Abstract
Purpose of review
To summarize recent advances in interleukin (IL)-4 and IL-13 blockade in the treatment of asthma.
Recent findings
Historically, anti-cytokine therapies have historically been unsuccessful in the treatment of asthma because of the heterogeneity of asthma. Recent advances in our understanding of asthma pathophysiology and our increased ability to phenotype patients has led to the identification of asthmatic subsets (endotypes) that are most likely to respond to anti-cytokine therapy. Several new biologic therapies targeting IL-13 or both IL-4 and IL-13 signaling are currently in clinical trials both types of therapies have demonstrated therapeutic benefit.
Summary
Anti-IL-4/13 therapies, guided by knowledge of an individuals’ underlying pathophysiology, are a promising class of therapies for treatment of asthma.
Keywords: Asthma, Interleukin (IL)-4, IL-13, monoclonal antibody
Introduction
Asthma is a clinically and physiologically heterogeneous disease characterized by chronic airway inflammation and obstruction. Recognition of distinct subclasses of asthma pathophysiologies have led to the notion of asthma as a collection of endotypes or disease pathophysiologies that share by overlapping physiologic manifestations[1]. The identification of specific asthma endotypes coupled with the expanding knowledge of airway inflammation, epithelial and immune responses to allergens and viral infections have provided new opportunities for the development and application of endotype specific therapies in the treatment of asthma. These same discoveries allow for a more informative manner of classifying asthma that goes beyond symptoms, lung function and response to medications and could allow truly tailored therapy for an individuals’ unique pathophysiology [2].
Allergic asthma, characterized by the presence of an immunogloublin (Ig)E-mediated sensitization to one or more environmental allergens, represents the most common asthma endotype. Although the initial preconditions necessary for allergic sensitization are only partly understood, it is known that a cascade of Th2 cytokines, including interleukin (IL)-4, IL-5, and IL-13 among others, are necessary to initiate and propagate the inflammation associated with allergy. These cytokines are needed to induce class switching of B-cells to produce allergen-specific IgE (meditated by IL-4 and IL-13), recruit mast cells (IL-9) and eosinophils (IL-5) to sites of allergic inflammation and induce goblet cell metaplasia (IL-4, IL-13) [3].
Inhaled corticosteroids (ICS) are a first-line medication in the treatment of asthma and are effective in a majority of patients. However, ICS therapy can be associated with significant side effects[4] and many asthmatics are not well controlled with ICS therapy alone[4]. Also, there remains a group of between 5-10% of asthmatics in whom corticosteroids in combination with long acting bronchodilators are not sufficient in providing adequate symptom control[5]. This subgroup of asthmatics refractory to standard therapy are candidates for medications that target specific immunologic pathways, which have the potential to be both more effective and less toxic than oral corticosteroids. However, the exquisite specificity of these medications require the development of biomarkers that will reliably identify patients that will maximally benefit from these therapies
One successful example of this approach is omalizumab, a humanized monoclonal antibody directed against free IgE, which was the first biologic asthma medication. Treatment with omalizumab is indicated for individuals with evidence of allergic sensitization to a perennial allergen plus detectable serum levels of free IgE within a pharmacologically determined range. This approach allowed targeted treatment for allergic asthma rather than “one drug fits all”. Using endotype specific biomarkers, which take into account the etiology and pathophysiological mechanisms of disease pathogenesis, a targeted individualized approach to asthma care can be extended beyond omalizumab. In this review, we will discuss recent advances in biological therapies for asthma and the emerging role for endotype specific biomarkers to predict response to therapy.
Anti-IL-4 therapy
IL-4 was simultaneously discovered in 1982 by groups led by Ellen Vitteta and and William Paul. It was described as a soluble factor capable of both inducing B-cell proliferation as well as class switching [6]. Later, as the Th1 vs Th2 paradigm developed, IL-4 was recognized as a key cytokine necessary to induce the differentiation of Th2 lymphocytes from naive T cells and helping sustain the allergic response over time. Naturally, asthma, as a recognized manifestation of Th2 inflammation, became a target of investigation for anti-IL-4 therapy. Altrakincept is a recombinant human IL-4 receptor delivered by aerosol and intended to act as an antagonist to IL-4 action. While preliminary studies of altrakincept in steroid-dependent, atopic asthmatics appeared promising[7], efficacy could not be demonstrated in phase III trials[8]. Likewise, a humanized monoclonal antibody targeting IL-4, pascolizumab[9], was found to be ineffective in treating asthma[8].
IL-13 blockade
The reasons that anti-IL-4 did not show efficacy in atopic asthma in large part was likely due to the biological redundancy provided by IL-13. IL-4 and IL-13 are highly homologous to one another, are expressed by many of the same immune cell types and share many signaling pathways. There are two known receptors for IL-4, which are both heterodimers (see Figure 1). The type 1 IL-4 receptor is comprised of the common gamma chain (γC) and the IL-4Rαchain. The type 2 IL-4 receptor is composed of the IL-4Rα chain and the IL-13Rα1 chain and mediates signaling in response to both IL-4 and IL-13. There is also a second IL-13 receptor, IL-13Rα2, which binds IL-13 (but not IL-4) and may act as an inhibitory receptor[10].
Fig. 1.
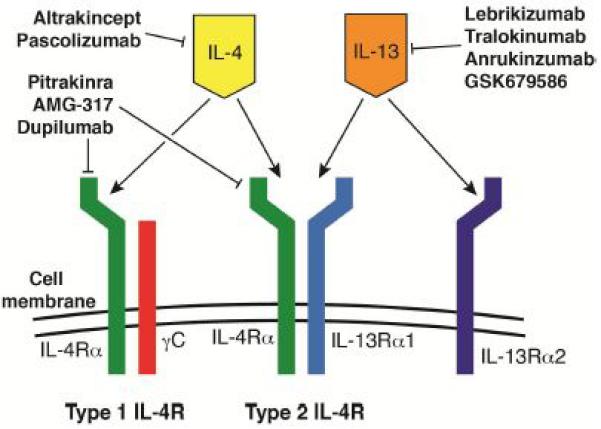
Diagram of IL-4 and IL-13 targeting medications
In addition to their shared functionality, IL-4 and IL-13 may play distinct roles in allergic inflammation. IL-4 is thought to signal primarily within the context of the immunologic synapse while IL-13 is responsible for more distant effects of allergic inflammation and may make IL-13 a more accessible therapeutic target[11]. The high degree of functional similarity between IL-4 and IL-13, in addition to the unique functions of IL-13, provided the scientific rationale for the development of a variety of anti-IL-13 therapies.
Perhaps the best studied of these therapies is lebrikizumab, a humanized IgG4 monoclonal antibody that binds to IL-13 and blocks its action. Initial studies of this drug demonstrated a reduced late phase asthmatic response in an allergen challenge study of 29 mild asthmatics [12,13]. A larger double-blind placebo controlled trial of 219 subjects with uncontrolled asthma while on ICS (80% of whom were also taking a long-acting beta-agonist (LABA)), showed that lebrikizumab improved FEV1 across all treated patients compared to controls although asthma symptom scores, as measured by ACQ5, were not improved. Significantly, periostin, a matricellular protein produced by respiratory epithelium in response to IL-13, differentiated individuals that responded to lebrikizumab. Those subjects with a high periostin level experienced a greater improvement in FEV1 than those with a low periostin level. There was also a trend toward improved exacerbations in the high periostin group[14].
A phase 2 study of 212 subjects investigated the role of IL-13 and the effect of different doses of lebrikizumab in mild asthmatic patients not receiving ICS. In contrast to uncontrolled asthmatics receiving ICS, mild asthmatics demonstrated no meaningful changes in FEV1 between lebrikizumab treated and placebo groups (the primary outcome of this study). There was a statistical difference favoring lebrikizumab as to risk of treatment failure (defined as worsening asthma symptoms with a decline in lung function or increased pharmacologic therapy) at all doses compared placebo (p < 0.001), although the degree of improvement would probably not be superior to ICS. Though subjects with high periostin tended to have greater improvements in FEV1, this finding was not statistically significant and there was no difference in any outcomes based on periostin levels[15].
The value of measuring periostin as a signature of the Th2 induced asthma endotype and its role as an indicator of expected beneficial response to therapy is an area of active investigation. Periostin has been demonstrated to correlate with airway eosinophilia in severe asthma [16]. Furthermore, in a study of 224 patients with asthma treated with ICS, individuals with the highest levels of periostin appear more likely to have declines in lung function (FEV1) [17]. Given that periostin expression levels tend to decline in the airway epithelium of patients treated with ICS[18], these studies suggest that high periostin in severe asthma may indicate ongoing Th2 inflammation despite ICS therapy. Studies addressing this hypothesis and the role of periostin in tracking treatment response will be needed.
Tralokinumab, another anti-IL-13 humanized monoclonal antibody, was investigated in a phase 2 clinical trial of 194 subjects ages 18-65 with moderate-to-severe, inadequately controlled (ACQ-6 of >1.5) asthma despite daily ICS treatment. Subjects were chosen to ensure >50% atopic subjects and received either placebo or one of 3 doses of tralokinumab. Induced sputum was collected at baseline and after three or eight weeks of treatment. The primary end point, a reduction in ACQ-6 of ≥0.5, was not met though patients receiving tralokinumab demonstrated less use of inhaled beta agonist. There was also a trend for an improvement in FEV1 (p = 0.072). As with lebrikizumab, subjects with evidence of active Th2 inflammation despite ICS therapy, in this case measured by sputum IL-13, tended to have the greatest response to tralokinumab [19].
A pair of humanized IgG1 monoclonal anti-IL-13 antibodies have also been developed for the treatment of asthma [20]. Both IMA-638 (anrukinzumab) and IMA-026 bind IL-13, but recognize different epitopes on the cytokine and consequently block IL-13 interaction with different receptors. IMA-638 blocks binding of IL-13 to the IL-4Rα whereas IMA-028 inhibits binding of IL-13 to IL-13Rα1. When tested in an allergen challenge model of asthma (n=27, 29), IMA-638 was able to attenuate both the early and late phase allergic response at 14 days while IMA-028 showed only a non-significant improvement in the late-phase response. The investigators concluded that specifically blocking binding of IL-13 to the IL4Rα1, rather that the IL13Rα1, may be more effective in preventing allergic responses after allergen challenge.
Not all clinical studies with anti-IL13 therapy have shown evidence of clinical improvement. GSK679586 is a humanized IgG1 monoclonal antibody that initially showed positive effects on measures of lung inflammation (FeNO) when studied in a phase I trial of mild asthmatic patients [21]. In a placebo-controlled study of 198 patients with poorly controlled, severe asthma on maximal doses of inhaled corticosteroids, patients were randomized to receive either GSK679586 or placebo [22]. Over the course of the study, there was no clinically significant improvement in the primary outcome (ACQ-7) or FEV1. Attempts to stratify the patients post hoc by serum IL-13 levels or blood eosinophil levels (both markers of Th2 inflammation) did not show an effect of GSK679586, although periostin and FeNO data were not collected. The authors provided several possible reasons for the contrast in efficacy of GSK679586 compared to other anti-IL-13 therapies. Chiefly, it appears that, in contrast to other studies of anti-IL-13 therapies discussed above, GSK679586 was directed at the most severe asthmatics receiving maximal doses of ICS who were less likely to respond to anti-IL-13 therapy. Another explanation was that GSK679586 inhibits binding of IL-13 to the IL13Rα1 and therefore, like IMA-028, may be less effective than therapies targeting IL-13 binding to the IL-4Rα1. Alternatively, the potency and/or dose of GSK679586 may have been inadequate to provide a therapeutic effect in this population.
Dual blockade of Anti IL-4 and IL-13
The overlapping nature of IL-4 and IL-13 signaling pathways also presents opportunities to inhibit the action of IL-4 and IL-13 simultaneously. One such medication is pitrakinra, a variant of the IL-4 protein that contains two amino acid changes that allows pitrakinra to bind the IL-4Rα chain, without allowing it to complex with either the γC or IL-13Rα1 chains. Binding by pitrakinra results in inhibition of both IL-4 and IL-13 signaling. In an allergen challenge study examining both subcutaneous injection (n=32) and nebulized pitrakinra (n=32), nebulized pitrakinra resulted in a decrease in the late phase allergic response measured by FEV1 [23]. A later study revealed a pharmacologic interaction between therapy and variation within the gene encoding the IL-4Rα chain (IL-4RA), identifying an asthma subgroup that was more responsive to pitrakinra [24].
In a larger study of 534 symptomatic moderate-to-severe adult (>18 years) asthmatics using corticosteroids, participants were randomized to inhaled pitrakinra or placebo. Subjects were stabilized for 4 weeks on LABA and ICS then randomized to pitrakinra or placebo for a 12 week treatment period. LABA was removed at day 28 and ICS were tapered starting on day 42 and halted on day 70. The results of the study revealed that although there was no therapeutic benefit for the entire population treated with pitrakinra compared to placebo, non-Hispanic white subjects with a common genotype had a significant dose-dependent reduction in asthma exacerbations along with decreased nocturnal awakenings and improved limited activity[25]. This larger study did not confirm the role of polymorphisms identified in the earlier study.
Another strategy that has been utilized to block Th2 signaling is to target IL-4Rα with a monoclonal antibody, and thus block both IL-4 and IL-13 signals. AMG-317 is a high affinity IgG2 monoclonal antibody targeting IL-4Rα. A phase 2, randomized, double-blind, placebo controlled trial was performed to test the effectiveness of AMG-317 in 294 moderate to severe asthmatics receiving ICS therapy [26]. After 12 weeks of therapy, all tested doses of AMG-317 failed to achieve a significant improvement in ACQ score (as the primary end point), although some benefit was noted in patients with the worst baseline ACQ scores and in the number of exacerbations experienced by patients receiving AMG-317. Interestingly, the authors speculated that heterogeneity of the study population was a contributor to the poor overall response to AMG-317 and that a subset of patients may benefit from therapy: patients with higher airway reversibility appeared to have better responses to therapy.
Like AMG-317, dupilumab is a fully humanized mAb to the IL-4Rα receptor that inhibits both IL-4 and IL-13[27]. A phase 2A study of 104 moderate to severe asthmatics, subjects were randomized to receive dupilumab or placebo. Interestingly, all subjects had to have a peripheral eosinophil count of 300cells/microLiter or ≥3% sputum eosinophils--a criteria that excluded over half of all patients assessed. After randomization, there was a 12 week intervention period that included a steroid reduction phase followed by an 8 week follow up period. The primary end point, occurrence of asthma exacerbation during the 12 week intervention phase, was met with an 87% reduction in the proportion of dupilumab treated subjects with an asthma exacerbation. FEV1 was improved at week 2 and was maintained through week 12 despite discontinuation of LABA and inhaled glucocorticosteroids. Multiple other outcomes met significance including change from baseline at week 12 in ACQ5, morning and evening asthma symptom scores, nocturnal awakenings and of inhalations of rescue beta agonist.
Conclusions and future of anti-IL-4/13 therapies
Anti-cytokine therapies for the treatment of atopic diseases may be a part of the future of allergy and particularly asthma care. Despite early setbacks, there are now several anti-IL-13 and anti-IL-4/13 therapies that appear promising. Demonstration of clinical efficacy of these medications is closely linked to the improved ability to define an individual's asthma endotype--or specific asthma pathogenesis. This new paradigm in the treatment of asthma is similar to the treatment of cancer in which a combination of phenotypic markers, such as histology, genetics and demographics can be used to optimize therapy.
The observation that the efficacy of pitrakinra is exquisitely sensitive to genetic polymorphisms within the IL-4Rα suggests that detailed genetic studies may help identify patients that will benefit from therapy. Dupilumab, like IL-4α, also acts by binding to IL-4Rα, raising the possibility that IL-4Rαpolymorphisms may have a pharmacologic interaction with dupilumab. Bispecific antibodies, antibodies that are able to inhibit the action of two substrates simultaneously, have been designed for IL-4 and anti IL-13 and could be an additional therapeutic option in individuals with an unfavorable IL-4Rα polymorphism [28].
Given the common pathogenesis of many atopic diseases, the potential application of anti-IL-4/13 therapies outside of asthma is exciting and reports of dupilumab in the treatment of atopic eczema are promising [29] but the roles of other anti-cytokine therapies in non-asthma atopic disease will need to be studied. Studies to better define the pathogenesis and propagation of non-atopic forms of asthma will need to be performed in order to find appropriate endotype specific biomarkers and identify new therapeutic drug targets. Likewise, anti-IL-4/13 therapies hold promise in the treatment of a variety of diseases in which Th2 cytokines have been implicated, such as interstitial lung disease [30].
Summary.
Anti-cytokine therapy, in particular IL-4 blockade, for asthma have been historically unsuccessful.
Increasing understanding of asthma endotypes has resulted in better identification of patients populations that are likely to respond to therapy.
Anti-IL-13 therapies have demonstrated efficacy in patients with moderate-severe asthma.
Medications that target the IL-4 receptor, and thus block signaling of both IL-4 and IL-13, have also shown promise in treating moderate-severe asthma.
Anti-IL-4/13 blockade appears to be most efficacious in patients with markers indicating underlying Th2 inflammation, such as increased eosinophils or periostin.
Acknowledgements
The authors would like to that Dr. H. James Wedner for his careful review of the manuscript. A.L.K. receives support from the NIH (F32DK091044) and holds stock in Gilead Pharmaceuticals (< $25,000). P.E.K. has participated in clinical trials and the publication of results sponsored by the following pharmaceutical companies: Genetech, Novartis, Boehringer Ingelheim, GlaxoSmithKline Merck and Pearl Pharmacetical. He has conducted clinical trials for Sanofi Aventis, Cephalon, Circassia, Mylan, Janssen and in the past participated in clinical trials with Actelion, Amgen, Amphstar, Electrocore, Shiongi and Watson. Wife owns stock in GlaxoSmithKline and Johnson&Johnson.
References
- 1.Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, Lemanske RF, Jr., Wardlaw AJ, Wenzel SE, Greenberger PA. Asthma endotypes: A new approach to classification of disease entities within the asthma syndrome. The Journal of allergy and clinical immunology. 2011;127(2):355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel SE. Asthma phenotypes: The evolution from clinical to molecular approaches. Nature medicine. 2012;18(5):716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 3.Martinez FD, Vercelli D. Asthma. Lancet. 2013;382(9901):1360–1372. doi: 10.1016/S0140-6736(13)61536-6. [DOI] [PubMed] [Google Scholar]
- 4.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, Pedersen SE, Group GI. Can guideline-defined asthma control be achieved? The gaining optimal asthma control study. American journal of respiratory and critical care medicine. 2004;170(8):836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 5.Kupczyk M, Wenzel S. U.S. And european severe asthma cohorts: What can they teach us about severe asthma? Journal of internal medicine. 2012;272(2):121–132. doi: 10.1111/j.1365-2796.2012.02558.x. [DOI] [PubMed] [Google Scholar]
- 6.Coffman RL. Converging discoveries: The first reports of il-4. Journal of immunology. 2013;190(3):847–848. doi: 10.4049/jimmunol.1203368. [DOI] [PubMed] [Google Scholar]
- 7.Borish LC, Nelson HS, Corren J, Bensch G, Busse WW, Whitmore JB, Agosti JM, Group I-RAS Efficacy of soluble il-4 receptor for the treatment of adults with asthma. The Journal of allergy and clinical immunology. 2001;107(6):963–970. doi: 10.1067/mai.2001.115624. [DOI] [PubMed] [Google Scholar]
- 8.Akdis CA. Therapies for allergic inflammation: Refining strategies to induce tolerance. Nature medicine. 2012;18(5):736–749. doi: 10.1038/nm.2754. [DOI] [PubMed] [Google Scholar]
- 9.Hart TK, Blackburn MN, Brigham-Burke M, Dede K, Al-Mahdi N, Zia-Amirhosseini P, Cook RM. Preclinical efficacy and safety of pascolizumab (sb 240683): A humanized anti-interleukin-4 antibody with therapeutic potential in asthma. Clinical and experimental immunology. 2002;130(1):93–100. doi: 10.1046/j.1365-2249.2002.01973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daines MO, Tabata Y, Walker BA, Chen W, Warrier MR, Basu S, Hershey GK. Level of expression of il-13r alpha 2 impacts receptor distribution and il-13 signaling. Journal of immunology. 2006;176(12):7495–7501. doi: 10.4049/jimmunol.176.12.7495. [DOI] [PubMed] [Google Scholar]
- 11.Steinke JW. Current prospective of anti-il-4, -il-9, and -il-13 therapies in allergic disease. Recent patents on inflammation & allergy drug discovery. 2010;4(3):222–230. doi: 10.2174/187221310793564281. [DOI] [PubMed] [Google Scholar]
- 12.Scheerens H, Arron JR, Su Z, Zheng Y, Putnam W, Erickson RW, Choy DF, Lee JH, Harris JM, Jarjour NN, Matthews JG. Predictive and pharmacodynamic biomarkers of interleukin-13 blockade: Effect of lebrikizumab on late phase asthmatic response to allergen challenge. Journal of Allergy and Clinical Immunology. 2011;127(2):AB164–AB164. [Google Scholar]
- 13.Scheerens H, Arron JR, Zheng Y, Putnam WS, Erickson RW, Choy DF, Harris JM, Lee J, Jarjour NN, Matthews JG. The effects of lebrikizumab in patients with mild asthma following whole lung allergen challenge. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2014;44(1):38–46. doi: 10.1111/cea.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, Mosesova S, et al. Lebrikizumab treatment in adults with asthma. The New England journal of medicine. 2011;365(12):1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 15.Noonan M, Korenblat P, Mosesova S, Scheerens H, Arron JR, Zheng Y, Putnam WS, Parsey MV, Bohen SP, Matthews JG. Dose-ranging study of lebrikizumab in asthmatic patients not receiving inhaled steroids. Journal of Allergy and Clinical Immunology. 2013;132(3):567–+. doi: 10.1016/j.jaci.2013.03.051. [A study of lebrikizumab in mild asthmatics showing that efficacy was reduced in this population.] [DOI] [PubMed] [Google Scholar]
- 16.Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, Shikotra A, Carter R, Audusseau S, Hamid Q, Bradding P, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. The Journal of allergy and clinical immunology. 2012;130(3):647–654. e610. doi: 10.1016/j.jaci.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanemitsu Y, Matsumoto H, Izuhara K, Tohda Y, Kita H, Horiguchi T, Kuwabara K, Tomii K, Otsuka K, Fujimura M, Ohkura N, et al. Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. Journal of Allergy and Clinical Immunology. 2013;132(2):305–+. doi: 10.1016/j.jaci.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 18.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, Erle DJ, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(40):15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piper E, Brightling C, Niven R, Oh C, Faggioni R, Poon K, She D, Kell C, May RD, Geba GP, Molfino NA. A phase ii placebo-controlled study of tralokinumab in moderate-to-severe asthma. European Respiratory Journal. 2013;41(2):330–338. doi: 10.1183/09031936.00223411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gauvreau GM, Boulet LP, Cockcroft DW, Fitzgerald JM, Carlsten C, Davis BE, Deschesnes F, Duong M, Durn BL, Howie KJ, Hui L, et al. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. American journal of respiratory and critical care medicine. 2011;183(8):1007–1014. doi: 10.1164/rccm.201008-1210OC. [DOI] [PubMed] [Google Scholar]
- 21.Hodsman P, Ashman C, Cahn A, De Boever E, Locantore N, Serone A, Pouliquen I. A phase 1, randomized, placebo-controlled, dose-escalation study of an anti-il-13 monoclonal antibody in healthy subjects and mild asthmatics. British Journal of Clinical Pharmacology. 2013;75(1):118–128. doi: 10.1111/j.1365-2125.2012.04334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Boever EH, Ashman C, Cahn AP, Locantore NW, Overend P, Pouliquen IJ, Serone AP, Wright TJ, Jenkins MM, Panesar IS, Thiagarajah SS, et al. Efficacy and safety of an anti-il-13 mab in patients with severe asthma: A randomized trial. Journal of Allergy and Clinical Immunology. 2014;133(4):989–+. doi: 10.1016/j.jaci.2014.01.002. [This negative outcome study raises questions concerning the likely effectiveness of IL-4 or IL-4/IL-13 inhibition achieving clinically meaningful improvement in severe asthma refractory to inhaled corticosteroids.] [DOI] [PubMed] [Google Scholar]
- 23.Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: Results of two phase 2a studies. Lancet. 2007;370(9596):1422–1431. doi: 10.1016/S0140-6736(07)61600-6. [DOI] [PubMed] [Google Scholar]
- 24.Slager RE, Hawkins GA, Ampleford EJ, Bowden A, Stevens LE, Morton MT, Tomkinson A, Wenzel SE, Longphre M, Bleecker ER, Meyers DA. Il-4 receptor alpha polymorphisms are predictors of a pharmacogenetic response to a novel il-4/il-13 antagonist. The Journal of allergy and clinical immunology. 2010;126(4):875–878. doi: 10.1016/j.jaci.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slager RE, Otulana BA, Hawkins GA, Yen YP, Peters SP, Wenzel SE, Meyers DA, Bleecker ER. Il-4 receptor polymorphisms predict reduction in asthma exacerbations during response to an anti-il-4 receptor alpha antagonist. The Journal of allergy and clinical immunology. 2012;130(2):516–522. e514. doi: 10.1016/j.jaci.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, Wenzel SE, Chon Y, Dunn M, Weng HH, Lin SL. A randomized, controlled, phase 2 study of amg 317, an il-4ralpha antagonist, in patients with asthma. American journal of respiratory and critical care medicine. 2010;181(8):788–796. doi: 10.1164/rccm.200909-1448OC. [DOI] [PubMed] [Google Scholar]
- 27.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, Wang L, Kirkesseli S, Rocklin R, Bock B, Hamilton J, et al. Dupilumab in persistent asthma with elevated eosinophil levels. New England Journal of Medicine. 2013;368(26):2455–2466. doi: 10.1056/NEJMoa1304048. [This study was particularly ground breaking in that their patient population was comprised of a single asthma endotype, which they describe as responding well to anti-IL4Rα therapy.] [DOI] [PubMed] [Google Scholar]
- 28.Spiess C, Bevers J, III, Jackman J, Chiang N, Nakamura G, Dillon M, Liu H, Molina P, Elliott JM, Shatz W, Scheer JM, et al. Development of a human igg4 bispecific antibody for dual targeting of interleukin-4 (il-4) and interleukin-13 (il-13) cytokines. Journal of Biological Chemistry. 2013;288(37):26583–26593. doi: 10.1074/jbc.M113.480483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beck L, Diamant T, Hamilton J, Graham N, Bieber T, Rocklin R, Ming J, Ren H, Kao R, Simpson E, Ardleanu M, et al. Dupilumab in adults with moderate-to-severe atopic dermatitis. New England Journal of Medicine. 2014;371(2):130–139. doi: 10.1056/NEJMoa1314768. [Describes the successful application of dupilumab to patients with atopic dermatitis, suggesting that biologics targeting Th2 cytokines may also be effective across a range of allergic diseases.] [DOI] [PubMed] [Google Scholar]
- 30.Datta A, Scotton CJ, Chambers RC. Novel therapeutic approaches for pulmonary fibrosis. British journal of pharmacology. 2011;163(1):141–172. doi: 10.1111/j.1476-5381.2011.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


