Abstract
Xylella fastidiosa is an important phytopathogenic bacterium that causes many serious plant diseases including Pierce’s disease of grapevines. X. fastidiosa is thought to induce disease by colonizing and clogging xylem vessels through the formation of cell aggregates and bacterial biofilms. Here we examine the role in X. fastidiosa virulence of an uncharacterized gene, PD1671, annotated as a two-component response regulator with potential GGDEF and EAL domains. GGDEF domains are found in c-di-GMP diguanylate cyclases while EAL domains are found in phosphodiesterases, and these domains are for c-di-GMP production and turnover, respectively. Functional analysis of the PD1671 gene revealed that it affected multiple X. fastidiosa virulence-related phenotypes. A Tn5 PD1671 mutant had a hypervirulent phenotype in grapevines presumably due to enhanced expression of gum genes leading to increased exopolysaccharide levels that resulted in elevated biofilm formation. Interestingly, the PD1671 mutant also had decreased motility in vitro but did not show a reduced distribution in grapevines following inoculation. Given these responses, the putative PD1671 protein may be a negative regulator of X. fastidiosa virulence.
Introduction
Xylella fastidiosa is a motile, xylem-limited bacterium transmitted to plants by xylem sap-feeding insects [1]. X. fastidiosa causes disease of many economically important crops, including Pierce’s disease in grapevines. It is proposed that once infection occurs, pathogenicity involves bacterial movement, aggregation, and occlusions of cells and biofilm formation, which leads to plugging and collapse of the xylem transport system [2].
X. fastidiosa moves against the transpiration stream using twitching motility [3], a flagella-independent movement involving the extension, attachment, and retraction of type IV pili [4]. X. fastidiosa translocation in plants requires degradation and movement through pit membranes [5,6], and plant primary cell walls are composed of celluloses, hemicelluloses, pectins, and proteins [7,8]. Once bacterial cells congregate, X. fastidiosa form biofilm, which is mediated by the type I pili [3] and non-fimbrial adhesins [9]. The biofilm is composed, in part, of extracellular polymeric substance (EPS) with fastidian gum, a derivative of xanthan gum, as the major constituent [10,11]. Fastidian gum production is regulated by the gum operon, of which gumD is postulated to initiate synthesis and gumJ plays a role in translocation of EPS [11,12]. Numerous molecular regulators are proposed to facilitate switching from motility to biofilm formation, such as the quorum sensing molecule diffusible signal factor (DSF) [13].
In many bacteria, movement and biofilm formation are inversely modulated by the intracellular second messenger, bis-(3’-5’)-cyclic dimeric guanosine monophosphate (c-di-GMP) [14]. C-di-GMP is synthesized by diguanylate cyclases (DGC) and hydrolyzed by phosphodiesterases (PDE); DGC catalyze two guanosine triphosphate (GTP) molecules to c-di-GMP using a GGDEF active site, and PDE degrade c-di-GMP to pGpG [5’-phosphogyanylyl-(3’-5’)-guanosine] using an EAL or HD-GYP domain. Hybrid proteins have been identified that contain both GGDEF and EAL domains [14–17].
X. fastidiosa has five putative proteins involved in production and turnover of c-di-GMP [17,18] three of which are characterized. CgsA has a GGDEF domain and is proposed to play a role in the transition of X. fastidiosa between vector and plant [13,19]. The Eal protein contains an EAL domain and was recently shown to be involved in antibiotic resistance and biofilm formation [20]. RpfG has an HD-GYP domain and is a response regulator involved in quorum sensing and biofilm formation [13]. The two uncharacterized proteins, PD1671 and PD1994, are annotated as containing both GGDEF and EAL domains [17]. We now report examination of the X. fastidiosa PD1671 protein, and provide evidence that its sequence is degenerative in both the GGDEF and EAL domains; however, it is involved in biofilm formation, presumably through regulation of gum gene expression and therefore EPS production, and subsequent Pierce’s disease development.
Materials and Methods
Bacterial strains
All studies involved X. fastidiosa strain Temecula 1, which was cultured at 28°C on modified periwinkle wilt (PW) agar [21] without phenol red and with 3.5 g L-1 bovine serum albumin (Life Technologies, Grand Island, NY). Mutants were cultured on modified PW containing 50 μg mL-1 kanamycin (Sigma Chemical Co., Saint Louis, MO). Complemented PD1671 strain was cultured on modified PW containing 50 μg mL-1 kanamycin and 5 μg mL-1 gentamycin (Sigma). Bacterial cultures were stored at -80°C on modified PW broth containing a final concentration of 7% DMSO (Sigma). Escherichia coli was cultured on Luria broth (LB) medium (Difco) with appropriate antibiotics (Sigma). Unless otherwise described, X. fastidiosa, mutants, and complemented mutant strains were cultured in spring-collected Vitis vinifera cv. Chardonnay xylem sap from California (kindly provided by Dr. A. Walker, University of California, Davis) for five days under agitation as previously described [22].
Bioinformatic analysis
The predicted PD1671 gene product was characterized with BLAST searches on GenBank at National Center for Biotechnology Information together with PFAM [23], Conserved Domain Database [24], KEGG [25], and SMART programs [26]. Amino acids coding for various domains were determined using Conserved Domain Database [24]. ClustalW2 was used to generate multiple protein alignments [27,28]. Sequence comparisons for the different domains were made to relevant subsets of the following: E. coli CheY (WP_000763867), Legionella pneumophila Lpl0329 (WP_011214592), Mycobacterium smegmatis MSDGC-1 (WP_003893571), Mycobacterium tuberculosis Rv1354c (NP_215870), Pseudomonas aeruginosa FimX (WP_003123576), Pseudomonas fluorescens LapD (WP_011331847), Pseudomonas putida PP2258 (AE016418), Rhodobacter sphaeroides BphG1 (WP_011331450), Vibrio parahaemolyticus ScrC (WP_005478021), and Xanthomonas oryzae Flip (WP_012444707). Sequence comparisons for the different X. fastidiosa Temecula proteins were the following: CgsA (WP_011097571), RpfG (WP_011097637), EAL (WP_011098186), PD1671 (WP_011098203), and PD1994 (WP_004087568). Sequence comparisons of PD1671 to X. fastidiosa orthologs included the following: 9a5c strain (NP_297691), Ann-1 strain (AIC10147), Dixon strain (EAO14097), EB92 strain (EGO81612), M12 strain (YP_001776351), and M23 strain (YP_001830444).
Identification of transposon insertion
We previously generated a transposon mutagenesis library (EZ-TN5TM Epicentre, Madison, WI) of X. fastidiosa Temecula 1 [29]. Mutants were screened for impaired twitching motility on agar plates. Flanking regions of one such mutant, subsequently identified as the PD1671 deleted mutant, were amplified and cloned. DNA sequencing was performed at the Core Laboratories Center at Cornell University (Ithaca, NY). DNA sequences were submitted to the X. fastidiosa comparative genomic database [30] and submitted to BLAST program. Tn5 insertion was also confirmed by PCR (polymerase chain reaction) (Table 1).
Table 1. Oligonucleotide primers used in this study.
| Primers | Sequence 5’-3’ | Use | Reference or source |
|---|---|---|---|
| KAN-2 FP-1 | ACCTACAAAGCTCTCATCAACC | Identify Tn insertion | Epicentre Biotech. |
| KAN-2 RP-1 | GCAATGTA CATCAGAGATTTTGAG | Identify Tn insertion | Epicentre Biotech. |
| PD1671.F | GAATTCTTATTCAATTGGGGGTTACT | Complementation | This study |
| PD1671.R | GAATTCTCTTGTTTGAGTTTGCTATG | Complementation | This study |
| PD1671.RecF | CTTCAAGGTGCTGGCATACATG | RT-PCR a PD1671 REC domain | This study |
| PD1671.RecR | GAAATCATCGGCACCACTATCA | RT-PCR PD1671 REC domain | This study |
| PD1671.GgF | TATGGTTACACTGCTTTCGAGC | RT-PCR PD1671 GGDEF domain | This study |
| PD1671.GgR | TTTAAAGCCTTGATTCAGCGGG | RT-PCR PD1671 GGDEF domain | This study |
| PD1671.EalF | CGTTCATCCACATAAACGTAGC | RT-PCR PD1671 EAL domain | This study |
| PD1671.EalR | GTTTGCAAGACGCAGTTATTCA | RT-PCR PD1671 EAL domain | This study |
| XP1275.RT.F | TTATGTAAGCGTCTTGGTGTGG | RT-PCR dnaQ | This study |
| XP1275.RT.R | GCACATGACCAGCGATCTTAC | RT-PCR dnaQ | This study |
| XP1455.RT.R | GGTGTGTGCATTTGCTTCTATG | RT-PCR gumJ | This study |
| XP1455.RT.F | GAGGAGAGTGAGGAAGGGATCT | RT-PCR gumJ | This study |
| XP1460.RT.R | TCTTCCGTGTCTTGGGATTC | RT-PCR gumD | This study |
| XP1460.RT.F | AATGACAGGCACATGACCAA | RT-PCR gumD | This study |
| RST31 | GCGTTAATTTTCGAAGTGATTCGATTGC | Basipetal movement | [31] |
| RST33 | CACCATTCGTATCCCGGTG | Basipetal movement | [31] |
a RT-PCR—reverse transcriptase-polymerase chain reaction.
Genetic complementation
Total genomic DNA was extracted from 5 mL cultures of X. fastidiosa Temecula 1 using the Mo Bio Ultraclean Microbial DNA kit (Mo Bio Laboratories Inc., Carlsbad, CA). The PD1671 gene with 377 bases upstream for the promoter region was PCR amplified with primers PD1671F and PD1671R (Table 1). The resulting 2.4 kb product was digested with EcoRI and cloned into pBBR1MCS-5 [32], sequenced (Core Laboratories Center, Cornell University, Ithaca, NY), and electroporated into X. fastidiosa PD1671 mutant cells [29,33]. Cells were plated on PW agar amended with kanamycin and gentamycin. After 10 days colonies were screened for wild-type phenotypes.
Bacterial growth and biofilm formation
Growth curves for wild-type, PD1671 mutant, and complemented PD1671 mutant were performed in V. vinifera xylem sap and biofilm formation was quantified with crystal violet staining as previously described [19,22]. Cells began at OD600 of 0.05 (1 x 106 cells mL-1) for the growth curve and 0.1 (3 x 106 cells mL-1) for biofilm assays. The growth curve was repeated five independent times with at least four replicates each time. Biofilm formation was measured in 96-well plates, and it was repeated with two independent experiments.
Twitching motility in microfluidic chambers
The speed at which wild-type and mutant cells migrate against media flow was assessed in microfluidic devices as previously described [34]. Flow speed of Pierce’s Disease 2 medium (PD2) [35] controlled by a syringe pump was maintained at 1 x 104 μm min-1. Cells attached to the glass surface of the microfluidic channels were observed microscopically using time-lapse image recordings every 30 s as previously reported [36]. Twenty five to thirty individual cells were tracked for the wild-type, PD1671 mutant, and complemented PD1671 mutant from three independent experiments. Data were analyzed with the Kruskal-Wallis test and means separated by the Kruskal-Wallis all pairwise comparison test.
Exopolysaccharide (EPS) production assay
X. fastidiosa wild-type, PD1671 mutant, and complemented PD1671 mutant were grown in one mL V. vinifera Chardonnay xylem sap for five days at 28°C, with agitation (200 rpm). EPS production was quantified by dry weight [37]. Pearson’s Chi-square analysis was performed to compare EPS production by wild-type, PD1671 mutant, and complemented PD1671 mutant. The experiment was performed three independent times with five replicates each.
Extracellular enzyme activity assays
The relative levels of carboxymethyl cellulase, endo-β-1,4-mannanase, polygalacturonase, and protease activity were assessed using radial diffusion assays, as described [37,38] with modifications. After cells were grown in one mL V. vinifera Chardonnay xylem sap for five days at 28°C, with agitation (200 rpm), and centrifuged, the supernatants were sterilized using filtration (0.2 μm). Three hundred microliters (6 x 50 μL) supernatant was then added to a 0.5 cm well that was created with a cork borer in the middle of a Petri plate. After 48 hr plates were stained (except protease assay) and enzyme activity was determined by measuring the zone diameter surrounding the supernatant cultures. Enzyme activity was visualized as clear or white halos surrounding the wells. Pearson’s Chi-square analysis was performed to compare the results. Assays were performed three times with five replicate each.
For the carboxymethyl cellulose assay, plates containing medium was used containing 0.1% carboxymethyl cellulose, 25 mM sodium phosphate, pH 7.0, and 0.8% agarose [37]. Plates were stained with 0.1% Congo red for 20 min and then were washed twice with 1 M NaCl. For the endo-β-1,4-mannanase assay, media was prepared by with 0.5 g locust bean gum galactomannan dissolved in 500 mL of McIlvaine buffer (pH 5) [37,38]. The suspension was heated to 80°C while constantly stirring for 2 hr, removed from heat, and stirred continually overnight. The suspension was centrifuged at 5,000g. Gelatin was added to the mix (250 mg L-1) and sterilized by autoclave. The plate was washed for 30 min in a 1.0:4.3 of 0.1 M citric acid and 0.2 M Na2HPO4 (pH 7.0), stained for 30 min in 0.5% (w/v) Congo red dye, washed in water for 2 min, fixed with 80% (v/v) ethanol for 10 min, washed in water again for 2 min, washed with three 20 min washes in McIlvaine buffer (pH 7), and developed after five washes with 1 M NaCl. To test for polygalacturonase activity, a medium was used containing 1.0% agarose and 0.1% polygalacturonic acid was dissolved in 100 mM potassium phosphate (pH 6.5) [37]. Surface of the medium was covered with 0.05% (w/v) ruthenium red for 20 min and rinsed 5 times in water. For the protease assay, activity was determined using PW plates containing 0.5% skimmed milk [37].
Semiquantitative RT-PCR (reverse transcriptase-PCR) analysis
Bacterial cultures were grown for four days in V. vinifera xylem sap to approximately 107 cell mL-1 (OD600≈1.0) and then harvested by centrifugation at 16,000g at 4°C for 5 minutes. The cells were suspended in RNeasy lysis buffer (Qiagen, Valencia, CA), and then lysed by Trizol reagent (Invitrogen, Carlsbad, CA). RNA was purified using RNeasy columns (Qiagen) and treated with DNase I (Invitrogen). RNA was quantified using a Nanodrop 1000 spectrophotometer (NanoDrop, Wilmington, DE) and qualitatively analyzed on agarose gels, then 5 μg of total RNA was used for cDNA synthesis using the first-strand cDNA using SuperScript III One-Step RT-PCR System with Platinum Taq polymerase according to the manufacturer’s protocol (Invitrogen). The resulting cDNA was utilized for regular PCR with gene or domain-specific primers (Table 1). Aliquots of each amplicon were electrophoresed on a 1.2% agarose gel and visualized by ethidium bromide staining. Gels were photographed using the Kodak Image Station 440CF (Eastman Kodak Company, Rochester, NY) and images densitometrically quantified with the Image J software (National Institutes of Health, Bethesda, MD) according to the manufacturer’s instructions. The experiment was performed three to six independent times with three replicates each.
Virulence assays in grapevines
The pathogenicity assay was performed [3,39,40] with modifications. A 20 μL aliquot of an OD600 = 2.0 (about 8 x 109 cells mL-1) suspension of wild-type and PD1671 mutant cells, cultured in V. vinifera xylem sap for four days or from PW plates, was inoculated individually by a needle puncture method (BD ultrafine short insulin pen needle, BD Biosciences, Franklin Lakes, NJ) into grapevines (V. vinifera L. cv. Cabernet Sauvignon) that had been grown for two months in the greenhouse. The cell suspension was inoculated into the stem at a position seven internodes up from the base of the shoot (ten plants per treatment). The negative control consisted of plants inoculated with succinate-citrate buffer [41]. Plants were scored weekly (until week twenty) for disease symptoms on a scale from zero to five [9]. The experiment was repeated three independent times and data were analyzed by repeated measures ANOVA.
Basipetal movement in plants
The basipetal movement of wild-type and PD1671 mutant in inoculated plants was performed as previously described [3] with modifications. Two month old V. vinifera L. cv. Cabernet Sauvignon plants varying from 60–100cm in height were inoculated by needle puncture, as described above. At least four shoots were examined after four, eight, and twelve weeks and the bacterial population determined by agar plate count. Shoots were cut from the main trunk and surface sterilized (70% ethanol for one min, 2% sodium hypochlorite solution for two min, and 70% ethanol for 30 s, followed by two rinses in sterile water). Subsequently, one cm sections were aseptically excised at measured distances from the original point of inoculation. The one cm sections were crushed in sterile polycarbonate bags containing 200 μL of sterile succinate-citrate buffer. The liquid phase resulting from the triturate was spread onto modified PW agar (wild-type X. fastidiosa) or PW agar containing kanamycin (PD1671 mutant). The plates were examined for the presence of X. fastidiosa after seven days at 28°C by measuring colony forming units (CFU) and PCR. PCR was performed using X. fastidiosa specific primer pairs RST31/RST33 (Table 1).
Statistical analysis
Statistical analyses were performed using the statistical software package SPSS version 18.0 or 19.0 (SPSS Inc., Chicago, IL). All quantitative assays, unless otherwise specified, were analyzed using one-way analysis of variance.
Results
Identifying a PD1671 mutant
A twitching-defective Tn5 mutant was identified with a disruption in open reading frame PD1671 at codon 468 of 566; PD1671 is predicted to encode a 566 amino acid protein. The Tn5 insertion abolished transcription from the gene segment examined, as determined by RT-PCR using dnaQ (PD1217) as an internal control (Table 2). The REC and GGDEF domains of PD1671 retained wild-type level expression. Whether the REC-GGDEF mRNA translated into functional and stable protein was not measured, however, we can assume PD1671 mutant phenotype findings reflected the PD1671 gene with a loss of the EAL domain.
Table 2. Relative X. fastidiosa RNA levels.
| Gene or domain a | Wild-type | PD1671 mutant | Complemented PD1671 |
|---|---|---|---|
| PD1671 REC domain | 1.11 ± 0.11 | 0.99 ± 0.07 | 1.78 ± 0.62 |
| PD1671 GGDEF domain | 0.96 ± 0.06 | 0.92 ± 0.18 | 1.20 ± 0.30 |
| PD1671 EAL domain | 0.96 ± 0.08 | 0.00 ± 0.00 b | 1.40 ± 0.62 |
| gumD | 1.18 ± 0.45 | 2.86 ± 0.31 b | 1.02 ± 0.01 |
| gumJ | 1.36 ± 0.33 | 2.61 ± 0.45 b | 1.25 ± 0.22 |
a RT-PCR (reverse transcriptase-polymerase chain reaction) experiments performed in Vitis vinifera xylem sap (three to six independent experiments with three replicates each). The standard deviations of the normalized means are shown. Expression of the gene regions was normalized to dnaQ gene expression [42]. Gene segments amplified: PD1671-REC domain (115 to 357bp), PD1671-GGDEF domain (601 to 858bp), PD1671-EAL domain (1325 to 1621bp), gumD (628–847bp), and gumJ (4–229bp).
b Statistically significant compared to wild-type (P<0.01).
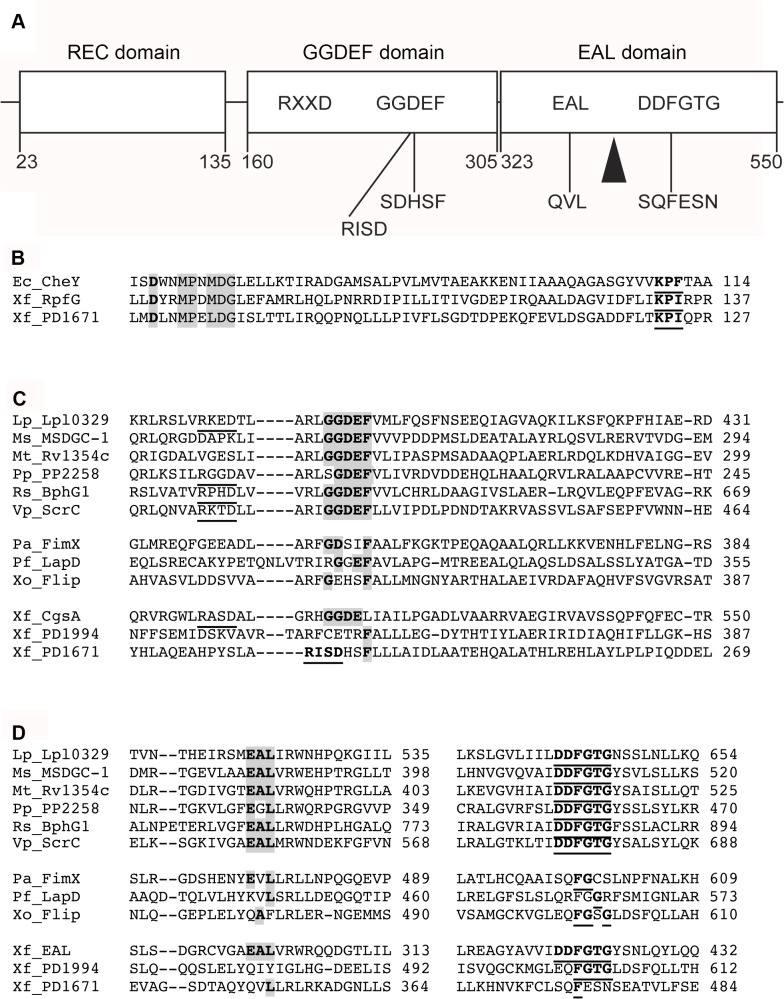
We examined the three major domains of PD1671: i) an N-terminal REC (response regulator, signal receiver, CheY-like receiver) domain, ii) a GGDEF domain, and iii) an EAL domain (Fig. 1A). i) We compared the PD1671 REC domain to the well-characterized E. coli CheY REC protein [43] and the relevant domain of X. fastidiosa REC-containing c-di-GMP protein RpfG [17] (Fig. 1B). The PD1671 REC domain has a predicted phosphorylation site, Mg2+ active site, and a dimerization interface. For the GGDEF and EAL domain comparisons, we aligned PD1671 with hybrid GGDEF-EAL domain-containing proteins with known enzymatic activity in both subunits [44–49], hybrid non-enzymatic GGDEF-EAL domain-containing proteins with alternative c-di-GMP function [50–52], and the four predicted Xylella fastidiosa DGC and/or PDE proteins [17]. ii) The putative Temecula 1 PD1671 GGDEF domain appears to lack the signature motif (Fig. 1C). iii) For the predicted X. fastidiosa PD1671 EAL domain, the conserved DDFGTG segment appears absent (Fig. 1D). For each of the three PD1671 domains, the critical conserved sequences described above for X. fastidiosa Temecula 1 are conserved across X. fastidiosa strains 9a5c, Ann-1, Dixon, EB92, M12, and M23 (S1 Fig).
Fig 1. Putative PD1671 domains.
A) Boxes represent the three PD1671 domains with domain names above the boxes and amino acid numbers below the boxes. Bacterial diguanylate cyclase and phosphodiesterase consensus sequences listed in boxes (X is any amino acid), and PD1671 aligned sequences listed below the boxes at their approximate locations. Arrow head denotes Tn5 insertion point. B) REC domain alignment. Xylella fastidiosa PD1671 REC domain alignment with functional REC protein and X. fastidiosa predicted c-di-GMP protein containing REC domain. Grey boxed/bold amino acids are the phosphorylation site, grey boxed/non-bold amino acids are the intermolecular recognition site, and bold/underlined amino acids are the dimerization interface. C) GGDEF domain. Top sequence group is hybrid GGDEF-EAL domain-containing proteins enzymatic in both domains, middle sequence group is non-enzymatic hybrid GGDEF-EAL domain-containing proteins, and bottom sequence group is X. fastidiosa predicted GGDEF domain proteins. Underlined amino acids are the allosteric I site, RxxD, and grey boxed/bold amino acids are the GGDEF sequences. Underlined/bold PD1671 residues denote a potential RxxD site. D) EAL alignment. Top sequence group is hybrid GGDEF-EAL containing proteins enzymatic in both subunits, middle sequence group is non-enzymatic hybrid GGDEF-EAL domain proteins, and bottom sequence group is X. fastidiosa predicted EAL proteins. Grey boxed/bold amino acids are signature EAL sequence and underlined/bold residues are DDFGTG sequences. Alignment comparison sequences: Ec = Escherichia coli, Lp = Legionella pneumophilia, Ms = Mycobacterium smegmatis, Mt = Mycobacterium tuberculosis, Pa = Pseudomonas aeruginosa, Pf = Pseudomonas fluorescens, Pp = Pseudomonas putida, Rs = Rhodobacter sphaeroides, Vp = Vibrio parahaemolyticus, Xf = Xylella fastidiosa, Xo = Xanthomonas oryzae.
Growth and motility
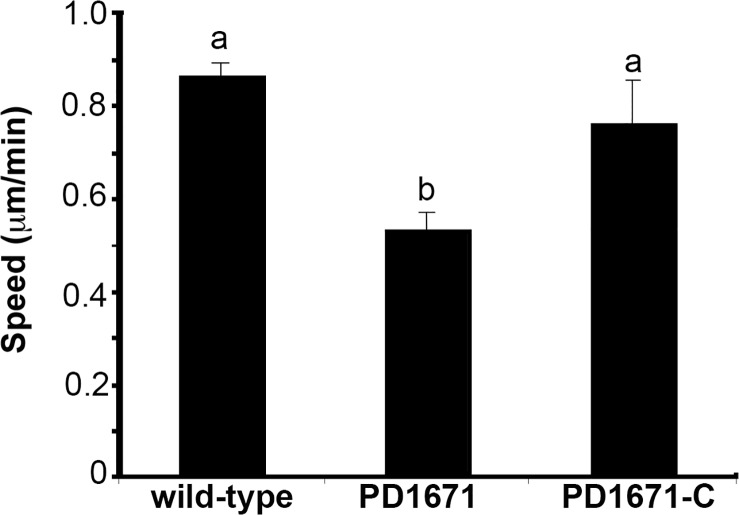
The PD1671 mutant, complemented PD1671 mutant, and wild-type cells grew equally in V. vinifera xylem sap (P = 0.927) (Fig. 2). Concerning motility, we examined the speed of strains under flow conditions in microfluidic chambers and found that the PD1671 mutant had reduced motility compared to wild-type or complemented mutant cells (P = 0.02) (Fig. 3).
Fig 2. Growth curve of the X. fastidiosa PD1671 mutant.
Growth of wild-type X. fastidiosa Temecula 1 (circle), PD1671 mutant (square), and complemented PD1671 mutant (PD1671-C) (triangle) strains in Vitis vinifera Chardonnay xylem sap. Cell densities were measured daily at OD600. Average and standard deviation of the averages of five independent experiments with a minimum of four replicates each (P = 0.927).
Fig 3. Movement of the X. fastidiosa PD1671 mutant in microfluidic chambers.
Twitching movement speed of wild-type X. fastidiosa Temecula 1, PD1671 mutant, and complemented PD1671 mutant (PD1671-C) cells in microfluidic flow chambers. Values shown are means and standard errors from three independent experiments. Letters above bars indicate significant differences by Kruskal-Wallis test and means were separated by the Kruskal-Wallis all pairwise comparison test (P = 0.02)
Expression of gum genes and extracellular enzyme activity
The gumD and gumJ genes in the mutant strain were upregulated approximately two-fold to that of the PD1671 complemented mutant and wild-type cells (P<0.01) (Table 2). By our assays, the PD1671 mutant strain had no effect on carboxymethyl cellulase (endo-1,4-β-glucanase) or undefined protease function, while endo-β-1,4-mannanase (a hemicellulase) and polygalacturonase (pectin-degrading enzyme) activity was not detected for any strains tested (Table 3).
Table 3. Relative X. fastidiosa exoenzyme activity.
| Extracellular enzyme a | Wild-type | PD1671 mutant | Complemented PD1671 |
|---|---|---|---|
| Carboxymethyl cellulase | 1.01 ± 0.20 | 1.10 ± 0.30 | 1.20 ± 0.25 |
| Endo-β-1,4-mannanase | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Polygalacturonase | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Protease | 2.12 ± 0.20 | 2.20 ± 0.30 | 2.10 ± 0.25 |
a Extracellular enzyme activities were estimated from the diameter (mm) of the halo zones of supernatant enzymatic activity surrounding each well. All assays were performed three times, with five replicate plates each. The standard deviations of the means for each enzyme are shown.
EPS production and biofilm formation
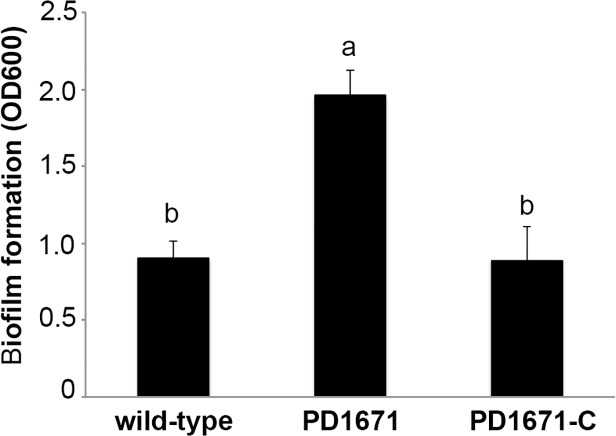
The amount of EPS was over two times greater in the PD1671 mutant in comparison to wild-type cells (P<0.0001) (Table 4). When biofilm formation was examined [19,22], we found that the PD1671 mutant formed more biofilm than wild-type cells (P = 0.001) (Fig. 4). For EPS production and biofilm formation assays the complement strain restored the phenotypes to those observed with the wild-type strain.
Table 4. Relative X. fastidiosa exopolysaccharide (EPS) production.
a Average dry weight of EPS (mg mL-1). The experiments were performed three times with five replicates each. The standard deviations are shown.
b Statistically significant compared to wild-type (P<0.0001).
Fig 4. Biofilm production by the X. fastidiosa PD1671 mutant.
Quantification of biofilm formation in Vitis vinifera xylem sap with agitation for wild-type X. fastidiosa Temecula 1, PD1671 mutant, and complemented PD1671 mutant (PD1671-C). Representative experiment shown. Different letters represent significant difference when means are compared (P = 0.001).
PD1671 mutant in planta
V. vinifera vines inoculated with the mutant and wild-type X. fastidiosa cells were examined for disease progression. When examined over time, both wild-type and PD1671 mutant strains were recovered from grapevine shoot sections upstream of the inoculation site with no significant differences in numbers (P = 0.40) (Fig. 5A). After twelve weeks both bacterial strains had completely translocated from the point of inoculation to the end of the shoots with comparable concentrations (1x108 CFU mL-1 for PD1671 mutant and 1.3x108 CFU mL-1 for wild-type). Plants inoculated with the PD1671 mutant and wild-type cells induced a similar overall pattern of disease progression, however, the mutant-induced disease was shifted; PD1671 mutant-inoculated plants showed disease symptoms three weeks earlier (P<0.005) and progressed to full disease rating three weeks sooner than plants inoculated with wild-type cells (Fig. 5B).
Fig 5. Movement and virulence in grapevines by the X. fastidiosa PD1671 mutant.
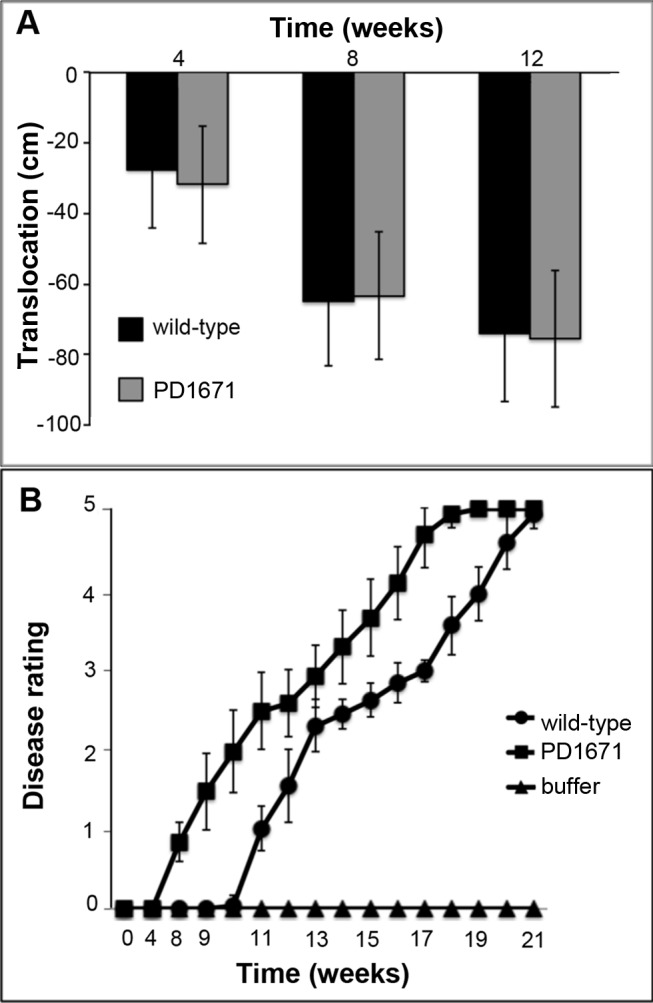
A) Basipetal translocation in planta of PD1671 mutant compared to wild-type X. fastidiosa Temecula 1. Average distances at which cells were recovered from vine regions upstream of the inoculation point (represented by 0 on the y axis) after four, eight, and twelve weeks post-inoculation. Error bars represent standard deviation of the means of at least four plants (P = 0.40). B) Severity of Pierce's disease. Average of at least 10 plants per treatment with Temecula 1 (circle), PD1671 mutant (square), and buffer negative control buffer (triangle). Experiment was repeated three times. Error bars represent standard deviation of the means of a representative experiment (P<0.005).
Discussion
In this report we demonstrate that a putative X. fastidiosa PD1671 protein is involved in gum gene expression, EPS production, biofilm formation, and Pierce’s disease development. The PD1671 gene is annotated as belonging to a two-component regulatory system [18], and is considered an orphan response regulator [53]. It contains an N-terminal REC domain of unknown function. Throughout bacterial genomes, REC domains are found as stand-alone proteins or as domains within larger proteins [54]. In GGDEF and EAL domain-containing proteins, phosphorylation of the REC domain is hypothesized to induce dimerization and function [15]. Given that the putative PD1671 REC domain has a predicted dimerization domain and the conserved aspartic residue for phosphorylation that aligns with the E. coli Asp57 [24,55,56], the domain may be functional.
Previous work identified the X. fastidiosa PD1671 as a hybrid GGDEF-EAL domain-containing protein [17]. When compared to known enzymatic GGDEF-EAL proteins [44–49], we found that the predicted PD1671 protein is lacking or degenerative in critical binding and active sites in both domains. Of note, the PD1671 EAL sequence is degenerate, however, a valine replacing alanine is the most common substitution in this motif [14]. GGDEF-EAL hybrid domain proteins are frequently inactive in one or both domains [15–17]. Those with degenerate sequences are found to have biological functions that appear to be either c-di-GMP dependent or independent [50–52,57]. PD1671 more closely aligns in the key conserved sequences with known non-enzymatic, c-di-GMP binding hybrid GGDEF-EAL proteins FimX, Flip, and LadD. Non-enzymatic GGDEF-EAL proteins can function as c-di-GMP receptors [58] by binding the ligand in either the EAL-like domain at an unknown position or in the GGDEF domain at the allosteric I RxxD site [51,59]. However, while the putative PD1671 has an RxxD sequence near the conserved position, it lacks the expected five residue linker between the RxxD sequence and the aligned GGDEF sequence [60] so it may not be functional.
Regulation of biofilm formation
X. fastidiosa forms biofilm that, along with increased bacterial concentration, is proposed to clog xylem vessels leading to disease symptoms [2]. X. fastidiosa produces EPS, which has been shown to be an important physical component of biofilm [61]. Based on homology to Xanthomonas species, the X. fastidiosa GumD protein is proposed to be part of a EPS polysaccharide assembly, and the GumJ protein is involved in EPS secretion [61–64]. Our results suggest that the uninterrupted putative PD1671 protein downregulates gum gene expression, which presumably limits EPS production and therefore reduces biofilm formation. Thus, when the PD1671 protein is fully expressed, it appears to be an anti-virulence regulator. In support of this, disruption of the X. fastidiosa gumD gene correlates with reduced EPS and biofilm production and an avirulent phenotype in grapevines [61].
Role in motility and exoenzyme activity
In microfluidic chambers, the PD1671 mutant migrated slower than wild-type cells. Reduced motility may suggest that in wild-type cells the PD1671 EAL domain may have a direct regulatory role in motility, and c-di-GMP is known to be important in type IV pili regulation and response [14]. Alternatively, the observed reduced motility may result from increased EPS production and biofilm formation that impedes movement. However, a difference in motility was not detected between the PD1671 mutant and wild-type cells in planta. The in vivo and in vitro environments may be distinct enough to produce different observed responses. For instance, results may reflect the difference in assay timeframes or that each environment induced unique gene expression profiles that altered findings [42,65–68].
Translocation requires degradation of pit membranes and two X. fastidiosa exoenzymes have been studied, polygalacturonase and endo-1,4-β-glucanase [69,70]. In our assays, these and the other the extracellular enzymes tested showed no differences in the PD1671 mutant compared to wild-type or complemented strains. Of note, polygalacturonase activity in media or xylem fluid has been reported to be unsuccessful or inconsistent [69,71]. Overall our findings suggest that the predicted PD1671 protein does not have an in vivo regulatory role in X. fastidiosa twitching motility or movement through pit membranes.
Effects on virulence
Motility and biofilm formation are thought to be key in X. fastidiosa-induced disease. Motility has been found to have a direct relationship with disease. X. fastidiosa mutants exhibiting increased motility are hypervirulent [9], while those with decreased motility have reduced pathogenicity [39,72]. While our PD1671 mutant strain was hypervirulent, it exhibited equal movement to wild-type cells in planta, suggesting that other factors explain the phenotype. Biofilm, composed in part by EPS, also has been found to have a direct relationship with symptom development. X. fastidiosa mutants with decreased biofilm have decreased virulence [39,61,72–74], suggesting that mutants with increased biofilm formation might be hypervirulent. The PD1671 mutant showed increased biofilm production and so might be expected to have increased pathogenicity. Therefore, we suspect that the hypervirulent phenotype of the PD1671 mutant resulted from upregulated gum gene expression that leads to increased EPS, which in turn increased biofilm production.
PD1671 involvement in regulating multiple responses
The observed phenotypes, along with degenerated protein sequences, suggest that the putative PD1671 plays a regulatory role, as other non-catalytic GGDEF-EAL hybrid domain-containing proteins have been implicated in regulation processes [57,58]. Motility and biofilm formation are regulated by a number of genes in X. fastidiosa. We previously identified a chemotaxis homologous operon, Pil-Chp, which regulates twitching motility [72]. In addition, the pilR/pilS two-component regulatory system regulates the transcription of pilA, the major pilin of the type IV pilus that controls twitching motility [29,75]. Biofilm formation and the pili genes are associated with sigma factor 54 (RpoN) [76] and the global regulators algU and gacA [73,77]. DSF and c-di-GMP have been shown to be involved in regulating biofilm and motility. High levels of DSF in X. fastidiosa are proposed to correlate with reduced motility, upregulated gum and adhesion genes, increased EPS production, and enhanced aggregation of cells with biofilm potentially by signaling through RpfG and altering c-di-GMP levels [13].
Given how enzymatically active GGDEF and EAL domain-containing proteins function, mutations in PDE proteins would be expected to show decreased motility and increased gum gene expression, EPS production, and biofilm formation, while mutations in DGC proteins would be predicted to exhibit the opposite phenotypes. While we cannot rule out a potential dominant-negative effect from the transposon insertion in the 3’ end of PD1671 [78–80], the phenotypes of our PD1671 mutant matches the predicted responses of the protein lacking an EAL domain. Therefore the observed responses of the PD1671 mutant suggest it might be directly or indirectly involved in decreasing c-di-GMP levels. However, the X. fastidiosa c-di-GMP proteins that have been directly examined exhibit unexpected phenotypes. Deletion of the X. fastidiosa eal PDE-encoding gene leads to decreased gum gene expression, EPS production, and biofilm formation and increased motility [20]. Mutation of the X. fastidiosa csgA DGC-encoding gene showed increased gum gene expression, EPS production, and biofilm formation but decreased virulence [19]. Whether the predicted PD1671 is involved in c-di-GMP levels and whether it associates with other X. fastidiosa regulatory systems are still areas of exploration. Understanding how the putative PD1671 regulates pathogenic responses will provide greater insight into the signaling mechanisms controlling biofilm-forming behaviors critical for Pierce’s disease development.
Supporting Information
Stars represent amino acids that are not conserved across the orthologs. A) REC domain alignment. Grey boxed/bold amino acids are the phosphorylation site, grey boxed/non-bold amino acids are the intermolecular recognition site, and bold/underlined amino acids are the dimerization interface. B) GGDEF alignment. Underlined/bold PD1671 residues note a potential RxxD site. Grey boxed/bold amino acids are the conserved residues matching the GGDEF sequence as seen in Fig. 1C. C) EAL alignment. Grey/boxed residues match the signature EAL sequence and the underlined/bold residues line with the DDFGTG sequences as seen in Fig. 1D.
(TIF)
Acknowledgments
We thank C.L. Reid and B. Dehaven for technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Support was provided by the University of California Pierce’s Disease Research Grants Program #2010-287 and #SA7465 to PM, HCH, and TJB [http://www.piercesdisease.org/grants/manage], and Nanobiotechnology Center, an STC program of the National Science Foundation, under agreement no. ECS-304 9876771 to HCH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Purcell AH, Hopkins DL. Fastidious xylem-limited bacterial plant pathogens. Annu Rev Phytopathol. 1996;34: 131–151. [DOI] [PubMed] [Google Scholar]
- 2. Chatterjee S, Almeida RP, Lindow S. Living in two worlds: the plant and insect lifestyles of Xylella fastidiosa . Annu Rev Phytopathol. 2008;46: 243–271. 10.1146/annurev.phyto.45.062806.094342 [DOI] [PubMed] [Google Scholar]
- 3. Meng Y, Li Y, Galvani CD, Hao G, Turner JN, Burr TJ, et al. Upstream migration of Xylella fastidiosa via pilus-driven twitching motility. J Bacteriol. 2005;187: 5560–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mattick JS. Type IV pili and twitching motility. Annu Rev Microbiol. 2002;56: 289–314. [DOI] [PubMed] [Google Scholar]
- 5. Newman KL, Almeida RP, Purcell AH, Lindow SE. Use of a green fluorescent strain for analysis of Xylella fastidiosa colonization of Vitis vinifera . Appl Environ Microbiol. 2003;69: 7319–7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellis EA, McEachern GR, Clark S, Cobb BG. Ultrastructure of pit membrane dissolution and movement of Xylella fastidiosa through pit membranes in petioles of Vitis vinifera . Botany. 2010;88: 596–600. [Google Scholar]
- 7. Sun Q, Greve LC, Labavitch JM. Polysaccharide compositions of intervessel pit membranes contribute to Pierce's disease resistance of grapevines. Plant Physiol. 2011;155: 1976–1987. 10.1104/pp.110.168807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol Cell Biol. 2005;6: 850–861. [DOI] [PubMed] [Google Scholar]
- 9. Guilhabert MR, Kirkpatrick BC. Identification of Xylella fastidiosa antivirulence genes: hemagglutinin adhesins contribute a biofilm maturation to X. fastidiosa and colonization and attenuate virulence. Mol Plant Microbe Interact. 2005;18: 856–868. [DOI] [PubMed] [Google Scholar]
- 10. Roper MC, Greve LC, Labavitch JM, Kirkpatrick BC. Detection and visualization of an exopolysaccharide produced by Xylella fastidiosa in vitro and in planta . Appl Environ Microbiol. 2007;73: 7252–7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. da Silva FR, Vettore AL, Kemper EL, Leite A, Arruda P. Fastidian gum: the Xylella fastidiosa exopolysaccharide possibly involved in bacterial pathogenicity. FEMS Microbiol Lett. 2001;203: 165–171. [DOI] [PubMed] [Google Scholar]
- 12. Simpson AJ, Reinach FC, Arruda P, Abreu FA, Acencio M, Alvarenga R, et al. The genome sequence of the plant pathogen Xylella fastidiosa. The Xylella fastidiosa consortium of the organization for nucleotide sequencing and analysis. Nature. 2000;406: 151–159. [DOI] [PubMed] [Google Scholar]
- 13. Chatterjee S, Wistrom C, Lindow SE. A cell-cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa . Proc Natl Acad Sci U S A. 2008;105: 2670–2675. 10.1073/pnas.0712236105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77: 1–52. 10.1128/MMBR.00043-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schirmer T, Jenal U. Structural and mechanistic determinants of c-di-GMP signalling. Nat Rev Microbiol. 2009;7: 724–735. 10.1038/nrmicro2203 [DOI] [PubMed] [Google Scholar]
- 16. Seshasayee AS, Fraser GM, Luscombe NM. Comparative genomics of cyclic-di-GMP signalling in bacteria: post-translational regulation and catalytic activity. Nucleic Acids Res. 2010;38: 5970–5981. 10.1093/nar/gkq382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryan RP, Fouhy Y, Lucey JF, Dow JM. Cyclic di-GMP signaling in bacteria: recent advances and new puzzles. J Bacteriol. 2006;188: 8327–8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Sluys MA, de Oliveira MC, Monteiro-Vitorello CB, Miyaki CY, Furlan LR, Camargo LEA, et al. Comparative analyses of the complete genome sequences of Pierce's disease and citrus variegated chlorosis strains of Xylella fastidiosa . J Bacteriol. 2003;185: 1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chatterjee S, Killiny N, Almeida RP, Lindow SE. Role of cyclic di-GMP in Xylella fastidiosa biofilm formation, plant virulence, and insect transmission. Mol Plant Microbe Interact. 2010;23: 1356–1363. 10.1094/MPMI-03-10-0057 [DOI] [PubMed] [Google Scholar]
- 20. de Souza AA, Ionescu M, Baccari C, da Silva AM, Lindow SE. Phenotype overlap in Xylella fastidiosa is controlled by the cyclic di-GMP phosphodiesterase Eal in response to antibiotic exposure and diffusible signal factor-mediated cell-cell signaling. Appl Environ Microbiol. 2013;79: 3444–3454. 10.1128/AEM.03834-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davis MJ, French WJ, Schaad NW. Axenic culture of the bacteria associated with phony disease of peach and plum leaf scald. Curr Microbiol. 1981;6: 309–314. [Google Scholar]
- 22. Zaini PA, De La Fuente L, Hoch HC, Burr TJ. Grapevine xylem sap enhances biofilm development by Xylella fastidiosa . FEMS Microbiol Lett. 2009;295: 129–134. 10.1111/j.1574-6968.2009.01597.x [DOI] [PubMed] [Google Scholar]
- 23. Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, et al. The Pfam protein families database. Nucleic Acids Res. 2012;40: D290–D301. 10.1093/nar/gkr1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, et al. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011;39: D225–D229. 10.1093/nar/gkq1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40: D109–114. 10.1093/nar/gkr988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Letunic I, Doerks T, Bork P. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012;40: D302–D305. 10.1093/nar/gkr931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, et al. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010;38: W695–699. 10.1093/nar/gkq313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 29. Li Y, Hao G, Galvani CD, Meng Y, De La Fuente L, Hoch HC, et al. Type I and type IV pili of Xylella fastidiosa affect twitching motility, biofilm formation and cell-cell aggregation. Microbiology. 2007;153: 719–726. [DOI] [PubMed] [Google Scholar]
- 30. Varani AM, Monteiro-Vitorello CB, de Almeida LG, Souza RC, Cunha OL, Lima WC, et al. Xylella fastidiosa comparative genomic database is an information resource to explore the annotation, genomic features, and biology of different strains. Genet Mol Biol. 2012;35: 149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Minsavage GV, Thompson CM, Hopkins DL, Leite RMCBC, Stall RE. Development of a polymerase chain reaction protocol for detection of Xylella fastidiosa in plant tissue. Phytopathology. 1994;84: 456–461. [Google Scholar]
- 32. Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM II, et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166: 175–176. [DOI] [PubMed] [Google Scholar]
- 33. Guilhabert MR, Hoffman LM, Mills DA, Kirkpatrick BC. Transposon mutagenesis of Xylella fastidiosa by electroporation of Tn5 synaptic complexes. Mol Plant Microbe Interact. 2001;14: 701–706. [DOI] [PubMed] [Google Scholar]
- 34. De La Fuente L, Montanes E, Meng Y, Li Y, Burr TJ, Hoch HC, et al. Assessing adhesion forces of type I and type IV pili of Xylella fastidiosa bacteria by use of a microfluidic flow chamber. Appl Environ Microbiol. 2007;73: 2690–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davis MJ, Purcell AH, Thomson SV. Isolation media for the Pierce’s disease bacterium. Phytopathology. 1980;70: 425–429. [Google Scholar]
- 36. De La Fuente L, Burr TJ, Hoch HC. Mutations in type I and type IV pilus biosynthetic genes affect twitching motility rates in Xylella fastidiosa . J Bacteriol. 2007;189: 7507–7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thowthampitak J, Shaffer BT, Prathuangwong S, Loper JE. Role of rpfF in virulence and exoenzyme production of Xanthomonas axonopodis pv. glycines, the causal agent of bacterial pustule of soybean. Phytopathology. 2008;98: 1252–1260. 10.1094/PHYTO-98-12-1252 [DOI] [PubMed] [Google Scholar]
- 38. Bourgault R, Bewley JD. Gel diffusion assays for endo-beta-mannase and pectin methylesterase can underestimate enzyme activity due to proteolytic degradation: a remedy. Anal Biochem. 2002;300: 87–93. [DOI] [PubMed] [Google Scholar]
- 39. Cursino L, Li Y, Zaini PA, De La Fuente L, Hoch HC, Burr TJ. Twitching motility and biofilm formation are associated with tonB1 in Xylella fastidiosa . FEMS Microbiol Lett. 2009;299: 193–199. 10.1111/j.1574-6968.2009.01747.x [DOI] [PubMed] [Google Scholar]
- 40. Hill BL, Purcell AH. Multiplication and movement of Xylella fastidiosa within grapevine and four other plants. Phytopathology. 1995;85: 1368–1372. [Google Scholar]
- 41. Hopkins DL, Thompson CM. Seasonal concentration of the Pierce’s disease bacterium in ‘Carlos’ and ‘Welder’ muscadine grapes compared with ‘Schuyler’ bunch grape. Hort Sci. 1984;19: 419–420. [Google Scholar]
- 42. Pashalidis S, Moreira LM, Zaini PA, Campanharo JC, Alves LM, Ciapina LP, et al. Whole-genome expression profiling of Xylella fastidiosa in response to growth on glucose. Omics. 2005;9: 77–90. [DOI] [PubMed] [Google Scholar]
- 43. Baker MD, Wolanin PM, Stock JB. Signal transduction in bacterial chemotaxis. Bioessays. 2006;28: 9–22. [DOI] [PubMed] [Google Scholar]
- 44. Levet-Paulo M, Lazzaroni JC, Gilbert C, Atlan D, Doublet P, Vianney A. The atypical two-component sensor kinase Lpl0330 from Legionella pneumophila controls the bifunctional diguanylate cyclase-phosphodiesterase Lpl0329 to modulate bis-(3'-5')-cyclic dimeric GMP synthesis. J Biol Chem. 2011;286: 31136–31144. 10.1074/jbc.M111.231340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bharati BK, Sharma IM, Kasetty S, Kumar M, Mukherjee R, Chatterji D. A full-length bifunctional protein involved in c-di-GMP turnover is required for long-term survival under nutrient starvation in Mycobacterium smegmatis . Microbiology. 2012;158: 1415–1427. 10.1099/mic.0.053892-0 [DOI] [PubMed] [Google Scholar]
- 46. Gupta K, Kumar P, Chatterji D. Identification, activity and disulfide connectivity of C-di-GMP regulating proteins in Mycobacterium tuberculosis . PLoS One. 2010;5: e15072 10.1371/journal.pone.0015072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Osterberg S, Aberg A, Herrera Seitz MK, Wolf-Watz M, Shingler V. Genetic dissection of a motility-associated c-di-GMP signalling protein of Pseudomonas putida . Environ Microbiol Rep. 2013;5: 556–565. 10.1111/1758-2229.12045 [DOI] [PubMed] [Google Scholar]
- 48. Tarutina M, Ryjenkov DA, Gomelsky M. An unorthodox bacteriophytochrome from Rhodobacter sphaeroides involved in turnover of the second messenger c-di-GMP. J Biol Chem. 2006;281: 34751–34758. [DOI] [PubMed] [Google Scholar]
- 49. Ferreira RB, Antunes LC, Greenberg EP, McCarter LL. Vibrio parahaemolyticus ScrC modulates cyclic dimeric GMP regulation of gene expression relevant to growth on surfaces. J Bacteriol. 2008;190: 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Navarro MV, De N, Bae N, Wang Q, Sondermann H. Structural analysis of the GGDEF-EAL domain-containing c-di-GMP receptor FimX. Structure. 2009;17: 1104–1116. 10.1016/j.str.2009.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Newell PD, Monds RD, O'Toole GA. LapD is a bis-(3',5')-cyclic dimeric GMP-binding protein that regulates surface attachment by Pseudomonas fluorescens Pf0-1. Proc Natl Acad Sci U S A. 2009;106: 3461–3466. 10.1073/pnas.0808933106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang F, Tian F, Li X, Fan S, Chen H, Wu M, et al. The degenerate EAL-GGDEF domain protein Filp functions as a cyclic di-GMP receptor and specifically interacts with the PilZ-domain protein PXO_02715 to regulate virulence in Xanthomonas oryzae pv. oryzae . Mol Plant Microbe Interact. 2014;27: 578–589. 10.1094/MPMI-12-13-0371-R [DOI] [PubMed] [Google Scholar]
- 53. Ulrich LE, Zhulin IB. The MiST2 database: a comprehensive genomics resource on microbial signal transduction. Nucleic Acids Res. 2010;38: D401–407. 10.1093/nar/gkp940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Galperin MY. Structural classification of bacterial response regulators: diversity of output domains and domain combinations. J Bacteriol. 2006;188: 4169–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sanders DA, Gillece-Castro BL, Stock AM, Burlingame AL, Koshland DE Jr. Identification of the site of phosphorylation of the chemotaxis response regulator protein, CheY. J Biol Chem. 1989;264: 21770–21778. [PubMed] [Google Scholar]
- 56. Bourret RB, Hess JF, Simon MI. Conserved aspartate residues and phosphorylation in signal transduction by the chemotaxis protein CheY. Proc Natl Acad Sci U S A. 1990;87: 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suzuki K, Babitzke P, Kushner SR, Romeo T. Identification of a novel regulatory protein (CsrD) that targets the global regulatory RNAs CsrB and CsrC for degradation by RNase E. Genes Dev. 2006;20: 2605–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sondermann H, Shikuma NJ, Yildiz FH. You've come a long way: c-di-GMP signaling. Curr Opin Microbiol. 2012;15: 140–146. 10.1016/j.mib.2011.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee VT, Matewish JM, Kessler JL, Hyodo M, Hayakawa Y, Lory S. A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol Microbiol. 2007;65: 1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Christen B, Christen M, Paul R, Schmid F, Folcher M, Jenoe P, et al. Allosteric control of cyclic di-GMP signaling. J Biol Chem. 2006;281: 32015–32024. [DOI] [PubMed] [Google Scholar]
- 61. Killiny N, Martinez RH, Dumenyo CK, Cooksey DA, Almeida RP. The exopolysaccharide of Xylella fastidiosa is essential for biofilm formation, plant virulence, and vector transmission. Mol Plant Microbe Interact. 2013;26: 1044–1053. 10.1094/MPMI-09-12-0211-R [DOI] [PubMed] [Google Scholar]
- 62. Scarpari LM, Lambais MR, Silva DS, Carraro DM, Carrer H. Expression of putative pathogenicity-related genes in Xylella fastidiosa grown at low and high cell density conditions in vitro . FEMS Microbiol Lett. 2003;222: 83–92. [DOI] [PubMed] [Google Scholar]
- 63. Katzen F, Ferreiro DU, Oddo CG, Ielmini MV, Becker A, Puhler A, et al. Xanthomonas campestris pv. campestris gum mutants: effects on xanthan biosynthesis and plant virulence. J Bacteriol. 1998;180: 1607–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim SY, Kim JG, Lee BM, Cho JY. Mutational analysis of the gum gene cluster required for xanthan biosynthesis in Xanthomonas oryzae pv oryzae . Biotechnol Lett. 2009;31: 265–270. 10.1007/s10529-008-9858-3 [DOI] [PubMed] [Google Scholar]
- 65. Shi X, Bi J, Morse JG, Toscano NC, Cooksey DA. Differential expression of genes of Xylella fastidiosa in xylem fluid of citrus and grapevine. FEMS Microbiol Lett. 2010;304: 82–88. 10.1111/j.1574-6968.2009.01885.x [DOI] [PubMed] [Google Scholar]
- 66. de Souza AA, Takita MA, Coletta-Filho HD, Caldana C, Goldman GH, Yanai GM, et al. Analysis of gene expression in two growth states of Xylella fastidiosa and its relationship with pathogenicity. Mol Plant Microbe Interact. 2003;16: 867–875. [DOI] [PubMed] [Google Scholar]
- 67. de Souza AA, Takita MA, Coletta-Filho HD, Caldana C, Yanai GM, Muto NH, et al. Gene expression profile of the plant pathogen Xylella fastidiosa during biofilm formation in vitro. FEMS Microbiol Lett. 2004;237: 341–353. [DOI] [PubMed] [Google Scholar]
- 68. Silva MS, De Souza AA, Takita MA, Labate CA, Machado MA. Analysis of the biofilm proteome of Xylella fastidiosa . Proteome Sci. 2011;9: 58 10.1186/1477-5956-9-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Roper MC, Greve LC, Warren JG, Labavitch JM, Kirkpatrick BC. Xylella fastidiosa requires polygalacturonase for colonization and pathogenicity in Vitis vinifera grapevines. Mol Plant Microbe Interact. 2007;20: 411–419. [DOI] [PubMed] [Google Scholar]
- 70. Perez-Donoso AG, Sun Q, Roper MC, Greve LC, Kirkpatrick B, Labavitch JM. Cell wall-degrading enzymes enlarge the pore size of intervessel pit membranes in healthy and Xylella fastidiosa-infected grapevines. Plant Physiol. 2010;152: 1748–1759. 10.1104/pp.109.148791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Aguero CB, Uratsu SL, Greve C, Powell AL, Labavitch JM, Meredith CP, et al. Evaluation of tolerance to Pierce's disease and Botrytis in transgenic plants of Vitis vinifera L. expressing the pear PGIP gene. Mol Plant Pathol. 2005;6: 43–51. 10.1111/j.1364-3703.2004.00262.x [DOI] [PubMed] [Google Scholar]
- 72. Cursino L, Galvani CD, Athinuwat D, Zaini PA, Li Y, De La Fuente L, et al. Identification of an operon, Pil-Chp, that controls twitching motility and virulence in Xylella fastidiosa . Mol Plant Microbe Interact. 2011;24: 1198–1206. 10.1094/MPMI-10-10-0252 [DOI] [PubMed] [Google Scholar]
- 73. Shi XY, Dumenyo CK, Hernandez-Martinez R, Azad H, Cooksey DA. Characterization of regulatory pathways in Xylella fastidiosa: genes and phenotypes controlled by gacA . Appl Environ Microbiol. 2009;75: 2275–2283. 10.1128/AEM.01964-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Matsumoto A, Huston SL, Killiny N, Igo MM. XatA, an AT-1 autotransporter important for the virulence of Xylella fastidiosa Temecula 1. Microbiologyopen. 2012;1: 33–45. 10.1002/mbo3.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hobbs M, Collie ES, Free PD, Livingston SP, Mattick JS. PilS and PilR, a two-component transcriptional regulatory system controlling expression of type 4 fimbriae in Pseudomonas aeruginosa . Mol Microbiol. 1993;7: 669–682. [DOI] [PubMed] [Google Scholar]
- 76. da Silva Neto JF, Koide T, Abe CM, Gomes SL, Marques MV. Role of sigma54 in the regulation of genes involved in type I and type IV pili biogenesis in Xylella fastidiosa . Arch Microbiol. 2008;189: 249–261. [DOI] [PubMed] [Google Scholar]
- 77. Shi XY, Dumenyo CK, Hernandez-Martinez R, Azad H, Cooksey DA. Characterization of regulatory pathways in Xylella fastidiosa: genes and phenotypes controlled by algU . Appl Environ Microbiol. 2007;73: 6748–6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Goymer P, Kahn SG, Malone JG, Gehrig SM, Spiers AJ, Rainey PB. Adaptive divergence in experimental populations of Pseudomonas fluorescens. II. Role of the GGDEF regulator WspR in evolution and development of the wrinkly spreader phenotype. Genetics. 2006;173: 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Malone JG, Williams R, Christen M, Jenal U, Spiers AJ, Rainey PB. The structure-function relationship of WspR, a Pseudomonas fluorescens response regulator with a GGDEF output domain. Microbiology. 2007;153: 980–994. [DOI] [PubMed] [Google Scholar]
- 80. Kulasakara H, Lee V, Brencic A, Liberati N, Urbach J, Miyata S, et al. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3'-5')-cyclic-GMP in virulence. Proc Natl Acad Sci U S A. 2006;103: 2839–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stars represent amino acids that are not conserved across the orthologs. A) REC domain alignment. Grey boxed/bold amino acids are the phosphorylation site, grey boxed/non-bold amino acids are the intermolecular recognition site, and bold/underlined amino acids are the dimerization interface. B) GGDEF alignment. Underlined/bold PD1671 residues note a potential RxxD site. Grey boxed/bold amino acids are the conserved residues matching the GGDEF sequence as seen in Fig. 1C. C) EAL alignment. Grey/boxed residues match the signature EAL sequence and the underlined/bold residues line with the DDFGTG sequences as seen in Fig. 1D.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.