Abstract
Air pollution epidemiology continues moving toward the study of mixtures and multi-pollutant modeling. Simultaneously, there is a movement in epidemiology to estimate policy-relevant health effects that can be understood in reference to specific interventions. Scaling regression coefficients from a regression model by an interquartile range (IQR) is one common approach to presenting multi-pollutant health effect estimates. We are unaware of guidance on how to interpret these effect estimates as an intervention. To illustrate the issues of interpretability of IQR-scaled air pollution health effects, we analyzed how daily concentration changes in two air pollutants (NO2 and PM2.5; nitrogen dioxide and particulate matter with aerodynamic diameter ≤ 2.5μm) related to one another within two seasons (summer and winter), within three cities with distinct air pollution profiles (Burbank, California; Houston, Texas; and Pittsburgh, Pennsylvania). In each city-season, we examined how realistically IQR-scaling in multipollutant lag-1 time-series studies reflects a hypothetical intervention that is possible given the observed data. We proposed 2 causal conditions to explicitly link IQR-scaled effects to a clearly defined hypothetical intervention. Condition 1 specified that the index pollutant had to experience a daily concentration change of greater than one IQR, reflecting the notion that the IQR is an appropriate measure of variability between consecutive days. Condition 2 specified that the co-pollutant had to remain relatively constant. We found that in some city-seasons, there were very few instances in which these conditions were satisfied (e.g., 1 day in Pittsburgh during summer). We discuss the practical implications of IQR scaling and suggest alternative approaches to presenting multi-pollutant effects that are supported by empirical data.
Air pollution epidemiology is increasingly moving toward analysis of mixtures and multi-pollutant modeling,1,2 which has motivated a robust discussion of the analytical methods and regulatory frameworks that are best-suited for this complex undertaking.3–5 Including multiple co-pollutants as independent variables in a regression model remains a common technique in time-series studies of acute effects.6–14 In this approach, the investigator often multiplies the regression coefficient by some large concentration change (e.g., an interquartile range [IQR]) to express the health effect of one pollutant, controlling for the co-pollutant(s).12,13,15 This scaling technique is a form of regression coefficient standardization.
Although the statistical challenges associated with highly correlated exposure variables are well-known in air pollution epidemiology,3,16 there has been less attention paid to the causal interpretation of health effects under this IQR scaling framework. Although there is a movement in epidemiology to estimate policy-relevant effects that can be defined in reference to specific interventions,17–19 investigators rarely explicitly state the intervention that IQR-scaled health effects correspond to. As researchers continue to improve communication between the fields of epidemiology and risk assessment, more explicit dialogue about the chosen modeling and scaling approach behind effect estimate calculation will enable more evidence-based regulatory policies.20 Regression coefficient standardization is argued to permit comparison of health effects of multiple exposures on one outcome, or one association across various populations.21,22 However, the technique has come under criticism for conflating specific characteristics of a given study (e.g., sampling design) and causal effects.23 For example, two hypothetical studies with identical populations and identical health effect estimates of a single pollutant but very different exposure distributions (say, due to sampling) will result in greatly different scaled effects. Thus, the scaling of the health effect estimate is very sensitive to the exposure profile of the sample(s), as well as to the effect of exposure.24 It has been argued that such scaling results in effect estimates without causal interpretation.25
In recent years, other sub-fields of epidemiology have grappled with how to explicitly define the intervention that a given effect estimate corresponds to. Social epidemiology, in particular, has articulated the challenges of defining interventions in the presence of immutable exposure variables, highly correlated independent variables, and social selection.26–28 In some cases, effect estimates have been demonstrated to rely on covariate strata with no variability in the exposure (non-positivity or “off-support” estimation),27,29,30 which prevents defining a counterfactual intervention, and may introduce bias.31 While post-model regression coefficient scaling does not threaten validity in the same manner as off-support estimation, it may present an analogous situation from a causal perspective: health effect estimates that represent hypothetical interventions that are poorly defined, unobserved in the empirical data, and perhaps impossible. While unfeasible/impossible interventions do not necessarily threaten the identifiability of a causal parameter,32 in epidemiology we often care about the practical applications of our findings, and the feasibility of effect estimates is central to this assessment.33,34
To illustrate the concerns related to unclearly defined or impossible health effect estimates in air pollution epidemiology, we analyze a simple exposure regimen of two ambient air pollutants, assessing how daily concentration changes in two pollutants relate to one another. Specifically, we analyze nitrogen dioxide (NO2) and particulate matter with aerodynamic diameter ≤ 2.5μm (PM2.5), co-pollutants that are frequently studied in multi-pollutant models.35–37 We analyze these co-pollutants in three US cities with different air pollution profiles, to illustrate how several factors (e.g., emission sources, local meteorology) may affect the extent to which an IQR-scaled effect estimate may be appropriate in a given dataset. Having described the conventional IQR-scaling approach for multi-pollutant models (hereafter called “the IQR approach”), we first identify one set of causal conditions that may link the IQR approach to an underlying real-world intervention. We then map the IQR approach onto several distinct air pollution profiles, to examine whether the intervention ostensibly represented by the IQR-scaled effect is empirically observed in various geographic regions and seasons. Our goal is to assess the extent to which the parameter estimate scaling commonly presented in the epidemiologic literature reflects real-world exposure scenarios for acute air pollution health effects. We also aim to provide examples of how researchers can assess the appropriateness of the air pollution effect estimates they present in acute health-effects studies. Our ultimate goal is to stimulate a discussion on best practices for air pollution health effect scaling, enabling calculation and presentation of more realistic health effect estimates for scientific and regulatory purposes.
Causal conditions underlying the IQR approach
Although IQR scaling is standard practice, we are unaware of a detailed explication of how to interpret and apply the effects it produces, in terms of real-world health effects. Linking a health effect estimate to an underlying causal parameter requires causal assumptions, which are separate from the statistical assumptions required for unbiased estimation from a regression model (e.g., linearity given the link function, correct specification of main terms and interaction terms, independent observations).32 We define one set of causal assumptions below, in the form of two separate but related causal conditions. We recognize that these causal conditions are unlikely to capture the causal meaning that each researcher attributes to her/his IQR-scaled air pollution effects; these are our attempt to express a potential intervention represented by IQR-scaled effects.
The first condition relates to using the IQR over the study period as a scaling interval. Here we focus on acute air pollution effects estimated in time-series studies, specifically lag 1 (consecutive days) effects. We use “lag-1 effect” to refer to the effect of the air pollution concentration on the previous day (day X-1) on a health outcome on the index day (day X). We use “daily difference” to refer to air pollution concentration changes between the day before the index day (day X-1) and the index day (day X). The daily difference and the lag-1 effect are distinct phenomena, and we posit that they are connected. Because the lag-1 health effects of one or more air pollutants are estimated between consecutive days, a hypothetical experiment corresponding to this lag would intervene on exposure to increase/decrease pollutant concentration(s) over a similar, temporally short time-frame. This implies that for intervention-relevant scaling of lag-1 health effects, the scaling interval applied to the regression coefficient should reflect an amount of variability that is possible in the exposure of interest, in this case an air pollutant, on consecutive days. Normally the IQR that is used for regression scaling is calculated from the full distribution of that air pollutant for the whole study period or across a season, thus including the extremes of exposure across the study period, regardless of how temporally close these extreme concentrations are. We hypothesized that the IQR across a season, which is often used to present results in this field, would often be larger than the IQR of daily differences within a season. This gives rise to the first causal condition, formalized below: that an IQR-sized concentration change should be observed on some consecutive days in the study period.
Second, when multiplying one pollutant’s coefficient by an IQR, controlling for co-pollutant(s), there is a tacit assumption that the concentration in one pollutant can change by a large interval while holding the co-pollutant(s) relatively constant (in cases where co-pollutants are modeled as main terms only, without interaction terms). This particular condition is seldom empirically examined, and so it is not known if days with a large concentration change in one pollutant and relatively small changes in the co-pollutant(s) actually exist. Given the strong correlation among many ambient air pollutants, assessing the feasibility of a hypothetical intervention defined by an IQR-scaled multi-pollutant regression coefficient requires examining this condition. The hypothetical intervention defined by these conditions (which the IQR approach estimates, in our opinion) is as follows: If we were to change the index pollutant by a large (IQR-sized) interval between day X-1 and day X, holding the co-pollutant constant between these consecutive days, what would be the effect on the health outcome on day X? We now test how frequently these conditions hold, using real-world data for two pollutants in three locations in the U.S.
Data source
We obtained ambient air pollution data from the U.S. Environmental Protection Agency’s Air Quality System for the years 2010 through 2012. We analyzed daily concentrations (24-hour averages) of NO2 in parts per billion (ppb) and PM2.5 in micrograms per cubic meter (μg/m3) from a central monitoring station in each of the following cities: Burbank, California; Houston, Texas; and Pittsburgh, Pennsylvania. These locations were chosen because they represent diverse air pollution profiles, emissions sources, and climatic conditions, enabling analysis of the joint NO2/PM2.5 exposure regimen across a variety of real-world settings of interest. NO2 and PM2.5 were chosen because both are associated with a host of acute adverse health outcomes,35,38 and because they are frequently analyzed together in multi-pollutant models.9,36,37 When there was more than one monitoring station in a city, we chose the monitoring station that had data for all of the EPA’s criteria air pollutants in the years we studied.
Methodological approach
We analyzed how daily concentration changes in one pollutant (the index pollutant) varied with daily concentration changes in the co-pollutant. We chose to focus on the daily differences between consecutive days in our analyses, corresponding to lag-1 health effects. In the literature, both PM2.5 and NO2 have been demonstrated to cause adverse health outcomes at lag 1.35,38 We calculated consecutive daily concentration differences by subtracting the previous day’s pollutant concentration from each daily concentration. Because the strength and direction of pollutant correlations may change between seasons, we analyzed concentration changes separately by season.16 We analyzed daily multi-pollutant concentration changes in summer (defined as June – August) and winter (defined as December – February) across the study years. We chose to use a consistent, simplified approach for defining seasons across cities to enable comparison of results. Multiple lag structures and combinations of lags may be of real-world interest; here we present only one combination, in order to focus the discussion and to enable comparison between cities and seasons.
Sources of variability
We analyzed two related sources of variability for each pollutant in each of the six city-seasons (3 cities × 2 seasons). First, we examined the distribution of daily pollutant concentrations across the study years, within each city-season. This distribution and its parameters are referred to as “study period” (e.g., study period IQR). Studies that employ IQR scaling typically use this study period IQR to scale effect estimates.
Separately, we analyzed daily concentration differences to represent a lag-1 effect. We refer to this distribution and its parameters as “daily difference,” e.g., the daily-difference IQR is the IQR of daily concentration changes in the air pollutant. This terminology distinguishes the daily-difference IQR from the study-period IQR, which is conventionally (though not exclusively39) analyzed in air pollution epidemiology.
Assessing whether causal conditions are met
We operationalized the two conditions discussed above, in order to quantify how many days within a city-season met each condition for scaling of lag 1 health effects. The first condition, that a study period IQR adequately reflects concentration differences between consecutive days, we refer to as Condition 1. This condition involves only one pollutant, and it is satisfied for a given day if the absolute value of the daily difference equals or exceeds the study period IQR for that pollutant in the given city-season. We opted to consider the absolute value of the daily difference between consecutive days because days with large concentration changes reflect the first condition regardless of the direction of the concentration change.
Condition 2 includes the rule defined in Condition 1 as well as a second condition, that the concentration of the co-pollutant remains relatively constant while the index pollutant of interest changes by a large interval (i.e., the IQR). Therefore, Condition 2 is a compound condition that involves both co-pollutants. We operationalized “remains relatively constant” as days with a low absolute value-daily difference (specifically, days whose absolute value-daily differences were in the lowest tertile). We chose the 33rd percentile as the cutoff for “low absolute daily difference” because it excludes days with high and moderately large concentration changes, while still capturing a reasonable proportion of days. In defining Condition 2, we imposed a dichotomous cutoff on what is a continuous phenomenon (variability in the co-pollutant); therefore we ran sensitivity analyses of Condition 2, fluctuating the “low daily difference” cutoff. We considered the 40th and 20th percentiles as alternative cutoffs and examined how results changed.
In a given pair of co-pollutants, one must consider changes in each individual pollutant and its co-pollutant as separate questions. If a day satisfies Condition 2 with NO2 as the main pollutant and PM2.5 as the co-pollutant, that day will by definition not satisfy Condition 2 when considering PM2.5 as the main pollutant and NO2 as the co-pollutant. Therefore, we ran all analyses twice, once considering NO2 as the index pollutant and PM2.5 as the co-pollutant, and the second time switching the two.
To provide a concrete example, in Pittsburgh during winter, the study period IQR for NO2 was 9.7 ppb. The 33rd percentile of absolute value-daily differences for PM2.5 was 2.0 μg/m3. Considering NO2 as the index pollutant and PM2.5 as the co-pollutant, days where the NO2 daily difference was more extreme than 9.7 ppb satisfy Condition 1 (large change in index pollutant). Among these days of large NO2 concentration change, the subset that had PM2.5 daily differences with absolute value less than 2.0 μg/m3 satisfy criterion 2.
Analysis
We compared the study-period distribution and the daily-difference distribution for both NO2 and PM2.5 in each city-season. We present median values, quartile values, and the IQR. We compared the study period IQR to the daily difference IQR by calculating a ratio of the two values. This enabled direct comparison of the two metrics of variability, and of how study period parameters map onto the distribution of daily differences. For both daily concentrations over the study period and daily differences, we calculated Pearson correlation coefficients for NO2 and PM2.5 for each city-season. We compared the study-period correlation to the daily-difference correlation using the method of Steiger for dependent correlations (specifically, Steiger’s modification of Dunn and Clark’s z; equation 1540 p.247).
To determine the extent to which the Conditions of the IQR method were met in each city-season, we present the number and percent of days that satisfied each, separately for NO2 and PM2.5 as the index pollutant. We represent these findings graphically, in daily difference scatterplots between NO2 and PM2.5 for each city-season. We use lines and shading to represent the two conditions. All analyses were conducted using R (version 2.13.1, R Foundation for Statistical Computing; Vienna, Austria).
Results
In all cities and seasons, greater NO2 variability occurred in the winter than summer, as indicated by IQRs (Table 1). Burbank had the largest IQRs for NO2, with values between 6.9 ppb (summer daily difference IQR) and 12.2 ppb (winter study period IQR). Interquartile ranges for PM2.5 were lowest in Houston, between 4.5 μg/m3 (summer daily difference IQR) and 6.1 μg/m3 (summer study period IQR). PM2.5 variability was higher during winter in Burbank (10.6 μg/m3, study period IQR), as compared with summer months.
Table 1.
Season-stratified study-period distribution daily difference distribution for ambient NO2 and PM2.5 in three US cities, 2010 – 2012
| NO2 (ppb) | PM2.5 (μg/m3) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Summer | Burbank (n=218 days) | Houston (n=265 days) | Pittsburgh (n=250 days) | Burbank (n=218 days) | Houston (n=265 days) | Pittsburgh (n=250 days) | |||||||
| SP | Diff. | SP | Diff. | SP | Diff. | SP | Diff. | SP | Diff. | SP | Diff. | ||
| 25th percentile | 14.4 | −3.5 | 8.4 | −1.9 | 6.2 | −1.8 | 16.3 | −2.3 | 9.6 | −2.2 | 9.5 | −3.5 | |
| Median | 19.8 | −0.6 | 10.5 | −0.4 | 8.9 | 0.3 | 19.1 | 0.5 | 11.7 | 0.0 | 12.9 | −0.1 | |
| 75th percentile | 25.3 | 3.4 | 13.1 | 2.3 | 11.9 | 2.5 | 22.8 | 2.9 | 15.6 | 2.3 | 17.3 | 3.0 | |
| IQR | 11.0 | 6.9 | 4.7 | 4.2 | 5.7 | 4.3 | 6.5 | 5.2 | 6.1 | 4.5 | 7.8 | 6.5 | |
| SP IQR: diff. IQR ratioa | 1.60 | 1.11 | 1.32 | 1.25 | 1.34 | 1.20 | |||||||
| Winter | Burbank (n=198 days) | Houston (n=265 days) | Pittsburgh (n=241 days) | Burbank (n=198 days) | Houston (n=265 days) | Pittsburgh (n=241 days) | |||||||
| SP | Diff. | SP | Diff. | SP | Diff. | SP | Diff. | SP | Diff. | SP | Diff. | ||
| 25th percentile | 20.5 | −5.3 | 12.0 | −5.1 | 9.2 | −4.2 | 11.5 | −4.5 | 8.4 | −2.8 | 7.0 | −3.8 | |
| Median | 26.9 | 0.6 | 16.5 | −0.1 | 13.8 | −0.3 | 15.8 | 0.6 | 10.9 | 0.1 | 10.7 | 0.2 | |
| 75th percentile | 32.7 | 4.6 | 20.5 | 4.8 | 19.0 | 4.0 | 22.0 | 4.5 | 13.4 | 2.8 | 15.0 | 3.6 | |
| IQR | 12.2 | 9.9 | 8.5 | 9.9 | 9.7 | 8.2 | 10.6 | 9.0 | 5.1 | 5.6 | 8.0 | 7.4 | |
| SP IQR: diff. IQR ratioa | 1.23 | 0.86 | 1.19 | 1.17 | 0.90 | 1.08 | |||||||
SP IQR: diff. IQR ratio expresses the ratio of the study period IQR to the daily difference IQR within each city-season
Abbreviations:
SP, Study period distribution; Diff., Daily difference distribution; IQR, interquartile range; NO2, nitrogen dioxide; PM2.5, particulate matter with aerodynamic diameter ≤2.5μm; ppb, parts per billion; μg/m3, micrograms per cubic meter
Generally, the study period IQR was greater than the daily difference IQR (10 of 12 city-seasons, Table 1), reflecting greater variability across the season than between consecutive days. In summer, study period IQRs were consistently larger than the daily difference IQRs. For example, in Burbank the study-period IQR of NO2 (11.0 ppb) was 60% larger than the daily difference IQR (6.9 ppb). Other differences were in the 20% – 35% range (except for Houston, 11%). In winter, no study-period IQR was more than 25% greater than its corresponding daily difference IQR.
The study-period correlations between NO2 and PM2.5 were similar to the daily- difference correlations between pollutants in all city-seasons (Table 2). For all cities, ambient NO2 and PM2.5 were more strongly correlated during winter months as compared with summer months. Pittsburgh exhibited the strongest correlations between NO2 and PM2.5 – approximately 0.45 during summer and 0.70 during winter. The study-period correlations between pollutants and daily-difference correlations between pollutants did not differ meaningfully within any city-season (P>0.05 for all comparisons).
Table 2.
Season-stratified correlations between ambient NO2 and PM2.5 for daily concentrations over the study period and daily differences
| Burbank | Houston | Pittsburgh | ||||
|---|---|---|---|---|---|---|
| Study period distribution | Daily difference distribution | Study period distribution | Daily difference distribution | Study period distribution | Daily difference distribution | |
| Summer | 0.054 | 0.163 | 0.206 | 0.164 | 0.447 | 0.436 |
| Winter | 0.392 | 0.439 | 0.411 | 0.444 | 0.766 | 0.697 |
In examining the number of days that met Condition 1, generally 10% to 25% of days per city-season had a daily difference exceeding the study-period IQR (Table 3). Considering NO2 as the index pollutant, summer days in Burbank and Pittsburgh had a smaller proportion: 10 days in Burbank (4.6%) and 17 in Pittsburgh (6.8%). Among these days of high concentration change in the index pollutant, the proportion that met the additional requirement of Condition 2 was always below 50%, in many cases much lower (less than 25% in 6 of 12 city-seasons). This demonstrates that a minority of high-difference days in the index pollutant exhibited small differences in the co-pollutant. The overall proportion of days that met Condition 2 never exceeded 7%, and in some cases was below 1%. During summertime, considering NO2 as the index pollutant, there were only 2 such days (0.9%) in Burbank, and 1 such day in Pittsburgh (0.4%).
Table 3.
Number and percent of days fulfilling Condition 1 and Condition 2 a across each city-season, with the index pollutant as NO2 and PM2.5 separately
| Index pollutant: NO2 | Index pollutant: PM2.5 | ||||||
|---|---|---|---|---|---|---|---|
| Burbank No. (%) | Houston No. (%) | Pittsburgh No. (%) | Burbank No. (%) | Houston No. (%) | Pittsburgh No. (%) | ||
| Summer | Condition 1 | 10 (4.6) | 47 (17.7) | 17 (6.8) | 26 (11.9) | 46 (17.4) | 39 (15.6) |
| Condition 2 | 2 (0.9) | 17 (6.4) | 1 (0.4) | 8 (3.7) | 17 (6.4) | 14 (5.6) | |
| Winter | Condition 1 | 21 (10.6) | 63 (23.8) | 37 (15.4) | 32 (16.2) | 68 (25.7) | 30 (12.4) |
| Condition 2 | 5 (2.5) | 16 (6.0) | 4 (1.7) | 9 (4.5) | 13 (4.9) | 2 (0.8) | |
Condition 1 stipulates that the absolute value-daily differences of the index pollutant exceed the study period-IQR; Condition 2 requires Condition 1 as well as stipulating that the absolute value of the co-pollutant’s daily difference is in the lower tertile of daily difference absolute values (when NO2 is the index pollutant, PM2.5 is the co-pollutant and vice versa).
Abbreviations:
NO2, nitrogen dioxide; PM2.5, particulate matter with aerodynamic diameter ≤2.5μm
A region may fail to meet the joint stipulations of Condition 2 because of failure to satisfy either component of this condition. There may be inadequate variability in daily differences for the index pollutant (i.e., not meeting Condition 1). Or, on such days of high variability, the co-pollutant may also generally exhibit high daily differences (i.e., not meeting Condition 2). Figures 1 and 2 graphically demonstrate two such instances in which few days met Condition 2. In Burbank during summer, there were relatively few days meeting Condition 1 for either index pollutant. Such days are represented by dots that fall outside of the dashed lines. In particular when NO2 was the index pollutant (Figure 1A), the large majority of days clustered near the x-axis origin. The shaded regions in the graph represent further restriction to days that satisfy Condition 2, i.e., days of low concentration change. Application of this Condition further restricts the number of days that are on-support for the IQR method, leaving only two days that satisfy both conditions.
Figure 1.
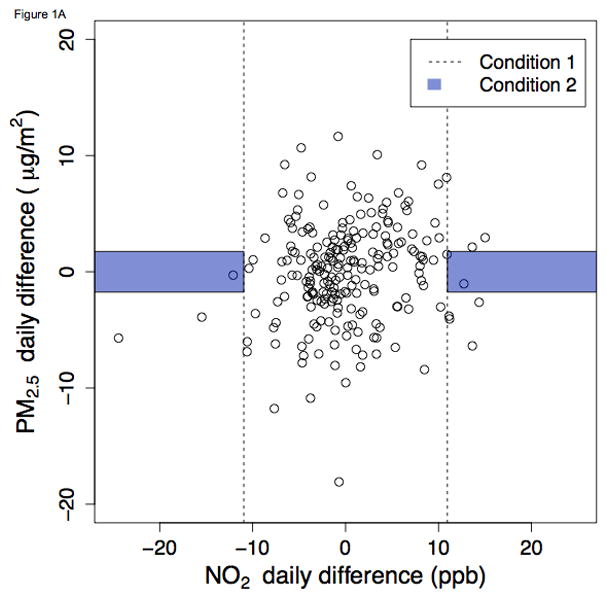
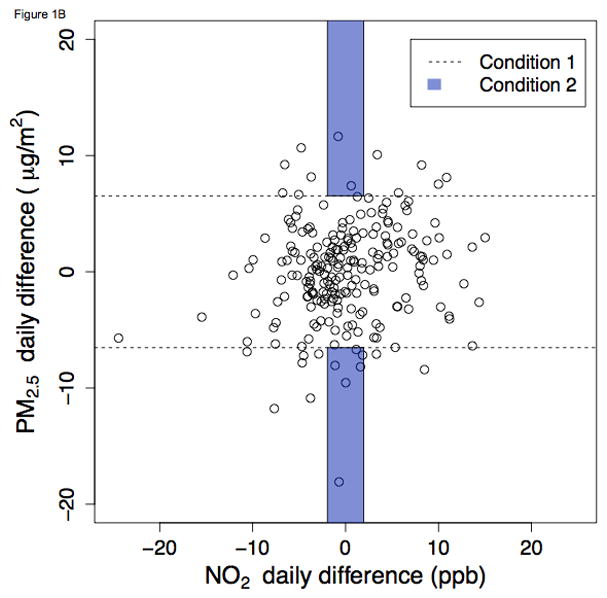
Ambient NO2 and PM2.5 daily differences during summer in Burbank, California, 2010 – 2012. Figures 1 and 2 graphically demonstrate which days met Conditions 1 and 2 for selected city-seasons (summer in Burbank and winter in Pittsburgh). The points in scatterplots are the same for A and B, with the index pollutant toggled (NO2 for A, PM2.5 for B). Days that satisfy Condition 1 (i.e., days with absolute value-daily differences exceeding the study period IQR) are represented by the points that fall outside of the dashed lines. Within this region beyond the dashed lines, days that satisfy Condition 2 are represented by the dots within the shaded region of “low daily difference in the co-pollutant” (i.e., the 33rd percentile of absolute value-daily differences). A. NO2 as index pollutant B. PM2.5 as index pollutant
Figure 2.
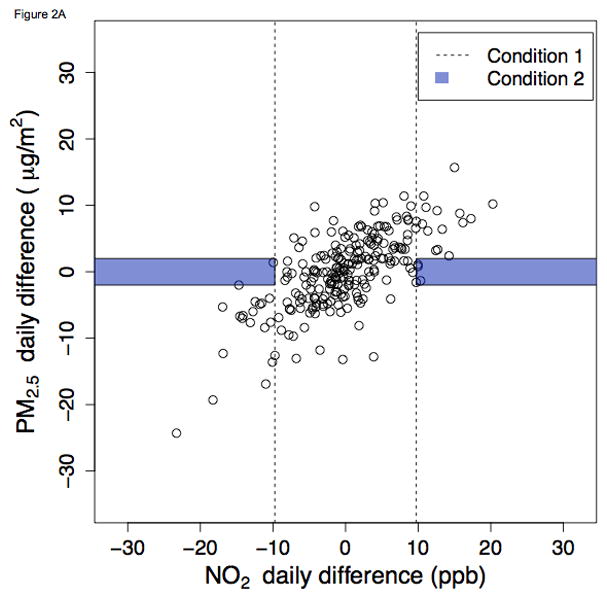
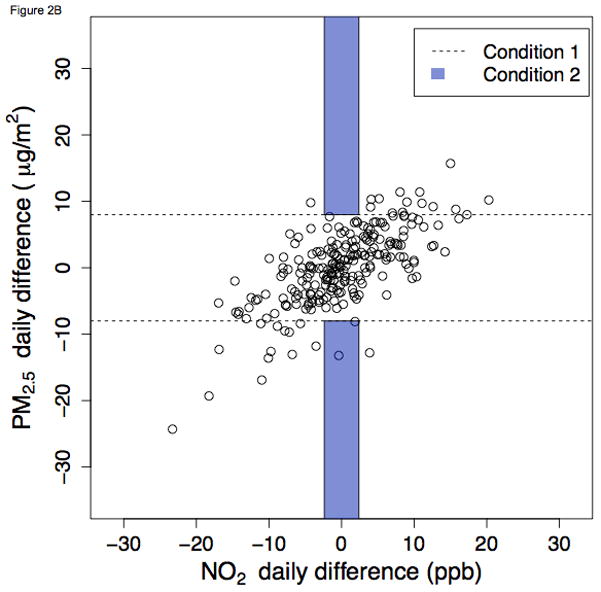
Ambient NO2 and PM2.5 daily differences during winter in Pittsburgh, Pennsylvania, 2010 – 2012 A. NO2 as index pollutant B. PM2.5 as index pollutant
Figure 2 shows daily differences during winter in Pittsburgh. In this instance, a moderate proportion of days met Condition 1 (dots outside of the dashed lines). However, due to the high correlation between PM2.5 and NO2, very few days satisfy Condition 2 (e.g., the two dots within the shaded region in Figure 2B). Complete graphics for all city-seasons and both index pollutants are presented in the web appendix. In sensitivity analyses examining alternative definitions of Condition 2, results were not greatly changed (eTable 1, eFigures 5 and 6). When the “low-concentration-change” cutoff was increased to the 40th percentile, the proportion of days satisfying Condition 2 still never exceeded 10%. When decreased to the 20th percentile, it was more frequently below 1%.
Discussion
We analyzed changes in daily concentrations in ambient PM2.5 and NO2 across three cities and two seasons to demonstrate how researchers may assess whether IQR scaling realistically reflects hypothetical interventions that are possible given the observed data. We attempted to demonstrate that the issues of time-scale of exposure variability, regression coefficient scaling, and correlation between co-pollutants have implications for the causal interpretation of multi-pollutant health effects. Ultimately, our goal was to determine whether IQR-scaling of multi-pollutant health effects reflects actual daily changes in multi-pollutant exposure regimens.
We believe that scaling air pollution effects to an interquartile interval implies that this is a reasonable concentration change in the time-window in which health effects are being estimated. In considering whether the study period IQR is an appropriate scaling interval for lag-1 health effects in time-series studies (i.e., Condition 1), we found that such variability was infrequently represented in season-stratified analyses. We also analyzed whether there were days in which a second co-pollutant’s concentration remained relatively constant, alongside the large daily difference in the index pollutant (i.e., Condition 2). We found that such daily concentration changes were adequately represented in very few real-data scenarios. For example, at least 5% of days in Houston exhibited this type of variability in the co-exposures of NO2 and PM2.5, making the IQR-scaled health effect estimate readily understandable as a hypothetical intervention. However, we also observed that such days are almost never observed in some city-seasons, for example Burbank during summer and Pittsburgh in both seasons. At least two factors contributed to this: (1) daily differences in pollutants rarely exceeded the study period IQR, and (2) days of high concentration changes in one pollutant also generally exhibited high concentration changes of the co-pollutant. In such scenarios, the hypothetical intervention, that of changing one pollutant a large amount while holding the other constant, is not supported by our data.
The approach that we used to characterize changes in multi-pollutant exposure regimens required several simplifications. First, air pollution health effects occur at various time lags in addition to lag 1.41,42 Second, although we used ambient pollutant concentrations from central site monitors, the true exposures of individuals are not necessarily represented by these central monitors. Exposure science continues to improve estimation of ambient air pollution for individual persons, where they spend their time.43 Third, our definition of “high change in concentration of the index pollutant” was based on the literature (i.e., a study period IQR), but our definition of “small change in the co-pollutant” was arbitrary; however, we examined alternative definitions. Fourth, precise definitions of season depend on local meteorology, and our simplified definitions may not optimally capture the full winter and summer air pollution profiles in our chosen cities. Finally, the three cities we selected represent distinct ambient air pollution profiles but do not represent the full range of ambient air pollution profiles. In light of this, it is worth noting that several of the correlations between NO2 and PM2.5 that we observed in this study were lower (i.e., <0.20 during summer in Burbank and Houston) than correlations commonly observed in many multi-pollutant studies (i.e., >0.50 during winter in Pittsburgh, and in other regions6,10). Higher co-pollutant correlations would decrease the probably that Condition 2 would be satisfied.
Nevertheless, our goal was to examine the practical interpretation of IQR-scaled health effects in multiple-pollutant modeling. Conducting this analysis across regions and seasons required uniform methods across the city-seasons; hence, our simplifying assumptions. We found evidence of ill-defined/unfeasible causal effects in some cases (given our particular definition of a hypothetical intervention). It is worth noting that (as in our examples), a single modeling or scaling approach may have a stronger empirical data basis in one region or season than others. It is our intent that this example should catalyze discussion of causal interpretation of health effects in environmental epidemiology, including how such effect estimates map onto interventions.18,19 Our general approach may also be applied using different pollutants, lag structures, time-scales, and exposure measurement methods. Although we addressed IQR scaling for single pollutants at temporally short lags (i.e., condition 1) and IQR scaling for multiple pollutants (i.e., condition 2) in one discussion, it is not a necessary feature of our approach to consider these issues in tandem.
Our approach of connecting IQR scaling to a hypothetical intervention is only one possible approach. With our causal conditions, we attempted to link commonly presented health effect estimates to a causal parameter of interest; however, these specific rules and this general approach are not broadly accepted by researchers presenting these health effects. Defining a hypothetical intervention is investigator-dependent; for example one could choose to hypothetically intervene on other variables in the multivariable model (e.g., temperature), while another investigator may consider them nuisance variables. Similarly, the failure to empirically observe an exposure-change scenario does not mean that such a scenario/intervention is not possible, and the health effects of such unobserved scenarios may well be of interest. However, investigators must be cautious in assigning causal interpretation to such interventions. In some cases, IQR scaling may violate the consistency assumption, which requires that “one needs to be able to explain how a certain level of exposure could be hypothetically assigned to a person exposed to a different level.”44 Although less broadly appreciated than the causal assumptions of positivity and exchangeability,31,45,46 consistency is nonetheless an important assumption for sharply defining causal contrasts.
Conclusions
The continued move toward mixtures and multi-pollutant research promises to more realistically reflect the ambient air pollution profile that humans are exposed to. This shift holds scientific promise as well as hurdles. We have aimed to elucidate one of these challenges, the importance of defining a hypothetical intervention that reflects actual air pollution scenarios that are contained in the dataset. The most immediate implication of our work is the suggestion that investigators should consider scaling air pollution health effects using distributions that reflect the temporal association between exposure and outcome (e.g., daily differences for lag-1 research; maximal concentration change over a 4-day window for lag-4 health effects; concentration changes within a day for lag-0 health effects), rather than the distribution over the entire study period. Different air pollution exposure periods (e.g., long-term health effects), modeling approaches (e.g., non-linearity or flexibly modeled exposure variables), and study designs (e.g., case-crossover) will likely require different approaches to defining a hypothetical intervention. We also believe that scaling co-pollutants simultaneously to reflect joint air pollution exposure changes is a promising future direction47,48 (e.g., through the use of the g-computation plug-in estimator).49,50 By more clearly defining a hypothetical intervention in reference to empirical data, we can aid in the interpretation of our presented health effect estimates, making them more readily applicable for scientific and regulatory purposes.
Supplementary Material
Acknowledgments
Sources of financial support:
This work was supported by grant R40 MC 25694-01-00 from the Maternal and Child Health Research Program, Maternal and Child Health Bureau—Health Resources and Services Administration; the California Air Resources Board (contract 99-322); the National Heart, Lung, and Blood Institute’s Division of Lung Diseases (grant number R01 HL081521); and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number K99 HD079658-01).
We acknowledge Kathleen Mortimer for her contributions to conceptualizing this topic and offering feedback on a draft of the manuscript.
Footnotes
Competing Financial Interests:
We declare that we have no competing financial interests.
SDC Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com). This content is not peer-reviewed or copy-edited; it is the sole responsibility of the author.
References
- 1.NRC (National Research Council) Air Quality Management in the United States. Washington, DC: National Academy Press; 2004. [Google Scholar]
- 2.U.S. EPA (U.S. Environmental Protection Agency) Air, Climate, and Energy Strategic Research Action Plan 2012–2016. 2012. [Google Scholar]
- 3.Dominici F, Peng RD, Barr CD, Bell ML. Protecting human health from air pollution: shifting from a single-pollutant to a multipollutant approach. Epidemiology. 2010;21(2):187–94. doi: 10.1097/EDE.0b013e3181cc86e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauderly JL, Burnett RT, Castillejos M, et al. Is the air pollution health research community prepared to support a multipollutant air quality management framework? Inhal Toxicol. 2010;22 (Suppl 1):1–19. doi: 10.3109/08958371003793846. [DOI] [PubMed] [Google Scholar]
- 5.Johns DO, Stanek LW, Walker K, et al. Practical advancement of multipollutant scientific and risk assessment approaches for ambient air pollution. Environ Health Perspect. 2012;120(9):1238–42. doi: 10.1289/ehp.1204939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu H, Yu IT, Tian L, et al. Effects of coarse particulate matter on emergency hospital admissions for respiratory diseases: a time-series analysis in Hong Kong. Environ Health Perspect. 2012;120(4):572–6. doi: 10.1289/ehp.1104002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson GB, Krall JR, Peng RD, Bell ML. Is the relation between ozone and mortality confounded by chemical components of particulate matter? Analysis of 7 components in 57 US communities. Am J Epidemiol. 2012;176(8):726–32. doi: 10.1093/aje/kws188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart JE, Garshick E, Dockery DW, Smith TJ, Ryan L, Laden F. Long-term ambient multipollutant exposures and mortality. Am J Respir Crit Care Med. 2011;183(1):73–8. doi: 10.1164/rccm.200912-1903OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in Los Angeles county, California. Environ Health Perspect. 2013;121(3):380–6. doi: 10.1289/ehp.1205827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesaroni G, Badaloni C, Gariazzo C, et al. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect. 2013;121(3):324–31. doi: 10.1289/ehp.1205862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin M, Schwartz J. The impact of secondary particles on the association between ambient ozone and mortality. Environ Health Perspect. 2008;116(4):453–8. doi: 10.1289/ehp.10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann B, Luttmann-Gibson H, Cohen A, et al. Opposing effects of particle pollution, ozone, and ambient temperature on arterial blood pressure. Environ Health Perspect. 2012;120(2):241–6. doi: 10.1289/ehp.1103647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strak M, Janssen NA, Godri KJ, et al. Respiratory health effects of airborne particulate matter: the role of particle size, composition, and oxidative potential-the RAPTES project. Environ Health Perspect. 2012;120(8):1183–9. doi: 10.1289/ehp.1104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billionnet C, Sherrill D, Annesi-Maesano I. Estimating the health effects of exposure to multi-pollutant mixture. Ann Epidemiol. 2012;22(2):126–41. doi: 10.1016/j.annepidem.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Krall JR, Anderson GB, Dominici F, Bell ML, Peng RD. Short-term exposure to particulate matter constituents and mortality in a national study of U.S. urban communities. Environ Health Perspect. 2013;121(10):1148–53. doi: 10.1289/ehp.1206185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito K, Thurston GD, Silverman RA. Characterization of PM2.5, gaseous pollutants, and meteorological interactions in the context of time-series health effects models. J Expo Sci Environ Epidemiol. 2007;17 (Suppl 2):S45–60. doi: 10.1038/sj.jes.7500627. [DOI] [PubMed] [Google Scholar]
- 17.Galea S. An argument for a consequentialist epidemiology. Am J Epidemiol. 2013;178(8):1185–91. doi: 10.1093/aje/kwt172. [DOI] [PubMed] [Google Scholar]
- 18.Westreich D. From Exposures to Population Interventions: Pregnancy and Response to HIV Therapy. Am J Epidemiol. 2014;179(7):797–806. doi: 10.1093/aje/kwt328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahern J, Hubbard A, Galea S. Estimating the effects of potential public health interventions on population disease burden: a step-by-step illustration of causal inference methods. Am J Epidemiol. 2009;169(9):1140–7. doi: 10.1093/aje/kwp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fann N, Bell ML, Walker K, Hubbell B. Improving the linkages between air pollution epidemiology and quantitative risk assessment. Environ Health Perspect. 2011;119(12):1671–5. doi: 10.1289/ehp.1103780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman TB, Browner WS. In defense of standardized regression coefficients. Epidemiology. 1991;2(5):383–6. doi: 10.1097/00001648-199109000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Cummings P. Arguments for and against standardized mean differences (effect sizes) Arch Pediatr Adolesc Med. 2011;165(7):592–6. doi: 10.1001/archpediatrics.2011.97. [DOI] [PubMed] [Google Scholar]
- 23.Greenland S, Schlesselman JJ, Criqui MH. The fallacy of employing standardized regression coefficients and correlations as measures of effect. Am J Epidemiol. 1986;123(2):203–8. doi: 10.1093/oxfordjournals.aje.a114229. [DOI] [PubMed] [Google Scholar]
- 24.Greenland S, Maclure M, Schlesselman JJ, Poole C, Morgenstern H. Standardized regression coefficients: a further critique and review of some alternatives. Epidemiology. 1991;2(5):387–92. [PubMed] [Google Scholar]
- 25.Morgenstern H, Thomas D. Principles of study design in environmental epidemiology. Environ Health Perspect. 1993;101 (Suppl 4):23–38. doi: 10.1289/ehp.93101s423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oakes JM. The (mis)estimation of neighborhood effects: causal inference for a practicable social epidemiology. Soc Sci Med. 2004;58(10):1929–52. doi: 10.1016/j.socscimed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Messer LC, Oakes JM, Mason S. Effects of socioeconomic and racial residential segregation on preterm birth: a cautionary tale of structural confounding. Am J Epidemiol. 2010;171(6):664–73. doi: 10.1093/aje/kwp435. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman JS, Cooper RS. Seeking causal explanations in social epidemiology. Am J Epidemiol. 1999;150(2):113–20. doi: 10.1093/oxfordjournals.aje.a009969. [DOI] [PubMed] [Google Scholar]
- 29.Ahern J, Cerda M, Lippman SA, Tardiff KJ, Vlahov D, Galea S. Navigating non-positivity in neighbourhood studies: an analysis of collective efficacy and violence. J Epidemiol Community Health. 2013;67(2):159–65. doi: 10.1136/jech-2012-201317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson PJ, Oakes JM, Anderton DL. Neighborhood poverty and American Indian infant death: are the effects identifiable? Ann Epidemiol. 2008;18(7):552–9. doi: 10.1016/j.annepidem.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen ML, Porter KE, Gruber S, Wang Y, van der Laan MJ. Diagnosing and responding to violations in the positivity assumption. Stat Methods Med Res. 2012;21(1):31–54. doi: 10.1177/0962280210386207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen ML, van der Laan MJ. Causal models and learning from data: integrating causal modeling and statistical estimation. Epidemiology. 2014;25(3):418–26. doi: 10.1097/EDE.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernan MA. Invited commentary: hypothetical interventions to define causal effects--afterthought or prerequisite? Am J Epidemiol. 2005;162(7):618–20. doi: 10.1093/aje/kwi255. discussion 621–2. [DOI] [PubMed] [Google Scholar]
- 34.Hernan MA, Taubman SL. Does obesity shorten life? The importance of well-defined interventions to answer causal questions. Int J Obes (Lond) 2008;32 (Suppl 3):S8–14. doi: 10.1038/ijo.2008.82. [DOI] [PubMed] [Google Scholar]
- 35.Bell ML, Zanobetti A, Dominici F. Evidence on vulnerability and susceptibility to health risks associated with short-term exposure to particulate matter: a systematic review and meta-analysis. Am J Epidemiol. 2013;178(6):865–76. doi: 10.1093/aje/kwt090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samoli E, Stafoggia M, Rodopoulou S, et al. Associations between fine and coarse particles and mortality in Mediterranean cities: results from the MED-PARTICLES project. Environ Health Perspect. 2013;121(8):932–8. doi: 10.1289/ehp.1206124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stafoggia M, Samoli E, Alessandrini E, et al. Short-term Associations between Fine and Coarse Particulate Matter and Hospitalizations in Southern Europe: Results from the MED-PARTICLES Project. Environ Health Perspect. 2013;121(9):1026–33. doi: 10.1289/ehp.1206151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect. 2010;118(4):449–57. doi: 10.1289/ehp.0900844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mann JK, Balmes JR, Bruckner TA, et al. Short-term effects of air pollution on wheeze in asthmatic children in Fresno, California. Environ Health Perspect. 2010;118(10):1497–502. doi: 10.1289/ehp.0901292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- 41.Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology. 2000;11(3):320–6. doi: 10.1097/00001648-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 42.Kim SY, Peel JL, Hannigan MP, et al. The temporal lag structure of short-term associations of fine particulate matter chemical constituents and cardiovascular and respiratory hospitalizations. Environ Health Perspect. 2012;120(8):1094–9. doi: 10.1289/ehp.1104721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarnat SE, Coull BA, Schwartz J, Gold DR, Suh HH. Factors affecting the association between ambient concentrations and personal exposures to particles and gases. Environ Health Perspect. 2006;114(5):649–54. doi: 10.1289/ehp.8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cole SR, Frangakis CE. The consistency statement in causal inference: a definition or an assumption? Epidemiology. 2009;20(1):3–5. doi: 10.1097/EDE.0b013e31818ef366. [DOI] [PubMed] [Google Scholar]
- 45.Westreich D, Cole SR. Invited commentary: positivity in practice. Am J Epidemiol. 2010;171(6):674–7. doi: 10.1093/aje/kwp436. discussion 678–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernan MA. Beyond exchangeability: the other conditions for causal inference in medical research. Stat Methods Med Res. 2012;21(1):3–5. doi: 10.1177/0962280211398037. [DOI] [PubMed] [Google Scholar]
- 47.Schildcrout JS, Sheppard L, Lumley T, Slaughter JC, Koenig JQ, Shapiro GG. Ambient air pollution and asthma exacerbations in children: an eight-city analysis. Am J Epidemiol. 2006;164(6):505–17. doi: 10.1093/aje/kwj225. [DOI] [PubMed] [Google Scholar]
- 48.Winquist A, Kirrane E, Klein M, et al. Joint effects of ambient air pollutants on pediatric asthma emergency department visits in atlanta, 1998–2004. Epidemiology. 2014;25(5):666–73. doi: 10.1097/EDE.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robins J. A new approach to causal inference in mortality studies with a sustained exposure period-application to control of the healthy worker survivor effect. Math Modelling. 1986;7(9–12):1393–1512. [Google Scholar]
- 50.Snowden JM, Rose S, Mortimer KM. Implementation of G-computation on a simulated data set: demonstration of a causal inference technique. Am J Epidemiol. 2011;173(7):731–8. doi: 10.1093/aje/kwq472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


