Abstract
Importance
Although considerable effort has been expended developing drug candidates for Alzheimer disease, none have yet succeeded owing to the lack of efficacy or to safety concerns. One potential shortcoming of current approaches to Alzheimer disease drug discovery and development is that they rely primarily on transformed cell lines and animal models that substantially overexpress wild-type or mutant proteins. It is possible that drug development failures thus far are caused in part by the limits of these approaches, which do not accurately reveal how drug candidates will behave in naive human neuronal cells.
Objective
To analyze purified neurons derived from human induced pluripotent stem cells from patients carrying 3 different presenilin 1 (PS1) mutations and nondemented control individuals in the absence of any overexpression. We tested the efficacy of γ-secretase inhibitor and γ-secretase modulator (GSM) in neurons derived from both normal control and 3 PS1 mutations (A246E, H163R, and M146L).
Design, Setting, and Participants
Adult human skin biopsies were obtained from volunteers at the Alzheimer Disease Research Center, University of California, San Diego. Cell cultures were treated with γ-secretase inhibitor or GSM. Comparisons of total β-amyloid (Aβ) and Aβ peptides 38, 40, and 42 in the media were made between vehicle- vs drug-treated cultures.
Main Outcomes and Measures
Soluble Aβ levels in the media were measured by enzyme-linked immunosorbent assay.
Results
As predicted, mutant PS1 neurons exhibited an elevated Aβ42:Aβ40 ratio (P <.05) at the basal state as compared with the nondemented control neurons. Treatment with a potent non–nonsteroidal anti-inflammatory druglike GSM revealed a new biomarker signature that differs from all previous cell types and animals tested. This new signature was the same in both the mutant and control neurons and consisted of a reduction in Aβ42, Aβ40, and Aβ38 and in the Aβ42:Aβ40 ratio, with no change in the total Aβ levels.
Conclusions and Relevance
This biomarker discrepancy is likely due to overexpression of amyloid precursor protein in the transformed cellular models. Our results suggest that biomarker signatures obtained with such models are misleading and that human neurons derived from human induced pluripotent stem cells provide a unique signature that will more accurately reflect drug response in human patients and in cerebrospinal fluid biomarker changes observed during GSM treatment.
Alzheimer disease (AD) is the leading cause of dementia in elderly individuals.1 A major problem in AD drug development is the high failure rate in clinical trials.2 Drug toxicity, the lack of suitable animal disease models, and the fact that drug exposure in humans begins only in clinical trials are some of the possible causes of failure.3,4 Improvement in the drug screening and development process could potentially lead to faster and more successful drug discovery for AD.
γ-Secretase modulators (GSMs), as opposed to γ-secretase inhibitors (GSIs), are a class of drugs that modify the cleavage at the γ sites and spare the ε-cleavage sites. It is the ε-cleavage sites that are responsible for the Notch processing that generates the notch intracellular domain, a peptide critical for cellular differentiation. The non–nonsteroidal anti-inflammatory drug(NSAID)–like GSM compounds are highly potent small molecules with 50% inhibitory concentrations (IC50s) in the low nanomolar range containing a bridged aromate scaffold. They offer significant improvement over the first-generation NSAID and second-generation NSAID-like GSM compounds.5,6 In animal and cell culture experiments, these second-generation non-NSAID–like GSMs have been shown to reduce the levels of β-amyloid (Aβ) 42 and to a lesser degree Aβ40 peptides, while concomitantly increasing Aβ38 and Aβ37 levels. And they are thought to increase processivity of amyloid precursor protein (APP)–C-terminal fragment γ-secretase substrates based on studies using reconstituted enzyme assay systems, APP-overexpressing cell lines, and APP transgenic mice.5,7 Their effectiveness in native human neurons is not known. Mutations in the genes encoding for presenilin 1 and 2 (PS1 and PS2) and APP cause fully penetrant dominantly inherited early-onset familial AD(EOFAD). It is also not known whether GSM will have differential effects based on specific PS1 mutations.
Neural stem cells (NSCs) and neurons derived from human induced pluripotent stem cells (iPSCs) have been used for screening of compounds in various neurologic diseases.8,9 Human iPSC models have the potential to accelerate the drug-discovery process by enabling candidate compounds to be exposed to human neuronal cells during the drug screening and optimization stages and not waiting until the phase 1 clinical trial.4 However, it is not known how biomarker readouts and potencies from the iPSC model systems would differ from the transformed mammalian cell culture model systems currently used in drug screening and optimization. Such information would provide valuable guidance during the drug screening and lead optimization process.
Methods
β-Amyloid Assays
Fibroblasts were plated at a density of 2.6 × 104 cells/cm2, sorted neurons at 4.9 × 105 cells/cm2, and iPSCs and NSCs at 1 × 105 cells/cm2. For fibroblasts and iPSC, media were concentrated using Amicon Ultra-0.5 Centrifugal Unit (Millipore UFC500396) by 4-fold. Media were collected on day 4 (fibroblasts) or day 5 (neurons) after plating for Aβ measurement. For drug-treated samples, media were collected 3 days after compound treatment. MesoScale human (4G8) Aβ 3-plex ultrasensitive kit K15141E-2 and Custom Human Total AβkitN45CA-1 were used to assay Aβ peptides. Statistical analyses were performed using 1-way analysis of variance and the Dunnett multiple comparison test. Informed consent was obtained from the participants under the University of California, San Diego, institutional review board protocol and guidelines.
Drug Treatment
Cells were treated with GSMs or GSIs at various concentrations in dimethyl sulfoxide (0.01% v/v) for 3 days prior to the media collection.
Remaining methods and reagents are detailed in the eAppendix in the Supplement.
Results
Generation of Induced Pluripotent Stem Cells From PS1 Mutations
We generated iPSC lines from nondemented control individuals and patients with PS1 mutations. Fibroblasts from 2 patients with the A246E mutation were obtained from Coriell (AG06840C and AG06848C). Although the PS1 mutations are nearly 100% penetrant, we chose to reprogram these 2 patients with familial AD (FAD) because the disease status was also confirmed by postmortem autopsy. In addition, skin biopsies were obtained from PS1 H163R and PS1 M146L mutation carriers and 2 nondemented control individuals followed up at the Shiley Marcos Alzheimer Disease Research Center at the University of California, San Diego. The nondemented volunteers were in their 80s and did not have any memory impairment or ADsymptoms.Oct3/4,Sox2, Klf4, and c-Myc were delivered to the fibroblasts using the Maloney Murine Leukemia Virus for reprogramming.10 To track fibroblasts that were transduced and to monitor retroviral reactivation in the iPSC, green fluorscent protein was coinfected with the 4 factors.11 Valproic acid was used briefly to improve the reprogramming efficiency.12
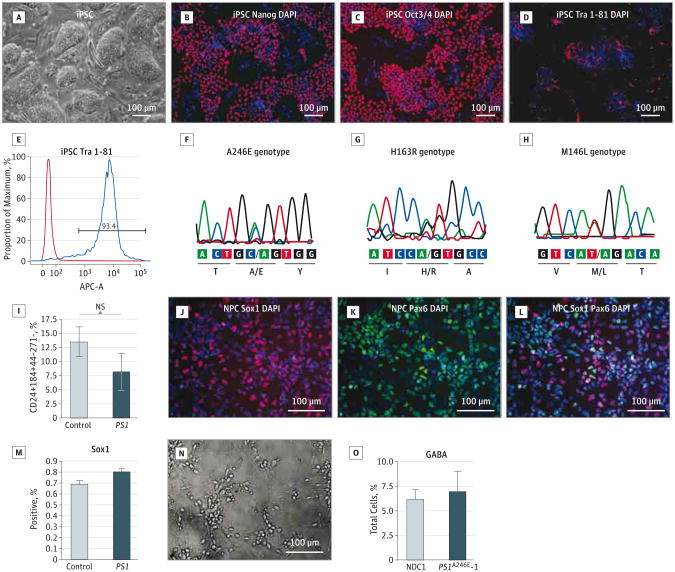
Patient samples and clinical information are summarized in the Table. Participant nondemented control (NDC) 1 was previously reported.13 The iPSCs formed condensed colonies and expressed pluripotent stem cell markers, such as Oct3/4, Sox2, and Tra 1-81, when examined by immunofluorescent staining (Figure 1A-D). Most of the cells were Tra 1-81 positive, as shown by flow cytometry (Figure 1E). We tested the ability of the iPSC lines to form 3 germ layers by generation of embryoid bodies. After replating the embryoid bodies, cells were stained with α-fetal protein, desmin, and Map2b as endodermal, mesodermal, and ectodermal markers, respectively (eFigure 1A-D in the Supplement). Additionally, the PS1 mutations were maintained after reprogramming, as shown by sequencing of genomic DNA (Figure 1F-H). Using quantitative polymerase chain reaction, we verified that the retroviruses were silenced in the iPSC (eFigure 1F in the Supplement).
Table. Summary of Patient Information.
| Patient | PS1 Mutation | ApoE Status | Age at Biopsy, y | Age at Onset, y | Mental Status | No. of iPSC Lines Studied |
|---|---|---|---|---|---|---|
| NDC1 | None | 2/3 | 85 | NA | Normal | 2 |
| NDC3 | None | 3/3 | 85 | NA | Normal | 2 |
| PS1A246E-1 | A246E | 3/3 | 56 | Approximately 50 | Demented | 3 |
| PS1A246E-2 | A246E | 3/4 | 56 | Approximately 46 | Vegetative state | 2 |
| PS1H163R | H163R | 3/3 | 41 | NA | Normal | 2 |
| PS1M146L | M146L | 4/4 | 42 | 36 | Demented | 1 |
Abbreviations: ApoE, apolipoprotein E; iPSC, induced pluripotent stem cell; NA, not applicable; NDC, nondemented control; PS1, presenilin 1.
Figure 1. Derivation and Differentiation of Induced Pluripotent Stem Cells (iPSCs).
A, Bright-field image of iPSCs. B-D, Images of iPSC stained with pluripotent stem cell markers: Nanog (B, red indicates Nanog and blue, DAPI), Oct3/4 (C, red indicates Oct3/4 and blue, DAPI), and Tra 1-81 (D, red indicates Tra 1-81 and blue, DAPI). E, Fluorescent-activated cell sorting quantification of percentage of positive Tra 1-81 cells in the iPSCs. F-H, Presenilin 1 (PS1) mutant genotype by polymerase chain reaction: A246E (F), H163R (G), and M146L (H). I, Quantitative analysis of the percentage of neural stem cell (NSC)–expressing Sox1 in the control and PS1 mutant lines. J and K, Immunofluorescent images of fluorescent-activated cell sorting–enriched NSCs express Sox1 (J, red indicates Sox1 and blue, DAPI) and Pax6 (K, green indicates Pax6 and blue, DAPI). L, Most of the Sox1 coexpress Pax6 (red indicates Sox1; green, Pax6; and blue, DAPI). M, Quantitative analysis of the percentage of NSCs expressing Sox1 in the control and PS1 lines. N, Bright-field image of sorted neurons at day 5 after plating. O, Evaluation of the subtype of neurons in the sorted neurons with anti–γ-aminobutyric acid antibody. Statistical analysis performed using the t test. NS indicates not statistically significant.
Generation of Neural Stem Cells and Neurons
We used a neural differentiation and sorting protocol that we previously developed14 to generate NSCs and neurons and to enrich the population of interest to a high degree of purity for downstream use in quantification and precise biochemical measurements. The iPSCs were differentiated to neural rosettes on PA6 stromal cells, then supplemented with Noggin and SB 431542 to inhibit TGFβ signaling (eFigure 1G in the Supplement).15,16 A combination of cell surface markers CD24+/CD184+/CD271−/CD44− was used to enrich the NSCs by fluorescent-activated cell sorting. We observed a lower neurogenic potential in the PS1 mutant lines compared with the NDC lines; however, this was not statistically significant (Figure 1I). The sorted NSCs were expanded and passaged in culture and expressed NSC markers Sox1, Pax6, and Nestin (Figure 1J-L; eFigure 1H-J in the Supplement). They were also Ki-67 positive, indicating that they were actively dividing (eFigure 1H-J in the Supplement). The percentages of the Sox1-positive cells were similar between the NDC and PS1 mutant lines (Figure 1M). The sorted NSCs were subsequently differentiated to neurons and glia by withdrawing basic fibroblast growth factor. After 3 weeks of differentiation, cell surface markers were used to select CD24+/CD184−/CD44− neurons from the differentiation cultures by fluorescent-activated cell sorting. The neurons developed a distinct dendritic and axonal morphology and expressed β-III tubulin and Map2b (Figure 1N; eFigure 1K-N in the Supplement). The percentages of the γ-aminobutyric acidergic cells as labeled by anti– γ-aminobutyric acid antibody in the control and mutant neurons were similar (Figure 1O). It has previously been shown that neurons generated by these methods produce a voltage-dependent action potential and electric currents, and they possess spontaneous synaptic activity, which could be reversibly blocked by a γ-aminobutyric acid type A receptor antagonist.13
Assessment of Secreted Aβ38, Aβ40, and Aβ42 Levels in Human Fibroblasts and Neurons
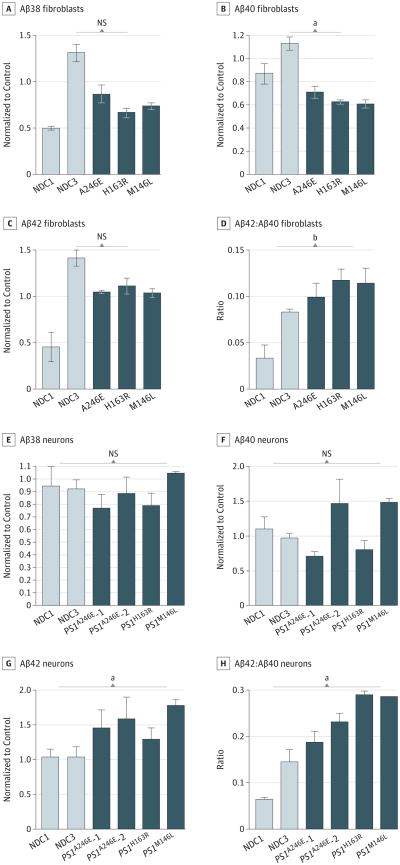
The EOFAD-linked PS1 mutations are known to cause an elevated Aβ42:Aβ40 ratio.17 We determined Aβ38, Aβ40, and Aβ42 levels in the conditioned media from the fibroblasts and neurons derived from iPSC by enzyme-linked immunosorbent assay. In the fibroblasts, the levels of Aβ38, Aβ40, and Aβ42 were low in the media from the cultures; therefore, the media were equivalently concentrated to detect the levels of Aβ38, Aβ40, and Aβ42. For all 3PS1 mutations tested (A246E, H163R, and M146L), we observed an elevation of the Aβ42:Aβ40 ratio (P <.001) in the fibroblast cultures compared with the control (Figure 2A-D). We then examined the purified neurons from 3-week–differentiated cultures. The PS1 mutant neurons from all 3 PS1 mutations exhibited an elevated Aβ42:Aβ40 ratio (P <.05) due to elevated levels of Aβ42 (Figure 2E-H).
Figure 2. β-Amyloid (Aβ) Peptide Variant Measurements in Conditioned Media From Fibroblasts and Neurons Derived From Nondemented Control (NDC) and Presenilin 1 (PS1) Mutant Induced Pluripotent Stem Cells.
A-D, β-Amyloid peptide variant measurements in human fibroblasts: Aβ38 (A), Aβ40 (B), Aβ42 (C), and Aβ42:Aβ40 ratio (D). E-H, β-Amyloid peptide variant measurements in human neurons derived from induced pluripotent stem cells: Aβ38 (E), Aβ40 (F), Aβ42 (G), and Aβ42:Aβ40 ratio (H). Statistical analysis performed using1-way analysis of variance and t test. NS indicates not statistically significant. The error bars indicate standard error of the mean.
aP < .05.
bP < .001.
Treatment With the γ-Secretase Inhibitor Semagacestat in Sorted Neurons
An important potential use of the human iPSC disease model system is preclinical drug candidate screening and evaluation. Previous AD iPSC and induced neuron studies examined the effects of first-generation GSI treatment13 and first-generation NSAID GSM treatment.18 However, the new generation of highly potent GSIs and GSMs, which are most clinically relevant, have not been tested in neurons derived from human iPSC.
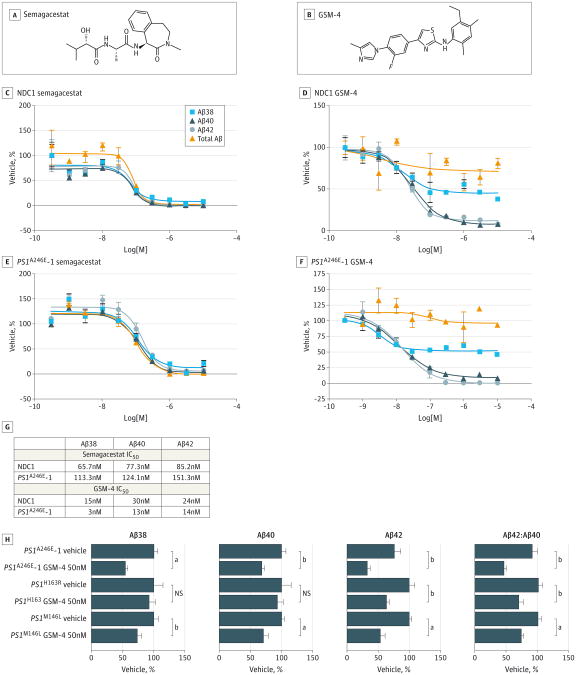
We generated 10-point concentration response curves for semagacestat, because it is one of the best-characterized GSIs of its class (Figure 3A), using purified neurons derived from human iPSC from both controls and a PS1 mutant. Consistent with its property of inhibiting γ-secretase cleavage at the ε site, semagacestat lowered the Aβ42, Aβ40, Aβ38, and total Aβ levels in a dose-dependent manner in both NDC1 and PS1A246E-1 neurons (Figure 3C and E). However, we found that the semagacestat IC50s assays using iPSCs derived in human neurons were higher than those previously reported in neurons from APPswe-overexpressing transgenic mice (Figure 3G).19
Figure 3. Treatment of Neurons Derived From Induced Pluripotent Stem Cells With γ-Secretase Inhibitor and γ-Secretase Modulator (GSM).
A, Chemical structure of γ-secretase inhibitor semagacestat. B, Chemical structure of GSM-4. C-F, Dose-response curves of semagacestat-treated nondemented control (NDC) 1 neurons (C), GSM-4–treated NDC1 neurons (D), semagacestat-treated presenilin 1 (PS1)A246E–1 mutant neurons (E), and GSM-4–treated PS1A246E-1 mutant neurons (F). G, Fifty percent inhibitory concentrations (IC50) of semagacestat and GSM-4 in NDC1 and PS1A246E-1 mutant neurons. H, β-Amyloid (Aβ) 38, Aβ40, and Aβ42 levels and Aβ42:Aβ40 ratios in neurons derived from 3 different PS1 mutations treated with GSM-4. Statistical analysis performed using 1-way analysis of variance and the Dunnett multiple comparison test. NS indicates not statistically significant. The error bars indicate the standard error of the mean. N = 3.
aP < .01.
bP < .05.
Treatment With γ-Secretase Modulator 4 in Sorted Neurons Reduces Aβ42, Aβ40, Aβ38, and the Aβ42:Aβ40 Ratio
We chose to examine GSM-4 because it is a potent second-generationnon-NSAID–like GSM(Figure 3B)7 and has been well characterized and tested in cell culture and transgenic mice overexpressing APP. Both structurally and functionally, GSM-4 is highly representative of the non-NSAID–like GSM class of compounds.20
We evaluated the dose-response curves in the sorted human neurons to assess GSM-4 potencies. Ten-point concentration response curves of GSM-4 were generated by treatment of NDC1 and PS1A246E-1 neurons (Figure 3D and F). The IC50s obtained were similar to previously reported values,7 which were in the low nanomolar range (Figure 3G). In these studies, treatment with GSM-4 lowered the levels of secreted Aβ42 and Aβ40, consistent with previously reported studies, but not Aβ38, which differed from previously reported studies using overexpressing human APP cell lines and transgenic mice.7 We also measured the total Aβ levels to ensure that the APP processing was not inhibited because the GSM-4 spares the ε site. As expected, total Aβ levels were unaffected (Figure 3D and F).
γ-Secretase modulator 4 was most potent at lowering Aβ42, followed by Aβ40 then Aβ38 in both control and PS1 mutant neurons. We found that the IC50 was lower in the PS1 mutant neurons compared with the NDC1 neurons, suggesting that GSM-4 is more effective against the PS1 A246E mutation than the wild-type PS1.
γ-Secretase Modulator 4 Reduces Aβ42, Aβ40, Aβ38, and the Aβ42:Aβ40 Ratio in Neurons Derived From Multiple PS1 Mutation Induced Pluripotent Stem Cell Lines
The exact mechanism by which the PS1 mutations affect γ-secretase function is not understood nor is it known whether high-potency GSM compounds would be effective against different PS1 mutations when tested in differentiated human neuronal cultures. To examine GSM efficacy against different PS1 mutations, we tested GSM-4 in neurons derived from 3 different mutations A246E, H163R, and M146L. γ-Secretase modulator 4 at a concentration of 50nM was effective in lowering Aβ42 and the Aβ42:Aβ40 ratio in neurons from all 3 mutant iPSC lines, although it was most effective against the A246E mutation (Figure 3H). Because GSM-4 was more effective in lowering Aβ42 than Aβ40, the Aβ42:40 ratio also decreased. This may have significance in EOFAD because an increased Aβ42:Aβ40 ratio has been thought to be a significant biomarker for most forms of EOFAD.21,22 Preferential inhibition of Aβ42 over Aβ40 would be beneficial in sporadic AD as well because Aβ42 is more likely to oligomerize and form toxic aggregates.
γ-Secretase Modulator 4Lowers Aβ42, Aβ40, and Aβ38 in Fibroblasts and Induced Pluripotent Stem Cells, Neural Stem Cells, and Differentiated Neuronal Cultures
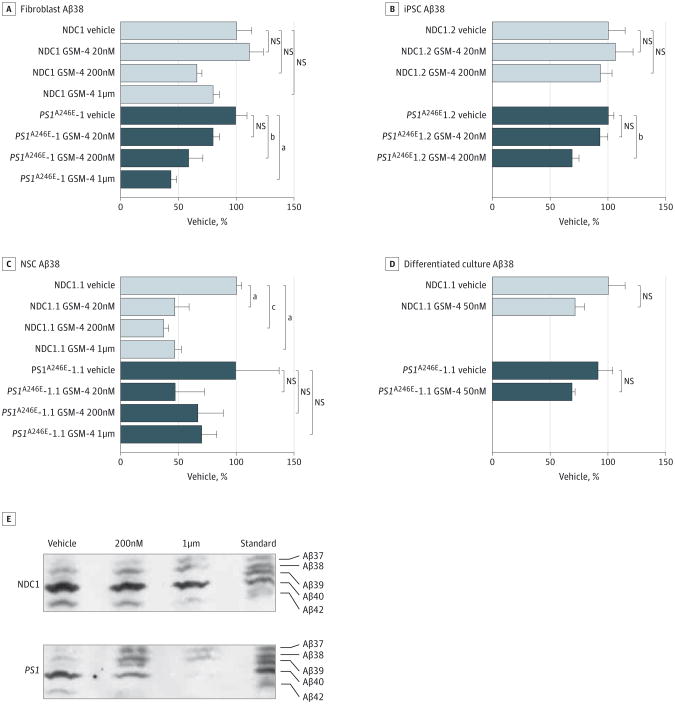
To determine whether our observations in differentiated neurons were cell-type specific, we further tested GSM-4 in human primary fibroblasts iPSCs, NSCs, and 3-week–differentiated neuronal cultures derived from human iPSC. We observed a similar pattern in all of the cell types tested (Figure 4A-D; eFigure 2 in the Supplement) that GSM-4 lowers Aβ38, Aβ40, Aβ42, and the Aβ42:Aβ40 ratio, without changing the levels of total Aβ peptides.
Figure 4. Treatment of Different Cell Types With γ-Secretase Modulator (GSM) 4.
A-D, β-Amyloid (Aβ) 38 from samples treated with GSM-4 at different concentrations: nondemented control (NDC) 1 and presenilin 1 (PS1)A246E–1 fibroblasts (A), NDC1 and PS1A246E-1 iPSCs (B), NDC1 and PS1A246E-1 NSCs (C), and differentiated neuronal cultures from NDC1 and PS1A246E-1 (D). E, Urea gel showing various Aβ species from conditioned media from 3-week– differentiated cultures of NDC and PS1A246E-1 treated with vehicle, 200nM, or 1μM of GSM-4. Statistical analysis performed using 1-way analysis of variance and the Dunnett multiple comparison test. NS indicates not statistically significant. The error bars indicate standard error of the mean. N = 3.
aP < .01.
bP < .05.
cP < .001.
The GSM-4 is not equally effective across the different cell types. For example, it is most efficacious in neurons, followed by NSCs or fibroblasts (eFigure 3 in the Supplement). It has been reported that γ-secretase activity could vary in different species and different cell types based on the composition of the various γ-secretase subunits.23 Therefore, it is possible that the level or type of GSM modulation of the γ-secretase enzyme complex could be cell-type dependent.
We next asked whether GSM-4 is effective at altering Aβ peptide variant profiles in human cells harboring an APP duplication mutation, which is similar to the case in patients with Down syndrome who develop AD later in life.24 We tested GSM-4 in NSCs derived from APP duplication mutation iPSCs, which contained an extra copy of APP.13 We found that GSM-4 is effective in lowering Aβ38, Aβ40, and Aβ42 without affecting total Aβ levels (eFigure 4 in the Supplement), suggesting that this GSM could be a potential candidate for ameliorating AD symptoms in patients with Down syndrome.
Aβ37 Generation Is Increased With γ-Secretase Modulator 4 Treatment
Our results suggested that other smaller Aβ peptides were being generated on treatment of these cells with GSM-4. To test our hypothesis, we performed a Western blot of Aβ peptides from conditioned media collected from GSM-4–treated 3-week– differentiated neuronal cultures derived from iPSCs using a urea gel system capable of resolving multiple Aβ peptide variants. On treatment of these cultures for 3 days with GSM-4, we observed a decrease in Aβ40 and Aβ42, more significantly in PS1 A246E mutation than NDC1, and an increased production of other smaller peptides such as Aβ39 and Aβ37 (Figure 4E), confirming that the GSM-4 treatment generated additional smaller Aβ peptide species. This is consistent with the lack of effect on total Aβ peptide levels in these cells on treatment with GSM-4. Previously published studies have shown that structurally related soluble GSMs promote the generation of multiple carboxy-truncated Aβ species including Aβ37, Aβ34, and Aβ33.25
Discussion
Our study reports the generation of iPSCs from multiple PS1 mutation carriers and the application of these lines for the evaluation of a promising class of therapeutic compounds. Our data revealed that these compounds are effective against a number of different PS1 mutations and that there are distinct differences between the effects of a particular diaryl aminothia-zole–containing GSM (GSM-4) in neurons derived from patients compared with those effects on Aβ peptide variants observed in APP-overexpressing cell lines. Although treatment times were longer in our study (3 days) compared with those described previously (1 day),7 in each of the latter cell-based assay systems, human APP was overexpressed and this alone may explain the different effects elicited by this particular GSM on Aβ peptide variant levels. It is possible that mutations alter the catalytic pocket of the γ-secretase complex, through slightly changing the enzyme's conformation, and this modifies the precise γ-site cleavages and thus affects the relative amounts of the various Aβ species. An alternative hypothesis is that non-NSAID–like GSMs, such as GSM-4, potentiate the γ-secretase processivity, lowering levels of Aβ42 and Aβ40 by increasing a carboxy-peptidaselike activity and chewing these longer Aβ peptide species into shorter ones, as previously suggested.26-28
In all cell types tested in this study, GSM-4 consistently preferentially lowered Aβ42 more efficaciously than either Aβ40 or Aβ38. By testing these types of compounds in human neuronal cells expressing the endogenous levels of APP and PS1 (either mutant or wild type), we showed that GSM-4 also lowers Aβ38 levels and generates other Aβ species such as Aβ39 and Aβ37. Furthermore, our observation that GSM-4 is most effective in purified neurons suggests that γ-secretase may have different efficiencies in different cell types. Collectively, our results suggested that there may be subtle differences in γ-secretase activities in different cell types and these differences could potentially be masked by overexpression of APP.
Previous studies have shown that the ratio of Aβ42:Aβ40 resulting from certain PS1 mutations is resistant to various types of GSM treatment.29 In this report, we tested a highly potent second-generation GSM and found lowering of Aβ42:Aβ40 ratios in multiple PS1 mutation–expressing neurons. However, we did not test the previously reported most-resistant PS1 mutations (eg, L166P).29 The observed discrepancies could be due to the type of mutation and/or overexpressed vs endogenous levels of APP and/or PS1 mutant proteins. Our data suggested that both of these factors are important. Our measurement of the IC50 of semagacestat using human neurons is about 5-fold higher than the reported IC50s previously published when tested in APP-overexpressing cell lines.19 These differences could be significant and could help to explain the failure of this and related compounds in human trials.30
Conclusions
A genetic study showed that a mutation at the β-site APP-cleaving enzyme 1 in APP, which resulted in decreased activity of β-site APP-cleaving enzyme 1, appeared to be protective against developing AD.31 This finding highlights the importance of continuing to develop drugs that affect APP-processing pathways as a mainstay of therapy. This human neuronal testing system may be particularly useful in preclinical drug discovery and development as either a primary or secondary screening/optimization tool.
In clinical studies, such as the scheduled prevention trials for patients at risk as FAD carriers, individualized testing against various FAD-linked mutations could be performed using this system to select the most effective compound prior to administration of the drug. This human neuronal model system would be ideal for identifying potentially resistant FAD-linked mutations that have in fact been reported in other less-sophisticated cell-based assay systems that use cells overexpressing either PS1, PS2, and/or APP wild type or mutant proteins in animal models.29 The human neuronal culture system described here in detail offers an opportunity to identify the most potent and most efficacious compounds preclinically and so will help to avoid failure in the more expensive in vivo animal or later developmental phases.
Supplementary Material
Acknowledgments
Funding/Support: This work was funded by National Institutes of Health grants U01-AG10483, U01-NS074501 (Dr Wagner), P50 AG005131-26 (Alzheimer's Disease Research Centers pilot grant to Dr Yuan), and P50-AG016750 (Dr Rungman); California Institute of Regenerative Medicine Comprehensive grant RC1-00116 (Dr Goldstein); Cure Alzheimer's Fund (Drs Tanzi and Wagner); and California Institute of Regenerative Medicine clinical training fellowship (Dr Yuan) and predoctoral fellowship (Dr Israel).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We thank the volunteers who donated skin tissues; the University of California, San Diego, Alzheimer's Disease Research Center for patient referral (AGO 5131); and Paul Aisen, MD (University of California, San Diego), for critical reading of the manuscript. He is funded by National Institutes of Health grant U01-AG10483.
Footnotes
Author Contributions: Dr Yuan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Tanzi, Koo, Ringman, Goldstein, Wagner, Yuan. Acquisition, analysis, or interpretation of data: Liu, Waltz, Woodruff, Ouyang, Israel, Herrera, Sarsoza, Goldstein, Wagner, Yuan.
Drafting of the manuscript: Goldstein, Yuan.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Liu, Waltz, Yuan.
Obtained funding: Goldstein, Wagner, Yuan.
Administrative, technical, or material support: Ouyang, Israel, Herrera, Ringman, Goldstein.
Study supervision: Tanzi, Koo, Goldstein, Wagner, Yuan.
Conflict of Interest Disclosures: Drs Tanzi and Wagner are shareholders and cofounders of a privately held company (Neurogenetic Pharmaceuticals Inc) that holds rights to a γ-secretase modulator currently in preclinical development. No other disclosures were reported.
References
- 1.Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the challenge of the second century. Sci Transl Med. 2011;3(77):sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berk C, Sabbagh MN. Successes and failures for drugs in late-stage development for Alzheimer's disease. Drugs Aging. 2013;30(10):783–792. doi: 10.1007/s40266-013-0108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3(8):711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 4.Grskovic M, Javaherian A, Strulovici B, Daley GQ. Induced pluripotent stem cells: opportunities for disease modelling and drug discovery. Nat Rev Drug Discov. 2011;10(12):915–929. doi: 10.1038/nrd3577. [DOI] [PubMed] [Google Scholar]
- 5.Chávez-Gutiérrez L, Bammens L, Benilova I, et al. The mechanism of γ-secretase dysfunction in familial Alzheimer disease. EMBO J. 2012;31(10):2261–2274. doi: 10.1038/emboj.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan SH, Shaner M. Bioengineered stem cells in neural development and neurodegeneration research. Ageing Res Rev. 2013;12(3):739–748. doi: 10.1016/j.arr.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kounnas MZ, Danks AM, Cheng S, et al. Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer's disease. Neuron. 2010;67(5):769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee G, Ramirez CN, Kim H, et al. Large-scale screening using familial dysautonomia induced pluripotent stem cells identifies compounds that rescue IKBKAP expression. Nat Biotechnol. 2012;30(12):1244–1248. doi: 10.1038/nbt.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng J, Liu Q, Rao MS, Zeng X. Using human pluripotent stem cell-derived dopaminergic neurons to evaluate candidate Parkinson's disease therapeutic agents in MPP+ and rotenone models. J Biomol Screen. 2013;18(5):522–533. doi: 10.1177/1087057112474468. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Chan EM, Ratanasirintrawoot S, Park IH, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27(11):1033–1037. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
- 12.Huangfu D, Maehr R, Guo W, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26(7):795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Israel MA, Yuan SH, Bardy C, et al. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature. 2012;482(7384):216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan SH, Martin J, Elia J, et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One. 2011;6(3):e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawasaki H, Mizuseki K, Nishikawa S, et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28(1):31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 16.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheuner D, Eckman C, Jensen M, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2(8):864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 18.Koch P, Tamboli IY, Mertens J, et al. Presenilin-1 L166P mutant human pluripotent stem cell-derived neurons exhibit partial loss of γ-secretase activity in endogenous amyloid-β generation. Am J Pathol. 2012;180(6):2404–2416. doi: 10.1016/j.ajpath.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Mitani Y, Yarimizu J, Saita K, et al. Differential effects between γ-secretase inhibitors and modulators on cognitive function in amyloid precursor protein-transgenic and nontransgenic mice. J Neurosci. 2012;32(6):2037–2050. doi: 10.1523/JNEUROSCI.4264-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oehlrich D, Berthelot DJC, Gijsen HJ. γ-Secretase modulators as potential disease modifying anti-Alzheimer's drugs. J Med Chem. 2011;54(3):669–698. doi: 10.1021/jm101168r. [DOI] [PubMed] [Google Scholar]
- 21.Kumar-Singh S, Theuns J, Van Broeck B, et al. Mean age-of-onset of familial alzheimer disease caused by presenilin mutations correlates with both increased Abeta42 and decreased Abeta40. Hum Mutat. 2006;27(7):686–695. doi: 10.1002/humu.20336. [DOI] [PubMed] [Google Scholar]
- 22.Potter R, Patterson BW, Elbert DL, et al. Increased in vivo amyloid-β42 production, exchange, and loss in presenilin mutation carriers. Sci Transl Med. 2013;5(189):189ra177. doi: 10.1126/scitranslmed.3005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serneels L, Van Biervliet J, Craessaerts K, et al. Gamma-secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer's disease. Science. 2009;324(5927):639–642. doi: 10.1126/science.1171176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lott IT, Head E. Down syndrome and Alzheimer's disease: a link between development and aging. Ment Retard Dev Disabil Res Rev. 2001;7(3):172–178. doi: 10.1002/mrdd.1025. [DOI] [PubMed] [Google Scholar]
- 25.Wagner SL, Zhang C, Cheng S, et al. Soluble γ-secretase modulators selectively inhibit the production of the 42-amino acid amyloid β peptide variant and augment the production of multiple carboxy-truncated amyloid β species. Biochemistry. 2014;53(4):702–713. doi: 10.1021/bi401537v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takami M, Nagashima Y, Sano Y, et al. Gamma-secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci. 2009;29(41):13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okochi M, Tagami S, Yanagida K, et al. γ-Secretase modulators and presenilin 1 mutants act differently on presenilin/γ-secretase function to cleave Aβ42 and Aβ43. Cell Rep. 2013;3(1):42–51. doi: 10.1016/j.celrep.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Crump CJ, Johnson DS, Li YM. Development and mechanism of γ-secretase modulators for Alzheimer's disease. Biochemistry. 2013;52(19):3197–3216. doi: 10.1021/bi400377p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kretner B, Fukumori A, Gutsmiedl A, et al. Attenuated Abeta42 responses to low potency gamma-secretase modulators can be overcome for many pathogenic presenilin mutants by second-generation compounds. J Biol Chem. 2011;286(17):15240–15251. doi: 10.1074/jbc.M110.213587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coric V, van Dyck CH, Salloway S, et al. Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch Neurol. 2012;69(11):1430–1440. doi: 10.1001/archneurol.2012.2194. [DOI] [PubMed] [Google Scholar]
- 31.Jonsson T, Atwal JK, Steinberg S, et al. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012;488(7409):96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.