Abstract
Objective: Many patients with bipolar disorder in the United States experience a deteriorating course of illness despite naturalistic treatment in the community. We examined a variety of factors associated with this pattern of illness progression.
Method: From 1995 to 2002, we studied 634 adult outpatients with bipolar disorder (mean age of 40 years) emanating from 4 sites in the United States. Patients gave informed consent and completed a detailed questionnaire about demographic, vulnerability, and course-of-illness factors and indicated whether their illness had shown a pattern of increasing frequency or severity of manic or depressive episodes. Fifteen factors previously linked in the literature to a poor outcome were examined for their relationship to illness progression using Kruskal-Wallis test, followed by a 2-sample Wilcoxon rank sum (Mann-Whitney) test, χ2, and logistical regression.
Results: All of the putative poor prognosis factors occurred with a high incidence, and, with the exception of obesity, were significantly (P < .05) associated with illness progression. These factors included indicators of genetic and psychosocial risk and loss of social support, early onset, long delay to first treatment, anxiety and substance abuse comorbidity, rapid cycling in any year, and the occurrence of more than 20 prior episodes prior to entering the network. A greater number of factors were linearly associated with the likelihood of a progressively worsening course.
Conclusions: Multiple genetic, psychosocial, and illness factors were associated with a deteriorating course of bipolar disorder from onset to study entry in adulthood. The identification of these factors provides important targets for earlier and more effective therapeutic intervention in the hope of achieving a more benign course of bipolar disorder.
Clinical Points
■ Bipolar illness in the United States is common, exceedingly complicated, often progressive, and in need of careful screening and follow-up.
■ A variety of vulnerability and poor prognosis factors occur in a high proportion of US patients and are thus targets for early and concerted treatment; these include childhood adversity; early onset of bipolar disorder in childhood or adolescence in two-thirds of outpatients; more anxiety, substance abuse, and medical comorbidity; more episodes and rapid cycling; and more treatment resistance.
■ Screening, treatment or referral, and careful longitudinal follow-up of manic and depressive symptoms and of comorbid medical conditions may help avert the very high incidence of illness progression and a poor long-term outcome of bipolar disorder in patients in the United States.
While many patients with bipolar disorder respond well to treatment, an increasingly large group have not only continuing difficulty, but also a course of illness characterized by increasing frequency or severity of manic or depressive episodes or what could be considered a pattern of illness deterioration or progression. We assessed the relationship of a host of factors that have previously been associated with a poor outcome in bipolar disorder to several patient-rated measures of illness progression that occurred prior to entry in our network in outpatients from the United States.
In the United States, the prevalence of bipolar disorder is in the range of 3%–5%,1and, in primary care, 9.8% of patients screened positive for bipolar disorder, but few were taking mood stabilizers or had a bipolar diagnosis entered into the record.2 Even in patients who were given a new diagnosis of bipolar disorder in another study,3 50% were treated with antidepressants, mostly in monotherapy (which is not recommended), and much fewer numbers of patients were treated with approved classes of medications such as lithium, mood stabilizers, or atypical antipsychotics.
The problem of poor recognition and treatment of bipolar disorder is particularly acute in the United States, where virtually every aspect of the illness is more difficult or severe than in European countries such as the Netherlands or Germany.4,5 These difficulties include more genetic vulnerability (parents and grandparents with bipolar and other illnesses), psychosocial stressors in childhood and over the course of illness, childhood onset (ie, 31% before age 13 and 69% before age 19 years), anxiety and substance comorbidity, manic and depressive episodes and rapid cycling, treatment nonresponse, and medical comorbidities. Childhood onset of bipolar disorder is associated with long delays to first treatment and a difficult course of illness into adulthood.6,7
Factors that had been previously associated in the literature and in our own studies with an adverse course of bipolar disorder were examined and included a history of childhood adversity (verbal, physical, or sexual abuse); early age at onset (prior to age 19 years); delay to first treatment for a manic or depressive episode; a history of anxiety or substance abuse comorbidity; rapid cycling; 20 or more prior episodes of depression or mania, being overweight (body mass index [BMI, kg/m2] > 26), having more than 2 medical comorbidities; poor social support, poor health care access; and employment difficulties.8 We wanted to see which of these factors individually and as a group might be associated with illness progression despite treatment in the community so that greater clinical attention could be paid to these factors and their amelioration. We postulated that each of these poor prognosis factors and the total number of them would be associated with a greater likelihood of a progressively worsening course of bipolar disorder.
METHOD
We examined self-reports of illness course and poor prognosis factors in 634 adult outpatients with bipolar disorder (mean age of 40 years) emanating from 1 of 4 sites in the United States, which included Los Angeles, California; Dallas, Texas; Cincinnati, Ohio; and Bethesda, Maryland. Patients gave verbal and written consent for participation in what was then called the Stanley Foundation Bipolar Network (1995–2002) and now continues as the Bipolar Collaborative Network.9–11
Given the international differences in the degree of treatment resistance and the very considerable differences in risk factors, age at onset, comorbidities, and course of illness variables in patients from the United States versus those from Europe,11–14 we examined the impact of the poor prognosis factors on illness progression only in the US patients in order to avoid the many clinical and statistical confounds that would be present in a divergent combined population.
The measures of illness progression/deterioration were taken from a questionnaire filled out by patients at network entry who answered “probably” or “definitely” to 3 questions on whether their illness was characterized by an increasing (1) severity of depression, (2) severity of mania, or (3) frequency of episode occurrence.
The poor prognosis factors examined included a childhood history of physical/sexual abuse, verbal abuse in childhood, onset of illness prior to age 19 years, delay to first treatment of more than 4 years, a lifetime history of anxiety or substance abuse comorbidity, rapid cycling, ≥ 20 prior episodes of mania and depression, a BMI (> 26) reflecting overweight or obesity, the experience of more than 2 medical comorbidities (from a list of 14 potential illnesses), poor social support (taken as a moderate or greater rating of lack of support on questions about a spouse, a confidant, the family, or the social network), poor health care access (moderate or greater difficulties with health care coverage or access), and employment difficulties (moderate or greater).6,15–17
Statistics
The influence of the putative poor prognosis factors on a deteriorating illness course was examined with a Kruskal-Wallis test, followed by a 2-sample Wilcoxon rank sum (Mann-Whitney) test. The relationship to illness progression of isolated vulnerability factors and other course-of-illness variables was evaluated with Person’s χ2. The independent contribution of the poor prognosis factors was assessed by linear regression analysis. This analysis was performed for each of the 3 measures of illness deterioration/progression separately, but reported in detail only for the combined measure of any evidence of illness deterioration based on a positive answer to any 1 of the 3 questions on the increasing severity of manic or depressive episodes or their increasing frequency of occurrence.
RESULTS
Table 1 lists the high incidence of each of the putative poor prognosis factors in this population of outpatients in the United States with bipolar illness. Each of these poor prognosis factors (with the exception of overweight/obesity) occurred in a higher percentage of patients who had a deteriorating course of illness compared to those who did not progress (Table 2) when any of the 3 measures of increasing frequency or severity of mania or depression were used. These poor prognosis factors consistently occurred in 78%–84% of patients who had illness progression, but in only 50%–70% of the patients without illness progression.
Table 1.
Measures of Illness Progression and Poor Prognosis Factors Present in a Large Portion of Patients With Bipolar Disorder in the United States
| Total N | Poor Prognosis Factor Present |
||||
| n | % | SE | 95% CI | ||
| Illness progression variables | |||||
| Increasing severity of depression | 655 | 416 | 63.5 | 0.02 | 0.60–0.67 |
| Increasing severity of mania | 653 | 356 | 54.5 | 0.02 | 0.50–0.59 |
| Increasing episode frequency | 660 | 350 | 53.0 | 0.02 | 0.49–0.57 |
| Any illness progression | 661 | 492 | 74.4 | 0.02 | 0.71–0.78 |
| Putative poor prognosis factors | |||||
| Parental history of depression | 602 | 326 | 54.2 | 0.02 | 0.50–0.58 |
| Parental history of bipolar disorder | 587 | 213 | 36.3 | 0.02 | 0.32–0.40 |
| Childhood physical or sexual abuse | 647 | 261 | 40.3 | 0.02 | 0.37–0.45 |
| Childhood verbal abuse | 647 | 374 | 57.8 | 0.02 | 0.54–0.62 |
| Early age at onset (before age 19 y) | 652 | 452 | 69.3 | 0.02 | 0.66–0.73 |
| Social stress at onset | 662 | 453 | 68.4 | 0.02 | 0.65–0.72 |
| Delay to treatment (≥ 4 y) | 608 | 408 | 67.2 | 0.02 | 0.63–0.71 |
| Anxiety disorder comorbidity | 675 | 321 | 47.5 | 0.02 | 0.43–0.52 |
| Substance abuse comorbidity | 675 | 330 | 48.9 | 0.02 | 0.45–0.53 |
| Rapid cycling (≥ 4 episodes/y) | 637 | 471 | 73.9 | 0.02 | 0.70–0.77 |
| > 20 Manic/depressive episodes | 638 | 379 | 59.5 | 0.02 | 0.55–0.63 |
| Body mass index (kg/m2) > 26 overweight or obese | 648 | 413 | 63.7 | 0.02 | 0.60–0.68 |
| > 2 Medical comorbidities | 668 | 298 | 44.6 | 0.02 | 0.41–0.49 |
| Poor health care access | 666 | 188 | 28.2 | 0.02 | 0.25–0.32 |
| Employment difficulties | 669 | 462 | 69.1 | 0.02 | 0.66–0.73 |
Table 2.
Relationship of Poor Prognosis Factors to Patterns of Illness Progressiona
| Factor | % Illness Progression if Poor Prognosis Factor is Absent | % Illness Progression if Poor Prognosis Factor is Present | χ2 | P | P Depression | P Mania | P Episode Frequency |
| Childhood physical or sexual abuse | 69.3 | 83.5 | 14.1 | 0 | .01 | 0 | 0 |
| Childhood verbal abuse | 63.4 | 82.5 | 30.0 | 0 | 0 | 0 | 0 |
| Early age at onset | 58.9 | 81.1 | 35.5 | 0 | 0 | 0 | 0 |
| Anxiety disorder | 66.1 | 84.2 | 28.3 | 0 | 0 | 0 | 0 |
| Substance abuse | 67.8 | 81.8 | 16.9 | 0 | 0 | 0 | .01 |
| Rapid cycling | 51.5 | 84.3 | 71.8 | 0 | 0 | 0 | 0 |
| > 20 Episodes | 60.2 | 85.6 | 53.6 | 0 | 0 | 0 | 0 |
| Body mass index overweight or obese | 76.0 | 73.2 | 0.6 | .45 | .21 | .77 | .88 |
| > 2 Comorbidities | 68.4 | 82.3 | 16.4 | 0 | 0 | 0 | 0 |
| Social stress | 67.5 | 78.1 | 8.3 | 0 | .01 | .32 | 0 |
| Health care | 70.8 | 83.9 | 12.0 | 0 | .03 | .01 | 0 |
| Employment | 65.7 | 78.6 | 12.3 | 0 | 0 | 0 | 0 |
| Delay to treatment (≥ 4y) | 56.1 | 82.8 | 49.6 | 0 | 0 | 0 | 0 |
| Family history of depression | 68.6 | 79.7 | 9.6 | 0 | 0 | 0 | .04 |
| Family history of bipolar disorder | 72.3 | 80.2 | 4.5 | .03 | .19 | .04 | 0 |
The second column of numbers indicates the % of patients who have a pattern of illness progression if that putative poor prognosis factor is absent (first column) or if it is present (third column); χ2 is in the next column, with statistical significance in the next. The last 3 columns indicate the significant relationship of each variable to a given pattern of illness progression evident in increasing severity of depression, severity of mania, or frequency of recurrence of episodes, respectively. As the relationships are similar to the other measure of any type of illness progression, the χ2 values are not presented for each individual measure. A P value of zero equals P < .001.
In the 3 columns on the right side of Table 2 are the significant relationships of each factor to the 3 measures of illness progression separately, ie, increasing severity of depression, increasing severity of mania, or increasing frequency of episode occurrence. As all of these relationships were in a similar range of statistical significance, we chose to present the percentages and χ2 values only for the single combined measure of illness progression on any 1 of the 3 measures.
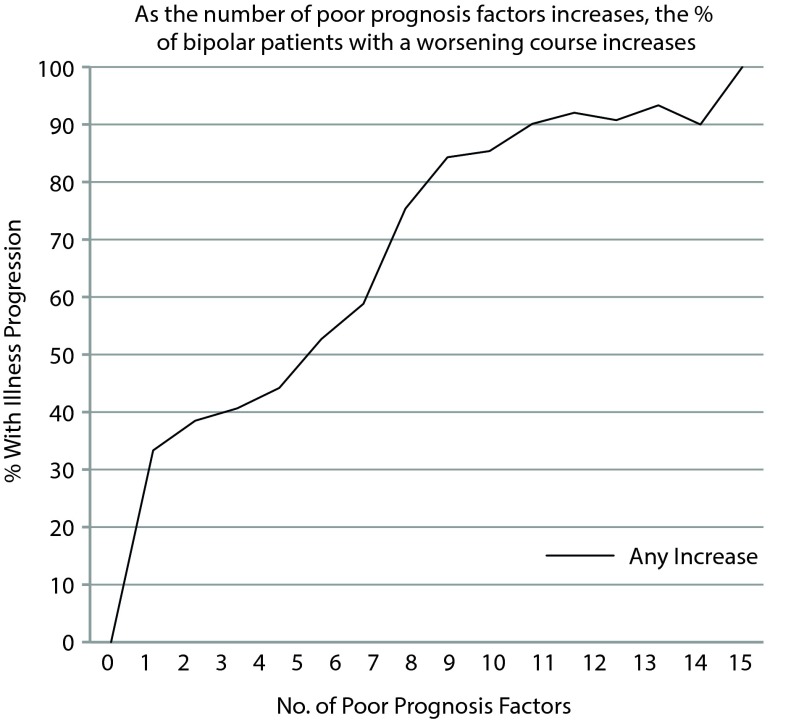
As illustrated in Figure 1, those with a greater number of poor prognosis factors showed an increasing likelihood of illness progression (n = 657, χ2 = 113.15, pseudo R2 = 0.152, P < .001). This relationship rose fairly linearly with increasing number of factors from 0 to 8 and then gradually leveled off, such that those with 9 to 14 poor prognosis factors were 85%–90% likely to show a pattern of illness worsening prior to network entry.
Figure 1.
More Poor Prognosis Factors Associated With Greater Illness Progression
When the independent contribution of each of the poor prognosis factors to illness progression was evaluated in a logistic regression (Table 3), the occurrence of rapid cycling remained highly significant, while early age at onset, substance abuse comorbidity, and a delay to first treatment of ≥ 4 years were at a trend level. These relationships were generally parallel to the magnitude of the χ2 values observed in the univariate analysis (Table 2).
Table 3.
Multivariate Analysis of Independent Contributors to Illness Progressiona
| Variable | Odds Ratio | SE | z | P | 95% CI |
| Physical or sexual abuse | 1.11 | 0.32 | 0.38 | .71 | 0.63–1.96 |
| Verbal abuse | 1.35 | 0.38 | 1.07 | .28 | 0.78–2.34 |
| Age at onset | 1.62 | 0.44 | 1.80 | .07 | 0.96–2.75 |
| Anxiety disorder | 1.36 | 0.36 | 1.19 | .23 | 0.82–2.27 |
| Substance abuse | 1.57 | 0.40 | 1.80 | .07 | 0.96–2.58 |
| Rapid cycling | 2.80 | 0.80 | 3.61 | 0 | 1.60–4.91 |
| > 20 Episodes | 1.46 | 0.42 | 1.32 | .19 | 0.83–2.58 |
| Body mass index (kg/m2) > 26 | 0.92 | 0.23 | 0.34 | .73 | 0.56–1.51 |
| > 2 Comorbidities | 1.22 | 0.32 | 0.77 | .44 | 0.73–2.03 |
| Poor social support | 1.18 | 0.31 | 0.63 | .53 | 0.71–1.97 |
| Poor health care | 1.32 | 0.41 | 0.91 | .36 | 0.72–2.42 |
| Poor employment | 1.32 | 0.35 | 1.05 | .3 | 0.79–2.21 |
| Family history of depression | 1.18 | 0.30 | 0.65 | .52 | 0.72–1.94 |
| Family history of mania | 0.95 | 0.26 | 0.19 | .85 | 0.55–1.63 |
| Delay to treatment > 3 y | 1.65 | 0.45 | 1.85 | .07 | 0.97–2.82 |
A logistic regression found poor prognosis factors successfully predicted illness progression (likelihood ratio χ2 = 96.46, P < .001, pseudo R2 = 0.1809, n = 480). A P value of zero equals P < .001.
DISCUSSION
These data underscore the large variety of negative factors that might contribute to a pattern of increasing severity or frequency of manic and depressive episodes despite how patients were treated in the community prior to network entry. Each factor has been previously linked to a more difficult course of illness in the literature,8 and most of these individual factors were present in a substantial portion of about 40%–70% of the US patients. With the exception of obesity, the presence as opposed to the absence of each of these putative poor prognosis factors was significantly related to a deteriorating course of illness as rated at network entry.
There was also a strong relationship of the total number or accumulation of these poor prognosis factors to a progressively deteriorating course of illness in terms of increasing frequency or severity of manic and depressive episodes (as illustrated in Figure 1). Each additional poor prognosis factor appeared to further increase the likelihood of having a deteriorating course. If a patient had ≥ 9 of these poor prognosis factors, there was an approximate 85% likelihood that they showed a pattern of illness progression. Both the χ2 in the univariate analysis and the logistic regression identified several of the poor prognosis factors as the most prominent contributors to the deteriorating course; these included a history of rapid cycling, an onset of illness in childhood or adolescence (prior to age 19 years), a > 4-year delay from illness onset to first treatment for either mania or depression, and a history of substance abuse comorbidity.
Childhood-onset, compared to adult-onset, bipolar disorder has been multiply associated with a more adverse course of illness.6,7,18–22 This poor outcome makes sense, as the illness in children is difficult to treat and stabilize and is associated with a high rate of relapse, with children being symptomatic about two-thirds of the time during long-term prospective follow-up.23–27 Thus, the occurrence of early illness, especially if it were not treated for an extended duration (at least 4 years), would negatively impact social, educational, and cognitive/emotional skills at important stages of development and be associated with the accumulation of mood episodes and psychosocial stressors, as well as the likelihood of substance abuse.6,7,28 Early onset illness has consistently also been linked to a greater genetic vulnerability,29 raising the possibility that early onset bipolar disorder is inherently more malignant than adult-onset bipolar disorder.
However, taken together, these findings are consistent with the view that stressors (both prior to and during the course of illness), episodes of illness, substance abuse, and a variety of other factors present in a high proportion of US patients contribute to the pattern of increasing severity/frequency of episodes of mania and depression. The repetition and recurrence of intermittent stressors, affective episodes, and bouts of substance use may cause not only an increase in the magnitude of behavioral response, reactivity, and pathology (ie, sensitization rather than tolerance), but also cross-sensitization to the others,30,31 resulting in a potential vicious positive feedback cycle. For example, recurrent stressors may precipitate new affective episodes or the relapse of substance use, each of which could further engender new stressors, and so on.
The literature is replete with examples of how early life stressors may provide the basis of a lasting increased vulnerability to episode recurrence and potentially an increased responsivity or sensitization to later stressors in adulthood.13,14,32–37 This increased vulnerability in those with childhood adversity to depression following the occurrence of new stressors in adulthood has been consistently demonstrated, even if a gene (such as the 5HT-T short allele)–environmental interaction is not always replicated.32,36,38 Such persisting and sensitizing effects of early stressors might occur via epigenetic mechanisms as demonstrated in animals and humans.31,39–46 Concurrent substance abuse has also been associated with a more difficult course of bipolar disorder,47 and it too carries the potential for sensitization via epigenetic mechanisms and cross-sensitization to both stressors and episodes.31
The occurrence of multiple episodes and rapid cycling has similarly been associated with a more treatment-nonresponsive illness to a great many different treatment modalities.30 A greater number of prior episodes has been independently linked to the vulnerability and latency to episode recurrence (a manifestation of episode sensitization),34,48 as well as to cognitive deterioration.30,49
In addition, childhood stressors and greater numbers of mood episode can each exert diverse adverse psychiatric and medical effects because of an increased percentage of short telomeres and/or decreases in telomerase activity.50–54
Many other adversities and course-of-illness characteristics may also accumulate and further contribute to illness deterioration and magnify stressor burden, adversity, and demoralization. For example, having ≥ 2 medical comorbidities, unemployment difficulties, lack of health care access, and lack of social support have been associated with an increased incidence of suicide attempts in patients with bipolar disorder,16,55 and each of these difficulties occurs in more than 50% of these patients from the United States. Having an anxiety disorder comorbidity has also consistently been associated with a poor outcome in patients with bipolar disorder.30,56
Clearly, those with a prior deteriorating course of bipolar illness have a greater number of these varied problems than those whose illness did not progress in this negative direction. Being overweight or obese by BMI measurement, even though it was not related to our indices of illness progression, has also been associated with other measures of a poor outcome in bipolar disorder,57 including cognitive difficulties and the accumulation of further medical comorbidities.
Limitations
A number of limitations need to be considered in the interpretation of these findings. All of the data are retrospective and based on patient self-report. The assessment of the main outcome measure of a progressive/deteriorating course of illness based on the report of an increasing frequency or severity of episodes of mania or depression is to some extent subjective and may not correlate highly with other more quantitative measures of poor outcome such as more time ill, less time euthymic, or more hospitalizations.
Some of the poor prognosis factors considered, such as rapid cycling or having had ≥ 20 prior episodes, may be intertwined with the definition of illness progression in terms of increasing frequency of episodes. However, it is also possible for patients to experience periods of rapid cycling or a high density of episodes and then respond well to treatment and show improvement or remission,8,10,58–60 so that these variables are not inextricably confounded. Also, the variables we chose to examine as likely poor prognosis factors from the literature do not represent an exhaustive list, and many other potential contributors to illness progression could also have been studied.
The findings are based on data exclusively from patients in the United States, and their generality to other populations and countries may not be warranted.4 A major limitation is that type and intensity of pharmacologic, somatic, and psychosocial treatment that patients received in the community prior to network entry was not accounted for. However, other measures of illness progression in the literature, such as the likelihood of episode recurrence as a function of the number of prior episodes, also were highly significant when the potential effects of treatment were not taken into account.61 Finally, more sophisticated modeling of causal mechanisms and sequences could not be performed, as the details and time of occurrence of the poor prognosis factors were not available.
Clinical and Public Health Implications
Despite these limitations, the findings identify a host of commonly occurring demographic, psychosocial, and illness characteristics associated with a pattern of illness progression or deterioration that was reported in more than two-thirds of the outpatients with bipolar disorder from the United States in our network. This result suggests that bipolar disorder as characterized in this population was in general poorly responsive to conventional treatment received in the community in a very high proportion of patients prior to network entry. These data converge with those in many other populations, indicating that bipolar disorder in the United States as it is currently treated both in children25–27,62 and in adults18,63–65 is characterized by an extraordinarily high degree of treatment resistance.
Many of the variables previously associated with a poor outcome in the literature and with illness progression in this study were present in a high proportion of our patients from the United States, and, thus, each potentially represents an important target for therapeutic intervention. There perhaps is little one can do presently about genetic vulnerability and a parental diagnosis of a mood disorder or an early onset of illness, but more rapidly instituting appropriate treatment as the illness emerges17 might render the illness more benign, especially since the duration of delay to first treatment is a contributor to a poor outcome,6,19,22,66 even independent of the adverse course associated with early onset.6
The research groups of Chang et al67 and Miklowitz et al68,69 have shown that family-focused therapy for children at high risk (by virtue of both having a relative with bipolar disorder and having a prodromal diagnosis of an anxiety or depressive disorder or bipolar disorder not otherwise specified) is associated with considerable improvement on a variety of measures compared to treatment as usual. Thus, family-focused therapy or related psychosocial interventions in conjunction with pharmacotherapy could help head off many of the difficulties identified here, particularly in the realm of recurrence and accumulation of stressors, episodes, and substance use. Family-focused therapy focuses on enhancing communication, social support, and symptom identification and monitoring, and these approaches could contribute to decreasing the incidence of verbal and emotional abuse and more rapidly attenuating anxiety disorder comorbidity.
In children at high risk by virtue of having a parent or first-degree relative with bipolar disorder, there appears to be a fairly consistent sequence of emerging diagnoses starting first with an anxiety disorder in childhood, then depression, and then much later bipolar disorder in adolescence or adulthood.70–73 In high-risk children in the United States, this progression of symptoms and diagnoses may be compressed or accelerated, with mania emerging earlier in childhood or adolescence in a more substantial percentage.74–78 Thus, psychotherapeutic approaches, such as family-focused therapy, and appropriate pharmacotherapy of these early appearing syndromes would seem especially important. Attempting to achieve primary or secondary prevention of substance abuse in adolescents at such high risk by virtue of their bipolar disorder79 should also be a consistent therapeutic endeavor. Thus, children at high risk of a mood or anxiety disorder by virtue of a parent with bipolar disorder should be carefully followed and treated and/or referred for psychiatric evaluation and treatment. If such a child has experienced verbal or other types of abuse, psychotherapy should also be considered, as this is an additional risk factor for not only early onset and more severe course of bipolar disorder, but also increases in a large array of medical illnesses in adolescence and adulthood.13,35 One can have parents of children rate depressive, anxiety, posttraumatic stress disorder, and bipolar symptoms longitudinally on instruments such as My Mood Monitor (www.whatsmym3.com) or on a personal calendar available at www.bipolarnews.org, which is a newsletter that also carries the latest information on treatment and research for physicians, patients, and family members.
Primary care physicians also have a critical role in the treatment of the medical comorbidities that so often accompany bipolar disorder. Some 40% of patients in the United States have metabolic syndrome, and monitoring and treatment of elevated lipids, blood pressure, waist circumference, and blood sugar will play an important role in reducing the 1 to 2 decades of lost life expectancy mainly from cardiovascular disease in those with bipolar disorder and related major psychiatric illness.80,81
Given the high prevalence of so many poor prognosis factors in outpatients with bipolar disorder in the United States and the very high rates of treatment resistance reflected in the measure of illness progression/deterioration and in many other measures,6,7,10,18,82 it would appear appropriate to reconceptualize the onset of bipolar disorder, particularly in childhood or adolescence, as a genuine medical emergency deserving the highest levels of both acute and long-term integrated and multimodal care (similar to that consistently given for childhood-onset diabetes). The importance of special and intensive follow-up care in bipolar disorder is also demonstrated by Kessing et al83 in their randomization of those with a first hospitalization for mania to either a specialty clinic (emphasizing illness education, monitoring, and early intervention) for 2 years or treatment as usual. Not only were there significantly few rehospitalizations in the specialty clinic group, but the differences between groups persisted and grew larger over the next 6 years (even though the specialty clinic treatment ended at 2 years).83 Without new approaches to the complexity and multiplicity of factors associated with a deteriorating course of bipolar disorder in the United States, the illness is likely to continue to rob overwhelming numbers of patients of their medical and psychiatric health and well-being.
Drug names: lithium (Lithobid and others).
Potential conflicts of interest: Dr Post has served on the speakers or advisory boards of AstraZeneca, Sunovion, Teva, and Validus. Dr Altshuler has served on the speakers or advisory boards of Sunovion (Health and Wellness Partners, LLC). Dr Nolen has received grants from Netherlands Organization for Health Research and Development, European Union, Stanley Medical Research Institute, AstraZeneca, GlaxoSmithKline, and Wyeth and has received honoraria or speaker’s fees from AstraZeneca and Lundbeck. Mr Grunze has served as a consultant to Lundbeck and Hoffmann Laroche and has served on the speakers or advisory boards of Lundbeck, BristolMyersSquibb, and Sanofi-Aventis. Dr Frye has received grant support from Assurex, Myriad, Pfizer, National Institute of Mental Health, National Institute of Alcohol Abuse and Alcoholism, and Mayo Foundation; has served as a consultant to Janssen Global Services, Mitsubishi Tanabe Pharma Corporation, Myriad, Sunovion, and Teva; and has received continuing medical education/travel support from CME Outfitters. Dr Suppes has received grant/research funding from National Institute of Mental Health, Sunovion, Elan Pharma International Limited, and VA Cooperative Studies Program; has served as a consultant or on the advisory boards of Sunovion and H. Lundbeck A/S; has received honoraria from International Society of Bipolar Disorders; has received continuing medical education honoraria from Healthmatters CME, Omnia-Prova Education Collaborative, and Medscape Education; has received royalties from Jones and Bartlett (formerly Compact Clinicals); and has received travel expenses from Sunovion, H. Lundbeck, Omnia-Prova Education Collaborative, American Psychiatric Association, and International Society of Bipolar Disorders. Dr McElroy has served as a consultant to Bracket, F. Hoffman-La Roche Ltd, and Shire; has received grant/research support from Cephalon, Forest, Marriott Foundation, Orexigen, Shire, and Takeda; and has served on the speakers or advisory boards of MedAvante, Naurex, Shire, and Sunovion. Dr Keck is presently or has been in the past year a principal or coinvestigator on research studies sponsored by Alkermes, AstraZeneca, Cephalon, Marriott Foundation, National Institute of Mental Health, Orexigen, Pfizer, and Shire; has been reimbursed for consulting to Sunovion, Alkermes, Shire, and Forest; and is a coinventor on US patent no. 6,387,956 (Shapira NA, Goldsmith TD, Keck PE Jr [University of Cincinnati]. Methods of treating obsessive-compulsive spectrum disorder comprises the step of administering an effective amount of tramadol to an individual.) filed March 25, 1999 and approved May 14, 2002. Dr Keck has received no financial gain from this patent. Drs Kupka and Rowe and Mr Leverich report no conflicts of interest related to the subject of this article.
Funding/support: This research was initially funded by the Stanley Medical Research Institute.
Role of the sponsor: The sponsor played no role in the design, interpretation, or approval of this study and manuscript.
References
- 1.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA. 2005;293(8):956–963. doi: 10.1001/jama.293.8.956. [DOI] [PubMed] [Google Scholar]
- 3.Baldessarini RJ, Leahy L, Arcona S, et al. Patterns of psychotropic drug prescription for US patients with diagnoses of bipolar disorders. Psychiatr Serv. 2007;58(1):85–91. doi: 10.1176/ps.2007.58.1.85. [DOI] [PubMed] [Google Scholar]
- 4.Post RM, Altschuler L, Kupka R, et al. More pernicious course of bipolar disorder in the United States than in many European countries: implications for policy and treatment. J Affect Disord. 2014;160:27–33. doi: 10.1016/j.jad.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Post RM, Altshuler LL, Leverich GS, et al. More medical comorbidities in patients with bipolar disorder from the United States than from the Netherlands and Germany. J Nerv Ment Dis. 2014;202(4):265–270. doi: 10.1097/NMD.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 6.Post RM, Leverich GS, Kupka RW, et al. Early onset bipolar disorder and treatment delay are risk factors for poor outcome in adulthood. J Clin Psychiatry. 2010;71(7):864–872. doi: 10.4088/JCP.08m04994yel. [DOI] [PubMed] [Google Scholar]
- 7.Perlis RH, Miyahara S, Marangell LB, et al. STEP-BD Investigators. Long-term implications of early onset in bipolar disorder: data from the first 1,000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Biol Psychiatry. 2004;55(9):875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Post RM, Leverich GS. Treatment of Bipolar Illness: A Case Book for Clinicians and Patients. New York, NY: WW Norton & Company, Inc; 2008. [Google Scholar]
- 9.Post RM, Nolen WA, Kupka RW, et al. The Stanley Foundation Bipolar Network, 1: rationale and methods. Br J Psychiatry suppl. 2001;178(41):s169–s176. doi: 10.1192/bjp.178.41.s169. [DOI] [PubMed] [Google Scholar]
- 10.Post RM, Altshuler LL, Frye MA, et al. Complexity of pharmacologic treatment required for sustained improvement in outpatients with bipolar disorder. J Clin Psychiatry. 2010;71(9):1176–1186. doi: 10.4088/JCP.08m04811yel. quiz 1252–1253. [DOI] [PubMed] [Google Scholar]
- 11.Post RM, Leverich GS, Altshuler LL, et al. Differential clinical characteristics, medication usage, and treatment response of bipolar disorder in the US versus the Netherlands and Germany. Int Clin Psychopharmacol. 2011a;26(2):96–106. doi: 10.1097/YIC.0b013e3283409419. [DOI] [PubMed] [Google Scholar]
- 12.Post RM, Luckenbaugh DA, Leverich GS, et al. Incidence of childhood-onset bipolar illness in the USA and Europe. Br J Psychiatry. 2008;192(2):150–151. doi: 10.1192/bjp.bp.107.037820. [DOI] [PubMed] [Google Scholar]
- 13.Post RM, Altshuler LL, Leverich GS, et al. Role of childhood adversity in the development of medical comorbidities associated with bipolar disorder. J Affect Disord. 2013a;147(1–3):288–294. doi: 10.1016/j.jad.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Post RM, Leverich GS, Kupka R, et al. Increased parental history of bipolar disorder in the United States: association with early age of onset. Acta Psychiatr Scand. 2014;129(5):375–382. doi: 10.1111/acps.12208. [DOI] [PubMed] [Google Scholar]
- 15.Post RM, Altshuler L, Leverich G, et al. More stressors prior to and during the course of bipolar illness in patients from the United States compared with the Netherlands and Germany. Psychiatry Res. 2013d;210(3):880–886. doi: 10.1016/j.psychres.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288–297. doi: 10.1016/s0006-3223(01)01239-2. [DOI] [PubMed] [Google Scholar]
- 17.Post RM, Altshuler L, Leverich GS, et al. The impact of verbal abuse on the course of bipolar disorder in patients from the United States. Bipolar Disord. 2013 In press. [Google Scholar]
- 18.Perlis RH, Dennehy EB, Miklowitz DJ, et al. Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: results from the STEP-BD study. Bipolar Disord. 2009;11(4):391–400. doi: 10.1111/j.1399-5618.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellivier F, Etain B, Malafosse A, et al. Age at onset in bipolar I affective disorder in the USA and Europe. World J Biol Psychiatry. 2014;15(5):369–376. doi: 10.3109/15622975.2011.639801. [DOI] [PubMed] [Google Scholar]
- 20.Carter TD, Mundo E, Parikh SV, et al. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37(4):297–303. doi: 10.1016/s0022-3956(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 21.Etain B, Lajnef M, Bellivier F, et al. Clinical expression of bipolar disorder type I as a function of age and polarity at onset: convergent findings in samples from France and the United States. J Clin Psychiatry. 2012;73(4):e561–e566. doi: 10.4088/JCP.10m06504. [DOI] [PubMed] [Google Scholar]
- 22.Suominen K, Mantere O, Valtonen H, et al. Early age at onset of bipolar disorder is associated with more severe clinical features but delayed treatment seeking. Bipolar Disord. 2007;9(7):698–705. doi: 10.1111/j.1399-5618.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 23.Strober M, DeAntonio M, Schmidt-Lackner S, et al. Early childhood attention-deficit/hyperactivity disorder predicts poorer response to acute lithium therapy in adolescent mania. J Affect Disord. 1998;51(2):145–151. doi: 10.1016/s0165-0327(98)00213-4. [DOI] [PubMed] [Google Scholar]
- 24.Geller B, Tillman R, Bolhofner K, et al. Pharmacological and nondrug treatment of child bipolar I disorder during prospective eight-year follow-up. Bipolar Disord. 2010;12(2):164–171. doi: 10.1111/j.1399-5618.2010.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009a;166(7):795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DelBello MP, Hanseman D, Adler CM, et al. Twelve-month outcome of adolescents with bipolar disorder following first hospitalization for a manic or mixed episode. Am J Psychiatry. 2007;164(4):582–590. doi: 10.1176/ajp.2007.164.4.582. [DOI] [PubMed] [Google Scholar]
- 27.Wozniak J, Petty CR, Schreck M, et al. High level of persistence of pediatric bipolar I disorder from childhood onto adolescent years: a four year prospective longitudinal follow-up study. J Psychiatr Res. 2011;45(10):1273–1282. doi: 10.1016/j.jpsychires.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin PI, McInnis MG, Potash JB, et al. Clinical correlates and familial aggregation of age at onset in bipolar disorder. Am J Psychiatry. 2006;163(2):240–246. doi: 10.1176/appi.ajp.163.2.240. [DOI] [PubMed] [Google Scholar]
- 29.Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2005;44(9):846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- 30.Post RM, Fleming J, Kapczinski F. Neurobiological correlates of illness progression in the recurrent affective disorders. J Psychiatr Res. 2012a;46(5):561–573. doi: 10.1016/j.jpsychires.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Post RM, Kalivas P. Bipolar disorder and substance misuse: pathological and therapeutic implications of their comorbidity and cross-sensitisation. Br J Psychiatry. 2013;202(3):172–176. doi: 10.1192/bjp.bp.112.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 33.Kendler KS, Thornton LM, Gardner CO. Genetic risk, number of previous depressive episodes, and stressful life events in predicting onset of major depression. Am J Psychiatry. 2001;158(4):582–586. doi: 10.1176/appi.ajp.158.4.582. [DOI] [PubMed] [Google Scholar]
- 34.Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry. 1992;149(8):999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- 35.Shonkoff JP, Garner AS. Committee on Psychosocial Aspects of Child and Family Health, Section on Developmental and Behavioral Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 36.Rutter M, Thapar A, Pickles A. Gene-environment interactions: biologically valid pathway or artifact? Arch Gen Psychiatry. 2009;66(12):1287–1289. doi: 10.1001/archgenpsychiatry.2009.167. [DOI] [PubMed] [Google Scholar]
- 37.Slavich GM, Monroe SM, Gotlib IH. Early parental loss and depression history: associations with recent life stress in major depressive disorder. J Psychiatr Res. 2011;45(9):1146–1152. doi: 10.1016/j.jpsychires.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Risch N, Herrell R, Lehner T, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsankova N, Renthal W, Kumar A, et al. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(5):355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 40.Roth TL, Lubin FD, Funk AJ, et al. Lasting epigenetic influence of early life adversity on the BDNF gene. Biol Psychiatry. 2009;65(9):760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGowan PO, Sasaki A, D’Alessio AC, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meaney MJ, Ferguson-Smith AC. Epigenetic regulation of the neural transcriptome: the meaning of the marks. Nat Neurosci. 2010;13(11):1313–1318. doi: 10.1038/nn1110-1313. [DOI] [PubMed] [Google Scholar]
- 43.Mehta D, Klengel T, Conneely KN, et al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2013;110(20):8302–8307. doi: 10.1073/pnas.1217750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishnan V, Han MH, Graham DL, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Labonté B, Suderman M, Maussion G, et al. Genome-wide methylation changes in the brains of suicide completers. Am J Psychiatry. 2013;170(5):511–520. doi: 10.1176/appi.ajp.2012.12050627. [DOI] [PubMed] [Google Scholar]
- 46.Xin Y, Ge Y. DNA methylation in brain aging and depression. Biol Psychiatry. 2012;71(8):13. [Google Scholar]
- 47.Strakowski SM, DelBello MP, Fleck DE, et al. The impact of substance abuse on the course of bipolar disorder. Biol Psychiatry. 2000;48(6):477–485. doi: 10.1016/s0006-3223(00)00900-8. [DOI] [PubMed] [Google Scholar]
- 48.Kessing LV, Søndergård L, Forman JL, et al. Lithium treatment and risk of dementia. Arch Gen Psychiatry. 2008;65(11):1331–1335. doi: 10.1001/archpsyc.65.11.1331. [DOI] [PubMed] [Google Scholar]
- 49.Kessing LV, Andersen PK. Does the risk of developing dementia increase with the number of episodes in patients with depressive disorder and in patients with bipolar disorder? J Neurol Neurosurg Psychiatry. 2004;75(12):1662–1666. doi: 10.1136/jnnp.2003.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elvsåshagen T, Vera E, Bøen E, et al. The load of short telomeres is increased and associated with lifetime number of depressive episodes in bipolar II disorder. J Affect Disord. 2011;135(1–3):43–50. doi: 10.1016/j.jad.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Entringer S, Epel ES, Kumsta R, et al. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A. 2011;108(33):E513–E518. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolkowitz OM, Reus VI, Mellon SH. Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin Neurosci. 2011;13(1):25–39. doi: 10.31887/DCNS.2011.13.1/owolkowitz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinsson L, Wei Y, Xu D, et al. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl Psychiatr. 2013;3(5):e261. doi: 10.1038/tp.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. J Clin Psychiatry. 2003;64(5):506–515. doi: 10.4088/jcp.v64n0503. [DOI] [PubMed] [Google Scholar]
- 56.Post RM, Leverich GS, Altshuler LL, et al. Relationship of prior antidepressant exposure to long-term prospective outcome in bipolar I disorder outpatients. J Clin Psychiatry. 2012;73(7):924–930. doi: 10.4088/JCP.11m07396. [DOI] [PubMed] [Google Scholar]
- 57.Fagiolini A, Kupfer DJ, Houck PR, et al. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry. 2003;160(1):112–117. doi: 10.1176/appi.ajp.160.1.112. [DOI] [PubMed] [Google Scholar]
- 58.Kupka RW, Luckenbaugh DA, Post RM, et al. Comparison of rapid-cycling and non–rapid-cycling bipolar disorder based on prospective mood ratings in 539 outpatients. Am J Psychiatry. 2005;162(7):1273–1280. doi: 10.1176/appi.ajp.162.7.1273. [DOI] [PubMed] [Google Scholar]
- 59.Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder. Demographics, diagnosis, family history, and course. Arch Gen Psychiatry. 1992;49(2):126–131. doi: 10.1001/archpsyc.1992.01820020046006. [DOI] [PubMed] [Google Scholar]
- 60.Kupka RW, Altshuler LL, Nolen WA, et al. Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar Disord. 2007;9(5):531–535. doi: 10.1111/j.1399-5618.2007.00467.x. [DOI] [PubMed] [Google Scholar]
- 61.Kessing LV, Mortensen PB, Bolwig TG. Clinical definitions of sensitization in affective disorder: a case register study of prevalence and prediction. J Affect Disord. 1998;47(1–3):31–39. doi: 10.1016/s0165-0327(97)00081-5. [DOI] [PubMed] [Google Scholar]
- 62.Geller B, Harms MP, Wang L, et al. Effects of age, sex, and independent life events on amygdala and nucleus accumbens volumes in child bipolar I disorder. Biol Psychiatry. 2009;65(5):432–437. doi: 10.1016/j.biopsych.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calabrese JR, Shelton MD, Rapport DJ, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry. 2005;162(11):2152–2161. doi: 10.1176/appi.ajp.162.11.2152. [DOI] [PubMed] [Google Scholar]
- 64.Kemp DE, Gao K, Fein EB, et al. Lamotrigine as add-on treatment to lithium and divalproex: lessons learned from a double-blind, placebo-controlled trial in rapid-cycling bipolar disorder. Bipolar Disord. 2012;14(7):780–789. doi: 10.1111/bdi.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nierenberg AA, Friedman ES, Bowden CL, et al. Lithium treatment moderate-dose use study (LiTMUS) for bipolar disorder: a randomized comparative effectiveness trial of optimized personalized treatment with and without lithium. Am J Psychiatry. 2013;170(1):102–110. doi: 10.1176/appi.ajp.2012.12060751. [DOI] [PubMed] [Google Scholar]
- 66.Drancourt N, Etain B, Lajnef M, et al. Duration of untreated bipolar disorder: missed opportunities on the long road to optimal treatment. Acta Psychiatr Scand. 2013;127(2):136–144. doi: 10.1111/j.1600-0447.2012.01917.x. [DOI] [PubMed] [Google Scholar]
- 67.Chang K, Howe M, Gallelli K, et al. Prevention of pediatric bipolar disorder: integration of neurobiological and psychosocial processes. Ann N Y Acad Sci. 2006;1094(1):235–247. doi: 10.1196/annals.1376.026. [DOI] [PubMed] [Google Scholar]
- 68.Miklowitz DJ. Family-focused treatment for children and adolescents with bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(2):95–101. [PMC free article] [PubMed] [Google Scholar]
- 69.Miklowitz DJ, Schneck CD, Singh MK, et al. Early intervention for symptomatic youth at risk for bipolar disorder: a randomized trial of family-focused therapy. J Am Acad Child Adolesc Psychiatry. 2013;52(2):121–131. doi: 10.1016/j.jaac.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grigoroiu-Serbănescu M, Christodorescu D, Jipescu I, et al. Psychopathology in children aged 10–17 of bipolar parents: psychopathology rate and correlates of the severity of the psychopathology. J Affect Disord. 1989;16(2–3):167–179. doi: 10.1016/0165-0327(89)90071-2. [DOI] [PubMed] [Google Scholar]
- 71.Duffy A, Alda M, Crawford L, et al. The early manifestations of bipolar disorder: a longitudinal prospective study of the offspring of bipolar parents. Bipolar Disord. 2007;9(8):828–838. doi: 10.1111/j.1399-5618.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- 72.Vandeleur C, Rothen S, Gholam-Rezaee M, et al. Mental disorders in offspring of parents with bipolar and major depressive disorders. Bipolar Disord. 2012;14(6):641–653. doi: 10.1111/j.1399-5618.2012.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hillegers MH, Reichart CG, Wals M, et al. Five-year prospective outcome of psychopathology in the adolescent offspring of bipolar parents. Bipolar Disord. 2005;7(4):344–350. doi: 10.1111/j.1399-5618.2005.00215.x. [DOI] [PubMed] [Google Scholar]
- 74.Chang KD, Steiner H, Ketter TA. Psychiatric phenomenology of child and adolescent bipolar offspring. J Am Acad Child Adolesc Psychiatry. 2000;39(4):453–460. doi: 10.1097/00004583-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 75.Birmaher B, Ehmann M, Axelson DA, et al. Schedule for Affective Disorders and Schizophrenia for School-age Children (K-SADS-PL) for the assessment of preschool children: a preliminary psychometric study. J Psychiatr Res. 2009;43(7):680–686. doi: 10.1016/j.jpsychires.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009b;66(3):287–296. doi: 10.1001/archgenpsychiatry.2008.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henin A, Biederman J, Mick E, et al. Psychopathology in the offspring of parents with bipolar disorder: a controlled study. Biol Psychiatry. 2005;58(7):554–561. doi: 10.1016/j.biopsych.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 78.Nurnberger JI, Jr, McInnis M, Reich W, et al. A high-risk study of bipolar disorder: childhood clinical phenotypes as precursors of major mood disorders. Arch Gen Psychiatry. 2011;68(10):1012–1020. doi: 10.1001/archgenpsychiatry.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilens TE, Biederman J, Kwon A, et al. Risk of substance use disorders in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2004;43(11):1380–1386. doi: 10.1097/01.chi.0000140454.89323.99. [DOI] [PubMed] [Google Scholar]
- 80.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42. [PMC free article] [PubMed] [Google Scholar]
- 81.Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794–1796. doi: 10.1001/jama.298.15.1794. [DOI] [PubMed] [Google Scholar]
- 82.Kessing LV, Hellmund G, Andersen PK. Predictors of excellent response to lithium: results from a nationwide register-based study. Int Clin Psychopharmacol. 2011;26(6):323–328. doi: 10.1097/YIC.0b013e32834a5cd0. [DOI] [PubMed] [Google Scholar]
- 83.Kessing LV, Hansen HV, Hvenegaard A, et al. Early Intervention Affective Disorders (EIA) Trial Group. Treatment in a specialized outpatient mood disorder clinic v. standard out-patient treatment in the early course of bipolar disorder: randomised clinical trial. Br J Psychiatry. 2013;202(3):212–219. doi: 10.1192/bjp.bp.112.113548. [DOI] [PubMed] [Google Scholar]