Abstract
Background
Neuronal guidance cues influence endothelial cell (EC) behavior to shape the embryonic vascular system. The repulsive neuronal guidance cue, Semaphorin 3E (Sema3E), is critical for creating avascular zones that instruct and subsequently pattern the first embryonic vessels, the paired dorsal aortae (DA). Sema3E−/− embryos develop highly branched plexus-like vessels during vasculogenesis, instead of smooth paired vessels. Unexpectedly, despite these severe DA patterning defects, mutant mice are viable throughout adulthood.
Results
Examination of Sema3E−/− mice reveals that the plexus-like DA resolve into single, unbranched vessels between embryonic (E) day E8.25 and E8.75. Although fusion of Sema3E−/− DA occurs slightly earlier than in heterozygotes, the DA are otherwise indistinguishable, suggesting a complete ‘rescue’ in their development. Resolution of the DA null plexuses occurs by remodeling rather than via changes in cell proliferation or death.
Conclusions
Normalization of Sema3E−/− DA patterning defects demonstrates resilience of embryonic vascular patterning programs. Additional repulsive guidance cues within the lateral plate mesoderm likely re-establish avascular zones lost in Sema3E−/− embryos and guide resolution of mutant plexus into branchless, parallel aortae. Our observations explain how Sema3E−/− mice survive throughout development and into adulthood, despite severe initial vascular defects.
Keywords: Sema3E, neuronal guidance cue, repulsive, endothelial, avascular, angiogenic remodeling, notochord, lateral plate mesoderm
INTRODUCTION
The vascular system consists of a highly organized and stereotyped network of blood vessels. Multiple signals from surrounding tissues coordinate and instruct vessels to form at precise locations within the embryo (Stone et al., 1995; Mukouyama et al., 2002; Hogan and Bautch, 2004; Reese et al., 2004; Adams and Eichmann, 2010). Key signaling components in this process are the neuronal guidance cues (NGCs) (Eichmann et al., 2005; Suchting et al., 2006). Originally termed for their ability to guide axonal growth, NGCs comprise a unique class of molecules that also have the ability to influence endothelial cell (EC) behavior in a positive or negative fashion, thereby directing blood vessel growth and patterning in the embryo. NGCs are categorized into four main classes, including Ephrins and Eph receptors, Semaphorins and Plexin receptors, Slits and Robo receptors and Netrins and Unc5 receptors (Dickson, 2002). NGCs are either secreted from, or membrane-tethered to, surrounding tissues, while cognate receptors are expressed on ECs. NGCs generally exhibit repulsive effects on ECs and direct blood vessel formation into areas lacking repulsive signals. Numerous loss-of-function studies in mice, frogs and zebrafish have demonstrated the profound influence of NGC signaling in shaping the embryonic vasculature (Wang et al., 1998; Helbling et al., 2000; Gitler et al., 2004; Lu et al., 2004; Torres-Vazquez et al., 2004; Bedell et al., 2005; Gu et al., 2005).
Semaphorins are secreted and/or membrane-tethered proteins that act as guidance molecules in both the nervous and vascular systems. We recently showed that Semaphorin 3E (Sema3E), a secreted member of the Semaphorin class 3 family, plays a significant role in guiding ECs during vasculogenesis (Meadows et al., 2012). Activation of the PlexinD1 receptor via Sema3E binding promotes intracellular signaling that results in the inhibition of EC migration towards the source of Sema3E, both in vivo and in vitro (Gu et al., 2005; Kigel et al., 2008; Sakurai et al., 2010; Meadows et al., 2012). Induction of this pathway leads to downstream inactivation of the small GTPase, R-Ras, causing rapid disassembly of integrin complexes and failure to adhere to the extracellular matrix (ECM) (Sakurai et al., 2010). This process is further promoted by the stimulation of another small GTPase Arf6, which induces internalization of integrins at focal adhesion sites in ECs. The loss of integrin-mediated adhesion culminates in the collapse of the actin cytoskeleton. Consequently, cellular extensions required for cell motility, such as filipodia and lamelipodia, retract from the Sema3E gradient thereby short-circuiting EC migration.
Ablation of Sema3E-PlexinD1 signaling in the embryo results in abnormal vascular patterning and angiogenesis (Gu et al., 2005; Zhang et al., 2009; Kim et al., 2011; Meadows et al., 2012). Mice lacking Sema3E show severe defects in the formation of the paired dorsal aortae (DA) and the sprouting intersomitic vessels (ISVs). Early during development, Sema3E plays a critical role in positioning the first blood vessels of the developing vascular network, the DA (Meadows et al., 2012). When Sema3E is absent, a highly branched plexus forms instead of the normally large and branchless aortae. This study demonstrated that Sema3E expressed from the notochord and lateral plate mesoderm provides repulsive signals that create avascular zones to define the DA boundaries and thereby patterning them. Later, Sema3E is expressed in forming somites, guiding angiogenic ISVs that sprout from the DA to grow between them. Mice or fish with defective Sema-PlexinD1 signaling display aberrantly branched ISVs that ectopically invade neighboring somites (Gu et al., 2005; Zygmunt et al., 2011). In addition, proper Sema3E-PlexinD1 signaling is required for normal Notch-dependent EC sprouting and vessel formation during angiogenesis, as seen in a model of conditional Sema3E ablation during retinal vessel growth (Kim et al., 2011). Together, these studies underline the significant impact of Sema3E during blood vessel formation in the developing embryo.
Despite the characterized vascular defects in Sema3E deficient embryos (Gu et al., 2005; Kim et al., 2011; Meadows et al., 2012), these mutant mice surprisingly survive into adulthood, are fertile and have life spans similar to wild-type or heterozygous Sema3E littermates. With so many defects in vascular patterning at different stages of embryonic and post-natal development, it is surprising that Sema3E null mice do not succumb to cardiovascular defects. This is especially peculiar considering the dramatic patterning defects seen in early embryos (Meadows et al., 2012), as the DA constitute the principal conduit for blood circulation in the early cardiovascular system.
Our studies set out to explore the seeming discrepancy between dramatic, defective vascular phenotypes observed in Sema3E null embryos and the resulting fertile, viable adult mice that emerge. We find that mice lacking Sema3E undergo a type of ‘regulative development’, or self-correction, whereby abnormally branched networks of aortic vessels remodel into normal, single aortae via morphogenetic cellular rearrangements, independent of changes in cell proliferation or death. Furthermore, our results suggest that additional repulsive guidance cues emanating from the lateral plate mesoderm are responsible for this resolution process. These observations spotlight the remodeling capacity of vessels and the redundancy of cues within embryonic tissues that together ensure proper vascular patterning and morphogenesis.
RESULTS
DA patterning defects in Sema3E−/− mice normalize over developmental time
By embryonic (E) day 8 to 8.5 (E8 - E8.5), the DA of wild-type and Sema3E heterozygous mice form as two distinct, smooth and parallel vessels separated by an intervening avascular midline and flanked by lateral avascular zones that isolate the DA from the extra-embryonic yolk sac vessels (Fig. 1A and B). These first vessels are stereotyped in appearance and morphology across individual embryos. By E9.5, these parallel vessels fuse into a single midline aorta along much of the length of the posterior trunk (Coffin et al., 1991).
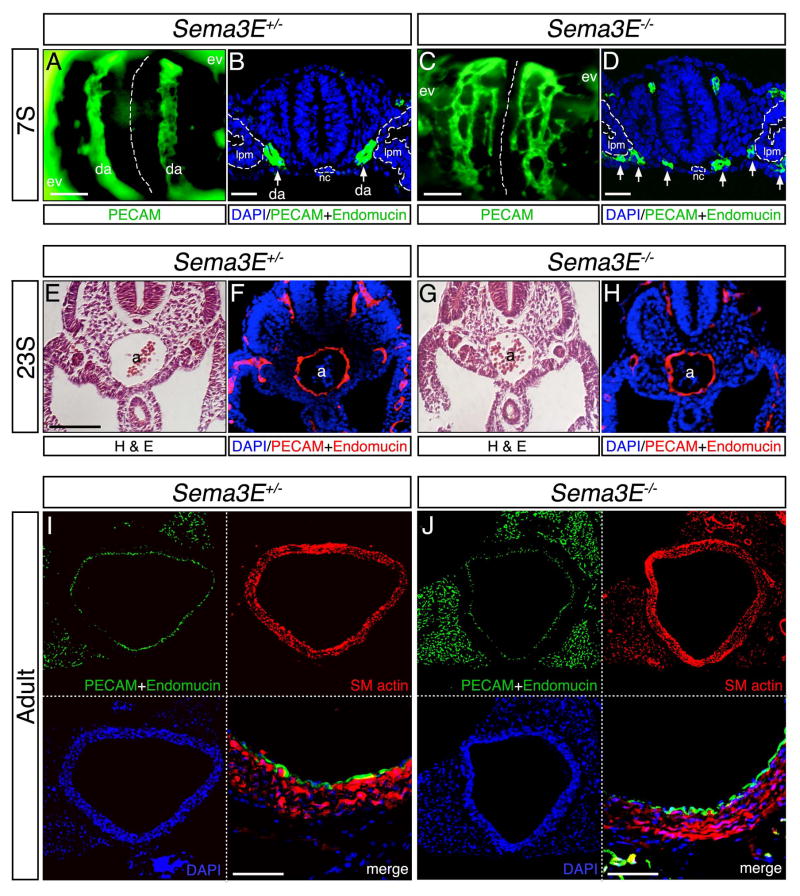
Figure 1. Dorsal aortae defects in Sema3E deficient embryos resolve over developmental time.
Sema3E+/− (A,B,E,F,I) and Sema3E−/− (C,D,G,H,J) embryos (A–H) and adults (I,J) in whole mount views stained for PECAM (A,C) and sections (B,D,E–J) stained with hematoxylin-eosin (H&E) (E,G) or for indicated antigens (B,D,F,H-J). Note that initial blood vessels in Sema3E−/− form aberrant plexus-like vascular structures (C,D), instead of smooth parallel vessels (A,B). Aortic vessels in Sema3E−/− are located much closer to midline notochord compared to Sema3E+/− (arrows). Dashed white lines indicate notochord in (A,C) and outline notochord and lateral plate mesoderm in (B,D). a, aorta; da, dorsal aortae; ev, extraembryonic vasculature; lpm, lateral plate mesoderm; nc, notochord; s, somite. Dorsal aorta diameter and morphology is indistinguishable between Sema3E+/− and Sema3E−/− 23S embryos (E–H) and adults (I,J). Note that ECs of normal appearance line the aortic lumen and layers of smooth muscle are of similar thickness in both genotypes. Scale bars: 100 μM (A,C), 12.5 μM (B,D) and 25 μM (lower right panels I and J)
Sema3E−/− embryos, by contrast, present presumptive DA as two plexus-like, highly branched networks of vessels, which extend into the normally lateral avascular areas and connect with the extra-embryonic vasculature (Fig. 1C and D, Meadows et al., 2012). In addition, the EC-free midline in Sema3E−/− mice is reduced in width, as mutant aortic structures form closer to the notochord than in Sema3E+/− embryos. As previously shown, the notochord is a source of multiple EC-repulsive guidance cues including Sema3E (Reese et al., 2004; Meadows et al., 2012) and the reduction in the midline vascular region is attributed to Sema3E absence in the notochord.
To better understand how Sema3E deficient mice are able to survive to adulthood in spite of dramatic DA abnormalities during embryogenesis, we first examined the DA of later staged Sema3E heterozygous and homozygous embryos for possible underlying defects. At E9.5, just after DA fusion at the midline, we observed no noticeable differences in expression of EC markers or overall morphology of Sema3E+/− and Sema3E−/− DA (Fig. 1E–H). Similarly, analysis of sectioned adult dorsal aorta showed that overall size and morphology remained indistinguishable between Sema3E+/− and Sema3E−/− mice (Fig. 1I and J). Staining for endothelial and smooth muscle markers indicated that Sema3E−/− mice express relatively normal levels of PECAM and Endomucin, and smooth muscle actin, respectively, in expected tissue locales. We did not observe obvious changes in thickness or organization of smooth muscle layers that surround aortic ECs. These observations indicated that Sema3E deficient mice apparently undergo DA fusion and form a morphogenetically and molecularly normal, and presumably functional, midline dorsal aorta.
Sema3E−/− aortic patterning defects resolve prior to DA fusion
Given that DA of Sema3E−/− mice are normal at E9.5, the patterning defects observed between E8 and E8.5 must resolve during the intervening developmental time period. We hypothesized that resolution of the aortae occurs during the fusion of the DA (from E9 to E9.5), whereby the two separate plexus-like structures fuse together to form a single blood vessel.
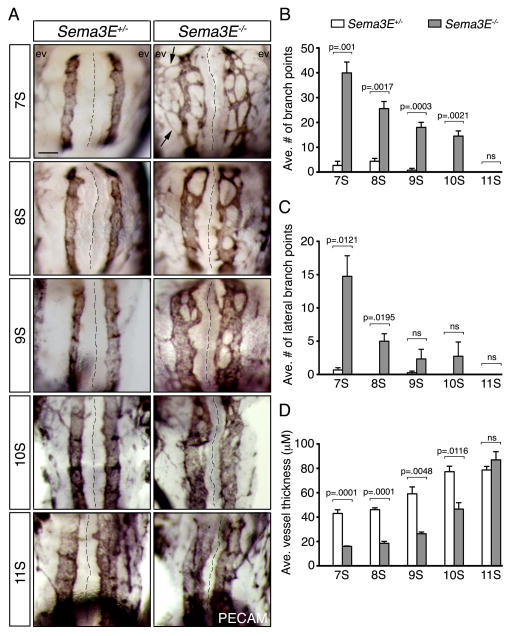
To address how normalization transpires, we examined the developing DA of somite (S) staged Sema3E heterozygous and null embryos. Our analysis shows that the network appearance of the Sema3E−/− DA does in fact resolve, however the mutant vessels normalize prior to fusion of the DA (Fig. 2A). As previously shown (Meadows et al., 2012), at 7S we observed numerous highly branched and honeycomb-like vessels in Sema3E−/− embryos (quantified by the number of branch points), including many vessels that spanned the normally lateral avascular zones (Fig. 2B and C). Conversely, by 11S (several somite stages before the DA fuse), no branching vessels were detected in the mutants and the DA appeared identical compared to Sema3E+/− DA. Although aortic vessels never crossed the embryonic midline, as previously reported (Meadows et al., 2011), the Sema3E−/− DA remained closer to the notochord than compared to heterozygote embryos.
Figure 2. Sema3E−/− aortic plexus-like vessels remodel into unbranched dorsal aortae by 11S.
(A) PECAM-DAB staining of Sema3E+/− (left hand column) and Sema3E−/− (right hand column) aortae. Note that Sema3E+/− DA are unbranched and initially wide set, while Sema3E−/− plexus vessels are initially highly branched, of smaller diameter, and closer to each other across the midline. These vessels rapidly converge between 7S and 11S to form two large unbranched vessels. Arrows highlight ectopic lateral vessels that connect aortic vessels to extraembryonic vessels. Black dashed lines mark the embryonic midline. Scale bar: 100 μM (A). (B–D) Quantification of parameters from (A), including average (ave.) number of total, or lateral branch points, as well as average vessel thickness. ev, extraembryonic vasculature; ns, not statistically significant; S, somite.
In addition to the rapid loss of branching complexity of these atypical vessels in Sema3E−/− embryos, we noticed that they underwent rapid changes in diameter, in stark contrast to wild-type DA that remained relatively constant in size. Sema3E−/− aortic vessels emerged as initially narrow vessels, only a fraction of the size of normal aortae, but increased in thickness as they converged and fused over developmental time. Indeed, our measurements indicated that the average vessel thickness of mutants dramatically increased with time, and by 11S no noticeable differences were detected between Sema3E+/− and Sema3E−/− embryos (Fig. 2D).
DA resolution is independent of changes in cell proliferation and death
Our studies suggested that normalization of the Sema3E−/− DA occurs via fusion or remodeling of the blood vessels within each plexus-like structure. However, it was possible that the observed reduction in the number of vessels and accompanying increase in vessel thickness from 7S - 11S occurred via different mechanisms. For example, a combination of apoptosis of regressing branches and/or an increase in EC proliferation of remaining vessels might account for this process.
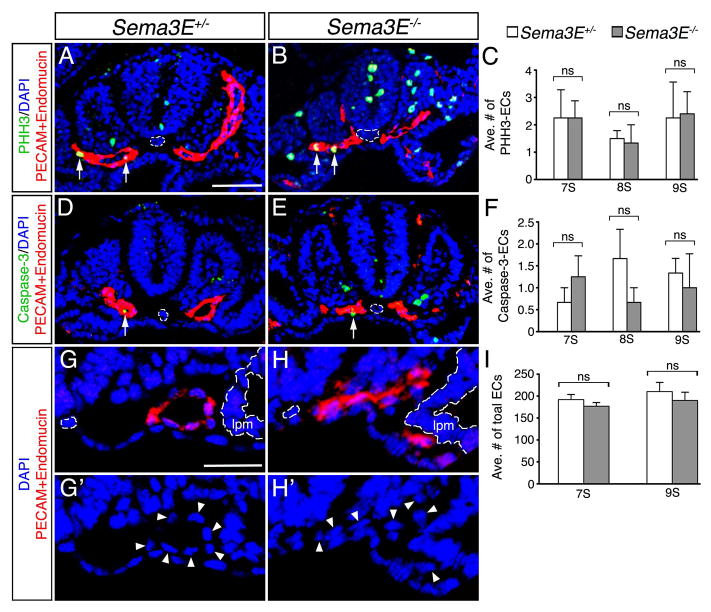
To assess these possibilities, we analyzed the rates of cell proliferation and death in 7S to 9S Sema3E+/− and Sema3E−/− aortic ECs, at the time when changes in DA patterning were most prevalent (Fig. 3). Our analysis shows that the rate of cell proliferation, as analyzed by the presence of phospho-histone H3 (PHH3), was similar between Sema3E heterozygous and null embryos (Fig. 3A–C). Similarly, no significant changes in the number of ECs expressing the apoptotic marker, cleaved Caspase-3 were observed between Sema3E+/− and Sema3E−/− embryos (Fig. 3D–F). Furthermore, the total number of ECs (spanning from both LPMs) remained consistent between Sema3E+/− and Sema3E−/− embryos (Fig. 3G–I), suggesting no effect on angioblast specification. Therefore, we conclude that morphogenetic rearrangement of ECs within the vessels within each vascular network, rather than changes in EC proliferation or death, are likely responsible for resolution of the Sema3E−/− aortic vessels into normal, parallel DA.
Figure 3. Resolution of dorsal aortae in Sema3E−/− embryos is not due to changes in cell proliferation or death.
Sections of Sema3E+/− (A,D,G,G′) and Sema3E−/− (B,E,H,H′) embryos assessing cell proliferation with PHH3 (A,B), cell death with staining for cleaved Caspase-3 (D,E) and total EC number with PECAM and Endomucin (G,H,). A,B,D,E: 9 somites (S) and G,G′,H,H′: 7S. Arrows mark positive ECs for indicated stainings and arrowheads mark ECs. Midline notochord and lateral plate mesoderm (lpm) are outlined by dashed white lines. (C,F,I) Quantification of PHH3 (C) and cleaved Caspase-3 (F) stained ECs, and the average (ave.) total number of ECs (I) reveals no significant changes in cell proliferation, programmed death and number. ns, not statistically significant. Scale bars: 25 μM (A,B,D,E) and 25 μM: 25 (G,G′,H,H′).
Blood circulates through select vessels in Sema3E−/− aortic plexus
Embryonic blood flow is not yet initiated during the earliest stages of blood vessel formation; however, at 7S, blood flow begins and is thereafter required for the embryo to develop and for proper vascular remodeling (Jones, 2011). Therefore, in Sema3E deficient mice, the two distinct aortic plexus-like networks form prior to blood flow and resolve as plasma and erythrocytes begin to circulate. How then does a Sema3E−/− embryo survive, given that blood circulation is required at a time when DA patterning defects are most prominent?
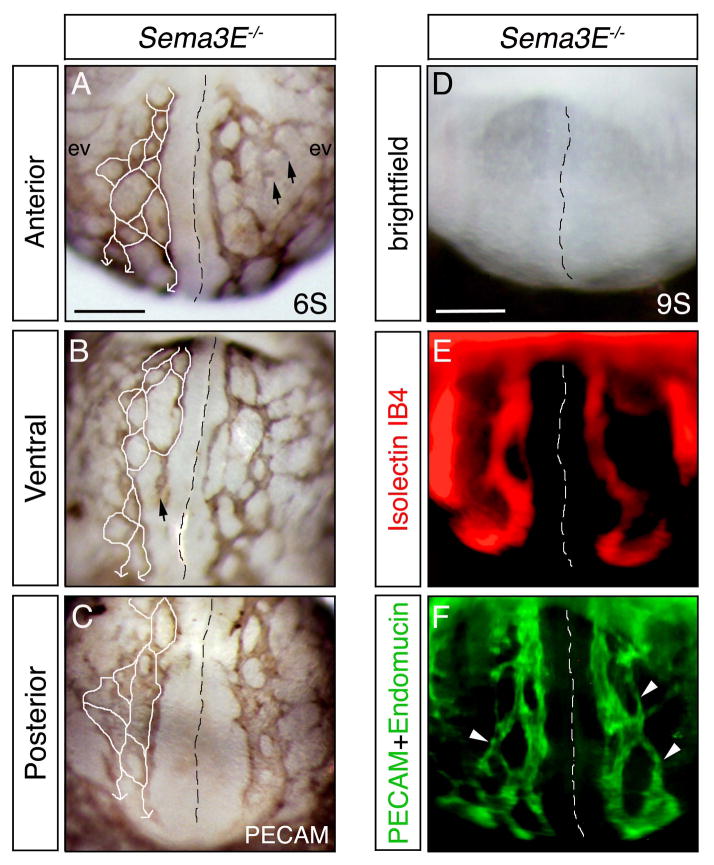
To address this question, we further examined the aortic vessels of Sema3E−/− embryos for connectivity and putative circulatory function. In all mutant embryos surveyed (stages 5S - 11S), we observed a continuous network of vessels that connected the heart to the presumptive umbilical cord (Fig. 4A–C). That is, we always observed a path for blood to circulate in mutants despite the increased complexity in blood vessel patterning (plexus-like morphology). The entire aortic network of vessels appeared to be connected as a closed ‘circuit’, with only a few vessels presenting as blunt-ended sprouts.
Figure 4. Sema3E null embryos form an interconnected aortic plexus, however select vessels carry blood flow.
(A–C) PECAM-DAB staining of a 6 somite (6S) Sema3E−/− embryo shown in anterior (A), ventral (B) and posterior views (C). White lines mark aortic vessels that connect to form a continuous circulatory loop. Black arrows indicate blunt ended vessels. (D–F) Anterior views of a 9S Sema3E−/− embryo injected with Isolectin IB4 and stained for PECAM and Endomucin. White arrowheads indicate interconnected vessels devoid of Isolectin IB4. Black and white dashed lines demarcate the embryonic midline. ev, extraembryonic vasculature. Scale bars: 100 μM (A–C) and 100 μM (D–F).
Interestingly, our previous studies suggested that blood flow occurs preferentially in select vessels of the mutant plexus, as indicated by expression of the blood flow dependent marker Connexin 40 (Meadows et al., 2013). To test this possibility, we injected Isolectin IB4 tracer into beating hearts of 8-9S stage Sema3E−/− embryos and assessed its presence in mutant aortic plexuses (Fig. 4D–F). We found that Isolectin IB4 travelled in a relatively linear manner, within a single vessel, within the aberrant highly branched vascular network in mutants. In addition, we observed that the vessels containing Isolectin IB4 were predominately the largest vessels within the plexus. These experiments indicate that although a continuous plexus is formed in Sema3E mutants, the majority of the blood is carried through select, large vessels.
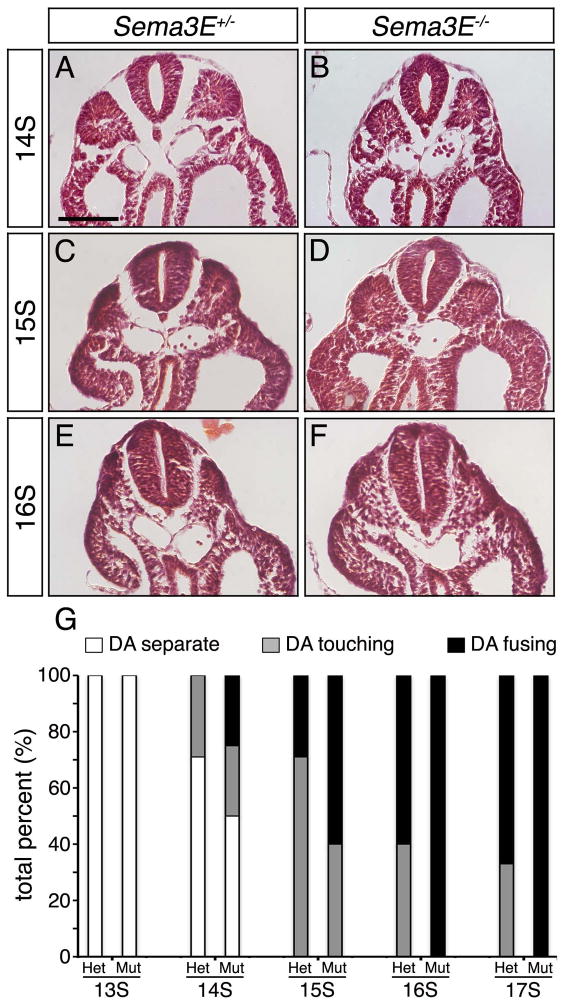
DA fusion occurs early in Sema3E−/− mice
Although Sema3E−/− DA patterning defects normalize rapidly, mutant aortic ECs remained closer to the notochord than in Sema3E+/− embryos throughout this ‘resolution period’. In fact, initial observation of mutants predicted that this midline displacement of the DA towards the notochord might result in precocious fusion of the dorsal aortae into a single midline vessel.
To investigate this possibility, somite-stage matched Sema3E+/− and Sema3E−/− embryos were sectioned, hematoxylin and eosin stained, and the DA were analyzed for midline fusion along their anteroposterior axis. Our results indicated that the DA of Sema3E+/− embryos typically began to fuse around 15-16S along the posterior trunk (Fig. 5A,C,E,G), often forming multiple points of fusion approximately 300 μm posterior to the heart. By contrast, the DA of Sema3E deficient embryos regularly came in contact with each other earlier than in Sema3E+/− embryos, often fusing hours earlier, at approximately 14-15S (Fig. 5B,D,F,G). By approximately 17S, DA fusion was found in the majority of Sema3E+/− and Sema3E−/− embryos examined with a higher percentage of Sema3E mutants undergoing fusion (Fig. 5G). These findings suggest that Sema3E acts as a notochord repulsive cue that holds aortic ECs at bay, restraining fusion of the paired DA at the embryonic midline.
Figure 5. Sema3E−/− dorsal aortae precociously fuse at the embryonic midline.
Representative images of Sema3E+/− (A,C,E) and Sema3E−/− (B,D,F) sectioned embryos stained for hematoxylin and eosin. Note early contact between the DA of Sema3E−/− embryos at 14 somite (14S) and precocious fusion of the DA at 15S, compared to Sema3E+/− embryos. Scale bar: 100 μM. (G) Graph of Sema3E+/− and Sema3E−/− DA categorized as separate, touching or fusing, as a total percentage of the embryos analyzed.
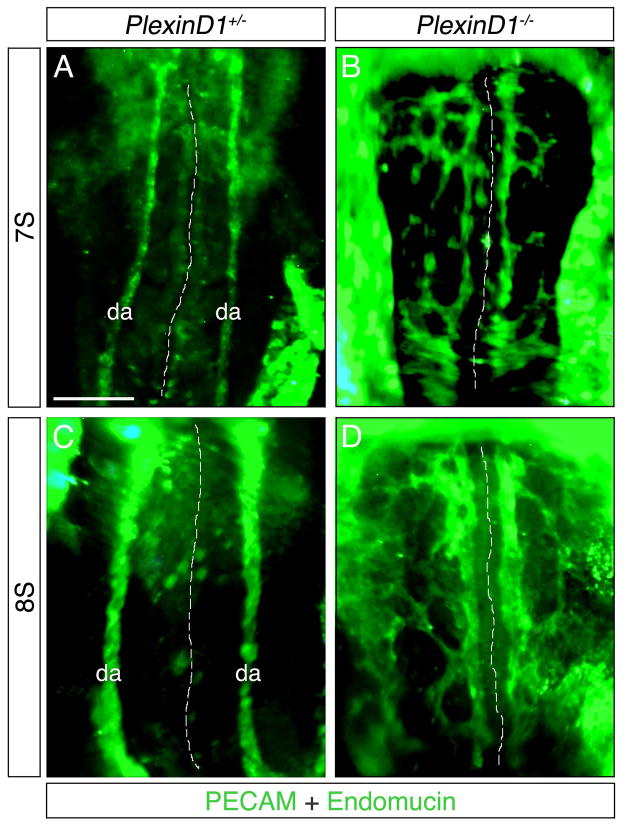
Loss of the Sema3E receptor, PlexinD1, results in DA defects
Prior studies have shown PlexinD1 and Sema3E null embryos to have effectively identical intersomitic blood vessel patterning defects at E11.5 (Gu et al., 2005), however it remains unclear whether a Sema3E-PlexinD1 relationship is present during earlier vascular development. To examine this question, we assessed the DA of early PlexinD1−/− embryos to determine if similar defects exist. Our results showed that the DA of PlexinD1+/− embryos appear as singular unbranched vessels (Fig. 6A and C), while PlexinD1−/− DA present as highly branched and disorganized vascular plexuses (Fig. 6B and D) similar to Sema3E deficient embryos (compare Fig. 1C and Fig. 2A to Fig. 6B and D). Moreover, many PlexinD1−/− aortic vessels, in a manner similar to Sema3E−/− embryos, form adjacent to the notochord and do not cross the midline. These observations strongly suggest a functional relationship between Sema3E and PlexinD1 and a central role for semaphorin-plexin signaling in controlling proper patterning and formation of the DA.
Figure 6. PlexinD1−/− embryos display similar dorsal aortae defects to Sema3E−/− embryos.
Ventral views of PlexinD1+/− (A,C) and PlexinD1−/− (B,D) embryos stained for PECAM and Endomucin. Note the branched, disorganized and plexus-like appearance of PlexinD1 mutant DA. Dashed white lines indicate the midline. da, dorsal aortae; S, somite. Scale bar: 100 μM.
DISCUSSION
Our previous work identified a significant role for Sema3E in shaping and patterning the endothelial progenitors that form the vertebrate dorsal aorta (Meadows et al., 2012). Embryos lacking Sema3E initially displayed strikingly disorganized DA, with vessels inappropriately connecting to the extra-embryonic vasculature, and consequent loss of stereotyped avascular zones. This phenotype predicted potentially lethal cardiovascular outcomes; however despite observed embryonic vascular defects (Gu et al., 2005), Sema3E−/− mice survive throughout adulthood in seemingly healthy condition. Here, we clarify the cellular mechanisms by which the abnormal embryonic vasculature of Sema3E−/− mice can yield a fully functional cardiovascular system as a result of vascular remodeling.
Given that Sema3E null mice survive into adulthood, it was possible that undetected cardiovascular abnormalities existed, stemming from early embryonic blood vessel patterning defects (E8 - E8.5). To identify potential adult aortic defects, we examined the DA of embryonic and adult Sema3E−/− mice. Our analysis showed no noticeable changes in expression of molecular markers, shape or morphology of the aorta when compared to heterozygous mice. Furthermore, smooth muscle layers, which envelop the mature dorsal aorta following embryonic midline fusion, were indistinguishable from Sema3E+/− mice. Our data suggested that, at least after fusion of the paired aortic vessels, the dorsal aorta is normal in Sema3E−/− mice. While we acknowledge stress or injury conditions (either in the aorta or peripheral vascular beds) might reveal underlying blood vessel defects, gross anatomical examination suggested no overt vascular defects or lesions.
In order for the embryo to survive throughout development, sufficient blood flow must be present to oxygenate tissues and support tissue growth. Given survival of Sema3E null mice to adulthood, their embryonic vessels clearly acquire early functionality despite patterning aberrations. We find that in all Sema3E−/− embryos examined, the aortic vascular networks formed an interconnected circuit that could allow for continued blood flow. Interestingly, previous experiments demonstrated that the arterial marker Cx40, whose expression depends on hemodynamic flow, is only present in a select few vessels of the mutant aortic plexuses (Meadows et al., 2012). This suggests that blood may be stochastically sent down a particular vessel, perhaps the path of least constraint to flow. Indeed, injection of Isolectin IB4 into the hearts of Sema3E−/− embryos revealed that blood flowed predominately through select, large vessels (likely the path of least resistance) within the mutant vascular plexuses. These observations provide an explanation as to why Sema3E−/− embryos survive past early development. We postulate that vessels selected to carry the bulk of circulatory flow might act as the fusion point for all other vessels, however this remains to be confirmed.
Our initial analysis indicated that the DA of Sema3E−/− mice resolved their aberrantly branched organization over time. This morphogenetic resolution can be likened to ‘regulative development’, where the embryo manages to bypass molecular or morphogenetic abnormalities to re-establish normal anatomy and/or function (Slack, 1991). Remarkably, resolution of the mutant vascular plexus back into two paired DA occurred in a rapid fashion prior to midline fusion. These initial tree-like aortic vessels resolved via remodeling and rearrangement, rather than through mechanisms that employ changes in cell proliferation or programmed death. Our data suggests that coalescence of these atypical vessels is likely the primary method for DA resolution in Sema3E null embryos. However, it remains unclear whether regression of vessels contributes to the overall remodeling and resolution of the aortae. Together, these observations uncover a unique type of vascular remodeling whereby a large plexus of blood vessels converge or coalesce to form a single, functional vessel. This is distinctive in that blood vessel remodeling has primarily been thought to occur via sprouting or splitting of pre-existing vessels, rather than through vessel convergence. It will be especially interesting to find out if this manner of vascular remodeling takes place in other areas during development or whether plexus coalescence is unique to the development of the DA in Sema3E−/− embryos.
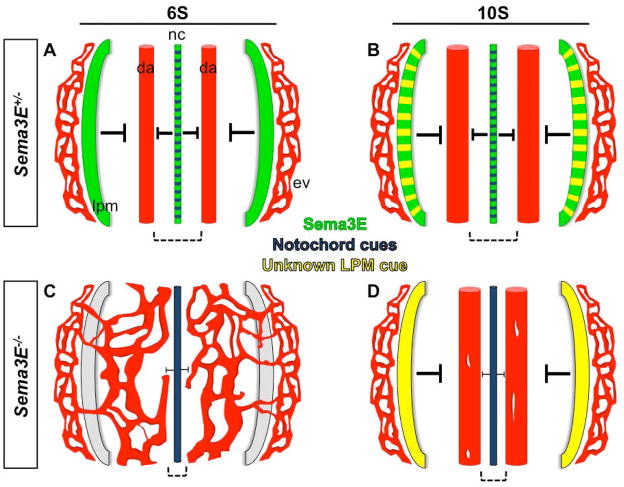
Interestingly, disappearance of the lateral Sema3E−/− vessels that inappropriately connect to the yolk sac vasculature suggested unknown signals emanating from the lateral plate mesoderm. These signals fundamentally rescued the Sema3E−/− lateral vascular defects (Fig. 7). However, despite extensive screening for potential candidates, we found no NGC altered at the right location or stages to account for the restoration of the lateral avascular regions. We therefore presume the existence of as yet unidentified repulsive cues. We speculate that the ISV blood vessel patterning defects originally characterized in the Sema3E deficient mice (Gu et al., 2005) are likely corrected over time by additional cues in a similar fashion. Together, these findings underline the intrinsic nature of redundant patterning cues in the embryo that work to prevent vascular abnormalities and ensure resilience in embryonic developmental programs.
Figure 7. Schematic representation of morphogenetic consequences of Sema3E ablation on dorsal aortae formation.
A) Sema3E from the midline notochord and lateral plate mesoderm, as well as a multiple signals from the notochord (including chordin, noggin, slit and netrin - blue), repel migrating angioblasts (precursor ECs) from 0S to 6S, constraining them to parallel locations where they coalesce and form two smooth aortic vessels that run along the anterioposterior axis. B) By 10S, the paired, parallel aortic tubes enlarge in diameter, remaining free of sprouting branches. C) In the absence of Sema3E, angioblasts migrate freely, except for a narrow domain near the notochord, and form a plexus on both sides of the embryo, that connects with extraembryonic vessels. D) Aortic vessels are rescued over time in Sema3E−/− embryos. Repulsion from the lateral plate mesoderm occurs between 8S and 11S, suggesting onset of expression of an as yet unknown cue. Aberrantly branched aortic plexus structures resolve as branches fuse into large smooth DA by 10-11S. All panels show ventral views, anterior is up; posterior is down. Blood vessels, red; da, dorsal aortae; ev, extraembryonic vasculature; Sema3E protein, green; notochord (nc) cues, blue; lateral plate mesoderm (lpm) unknown cue, yellow. Dashed black brackets indicate the midline avascular space between aortic vessels.
Overall, this study advances our understanding of the molecules that affect the formation and patterning of forming blood vessels, while drawing attention to the multiplicity of molecules that affect EC behavior. It remains an open question as to what factor(s) or forces rescue the Sema3E−/− DA phenotype. Identifying the full array of repulsive cues that shape developing blood vessels will reveal how coordinated signals sculpt forming blood vessels.
Conclusion
Given the dramatic changes in the shape of the DA of Sema3E−/− embryos, it is surprising that mutant adult mice are viable and fertile (Gu et al., 2005). We demonstrate that DA patterning defects resolve via fusion of the vascular networks back into single paired DA, effectively rescuing the phenotype. These studies highlight the inherent ability of the embryo to utilize multiple EC guidance cues from various tissues thereby ensuring that the vascular system is properly and precisely shaped and patterned.
EXPERIMENTAL PROCEDURES
Histology
Embryos fixed overnight in 4% Paraformaldehyde (PFA), were processed for paraffin sectioning using standard procedures. Briefly, embryos were dehydrated with 2X washes of 100% EtOH followed by 2X washes in Xylenes. Following Xylenes, embryos were incubated in 50% Xylenes/50% paraffin wax at 65°C. Embryos were then incubated in 4X 100% paraffin wax washes 1 hour (hr) each at 65°C and embedded. For Hematoxylin and Eosin staining on sections, the following washes were performed in order: 2X, 7 minutes in Xylenes; 2X, 2 minutes 100% EtOH; 2X, 2 minutes in 95% EtOH; 1X, 2 minutes in H2O; 1X, 7 minutes in Hematoxylin; 1X, 2 minutes in in H2O; 1X, 5 seconds in 99 mLs of 70% EtOH plus 1 mL HCl; 1X, 5 minutes in H2O; 1X, 3 minutes in Eosin (25 mLs diluted in 74 mLs of 100% EtOH and 1 mL of Acetic acid); 2X, 2 minutes in 95% EtOH; 2X, 2 minutes in 100% EtOH; 2X, 2 minutes in Xylenes and mounted with Permount (FisherChemicals) on coverslips.
PECAM DAB staining
Embryos were fixed overnight at 4°C in 4% PFA in PBS. The following day, embryos were washed in PBS, transferred to 0.25% Trypsin (Hyclone) for 2 min, rinsed in PBS, and blocked in CAS-Block (Invitrogen) for 1 hr at room temperature (RT). Following block, embryos were incubated overnight with PECAM antibody (BD Pharmingen; 1:300) in PBST (PBS + 0.1% Triton-X-100) at 4°C. The next day, embryos were washed with PBST and stained with DAB solution as per kit instructions (Vector labs). The staining reaction was stopped by rinsing in water and fixation in 4% PFA.
Immunofluorescent antibody staining
Paraffin sections were washed 2X for 5 minutes in Xylenes, rehydrated in a series of 1 minute EtOH washes (2X-100%, 95%, 90%, 80%, 70% and 40%), followed by 2X, 3 minute PBS washes. Sections were then permeabilzed for 5 minutes using PBST (PBS + 0.1% Triton-X-100). Then samples were rinsed with PBS and blocked with parafilm coverslips and CAS-block (Invitrogen) for 1 hr in a humidity chamber. Primary antibodies were diluted in CAS-block at 1:100, coverslipped with parafilm and incubated in a humidity chamber overnight at 4°C. The following day, the samples were washed with 1X, PBST for 5 minutes and 3X in PBS for 3 minutes each. Alexa Fluor (Invitrogen) secondary antibodies were diluted in CAS-block at 1:500, parafilm coverslipped and incubated in a humidity chamber for 2 hrs at RT. Sections were rinsed with PBS, washed in PBS 3X for 5 minutes and mounted with ProLong Gold antifade reagent plus DAPI (Invitrogen). For whole-mount antibody stainings, embryos were washed in PBST (PBS + 0.1% Triton-X-100) for 1 hr and blocked in CAS-block for 1 hr at RT followed by incubation with primary antibodies diluted in CAS-block (1:100) at 4°C overnight. Next, embryos were washed 5X in PBS for 30 minutes each and incubated with secondary antibodies diluted 1:500 in CAS-block at 4°C overnight. The following day, embryos were washed 5X in PBS for 1 hr each and staining visualized. The following antibodies were used: PECAM (BD Pharmigen), Endomucin (Santa Cruz Biotech), phospo histone-H3 (Millipore), cleaved Caspase-3 (Cell Signaling). For cleaved Caspase-3 immunostaining, sections were subjected to antigen retrival using a 2100 Retriever and buffer A (Electron Microscopy Sciences) after the rehydration steps.
Isolectin IB4 injections
Dissected Sema3E+/− and −/− embryos, 8S-9S, were pinned onto Sylgard coated dishes in PBS, and undiluted Isolectin GS-IB4 from Griffonia simplicifolia - Alexa Fluor 594 conjugate (Invitrogen) was injected into beating hearts using a mouth pipette constructed with a flame pulled glass needle and tygon tubing. Embryos were immediately imaged after injections.
Statistical analyses
Sema3E+/− and −/− vasculature was assessed for total branch points, lateral branch points and vessel thickness within a defined 400 μm2 area using ImageJ. In all instances, each DA and mutant plexus from 3–5 embryos per somite stage (7-11S) were counted. Lateral branch points were defined as those branch points extending into the normally avascular space from the lateral most continuous vessel. For vessel thickness, 10 measurements at the thickest parts of the aortae of Sema3E+/− embryos were averaged per embryo, while the thickest part of each vessel within the Sema3E−/− aortic plexus were averaged per embryo (only the left aortae or plexus were counted). To assess cell proliferation (phospho histone-H3) and death (cleaved Caspase-3) in Sema3E+/− and −/− aortic vessels, 3 embryos per somite stage (7-9S) were sectioned, stained and counted. For total EC numbers, 3 embryos per 7S and 9S were sectioned, stained and counted. 4–7 embryos per somite stage were analyzed for categorizing the DA as separate, touching or fusing
Mouse lines and genotyping primers
Sema3E mice were maintained on C57BL/6-CD1 backgrounds and genotyped as previously described (Gu et al., 2005). PlexinD1 mice were genotyped as previously described (Gitler et al., 2004).
Embryonic dorsal aortae patterning defects in Sema3E−/− mice resolve during subsequent development.
Resolution of Sema3E−/− dorsal aortae occurs via angiogenic remodeling.
Sema3E is not required for fusion of the paired aortae into a single midline aorta.
Acknowledgments
We thank Chenghua Gu for the Sema3E mice and Stephen Fu for assistance with immunofluorescent staining. We also thank Russell Ratliff, Jayme Lauderdale, Kynlee Meadows and Emmett Carroll for support. We are grateful for grant support NIH HL007360-32 and AHA Postdoctoral fellowship (SM), and NIH DK079862 and AHA Grant-in-Aid 0755054Y (OC).
References
- Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harbor perspectives in biology. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell VM, Yeo SY, Park KW, Chung J, Seth P, Shivalingappa V, Zhao J, Obara T, Sukhatme VP, Drummond IA, Li DY, Ramchandran R. roundabout4 is essential for angiogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102:6373–6378. doi: 10.1073/pnas.0408318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin JD, Harrison J, Schwartz S, Heimark R. Angioblast differentiation and morphogenesis of the vascular endothelium in the mouse embryo. Developmental biology. 1991;148:51–62. doi: 10.1016/0012-1606(91)90316-u. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Eichmann A, Makinen T, Alitalo K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005;19:1013–1021. doi: 10.1101/gad.1305405. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7:107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- Helbling PM, Saulnier DM, Brandli AW. The receptor tyrosine kinase EphB4 and ephrin-B ligands restrict angiogenic growth of embryonic veins in Xenopus laevis. Development. 2000;127:269–278. doi: 10.1242/dev.127.2.269. [DOI] [PubMed] [Google Scholar]
- Hogan KA, Bautch VL. Blood vessel patterning at the embryonic midline. Curr Top Dev Biol. 2004;62:55–85. doi: 10.1016/S0070-2153(04)62003-5. [DOI] [PubMed] [Google Scholar]
- Kigel B, Varshavsky A, Kessler O, Neufeld G. Successful inhibition of tumor development by specific class-3 semaphorins is associated with expression of appropriate semaphorin receptors by tumor cells. PloS one. 2008;3:e3287. doi: 10.1371/journal.pone.0003287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C. Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes & development. 2011;25:1399–1411. doi: 10.1101/gad.2042011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Breant C, Claes F, De Smet F, Thomas JL, Autiero M, Carmeliet P, Tessier-Lavigne M, Eichmann A. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- Meadows SM, Fletcher PJ, Moran C, Xu K, Neufeld G, Chauvet S, Mann F, Krieg PA, Cleaver O. Integration of repulsive guidance cues generates avascular zones that shape mammalian blood vessels. Circulation research. 2012;110:34–46. doi: 10.1161/CIRCRESAHA.111.249847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Reese DE, Hall CE, Mikawa T. Negative regulation of midline vascular development by the notochord. Dev Cell. 2004;6:699–708. doi: 10.1016/s1534-5807(04)00127-3. [DOI] [PubMed] [Google Scholar]
- Sakurai A, Gavard J, Annas-Linhares Y, Basile JR, Amornphimoltham P, Palmby TR, Yagi H, Zhang F, Randazzo PA, Li X, Weigert R, Gutkind JS. Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras. Mol Cell Biol. 2010;30:3086–3098. doi: 10.1128/MCB.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JM. From Egg to Embryo: Regional Specification in Early Development. New York: Cambridge University Press; 1991. [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchting S, Bicknell R, Eichmann A. Neuronal clues to vascular guidance. Exp Cell Res. 2006;312:668–675. doi: 10.1016/j.yexcr.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Van NP, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7:117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Singh MK, Degenhardt KR, Lu MM, Bennett J, Yoshida Y, Epstein JA. Tie2Cre-mediated inactivation of plexinD1 results in congenital heart, vascular and skeletal defects. Developmental biology. 2009;325:82–93. doi: 10.1016/j.ydbio.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt T, Gay CM, Blondelle J, Singh MK, Flaherty KM, Means PC, Herwig L, Krudewig A, Belting HG, Affolter M, Epstein JA, Torres-Vazquez J. Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1. Developmental Cell. 2011;21:301–314. doi: 10.1016/j.devcel.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]