SUMMARY
Background
Inflammation may reduce hippocampal volume by blocking neurogenesis and promoting neurodegeneration. Posttraumatic stress disorder (PTSD) has been linked with both elevated inflammation and reduced hippocampal volume. However, few studies have examined associations between inflammatory markers and hippocampal volume, and none have examined these associations in the context of PTSD.
Methods
We measured levels of the inflammatory markers interleukin-6 (IL-6) and soluble receptor II for tumor necrosis factor (sTNF-RII) as well as hippocampal volume in 246 Gulf War veterans with and without current and past PTSD as assessed with the Clinician Administered PTSD Scale (CAPS). Enzyme-linked immunosorbent assays were used to measure inflammatory markers, and 1.5 Tesla magnetic resonance imaging (MRI) and Freesurfer version 4.5 were used to quantify hippocampal volume. Hierarchical linear regression and analysis of covariance models were used to examine if hippocampal volume and PTSD status would be associated with elevated levels of IL-6 and sTNF-RII.
Results
Increased sTNF-RII, but not IL-6, was significantly associated with reduced hippocampal volume (β = −.14, p = .01). The relationship between sTNF-RII and hippocampal volume was independent of potential confounds and covariates, including PTSD status. Although we observed no PTSD diagnosis-related differences in either IL-6 or sTNF-RII, higher PTSD severity was associated with significantly increased sTNF-RII (β = .24, p = .04) and reduced IL-6 levels (β = −.24, p = .04).
Conclusions
Our results indicate that specific inflammatory proteins may be associated with brain structure and function as indexed by hippocampal volume and PTSD symptoms.
Keywords: hippocampus, inflammation, posttraumatic stress disorder, structural magnetic resonance imaging, trauma, veterans
1. INTRODUCTION
The immune system has the potential to profoundly influence learning and memory through effects on brain function and structure (Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Perry, Cunningham, & Holmes, 2007; Yirmiya & Goshen, 2011). The hippocampus, a brain area that plays a critical role in learning and memory, appears highly sensitive to inflammation (Devito & Eichenbaum, 2011; Eichenbaum, 2004). High levels of inflammation block neurogenesis, the growth of new neurons, in the hippocampus (Ben Menachem-Zidon et al., 2008; Monje, Toda, & Palmer, 2003), which in turn is critical for hippocampus-mediated learning and memory (van Praag et al., 2002; Winocur, Wojtowicz, Sekeres, Snyder, & Wang, 2006). There is also evidence that high levels of inflammation can cause atrophy in the hippocampus by promoting neuronal death (Cunningham, Wilcockson, Campion, Lunnon, & Perry, 2005). Despite this evidence, few studies have examined the relationship between inflammation and hippocampal volume in humans.
The effects of inflammation on the brain are due to neuroinflammation and not systemic inflammation per se. However, peripheral inflammatory proteins called cytokines and their receptors may play an important role in driving neuroinflammation (Banks, 2001; Dantzer et al., 2008; Raison, Capuron, & Miller, 2006). Cytokines are typically too large to cross the blood-brain barrier (BBB) efficiently. However, they can influence the brain by transmitting signals through interactions between brain endothelial cells and perivascular macrophages, activating afferent nerves, passing through the BBB when it loses structural integrity due to inflammation or sepsis, and entering brain areas without BBB (Banks, 2001; Dantzer et al., 2008; Miller, Haroon, Raison, & Felger, 2013). Recent evidence indicates that an antagonist for the inflammatory cytokine tumor necrosis factor-α (TNF-α) which works exclusively in the periphery—reduced symptoms of depression in individuals with high levels of systemic inflammation (Raison et al., 2013), suggesting that alterations in peripheral cytokines can impact brain function. Thus, peripheral inflammatory cytokines may provide a useful index related to neuroinflammation.
Small studies have reported associations between peripheral inflammatory markers and hippocampal volume. Specifically, reduced hippocampal volume has been linked with elevated peripheral levels of IL-6 in samples of critically ill patients (Lindlau et al., 2014), depressed patients (Frodl et al., 2012), and healthy individuals (Marsland, Gianaros, Abramowitch, Manuck, & Hariri, 2008), and with elevated peripheral levels of the inflammatory cytokine tumor necrosis factor-alpha (TNF-α) in breast cancer survivors (Kesler et al., 2013). The soluble receptor II for tumor necrosis factor (sTNF-RII) has recently been identified as a potential peripheral biomarker for mild cognitive impairment and Alzheimer’s Disease (Buchhave et al., 2010; Jiang et al., 2011; Zhang, Peng, & Jia, 2014), both of which have been linked to reduced hippocampal volume (Leung et al., 2010). However, to our knowledge, no studies have examined the relationship between sTNF-RII and hippocampal volume in humans.
Posttraumatic stress disorder (PTSD) has also been linked with elevated inflammation and reduced hippocampal volume. A number of studies with small samples and mixed samples of traumatized and non-traumatized controls have documented elevated levels of inflammatory cytokines and increased pro-inflammatory signaling through the transcription factor nuclear factor-κB (NF-κB) in PTSD (Hoge et al., 2009; O’Donovan et al., 2011b; Pace et al., 2012; Spitzer et al., 2010). In fact, one recent large-scale study identified the inflammatory marker C-reactive protein as a predictor of PTSD onset in a prospective study of war zone-deployed Marines (Eraly et al., 2014). Moreover, strong evidence now implicates inflammation as a causal factor in the development of symptoms associated with PTSD (Dantzer et al., 2008; O’Donovan et al., 2013; Raison, Capuron, & Miller, 2006; Raison et al., 2013; Slavich, Way, Eisenberger, & Taylor, 2010), including memory-related symptoms considered to be mediated by the hippocampus (Cohen et al., 2003; Yirmiya & Goshen, 2011). However, other studies have documented similar or lower levels of inflammatory markers in individuals with compared to without PTSD (McCanlies et al., 2011; Sondergaard, Hansson, & Theorell, 2004), highlighting that some but not all individuals with PTSD display elevated inflammation.
Numerous structural magnetic resonance imaging (MRI) studies have reported reduced hippocampal volume in patients with PTSD relative to controls (Bremner et al., 1995; Vermetten, Vythilingam, Southwick, Charney, & Bremner, 2003; Wang et al., 2010; Woodward et al., 2006), and in individuals exposed to trauma who do not develop PTSD (Karl et al., 2006; Vythilingam et al., 2002). However, effect sizes are generally small and not all studies have replicated the effect of reduced hippocampal volume in PTSD (Mohlenhoff, Chao, Buckley, Weiner, & Neylan, 2014; Schuff et al., 2001). Moreover, while some studies suggest that smaller hippocampal volume is a risk factor for PTSD (Gilbertson et al., 2002), others indicate that it is a correlate of current PTSD that may resolve when symptoms resolve (Apfel et al., 2011).
Despite strong reasons to predict that elevated inflammation could be associated with reduced hippocampal volume and PTSD symptoms, no studies have examined associations among inflammatory markers, hippocampal volume, and PTSD in a single study. In the present study, we examined levels of IL-6 and sTNF-RII as well as hippocampal volume in Gulf War veterans with and without current and past PTSD. We hypothesized that levels of IL-6 and sTNF-RII would be associated with reduced hippocampal volume independent of PTSD, and that PTSD would also be associated with elevated inflammation.
2. METHODS
2.1 Sample and clinical assessment
We conducted a secondary analysis of imaging and clinical data in 206 Gulf War veterans selected from a total sample of 279 based on the availability of secondary analysis of imaging, inflammatory marker, and clinical data. All veterans were enrolled in a cross-sectional study of the effects of service in the Persian Gulf War on the brain. Clinical and imaging data from the study have been reported in previous publications on relationship between Gulf War Illness (GWI), brain N-acetylaspartate, and PTSD (Weiner et al., 2010), the effects of current versus lifetime PTSD on hippocampal volume (Apfel et al. 2011), the effects of suspected low-level sarin exposure on brain structure and function (Chao, Rothlind, Cardenas, Meyerhoff, & Weiner, 2010), and the relationship of brain volume to sleep quality (Chao, Mohlenhoff, Weiner, & Neylan, 2014). Details of the original study design, recruitment, and participant characteristics have been described elsewhere (Weiner et al., 2011). Inclusion was based on being a United States veteran of the First Persian Gulf War. Exclusion criteria were severe physical impairment or medical illness, current or lifetime history of psychosis, current suicidal or homicidal ideation, current substance dependence, history of neurological or systemic illness affecting central nervous system functioning, history of head injury with loss of consciousness for at least 10 minutes, presence of severe claustrophobia or ferro-metallic objects in the body, and evidence of cholesteatoma or tympanic membrane perforation. All research was approved by the University of California, San Francisco and the Veterans Affairs Committees on Human Research and the Department of Defense Human Subjects’ Research Review Board. All participants provided written informed consent.
2.2 Inflammatory markers
Morning fasting venous blood samples were collected between 7AM and 10AM and used for assessment of inflammatory markers. Patients ate a low tyrosine and tyramine dinner on the evening before blood samples were taken and then fasted overnight. The human IL-6 Quantikine high sensitivity enzyme-linked immunosorbent assay (hsELISA) and human sTNF-RII Quantikine hsELISA were used to measure IL-6 and sTNF-RII respectively (R&D Systems, USA). The lower limits of detection were 0.12 pg/ml for IL-6 and 0.2 pg/ml for sTNF-RII. Where duplicates differed more than 20%, samples were repeated in duplicate. Intra-assay coefficients of variation are <10% for both IL-6 and sTNF-RII. Fourteen samples had IL-6 levels below the lowest detectable limit of the assays; these samples were all recoded as being one unit below the lowest detectable limit. All samples had sTNF-RII levels within the range of the assay.
2.3 Image acquisition and processing
All subjects were scanned on a 1.5 Tesla Vision, Siemens MRI scanner (Siemens Medical Systems, Iselin, New Jersey). A T1-weighted 3D volumetric magnetization-prepared rapid gradient echo (MPRAGE) sequence was acquired with the following parameters: repetition time/spin-echo time/inversion time = 10/4/300 msec, 1 × 1 mm2 in-plane resolution, and 1.5-mm slab thickness, angulated perpendicular to the long axis of the hippocampus. We used the publically available Freesurfer version 4.5 (http://surfer.nmr.mgh.harvard.edu/) to estimate each subjects’ right and left hippocampal volume and intracranial volume (ICV, (Buckner et al., 2004). Freesurfer morphometric procedures have been demonstrated to show good test-retest reliability across scanner manufacturers and across field strengths (Han et al., 2006; Reuter, Schmansky, Rosas, & Fischl, 2012). All regional volumes were visually inspected for errors.
2.4 Psychiatric diagnoses
The Clinician Administered PTSD Scale (CAPS; (Blake et al., 1995) was used to diagnose PTSD, and the Structured Clinical Interview for DSM-IV Diagnosis (SCID; (Spitzer, Williams, Gibbon, & First, 1992) was used to diagnose psychiatric disorders other than PTSD, including the exclusionary diagnoses of lifetime psychotic disorders and bipolar disorder and current diagnosis of substance dependence. Trained clinical interviewers who calibrated their assessments at weekly case-consensus meetings made all diagnoses. Based on the CAPS, current PTSD was defined as meeting DSM-IV criteria for PTSD in the past month, and past PTSD was defined as meeting DSM-IV criteria for PTSD during a one-month period in the past but not currently. One participant without current PTSD was missing the lifetime CAPS assessment and was excluded from PTSD-group analyses. Severity of depressive symptoms was assessed with the Beck Depression Inventory-II, a 21-item widely used, validated, self-report measure in a subsample of 198 veterans (Beck, Steer, & Garbin, 1988).
2.5 Trauma exposure
The Life Stressor Checklist-Revised (LSC-R) was used to assess exposure to 21 different traumatic events involving experiencing or witnessing threat to life or physical integrity (Wolfe, Kimerling, Brown, Chresman, & Levin, 1996). Lifetime trauma exposure was defined as the number of different categories of trauma exposure experienced across the lifespan. Childhood trauma was defined as exposure to any of the following five traumatic events before age 14: physical neglect; family violence; physical abuse; forced sexual touch; or forced sexual intercourse (O’Donovan et al., 2011a; Otte et al., 2005).
2.6 Covariates
Education was assessed by self-report and body mass index (BMI) was calculated by computing weight divided by height in meters squared. GWI was defined by the Center for Disease Control and Prevention’s criteria (Fukuda et al., 1998) as the presence of one or more symptoms from at least two of the following symptom clusters: general fatigue (cluster A); mood and cognitive abnormalities (cluster B); and musculoskeletal pain (cluster C). To be considered GWI for this study, symptoms must have developed within the year after leaving the Gulf region and have lasted for six months or longer. Participants were asked to list all current prescription and nonprescription medications and we selected medications as having psychotropic, anti-inflammatory, and immunomodulating properties for adjustment in this study.
2.7 Data analysis
Hierarchical linear regression models were used to examine associations of IL-6 and sTNF-RII with hippocampal volume. Covariates including age, gender, and ICV were entered in the first step and each inflammatory marker was entered in the second step of separate regression models. Analysis of covariance (ANCOVA) models adjusted for age and gender were used to examine group differences in inflammatory markers by PTSD status and planned contrasts were used to examine if individuals with current PTSD had levels of inflammatory markers that were significantly different than those in either the past PTSD or no PTSD group. We also used hierarchical linear regression models to examine if higher severity of PTSD would be associated with IL-6 and sTNF-RII in individuals with current or past PTSD. In secondary analyses, we examined if significant associations were independent of potential confounding and mediating factors including PTSD status entered as dummy variables for current and past PTSD, BMI, GWI, depression symptoms, childhood and lifetime trauma exposure, and use of psychotropic, anti-inflammatory, or immunomodulating medications. Distributions of raw IL-6, sTNF-RII, hippocampal volume, and ICV data were non-normal and these variables were natural log transformed. Cases less or greater than three standard deviations from the mean for both log-transformed IL-6 (n = 2) and log-transformed sTNF-RII (n = 2) were excluded leaving a sample of 206 veterans with complete inflammatory marker and hippocampal volume data. The threshold for statistical significance was set at p ≤ .05. All analyses were conducted in SPSS 21.0 (IBM Inc.).
3. RESULTS
3.1 Descriptive statistics
Sample characteristics are displayed in Table 1. The sample was 82.5% male and ranged in age from 31 to 71 years. As predicted, IL-6 and sTNF-RII were positively, albeit weakly, correlated with each other (r = .19, p = .008), and with older age (IL-6: r = .14, p = .048; sTNF-RII: r = .19, p = .006), and higher BMI (IL-6: r = .25, p < .001; sTNF-RII: r = .21, p = .002). Women had slightly smaller ICV- and age-adjusted total hippocampal volume than males, but this difference was not statistically significant [F(1,202) = 1.79, p = .18].
Table 1.
Sample characteristics
| n | Mean/Median/% (SD/IQR) |
Range | |
|---|---|---|---|
| Age | 206 | 44.28 (9.76) | 31–71 |
| Male Gender | 170 | 82.5% | |
| Ethnicity | |||
| White | 134 | 65.0% | |
| Black | 31 | 15.0% | |
| Hispanic | 15 | 7.3% | |
| Other | 17 | 8.3% | |
| Years of Education | 197 | 14.67 (2.02) | 10–20 |
| BMI | 206 | 27.94 (4.31) | 17.71–42.98 |
| Medications | |||
| Psychotropic | 43 | 20.9% | |
| Anti-inflammatory | 42 | 20.4% | |
| Immunomodulating | 21 | 10.2% | |
| Childhood Traumaa | 45 | 21.8% | |
| Lifetime Traumab | 202 | 8.39 (3.67) | 1–20 |
| CAPS Lifetime | 205 | 32.28 (36.12) | 0–125 |
| CAPS Current | 206 | 17.74 (25.32) | 0–108 |
| Depression Symptomsd | 198 | 10.77 (9.67) | 0–51 |
| Gulf War Illnessc | 70 | 34.0% | |
| ICV, mLe | 206 | 1584k (148k) | 1216k–1931k |
| Hippocampal Volume, mLe | 206 | 8889 (907) | 6582–11480 |
| IL-6 (pg/ml)e | 206 | .50 (.46) | .03–2.70 |
| sTNF-RII (pg/ml)e | 206 | 2.21 (.61) | 1.23–4.54 |
Notes: BMI = body mass index; CAPS = Clinician Administered PTSD Scale; ICV = intracranial volume; IL-6 = interleukin-6; sTNF-RII = soluble receptor for tumor necrosis factor;
Based on presence/absence of five categories of severe trauma from the Life Stressor Checklist-Revised (LSC-R) before age 14 (Wolfe, Kimerling, Brown, Chresman, & Levin, 1996);
Based on number of categories reported on LSC-R (Wolfe et al., 1996);
Based on standard criteria (Fukuda et al., 1998);
Based on Beck Depression-II (Beck, Steer, & Garbin, 1988);
Raw values are presented as median and IQR for IL-6, sTNF-α-RII, hippocampal volume and ICV.
3.2 Inflammation and hippocampal volume
First, we examined if IL-6 or sTNF-RII were associated with left or right hippocampal volumes adjusting for age, gender, and ICV in linear regression models. Results indicated that IL-6 was not significantly associated with right hippocampal volume [FChange(1, 201) = .67, β = −.05, p = .41], or left hippocampal volume [FChange(1, 201) = .23, β = −.026, p = .63]. However, sTNF-RII was significantly associated with both right hippocampal volume [FChang (1, 201) = 6.63, β = −.14, p = .01] (Figure 1a), and left hippocampal volume [FChange(1, 201) = 4.93, β = −.12, p = 03] (Figure 1b). Similarly, whereas IL-6 was not associated with overall hippocampal volume [FChange(1, 201) = .46, β = −.04, p = .50), higher sTNF-RII was associated with reduced overall hippocampal volume [FChange(1, 201) = 6.56, β = −.14, p = .01] (Figure 1).
Figure 1.
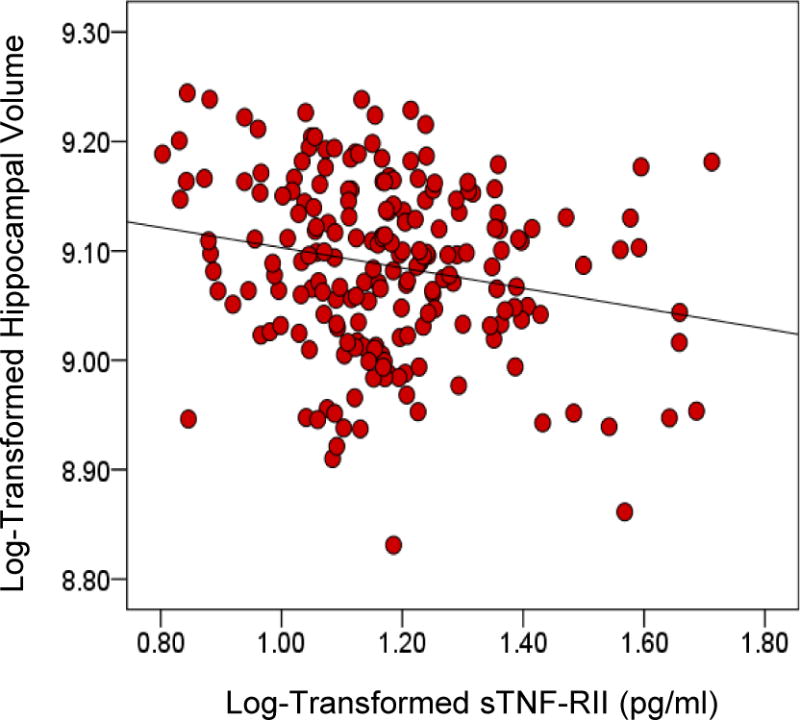
Figure 1 illustrates the significant positive association between soluble receptor-II for tumor necrosis factor (sTNF-RII) and overall intracranial volume-adjusted hippocampal volume (β = − 0.14, p = 0.01).
Table 2 shows associations between various potential mediating and confounding factors and right and left hippocampal volume in our sample. In secondary analyses, we found that the relationship between sTNF-RII and hippocampal volume was independent of potential mediating and confounding factors including PTSD status, BMI, GWI, depression, childhood and lifetime trauma exposure and medication use (p’s ≤ .02).
Table 2.
Associations between potential mediating and confounding factors and right and left hippocampal volume.
| Characteristic | Right Hippocampal Volume r or t | P | Left Hippocampal Volume r or t | P |
|---|---|---|---|---|
| Age | −.20 | .005 | −.18 | .009 |
| Gender | 5.71 | .001 | 5.90 | .001 |
| Years of Education | .11 | .11 | .13 | .08 |
| BMI | −.06 | .39 | −.11 | .11 |
| Medications | ||||
| Psychotropic | 1.02 | .31 | 1.31 | .20 |
| Anti-inflammatory | −.26 | .80 | −.40 | .69 |
| Immunomodulating | .14 | .89 | .55 | .59 |
| Childhood Traumaa | 1.40 | .17 | 1.14 | .26 |
| Lifetime Traumab | −.01 | .91 | −.004 | .95 |
| CAPS Lifetime | −.12 | .09 | −.09 | .18 |
| CAPS Current | −.10 | .14 | −.06 | .38 |
| Depression Symptomsc | .001 | .99 | .01 | .88 |
| Gulf War Illnessd | −1.52 | .13 | −2.33 | .02 |
Notes: BMI = body mass index; CAPS = Clinician Administered PTSD Scale;
Based on presence/absence of trauma before age 14 on Life Stressor Checklist-Revised (LSC-R) (Wolfe et al., 1996);
Based on number of categories reported on LSC-R (Wolfe et al., 1996);
Based on Beck Depression Inventory-II (Beck, Steer, & Garbin, 1988);
Based on standard criteria (Fukuda et al., 1998); Statistics are r values from Pearson’s correlations for age, years of education, BMI, lifetime trauma exposure and CAPS scores; Statistics are t values from Student’s t-tests for gender, childhood trauma and GWI status.
3.3 PTSD-related differences in inflammatory markers
Table 3 displays sample characteristics for groups with and without current and past PTSD. Individuals with current PTSD were slightly younger and veterans with past PTSD tended to have higher BMI than those without a history of PTSD. Unsurprisingly, the current PTSD group had significantly higher childhood and lifetime trauma exposure, CAPS scores, depressive symptoms, and likelihood of GWI. However, there were no significant gender differences among the groups.
Table 3.
Sample characteristics by PTSD status
| No PTSD n = 132 n(%)/M(SD) |
Past PTSD n = 33 n(%)/M(SD) |
Current PTSD n = 40 n(%)/M(SD) |
F or χ2 | P | |
|---|---|---|---|---|---|
| Age | 45.02 (10.1) | 43.88 (9.76) | 42.12 (8.63) | 1.38 | .26 |
| Gender | 113 M/19 F | 25 M/8 F | 31 M/9 F | 2.61 | .27 |
| Years of Education | 14.82 (2.08) | 14.53 (1.68) | 14.28 (2.08) | 1.16 | .32 |
| BMI | 27.47 (4.15) | 29.76 (4.71) | 28 (4.26) | 3.83 | .02 |
| Medications | |||||
| Psychotropic | 29 (14%) | 8 (4%) | 6 (3%) | 1.15 | .56 |
| Anti-Inflammatory | 28 (14%) | 5 (2%) | 9 (4%) | .72 | .70 |
| Immunomodulating | 11 (5%) | 4 (2%) | 6 (3%) | 1.64 | .44 |
| Childhood Traumaa | 18 (40%) | 8 (18%) | 19 (42%) | 21.41 | <.001 |
| Lifetime Traumab | 7.32 (3.24) | 9.03 (2.99) | 11.49 (3.67) | 24.60 | <.001 |
| CAPS Lifetime | 8.75 (13.02) | 65.94 (18.59) | 82.15 (24.28) | 376.95 | < .001 |
| CAPS Current | 3.74 (7.96) | 18.70 (12.81) | 63.23 (15.38) | 481.78 | < .001 |
| Depression Symptomsc | 7.69 (7.08) | 9.65 (7.35) | 22.11 (10.80) | 48.37 | <.001 |
| Gulf War Illnessd | 59 (84%) | 9 (13%) | 2 (3%) | 22.99 | <.001 |
| IL-6e | .44 (.25) | .52 (.30) | .41 (.18) | 1.94 | .15 |
| sTNF-RIIe | 1.18 (.18) | 1.14 (.16) | 1.19 (.16) | .82 | .44 |
Notes: BMI = body mass index; CAPS = Clinician Administered PTSD Scale; IL-6 = interleukin-6; sTNF-RII = soluble receptor for tumor necrosis factor;
Based on presence/absence of trauma before age 14 on Life Stressor Checklist-Revised (LSC-R) (Wolfe et al., 1996);
Based on number of categories reported on LSC-R (Wolfe et al., 1996);
Based on Beck Depression-II (Beck, Steer, & Garbin, 1988): n = 128 for No PTSD, n = 31 for Lifetime PTSD, and n = 38 for Current PTSD;
Based on standard criteria (Fukuda et al., 1998);
Raw values are presented as median and IQR for IL-6 and sTNF-α-RII.
Using ANCOVA models adjusted for age and gender, we examined differences among PTSD groups in IL-6 and sTNF-RII. In contrast with our predictions, PTSD status was not associated either IL-6 [F(2,200) = 2.05, p = .13] or sTNF-RII [F(2,201) = .95, p = .39]. Furthermore, planned contrasts suggested that the group with current PTSD did not have significantly different levels of either IL-6 or sTNF-RII compared to either of the other groups. Figures 2a and 2b illustrate levels of IL-6 and sTNF-RII in groups differing in trauma exposure and PTSD status. Adjusting for BMI, medication use, childhood and lifetime trauma, depressive symptoms, and GWI did not change this pattern of results.
Figure 2.
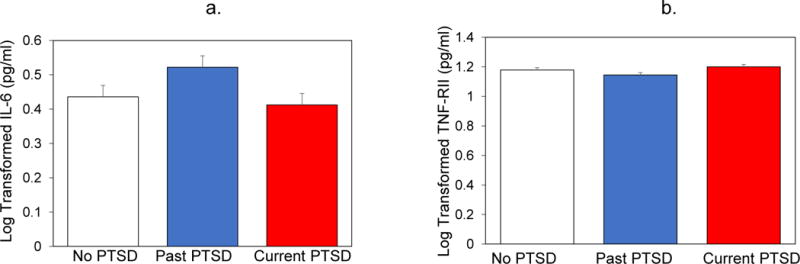
(a) and (b) illustrate mean (standard error of mean) levels of IL-6 and STNF-RII in the three groups with and without current and past PTSD. Results indicated no significant differences among the groups on either marker of inflammatory activity.
Although there were no significant PTSD-related group differences in inflammation, we nonetheless examined if there were associations between current PTSD severity scores and inflammatory markers, including only veterans with current or past PTSD. In this subsample, IL-6 and sTNF-RII were not correlated with one another (r = .04, p = .77); higher sTNF-RII (r = .25, p = .03) but not IL-6 (r = .15, p = .21) was significantly associated with older age; higher IL-6 (r = .26, p = .03) but not sTNF-RII (r = .12, p = .31) was associated with higher BMI; and neither IL-6 (r = −.12, p = .33) nor sTNF-RII (r = .06, p = .61) were associated with depressive symptoms. There were no differences in IL-6 by GWI status (t = .76, df = 13.35, p = .46), and a non-significant trend towards higher sTNF-RII in those with GWI (t = −1.86, df = 14.81, p = .08).
Adjusting for age and gender, individuals with higher overall PTSD severity had significantly lower levels of IL-6 [F(1,69) = 4.28, β = −.24, p = .04] and significantly higher levels of sTNF-RII [F(1,69) = 4.37, β = .24, p = .04], suggesting altered inflammatory activity in individuals with higher compared to lower PTSD severity. These results remained significant in separate models adjusted for years of education, medication use, and childhood trauma exposure (p’s ≤ .05). The relationship between PTSD severity and IL-6 became marginally non-significant when adjusting for BMI (β = −.20, p = .09) and lifetime trauma exposure (β = −.24, p = .06), but remained significant when adjusting for GWI and depression severity. In contrast, the relationship between PTSD severity and sTNF-RII became non-significant when adjusting for GWI (β = .19, p = .11) and depression severity (β = .22, p = .16), but remained significant when adjusting for BMI and lifetime trauma exposure. In all cases, the effect size remained similar to the original effect size and the greatest change in effect size was observed with the adjustment for BMI in the IL-6 analyses and GWI in the sTNF-RII analyses. PTSD severity was not significantly associated with hippocampal volume in this sample [F(1,68) = .11, β = −.03, p = .74]. Thus, lacking the main effect of an association between PTSD severity and hippocampal volume, we did not proceed with mediation models.
4. DISCUSSION
In our sample of Gulf War veterans, higher levels of sTNF-RII were significantly associated with reduced overall, right, and left hippocampal volume. Previous preclinical research suggests that inflammation has the potential to block neurogenesis (Monje, Toda, & Palmer, 2003) and promote neurodegeneration (Cunningham et al., 2005), which could reduce hippocampal volume and drive impairments in learning and memory. We found no differences in levels of the inflammatory markers IL-6 and sTNF-RII among groups with and without current and past PTSD. However, greater severity of PTSD was associated with lower IL-6 and higher sTNF-RII levels, indicating alterations in inflammatory activity associated with PTSD severity. Overall, our findings are consistent with the idea that aspects of inflammation are associated with reduced hippocampal volume, independent of PTSD status. Our data also tentatively suggest that peripheral inflammatory activity may act as a marker of inflammatory processes associated with brain structure as indexed by hippocampal volume, and brain function as indexed by PTSD symptoms.
In contrast with prior research on IL-6 levels in critically ill and healthy people (Lindlau et al., 2014; Marsland et al., 2008), levels of IL-6 were not significantly associated with hippocampal volume in our sample. However, our finding that elevated levels of sTNF-RII were associated with smaller hippocampal volume is consistent with and extends the prior finding that elevated TNF-α is associated with reduced hippocampal volume in breast cancer survivors (Kesler et al., 2013). Although strongly positively correlated with TNF-α, sTNF-RII may provide a more stable estimate of inflammatory activity because TNF-α and IL-6 are cleared from the system more rapidly than its soluble receptors and levels of TNF-α can also be masked by both soluble and membrane-bound TNF-α receptors (Brockhaus, 1997; MacEwan, 2002). Moreover, soluble TNF-α receptors play a key role in modulating the effects of TNF-α (Horiuchi, Mitoma, Harashima, Tsukamoto, & Shimoda, 2010).
Elevated inflammation may influence hippocampal volume by blocking neurogenesis. For example, in one study, administration of the non-steroidal anti-inflammatory drug indomethacin restored neurogenesis following endotoxin-induced inflammation, and increased neurogenesis following cranial irradiation in female rodents (Monje, Toda, & Palmer, 2003). Targeted anti-inflammatory treatments may also have the potential to reduce the negative effects of inflammation in humans. In particular, given that inflammation, and particularly TNF-α, has been implicated in post-operative cognitive decline (Terrando et al., 2010), it is possible that inflammation influences cognitive function through inflammatory pathways and that targeting specific abnormalities in inflammatory signaling could yield cognitive benefits. Elevated inflammation may also drive neuronal death and reducing inflammation may prevent neurodegeneration (Cunningham et al., 2005). Psychotropic medications, including selective serotonin reuptake inhibitors (SSRIs), appear to reduce inflammation and enhance functioning in patients with psychiatric disorders (Hannestad, DellaGioia, & Bloch, 2011). Thus, it is possible that reduced inflammation is one pathway mediating hippocampal volume increases following treatment with SSRIs (Bremner, 2006; Vermetten et al., 2003). However, more research is needed before translating these findings to new treatment approaches for low-grade inflammation in humans.
Our finding that levels of inflammatory markers were similar across groups varying in PTSD status is inconsistent with previous small studies that have demonstrated that inflammation is elevated in individuals with current PTSD, and that PTSD is characterized by elevated pro- and reduced anti-inflammatory signaling to immune cells (Hoge et al., 2009; O’Donovan et al., 2011b; Pace et al., 2012; Spitzer et al., 2010). The present study differed from prior work in several important ways. First, almost all of the veterans in our sample had experienced some traumatic event in their lifetime. Traumatic stress exposure per se has been linked with elevated inflammation in prior studies, independent of psychiatric disorders, including PTSD (Danese, Pariante, Caspi, Taylor, & Poulton, 2007; O’Donovan, Neylan, Metzler, & Cohen, 2012). Thus, our sample overall may have trauma-related elevated inflammation even in the absence of PTSD. Second, it is also possible that the reason for the inconsistency across studies is related to the heterogeneity of PTSD. Just as not all patients with depression show elevated levels of inflammatory proteins, it is likely that only a subset of patients with PTSD have elevated inflammation (O’Donovan, 2014). In fact, our data linking PTSD severity with lower IL-6 and higher sTNF-RII supports this hypothesis, suggesting that only a subset of patients might display elevated levels of specific inflammatory markers. Our pattern of findings was surprising in that those with the most severe symptoms had higher sTNF-RII as expected, but lower IL-6. These data highlight the need to think about the complexity of inflammatory signaling pathways and the multiple interacting proteins and receptors involved. Finally, our study is larger than most previous studies. Future studies should focus on uncovering the symptom profiles most closely associated with inflammation in patients with PTSD, which may shed light on specific subpopulations of patients with PTSD with elevated inflammation.
A number of limitations of the current study should be considered. First, levels of only two inflammatory markers were measured at a single time point, which likely does not provide an adequate index of all inflammatory proteins and receptors, or of exposure to inflammatory activity over years and decades. Second, neither the present cross-sectional study nor previous observational studies in humans have the ability to clarify causal direction in the relationship between inflammation and hippocampal volume. It remains possible that other genetic or environmental factors contribute to levels of both inflammatory activity and hippocampal volume, or that the hippocampus influences systemic inflammation. Third, individuals in our sample were recruited for a study on GWI, which may have been a confounding factor that led to different exposures and differential medication use across groups. While 56% of those in the current PTSD group reported symptoms consistent with the diagnosis of GWI, only 20% and 25% of those in the no PTSD and past PTSD groups received such a diagnosis. While our overall pattern of findings remained the same when adjusting for psychotropic, anti-inflammatory, and immunomodulating medication use, as well as GWI, the relationship between PTSD severity and sTNF-RII became non-significant when adjusting for GWI. Many symptoms of GWI overlap with symptoms of PTSD and it is therefore difficult to tease them apart. Fourth, although study participation and data had no influence on compensation or clinical care in the Veterans Affairs system, the focus of the study on veterans may have influenced responding to self-report and interview measures (McNally & Frueh, 2013). Other study limitations include the low percentage of women, which limits generalization of our findings to females, and the lack of information about the socioeconomic and smoking status of the participants. These limitations notwithstanding, the present results are consistent with the idea that elevated sTNF-RII may index a process that has adverse effects on hippocampal volume.
Conclusion
The present study reports a relationship between the inflammatory marker sTNF-RII and hippocampal volume in a sample of Gulf War veterans independent of PTSD status, and an association of more severe current PTSD symptoms with lower IL-6 and higher sTNF-RII in veterans with past and current PTSD. Future studies employing longitudinal or experimental study designs and/or anti-inflammatory interventions will be necessary to confirm causality in the relationship between inflammation and hippocampal volume. Taken together with prior preclinical and clinical research, our data support elevated inflammation as a potential mechanism of reduced hippocampal volume in humans.
HIGHLIGHTS.
Our sample includes veterans with and without posttraumatic stress disorder (PTSD).
We examine if inflammatory markers are associated with hippocampal volume.
Higher sTNF-RII, but not IL-6, was associated with reduced hippocampal volume.
Neither current nor past PTSD diagnoses were associated with sTNF-RII or IL-6.
More severe PTSD symptoms were associated with elevated sTNF-RII and lower IL-6.
Acknowledgments
This study was generously supported by grants from the Lightfighter Foundation, the US Army Military Operational Medicine Research Program, Department of Defense W81XWH-11-2-0189 entitled, “Investigation of Links Between PTSD and Dementia,” awarded to the Northern California Institute for Research and the Mental Illness Research and Education Clinical Center (MIRECC) of the U.S. Veterans Health Administration and the National Institute on Aging (K24 AG031155) as well as by a National Center for Advancing Translational Sciences Career Development Award (KL2 TR000143) and a Society in Science – Branco Weiss Fellowship (to AOD). Resources and the use of facilities were provided by the Veterans Administration Medical Center, San Francisco, California. This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of any of the funding bodies. The authors thank Jennifer Hlavin, Mark Pacult, Natasha Sukerkar, and Kevin Kim for assistance with this study and manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apfel BA, Ross J, Hlavin J, Meyerhoff DJ, Metzler TJ, Marmar CR, Neylan TC. Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol Psychiatry. 2011;69(6):541–548. doi: 10.1016/j.biopsych.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA. Cytokines, CVOs, and the blood-brain barrier. In: Ader R, Felten DL, Cohen DN, editors. Psychoneuroimmunology. 3. San Diego: Academic Press; 2001. pp. 483–499. [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- Ben Menachem-Zidon O, Goshen I, Kreisel T, Ben Menahem Y, Reinhartz E, Ben Hur T, Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacol. 2008;33(9):2251–2262. doi: 10.1038/sj.npp.1301606. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinican-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bremner JD. The relationship between cognitive and brain changes in posttraumatic stress disorder. Ann NY Acad Sci. 2006;1071:80–86. doi: 10.1196/annals.1364.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus M. Soluble TNF receptor: what is the significance? Intensive Care Med. 1997;23(8):808–809. doi: 10.1007/s001340050416. [DOI] [PubMed] [Google Scholar]
- Buchhave P, Zetterberg H, Blennow K, Minthon L, Janciauskiene S, Hansson O. Soluble TNF receptors are associated with Abeta metabolism and conversion to dementia in subjects with mild cognitive impairment. Neurobiol Aging. 2010;31(11):1877–1884. doi: 10.1016/j.neurobiolaging.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Chao LL, Mohlenhoff BS, Weiner MW, Neylan TC. Associations between subjective sleep quality and brain volume in Gulf War veterans. Sleep. 2014;37(3):445–452. doi: 10.5665/sleep.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Rothlind JC, Cardenas VA, Meyerhoff DJ, Weiner MW. Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in US veterans. Neurotoxicol. 2010;31:493–501. doi: 10.1016/j.neuro.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen O, Reichenberg A, Perry C, Ginzberg D, Pollmacher T, Soreq H, Yirmiya R. Endotoxin-induced changes in human working and declarative memory associate with cleavage of plasma “readthrough” acetylcholinesterase. J Mol Neurosci. 2003;21(3):199–212. doi: 10.1385/jmn:21:3:199. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25(40):9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devito LM, Eichenbaum H. Memory for the order of events in specific sequences: contributions of the hippocampus and medial prefrontal cortex. J Neurosci. 2011;31(9):3169–3175. doi: 10.1523/JNEUROSCI.4202-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44(1):109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, Marine Resiliency Study T. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71(4):423–431. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Carballedo A, Hughes MM, Saleh K, Fagan A, Skokauskas N, Connor TJ. Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: high IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Transl Psychiatry. 2012;2:e88. doi: 10.1038/tp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, Reeves WC. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA. 1998;280:981–988. doi: 10.1001/jama.280.11.981. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neurosci. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacol. 2011;36(12):2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26(5):447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatol. 2010;49(7):1215–1228. doi: 10.1093/rheumatology/keq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Hampel H, Prvulovic D, Wallin A, Blennow K, Li R, Shen Y. Elevated CSF levels of TACE activity and soluble TNF receptors in subjects with mild cognitive impairment and patients with Alzheimer’s disease. Mol Neurodegener. 2011;6:69. doi: 10.1186/1750-1326-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30(7):1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30(Suppl):S109–116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KK, Barnes J, Ridgway GR, Bartlett JW, Clarkson MJ, Macdonald K, Alzheimer’s Disease Neuroimaging, I Automated cross-sectional and longitudinal hippocampal volume measurement in mild cognitive impairment and Alzheimer’s disease. NeuroImage. 2010;51(4):1345–1359. doi: 10.1016/j.neuroimage.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindlau A, Widmann CN, Putensen C, Jessen F, Semmler A, Heneka MT. Predictors of hippocampal atrophy in critically ill patients. Eur J Neurol. 2014 doi: 10.1111/ene.12443. in press. [DOI] [PubMed] [Google Scholar]
- MacEwan DJ. TNF ligands and receptors–a matter of life and death. Br J Pharmacol. 2002;135(4):855–875. doi: 10.1038/sj.bjp.0704549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64(6):484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCanlies EC, Araia SK, Joseph PN, Mnatsakanova A, Andrew ME, Burchfiel CM, Violanti JM. C-reactive protein, interleukin-6, and posttraumatic stress disorder symptomology in urban police officers. Cytokine. 2011;55(1):74–78. doi: 10.1016/j.cyto.2011.03.025. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Frueh BC. Why are Iraq and Afghanistan War veterans seeking PTSD disability compensation at unprecedented rates? J Anx Disord. 2013;27(5):520–526. doi: 10.1016/j.janxdis.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30(4):297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohlenhoff BS, Chao LL, Buckley ST, Weiner MW, Neylan TC. Are hippocampal size differences in posttraumatic stress disorder mediated by sleep pathology? Alzheimers Dement. 2014;10(3 Suppl):S146–154. doi: 10.1016/j.jalz.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- O’Donovan A. Inflammation and depression: unraveling the complex interplay in inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;58(5):541–542. doi: 10.1097/MPG.0000000000000292. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, Neylan TC. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011a;70(5):465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Neylan TC, Metzler T, Cohen BE. Lifetime exposure to traumatic psychological stress is associated with elevated inflammation in the Heart and Soul Study. Brain Behav Immun. 2012;26(4):642–649. doi: 10.1016/j.bbi.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Rush G, Hoatam G, Hughes BM, McCrohan A, Kelleher C, Malone KM. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress Anx. 2013;30(4):307–314. doi: 10.1002/da.22087. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, Neylan T. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis Markers. 2011b;30(2–3):123–132. doi: 10.3233/DMA-2011-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Neylan TC, Pole N, Metzler T, Best S, Henn-Haase C, Marmar CR. Association between childhood trauma and catecholamine response to psychological stress in police academy recruits. Biol Psychiatry. 2005;57(1):27–32. doi: 10.1016/j.biopsych.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun. 2012;26(1):13–17. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7(2):161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, Weiner MW. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):952–959. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Natl Acad Sci U S A. 2010;107(33):14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondergaard HP, Hansson LO, Theorell T. The inflammatory markers C-reactive protein and serum amyloid A in refugees with and without posttraumatic stress disorder. Clin Chim Acta. 2004;342(1–2):93–98. doi: 10.1016/j.cccn.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Spitzer C, Barnow S, Volzke H, Wallaschofski H, John U, Freyberger HJ, Grabe HJ. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. J Psychiatr Res. 2010;44(1):15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbon M, First M. The structured clinical interview for DSM-IV Diagnosis (SCID) Washington, D.C.: American Psychiatric Press; 1992. [Google Scholar]
- Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107(47):20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54(7):693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Bremner JD. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159(12):2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, Schuff N. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Meyerhoff DJ, Neylan TC, Hlavin J, Ramage ER, McCoy D, McCarthy C. The relationship between Gulf War illness, brain N-acetylaspartate, and post-traumatic stress disorder. Mil Med. 2010;176:896–902. doi: 10.7205/milmed-d-10-00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16(3):296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Kimerling R, Brown P, Chresman K, Levin K. Psychometric review of the life stressor checklist-revised. In: Stamm BH, editor. Instrumentation in stress, trauma, and adaptation. Lutherville, MD: Sidran Press; 1996. pp. 144–151. [Google Scholar]
- Woodward SH, Kaloupek DG, Streeter CC, Kimble MO, Reiss AL, Eliez S, Arsenault NJ. Hippocampal volume, PTSD, and alcoholism in combat veterans. Am J Psychiatry. 2006;163:674–681. doi: 10.1176/ajp.2006.163.4.674. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Zhang J, Peng M, Jia J. Plasma Amyloid-beta oligomers and soluble tumor necrosis factor receptors as potential biomarkers of AD. Curr Alzheimer Res. 2014 doi: 10.2174/1567205011666140317103222. In Press. [DOI] [PubMed] [Google Scholar]


