Abstract
Oxidative stress contributes to the formation of cataracts. The leucine rich repeat containing G protein-coupled receptor 4 (LGR4, also known as GPR48), is important in many developmental processes. Since deletion of Lgr4 has previously been shown to lead to cataract formation in mice, we sought to determine the specific role that Lgr4 plays in the formation of cataracts. Initially, the lens opacities of Lgr4−/− mice at different ages without ocular anterior segment dysgenesis (ASD) were evaluated with slit-lamp biomicroscopy. Lenses from both Lgr4−/− and wild-type mice were subjected to oxidation induced protein denaturation to assess the ability of the lens to withstand oxidation. The expression of antioxidant enzymes was evaluated with real-time quantitative PCR. Phenotypically, Lgr4−/− mice showed earlier onset of lens opacification and higher incidence of cataract formation compared with wild-type mice of similar age. In addition, Lgr4−/− mice demonstrated increased sensitivity to environmental oxidative damage, as evidenced by altered protein expression. Real-time quantitative PCR showed that two prominent antioxidant defense enzymes, catalase (CAT) and superoxidase dismutase-1 (SOD1), were significantly decreased in the lens epithelial cells of Lgr4−/− mice. Our results suggest that the deletion of Lgr4 can lead to premature cataract formation, as well as progressive deterioration with aging. Oxidative stress and altered expression of several antioxidant defense enzymes contribute to the formation of cataracts.
Introduction
Age-related cataracts are the most frequent cause of treatable blindness in the world [1]. Cataract formation entails a progressive opacification of the ocular lens, leading to deterioration of the optic image quality formed on the retina, and eventually causing blindness. With increasing life expectancy, the incidence of age-related cataracts will likely increase and produce both a significant medical challenge and socio-economic burden worldwide [2].
Oxidative insult appears to be the most important risk factor for age-related cataract formation [3]. The lens of the vertebrate eye is comprised of a single layer of epithelial cells on its anterior hemispheric surface, with fiber cells that differentiated from epithelial cells making up the bulk of its volume. Most of the metabolic, synthetic, and active transport machineries in the lens are localized to lens epithelial (nucleated) cells. Environmental insults such as oxidative stress and UV radiation are mitigated by epithelial cells through multiple cellular defense mechanisms that begin with altered gene expression [4,5]. The numerous protective mechanisms that have evolved against oxidative stress in the ocular lens make it an excellent model for studying both the biology of aging and the molecular mechanisms associated with oxidative stress.
Several mechanisms have been developed to maintain the redox state of the lens. Aging of the lens is characterized by accumulation of oxidized lens components (especially reactive oxygen species, ROS) and diminished activities of deoxidizing enzymes [6]. Many of the protein and membrane alterations observed in human cataract lenses are due to oxidative origin, and incubation of animal lenses in the presence of hydrogen peroxide (or other oxidants) reproduces many of those changes. Previous works have provided evidence that human lens epithelium is capable of responding to the presence of oxidative stress through the altered expression of numerous genes, including superoxide dismutase, catalase, and glutathione peroxidase [7,8]. A caveat to those findings is that numerous investigations into antioxidants and deoxidizing enzymes are undertaken with the intent of preventing or reversing the damages associated with age-related cataract formation.
Lgr4 (leucine-rich repeat containing G protein-coupled receptor 4), also known as Gpr48 (G protein-coupled receptor 48), is one of the genes under active investigation in our laboratory. LGR4 is widely expressed in multiple organs such as intestines, heart, kidneys, cartilage, reproductive tracts and the nervous system, with LGR4 playing important roles in the development of these organs in mice [9–14]. Specifically, we have previously demonstrated that inactivation of Lgr4 induced an eye open at birth phenotype by reducing epithelial cell proliferation and migration through HB-EGF mediated EGFR activation [15,16]. In another study, we found that deletion of Lgr4 led to ocular anterior segment dysgenesis (ASD) [17]. Previously, we have described that about 26% of Lgr4 knockout mice have cataracts, lens fiber disorganization and abnormal protein deposition in lenses [17]. However, its mechanism was unclear.
In this study, we first verified that Lgr4 null mice had early onset of cataracts, along with description of their phenotype with regards to the cataracts. Subsequently, we determined that Lgr4 knockout led to age-related cataract formation in mice. We then performed studies to investigate if Lgr4 deletion attenuated the antioxidative damage ability of lens epithelial cells. Our results will hopefully lead to the identification of novel targets for the prevention and treatment of age-related cataract formation.
Materials and Methods
Mice
Lgr4 +/− mice were generated as previously described [17]. These mice were maintained on a mixed 129 x C57BL/6 background. Heterozygous mice were intercrossed to generate homozygous Lgr4 −/− mice and wild-type littermate controls. All studies and procedures were approved by the Wenzhou Medical University Animal Care and Use Committee.
Genomic DNA was extracted from mouse tails, and genotypes were determined by PCR analysis using three primers as previously described [17]. Six-week-old Lgr4 knockout mice that had no ocular ASD were selected and raised for the following experiments. Age-matched wild-type mice were used as negative control. Mice were sacrificed by neck dislocation prior to experiments.
X-gal staining
To perform X-gal staining, embryonic mice heads or eyes of adult mice were embedded in frozen section medium (Neg-50; Richard-Allen Scientific, Kalamazoo, MI) in preparation for making 10 μm thick frozen sections. The frozen sections were then placed on the slides. The slides were immediately incubated with the β-glycosidase fixative buffer (0.2% glutaraldehyde, 1.5% formaldehyde, 2 mM MgCl2, 5 mM EDTA, 0.1 M sodium phosphate buffer, pH 8.0) for 30 minutes and soaked three times for 15 minutes each in the washing buffer (0.1 M sodium phosphate buffer, 2 mM MgCl2, 5 mM EDTA, 0.01% sodium deoxycholate, 0.02% Nonidet P40, pH 8.0). Subsequently, the slides were incubated in the staining solution (washing buffer containing 1 mg/mL X-gal, 5 mM K3[Fe(CN)6], 5 mM K4[Fe(CN)6]x3H2O) until results were optimized. The slides were then counterstained with eosin (Shanghai SSS Reagent Co., Shanghai, China) before they were evaluated and photographed under a microscope (Imager Z1; Carl Zeiss, Oberkochen, Germany).
Slit-Lamp Biomicroscopy
Selected mice were raised to 70 weeks and the lens changes were observed every week. Mice pupils were dilated with eye drops containing 0.5% tropicamide and 0.5% phenylephrine hydrochloride. Approximately 10 minutes later, the mice were examined with a slit lamp (Photo Slit-Lamp; Haag-Streit AG International, Koeniz, Switzerland). The pictures were analyzed using Eye CapTM Image Capture Software (Haag-Streit AG International).
Oxidation Exposure
Fresh clear lenses procured from variably aged mice were pre-incubated in TC-199 medium for 2 hours in a CO2 incubator. Lenses were then transferred onto 96-well plates containing 200 μL of fresh TC-199 medium containing 0.1 mM H2O2 and 2.31 units glucose oxidase (GO) to achieve a final concentration of 0.2 mM H2O2 and incubated for 24 hours [18]. Lens images were captured from culture plates at 0, 12, and 24 hours after H2O2 treatment using a dark field microscopy (Olympus BX40; Olympus, Tokyo, Japan).
For the protein denaturation assay, fresh clear lenses from knockout and wild-type mice at different weeks were disrupted in 1,000 μL Tris-HCl buffer (pH 7.4) containing 0.1% glucose, 0.1 mM H2O2, and 2.31 units GO. The homogenate was centrifuged for 20 minutes at 4°C in a 10,000g microfuge twice. Protein concentrations of the supernatant were determined by BCA assay (Pierce, Rockford, IL) and samples were diluted to 1 mg/ml protein. Samples were then placed in a 37°C water bath for indicated times and measured by a spectrophotometer at 360 nm.
RNA Isolation and cDNA Synthesis
The lens epithelium was removed by grasping the capsule with fine forceps and carefully peeling it away from the underlying fibrous cell mass as described [19]. RNA isolation was performed using standard protocol. Subsequently, it was treated with DNase I, and reverse-transcribed with RevertAid M-MuLV Reverse Transcriptase using oligo (dT) primers to synthesize the cDNA.
Determination of mRNA Levels
Gene expression was determined with an ABI Prism 7700 Sequence Detection system (Applied Biosystems, Foster City, CA) using SYBR-green technology. PCR primers were designed using Primer Express 1.5 Software with the manufacturer’s default settings. After PCR amplification, dissociation curves were constructed to confirm formation of the intended PCR products. For quantification, mRNA expression was normalized to the expressed housekeeping gene GAPDH. Relative expression levels were calculated with the Ct rule (Applied Biosystems, User Bulletin No. 2).
Statistical Analysis
Each assay was performed in triplicate, and all data are expressed as mean ± SD or mean ± SEM. Statistical analyses were performed using independent samples and Student’s t-test or Fisher’s exact test. Two-tailed values of P < 0.05 were considered statistically significant. The software package SPSS version 11.0 (SPSS Inc) was used for statistical analyses.
Results
Genotypic and phenotypic characterization of knockout mice
The presence of the mutated gene in the Lgr4 null offspring was initially verified. As shown in Fig. 1A, PCR analysis of the null mouse genes showed that the expected recombination event had occurred. The size of the PCR product was approximately 1 kb for the wild-type allele and approximately 700 bp for the targeted allele.
Fig 1. Deletion of Lgr4 in mice causes different types of age-related cataract formation.
(A) Disruption of the mouse Lgr4 gene. Genotype analysis of Lgr4 +/+, Lgr4 −/−, and Lgr4 +/− mice. +/+, −/−, and +/− represent genomic DNAs isolated from Lgr4 +/+, Lgr4 −/−, and Lgr4 +/− mice, respectively. The 1 kb band is derived from the wild-type allele, and the 700 bp band is derived from the mutated allele. (B) Slit-lamp biomicroscopy examination of wild-type and Lgr4 knockout lenses. At 20 weeks of age, diverse morphologies and various degrees of lens opacification were observed in the anterior portion of the cortex in Lgr4 knockout mice, while most of the wild-type mice remained clear (a,b). Punctate anterior cortical cataract (c). Lamellar cortical cataract (d). The cataract region includes the Y-sutures and looks like a “fishbone” pattern (e). The whole lens becomes opaque (f). (C) Expression profile of Lgr4 in mouse lens. LacZ staining of heterozygous lenses showed strong Lgr4 expression in lens epithelial cells from both the embryonic and adult mice at different ages.
Mice heterozygous for Lgr4 and the wild-type mice had no apparent abnormalities. As we have reported [17], many homozygous Lgr4 −/− mice showed ocular ASD of varying degrees (data not shown). To avoid the possibility that the onset of cataracts is a secondary result of ASD, we selected and raised mice without obvious ocular ASD and their wild-type littermates for the subsequent experiments. Slit-lamp biomicroscopy results showed that Lgr4 −/− mice developed a variety of cataracts whereas most of the lenses from the wild-type mice remained clear (Fig. 1B). As lens epithelial cells play important roles in the development of cataracts, we investigated the detailed expression profile of Lgr4 in lens epithelial cells. LacZ staining of heterozygous lenses confirmed the presence of the mutated gene in lens epithelial cells from both the embryonic and adult mice with strong staining using β-galactosidase (Fig. 1C). The expression of β-galactosidase translates into strong Lgr4 expression assuming that the presence of the marker is a reflection of Lgr4 expression.
Lgr4 deletion leads to the early onset of age-related cataract
To further analyze the cataract phenotype of Lgr4 −/− mice, both direct ophthalmic examination and slit lamp microscopy were performed on lenses of homozygous Lgr4 −/− and wild-type animals. A total of 112 Lgr4 −/− mice and 164 wild-type mice, ranging from 6 weeks to 70 weeks, were examined. Homozygous Lgr4 −/− mice with nontransparent lens accounted for about 47.3% (53/112) of total mutant adults, whereas only 17.7% (29/164) wild-type mice showed cataracts. More importantly, we were able to confirm that risk of cataract formation is progressive with increasing age well pass maturity for the mice, suggesting that Lgr4 in cataract formation is maintained beyond the developmental stage. Many Lgr4 knockout murine lenses showed opacification, starting at the age of 9–16 weeks (Fig. 2), with the incidence progressively worsening with advancing age. In mice older than 24 weeks, more than half of the null mice (64.4%) suffered from cataract formation in comparison to only 25.8% of the wild-type animals. These results indicated that Lgr4 deletion leads to early onset and increased incidence of age-related cataract.
Fig 2. Lgr4 deletion leads to the early onset of age-related cataract.
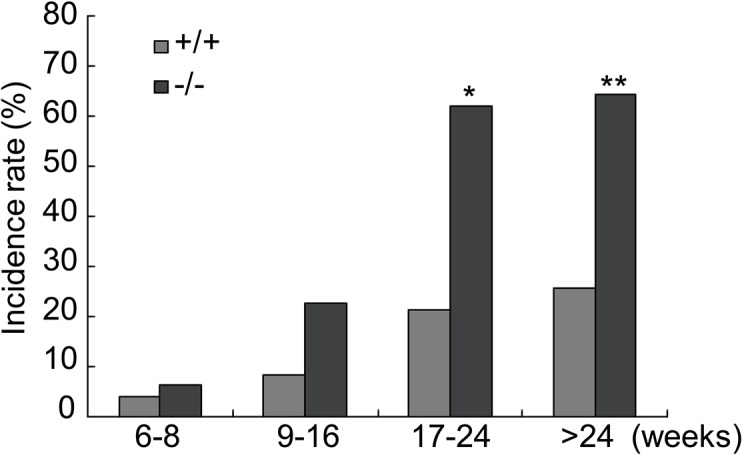
At the age of 6 to 8 weeks (wild-type: n = 24; Lgr4 −/−: n = 16), Lgr4 −/− mice showed no increased incidence of cataract formation. In the second stage (9 to 16 weeks, wild-type: n = 36; Lgr4 −/−: n = 22), the incidence of cataract formation was increased in Lgr4 −/− mice. The incidence of cataracts in Lgr4 −/− mice was significantly increased by maturity (17 to 24 weeks, wild-type: n = 42; Lgr4 −/−: n = 29) and older than 24 weeks (wild-type: n = 62; Lgr4 −/−: n = 45). *P < 0.001, **P < 0.0001.
The external morphologies of lenses from the young animals (6–16 weeks) showed no obvious difference between the two groups. However, a mild loss of lens clarity with different types in mutant animals was observed after 16 weeks of age, which progressed in severity with aging. Cortical cataracts located in the anterior portion of the lenses were evident by 20 weeks of age and becomes progressive. Formation of the Y-structure along with a white nontransparent fishbone pattern occurred in the lenses by 6 months of age. In contrast, wild-type littermates were usually clear with only minor opacifications (Fig. 1B).
Lgr4 knockout attenuates the antioxidative ability of the lens epithelial cells
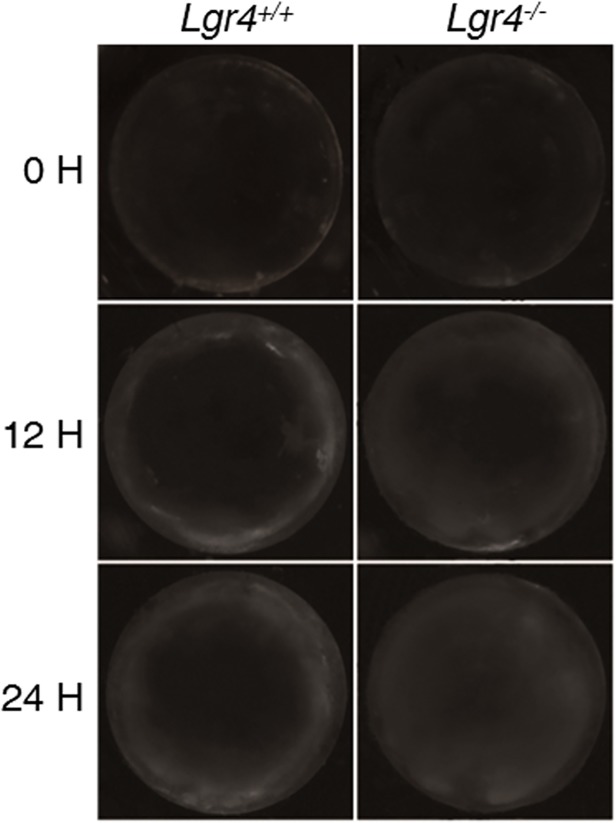
To investigate the mechanisms of Lgr4 deletion induced age-related cataract formation, we studied the ability of lens proteins to resist oxidative stress. Whole lenses from Lgr4 −/− mice and their wild-type littermates at different ages were treated with 0.2 mM H2O2, and the lens opacity was observed at different times. The results showed that 10-week old animals did not display significant differences in the degree of opacity; both groups remained clear during the 24-hour procedure (data not shown). However, phenotypically normal lenses from mice older than 24 weeks (both knockout and wild-type) exposed to 0.2 mM H2O2 displayed variability in their tolerance to oxidative stress. Both lenses maintained transparency in the first 6 hours of culture. Lenses derived from Lgr4 −/− mice started showing opacification at 12 hours, initially in the peripheral cortical regions. With prolonged exposure, haziness expanded to involve the entire cortex. The lenses from the Lgr4 −/− mice were cloudy throughout, whereas the lenses from wild-type mice showed only slight cloudiness (Fig. 3).
Fig 3. Morphologic changes of murine lenses cultured in the presence of H2O2.
Lenses removed from 30-week-old mice were subjected to peroxidase damage (H2O2) and incubated for 24 hours. Lens images were captured on culture plates at 0, 12, and 24 hours after H2O2 treatment using dark field microscopy. Representative images show that the degree of lens opacity in the knockout mice was significantly more than in wild-type mice. All images are representative of at least 3 independent experiments.
To further confirm that Lgr4 deletion would attenuate the antioxidative ability of lens epithelial cells, we performed protein denaturation assay. Fresh transparent lens homogenates from Lgr4 −/− mice and wild-type mice at different weeks were treated with 0.2 mM H2O2 and incubated at 37°C for indicated times. The turbidity was examined using spectrophotometry to illustrate amount of denaturation. Similar to the whole lens incubation assay, there was no significant difference between young Lgr4 −/− mice and their wild-type littermates (<10 weeks). However, lens homogenates of Lgr4 −/− mice denatured much faster than their comparable littermates at older age (>18 weeks) (Fig. 4). These results indicate that the deletion of Lgr4 significantly attenuates the antioxidative ability of lens epithelial cells.
Fig 4. Oxidation-induced denaturation of proteins in Lgr4 knockout lenses.
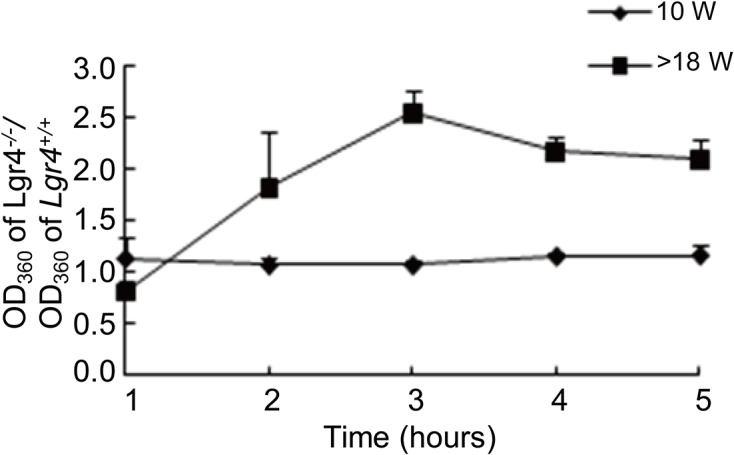
Lens supernatants of 10 weeks (10 W) and >18 weeks (>18 W) diluted to 1 mg/mL protein using the TC-199 medium were subjected to oxidative damage with 0.1 mM H2O2 and 2.31 units of glucose oxidase (GO) for indicated times. Sample absorbance was measured at 360 nM. Supernatants from Lgr4 knockout lenses showed a more rapid increase in turbidity (and therefore, protein denaturation), suggesting that the knockout lens had less overall resistance to oxidation than wild-type. Data are expressed as mean ± SD (n = 3). These results are representative of 3 independent experiments.
Lgr4 knockout down-regulates the expression of antioxidant enzymes in lens epithelial cells
To evaluate the expression of antioxidant enzymes in lens epithelial cells, the lens epithelium from Lgr4 −/− mice and their wild-type littermates of 24 weeks old was removed as described. Total RNA was extracted and the gene expression was measured by real-time PCR. Of the three antioxidant defense enzymes, the expressions of catalase (CAT) and superoxidase dismutase-1 (SOD1) were significantly down-regulated in Lgr4 −/− mice compared to those in wild-type animals. The gene glutathione peroxidase-1 (GPX1) was not changed under the present experimental conditions (Fig. 5).
Fig 5. The gene expression levels of antioxidant enzymes are reduced in Lgr4 −/− mice older than 24 weeks.
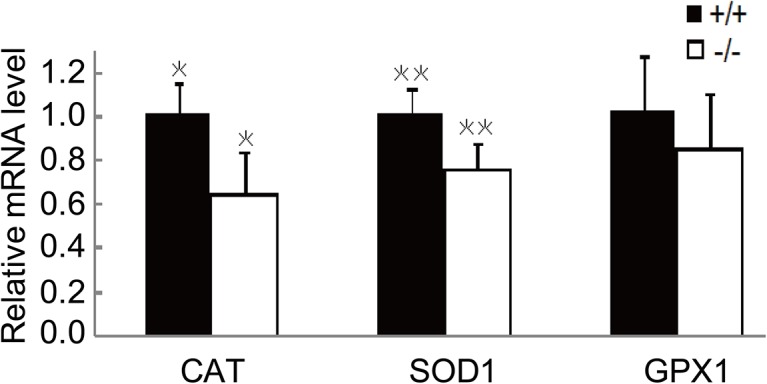
The lens epithelium from Lgr4 −/− mice and their wild-type littermates was isolated as described in Materials and Methods. Total RNA was extracted and the gene expression was measured by real-time PCR. The data were normalized to the level in wild-type mice. Results are expressed as mean ± SEM. n = 3, *P < 0.01.
Discussion
LGR4 has been implicated in many physiological processes including embryonic development, cell motility, and tumor metastasis [10–15,20–27]. Recently, a nonsense mutation (c.376C>T) of LGR4, which abolishes the signal transduction of LGR4, has been reported in humans to be strongly associated with low bone mineral density, osteoporotic features, electrolyte imbalances, reduced testosterone levels, and increased risk of cutaneous squamous cell cancers and biliary tract cancers [28]. Interestingly, these characteristics overlap with those of Lgr4 knockout mice [28]. This supports the notion that LGR4 may be important in human development as well. The role of LGR4 in mice has been well established; yet, its roles in vivo under normal physiological conditions remain poorly understood with regards to the pathogenesis of lens opacification.
In this study, we sought to investigate the role of Lgr4 in cataract formation using a line of Lgr4 knockout mice. We found Lgr4 was strongly expressed in lens epithelial cells from both the embryonic and adult mice. We selected and raised Lgr4 −/− mice without obvious ASD and their wild-type littermates for experiments to avoid the possibility that the onset of cataract is a secondary result of ASD. We found that Lgr4 −/− mice established early onset cataract, and the lens opacification was progressive in severity. More importantly, the incidence of cataract formation was much higher in Lgr4 −/− mice compared with wild-type of the same age. These results indicated that Lgr4 mitigates the onset of age-related cataract formation.
To elucidate the mechanisms of Lgr4 mediated age-related cataract formation, the lens from Lgr4 knockout mice was treated with H2O2, and the resultant lens opacification was imaged. Moreover, we also tested the stability of the total lens protein, most of which are crystallins, in younger and older Lgr4 −/− mice. All the results indicated that Lgr4 deletion attenuates the antioxidant capacity of the lens.
We performed early initial investigations into the cause of Lgr4 related cataract formation with analysis of the lens gap junctions. Lens epithelial and fiber cells contain distinct gap junctions, oligomeric proteins called connexins (Cx) [29]. There are three connexins identified in the lens: Cx43 is expressed only in epithelial cells, Cx46 is expressed only in fiber cells, and Cx50 is expressed in both epithelial cells and fiber cells [30]. Using Western blot analysis and immunofluorescence assay, no marked defects of the three connexins were observed in the lenses of Lgr4 knockout compared with wild-type (data not shown). These results suggest that knockout of Lgr4 gene does not increase cataract formation through disruption of the gap junction channels.
One of the mechanisms by which mammals prevent age-related cataract formation is up-regulation of antioxidant defense enzymes, which are mainly expressed by the lens epithelial cells. We isolated the lens epithelium from Lgr4 −/− mice and their wild-type littermates, and the expression of three main antioxidant defense enzymes (CAT, SOD1, GPX1) was evaluated by real-time quantitative PCR. The results showed that CAT and SOD1 were significantly reduced due to Lgr4 deletion. Reactive oxygen species (ROS) including the superoxide anion (O2 -), hydroxyl free radicals (OH-), and H2O2 may interfere with the redox state of the lens, and contribute to cataract formation [31]. SOD1 catalyzes the conversion of O2 - to H2O2. H2O2 can be degraded enzymatically by CAT, an enzyme that specifically uses H2O2 as a substrate, or by GPX1, a selenoenzyme that reduces peroxides and requires glutathione [32]. Thus, our data indicate that Lgr4 deletion may accelerate the onset of age-related cataract formation through down-regulation of SOD1 and CAT.
The process of LGR4 regulation of the antioxidant enzymes in lens epithelial cells remains elusive. Several pathways have been suggested for LGR4 regulation of antioxidant enzymes. LGR4 has long been considered an orphan receptor until recent discoveries identified R-spondin as the extracellular ligand of LGR4 [33–35]. R-spondin binding to LGR4 potentiates Wnt signaling pathway by inhibiting ZNR3 and RNF43, which promotes degradation of the Wnt receptor Frz and LRP5/6 [36]. LGR4 mediated Wnt signaling pathway has been implicated in stem cell maintenance in many tissues [34]. In addition, LGR4 has been shown to signal through classical G protein-coupled receptor signaling in multiple tissues. In this pathway, cyclin AMP (cAMP) is activated due to activation of LGR4, and cAMP activates protein kinase A (PKA) which in turn phosphorylates the transcription factor Cre-binding protein leading to expression of target genes. Reported LGR4 targets regulated by cAMP/PKA pathway include the estrogen receptor α, ATF4, Pitx2, and mineralocorticoids [37]. However, the ligands that activate cAMP/PKA signaling pathway remain unidentified. Our group has previously reported that LGR4 regulates the proliferation and migration of keratinocyte through HB-EGF mediated EGFR transactivation [15]. Wnt, cAMP/PKA and EGFR signaling pathways have all been reported to be involved in the regulation of antioxidative stress ability in lens epithelial cells [38,39]. However, the pathway responsible for LGR4 mediated development of age-related cataract still needs further investigation.
In conclusion, Lgr4 knockout mice developed age-related cataract at 17 to 24 weeks after birth. The opacification begins in the anterior and peripheral cortical zone and the nucleus of the lens, and progresses both in extent and severity with increasing age. The lens proteins showed reduced resistance to oxidative stress. Expression levels of CAT and SOD1 are decreased in Lgr4 −/− lens. These results suggest murine knockout of Lgr4 gene induces age-related cataract by down-regulating the gene expression of antioxidant defense enzymes in lens epithelial cells.
Acknowledgments
The authors thank Xiaogang Chen for assistance with PCR and Jun Gao for assistance with LacZ staining.
Data Availability
All relevant data are within the paper.
Funding Statement
Support was provided by National Natural Science Foundation of China Grants 81100671 for Q. Hou and 81201657 for Z. Wang; Zhejiang Provincial Natural Science Foundation of China Grant Y2110609 for Q. Hou. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bastawrous A, Dean WH, Sherwin JC. Blindness and visual impairment due to age-related cataract in sub-Saharan Africa: a systematic review of recent population-based studies. Br J Ophthalmol. 2013; 97: 1237–1243. 10.1136/bjophthalmol-2013-303135 [DOI] [PubMed] [Google Scholar]
- 2. Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012; 96: 614–618. 10.1136/bjophthalmol-2011-300539 [DOI] [PubMed] [Google Scholar]
- 3. Beebe DC, Holekamp NM, Shui YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010; 44: 155–165. 10.1159/000316481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mathias RT, Rae JL. The lens: local transport and global transparency. Exp Eye Res. 2004; 78: 689–698. [DOI] [PubMed] [Google Scholar]
- 5. Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004; 82: 844–851. [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng Y, Liu Y, Ge J, Wang X, Liu L, Bu Z, et al. Resveratrol protects human lens epithelial cells against H2O2-induced oxidative stress by increasing catalase, SOD-1, and HO-1 expression. Mol Vis. 2010; 16: 1467–1474. [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Y, Zhang L, Sun D, Li Z, Wang L, Liu P. Genetic polymorphisms of superoxide dismutases, catalase, and glutathione peroxidase in age-related cataract. Mol Vis. 2011; 17: 2325–2332. [PMC free article] [PubMed] [Google Scholar]
- 8. Bhabak KP, Mugesh G. Functional mimics of glutathione peroxidase: bioinspired synthetic antioxidants. Acc Chem Res. 2010; 43: 1408–1419. 10.1021/ar100059g [DOI] [PubMed] [Google Scholar]
- 9. Du B, Luo W, Li R, Tan B, Han H, Lu X, et al. Lgr4/Gpr48 negatively regulates TLR2/4-associated pattern recognition and innate immunity by targeting CD14 expression. J Biol Chem. 2013; 288: 15131–15141. 10.1074/jbc.M113.455535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mazerbourg S, Bouley DM, Sudo S, Klein CA, Zhang JV, Kawamura K, et al. Leucine-rich repeat-containing, G protein-coupled receptor 4 null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality. Mol Endocrinol. 2004; 18: 2241–2254. [DOI] [PubMed] [Google Scholar]
- 11. Yi T, Weng J, Siwko S, Luo J, Li D, Liu M. LGR4/GPR48 inactivation leads to aniridia-genitourinary anomalies-mental retardation syndrome defects. J Biol Chem. 2014; 289: 8767–8780. 10.1074/jbc.M113.530816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang F, Zhang X, Wang J, Chen M, Fan N, Ma Q, et al. LGR4 acts as a link between the peripheral circadian clock and lipid metabolism in liver. J Mol Endocrinol. 2014; 52: 133–143. 10.1530/JME-13-0042 [DOI] [PubMed] [Google Scholar]
- 13. Pan H, Cui H, Liu S, Qian Y, Wu H, Li L, et al. Lgr4 gene regulates corpus luteum maturation through modulation of the WNT-mediated EGFR-ERK signaling pathway. Endocrinology. 2014; 155: 3624–3637. 10.1210/en.2013-2183 [DOI] [PubMed] [Google Scholar]
- 14. Kinzel B, Pikiolek M, Orsini V, Sprunger J, Isken A, Zietzling S, et al. Functional roles of Lgr4 and Lgr5 in embryonic gut, kidney and skin development in mice. Dev Biol. 2014; 390: 181–190. 10.1016/j.ydbio.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 15. Wang Z, Jin C, Li H, Li C, Hou Q, Liu M, et al. GPR48-Induced keratinocyte proliferation occurs through HB-EGF mediated EGFR transactivation. FEBS Lett. 2010; 584: 4057–4062. 10.1016/j.febslet.2010.08.028 [DOI] [PubMed] [Google Scholar]
- 16. Jin C, Yin F, Lin M, Li H, Wang Z, Weng J, et al. GPR48 regulates epithelial cell proliferation and migration by activating EGFR during eyelid development. Invest Ophthalmol Vis Sci. 2008; 49: 4245–4253. 10.1167/iovs.08-1860 [DOI] [PubMed] [Google Scholar]
- 17. Weng J, Luo J, Cheng X, Jin C, Zhou X, Qu J, et al. Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proc Natl Acad Sci U S A. 2008; 105: 6081–6086. 10.1073/pnas.0708257105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang J, Li J, Huang C, Xue L, Peng Y, Fu Q, et al. Targeted knockout of the mouse betaB2-crystallin gene (Crybb2) induces age-related cataract. Invest Ophthalmol Vis Sci. 2008; 49: 5476–5483. 10.1167/iovs.08-2179 [DOI] [PubMed] [Google Scholar]
- 19. Zandy AJ, Lakhani S, Zheng T, Flavell RA, Bassnett S. Role of the executioner caspases during lens development. J Biol Chem. 2005; 280: 30263–30272. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Dong J, Li D, Lai L, Siwko S, Li Y, et al. Lgr4 regulates mammary gland development and stem cell activity through the pluripotency transcription factor Sox2. Stem Cells. 2013; 31: 1921–1931. 10.1002/stem.1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoshii T, Takeo T, Nakagata N, Takeya M, Araki K, Yamamura K. LGR4 Regulates the Postnatal Development and Integrity of Male Reproductive Tracts in Mice. Biol Reprod. 2007; 76: 303–313. [DOI] [PubMed] [Google Scholar]
- 22. Kato S, Matsubara M, Matsuo T, Mohri Y, Kazama I, Hatano R, et al. Leucine-rich repeat-containing G protein-coupled receptor-4 (LGR4, Gpr48) is essential for renal development in mice. Nephron Exp Nephrol. 2006; 104: e63–75. [DOI] [PubMed] [Google Scholar]
- 23. Yamashita R, Takegawa Y, Sakumoto M, Nakahara M, Kawazu H, Hoshii T, et al. Defective development of the gall bladder and cystic duct in Lgr4- hypomorphic mice. Dev Dyn. 2009; 238: 993–1000. 10.1002/dvdy.21900 [DOI] [PubMed] [Google Scholar]
- 24. Mendive F, Laurent P, Van Schoore G, Skarnes W, Pochet R, Vassart G. Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev Biol. 2006; 290: 421–434. [DOI] [PubMed] [Google Scholar]
- 25. Gao Y, Kitagawa K, Hiramatsu Y, Kikuchi H, Isobe T, Shimada M, et al. Up-regulation of GPR48 induced by down-regulation of p27Kip1 enhances carcinoma cell invasiveness and metastasis. Cancer Res. 2006; 66: 11623–11631. [DOI] [PubMed] [Google Scholar]
- 26. Zhu YB, Xu L, Chen M, Ma HN, Lou F. GPR48 Promotes Multiple Cancer Cell Proliferation via Activation of Wnt Signaling. Asian Pac J Cancer Prev. 2013; 14: 4775–4778. [DOI] [PubMed] [Google Scholar]
- 27. Wu J, Xie N, Xie K, Zeng J, Cheng L, Lei Y, et al. GPR48, a poor prognostic factor, promotes tumor metastasis and activates beta-catenin/TCF signaling in colorectal cancer. Carcinogenesis. 2013; 34: 2861–2869. 10.1093/carcin/bgt229 [DOI] [PubMed] [Google Scholar]
- 28. Styrkarsdottir U, Thorleifsson G, Sulem P, Gudbjartsson DF, Sigurdsson A, Jonasdottir A, et al. Nonsense mutation in the LGR4 gene is associated with several human diseases and other traits. Nature. 2013; 497: 517–520. 10.1038/nature12124 [DOI] [PubMed] [Google Scholar]
- 29. Berthoud VM, Beyer EC. Oxidative stress, lens gap junctions, and cataracts. Antioxid Redox Signal. 2009; 11: 339–353. 10.1089/ars.2008.2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007; 94: 120–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spector A. Review: Oxidative stress and disease. J Ocul Pharmacol Ther. 2000; 16: 193–201. [DOI] [PubMed] [Google Scholar]
- 32. Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001; 52: 35–41. [DOI] [PubMed] [Google Scholar]
- 33. Glinka A, Dolde C, Kirsch N, Huang YL, Kazanskaya O, Ingelfinger D, et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep. 2011; 12: 1055–1061. 10.1038/embor.2011.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011; 476: 293–297. 10.1038/nature10337 [DOI] [PubMed] [Google Scholar]
- 35. Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2011; 108: 11452–11457. 10.1073/pnas.1106083108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xie Y, Zamponi R, Charlat O, Ramones M, Swalley S, Jiang X, et al. Interaction with both ZNRF3 and LGR4 is required for the signalling activity of R-spondin. EMBO Rep. 2013; 14: 1120–1126. 10.1038/embor.2013.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siwko S, Lai L, Weng J, Liu M. Lgr4 in ocular development and glaucoma. J Ophthalmol. 2013; 2013: 987494 10.1155/2013/987494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen KC, Zhou Y, Zhang W, Lou MF. Control of PDGF-induced reactive oxygen species (ROS) generation and signal transduction in human lens epithelial cells. Mol Vis. 2007; 13: 374–387. [PMC free article] [PubMed] [Google Scholar]
- 39. Chong CC, Stump RJ, Lovicu FJ, McAvoy JW. TGFbeta promotes Wnt expression during cataract development. Exp Eye Res. 2009; 88: 307–313. 10.1016/j.exer.2008.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.