Abstract
IMPORTANCE
Severely injured patients experiencing hemorrhagic shock often require massive transfusion. Earlier transfusion with higher blood product ratios (plasma, platelets, and red blood cells), defined as damage control resuscitation, has been associated with improved outcomes; however, there have been no large multicenter clinical trials.
OBJECTIVE
To determine the effectiveness and safety of transfusing patients with severe trauma and major bleeding using plasma, platelets, and red blood cells in a 1:1:1 ratio compared with a 1:1:2 ratio.
DESIGN, SETTING, AND PARTICIPANTS
Pragmatic, phase 3, multisite, randomized clinical trial of 680 severely injured patients who arrived at 1 of 12 level I trauma centers in North America directly from the scene and were predicted to require massive transfusion between August 2012 and December 2013.
INTERVENTIONS
Blood product ratios of 1:1:1 (338 patients) vs 1:1:2 (342 patients) during active resuscitation in addition to all local standard-of-care interventions (uncontrolled).
MAIN OUTCOMES AND MEASURES
Primary outcomes were 24-hour and 30-day all-cause mortality. Prespecified ancillary outcomes included time to hemostasis, blood product volumes transfused, complications, incidence of surgical procedures, and functional status.
RESULTS
No significant differences were detected in mortality at 24 hours (12.7% in 1:1:1 group vs 17.0% in 1:1:2 group; difference, −4.2% [95% CI, −9.6% to 1.1%]; P = .12) or at 30 days (22.4% vs 26.1%, respectively; difference, −3.7% [95% CI, −10.2% to 2.7%]; P = .26). Exsanguination, which was the predominant cause of death within the first 24 hours, was significantly decreased in the 1:1:1 group (9.2% vs 14.6% in 1:1:2 group; difference, −5.4% [95% CI, −10.4% to −0.5%]; P = .03). More patients in the 1:1:1 group achieved hemostasis than in the 1:1:2 group (86% vs 78%, respectively; P = .006). Despite the 1:1:1 group receiving more plasma (median of 7 U vs 5 U, P < .001) and platelets (12 U vs 6 U, P < .001) and similar amounts of red blood cells (9 U) over the first 24 hours, no differences between the 2 groups were found for the 23 prespecified complications, including acute respiratory distress syndrome, multiple organ failure, venous thromboembolism, sepsis, and transfusion-related complications.
CONCLUSIONS AND RELEVANCE
Among patients with severe trauma and major bleeding, early administration of plasma, platelets, and red blood cells in a 1:1:1 ratio compared with a 1:1:2 ratio did not result in significant differences in mortality at 24 hours or at 30 days. However, more patients in the 1:1:1 group achieved hemostasis and fewer experienced death due to exsanguination by 24 hours. Even though there was an increased use of plasma and platelets transfused in the 1:1:1 group, no other safety differences were identified between the 2 groups.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01545232
In the United States, injury is the leading cause of death among individuals between the ages of 1 and 44 years, it is the leading cause of years of life lost for those younger than 75 years, and it is the third leading cause of death overall.1 Deaths from injury have increased 23% during the last decade.2 Approximately 20% to 40% of trauma deaths occurring after hospital admission involve massive hemorrhage from truncal injury and are potentially preventable with rapid hemorrhage control and improved resuscitation techniques.3
Damage control resuscitation is defined as rapid hemorrhage control through early administration of blood products in a balanced ratio (1:1:1 for units of plasma to platelets to red blood cells [RBCs]; a ratio that is the closest approximation to reconstituted whole blood), prevention and immediate correction of coagulopathy, and minimization of crystalloid fluids.4 Damage control resuscitation was developed to treat intravascular volume deficits, the acute coagulopathy of trauma, preserve oxygen-carrying capacity, repair the endothelium, and prevent dilutional coagulopathy.4,5
Damage control resuscitation was codified as a US Department of Defense clinical practice guideline in 20046 and has become the standard of care for battlefield resuscitation that is now used in many civilian trauma centers. Damage control resuscitation principles have been associated with improved outcomes compared with more traditional transfusion practices.7–12 Conversely, other studies have reported beneficial outcomes across a wider range of blood product ratios or goal-directed approaches.13,14 However, concerns about the safety of exposing injured patients to large amounts of plasma-containing blood products were difficult to address in previous retrospective studies.
There are no large, multicenter, randomized clinical trials with survival as a primary end point that support optimal trauma resuscitation practices with approved blood products. As a result, there are multiple and often conflicting recommendations promulgated by various organizations.15–18 The Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study demonstrated that clinicians generally were transfusing patients with a blood product ratio of 1:1:1 or 1:1:2 and that early transfusion of plasma (within minutes of arrival to a trauma center) was associated with improved 6-hour survival after admission.10,19
The Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial was designed to address the effectiveness and safety of a 1:1:1 transfusion ratio compared with a 1:1:2 transfusion ratio in patients with trauma who were predicted to receive a massive transfusion.
Methods
Study Design and Intervention
A pragmatic, phase 3, multisite, randomized trial, the PROPPR study compared the effectiveness and safety of a 1:1:1 transfusion ratio of plasma, platelets, and RBCs to a 1:1:2 ratio.20 Patients were randomized within each site, and the intervention consisted of containers of blood products prepared by each site’s blood bank and delivered to the bedside within 10 minutes (DJ Novak et al and the PROPPR Study Group, unpublished data, 2015; Supplement 1). The initial container was sealed to blind the physicians to treatment assignment. The patient was declared randomized when the seal was broken. The blood products were transfused in a prespecified order designed to maintain the appropriate assigned ratio.
All containers for the 1:1:1 group included 6 U of plasma, 1 dose of platelets (a pool of 6 U on average), and 6 U of RBCs, which were transfused in the following order: platelets first, then alternating RBC and plasma units. The initial and all subsequent odd-numbered containers for the 1:1:2 group included 3 U of plasma, 0 doses of platelets, and 6 U of RBCs, which were transfused in the following order: alternating 2 U of RBCs and 1 U of plasma. The second and all subsequent even-numbered containers included 3 U of plasma, 1 dose of platelets (a pool of 6 U on average), and 6 U of RBCs, which were transfused in the following order: platelets first, then alternating 2 U of RBCs and 1 unit of plasma. Patients with multiple intravenous lines could receive blood products simultaneously, otherwise patients received products sequentially.
Transfusion of all study blood products was stopped when clinically indicated, irrespective of ratio or partial blood container use.20 Transfusion of study blood products ended in several ways: achievement of hemostasis, death, declaration of treatment futility, no need for further blood products after randomization, or protocol violations.
No other resuscitation, pharmacological, or clinical treatment was controlled by the trial protocol (Supplement 1). The study was approved by the US Food and Drug Administration (FDA) (Investigational New Drug No. 14929), Health Canada, the Department of Defense, and all site institutional review boards. In addition, the study was monitored by an external data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute and used exception from informed consent, including community consultation with delayed patient or legally authorized representative consent.21
Study Population
Patients included in the PROPPR trial were severely injured and met the local criteria for highest level trauma activation at 1 of 12 participating level I trauma centers in North America. These site-specific criteria, reviewed by the American College of Surgeons, are based on heart rate, blood pressure, respiratory rate, and mechanism of injury and are used clinically to ensure trauma teams are present before these critically injured patients arrive at the emergency department. The research personnel were notified along with the trauma teams. The goal was to rapidly enroll patients with severe hemorrhage who were nonmoribund, regardless of injury type.
To facilitate rapid identification of patients with severe bleeding, inclusion criteria included the patient having at least 1 U of any blood component transfused prior to hospital arrival or within 1 hour of admission and prediction by an Assessment of Blood Consumption score22 of 2 or greater or by physician judgment of the need for a massive transfusion (defined as =10 U of RBCs within 24 hours). The complete inclusion and exclusion criteria are listed in the Box.
Box. Inclusion and Exclusion Criteria for the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial.
Eligible Patients Met All of the Following
Highest trauma level activation
Estimated age of 15 years or older or weight of 50 kg or greater if age unknown
Received directly from the injury scene
Initiated transfusion of at least 1 U of blood component within the first hour of arrival or during prehospital transport
Predicted to receive a massive transfusion by exceeding the threshold score of either the Assessment of Blood Consumption score of 2 or greater or based on the attending trauma physician’s judgment
Patients Who Were Ineligible Met at Least 1 of the Following
Received a lifesaving intervention from an outside hospital or health care facility
Had devastating injuries and expected to die within 1 hour of admission (eg, lethal traumatic brain injury)
Directly admitted from a correctional facility
Required a thoracotomy prior to receiving randomized blood products in the emergency department
Younger than 15 years or weighed less than 50 kg if age unknown
Known pregnancy in the emergency department
Had burns covering greater than 20% total body surface area
Suspected inhalation injury
Received greater than 5 consecutive minutes of cardiopulmonary resuscitation (with chest compressions) prior to arriving at the hospital or within the emergency department
Known do-not-resuscitate order prior to randomization
Enrolled in a concurrent, ongoing, interventional, randomized clinical trial
Activated the opt-out process for the PROPPR trial (usually by wearing a bracelet given out at a community consent presentation)
More than 3 U of red blood cells given before randomization
Outcomes and Other Variables of Interest
The primary outcomes included absolute percentage group differences for 24-hour and 30-day mortality. These 2 outcome measures tested 2 separate questions regarding short-term effectiveness and long-term safety without adjustment for multiple comparisons per protocol.23 Each death was adjudicated by a clinician blinded to group assignment and external to the trial site and 1 or more causes of death were assigned.
Ancillary outcomes were prespecified to evaluate the effectiveness and safety of the transfusion ratios and included (1) time to hemostasis; (2) the number and type of blood products used from randomization until hemostasis was achieved; (3) the number and type of blood products used after hemostasis was achieved up to 24 hours postadmission; (4) 23 complications; (5) hospital-, ventilator-, and ICU-free days (within the first 30 days or hospital discharge, whichever occurred first); (6) incidence of major surgical procedures; and (7) functional status at hospital discharge or 30 days, whichever occurred first, as measured by discharge destination and Glasgow Outcome Scale-Extended.
Blood product ratios were calculated as 2 separate ratios: plasma to RBCs and platelets to RBCs. For example, a 1:1 ratio of plasma to RBCs is equivalent to 1.0 and represents equal total units of plasma and RBCs within the specified interval. A 1:2 ratio is equivalent to 0.5 and represents twice as many total RBC units as plasma units. Ratios for patients who received no RBCs within a specified interval cannot be calculated because the denominator is zero, and therefore are not included in the calculation of cumulative ratios of blood products in that interval.
Race and Hispanic ethnicity were collected by patient self-report or hospital staff determination and were included to identify disparities in treatment or outcome. The Injury Severity Score is an anatomic scoring system used for patients with multiple injuries, correlates with mortality, and has a range of 0 (uninjured) to 75 (usually unsurvivable injuries).24 The critical administration threshold represents the trauma subset at highest risk of hemorrhagic mortality25 and denotes patients receiving more than 3 U of RBCs within at least 1 hour during the first 24 hours after admission. The Assessment of Blood Consumption score has a range of 0 to 4 with scores of 2 or greater associated with the need for a massive transfusion.22
Anatomic hemostasis in the operating room was defined as an objective assessment by the surgeon indicating that bleeding within the surgical field was controlled and no further hemostatic interventions were anticipated. In the interventional radiology suite, anatomic hemostasis was defined as achieving resolution of contrast blush after embolization.
Sample Size
The initial sample size of 580 was planned to detect a clinically meaningful 10% difference in 24-hour mortality (11% vs 21%) and a 12% difference in 30-day mortality (23% vs 35%), which was supported by prior data.26,27 Sample size was increased to 680 by the data and safety monitoring board according to the trial’s adaptive design. With 680 patients and given the final observed mortality proportions in the 1:1:1 group, the PROPPR trial had 95% power to detect the prespecified 10% difference at 24 hours and 92% power to detect the prespecified 12% difference at 30 days, if such differences existed.
Statistical Analysis
The primary analysis separately compared 24-hour and 30-day mortality in the 2 transfusion ratio groups using a 2-sided Mantel-Haenszel test adjusting for site. For the 4 patients missing a primary outcome, a sensitivity analysis using all possible combinations (n = 16) of outcomes was performed and a range of intent-to-treat P values for the hypothetical Mantel-Haenszel tests are presented.28 The critical level for significance (P ≤ .044) was adjusted for 2 interim analyses, and all tests were conducted using 2-sided tests.29 In Cox analyses, the 4 patients missing a 30-day outcome were censored at the last known follow-up time.30 Lack of protocol compliance was measured by the per-patient percentage of blood products given out of order. A sensitivity analysis compared treatment groups excluding these patients.
All analyses were generated using SAS version 9.3 (SAS Institute Inc). Additional details regarding the study design and analysis were published previously.20
Results
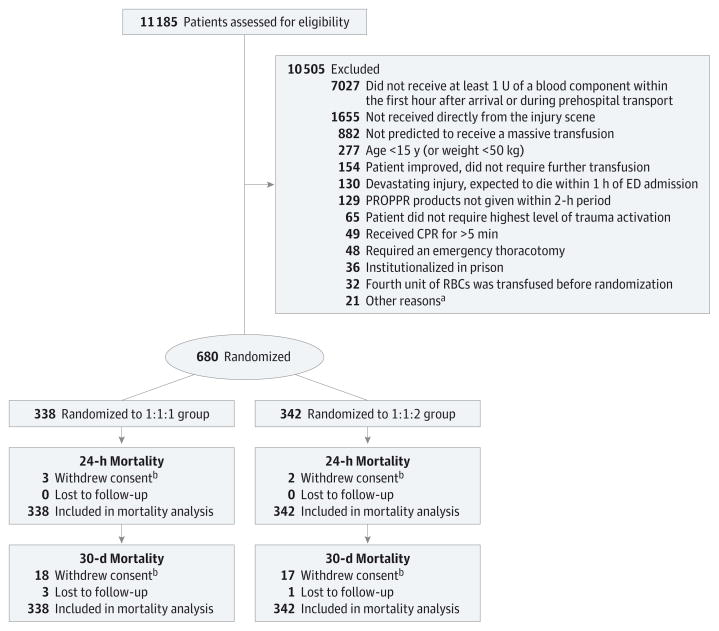
From August 3, 2012, to December 2, 2013, a total of 14 313 highest-level trauma activations occurred at the 12 enrolling sites, of which 78% were screened. A total of 680 patients were randomized (338 to the 1:1:1 group and 342 to the 1:1:2 group; Figure 1). Randomized blood products were transfused to 669 patients. No differences were detected between treatment groups in baseline characteristics (Table 1).
Figure 1. Flow of Patients in the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial.
CPR indicates cardiopulmonary resuscitation; ED, emergency department; RBC, red blood cell.
aIncluded patients with the following: 6 known pregnancies, 5 with physicians who refused to randomize, 4 with known do-not-resuscitate order prior to randomization, 3 with burns covering more than 20% of total body surface area, 1 with a documented inhalation injury, 1 who opted out upon arrival to the ED, 1 unknown reason.
bThe vital statistic data were obtained for patients who withdrew consent when available. Patients who withdrew consent at 24 hours are included in the count of those who withdrew at 30 days.
Table 1.
Patient Characteristics by Treatment Group
| 1:1:1 Group (n = 338) | 1:1:2 Group (n = 342) | |
|---|---|---|
| Age, median (IQR), ya | 34.5 (25 to 51) | 34 (24 to 50) |
| Male sex, No. (%) | 263 (77.8) | 283 (82.7) |
| Race, No. (%)b | ||
| White | 210 (62.1) | 224 (65.5) |
| Black | 94 (27.8) | 93 (27.2) |
| Other | 35 (10.4) | 25 (7.3) |
| Hispanic ethnicity, No. (%)c | 61 (18.0) | 59 (17.3) |
| Glasgow Coma Scale score, median (IQR) | 14 (3 to 15) | 14 (3 to 15) |
| Systolic blood pressure, No. of patients | 330 | 328 |
| Median (IQR), mm Hgd | 102 (81 to 126) | 102 (80 to 125) |
| No. (%) with ≤90 mm Hg | 127 (38.5) | 128 (39.0) |
| Diastolic blood pressure, No. of patients | 284 | 279 |
| Median (IQR), mm Hgd | 70 (53 to 90) | 68 (50 to 91) |
| Heart rate, No. of patients | 336 | 341 |
| Median (IQR), beats/mind | 115 (97 to 135) | 113 (93 to 130) |
| No. (%) with ≥120 beats/min | 148 (44.0) | 152 (44.6) |
| Respiratory rate, No. of patients | 308 | 313 |
| Median (IQR), breaths/min | 20 (17.5 to 26.0) | 20 (17 to 26) |
| Assessment of Blood Consumption score ≥2, No. (%)22,e | 215 (63.6) | 223 (65.2) |
| Mechanism of injury, No. (%) | ||
| Any blunt injury | 185 (54.7) | 173 (50.6) |
| Any penetrating injury | 157 (46.4) | 173 (50.6) |
| Time to randomization, median (IQR), min | 27.5 (17 to 47) | 25.5 (16 to 41) |
| Hemoglobin level, No. of patients | 327 | 325 |
| Median (IQR), g/dL | 11.7 (10.1 to 13.4) | 11.9 (10.1 to 13.2) |
| No. (%) with ≤11 g/dL | 121 (37.0) | 126 (38.8) |
| International normalized ratio, No. of patients | 218 | 218 |
| Median (IQR) | 1.3 (1.2 to 1.5) | 1.3 (1.2 to 1.5) |
| No. (%) with ratio >1.5 | 57 (26.1) | 59 (27.1) |
| Thromboelastography R time, No. of patients | 276 | 279 |
| Median (IQR), min | 3.8 (2.9 to 4.6) | 3.8 (2.8 to 4.7) |
| No. (%) with time >8 min | 12 (4.3) | 12 (4.3) |
| Platelet count, No. of patients | 317 | 317 |
| Median (IQR), in thousands | 213 (164 to 261) | 212 (164 to 264) |
| No. (%) with count <150 in thousands | 54 (17.0) | 60 (18.9) |
| Base excess, No. of patients | 318 | 301 |
| Median (IQR), mmol/L | −8 (−12.5 to −3.8) | −8.5 (−12.8 to −4.7) |
| No. (%) with score ≤−4 mmol/L | 238 (74.8) | 239 (79.4) |
| Injury Severity Score, median (IQR)f | 26.5 (17 to 41) | 26 (17 to 38) |
| Revised Trauma Score, No. of patientsg | 303 | 304 |
| Median (IQR) | 6.8 (4.1 to 7.8) | 6.4 (4.1 to 7.8) |
| Resuscitation indicators, No. (%) | ||
| Massive transfusionh | 153 (45.3) | 160 (46.8) |
| Critical administration thresholdi | 281 (83.1) | 314 (91.8) |
Abbreviations: IQR, interquartile range; RBC, red blood cell.
One patient was missing a verified age so it was imputed using the median of the interval for estimated age.
More than 1 race could be selected per patient, therefore percentages may exceed 100%. Other included American Indian/Alaskan Native/Aboriginal, Asian, Native Hawaiian/other Pacific Islander, other, and unknown.
Determined by either self-report from the patient or family or direct observation by medical staff.
Patients with blood pressure and heart rate that was not recorded, measured, detectable, or palpable were excluded from the median calculations and the Wilcoxon rank sum test.
The score range was 0 to 4. Patients with a score of 0 (n = 50) and 1 (n = 192) were enrolled in the trial as physician overrides, which was defined as a score of less than 2 and attending physician determination that a massive transfusion was needed.
The score range was 0 to 75. A score greater than 15 indicates major trauma.
The score range was 0 to 7.8. A higher score is associated with better survival probability.
Defined as 10 U or greater of RBCs received within first 24 hours. Includes observations made postrandomization.
Defined as 3 U or greater of RBCs received at least once per 1-hour interval during the first 24 1-hour periods. One patient in each treatment group did not receive any RBCs. Includes observations made postrandomization.
The majority of patients were male with similar ages in both groups. Patients in both groups were profoundly injured with a median Injury Severity Score of 26 and severely bleeding based on the critical administration threshold (87% positive based on this threshold overall). The initial hemoglobin level was 11.7 g/dL (37% had hemoglobin levels <11 g/dL) in the 1:1:1 group and 11.9 g/dL (38.8% had hemoglobin levels <11 g/dL) in the 1:1:2 group. Seventy-five percent of patients required an interventional radiology or operating room procedure within 2 hours of admission (data not shown).
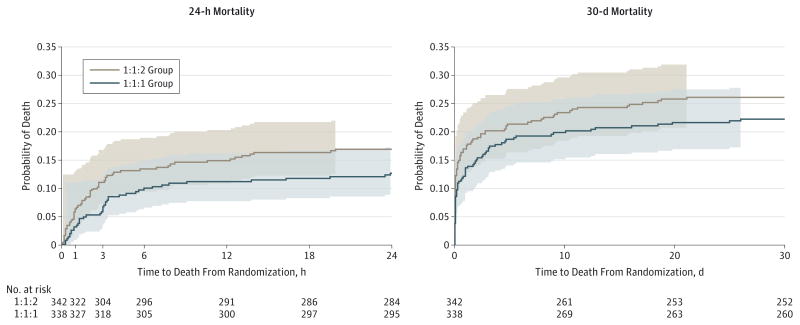
The primary trial outcomes of mortality at 24 hours and 30 days were obtained on 100% and 99.4% of patients, respectively. No significant differences in mortality were detected at 24 hours (12.7% in the 1:1:1 group vs 17.0% in the 1:1:2 group; difference, −4.2% [95% CI, −9.6% to 1.1%) or at 30 days (22.4% vs 26.1%, respectively; difference, −3.7% [95% CI, −10.2% to 2.7%) (Table 2).31 The range of intent-to-treat P values computed for all possible combinations of 30-day outcomes for the 4 patients with missing values did not change these results. The P values ranged from 0.21 to 0.36 (eTable 1 in Supplement 2). The Kaplan-Meier curves (Figure 2) show a separation in survival between the 2 treatment groups across the follow-up period, but the difference was not significant (unadjusted log-rank test, P = .21).
Table 2.
Trial Outcomes by Treatment Group
| 1:1:1 Group (n = 338) | 1:1:2 Group (n = 342) | Difference (95% CI), % | Adjusted RR (95% CI) | P Valuea | |
|---|---|---|---|---|---|
| 24-h Mortality, No. (%)b | 43 (12.7) | 58 (17.0) | −4.2 (−9.6 to 1.1) | 0.75 (0.52 to 1.08) | .12 |
|
| |||||
| 30-d Mortality, No. (%)b | 75 (22.4) | 89 (26.1) | −3.7 (−10.2 to 2.7) | 0.86 (0.65 to 1.12) | .26 |
|
| |||||
| Achieved hemostasis | |||||
|
| |||||
| No. (%) | 291 (86.1) | 267 (78.1) | .006 | ||
|
| |||||
| Anatomic, median (IQR), minc | 105 (64 to 179) | 100 (56 to 181) | .44 | ||
|
| |||||
| Hospital-free days, median (IQR)c,d | 1 (0 to 17) | 0 (0 to 16) | .83 | ||
|
| |||||
| Ventilator-free daysd | |||||
|
| |||||
| Total No. of patients | 337 | 340 | |||
|
| |||||
| Median (IQR)c | 8 (0 to 16) | 7 (0 to 14) | .14 | ||
|
| |||||
| ICU-free daysd | |||||
|
| |||||
| Total No. of patients | 337 | 340 | |||
|
| |||||
| Median (IQR)c | 5 (0 to 11) | 4 (0 to 10) | .10 | ||
|
| |||||
| Incidence of primary surgical procedure | 290 (85.8) | 284 (83.0) | 2.8 (−2.8 to 8.3) | ||
|
| |||||
| Disposition at 30 d, No. (%)e | |||||
|
| |||||
| Home | 118 (34.9) | 105 (30.7) | .37 | ||
|
| |||||
| Remained hospitalized | 82 (24.3) | 77 (22.5) | |||
|
| |||||
| Otherf | 59 (17.5) | 71 (20.8) | |||
|
| |||||
| Morgue | 75 (22.2) | 89 (26.0) | |||
|
| |||||
| Unknown | 4 (1.2) | 0 | |||
|
| |||||
| Glasgow Outcome Scale-Extended score | |||||
|
| |||||
| Total No. of patientsg | 30 | 28 | |||
|
| |||||
| Median (IQR)c | 4 (3 to 6) | 4.5 (3.5 to 7.0) | .11 | ||
Calculated using the Mantel-Haenszel test for binary outcomes measured from randomization, adjusting for site.
Breslow-Day test for homogeneity, : 24-hour P = .51, 30-day P = .65.
The van Elteren test31 was used to compare medians, adjusting for site.
Individuals who died within the first 24 hours from admission were assigned zero ICU-, ventilator-, and hospital-free days.
A generalized logit model was fit to test for treatment differences.
Includes long-term care facility, skilled nursing facility, rehabilitation facility, acute care hospital, assisted living, psychiatric facility, and jail.
Obtained only on discharged patients who had a head injury.
Figure 2. Kaplan-Meier Failure Curves for Mortality at 24 Hours and 30 Days.
The colored areas indicate 95% confidence bands, which were calculated using the Hall-Wellner method. The Hall-Wellner bands extend to the last event (death) in each group. For 24-hour mortality, the Cox proportional hazards regression model, adjusted for site as a random effect, produced a hazard ratio (HR) of 0.72 (95% CI, 0.49–1.07). There were no patients lost to follow-up during the first 24 hours from randomization. For 30-day mortality, the Cox proportional hazards regression model, adjusted for site as a random effect, produced an HR of 0.83 (95% CI, 0.61–1.12). Between 24 hours and 30 days, 4 patients were lost to follow-up and were censored when they withdrew consent or were last known to be alive (3 in the 1:1:1 group and 1 in the 1:1:2 group).
Sensitivity analyses excluding patients who received blood products given out of order yielded results similar to the main analysis. The mean percentages of intervention units given out of order per patient (protocol noncompliance) were significantly lower in the 1:1:1 group (4%; 95% CI, 3.2%–5.7%) vs the 1:1:2 group (7%; 95% CI, 6.1% to 8.5%) (P = .01).
Exsanguination, the predominant cause of death within the first 24 hours, was decreased in the 1:1:1 group (9.2%) vs the 1:1:2 group (14.6%) (difference, −5.4% [95% CI, −10.4% to −0.5%], P = .03); the median time to death due to exsanguination was 106 minutes (interquartile range [IQR], 54 to 198 minutes) and 96 minutes (IQR, 43 to 194 minutes), respectively. From 24 hours through 30 days, the numbers of additional all-cause deaths were similar (32 for the 1:1:1 group vs 31 for the 1:1:2 group). Over 30 days, deaths due to exsanguination occurred in 10.7% of patients in the 1:1:1 group vs 14.7% in the 1:1:2 group, whereas deaths due to traumatic brain injury were 8.1% vs 10.3%, respectively. Additional causes of death were infrequent and are shown in Table 3. More patients achieved anatomic hemostasis in the 1:1:1 group (86.1% vs 78.1% in the 1:1:2 group, P = .006) with a median time of 105 minutes (IQR, 64 to 179 minutes) vs 100 minutes (IQR, 56 to 181 minutes), respectively (P = .44) in those who achieved anatomic hemostasis (Table 2).
Table 3.
Adjudicated Cause of Death by Treatment Group and Period From Randomization
| First 24 Hours
|
30 Days
|
|||||
|---|---|---|---|---|---|---|
| No. (%)
|
Difference (95% CI),%a | No. (%)
|
Difference (95% CI), %a | |||
| 1:1:1 Group (n = 338) | 1:1:2 Group (n = 342) | 1:1:1 Group (n = 335) | 1:1:2 Group (n = 341) | |||
| Total No. of deaths | 43 | 58 | 75 | 89 | ||
|
| ||||||
| Cause of deathb | ||||||
|
| ||||||
| Exsanguination | 31 (9.2) | 50 (14.6) | −5.4 (−10.4 to −0.5) | 36 (10.7) | 50 (14.7) | −3.9 (−9.1 to 1.2) |
|
| ||||||
| Traumatic brain injury | 11 (3.3) | 12 (3.5) | −0.3 (−3.2 to 2.7) | 27 (8.1) | 35 (10.3) | −2.2 (−6.7 to 2.2) |
|
| ||||||
| Respiratory, pulmonary contusion, or tension pneumothorax | 3 (0.9) | 1 (0.3) | 0.6 (−0.9 to 2.4) | 5 (1.5) | 2 (0.6) | 0.9 (−0.8 to 3.0) |
|
| ||||||
| Sepsis | 0 | 0 | 0 (−1.1 to 1.1) | 1 (0.3) | 2 (0.6) | −0.3 (−1.9 to 1.2) |
|
| ||||||
| Multiple organ failure | 0 | 0 | 0 (−1.1 to 1.1) | 10 (3.0) | 8 (2.3) | 0.6 (−2.0 to 3.4) |
|
| ||||||
| Type of cardiovascular event | ||||||
|
| ||||||
| Stroke | 0 | 1 (0.3) | −0.3 (−1.7 to 0.9) | 2 (0.6) | 1 (0.3) | 0.3 (−1.1 to 1.9) |
|
| ||||||
| Myocardial infarction | 1 (0.3) | 1 (0.3) | 0 (−1.4 to 1.4) | 1 (0.3) | 2 (0.6) | −0.3 (−1.9 to 1.2) |
|
| ||||||
| Pulmonary embolism | 0 | 1 (0.3) | −0.3 (−1.7 to 0.9) | 0 | 1 (0.3) | −0.3 (−1.7 to 0.9) |
|
| ||||||
| Transfusion-related fatality | 0 | 0 | 0 (−1.1 to 1.1) | 1 (0.3) | 0 | 0.3 (−0.8 to 1.7) |
|
| ||||||
Calculated using exact unconditional methods based on the Farrington-Manning score statistic.
A patient may have had more than 1 cause of death.
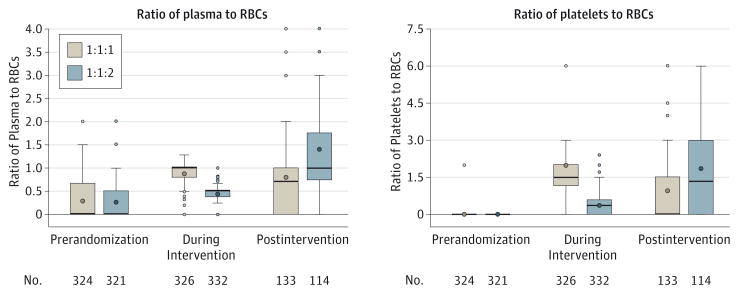
Cumulative transfusion ratios and the distribution of blood product amounts (prerandomization, during the intervention, and postintervention) are shown in Figure 3 and Figure 4. During the intervention, patients received median ratios of plasma to RBCs of 1.0 in the 1:1:1 group and 0.5 in the 1:1:2 group. The median ratios of platelets to RBCs during the intervention were 1.5 for the 1:1:1 group and 0.4 for the 1:1:2 group. Higher cumulative plasma and platelet ratios in the 1:1:2 group vs the 1:1:1 group were seen during the postintervention period.
Figure 3. Distribution of Cumulative Blood Product Ratios Within Period up to 24 Hours After Admission.
Prerandomization blood products include those given prior to hospital arrival. Patients who received no red blood cells (RBCs) within an interval were excluded because RBCs are in the ratio denominator. The lower and upper edges of the boxes are the 25th and 75th percentiles, the whiskers extend to ±1.5 × the interquartile range, and the points outside are the outliers. The thick line inside the box represents the median and the circle is the mean.
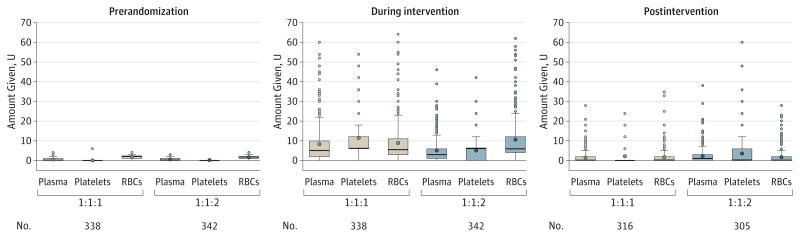
Figure 4. Distribution of Blood Product Amounts Within Period up to 24 Hours After Admission.
Prerandomization blood products include those given prior to hospital arrival. The lower and upper edges of the boxes are the 25th and 75th percentiles, the whiskers extend to ±1.5 × the interquartile range, and the points outside are the outliers. The thick line inside the box represents the median and the circle is the mean. Five or 6 U pools of whole blood–derived platelets were considered equivalent to 1 U of apheresis platelets (eg, an adult dose of platelets).
Similar amounts of total blood products (median of 2 U) were delivered prerandomization to both groups (eFigure in Supplement 2). The median total blood product amounts transfused were 16 U in the 1:1:1 group and 15 U in the 1:1:2 group during the intervention period. Patients in the 1:1:1 group received fewer blood products during the postintervention period than the 1:1:2 group (median of 1 U vs 2 U, respectively). The median total for blood products transfused up to 24 hours after admission was 25.5 U in the 1:1:1 group and 19 U in the 1:1:2 group. Total plasma (median of 7 U in the 1:1:1 group vs 5 U in the 1:1:2 group, P < .001) and platelets (12 U vs 6 U, respectively, P < .001) transfused within the first 24 hours were higher in the 1:1:1 group, but similar for RBCs (9 U) (eTable 2 in Supplement 2). Use of tranexamic acid and other procoagulants was similar.
Differences were not detected in any of the 23 complications at 30 days (Table 4), including acute respiratory distress syndrome, multiple organ failure, venous thromboembolism, sepsis, and transfusion-related complications. The overall rate of complications was high (89% of patients). One patient in the 1:1:1 group died from transfusion-associated circulatory overload. Significant differences between groups in the other ancillary outcomes focusing on safety were not detected and are shown in Table 2.
Table 4.
Incidence of Prespecified Complications by Treatment Group
| 1:1:1 Group (n = 338)
|
1:1:2 Group (n = 342)
|
Difference Between Groups in Percentage of Patients With Event, % (95% CI)c | |||
|---|---|---|---|---|---|
| Total No. of Eventsa | No. (%) of Patientsb | Total No. of Eventsa | No. (%) of Patientsb | ||
| Systemic inflammatory response syndrome | 265 | 231 (68.3) | 239 | 216 (63.2) | 5.2 (−2.1 to 12.3) |
|
| |||||
| Sepsis | 110 | 99 (29.3) | 102 | 91 (26.6) | 2.7 (−4.2 to 9.5) |
|
| |||||
| Infection (urinary tract infection, wound, line, other) | 155 | 98 (29.0) | 146 | 106 (31.0) | −2.0 (−8.9 to 5.0) |
|
| |||||
| Death | 75 | 75 (22.2) | 89 | 89 (26.0) | −3.8 (−10.3 to 2.7) |
|
| |||||
| Acute kidney injury | 87 | 74 (21.9) | 93 | 85 (24.9) | −3.0 (−9.4 to 3.5) |
|
| |||||
| Ventilator-associated pneumonia | 70 | 62 (18.3) | 65 | 58 (17.0) | 1.4 (−4.4 to 7.2) |
|
| |||||
| Transfusion-related metabolic complication (hypocalcemia or hyperkalemia) | 53 | 53 (15.7) | 60 | 59 (17.3) | −1.6 (−7.2 to 4.1) |
|
| |||||
| Acute lung injury | 56 | 47 (13.9) | 66 | 57 (16.7) | −2.8 (−8.3 to 2.7) |
|
| |||||
| Acute respiratory distress syndrome | 55 | 46 (13.6) | 57 | 48 (14.0) | −0.4 (−5.7 to 4.9) |
|
| |||||
| Deep vein thrombosis | 28 | 25 (7.4) | 24 | 24 (7.0) | 0.4 (−3.6 to 4.4) |
|
| |||||
| Abdominal complication | 29 | 24 (7.1) | 23 | 22 (6.4) | 0.7 (−3.3 to 4.6) |
|
| |||||
| Cardiac arrest | 25 | 23 (6.8) | 30 | 27 (7.9) | −1.1 (−5.2 to 3.0) |
|
| |||||
| Multiple organ failure | 24 | 20 (5.9) | 18 | 15 (4.4) | 1.5 (−1.9 to 5.1) |
|
| |||||
| Symptomatic pulmonary embolism | 14 | 14 (4.1) | 13 | 13 (3.8) | 0.3 (−2.8 to 3.5) |
|
| |||||
| Additional bleeding after hemostasis requiring interventional radiology or operating room procedure | 13 | 13 (3.8) | 18 | 16 (4.7) | −0.8 (−4.1 to 2.4) |
|
| |||||
| Asymptomatic pulmonary embolism | 11 | 11 (3.3) | 11 | 11 (3.2) | 0 (−2.8 to 2.9) |
|
| |||||
| Stroke | 9 | 8 (2.4) | 11 | 11 (3.2) | −0.8 (−3.6 to 1.8) |
|
| |||||
| Abdominal compartment syndrome | 3 | 3 (0.9) | 3 | 3 (0.9) | 0 (−1.8 to 1.8) |
|
| |||||
| Delayed serological transfusion reaction | 2 | 2 (0.6) | 0 | 0 | 0.6 (−0.5 to 2.1) |
|
| |||||
| Transfusion-related allergic reactions | 2 | 2 (0.6) | 1 | 1 (0.3) | 0.3 (−1.1 to 1.9) |
|
| |||||
| Hypernatremia (associated with hypertonic saline) | 1 | 1 (0.3) | 4 | 4 (1.2) | −0.9 (−2.7 to 0.6) |
|
| |||||
| Febrile nonhemolytic transfusion reaction | 1 | 1 (0.3) | 1 | 1 (0.3) | 0 (−1.4 to 1.4) |
|
| |||||
| Transfusion-associated circulatory overload | 1 | 1 (0.3) | 0 | 0 | 0.3 (−0.8 to 1.7) |
|
| |||||
| Myocardial infarction | 0 | 0 | 2 | 2 (0.6) | −0.6 (−2.1 to 0.6) |
|
| |||||
| Any prespecified complications | 1089 | 297 (87.9) | 1076 | 310 (90.6) | −2.8 (−7.6 to 1.9) |
|
| |||||
A patient may have had multiple complications of the same type.
Percentages may add to more than 100% because a patient may have had more than 1 complication.
Calculated using exact unconditional methods based on the Farrington-Manning score statistic.
Discussion
Transfusion for patients with severe trauma and major bleeding has been predominantly guided by tradition rather than evidence from large, multicenter randomized trials. Over the last decade, transfusion therapy has undergone a significant change with many patients receiving less crystalloid and early, more balanced transfusion ratios attempting to reconstitute whole blood.4–12,27,32–41 This change has largely been associated with decreased transfusion amounts, fewer inflammatory complications, and improved survival.4–12,27,32–41
To our knowledge, the PROPPR trial was the first multi-center randomized trial using approved blood products to compare 2 transfusion ratios with mortality as the primary end point. Among the 680 patients predicted to receive a massive transfusion and transfused with a 1:1:1 or 1:1:2 ratio, no significant differences in overall mortality at 24 hours or 30 days were detected. However, more patients achieved hemostasis in the 1:1:1 group, fewer patients died of exsanguination, and this transfusion ratio appears to be safe. Results from the PROMMTT study showed that earlier use of higher amounts of plasma and platelets (albeit without consistent ratios) was associated with improved survival during the first 6 hours after admission.10,19 Data from the PROPPR trial evaluated the effect of early transfusion of different but consistent ratios in patients predicted to receive a massive transfusion. Taken together, these data support early (within minutes of hospital arrival) use of a 1:1:1 transfusion ratio in patients with rapid bleeding.
Despite significant concerns that the 1:1:1 group would experience higher rates of multiple inflammatory-mediated complications such as acute respiratory distress syndrome, multiple organ failure, infection, venous thromboembolism, and sepsis,13,14,42–45 no differences were detected between the 2 treatment groups. Furthermore, the rates of multiple organ failure (5%) and acute respiratory distress syndrome (14%) were lower than in recent studies in similarly injured patient populations,46,47 which may be attributable to delivering blood to the bedside earlier (median of 8 minutes)20 and limited crystalloid exposure (median, 6.3–6.6 L) during the first 24 hours of care. In this trial, the early availability of blood products administered within minutes of arrival using a transfusion ratio of 1:1:1 was associated with more patients achieving hemostasis and decreased hemorrhage-related deaths over the first 24 hours with no differences in complications. Therefore, patient safety was not compromised over 30 days.
Transfusing patients based on an empirical ratio rather than guided solely by laboratory data (goal-directed) is considered controversial by some researchers.44,45,48 This trial was not designed to study this question. However, after the controlled, ratio-driven intervention was completed, clinicians treated patients based on local laboratory-guided standard-of-care practice.49 It appears that laboratory-directed catching up occurred in the 1:1:2 group with plasma and platelets approaching a cumulative ratio of 1:1:1. Other studies have shown similar results with laboratory-directed resuscitation.11 This catching up after the completion of randomized blood product transfusion may have decreased the ability to detect differences in mortality at 24 hours and 30 days or in the prespecified ancillary outcomes.
The concepts of damage control resuscitation and data from the PROMMTT study formed the biological basis of the PROPPR trial, ie, both early initiation (within minutes of arrival) and increased ratios of plasma and platelets would decrease death from hemorrhage by improving hemostasis.4–12,27,32–41 Recent trauma resuscitation studies have demonstrated that most early deaths due to hemorrhage occur within 2 to 3 hours.3,10,27,50,51 The PROMMTT study demonstrated a median time to hemorrhagic death from admission of 2.6 hours,10 and in the PROPPR trial, the median time was 2.3 hours. In recognition of the known physiology of patients with major bleeding, the FDA recently recommended moving the end point of hemostasis in a pivotal phase 3 prothrombin complex concentrate trial to within 4 hours of the intervention.52 These data support recent recommendations by the FDA to include a 3-hour end point for intervention studies focusing on traumatic hemorrhage.53
In the current study, the FDA only allowed 2 separate primary end points (24 hours and 30 days) in recognition of the assumed time frame of death from hemorrhage after injury.3,10,54 However, most outcomes relevant to hemorrhage control occurred early (within the initial 2–3 hours after randomization). Thereafter, the number of patients who died was similar between groups, explaining the diminished effects at 24 hours and 30 days. This pattern of traumatic death is consistent with previous randomized resuscitation studies.51,55,56
This trial had a number of strengths. The trial addressed most of the limitations found in previous randomized trauma resuscitation trials, including lack of blinded treatment assignment, enrollment after bleeding slowed, survival and selection biases, and small sample size.48,55–61 The trial was performed under exception from informed consent so that patients with severe bleeding could be enrolled rapidly and required that all blood products be immediately available for infusion within 10 minutes of calling the blood bank (Supplement 1). The selection criteria used in this study resulted in the rapid enrollment of patients who were severely bleeding, critically injured, in shock, and transfused with a median greater than 19 U of blood products. Separation of the ratio groups was maintained during the intervention period.
Another strength of the trial was the high degree of compliance with treatment protocols while simultaneously caring for patients with severe injuries. Follow-up at 24 hours was complete in both intervention groups, and only 4 patients were lost to follow-up at 30 days. Additionally, we blinded clinicians to treatment assignment until infusion of randomized products and used direct observation for accurate data collection of blood product delivery.
Limitations include power to detect differences smaller than the effect size we considered to be both clinically meaningful and affordable to study when we designed the trial. The PROPPR trial had 95% power to detect the prespecified 10% difference at 24 hours and 92% power to detect the prespecified 12% difference at 30 days, if such differences existed. As in many studies, observed mortality in the comparison group (1:1:2) was lower than expected, whereas in the 1:1:1 group, observed mortality was similar to what was projected. A total sample size of 2968 would have been required to detect the observed difference of 4.2% given the observed 24-hour mortality of 12.7% in the 1:1:1 group with 90% power. A further limitation is the inability to independently examine the effects of plasma and platelets on outcomes. To enroll patients with massive bleeding, the protocol required transfusion of at least 1 U of any blood product and no more than 3 U of RBCs prior to randomization, resulting in an inability to use randomized blood products starting with the first transfusion.
Even though the study was blinded until the opening of the containers, another limitation was that clinicians could not be blinded after the containers were opened without altering patient care. This trial was also limited by an inability to completely exclude patients with an unsurvivable brain injury; 23% of deaths at 24 hours and 38% of all deaths at 30 days were associated with traumatic brain injury. Last, the issue of competing risks of death from hemorrhage and traumatic brain injury in trauma studies that require rapid enrollment before definitive diagnosis of all major injuries is well-known and will continue to be an issue in future trauma studies unless novel regulatory, study design, or technological solutions are developed to solve this issue.3,54
Given the lower percentage of deaths from exsanguination and our failure to find differences in safety, clinicians should consider using a 1:1:1 transfusion protocol, starting with the initial units transfused while patients are actively bleeding, and then transitioning to laboratory-guided treatment once hemorrhage control is achieved. Future studies of hemorrhage control products, devices, and interventions should concentrate on the physiologically relevant period of active bleeding after injury and use acute complications and later deaths (24 hours and 30 days) as safety end points.
Conclusions
Among patients with severe trauma and major bleeding, early administration of plasma, platelets, and RBCs in a 1:1:1 ratio compared with a 1:1:2 ratio did not result in significant differences in mortality at 24 hours or at 30 days. However, more patients in the 1:1:1 group achieved hemostasis and fewer experienced death due to exsanguination by 24 hours. Even though there was an increased use of plasma and platelets transfused in the 1:1:1 group, no other safety differences were identified between the 2 groups.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported with grant U01HL077863 from the US National Heart, Lung, and Blood Institute and funding from the US Department of Defense, the Defence Research and Development Canada in partnership with the Canadian Institutes of Health Research-Institute of Circulatory and Respiratory Health (grant CRR-120612).
Group Information
The PROPPR Study Group: Clinical Coordinating Center, University of Texas Health Science Center, Houston: John B. Holcomb, MD, Charles E. Wade, PhD, Deborah J. del Junco, PhD, Erin E. Fox, PhD, Nena Matijevic, PhD (laboratory committee co-chair), Jeanette M. Podbielski, RN, Angela M. Beeler, BS. Data Coordinating Center, University of Texas Health Science Center, Houston: Barbara C. Tilley, PhD, Sarah Baraniuk, PhD, Stacia M. DeSantis, PhD, Hongjian Zhu, PhD, Joshua Nixon, MS, Roann Seay, MS, Savitri N. Appana, MS, Hui Yang, MS, Michael O. Gonzalez, MS. Core Laboratory, University of Texas Health Science Center, Houston: Lisa Baer, MS, Yao-Wei Willa Wang, MD, Brittany S. Hula, MS, Elena Espino, BS, An Nguyen, BS, Nicholas Pawelczyk, BS, Kisha D. Arora-Nutall, BS, Rishika Sharma, MD, Jessica C. Cardenas, PhD, Elaheh Rahbar, PhD, Tyrone Burnett Jr, BS, David Clark, BS. Resuscitation Outcomes Consortium, University of Washington: Gerald van Belle, PhD, Susanne May, PhD, Brian Leroux, PhD, David Hoyt, MD, Judy Powell, BSN, RN, Kellie Sheehan, BSN. Systems Biology Committee, University of California, Berkeley: Alan Hubbard, PhD (co-chair), Adam P. Arkin, PhD. Transfusion Committee: John R. Hess, MD, MPH (co-chair, University of Washington), Jeannie L. Callum, MD (co-chair, Sunnybrook Health Sciences Centre). Anesthesiology Committee: Jean-Francois Pittet, MD (chair, University of Alabama, Birmingham). Emergency Medicine Committee: Christopher N. Miller, MD (chair, University of Cincinnati). PROPPR Clinical Sites (listed in order of number of patients enrolled): University of Texas Health Science Center, Houston: Bryan A. Cotton, MD, MPH, Laura Vincent, BSN, RN, CCRP, Timothy Welch, Tiffany Poole, DC, Evan G. Pivalizza, MD, Sam D. Gumbert, MD, Yu Bai, MD, PhD, James J. McCarthy, MD, Amy Noland, MD, Rhonda Hobbs, MT(ASCP)SBB. University of Washington: Eileen M. Bulger, MD, Patricia Klotz, RN, Lindsay Cattin, BA, Keir J. Warner, BS, Angela Wilson, BA, David Boman, BA, Nathan White, MD, MS, Andreas Grabinsky, MD, Jennifer A. Daniel-Johnson, MBBS. University of California, San Francisco: Mitchell Jay Cohen, MD (systems biology and laboratory committee co-chair), Rachael A. Callcut, MD, MSPH, Mary Nelson, RN, MPA, Brittney Redick, BA, Amanda Conroy, BA, Marc P. Steurer, MD, DESA, Preston C. Maxim, MD, Eberhard Fiebig, MD, Joanne Moore, Eireen Mallari, MT. University of Cincinnati: Peter Muskat, MD, Jay A. Johannigman, MD, Bryce R. H. Robinson, MD, Richard D. Branson, MSc, RRT, Dina Gomaa, BS, RRT, Christopher Barczak, BS, MT (ASCP), Suzanne Bennett, MD, Patricia M. Carey, MD, Helen Hancock, BS, MT(ASCP), Carolina Rodriguez, BA. University of Southern California: Kenji Inaba, MD, Jay G. Zhu, MD, Monica D. Wong, MS, Michael Menchine, MD, MPH, Kelly Katzberg, MD, FACEP, Sean O. Henderson, MD, Rodney McKeever, MD, Ira A. Shulman, MD, Janice M. Nelson, MD, Christopher W. Tuma, BA, MT(ASCP), SBB, Cheryl Y. Matsushita, BS, MT(ASCP). Shock, Trauma and Anesthesiology Research-Organized Research Center, R. Adams Cowley Shock Trauma Center, University of Maryland Medical Center: Thomas M. Scalea, MD, Deborah M. Stein, MD, MPH, Cynthia K. Shaffer, MS, MBA, Christine Wade, BA, Anthony V. Herrera, MS, Seeta Kallam, MBBS, Sarah E. Wade, BS, Samuel M. Galvagno Jr, DO, PhD, Magali J. Fontaine, MD, PhD, Janice M. Hunt, BS, MT(ASCP) SBB, Rhonda K. Cooke, MD. University of Tennessee Health Science Center, Memphis: Timothy C. Fabian, MD, Jordan A. Weinberg, MD, Martin A. Croce, MD, Suzanne Wilson, RN, Stephanie Panzer-Baggett, RN, Lynda Waddle-Smith, BSN, Sherri Flax, MD. Medical College of Wisconsin: Karen J. Brasel, MD, MPH, Pamela Walsh, AS, CCRC, David Milia, MD, Allia Nelson, BS, BA, Olga Kaslow, MD, PhD, Tom P. Aufderheide, MD, MS, Jerome L. Gottschall, MD, Erica Carpenter, MLS(ASCP). University of Arizona: Terence O’Keeffe, MBChB, MSPH, Laurel L. Rokowski, RN, BSN, MKT, Kurt R. Denninghoff, MD, Daniel T. Redford, MD, Deborah J. Novak, MD, Susan Knoll, MS, MT(ASCP)SBB. University of Alabama, Birmingham: Jeffrey D. Kerby, MD, PhD, Patrick L. Bosarge, MD, Albert T. Pierce, MD, Carolyn R. Williams, RN, BSN, BSME, Shannon W. Stephens, EMTP, Henry E. Wang, MD, MS, Marisa B. Marques, MD. Oregon Health & Science University: Martin A. Schreiber, MD, Jennifer M. Watters, MD, Samantha J. Underwood, MS, Tahnee Groat, MPH, Craig Newgard, MD, MPH, Matthias Merkel, MD, PhD, Richard M. Scanlan, MD, Beth Miller, MT(ASCP)SBB. Sunnybrook Health Science Center: Sandro Rizoli, MD, PhD, Homer Tien, MD, Barto Nascimento, MD, MSc, CTBS, Sandy Trpcic, Skeeta Sobrian-Couroux, RN, CCRP, BHA, Marciano Reis, Adic Pérez, MD, Susan E. Belo, MD, PhD, Lisa Merkley, BA, MLT, CBTS, Connie Colavecchia, BSc, MLT.
Footnotes
Disclaimer: The content is the sole responsibility of the authors and should not be construed as official or as reflecting the views of any of the sponsors.
Additional Information: We dedicate this work to the US Soldiers, Sailors, Airmen, and Marines who put their lives on the line every day. We hope this effort will help improve the care of seriously injured patients, both military and civilian.
Role of the Funder/Sponsor: The US National Heart, Lung, and Blood Institute (NHLBI) and the US Department of Defense had a role in the study design but had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. However, Dr Pearson is employed by the NHLBI and she did participate in the review and approval of the manuscript.
Author Contributions: Drs Tilley and Baraniuk had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Holcomb, Tilley, Baraniuk, Wade, del Junco, Bulger, Cohen, Cotton, Kerby, Muskat, Rizoli, Robinson, Scalea, Schreiber, Stein, Callum, Hess, Miller, Pittet, Hoyt, Pearson, Leroux, van Belle.
Acquisition, analysis, or interpretation of data: Tilley, Baraniuk, Fox, Wade, Podbielski, del Junco, Brasel, Bulger, Callcut, Cohen, Cotton, Fabian, Inaba, Kerby, Muskat, O’Keeffe, Rizoli, Robinson, Scalea, Schreiber, Stein, Weinberg, Callum, Hess, Matijevic, Miller, Pittet, Hoyt, Leroux, van Belle.
Drafting of the manuscript: Holcomb, Tilley, Baraniuk, Fox, Wade, Podbielski, Callcut, Cohen, Cotton, Robinson, Stein, Hess, Pearson, van Belle.
Critical revision of the manuscript for important intellectual content: Tilley, Baraniuk, Fox, Wade, del Junco, Brasel, Bulger, Callcut, Cohen, Cotton, Fabian, Inaba, Kerby, Muskat, O’Keeffe, Rizoli, Robinson, Scalea, Schreiber, Stein, Weinberg, Callum, Hess, Matijevic, Miller, Pittet, Hoyt, Pearson, Leroux, van Belle.
Statistical analysis: Tilley, Baraniuk, Fox, Wade, Fabian, van Belle.
Obtained funding: Holcomb, Wade, Cotton, Schreiber, van Belle.
Administrative, technical, or material support: Tilley, Wade, Podbielski, del Junco, Bulger, Callcut, Cohen, Cotton, Kerby, Muskat, O’Keeffe, Rizoli, Robinson, Scalea, Stein, Weinberg, Callum, Hess, Matijevic, Miller, Pittet, Hoyt, Pearson, Leroux,
Study supervision: Holcomb, Tilley, Wade, Bulger, Callcut, Muskat, Rizoli, Robinson, Schreiber, Weinberg, Callum, Hess, Matijevic, Pittet, Hoyt, van Belle.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Rizoli reported receiving grant funding from TEM International and CSL Behring. Dr Stein reported serving as an advisor for Decisio Health for which she receives travel reimbursement. No other disclosures were reported.
Additional Contributions: We thank the members of the data and safety monitoring board (Lance Becker, Charles Cairns, Ralph D’Agostino, Karl Jern, Nigel Key, Laurence McCullough, Jeremy Perkins, Herbert Wiedemann, Janet Wittes, and Jay Mason) and the external advisory committee (Kenneth G. Mann, Kathleen Brummel, Beth Hartwell, Charles Esmon, Morris Blajchman, Andrew P. Cap, Andrei Kindzelski, and Anthony E. Pusateri) for their time and effort. We also thank the Resuscitation Outcomes Consortium protocol review committee for their important contributions as well as COL Dallas Hack and COL Robert Vandre for their extraordinary commitment and unwavering support for this trial. We could not have successfully completed this study without the help of hundreds of unnamed clinical personnel and we thank them for their sustained efforts.
References
- 1.US Centers for Disease Control and Preventionl. [Accessed December 21, 2014];Injury prevention and control: data and statistics. 2012 http://webappa.cdc.gov/cgi-bin/broker.exe.
- 2.Rhee P, Joseph B, Pandit V, et al. Increasing trauma deaths in the United States. Ann Surg. 2014;260(1):13–21. doi: 10.1097/SLA.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 3.Tisherman SA, Schmicker RH, Brasel KJ, et al. Detailed description of all deaths in both the Shock and Traumatic Brain Injury Hypertonic Saline Trials of the Resuscitation Outcomes Consortium [published online July 28, 2014] Ann Surg. doi: 10.1097/SLA.0b013e3181df0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62(2):307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 5.Holcomb JB, Pati S. Optimal trauma resuscitation with plasma as the primary resuscitative fluid: the surgeon’s perspective. Hematology Am Soc Hematol Educ Program. 2013;2013:656–659. doi: 10.1182/asheducation-2013.1.656. [DOI] [PubMed] [Google Scholar]
- 6.US Army Institute of Surgical Research. [Accessed December 21, 2014];Joint Theater Trauma System Clinical Practice Guideline: damage control resuscitation at level IIb and III treatment facilities. http://www.usaisr.amedd.army.mil/assets/cpgs/Damage%20Control%20Resuscitation%20-%201%20Feb%202013.pdf.
- 7.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 8.Shaz BH, Dente CJ, Nicholas J, et al. Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion. 2010;50 (2):493–500. doi: 10.1111/j.1537-2995.2009.02414.x. [DOI] [PubMed] [Google Scholar]
- 9.Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254(4):598–605. doi: 10.1097/SLA.0b013e318230089e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holcomb JB, del Junco DJ, Fox EE, et al. PROMMTT Study Group. The Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–136. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson PI, Sørensen AM, Larsen CF, et al. Low hemorrhage-related mortality in trauma patients in a level I trauma center employing transfusion packages and early thromboelastography-directed hemostatic resuscitation with plasma and platelets. Transfusion. 2013;53(12):3088–3099. doi: 10.1111/trf.12214. [DOI] [PubMed] [Google Scholar]
- 12.Langan NR, Eckert M, Martin MJ. Changing patterns of in-hospital deaths following implementation of damage control resuscitation practices in US forward military treatment facilities. JAMA Surg. 2014;149(9):904–912. doi: 10.1001/jamasurg.2014.940. [DOI] [PubMed] [Google Scholar]
- 13.Scalea TM, Bochicchio KM, Lumpkins K, et al. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg. 2008;248(4):578–584. doi: 10.1097/SLA.0b013e31818990ed. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JL, Moore EE, Kashuk JL, et al. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch Surg. 2010;145(10):973–977. doi: 10.1001/archsurg.2010.216. [DOI] [PubMed] [Google Scholar]
- 15.American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105(1):198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 16.Dzik WH, Blajchman MA, Fergusson D, et al. Clinical review: Canadian National Advisory Committee on Blood and Blood Products—massive transfusion consensus conference 2011: report of the panel. Crit Care. 2011;15(6):242. doi: 10.1186/cc10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Society of Anesthesiologists. [Accessed September 1, 2014];Standards, guidelines, statements and other documents. https://www.asahq.org/For-Members/Standards-Guidelines-and-Statements.aspx.
- 18.Spahn DR, Bouillon B, Cerny V, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17(2):R76. doi: 10.1186/cc12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.del Junco DJ, Holcomb JB, Fox EE, et al. PROMMTT Study Group. Resuscitate early with plasma and platelets or balance blood products gradually: findings from the PROMMTT study. J Trauma Acute Care Surg. 2013;75(1 suppl 1):S24–S30. doi: 10.1097/TA.0b013e31828fa3b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baraniuk S, Tilley BC, del Junco DJ, et al. PROPPR Study Group. Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial: Design, rationale and implementation. Injury. 2014;45(9):1287–1295. doi: 10.1016/j.injury.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services; US Food and Drug Administration. [Accessed September 1, 2014];Guidance for institutional review boards, clinical investigators, and sponsors: exception from informed consent requirements for emergency research. 2013 http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM249673.pdf.
- 22.Nunez TC, Voskresensky IV, Dossett LA, Shinall R, Dutton WD, Cotton BA. Early prediction of massive transfusion in trauma: simple as ABC (Assessment of Blood Consumption)? J Trauma. 2009;66(2):346–352. doi: 10.1097/TA.0b013e3181961c35. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien PC. The appropriateness of analysis of variance and multiple-comparison procedures. Biometrics. 1983;39(3):787–794. [PubMed] [Google Scholar]
- 24.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 25.Savage SA, Zarzaur BL, Croce MA, Fabian TC. Redefining massive transfusion when every second counts. J Trauma Acute Care Surg. 2013;74(2):396–400. doi: 10.1097/TA.0b013e31827a3639. [DOI] [PubMed] [Google Scholar]
- 26.Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med. 1990;9(12):1447–1454. doi: 10.1002/sim.4780091208. [DOI] [PubMed] [Google Scholar]
- 27.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 28.Hollis S. A graphical sensitivity analysis for clinical trials with non-ignorable missing binary outcome. Stat Med. 2002;21(24):3823–3834. doi: 10.1002/sim.1276. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–556. [PubMed] [Google Scholar]
- 30.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. 2. New York, NY: Springer; 2003. [Google Scholar]
- 31.van Elteren PH. On the combination of independent two-sample tests of Wilcoxon. Bull Int Stat Inst. 1960;37:351–361. [Google Scholar]
- 32.Ho AM, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation? Am J Surg. 2005;190(3):479–484. doi: 10.1016/j.amjsurg.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 33.Huber-Wagner S, Qvick M, Mussack T, et al. Working Group on Polytrauma of German Trauma Society (DGU) Massive blood transfusion and outcome in 1062 polytrauma patients: a prospective study based on the Trauma Registry of the German Trauma Society. Vox Sang. 2007;92 (1):69–78. doi: 10.1111/j.1423-0410.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- 34.Duchesne JC, Hunt JP, Wahl G, et al. Review of current blood transfusion strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma. 2008;65(2):272–276. doi: 10.1097/TA.0b013e31817e5166. [DOI] [PubMed] [Google Scholar]
- 35.Sperry JL, Ochoa JB, Gunn SR, et al. Inflammation and the Host Response to Injury Investigators. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65(5):986–993. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 36.Johansson PI, Stensballe J. Effect of haemostatic control resuscitation on mortality in massively bleeding patients: a before and after study. Vox Sang. 2009;96(2):111–118. doi: 10.1111/j.1423-0410.2008.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perkins JG, Cap AP, Spinella PC, et al. An evaluation of the impact of apheresis platelets used in the setting of massively transfused trauma patients [published correction appears J Trauma, 2009, 67(6)1453] J Trauma. 2009;66(4 suppl):S77–S84. doi: 10.1097/TA.0b013e31819d8936. [DOI] [PubMed] [Google Scholar]
- 38.Holcomb JB, Zarzabal LA, Michalek JE, et al. Trauma Outcomes Group. Increased platelet: RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(2 suppl 3):S318–S328. doi: 10.1097/TA.0b013e318227edbb. [DOI] [PubMed] [Google Scholar]
- 39.Kautza BC, Cohen MJ, Cuschieri J, et al. Inflammation and the Host Response to Injury Investigators. Changes in massive transfusion over time: an early shift in the right direction? J Trauma Acute Care Surg. 2012;72(1):106–111. doi: 10.1097/TA.0b013e3182410a3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radwan ZA, Bai Y, Matijevic N, et al. An emergency department thawed plasma protocol for severely injured patients. JAMA Surg. 2013;148 (2):170–175. doi: 10.1001/jamasurgery.2013.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson BR, Cotton BA, Pritts TA, et al. PROMMTT study group. Application of the Berlin definition in PROMMTT patients: the impact of resuscitation on the incidence of hypoxemia. J Trauma Acute Care Surg. 2013;75(1 suppl 1):S61–S67. doi: 10.1097/TA.0b013e31828fa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson GA, Sperry JL, Rosengart MR, et al. Inflammation and Host Response to Injury Investigators. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67(2):221–227. doi: 10.1097/TA.0b013e3181ad5957. [DOI] [PubMed] [Google Scholar]
- 43.Roback JD, Caldwell S, Carson J, et al. American Association for the Study of Liver; American Academy of Pediatrics; US Army; American Society of Anesthesiology; American Society of Hematology. Evidence-based practice guidelines for plasma transfusion. Transfusion. 2010;50(6):1227–1239. doi: 10.1111/j.1537-2995.2010.02632.x. [DOI] [PubMed] [Google Scholar]
- 44.Pieracci FM, Kashuk JL, Moore EE. Postinury hemotherapy and hemostasis. In: Mattox KL, Moore EE, Feliciano DV, editors. Trauma. 7. New York, NY: McGraw-Hill; 2012. pp. 216–235. [Google Scholar]
- 45.Kelly JM, Callum JL, Rizoli SB. 1:1:1-warranted or wasteful? even where appropriate, high ratio transfusion protocols are costly: early transition to individualized care benefits patients and transfusion services. Expert Rev Hematol. 2013;6 (6):631–633. doi: 10.1586/17474086.2013.859520. [DOI] [PubMed] [Google Scholar]
- 46.Park PK, Cannon JW, Ye W, et al. Transfusion strategies and development of acute respiratory distress syndrome in combat casualty care. J Trauma Acute Care Surg. 2013;75(2 suppl 2):S238–S246. doi: 10.1097/TA.0b013e31829a8c71. [DOI] [PubMed] [Google Scholar]
- 47.Sauaia A, Moore EE, Johnson JL, et al. Temporal trends of postinjury multiple-organ failure: still resource intensive, morbid, and lethal. J Trauma Acute Care Surg. 2014;76(3):582–592. doi: 10.1097/TA.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nascimento B, Callum J, Tien H, et al. Effect of a fixed-ratio (1:1:1) transfusion protocol versus laboratory-results-guided transfusion in patients with severe trauma: a randomized feasibility trial. CMAJ. 2013;185(12):583–589. doi: 10.1503/cmaj.121986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansson PI, Stensballe J, Oliveri R, Wade CE, Ostrowski SR, Holcomb JB. How I treat patients with massive hemorrhage. Blood. 2014;124(20):3052–3058. doi: 10.1182/blood-2014-05-575340. [DOI] [PubMed] [Google Scholar]
- 50.Hoyt DB, Bulger EM, Knudson MM, et al. Death in the operating room: an analysis of a multi-center experience. J Trauma. 1994;37(3):426–432. [PubMed] [Google Scholar]
- 51.Bulger EM, May S, Kerby JD, et al. ROC Investigators. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg. 2011;253(3):431–441. doi: 10.1097/SLA.0b013e3181fcdb22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarode R, Milling TJ, Jr, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128(11):1234–1243. doi: 10.1161/CIRCULATIONAHA.113.002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.US Food and Drug Administration. [Accessed December 21, 2014];Product development program for interventions in patients with severe bleeding due to trauma or other causes. 2010 http://www.fda.gov/BiologicsBloodVaccines/NewsEvents/WorkshopsMeetingsConferences/ucm241913.htm.
- 54.Holcomb JB, Weiskopf R, Champion H, et al. Challenges to effective research in acute trauma resuscitation: consent and endpoints. Shock. 2011;35(2):107–113. doi: 10.1097/SHK.0b013e3181f7fd01. [DOI] [PubMed] [Google Scholar]
- 55.Moore EE, Moore FA, Fabian TC, et al. Poly Heme Study Group. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208(1):1–13. doi: 10.1016/j.jamcollsurg.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 56.Hauser CJ, Boffard K, Dutton R, et al. CONTROL Study Group. Results of the CONTROL trial: efficacy and safety of recombinant activated factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69(3):489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 57.Silverman T, Aebersold P, Landow L, Lindsey K. Regulatory perspectives on clinical trials for trauma, transfusion, and hemostasis. Transfusion. 2005;45(1 suppl):14S–21S. doi: 10.1111/j.0041-1132.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 58.Dutton R, Hauser C, Boffard K, et al. CONTROL Steering Committee. Scientific and logistical challenges in designing the CONTROL trial: recombinant factor VIIa in severe trauma patients with refractory bleeding. Clin Trials. 2009;6(5):467–479. doi: 10.1177/1740774509344102. [DOI] [PubMed] [Google Scholar]
- 59.Snyder CW, Weinberg JA, McGwin G, Jr, et al. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma. 2009;66 (2):358–362. doi: 10.1097/TA.0b013e318196c3ac. [DOI] [PubMed] [Google Scholar]
- 60.Ho AM, Dion PW, Yeung JH, et al. Prevalence of survivor bias in observational studies on fresh frozen plasma: erythrocyte ratios in trauma requiring massive transfusion. Anesthesiology. 2012;116(3):716–728. doi: 10.1097/ALN.0b013e318245c47b. [DOI] [PubMed] [Google Scholar]
- 61.del Junco DJ, Fox EE, Camp EA, Rahbar MH, Holcomb JB PROMMTT Study Group. Seven deadly sins in trauma outcomes research: an epidemiologic post mortem for major causes of bias. J Trauma Acute Care Surg. 2013;75(1 suppl 1):S97–S103. doi: 10.1097/TA.0b013e318298b0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.