Abstract
Purpose
A persistent stone burden after renal stone treatment may result in future patient morbidity and potentially lead to additional surgery. This problem is particularly common after treatment of lower pole stones. We describe a potential noninvasive therapeutic option using ultrasound waves to create a force sufficient to aid in stone fragment expulsion.
Materials and Methods
Human stones were implanted by retrograde ureteroscopy or antegrade percutaneous access in a live porcine model. The calibrated probe of a system containing ultrasound imaging and focused ultrasound was used to target stones and attempt displacement. To assess for injury an additional 6 kidneys were exposed for 2 minutes each directly to the output used for stone movement. Another 6 kidneys were exposed to more than twice the maximum output used to move stones. Renal tissue was analyzed histologically with hematoxylin and eosin, and nicotinamide adenine dinucleotide staining.
Results
Stones were moved to the renal pelvis or ureteropelvic junction by less than 2 minutes of exposure. Stone velocity was approximately 1 cm per second. There was no tissue injury when tissue was exposed to the power level used to move stones. Localized thermal coagulation less than 1 cm long was observed in 6 of 7 renal units exposed to the level above that used for ultrasonic propulsion.
Conclusions
Transcutaneous ultrasonic propulsion was used to expel calculi effectively and safely from the kidney using a live animal model. This study is the first step toward an office based system to clear residual fragments and toward use as a primary treatment modality in conjunction with medical expulsive therapy for small renal stones.
Keywords: kidney, kidney calculi, ultrasonography, lithotripsy, ureteroscopy
The goal of elective stone treatment is to render the patient stone free. However, stone size and site, composition and collecting system anatomical factors, such as the infundibulopelvic angle and length, and the spatial orientation of caliceal anatomy, have been identified as factors that may prospectively predict success of primary stone treatment.1 As a result, optimal surgical management of renal calculi remains a controversial topic as well as a treatment dilemma for the practicing urologist.
The goal of rendering a patient stone free is particularly problematic when treating lower pole caliceal stones.2,3 Percutaneous nephrostolithotomy provides a greater SFR than SWL, although it is a more invasive option.2,4 The estimated probability that residual stones after SWL cause further intervention and re-treatment at 5 years is 24% and 52%, respectively.5
Despite the evolution of our technology driven surgical approaches successful surgical management of lower pole stones principally depends on 2 processes, including stone fragmentation and residual fragment clearance. While residual fragment clearance using percussion, diuresis and inversion therapy have been described, success with these measures is limited.6–8 With advancements in ureteroscope technology, ureteral access sheaths and small nitinol baskets there is increased ability to remove fragments, although this can be a tedious process. To our knowledge there is no prior research on focused ultrasound technology directed at stone fragments as a mechanism to improve stone clearance and SFR after surgical treatment.
Our group recently reported the ability to move stones with focused ultrasound in a phantom model.9 In the current study we describe the development and initial testing of a prototype device using noninvasive ultrasound image guidance and focused ultrasound technology to move stones in a porcine renal collecting system. The goal of this new device is to guide small stones or residual stone fragments out of a low caliceal position into the renal pelvis or UPJ to facilitate spontaneous clearance.
Materials and Methods
Figure 1 shows the new Model H-106 device (Sonic Concepts, Bothell, Washington) that was developed, consisting of a 6 cm diameter, 2 MHz, 8 element annular array curved to a natural focus of 6 cm. The 8 elements were excited by the synchronized outputs of 8 signals from an SC-200 radio frequency synthesizer (Sonic Concepts) and amplified by 8, 100 W IC-706MKIIG amplifiers (Icom®). A laptop computer controlled the excitation timing of each element, which allowed focal depth to be varied from 4.5 to 8.5 cm.
Figure 1.
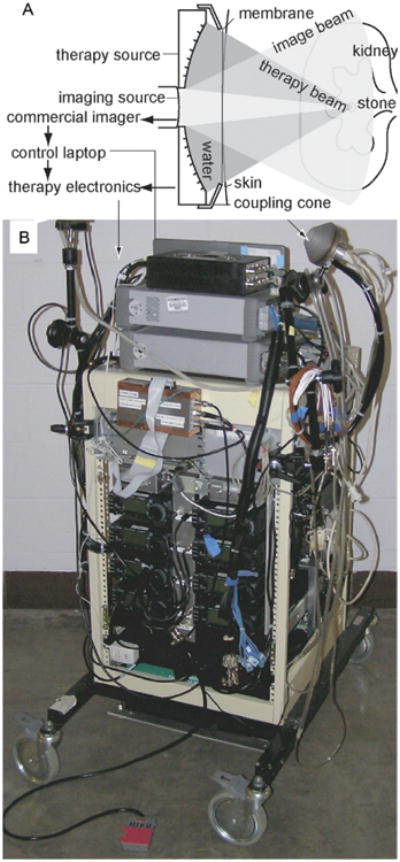
New experimental ultrasound system. Imaging probe is placed in therapy probe central aperture (A). Probes are held in water filled coupling cone with acoustically transparent window, which is placed in contact with skin. Electronics and probe with longer coupling cone (B). Ultrasound imager is not shown.
The computer also collected the image from the HDI-5000 ultrasound imaging system (Philips ATL®) and the selected focusing depth was overlaid on the image. This allowed the operator to visually align the stone at the focus. A foot switch turned the focused ultrasound on for about 50 milliseconds and off for about 50 milliseconds while the switch was closed. Total exposure in a burst of pulses was 1 to 4 seconds and no more than 10 bursts were used to move 1 stone.
The acoustic beam is shaped as an hourglass with the greatest energy concentration and highest pressure in the narrow focus region. The region over which pressure is within half peak pressure is only 1 cm long and about 1 mm wide. Time averaged acoustic intensity measured in water and derated to the 6.5 cm penetration depth in tissue was 225, 450 and 900 W/cm2, respectively.
All animal studies were approved by the University of Washington institutional animal care and use committee. A total of 12 common domestic female pigs weighing 50 to 60 kg underwent endoscopic and/or open surgery after general anesthesia was induced. In 6 pigs artificial stones (radiopaque glass/metal beads 3 and 5 mm in diameter, respectively) and human kidney stones (cystine, calcium oxalate monohydrate or calcium phosphate 1 to 8 mm in diameter) were endoscopically placed into the lower or mid pole calyx by retrograde ureteroscopy or antegrade percutaneous access using a nitinol basket. Stone position was visually confirmed endoscopically and fluoroscopically. Focused ultrasound energy was used to displace these stones/beads out of the calyx.
Six anesthetized pigs served as controls to assess for thermal injury using this focused ultrasound energy. Each underwent a midline laparotomy to expose the kidneys. A longer coupling cone was added to the array to place the focus 0.5 to 1 cm beyond the end of the cone. The abdomen was filled with saline to ensure coupling and the cone was placed in direct contact with the kidney. Five regions on each kidney were targeted and the surface location was marked with ink. Control regions of the kidney received no ultrasound exposure. Other regions received 2-minute total exposure at 50% duty cycle at a time averaged intensity of 325 and 1,900 W/cm2, respectively. The 325 W/cm2 exposure represents average therapeutic exposure. The 1,900 W/cm2 exposure, which is more than twice the maximum therapeutic exposure, served as a positive control.
The kidneys were harvested immediately after ultrasound exposure. Each kidney was freshly sectioned for gross examination and formalin preserved. Microscopic examination was done using separate samples stained with hematoxylin and eosin, and nicotinamide adenine dinucleotide stain. Any signs of thermal or mechanical injury to the renal parenchyma were assessed by observers blinded to exposure conditions.
Results
Stone motion was observed by ultrasound and fluoroscopy. Figure 2 shows a representative image of ultrasonic stone propulsion. In this example a 1-second ultrasound burst pushed the 5 mm bead from the lower pole to the UPJ and ultimately down an indwelling ureteral access sheath. The bead moved several cm in 1.3 seconds (fig. 2).
Figure 2.
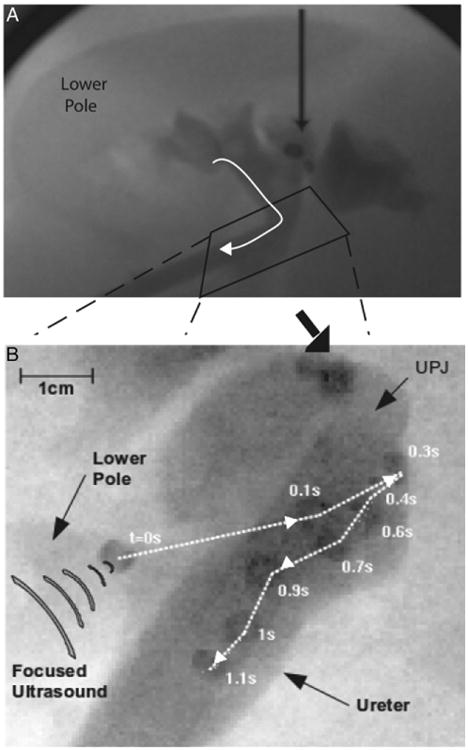
Superimposed frames of fluoroscopic movie tracking ultrasonic expulsion of bead (A and B). Images show 5 mm bead moved about 3 cm in 1.3 seconds (s), traveling from lower pole through UPJ into ureter.
Stones or beads were moved to the renal pelvis and UPJ in all 6 pigs. No more than 10 minutes per stone relocation event were required and focused ultrasound total exposure was shorter than 2 minutes. Most of the effort involved visualizing the stone at an appropriate angle to push it through the infundibulum toward the renal pelvis and UPJ.
Stone motion was not observed at all angles of focused ultrasound delivery. If stones were pushed toward the papillae, the stone ricocheted in a direction along the interface. Larger fluid spaces created by hydrodistention of the intrarenal collecting system made propulsion easier. Some stones were resistant to repositioning despite graduated increases in the power output. Angles of focus that were parallel to the axis of the infundibulum resulted in larger displacement of the stone. There was no difference in the ability to move stones of varying compositions.
Figure 3 shows renal histology. There was no evidence of thermal or mechanical injury in the control or in the kidney exposed to 325 W/cm2. The sample exposed to 1,900 W/cm2 showed damaged regions consistent with thermal coagulation. Blinded histological review revealed no thermal injury in any control or in the 5 samples exposed to approximately 325 W/cm2. Six of the 7 samples that received 1,900 W/cm2 showed thermal injury but the lesion created was localized to within 1 cm in its longest dimension.
Figure 3.
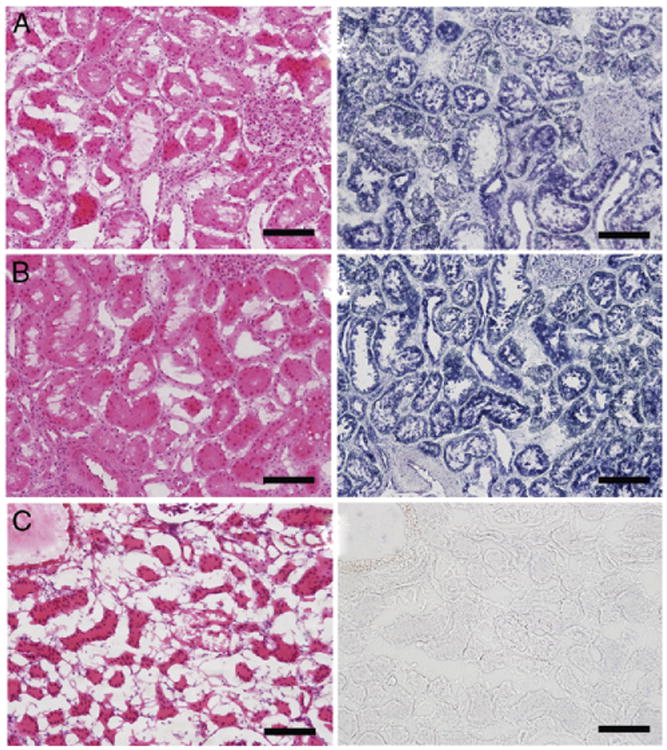
Sections of porcine kidney not exposed to ultrasound as control (A), and exposed to 325 W/cm2 for ultrasonic stone propulsion (B) and to 1,900 W/cm2, well above that for stone propulsion (C). Note thermal injury (C). H & E and nicotinamide adenine dinucleotide stain, scale bar represents 100 μm.
Discussion
Residual stone fragments are a potential cause of significant morbidity, such as symptomatic episodes and/or the need for further intervention.5,10 Unfortunately residual stone fragments are common. A randomized trial showed a surprisingly low SFR after ureteroscopy (50%) and SWL (35%) for lower pole calculi smaller than 1 cm.3 Technology has advanced since this study, and smaller flexible ureteroscopes that allow greater deflection capabilities along with smaller nitinol baskets theoretically would impact the ureteroscopy SFR if repeated. Although percutaneous nephrostolithotomy provides a higher SFR for lower pole calculi, many believe this to be an overly invasive option, especially for stones 1 cm or less.
We report a safe, effective device and method to expel small stones or fragments from the kidney in a completely noninvasive manner. This technology is portable and could potentially be used in the clinical or operative setting. The study simulated the expulsion of residual fragments or small stones primarily residing in the lower pole. Potential applications for this device also include adjunctive use with primary medical expulsive therapy and the management of obstructing UPJ stones by pushing the stone back into a nonobstructing location. In this manner this device may obviate the need for urinary diversion via a stent or percutaneous nephrostomy tube in a patient with a symptomatic obstructing UPJ stone.
The device may also be used in patients with known infection stones, in whom complete stone clearance is essential to prevent further stone formation and decrease the likelihood of recurrent infection. This ultrasound based device may also have a role in the pediatric and pregnant populations, in which there is even greater concern over the effects of ionizing radiation common to most ureteroscopic, SWL and percutaneous stone managements. The ability to move stones might even offer benefit to diagnostic ultrasound imaging, for example to distinguish between a single large stone and a cluster of multiple stones. Such a distinction may be an important branch point in the clinical decision making algorithm.
Limitations of this device include the difficult visualization of small stones and/or renal anatomy on ultrasound. Stones were not propelled at all angles and aligning ultrasound energy in the direction of the infundibulum appeared to be most effective. Stone composition was not a determining factor. The described version could only move stones at a depth of less than 8.5 cm, often less than that in many patients with stones. The large probe size restricted access below the ribs and made the beam narrower than needed. Although thermal injury could be produced with this device, it was seen only at energy levels well above those used for stone repositioning.
In this study the skills of an experienced ultrasound technologist were used. However, increased education on ultrasound imaging will add to the urologist skill set and allow the rapid translation of this technology to the clinical arena.
We believe that this is exciting technology with potential applications, as described. Advantages of this new technology include portability, reusability and no need for sterilization. It is potentially a painless procedure that could ultimately be used in patients without anesthesia. Ongoing research is being done, including the construction of a second generation prototype device with new applications and algorithms to improve stone detection and potentially facilitate stone movement. At the time of this study a new device in development has overcome the described limitation of moving stones at a skin-to-stone distance of less than 8.5 cm. Research is also under way to establish a threshold for thermal and mechanical injury using this technology.
Conclusions
A novel, image guided therapy using ultrasound technology has been developed to aid in stone fragment expulsion. Stone repositioning with the device was efficient and reproducible. This has many potential applications for kidney stones and could be used in the clinic, emergency room or operative setting.
Acknowledgments
Drs. Oleg A. Sapozhnikov and John C. Kucewicz developed image guidance, Frank L. Starr, III managed animals and Tatiana D. Khokhlova measured output (each from Center for Industrial and Medical Ultrasound). Drs. Hunter B. Wessells and Mathew S. Sorensen, Department of Urology, University of Washington, initiated our collaboration.
Supported by National Institutes of Health Grants DK48331, NIH DK086371 and NIH DK092197, and the National Space Biomedical Research Institute through National Aeronautical and Space Administration NCC 9-58.
Abbreviations and Acronyms
- SFR
stone-free rate
- SWL
shock wave lithotripsy
- UPJ
ureteropelvic junction
Footnotes
Study received institutional animal care and use committee approval.
References
- 1.Sampaio FJ, Aragao AH. Limitations of extra-corporeal shockwave lithotripsy for lower caliceal stones: anatomic insight. J Endourol. 1994;8:241. doi: 10.1089/end.1994.8.241. [DOI] [PubMed] [Google Scholar]
- 2.Albala DM, Assimos DG, Clayman RV, et al. Lower pole I: a prospective randomized trial of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy for lower pole nephrolithiasis-initial results. J Urol. 2001;166:2072. doi: 10.1016/s0022-5347(05)65508-5. [DOI] [PubMed] [Google Scholar]
- 3.Pearle MS, Lingeman JE, Leveillee R, et al. Prospective randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. J Urol. 2008;179:S69. doi: 10.1016/j.juro.2008.03.140. [DOI] [PubMed] [Google Scholar]
- 4.Lingeman JE, Siegel YI, Steele B, et al. Management of lower pole nephrolithiasis: a critical analysis. J Urol. 1994;151:663. doi: 10.1016/s0022-5347(17)35042-5. [DOI] [PubMed] [Google Scholar]
- 5.Chen RN, Streem SB. Extracorporeal shock wave lithotripsy for lower pole calculi: long-term radiographic and clinical outcome. J Urol. 1996;156:1572. [PubMed] [Google Scholar]
- 6.Chiong E, Hwee ST, Kay LM, et al. Randomized controlled study of mechanical percussion, diuresis and inversion therapy to assist passage of lower pole renal calculi after shock wave lithotripsy. Urology. 2005;65:1070. doi: 10.1016/j.urology.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 7.Kekre NS, Kumar S. Optimizing the fragmentation and clearance after shock wave lithotripsy. Curr Opin Urol. 2008;18:205. doi: 10.1097/MOU.0b013e3282f46b24. [DOI] [PubMed] [Google Scholar]
- 8.Pace KT, Tariq N, Dyer SJ, et al. Mechanical percussion, inversion and diuresis for residual lower pole fragments after shock wave lithotripsy: a prospective, single blind, randomized controlled trial. J Urol. 2001;166:2065. [PubMed] [Google Scholar]
- 9.Shah A, Owen NR, Lu W, et al. Novel ultrasound method to reposition kidney stones. Urol Res. 2010;38:491. doi: 10.1007/s00240-010-0319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Streem SB, Yost A, Mascha E. Clinical implications of clinically insignificant store fragments after extracorporeal shock wave lithotripsy. J Urol. 1996;155:1186. [PubMed] [Google Scholar]


