Abstract
Background
Platelets play a central role in hemostasis after trauma. However, the platelet count of most trauma patients does not fall below the normal range (100-450 ×109/L), and as a result, admission platelet count has not been adequately investigated as a predictor of outcome. The purpose of this study was to examine the relationship between admission platelet count and outcomes after trauma.
Methods
Retrospective cohort study of 389 massively transfused trauma patients. Regression methods and the Kruskal-Wallis test were used to test the association between admission platelet count and 24 hour mortality and units of PRBCs transfused.
Results
For every 50 × 109/L increase in admission platelet count, the odds of death decreased 17% at 6 hours (p=0.03, 95% CI 0.70-0.99) and 14% at 24 hours (p=0.02, 95% CI 0.75-0.98). The probability of death at 24 hours decreased with increasing platelet count. For every 50 × 109/L increase in platelet count, patients received 0.7 fewer units of blood within the first 6 hours (p=0.01, 95% CI -1.3- -0.14) and one less unit of blood within the first 24 hours (p=0.002, 95% CI -1.6 - -0.36). The mean number of units of PRBCs transfused within the first 6 and 24 hours decreased with increasing platelet count.
Conclusions
Admission platelet count was inversely correlated with 24 hour mortality and transfusion of PRBCs. A normal platelet count may be insufficient after severe trauma, and as a result, these patients may benefit from a lower platelet transfusion threshold. Future studies of platelet number and function after injury are needed.
Introduction
Uncontrolled hemorrhage is a major cause of mortality in both civilian and military trauma patients (1, 2). Only 2% of severely injured trauma patients arrive to the hospital in hemorrhagic shock requiring a massive transfusion (1), defined as transfusion of ≥10 units of packed red blood cells (PRBCs) over 24 hours. However, the mortality rate in this subset of trauma patients is about 40%, with half of these deaths occurring within the first 24 hours (3). Several studies indicate that many of these traumatic deaths due to hemorrhage are preventable (2, 4), and therefore a significant amount of research has been done to investigate resuscitation strategies and other therapeutic measures that may lead to improved survival in these patients. Most of these studies have focused exclusively on determining the optimal ratio of fresh frozen plasma (FFP) to PRBCs needed to prevent and reverse the coagulopathy of trauma (5-7). However, one landmark study examined platelet:PRBC ratio in addition to FFP:PRBC ratio and demonstrated that patients who received an FFP:PRBC ratio ≥1:2 in addition to a platelet:PRBC ratio ≥1:2 had the greatest overall survival, compared to patients who received lower ratios (1).
Platelets serve two critical functions of the coagulation process. Platelet adhesion and aggregation at the site of endothelial injury forms a hemostatic plug, and platelets enhance activation of coagulation proteases leading to thrombus formation (8, 9). Despite these important roles, there are very few studies investigating the effects of platelet function in severely injured trauma patients (9, 10) and even fewer studies investigating the effect of platelet count on trauma outcomes (11). We investigated the effect of admission platelet count on mortality and the number of units of PRBCs transfused in a cohort of massively transfused civilian trauma patients. In addition, we examined whether platelet count is associated with injury severity and coagulopathy on admission.
Methods
The cohort for this study included 389 massively transfused (≥10 units of PRBCs within the first 24 hours of admission) trauma patients. These patients are a subset of patients from an Institutional Review Board approved, retrospective, multicenter study that included adult trauma patients who arrived from the scene and received at least one unit of PRBCs in the Emergency Department (ED) (1). The multicenter trial included patients from sixteen level I trauma centers between July 2005 and June 2006.
The primary outcome of this study was mortality. We explored mortality at two time points: 6 hours and 24 hours, but chose to focus on mortality at 24 hours because interventions such as platelet transfusions likely have more of an impact on outcome compared to mortality at 6 hours. Secondary outcomes included the number of units of PRBCs transfused and the association of admission platelet count with injury severity and coagulopathy on admission. We examined the relationship between admission platelet count and admission International Normalized Ratio (INR) and partial thromboplastin time (PTT). Admission INR and PTT were analyzed as continuous predictors. However, in one analysis, we defined coagulopathy as an INR ≥1.4.
Patients were categorized according to admission platelet count into one of the following arbitrary categories: 0-100 × 109/L, 101-200 × 109/L, 201-300 × 109/L, and ≥301 × 109/L. Patients were further categorized into four injury severity groups according to Injury Severity Score (ISS) and admission base deficit: 1) ISS ≥25, base deficit ≥-6, 2) ISS ≥25, base deficit <-6, 3) ISS <25, base deficit ≥-6, and 4) ISS <25, base deficit <-6. These cutoffs for ISS and base deficit have been used in prior studies (12-16).
The chi-squared test was used to test for an association between nominal categorical variables. The Kruskal-Wallis test was used to test for an association between nominal categorical variables and non-normally distributed continuous variables. Spearman's correlation coefficient was used to test for an association between two non-normally distributed continuous variables. Logistic regression was used for the dichotomous outcomes mortality at 6 hours and at 24 hours. Linear regression was used for the continuous outcome number of units of packed red blood cells (PRBCs) transfused. A lowess curve was used to graph the association between admission platelet count and admission INR and PTT. A multivariable logistic regression model with mortality at 24 hours as the outcome and admission platelet count, ISS, glasgow coma score (GCS), and admission base deficit was built. We controlled for ISS GCS, and admission base deficit because these are well established predictors of mortality in trauma patients. The model was tested using the linktest and the Hosmer-Lemeshow goodness-of-fit test, the area under the receiver operator characteristic curve was determined, and the model was graphed. STATA SE 10.1 (College Station, TX) was used for all statistical analyses.
Results
There were 389 patients in the cohort (Table 1). Overall mortality was 14% (54 patients) at 6 hours and 25% (96 patients) at 24 hours. There were 45 patients (12%) with an admission platelet count between 0-100 × 109/L, 149 patients (38%) with 101-200 × 109/L, 138 patients (35%) with 201-300 × 109/L, and 57 patients (15%) with ≥301 × 109/L.
Table 1. Baseline Characteristics of the Trauma Cohort (n=389).
| Characteristic | All Trauma Patients |
|---|---|
| Age | 36 (25-50) |
| Male gender | 287 (74%) |
| Mechanism of Injury | |
| Penetrating | 129 (33%) |
| Blunt | 259 (67%) |
| Injury Severity Score | 32 (22-43) |
| Glasgow Coma Score | 10 (3-15) |
| Laboratory Values | |
| Base Deficit | -10 (-15 - -6) |
| INR | 1.4 (1.2-1.7) |
| PTT | 31.1 (25.6-44.8) |
| Platelet Count | 201(138-263) |
| Vital Signs | |
| Heart Rate | 118 (97-137) |
| Systolic Blood Pressure | 100 (80-119) |
| Respiratory Rate | 16 (20-26) |
| Temperature (C) | 36.1 (35.5-36.7) |
| Blood Product Transfusion | |
| Units of PRBCs | |
| within first 6 hours | 12 (9-20) |
| within first 24 hours | 15 (12-24) |
| Units of FFP | |
| within first 6 hours | 7 (4-11) |
| within first 24 hours | 9 (6-15) |
| Units of Platelets | |
| within first 6 hours | 2 (0-8) |
| within first 24 hours | 6 (2-12) |
| Liters of crystalloid | |
| within first 6 hours | 7 (4-11) |
| within first 24 hours | 10 (7-17) |
Median (Interquartile Range)
Platelet Count and Mortality
For every 50 × 109/L increase in admission platelet count, the odds of death at 6 hours decreased 17% (p=0.03, 95% CI 0.70-0.99) and the odds of death at 24 hours decreased 14% (p=0.02, 95% CI 0.75-0.98). For those who received platelets within the first 24 hours, the odds of death at 24 hours were 67% lower compared to those who did not receive platelets (p<0.001, 95% CI 0.20-0.57). Of those with an admission platelet count between 0-100 × 109/L, 33% died within 24 hours, 101-200 × 109/L, 30% died, 201-300 × 109/L, 21% died, and ≥301 × 109/L, 14% died. The probability of death at 24 hours decreased with increasing platelet count (Figure 1).
Figure 1. Probability of Death at 24 Hours by Admission Platelet Count.
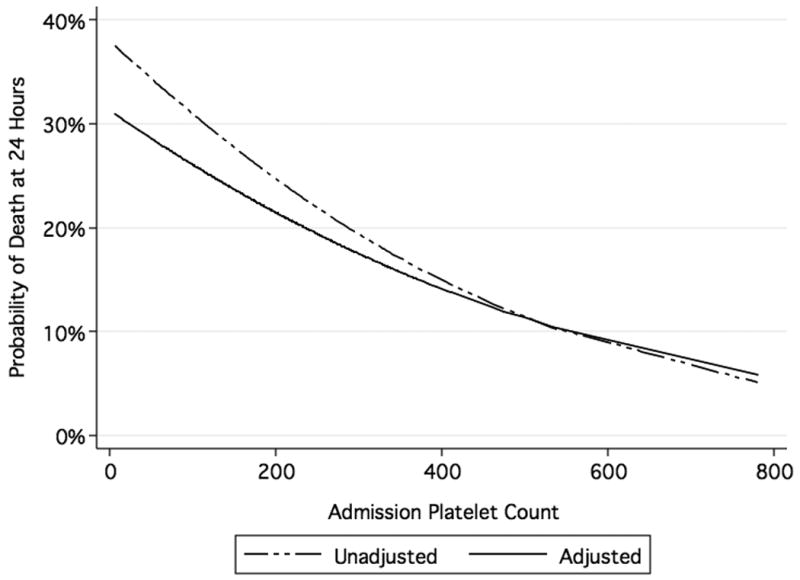
Unadjusted logistic regression model includes admission platelet count. Adjusted logistic regression model includes admission platelet count, Injury Severity Score, Glasgow Coma Score, and admission base deficit.
In a logistic regression model predicting mortality at 24 hours, the odds of death decreased 12% (p=0.08, 95% CI 0.76-1.02) for every 50 × 109/L increase in platelet count. This model controlled for other well established predictors of mortality in trauma patients including ISS, GCS, and admission base deficit (Figure 1 and Table 2).
Table 2. Multivariable Analysis: Predictors of Mortality at 24 Hours1.
| Predictor | Adjusted Odds Ratio | p value | 95% CI |
|---|---|---|---|
| Injury Severity Score | 1.05 | <0.001 | (1.03-1.07) |
| Glasgow Coma Score | 0.99 | 0.80 | (0.94-1.05) |
| Admission Base Deficit | 0.92 | <0.001 | (0.89-0.96) |
| Admission Platelet Count2 | 0.88 | 0.08 | (0.76-1.02) |
Area under the ROC curve=0.76 for this prediction model
per 50 × 109/L increase in platelet count
Blood Product Transfusions
There were 147 patients (38%) who did not receive any platelet transfusions within the first 6 hours and 74 patients (19%) who did not receive any platelet transfusions within the first 24 hours. There were 7 patients with a platelet count <50 × 109/L and 4 of them (57%) received a platelet transfusion within the first 6 hours and the first 24 hours. There were 42 patients with a platelet count <100 × 109/L; 30 of these patients (71%) received a platelet transfusion within the first 6 hours and 34 (81%) received a platelet transfusion within the first 24 hours. The platelet count on admission was associated with whether platelets were transfused within the first 6 hours, but not within the first 24 hours (Table 3).
Table 3. Admission Platelet Count and Platelet Transfusion within the First 6 and 24 Hours.
| Platelet Transfusion | ||||
|---|---|---|---|---|
| Platelet Count | Within First 6 Hours | Within First 24 Hours | ||
| Yes | No | Yes | No | |
| 0-100 × 109/L | 73% | 27% | 82% | 18% |
| 101-200 × 109/L | 70% | 30% | 85% | 15% |
| 201-300 × 109/L | 51% | 49% | 78% | 22% |
| ≥301 × 109/L | 60% | 40% | 75% | 25% |
| p=0.0051 | p=0.3 | |||
p-value for chi-squared test
Patients received 0.7 fewer units of blood within the first 6 hours for every 50 × 109/L increase in platelet count (p=0.01, 95% CI -1.3- -0.14). Patients received one fewer unit of blood within the first 24 hours for every 50 × 109/L increase in platelet count (p=0.002, 95% CI -1.6 - -0.36). The mean number of units of PRBCs transfused within the first 6 hours and the first 24 hours decreased with increasing platelet count (Table 4).
Table 4. Number of Units of Packed Red Blood Cells (PRBCs) Transfused by Admission Platelet Count.
| Platelet Count | Number of Units of PRBCs Transfused | |
|---|---|---|
| Within First 6 Hours | Within First 24 Hours | |
| 0-100 × 109/L | 19.4 ± 11.8 | 25.8 ± 17.7 |
| 101-200 × 109/L | 16.4 ± 10.3 | 20.5 ± 12.7 |
| 201-300 × 109/L | 13.6 ± 9.6 | 17.5 ± 9.0 |
| ≥301 × 109/L | 15.1 ± 11.2 | 18.8 ± 10.0 |
| p=0.0011 | p=0.01 | |
p-value for Kruskal-Wallis test
Injury Severity and Coagulopathy
There were 194 patients (50%) with an ISS >25 and admission base deficit ≥-6, 107 patients (28%) with an ISS ≤25 and base deficit ≥-6, 52 patients (13%) with an ISS >25 and base deficit <-6, and 36 patients (9%) with an ISS ≤25 and base deficit <-6. In the two injury severity groups with a base deficit ≥-6, there were similar percentages of patients in each of the platelet count categories (Figure 2). In these two injury severity groups, greater than 50% of patients had an admission platelet count ≤200 × 109/L. In the two injury severity groups with a base deficit <-6, greater than 50% of patients had an admission platelet count ≥201 × 109/L. When ISS and admission base deficit were analyzed separately, admission platelet count was not associated with ISS (r2=-0.06, p=0.25) or admission base deficit (r2=0.18, p<0.001).
Figure 2. Platelet Count by Injury Severity Score and Admission Base Deficit Categories.
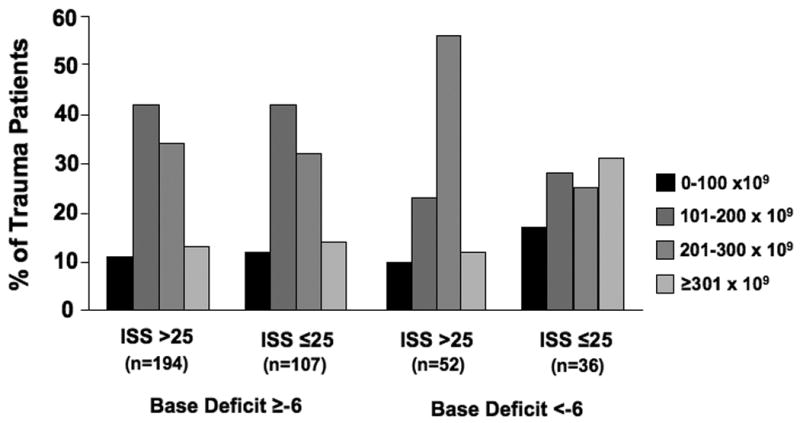
chi-squared test p=0.01
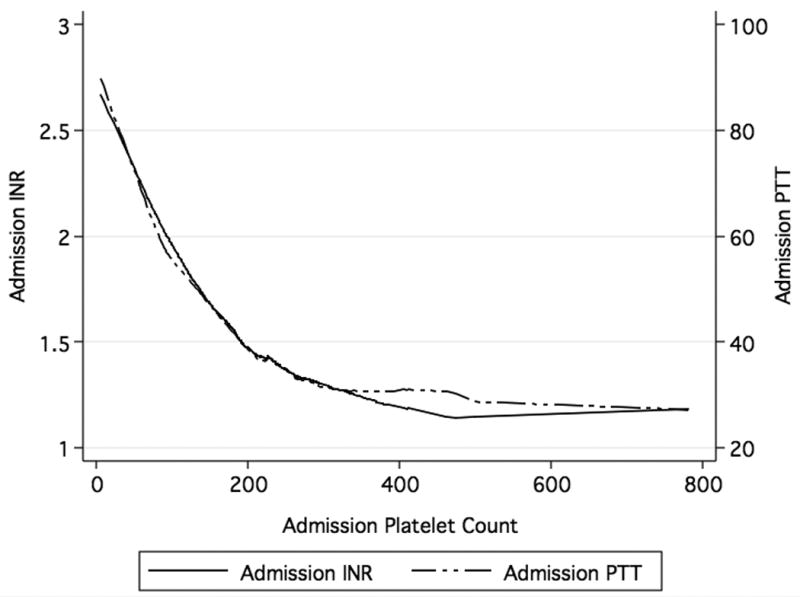
Admission INR and PTT were associated with admission platelet count (r2= -0.48, p<0.001 and r2= -0.46, p<0.001). As the admission platelet count increased, admission INR and PTT decreased (Figure 3). Of those with a platelet count 0-100 × 109/L, 88% also had coagulopathy (INR ≥1.4), 101-200 × 109, 60% were coagulopathic, 201-300 × 109, 42% were coagulopathic, ≥301 × 109, 24% were coagulopathic (p<0.001). Similar to admission platelet count, admission INR and PTT were not associated with injury severity (r2= 0.14, p=0.007 and r2= 0.23, p<0.001). Admission INR was not associated with base deficit (r2= -0.18, p<0.001), but admission PTT was associated with base deficit (r2= -0.30, p<0.001).
Figure 3. Association Between Admission Platelet Count and Admission INR and Admission Platelet Count and Admission PTT.
Discussion
In this cohort of massively transfused trauma patients, our results indicate that as the admission platelet count increases, the probability of death at 24 hours decreases. Furthermore, admission platelet count shows a trend towards prediction of early mortality in an adjusted analysis including other well established predictors of mortality in these patients. In addition, patients with a higher admission platelet count receive fewer units of PRBCs within both the first 6 hours and 24 hours after admission. Lastly, platelet count is associated with the combination of ISS and admission base deficit and also with coagulopathy on admission.
As admission platelet count increases, the probability of death at 24 hours decreases. This platelet count represents a baseline platelet count after injury that is present before the start of major resuscitation. Once two blood volumes have been replaced by crystalloid or PRBCs, the platelet count may decrease to approximately 50 × 109/L due to dilution (17, 18). This dilutional thrombocytopenia leads to microvascular bleeding. Therefore, a low platelet count on admission followed by dilution leads to an even lower platelet count that may then result in poorer outcomes. Several other studies, each examining different types of patients, support our finding that decreased platelet counts are associated with increased mortality. One study at a major level I trauma center showed a stepwise, dose response association between the severity of coagulopathy (indicated by platelet count, PTT, INR, and fibrinogen) present on admission and in-hospital mortality (11). In patients with traumatic brain injury, low platelet counts were associated with mortality and vegetative state at 6 months (19). However, it was unclear whether the platelet counts used for the analyses in that study were from the time of admission or from some other time point during hospitalization. Finally, one study reported that increased amounts of plasma and platelets given in the operating room in predetermined ratios in patients undergoing operation for ruptured abdominal aortic aneurysm improved survival (20). That study demonstrated a stepwise, dose response association between platelet count and survival. There was 30% survival in patients with a platelet count <50 × 109/L upon admission to the ICU after surgery, 45% in those with a platelet count between 50 × 109/L and 100 × 109/L, and 69% in those with a platelet count >100 × 109/L. Interestingly, although guidelines recommend maintaining the platelet count >50 × 109/L, in that study, patients with an even greater platelet count (>100 × 109/L) had better survival than those with a normal platelet count of 50 × 109/L-100 × 109/L.
Current transfusion guidelines recommend maintaining a platelet count greater than 50 × 109/L and greater than 100 × 109/Lfor patients with multiple trauma or CNS injury (18). Interestingly, only 57% of patients with an admission platelet count <50 × 109/L in our trauma cohort received a platelet transfusion during the first 24 hours and 81% of patients with an admission platelet count <100 × 109/L received a platelet transfusion during the first 24 hours. Although we do not have data on platelet counts later in the resuscitation, this is a cohort of massively transfused trauma patients, and therefore, without any platelet transfusion the platelet count on admission can be expected to decline over the course of resuscitation. It may be important to anticipate which trauma patients are likely to need a massive transfusion because these patients are at greater risk of developing dilutional thrombocytopenia. In addition, there is individual variability in response to injury. Most trauma patients are young, otherwise healthy individuals and may be able to tolerate a lower platelet count. However, many trauma patients are elderly and may have underlying comorbidities that contribute to decreased platelet function. These patients may have a lower transfusion threshold than younger patients with no comorbidities.
While platelet count appears to be a predictor of mortality, it is important to recognize that not all platelets accounted for in the platelet count are functional. Major trauma affects platelet function. One study measured both platelet function and platelet activation parameters in Level I and II trauma patients (9). That study demonstrated that platelet function and activation increase immediately after trauma and then return to normal over the next few days. Importantly, non-survivors were likely to have a combination of decreased platelet function and increased platelet activation. In addition, many severely injured trauma patients are hypothermic and have acidemia. Acidosis inhibits platelet aggregation (21) and hypothermia reversibly decreases platelet function (22). Furthermore, a fraction of the platelets within each transfusion unit are non-viable. The recovery rate of five-day old platelets after transfusion is about 50% (17). Non-viable platelets are sequestered in the spleen and it is unknown how quickly these non-viable platelets are removed from the circulation (17). A normal platelet count may provide the clinician with a false sense of security, because not all platelets are functional. In our analysis, we observed that patients with a normal platelet count had an increased risk of death compared to those with higher platelet counts.
While it is easy to determine platelet count, assessing platelet function is more challenging. Rotational thromboelastography (TEG) can provide evidence of reduced platelet function, but lacks the ability to determine the mechanism of decreased function. Several investigators have evaluated the use of TEG to guide platelet transfusion, however, to date there are no codified criteria on its use. Platelet function can be determined by flow cytometry or other functional analyses some of which are currently commercially available, but there are no comprehensive studies of platelet function after trauma. In light of our finding that even normal platelet counts are often inadequate, the study of platelet function represents an important focus for future investigation.
Several retrospective trauma studies have investigated the effect of platelet:PRBC transfusion ratios on survival. Two of these studies demonstrated that trauma survivors received a platelet to PRBC transfusion ratio of 0.8 compared to 0.5 in non-survivors (4, 16). The most recent study to examine transfusion ratios in massively transfused trauma patients investigated both the FFP:PRBC ratio and the platelet:PRBC ratio (1). Overall survival was 71% in patients who received both an FFP:PRBC ratio ≥1:2 and a platelet:PRBC ratio ≥1:2, compared to 41% in patients who received both an FFP:PRBC ratio <1:2 and a platelet:PRBC ratio <1:2. These studies have provided important insight into the association between resuscitation strategies and trauma outcomes. Guidelines for platelet transfusions are based on observational studies like these and expert opinion. Prospective, randomized studies using predetermined platelets:PRBC ratios and FFP:PRBC ratios versus standard routine resuscitation are needed to determine whether improved outcomes can be achieved in massively transfused trauma patients.
Ten to fifteen percent of all PRBCs are used for injured patients (3). If traumatic hemorrhage can be stopped early, then each trauma patient may require fewer units of PRBCs. Our results indicate that as admission platelet count increases, the number of units of PRBCs transfused decreases. Ideally, a prospective study of massively transfused trauma patients that records baseline admission data and then follows each patient's clinical course throughout the resuscitation phase, the operation, and finally the intensive care unit is needed to determine how the timing of blood product transfusions affects lab values and more importantly, mortality and other trauma outcomes. In addition, this study design may help to determine lab value cut points that may serve as transfusion triggers for these patients.
In our study, as admission base deficit increased, platelet count decreased. This is consistent with the results of a study of early coagulopathy in military trauma patients (23). In that study, coagulopathy was defined as an INR ≥1.5 and within each ISS category, those with a greater base deficit were more likely to be coagulopathic. Our findings are concerning since it is well known that platelet function is impaired in the setting of acidosis. Therefore, greater base deficit may be associated with both decreased platelet count and impaired function. Prior studies have shown a clear association between coagulopathy, defined by INR, and injury severity (11, 24). The prevalence of coagulopathy increases with increasing injury severity. However, in our analysis, admission platelet count, INR, and PTT were not associated with injury severity.
Most authors define acute traumatic coagulopathy using either an INR or a PTT cut point. The role of platelets in acute traumatic coagulopathy is unclear. One reason for this may be that there is great variability in the critical number of platelets required for clot initiation (25, 26). However, since platelets play a critical role in the coagulation process, defining acute traumatic coagulopathy using only INR and PTT parameters may overlook important deficiencies that may be treatable by transfusing platelets.
In conclusion, admission platelet count is inversely correlated with 24 hour mortality and transfusion of PRBCs. A normal platelet count may be insufficient after severe trauma, and as a result, these patients may benefit from a lower platelet transfusion threshold. Future studies of platelet count and function after injury are needed to determine which transfusion strategies will improve outcomes after major trauma.
Acknowledgments
The authors wish to acknowledge the support of the numerous research coordinators at the various centers. This work was funded in part by a grant from the US Army to the University of Texas Health Science Center San Antonio, W81XWH-07-1-0717.
Lisa M. Brown, MD was supported by NIH T32 GM008258-21
Footnotes
This paper was presented at the American Association for the Surgery of Trauma meeting September 22-25, 2010, Boston, MA
The additional authors are:
- JB Holcomb and CE Wade from United States Army Institute of Surgical Research and Brooke Army Medical Center, San Antonio TX
- KJ Brasel from the Medical College of Wisconsin, Milwaukee, WI
- G Vercruysse and J MacLeod from Emory University School of Medicine, Atlanta, GA
- RP Dutton and JR Hess from Maryland School of Medicine, Baltimore, MD
- JC Duchesne and NE McSwain from Tulane University, New Orleans, LA
- P Muskat and J Johannigamn from University Hospital, Cincinnati, OH
- HM Cryer and A Tillou from University of California Los Angeles School of Medicine, Los Angeles, CA
- JF Pittet from the University of California, San Francisco, CA
- MA De Moya from Massachusetts General Hospital, Boston, MA
- MA Schreiber and B Tieu from Oregon Health Science Center, Portland, OR
- S Brundage from Stanford University Medical Center. Stanford, CA
- LM Napolitano, M Brunsvold, and KC Sihler from the University of Michigan Health System, Ann Arbor, MI
- G Beilman from the University of Minnesota, Rochester, MN
- AB Peitzman, MS Zenait, J Sperry and L Alarcon from the University of Pittsburg Medical Center, Pittsburg, PA
- MA Croce from the University of Tennessee Health Science Center, Memphis, TN
- JP Minei from University of Texas Southwestern Medical Center, Dallas, TX
- R Kozar and EA Gonzalez from the University of Texas Health Science Center, Houston, TX
- RM Stewart, SM Cohn and JE Mickalek from the University of Texas Health Science Center, San Antonio, TX
- EM Bulger from the University of Washington, Seattle, WA
- BA Cotton and TC Nunez from Vanderbilt University Medical Center, Nashville, TN
- R Ivatury from Virginia Commonwealth University, Richmond, VA
- JW Meredith, P Miller, GJ Pomper, and B Marin from Wake Forest University School of Medicine, Winston Salem, NC
References
- 1.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 2.Spinella PC, Holcomb JB. Resuscitation and transfusion principles for traumatic hemorrhagic shock. Blood Rev. 2009 doi: 10.1016/j.blre.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Como JJ, Dutton RP, Scalea TM, et al. Blood transfusion rates in the care of acute trauma. Transfusion. 2004;44:809–813. doi: 10.1111/j.1537-2995.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- 4.Cinat ME, Wallace WC, Nastanski F, et al. Improved survival following massive transfusion in patients who have undergone trauma. Arch Surg. 1999;134:964–968. doi: 10.1001/archsurg.134.9.964. discussion 968-970. [DOI] [PubMed] [Google Scholar]
- 5.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 6.Sperry JL, Ochoa JB, Gunn SR, et al. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–993. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez EA, Moore FA, Holcomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 8.Davenport RA, Brohi K. Coagulopathy in trauma patients: importance of thrombocyte function? Curr Opin Anaesthesiol. 2009;22:261–266. doi: 10.1097/ACO.0b013e328325a6d9. [DOI] [PubMed] [Google Scholar]
- 9.Jacoby RC, Owings JT, Holmes J, et al. Platelet activation and function after trauma. J Trauma. 2001;51:639–647. doi: 10.1097/00005373-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Boldt J, Menges T, Wollbruck M, et al. Platelet function in critically ill patients. Chest. 1994;106:899–903. doi: 10.1378/chest.106.3.899. [DOI] [PubMed] [Google Scholar]
- 11.Hess JR, Lindell AL, Stansbury LG, et al. The prevalence of abnormal results of conventional coagulation tests on admission to a trauma center. Transfusion. 2009;49:34–39. doi: 10.1111/j.1537-2995.2008.01944.x. [DOI] [PubMed] [Google Scholar]
- 12.Asensio J, Trunkey D. Current Therapy of Trauma and Surgical Critical Care. Philadelphia: Mosby Elsevier; 2008. [Google Scholar]
- 13.Lustenberger T, Talving P, Kobayashi L, et al. Early Coagulopathy After Isolated Severe Traumatic Brain Injury: Relationship With Hypoperfusion Challenged. J Trauma. doi: 10.1097/TA.0b013e3181cdae81. [DOI] [PubMed] [Google Scholar]
- 14.Larson CR, White CE, Spinella PC, et al. Association of shock, coagulopathy, and initial vital signs with massive transfusion in combat casualties. J Trauma. 69(1):S26–32. doi: 10.1097/TA.0b013e3181e423f4. [DOI] [PubMed] [Google Scholar]
- 15.Bilello JF, Davis JW, Lemaster D, et al. Prehospital Hypotension in Blunt Trauma: Identifying the “Crump Factor”. J Trauma. 2009 doi: 10.1097/TA.0b013e31819638d0. [DOI] [PubMed] [Google Scholar]
- 16.Cosgriff N, Moore EE, Sauaia A, et al. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma. 1997;42:857–861. doi: 10.1097/00005373-199705000-00016. discussion 861-852. [DOI] [PubMed] [Google Scholar]
- 17.Ketchum L, Hess JR, Hiippala S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J Trauma. 2006;60:S51–58. doi: 10.1097/01.ta.0000199432.88847.0c. [DOI] [PubMed] [Google Scholar]
- 18.Stainsby D, MacLennan S, Thomas D, et al. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135:634–641. doi: 10.1111/j.1365-2141.2006.06355.x. [DOI] [PubMed] [Google Scholar]
- 19.Van Beek JG, Mushkudiani NA, Steyerberg EW, et al. Prognostic value of admission laboratory parameters in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:315–328. doi: 10.1089/neu.2006.0034. [DOI] [PubMed] [Google Scholar]
- 20.Johansson PI, Stensballe J, Rosenberg I, et al. Proactive administration of platelets and plasma for patients with a ruptured abdominal aortic aneurysm: evaluating a change in transfusion practice. Transfusion. 2007;47:593–598. doi: 10.1111/j.1537-2995.2007.01160.x. [DOI] [PubMed] [Google Scholar]
- 21.Marumo M, Suehiro A, Kakishita E, et al. Extracellular pH affects platelet aggregation associated with modulation of store-operated Ca(2+) entry. Thromb Res. 2001;104:353–360. doi: 10.1016/s0049-3848(01)00374-7. [DOI] [PubMed] [Google Scholar]
- 22.Valeri CR, Feingold H, Cassidy G, et al. Hypothermia-induced reversible platelet dysfunction. Ann Surg. 1987;205:175–181. doi: 10.1097/00000658-198702000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niles SE, McLaughlin DF, Perkins JG, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64:1459–1463. doi: 10.1097/TA.0b013e318174e8bc. discussion 1463-1455. [DOI] [PubMed] [Google Scholar]
- 24.Brohi K, Singh J, Heron M, et al. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 25.Bowbrick VA, Mikhailidis DP, Stansby G. Influence of platelet count and activity on thromboelastography parameters. Platelets. 2003;14:219–224. doi: 10.1080/0953710031000118849. [DOI] [PubMed] [Google Scholar]
- 26.Oshita K, Az-ma T, Osawa Y, et al. Quantitative measurement of thromboelastography as a function of platelet count. Anesth Analg. 1999;89:296–299. doi: 10.1097/00000539-199908000-00006. [DOI] [PubMed] [Google Scholar]


