Abstract
Coordination of cell growth with nutrient availability, in particular amino acids, is a central problem that has been solved by the implementation of complex regulatory cascades. Although the specific regulatory mechanisms differ between kingdoms and species, a common theme is the use of tRNA molecules as sensors and transducers of amino acid starvation. In many bacteria, amino acid starvation leads to high levels of uncharged tRNAs, a signal for the synthesis of the stringent response’s alarmones, halting transcription of stable RNAs and inducing the synthesis of amino acid synthesis pathways 1. In gram-positive Bacteria (as well as the Deinococcus-Thermus clade), uncharged tRNAs bind structures (T-boxes) in the leader sequences of mRNA encoding gene, activating the expression of genes involved in amino acid metabolism 2. In eukaryotes, the conserved General Amino Acid Control (GAAC) response is triggered by shortage of amino acids that leads to the binding of uncharged tRNAs to Gcn2 kinase and, through a cascade of events, to the activation of the central activator of amino acid synthesis genes, Gcn4 3. As the study by Scheidt et al. 4 and several other recent studies in this field reveal, variations in charging levels are not the only mechanism by which tRNAs play a role in amino acid starvation responses; levels of post-transcriptional modifications also seem to play major roles.
Keywords: tRNA, GCN4, thiolation, UPR, TOR
The anticodon-stem-loop (ASL) of tRNAs drives decoding by interacting directly with the mRNA codon (Fig. 1). Modifications of the ASL are the most distinct and chemically complex of all RNA modifications 5. They are required for accurate codon recognition and translocation, enhance aminoacylation properties of tRNAs, and prevent ribosomal frameshifting 5. In eukaryotes, two of the most complex ASL modifications are N6-threonylcarbamoyladenosine (t6A) and 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U). The first modification is found at position 37 of most tRNAs decoding ANN codons, just before the residue that interacts with the first base of the codon. The second modification is found at position 34, the residue that decodes the 3rd base of the codon, of tRNALysUUU, tRNAGluUUC, and tRNAGlnUUG (Fig. 1). Both these modifications are found in tRNALysUUU and affect translation efficiency 5. In addition, several other striking parallels can be made between the two.
t6A and mcm5s2U are both synthesized by elaborate multimeric complexes that were first thought to be transcription factors. The KEOPS complex (Kinase, putative Endopeptidase and Other Proteins of Small size) in combination with Sua5 is involved in the synthesis of t6A 6, and the Elongator (ELP) complex in combination with the URM1 pathway enzymes (Uba4, Urm1, Ncs2/Ncs6) is involved in the synthesis of mcm5s2U 5. Saccharomyces cerevisiae mutants lacking t6A or mcm5s2U display many similar phenotypes. For example, telomere shortening is observed in the absence of either modification 7,8. In the case of ELP, it is now firmly established that most of the ELP phenotypes are due to the absence of the modified base, as the phenotypes are suppressed by overexpressing the tRNA targets 7,8,9. For example, the levels of the Sir4, a regulator involved in telomere maintenance and enriched in AAA (Lys) codons, are decreased in mcm5s2U deficient strains. Overexpression of tRNALysUUU suppresses the telomere shortening phenotype 8. In Schizosaccharomyces pombe, key regulators, including the two subunits Atf1 and Pcr1 of the central regulator of the core environmental stress response 10 and the cell division regulator Cdr2 11, have an over-representation of AAA codons over other Lys codons, and their efficient translation is dependent on mcm5s2U levels. More generally, proteins involved in translation initiation and elongation are enriched in AAA codons, and reduced in cells lacking mcm5s2U 12,13. It is not yet known if the levels of these proteins enriched in AAA codons also require t6A for efficient translation in yeast, but it has been shown in mammals that a decrease in sulfur modified form of t6A (ms2t6A) on tRNALysUUU leads to lower levels of proinsulin 14.
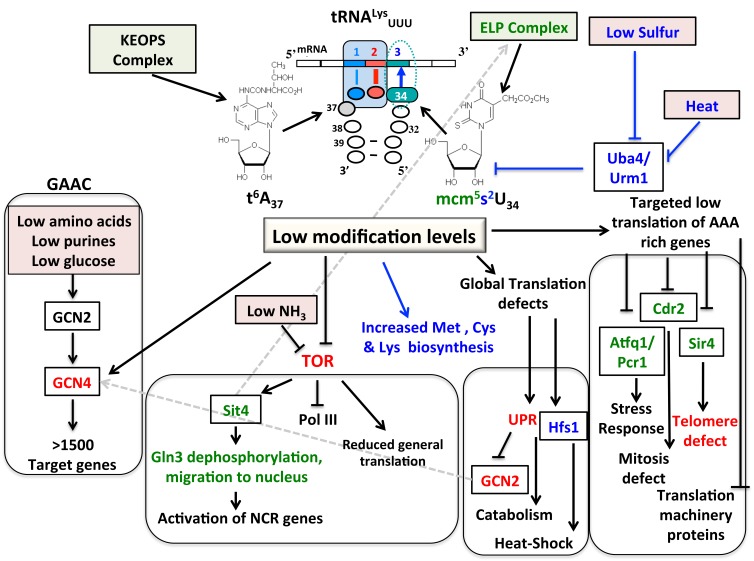
Figure 1. FIGURE 1: Possible cascade of events triggered when s2U, mcm5U or t6A levels are reduced in tRNALysUUU.
Alteration in the levels of mcm5s2U occurring at position 34 of the tRNA (wobble position) and t6A occurring at position 37 (adjacent to the first base of the anti-codon) have global cellular affects. Reduction in either modification alters many regulatory cascades with the text color indicating the modification responsible for the change (red indicates expression change seen in t6A and mcm5s2U mutants). Solid lines represent experimental data; dashed lines indicated potential points of regulation
Another phenotype shared by a t6A or a mcm5s2U deficient S. cerevisiae derivatives is the GCN2 independent activation of GCN4 15,16. As the activation of the GAAC leads to a major reprograming of transcription (>500 genes are induced and >1000 are repressed) 17, this makes it difficult to interpret whether specific phenotypes observed in these tRNA modification mutants are due to direct or indirect effects. This is made all the more problematic given that another central regulator, the Target of Rapamycin (or TOR), is also affected by both t6A and mcm5s2U.
The TOR kinases regulate the balance between protein synthesis and protein degradation in response to nutrient quality and TOR activity is inhibited by low nitrogen conditions, caffeine or rapamycin 18. In yeast, reduced TOR levels increase levels of the Sit4 phosphatase, which dephosphorylates TOR targets such as the regulator Gln3. Unphosporylated Gln3 will then relocate to the nucleus to activate genes required for growth on low quality nitrogen sources. The following links between TOR and mcm5s2U have previously been made: 1) ELP mutants are sensitive to rapamycin and caffeine 16; 2) deletion of SIT4 leads to rapamycin hypersensitivity and resistance to zymocin (a tRNase that recognizes mcm5s2U and cleaves tRNALysUUU leading to cell death) 19 because Sit4 activates the ELP complex by phosphorylation 20. As shown by Scheidt et al. 4, the Elongator mutants mislocate Gln3 leading to rapamycin hypersensitivity, a phenotype that can be suppressed by over-expression of the tRNAs modified by mcm5s2U, suggesting that proper tRNA modification affects Gln3 localization and subsequent activation of the nitrogen catabolite repression (NCR) response. Mislocalization of Gln3 occurs both in elp3∆ (removing the mcm5 moiety) and in urm∆ (s2 moiety). A link between t6A synthesis enzymes and TOR has also recently been seen in flies. The Glavic group showed that lowering levels of one of the subunits of the KEOPS complex (Bud32) reduces TOR phosphorylation of S6K (SCH9 in yeast) required for TOR dependent regulation of ribosome biogenesis 21.
How Gcn4 and TOR signaling depend on t6A and mcm5s2U is still far from understood at the molecular level. Are the Gcn4 activation and TOR repression in strains lacking these modifications due to direct effects caused by poor translation of specific proteins or are they part of general stress responses caused by translation inaccuracy and protein misfolding? The reality might lie in a combination of responses as in addition to the targeted effects described above, low mcm5s2U increases levels of proteins involved in proteasomal degradation 12. In addition, s2 synthesis proteins in S. cerevisiae are unstable at high temperature and reduced levels of the modification lead to activation of the heat-shock response regulator (Hsf1) through the synthesis of unfolded proteins (Fig. 1) 22. Finally, silencing both t6A synthesis genes Bud32 and Kae1 in flies activates the Unfolded Protein Response (UPR) 23 and mutations of the thiolation enzyme leading to the formation of ms2i6A in mouse led to an increase of the Endoplasmic Reticulum (ER) stress response 14.
Because the synthesis of the t6A and mcm5s2U modifications of tRNALysUUU draw on primary metabolism intermediates 24, it is tempting to propose that these could serve as sensing signals linking metabolism and translation. One recent example of such an integration is found in the mcm5s2U thiolation pathway; sulfur starvation reduces the levels of the Uba4 thiolation enzyme and hence the levels of mcm5s2U in yeast 13 (Fig. 1). Even if the underlying molecular mechanisms are not fully understood, low mcm5s2U levels trigger an adaptive response: 1) reduced protein expression due to general slow-down of translation of lysine rich proteins that are found predominantly in the ribosomal machinery; 2) increased levels of methionine, cysteine, and lysine synthesis proteins 13.
The complexity of the responses with the interplay of central regulators such as GCN4 and TOR (Fig. 1), make the dissection of the roles of t6A and mcm5s2U a delicate exercise that will require combining of classical genetic and biochemical studies with the genome wide bioinformatics, proteomic and profiling studies that are now available. These studies are all the more critical as derivatives of both modifications have been linked to human diseases as defects in the ms2t6A synthesis enzyme have been linked to type 2 diabetes 14, and familial dysautonomia patients have reduced levels of mcm5s2U 25.
Funding Statement
This work was supported by the National Institutes of Health (grant number R01 GM70641 to V.D.C.-L.), P.C.T. was funded in part by a Chateaubriand Fellowship from the French Embassy in the United States. We thank Jennifer Thiaville for editing the manuscript.
References
- 1.Dalebroux ZD, Swanson MS. ppGpp: magic beyond RNA polymerase. Nat Rev Microbiol. 2012;10(3):203–212. doi: 10.1038/nrmicro2720. [DOI] [PubMed] [Google Scholar]
- 2.Green NJ, Grundy FJ, Henkin TM. The T box mechanism: tRNA as a regulatory molecule. FEBS Lett. 2010;584(2):318–324. doi: 10.1016/j.febslet.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 4.Scheidt V, Judes A, Bar C, Klassen R, Schaffrath R. Loss of wobble uridine modification in tRNA anticodons interferes with TOR pathway signaling. 2014;(12):416–424. doi: 10.15698/mic2014.12.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Yacoubi B, Bailly M, de Crécy-Lagard V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu Rev Genet. 2012;46:69–95. doi: 10.1146/annurev-genet-110711-155641. [DOI] [PubMed] [Google Scholar]
- 6.Perrochia L, Crozat E, Hecker A, Zhang W, Bareille J, Collinet B, van Tilbeurgh H, Forterre P, Basta T. In vitro biosynthesis of a universal t6A tRNA modification in Archaea and Eukarya. Nucleic Acids Res. 2013;41(3):1953–1964. doi: 10.1093/nar/gks1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng F-L, Hu Y, Shen N, Tong X-J, Wang J, Ding J, Zhou J-Q. Sua5p a single-stranded telomeric DNA-binding protein facilitates telomere replication. Embo J. 2009;28(10):1466. doi: 10.1038/emboj.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Huang B, Eliasson M, Rydén P, Byström AS. Elongator complex influences telomeric gene silencing and DNA damage response by its role in wobble uridine tRNA modification. PLoS Genet. 2011;7(9):e1002258. doi: 10.1371/journal.pgen.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esberg A, Huang B, Johansson MJO, Byström AS. Elevated levels of two tRNA species bypass the requirement for elongator complex in transcription and exocytosis. Molecular Cell. 2006;24(1):139. doi: 10.1016/j.molcel.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Vazquez J, Vargas-Perez I, Sanso M, Buhne K, Carmona M, Paulo E, Hermand D, Rodriguez-Gabriel M, Ayte J, Leidel S, Hidalgo E. Modification of tRNA(Lys) UUU by elongator is essential for efficient translation of stress mRNAs. PLoS Genet. 2013;9(7):e1003647. doi: 10.1371/journal.pgen.1003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer F, Matsuyama A, Candiracci J, Dieu M, Scheliga J, Wolf DA, Yoshida M, Hermand D. Translational control of cell division by Elongator. Cell Rep. 2012;1(5):424–433. doi: 10.1016/j.celrep.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rezgui VAN, Tyagi K, Ranjan N, Konevega AL, Mittelstaet J, Rodnina MV, Peter M, Pedrioli PGA. tRNA tKUUU, tQUUG, and tEUUC wobble position modifications fine-tune protein translation by promoting ribosome A-site binding. Proc Natl Acad Sci U S A. 2013;110(30):12289–12294. doi: 10.1073/pnas.1300781110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laxman S, Sutter BM, Wu X, Kumar S, Guo X, Trudgian DC, Mirzaei H, Tu BP. Sulfur amino acids regulate translational capacity and metabolic homeostasis through modulation of tRNA thiolation. Cell. 2013;154(2):416–429. doi: 10.1016/j.cell.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei FY, Suzuki T, Watanabe S, Kimura S, Kaitsuka T, Fujimura A, Matsui H, Atta M, Michiue H, Fontecave M, Yamagata K, Tomizawa K. Deficit of tRNA(Lys) modification by Cdkal1 causes the development of type 2 diabetes in mice. J Clin Invest. 2011;121(9):3598–3608. doi: 10.1172/JCI58056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daugeron MC, Lenstra TL, Frizzarin M, El Yacoubi B, Liu X, Baudin-Baillieu A, Lijnzaad P, Decourty L, Saveanu C, Jacquier A, Holstege FC, de Crécy-Lagard V, van Tilbeurgh H, Libri D. Gcn4 misregulation reveals a direct role for the evolutionary conserved EKC/KEOPS in the t6A modification of tRNAs. Nucleic Acids Res. 2011;39(14):6148–60. doi: 10.1093/nar/gkr178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zinshteyn B, Gilbert WV. Loss of a conserved tRNA anticodon modification perturbs cellular signaling. PLoS genetics. 2013;9(8):e1003675. doi: 10.1371/journal.pgen.1003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natarajan K, Meyer MR, Jackson BM, Slade D, Roberts C, Hinnebusch AG, Marton MJ. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol Cell Biol. 2001;21(13):4347–4368. doi: 10.1128/MCB.21.13.4347-4368.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardenas ME, Cutler NS, Lorenz MC, Di Como CJ, Heitman J. The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev. 1999;13(24):3271–3279. doi: 10.1101/gad.13.24.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jablonowski D, Taubert JE, Bar C, Stark MJ, Schaffrath R. Distinct subsets of Sit4 holophosphatases are required for inhibition of Saccharomyces cerevisiae growth by rapamycin and zymocin. Eukaryot Cell. 2009;8(11):1637–1647. doi: 10.1128/EC.00205-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jablonowski D, Fichtner L, Stark MJ, Schaffrath R. The yeast elongator histone acetylase requires Sit4-dependent dephosphorylation for toxin-target capacity. Mol Biol Cell. 2004;15(3):1459–1469. doi: 10.1091/mbc.E03-10-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibar C, Cataldo VF, Vasquez-Doorman C, Olguin P, Glavic A. Drosophila p53-related protein kinase is required for PI3K/TOR pathway-dependent growth. Development. 2013;140(6):1282–1291. doi: 10.1242/dev.086918. [DOI] [PubMed] [Google Scholar]
- 22.Damon JR, Pincus D, Ploegh HL. tRNA thiolation links translation to stress responses in Saccharomyces cerevisiae. Mol Biol Cell. 2014;26(2):270–82. doi: 10.1091/mbc.E14-06-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rojas-Benitez D, Ibar C, Glavic A. The Drosophila EKC/KEOPS complex: roles in protein synthesis homeostasis and animal growth. Fly (Austin) 2013;7(3):168–172. doi: 10.4161/fly.25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helm M, Alfonzo Juan D. Posttranscriptional RNA modifications: playing metabolic games in a cell’s chemical legoland. Chem Biol. 2014;21(2):174–185. doi: 10.1016/j.chembiol.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlsborn T, Tukenmez H, Chen C, Byström AS. Familial dysautonomia (FD) patients have reduced levels of the modified wobble nucleoside mcm5s2U in tRNA. Biochem Biophys Res Commun. 2014;454(3):441–445. doi: 10.1016/j.bbrc.2014.10.116. [DOI] [PubMed] [Google Scholar]