiotensin converting enzyme; CCB, calcium channel blocker.
Relationship of CAC Scores with Nonobstructive CAD
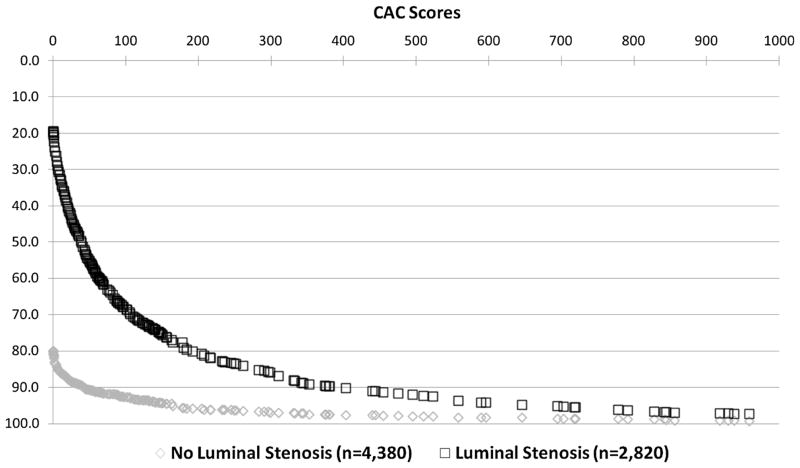
Of the 4,380 patients without a luminal stenosis, 86% had a CAC score of <10 (Figure 1). Of the 322 patients without a luminal stenosis and a CAC score ≥100, 77% were <60 years of age. By comparison, for those with a luminal stenosis, only 19.8% had a CAC score of 0 while 21.9% and 10.0% of patients had a CAC score of 100–399 and ≥400. Of the 885 patients with a luminal stenosis and a CAC score ≥100, ~70% were 60 years of age and older.
Figure 1.
Prevalence of increasing CAC scores among 7,200 symptomatic patients with no and with luminal stenosis. This figure illustrates the higher prevalence of CAC and more extensive CAC scores among patients with mild CAD.
The odds of detectable CAC >0 were elevated 17.38-fold (95% CI 15.41–19.61) for patients with a luminal stenosis as compared to those without a luminal stenosis (P < .0001). Similarly, the odds of a CAC score ≥100 or ≥400 were elevated 5.79-fold (95% CI 5.04–6.65, P < .0001) and 4.42-fold (95% CI 3.52–5.56, P < .0001) for patients with as compared to those without a luminal stenosis. This relationship of higher odds of CAC in patients with a luminal stenosis as compared to those without a luminal stenosis was maintained in multivariable logistic regression models that included age and other cardiac risk factors as well as presenting symptoms.
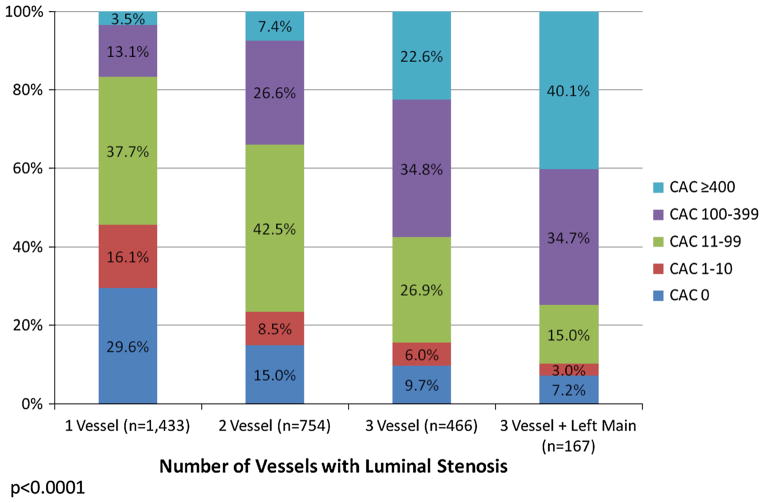
In Figure 2, the representation of CAC subsets by the number of vessels with luminal stenosis is reported. The rate of more extensive CAC increased proportionally with the number of vessels with a luminal stenosis. The frequency of a CAC score ≥400 ranged from 3.5% for patients with 1 vessel to 40.1% for patients with luminal stenosis in 3 vessels and the left main coronary artery. For patients with 3-vessel involvement including a left main luminal stenosis, nearly three-fourths of this subset had a CAC score of 100 or higher. Conversely, there was a decline in the prevalence of a zero CAC score such that nearly 30% of patients with 1 vessel luminal stensosis and only 7.2% of patients with a luminal stenosis in all 3 vessels plus the left main artery had a zero CAC score.
Figure 2.
Prevalence of CAC scores by the number of vessels with luminal stenosis in 2,820 symptomatic patients.
Prognosis in Patients with a Luminal Stenosis
At 4 years of follow-up, overall mortality was 1.9%. Four-year mortality was 1.1% and 3.4% for patients without and with a luminal stenosis on CCTA (P < .0001). The relative hazard was 3.33 (95% CI 2.07–5.35) for patients with as compared to those without a luminal stenosis (P < .0001). The overall mortality hazard for patients with 2 or more vessels with a luminal stenosis was elevated ~5.6-fold when compared to patients without a luminal stenosis (P < .0001).
Prognosis by CAC Scores
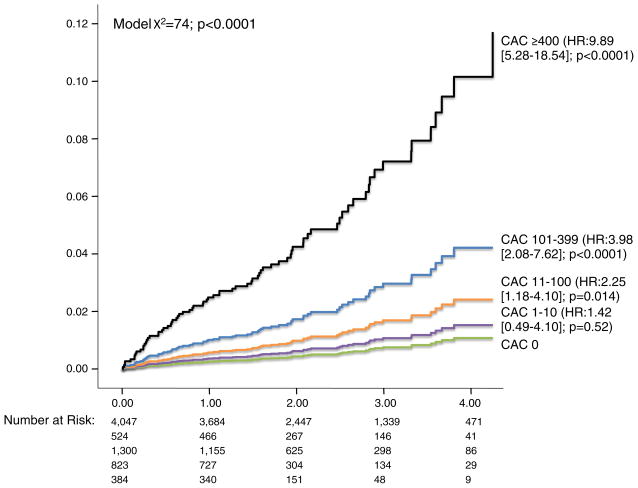
Overall mortality at 4 years was 1.1%, 1.5%, 2.4%, 4.1%, and 10.4%, respectively, for patients with a CAC score of 0, 1–10, 11–99, 100–399, and ≥400 (Figure 3, P < .0001). Even in a risk-adjusted model that included age and other risk factors and symptom covariates, CAC remained an independent estimator of all-cause mortality (Table 2, P = .001). The adjusted hazard for a CAC score ≥400 was 2.14 (95% CI 2.06–8.10, P < .0001). Similarly, in a death or MI model, CAC was predictive (P = .001). In this latter death or MI model, a CAC score of 400 or higher was associated with a relative hazard of 3.24 (95% CI 1.67–6.27, P < .0001).
Figure 3.
Cumulative all-cause mortality in 7,200 symptomatic patients with nonobstructive CAD based on the CAC score (n = 75 deaths).
Table 2.
Multivariable model estimating all-cause mortality in 7,200 symptomatic patients with nonobstructive CAD
| Hazard ratio | 95.0% CI for HR
|
Wald χ2 | P value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Univariable model | |||||
| CAC score (per category)* | 2.13 | 1.75 | 2.60 | 56 | <.0001 |
| CAC | 57 | ||||
| 1–10 | 1.46 | 0.51 | 4.21 | <1 | .48 |
| 11–99 | 2.32 | 1.21 | 4.42 | 6 | .011 |
| 100–399 | 4.09 | 2.14 | 7.83 | 18 | <.0001 |
| ≥400 | 10.17 | 5.43 | 19.06 | 52 | <.0001 |
| Multivariable model | |||||
| CAC score (per category)* | 1.55 | 1.24 | 1.95 | 15 | <.0001 |
| CAC score | 18 | .001 | |||
| 1–10 | 0.93 | 0.32 | 2.71 | <1 | .90 |
| 11–99 | 1.34 | 0.69 | 2.59 | <1 | .38 |
| 100–399 | 1.68 | 0.83 | 3.40 | 2 | .15 |
| ≥400 | 2.14 | 2.06 | 8.10 | 16 | <.0001 |
Overall multivariable model χ2 = 138, P < .0001; covariates: age and other cardiac risk factors and presenting symptoms (overall mortality at 4 years: 2.2%, 76 deaths).
Per category: 0 (comparator) vs 1–10, 11–99, 100–399, and ≥400.
Estimating Risk by CAC Among CAD Patient Subsets
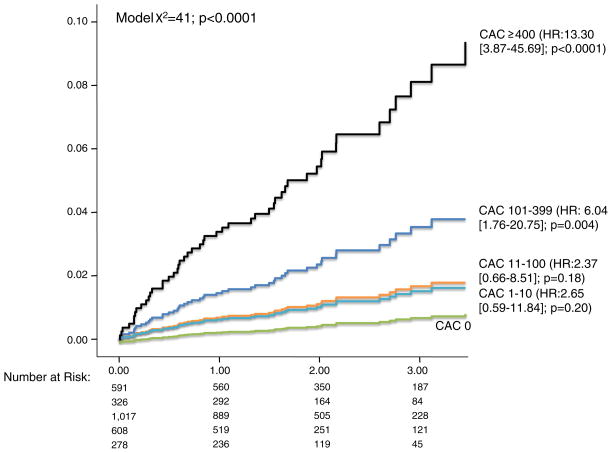
In a subset analysis of patients without a luminal stenosis, CAC was not a univariable estimator of all-cause mortality (P = .44). Of the 815 patients without a luminal stenosis and detectable CAC, only 1 death (0.1%) was observed during follow-up. However, among patients with a luminal stenosis, the relative hazard for all-cause mortality was 6.04 (P = .004) and 13.30 (P < .0001) for patients with a CAC score of 100–399 and ≥400 (Figure 4). In this same subset with a luminal stenosis, CAC remained predictive of all-cause mortality (P < .0001) within a multivariable model containing age and other risk factors and presenting symptoms (Table 3). When assessing the independent prognostic ability of CAC in patients with a luminal stenosis, a CAC score of 100–399 and ≥400 had an elevated mortality hazard of 2.88 (P = .002) and 6.76 (P < .0001). CAC scores of 100 or higher were also independently predictive of death or MI (P = .002). By comparison, mortality risk was similar for patients with CAC scores <100 (P < .25). In an ROC analysis, CAC also had improved classification of mortality when compared to the segment involvement score (AUC: 0.70 [95% CI 0.63–0.78] vs 0.61 [95% CI 0.53–0.69], P < .0001).
Figure 4.
Cumulative all-cause mortality in 2,820 symptomatic patients with luminal stenosis (>0% but <50% stenosis) based on the coronary artery calcium score (n = 50 deaths).
Table 3.
Multivariable model estimating all-cause mortality in 2,820 symptomatic patients with a luminal stenosis
| Hazard ratio | 95% CI for HR
|
Wald χ2 | P value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Univariable models estimating all-cause mortality | |||||
| CAC (per category)* | 1.85 | 1.35 | 2.53 | 15 | <.0001 |
| CAC | 39 | <.0001 | |||
| 100–399 | 2.88 | 1.45 | 5.72 | 9 | .002 |
| ≥400 | 6.76 | 3.45 | 13.27 | 31 | <.0001 |
| Multivariable model estimating all-cause mortality | |||||
| CAC (per category)* | 1.99 | 1.41 | 2.82 | 15 | <.0001 |
| CAC | 15 | .001 | |||
| 100–399 | 2.10 | 1.05 | 4.21 | 4 | .036 |
| ≥400 | 3.87 | 1.91 | 7.82 | 14 | <.0001 |
| Multivariable model estimating death or MI | |||||
| CAC (per category)* | 1.81 | 1.30 | 2.50 | 13 | <.0001 |
| CAC | 12 | .002 | |||
| 100–399 | 2.05 | 1.09 | 3.83 | 5 | .025 |
| ≥400 | 3.22 | 1.65 | 6.28 | 12 | <.0001 |
For these models, CAC scores < 100 were collapsed into one category as they had similar event rates. Multivariable mortality model χ2 = 92, P < .0001, multivariable death or MI model χ2 = 84, P < .0001; clinical covariates: age and other cardiac risk factors (overall mortality at 4 years: 2.8%, 50 deaths, death or MI at 4 years: 3.3%, 58 events).
Per category: 0 (comparator) vs <100, 100–399, and ≥400.
In this subset of patients with a luminal stenosis, the mortality risk was significantly elevated for patients with a CAC score ≥100 at 6 months of follow-up (Table 4). Mortality rates continued to increase throughout 4 years of follow-up with a greater separation in rates between patients with a CAC score <100 and 100–399. By 1 year of follow-up, there was a significant increase in risk between patients with a CAC score of 100–399 and ≥400 (P = .012).
Table 4.
Life table analysis among 2,820 CAC patient subsets with a luminal stenosis
| Time to follow-up (in years)
|
|||||
|---|---|---|---|---|---|
| 0.5 year | 1.0 year | 2.0 years | 3.0 years | 4.0 years | |
| CAC subset | |||||
| <100 (n = 1,934) (%) | 0.0 | 0.4 | 0.8 | 1.0 | 1.4 |
| 100–399 (n = 608) (%) | 0.6 | 1.3 | 2.3 | 2.1 | 4.4 |
| ≥400 (n = 278) (%) | 1.8 | 4.1 | 4.3 | 7.5 | 12.2 |
| P value | |||||
| <100 vs 100–399 | .052 | .024 | .026 | .002 | .003 |
| <100 vs ≥400 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| 100–399 vs ≥400 | .096 | .012 | .003 | .013 | .013 |
Mortality Risk by CAC Scores Across Varied Patient Ages
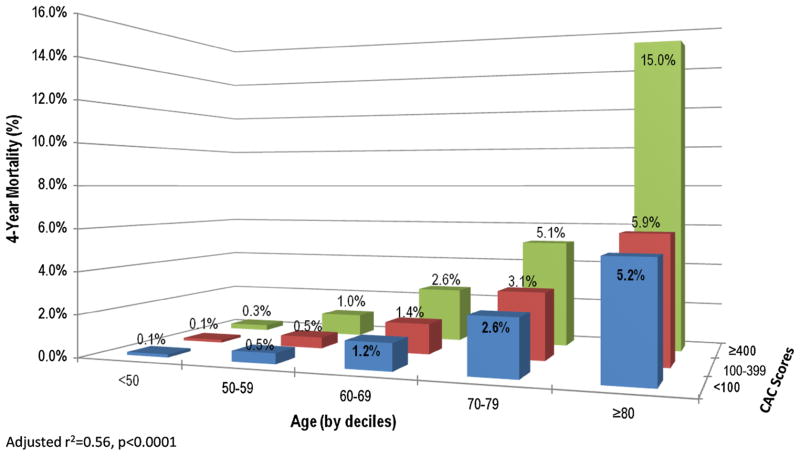
Figure 5 plots the predicted mortality of CAC patient subsets across varying age groups with a luminal stenosis. There was a proportional relationship between CAC and increasing mortality rates for patients of advanced ages. In patients <60 years of age, the predicted mortality rate was <1% across the range of CAC score subsets. By comparison, mortality rates increased for older patients and by CAC scores. For patients who are 70–79 years of age, the mortality rates ranged from 2.6% to 5.1% for CAC scores from 0 to ≥400. For patients who are ≥80 years of age, the mortality rates ranged from 5.2% to 15.0% for CAC scores from 0 to ≥400.
Figure 5.
Interactive relationship between the extent of CAC scores, age (by decile), and the overall predicted mortality. These results reveal a low mortality rate for patients <60 years of age with increasing mortality rates proportionally with advanced ages and CAC scores.
Estimating the Proportion of Excess Risk with CAC Scores
In a series of Cox models, we assessed the proportion of excess risk explained by selected clinical factors and anatomic markers in patients with luminal stenosis (Table 5). In general, clinical factors appeared to explain a greater proportion of the excess risk associated with CAC. For example, the proportion of excess risk with CAC that may be explained by age was estimated at ~34%. In additional models, the proportion of excess risk with CAC that may be explained by hypertension, diabetes, and presenting symptoms increased to 47%. By comparison, the proportion of excess risk for CAC that may be explained by anatomic markers only increased to slightly more than 25% when considering the number of vessels with luminal stenosis, left main, or even the extent of proximal luminal stenoses. A similar pattern was noted for death or MI models.
Table 5.
The proportion of excess risk* explained by clinical, symptomatic, and other anatomic CAD factors when estimating the predictive ability of CAC among 2,820 patients with a luminal stenosis
| A. Models estimating all-cause mortality
| ||||
|---|---|---|---|---|
| Clinical risk adjustment**
| ||||
| Unadjusted HR | Age-adjusted HR | +Risk factor-adjusted HR | +Symptom-adjusted HR | |
| CAC (per category)* | 2.61 | 2.07 | 1.98 | 1.86 |
| P value | <.0001 | <.0001 | <.0001 | <.0001 |
| Proportion of excess risk | – | 33.7% | 38.9% | 46.5% |
|
| ||||
|
Anatomic risk adjustment***
| ||||
| Incremental models | Unadjusted HR | +Luminal stenosis extent-adjusted HR | +LM luminal stenosis-adjusted HR | +Proximal luminal stenosis extent-adjusted HR |
|
| ||||
| CAC (per category)* | 2.61 | 2.43 | 2.40 | 2.19 |
| P value | <.0001 | <.0001 | <.0001 | <.0001 |
| Proportion of Excess Risk | – | 11.3% | 12.8% | 25.8% |
| B. Models estimating death or MI
| ||||
|---|---|---|---|---|
| Clinical risk adjustment**
| ||||
| Unadjusted HR | Age-adjusted HR | +Risk factor-adjusted HR | +Symptom-adjusted HR | |
| CAC (per category)* | 2.36 | 1.89 | 1.82 | 1.71 |
| P value | <.0001 | <.0001 | <.0001 | <.0001 |
| Proportion of excess risk | – | 34.7% | 39.8% | 47.8% |
|
| ||||
|
Anatomic risk adjustment***
| ||||
| Incremental models | Unadjusted HR | +Luminal stenosis extent-adjusted HR | +LM luminal stenosis-adjusted HR | +Proximal luminal stenosis extent-adjusted HR |
|
| ||||
| CAC (per category)* | 2.362 | 2.23 | 2.23 | 2.06 |
| P value | <.0001 | <.0001 | <.0001 | <.0001 |
| Proportion of excess risk | – | 9.4% | 9.8% | 22.1% |
The first set of models estimate all-cause mortality and the second set—death or MI.
The proportion of excess risk explained was calculated using the equation: [HRU − HRA]/[HRU − 1] where HR is the hazard ratio and HRU is an unadjusted HR and HRA is a risk-adjusted HR.22,23 For this calculation, HR were continued until the third decimal place.
For the clinical risk adjustment, risk factors included age, hypertension, and diabetes mellitus.
For the anatomic risk adjustment, luminal stenosis extent was defined as the number of vessels with luminal stenosis, LM luminal stenosis was defined as left main luminal stenosis, and proximal luminal stenosis extent was defined as the number of vessels with proximal luminal stenosis.
DISCUSSION
In patients without obstructive CAD, the current analysis revealed that CAC was highly and independently predictive of all-cause mortality and death or MI; particularly among patients with evidence of a luminal stenosis. This data contributes to our unfolding knowledge with regards to the adverse event risk associated in patients without obstructive CAD.14,17 Heretofore, non-obstructive atherosclerosis was largely defined as the absence of obstructive CAD or by visual estimation of luminal irregularities.7,17,21 Evidence is abundant that CAC is a well-established prognostic tool that may be applied for cardiovascular screening of asymptomatic, apparently healthy individuals.2,3,6,20 Although prior research indicates that CCTA may prove useful for the detection of atherosclerotic plaque in the absence of an obstructive coronary stenosis, the current analysis defined a near-term prompt separation in mortality risk, as early as 6 months, for patients with a luminal stenosis and elevated CAC scores.14,17,24 Importantly, CAC was not predictive of mortality in the setting of no luminal stenosis (P = .44) but was most beneficial in terms of risk stratification for patients with mild but nonobstructive luminal stenosis. In patients with a luminal stenosis, a high risk CAC score ≥400 was associated with a 13-fold elevated hazard for death when compared to patients with a zero CAC score. Moreover, a CAC score improved the estimation of mortality when compared to the segment involvement score.
Risk Stratification in Patients Without a Luminal Stenosis
In the absence of any luminal stenosis, it remains plausible that detectable CAC could impart risk as a subcomponent of expanded and remodeled coronary arteries but non-intrusive to the lumen.25,26 However, in this subset without a luminal stenosis, the vast majority (i.e., 86%) of patients had very low CAC scores<10 and this cohort were largely younger than 60 years of age. In fact, only 1 death was observed among the 815 patients without a luminal stenosis but with detectable CAC (i.e., a CAC score >0). Thus, it is likely that the low prevalence and extent of CAC was insufficient to impart risk or elicit a separation in risk among higher risk CAC patient subsets. In a similar analysis of patients with a 0 CAC score, the majority of patients have normal stress myocardial perfusion scans; thus excluding flow-limiting CAD.27
Prognosis in the Setting of Mild but Nonobstructive Luminal Stenosis
However, CAC was highly predictive of mortality and death or MI for patients with a luminal stenosis. Several critical factors are noteworthy. The extensive nature of CAC in the setting of a luminal stenosis is important and signifies variable atherosclerotic disease burden.28 In our cohort, nearly 80% of patients had detectable CAC with approximately one-third having a CAC score ≥100. Prior evidence reports a similarly high prevalence of CAC in those without obstructive CAD. Importantly, prior research has noted a diminished diagnostic accuracy of CAC to detect obstructive CAD but, in these reports, the ensuing risk of calcified plaque in the absence of an obstructive coronary stenosis was not evaluated such as in our in large prognostic series.28,29 This sizeable burden of CAC for patients with luminal stenosis is one factor promoting worsening mortality; especially when contrasted to the low prevalence of CAC in those without a luminal stenosis.
For those with a luminal stenosis and a high risk CAC score ≥400, there was a near-term separation in risk within 6 months of follow-up. This separation in risk continued to expand during follow-up for those with a high CAC score (i.e., ≥400), such that the mortality rates increased to a high of ~12% at 4 years of follow-up. A similar pattern was noted for CAC scores of 100–399 but the mortality risk achieved statistical significance and risk separation at 1 year of follow-up. Importantly, throughout follow-up, there was no significant difference between patients with a CAC score of 0, 1–10, or 11–99, respectively (Figure 4). This pattern of prognostic findings may signify that higher risk CAC scores may serve as a marker for more diffuse and extensive atherosclerosis. Diffuse, nonobstructive CAD has been reported to double-mortality risk among stable angina patients when compared to asymptomatic individuals.30 Similarly, in a relatively large series of 2,583 patients with <50% stenosis, 3-year mortality was elevated to as a high as a sixfold for patients with 3 vessels with positive luminal stenosis.17 Moreover, similar reports have noted that nearly one in 3 patients with a high risk CAC score ≥1,000 have an abnormal myocardial perfusion scan.31 Information regarding coronary flow reserve may prove additionally useful to risk stratify patients; as a recent reported noted high risk status for patients with a CAC score of 0 and impaired flow reserve.12
Determining the Excess Risk of CAC
We also attempted to perform analyses that explained the excess risk associated with CAC in the setting of a luminal stenosis. As has been observed, there is a strong relationship between CAC and aging. We reported a gradient relationship between mortality, CAC scores, and aging (Figure 5). Similar findings have been reported with CAC and all-cause mortality in prior asymptomatic patient series.6 Thus, it remained important to evaluate the unique contribution that CAC imparts to any risk estimation by eliminating or adjusting the effect of age and other covariates in prognostic models. In our analyses, the proportion of excess risk for CAC that was explained by age was high for both all-cause mortality (i.e., ~34%) and death or MI (i.e., ~35%) models. This data is consistent with prior multivariable models which uniformly identify age as an important component of CAC risk analyses and present age-based nomograms for CAC.2,26,29,32,33 Importantly, though risk may be partially explained by aging, the impact and unique contribution of CAC extent to prognostic models defines a high risk marker of atherosclerotic disease burden.
Study Limitations
Our registry includes a consecutive series of patients referred to CCTA across many institutions. Institutional test-preference and other selection biases may have influenced our findings. There is also a possibility that additional information may be garnered if we had information as to the cause of death. The observational nature of registry data and the ascertainment of outcome data may fail to identify all at-risk patients with hospitalized acute coronary syndromes for patients presenting at remote locations or for patients moving far away from the enrolling site. We limited our multivariable models due to concerns over model overfitting. Thus, it remains likely that the inclusion of alternative and unmeasured covariates may have altered our prognostic findings. Not every patient had a CAC score and this may have biased the current analysis. Importantly, death and death or MI rates were similar for those with and without CAC scores (P >.40). As well, patients who had a CAC score were similar by age, smoking status, and diabetes prevalence when compared to those without CAC scores; although family history and dyslipidemia were more prevalent in those with CAC scores. Finally, a more detailed assessment of non-calcified plaque may provide further insight into the relationship between CAC and ensuing prognostic risk.
NEW KNOWLEDGE GAINED
For patients that undergo an angiographic evaluation, the vast majority of patients have nonobstructive coronary artery disease (CAD). Clinically, many of these patients are considered to be low risk. Recent evidence suggests that atherosclerotic plaque in patients without any obstructive lesions are at elevated prognostic risk. The current evaluation examined the prognostic utility of the extent of CAC provide important clues as to a patient’s risk of death or MI, particularly for patients with a luminal stenosis on CCTA. Importantly, CAC scoring was not helpful for risk stratification purposes in the setting of no identifiable luminal stenosis. For patients with detectable, yet mild luminal stenosis, a CAC score provided important information as to a patient’s long-term mortality or MI risk. For the patients with a luminal stenosis, a CAC score ≥400 was associated with an immediate risk elevation and clear delineation of high risk status. These results can guide further identification of at-risk patients for those frequently identified as having nonobstructive CAD.
CONCLUSION
There is unfolding evidence as to the prognostic significance of atherosclerotic plaque absent of any obstructive lesions. Data from the CONFIRM registry validates prior findings in asymptomatic individuals that the extent of CAC can provide important clues as to a patient’s risk of death or MI, particularly for patients with a luminal stenosis on CCTA. Importantly, CAC scoring was not helpful for risk stratification purposes in the setting of no identifiable luminal stenosis. For patients with detectable, yet mild luminal stenosis, a CAC score provided important information as to a patient’s long-term mortality or MI risk. For the patients with a luminal stenosis, a CAC score ≥400 was associated with a clear delineation of high risk status. For this patient with a luminal stenosis, the detection of a high risk CAC score identifies a patient at significantly elevated mortality risk that may benefit from more intensive, preventive strategies.
Footnotes
This registry was performed independently by the participating institutions and without any grant or in-kind support from any equipment or pharmaceutical manufacturer.
DISCLOSURES
The following authors declared conflicts of interest: Stephan Achenbach: (Grant Support: Siemens and Bayer Schering Pharma; Consultant: Servier); Mouaz Al-Mallah (Consultant: Astellas); Matthew Budoff (Consultant: GE Healthcare); Filippo Cademartiri (Speaker’s Bureau: Bracco Diagnostics; Consultant: Guerbet); Kavitha Chinnaiyan (Grant Support: Bayer Phama, Blue Cross Blue Shield of Michigan); Benjamin Chow (Grant Support and Consultant: GE Healthcare; Grant Support: Servier; Educational Support: TeraRecon); Ricardo Cury (Consultant: Astellas Pharma, GE Healthcare, Novartis); Joerg Hausleiter (Grant Support: Siemens Medical Systems); Philipp Kaumann (Grant Support: GE Healthcare); Jonathon Leipsic (Speaker’s Bureau: GE Healthcare, Edwards Lifesciences, Heartflow, and Circle CVI); Gilbert Raff (Grant Support: Siemens, Bayer Pharma). The remainder of the authors have no disclosures.
References
- 1.Rozanski A, Gransar H, Shaw LJ, Kim J, Miranda-Peats L, Wong ND, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the Eisner (early identification of subclinical atherosclerosis by noninvasive imaging research) prospective randomized trial. J Am Coll Cardiol. 2011;57:1622–32. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 4.Erbel R, Mohlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: The Heinz Nixdorf recall study. J Am Coll Cardiol. 2010;56:1397–406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–70. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 6.Raggi P, Gongora MC, Gopal A, Callister TQ, Budoff M, Shaw LJ. Coronary artery calcium to predict all-cause mortality in elderly men and women. J Am Coll Cardiol. 2008;52:17–23. doi: 10.1016/j.jacc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Shaw LJ, Min JK, Narula J, Lin F, Bairey-Merz CN, Callister TQ, et al. Sex differences in mortality associated with computed tomographic angiographic measurements of obstructive and non-obstructive coronary artery disease: An exploratory analysis. Circ Cardiovasc Imaging. 2010;3:473–81. doi: 10.1161/CIRCIMAGING.109.860981. [DOI] [PubMed] [Google Scholar]
- 8.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah MH, Berman DS, et al. Rationale and design of the confirm (coronary ct angiography evaluation for clinical outcomes: An international multicenter) registry. J Cardiovasc Comput Tomogr. 2011;5:84–92. doi: 10.1016/j.jcct.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Al-Mallah MH, Qureshi W, Lin FY, Achenbach S, Berman DS, Budoff MJ, et al. Does coronary ct angiography improve risk stratification over coronary calcium scoring in symptomatic patients with suspected coronary artery disease? Results from the prospective multicenter international confirm registry. Eur Heart J Cardiovasc Imaging. 2013 doi: 10.1093/ehjci/jet148. [DOI] [PubMed] [Google Scholar]
- 11.Liu YC, Sun Z, Tsay PK, Chan T, Hsieh IC, Chen CC, Wen MS, Wan YL. Significance of coronary calcification for prediction of coronary artery disease and cardiac events based on 64-slice coronary computed tomography angiography. BioMed Res Int. 2013;2013:472347. doi: 10.1155/2013/472347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naya M, Murthy VL, Foster CR, Gaber M, Klein J, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Prognostic interplay of coronary artery calcification and underlying vascular dysfunction in patients with suspected coronary artery disease. J Am Coll Cardiol. 2013;61:2098–106. doi: 10.1016/j.jacc.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the international multicenter confirm (coronary ct angiography evaluation for clinical outcomes: An international multicenter registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–60. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 15.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 17.Lin FY, Shaw LJ, Dunning AM, Labounty TM, Choi JH, Weinsaft JW, et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: A prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol. 2011;58:510–9. doi: 10.1016/j.jacc.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 18.Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L, et al. SCCT guidelines for performance of coronary computed tomographic angiography: A report of the society of cardiovascular computed tomography guidelines committee. J Cardiovasc Comput Tomogr. 2009;3:190–204. doi: 10.1016/j.jcct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–33. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 20.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–9. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 21.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–70. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 22.Shishehbor MH, Litaker D, Pothier CE, Lauer MS. Association of socioeconomic status with functional capacity, heart rate recovery, and all-cause mortality. J Am Med Assoc. 2006;295:784–92. doi: 10.1001/jama.295.7.784. [DOI] [PubMed] [Google Scholar]
- 23.Shaw LJ, Wilson PW, Hachamovitch R, Hendel RC, Borges-Neto S, Berman DS. Improved near-term coronary artery disease risk classification with gated stress myocardial perfusion SPECT. JACC Cardiovasc Imaging. 2010;3:1139–48. doi: 10.1016/j.jcmg.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Achenbach S, Moselewski F, Ropers D, Ferencik M, Hoffmann U, MacNeill B, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: A segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–7. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 25.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, et al. Insights from the NHLBI-sponsored women’s ischemia syndrome evaluation (wise) study: Part II: Gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–9. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 26.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the american college of cardiology foundation clinical expert consensus task force (ACCF/AHA writing committee to update the 2000 expert consensus document on electron beam computed tomography) developed in collaboration with the society of atherosclerosis imaging and prevention and the society of cardiovascular computed tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Mouden M, Timmer JR, Reiffers S, Oostdijk AH, Knollema S, Ottervanger JP, et al. Coronary artery calcium scoring to exclude flow-limiting coronary artery disease in symptomatic stable patients at low or intermediate risk. Radiology. 2013;269:77–83. doi: 10.1148/radiol.13122529. [DOI] [PubMed] [Google Scholar]
- 28.Leber AW, Knez A, von Ziegler F, Becker A, Nikolaou K, Paul S, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: A comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147–54. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 29.O’Rourke RA, Brundage BH, Froelicher VF, Greenland P, Grundy SM, Hachamovitch R, et al. American College of Cardiology/American Heart Association expert consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. J Am Coll Cardiol. 2000;36:326–40. doi: 10.1016/s0735-1097(00)00831-7. [DOI] [PubMed] [Google Scholar]
- 30.Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–44. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 31.Mouden M, Ottervanger JP, Timmer JR, Reiffers S, Oostdijk AH, Knollema S, et al. Myocardial perfusion imaging in stable symptomatic patients with extensive coronary atherosclerosis. Eur J Nucl Med Mol Imaging. 2014;41:136–43. doi: 10.1007/s00259-013-2539-z. [DOI] [PubMed] [Google Scholar]
- 32.Sirineni GK, Raggi P, Shaw LJ, Stillman AE. Calculation of coronary age using calcium scores in multiple ethnicities. Int J Cardiovasc Imaging. 2008;24:107–11. doi: 10.1007/s10554-007-9233-9. [DOI] [PubMed] [Google Scholar]
- 33.Haberl R, Becker A, Leber A, Knez A, Becker C, Lang C, et al. Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: Results of 1,764 patients. J Am Coll Cardiol. 2001;37:451–7. doi: 10.1016/s0735-1097(00)01119-0. [DOI] [PubMed] [Google Scholar]