Abstract
Crystallins are the major proteins in the lens of the eye and function to maintain transparency of the lens. Of the human crystallins, α, β, and γ, the β-crystallins remain the most elusive in their structural significance due to their greater number of subunits and possible oligomer formations. The β-crystallins are also heavily modified during aging. This review focuses on the functional significance of deamidation and the related modifications of racemization and isomerization, the major modifications in β-crystallins of the aged human lens. Elucidating the role of these modifications in cataract formation has been slow, because they are analytically among the most difficult post-translational modifications to study. Recent results suggest that many amides deamidate to similar extent in normal aged and cataractous lenses, while others may undergo greater deamidation in cataract. Mimicking deamidation at critical structural regions induces structural changes that disrupt the stability of the β-crystallins and lead to their aggregation in vitro. Deamidations at the surface disrupt interactions with other crystallins. Additionally, the α-crystallin chaperone is unable to completely prevent deamidated β-crystallins from insolubilization. Therefore, deamidation of β-crystallins may enhance their precipitation and light scattering in vivo contributing to cataract formation.
Future experiments are needed to quantify differences in deamidation rates at all Asn and Gln residues within crystallins from aged and cataractous lenses, as well as racemization and isomerization which potentially perturb protein structure greater than deamidation alone. Quantitative data is greatly needed to investigate the importance of these major age-related modifications in cataract formation.
Keywords: Beta-crystallins, Deamidation, Proteomics, Cataracts, Aging, Post-translational modification
1. Introduction
Several recent reviews have focused on the numerous modifications of crystallins associated with aging and cataracts (Moreau and King, 2012; Sharma and Santhoshkumar, 2009; Wilmarth et al., 2010). The aim of this review is to focus on the mechanism and functional significance of deamidation, the major modification in crystallins of the aged human lens. We will review several studies examining the functional significance of deamidation, with special emphasis on how deamidation alters β-crystallin structure. We will also discuss the related modifications of racemization and isomerization, as well as non-enzymatic cleavages that can occur during such processes. These modifications have been described in lens for nearly 40 years (Van Kleef et al., 1975). However, elucidating their role in cataract formation has been slow, because they are analytically among the most difficult post-translational modifications to study. We will review recent progress in detecting and quantifying these modifications.
2. Mechanism of deamidation and associated modifications in lens
Deamidation, which converts an amide to an acid, is a ubiquitous protein modification. Not surprisingly, it is the major modification to long-lived β-crystallins in aged and cataractous lenses. Deamidation at L-Asn residues in proteins occurs primarily by formation of a L-succinimide ring intermediate that forms via attack of the backbone amide on the side chain carbonyl to displace ammonia (Fig. 1A, step A). Following its formation, the L-succinimide ring is hydrolyzed, leaving a carboxylate group that introduces a negative charge in the protein in a newly formed L-Asp residue (Fig. 1A, step B). This hydrolysis can also result in the formation of an L-isoAsp (Fig. 1A, step C), where the protein backbone is elongated by insertion of an extra methylene group in the peptide backbone. The process also induces racemization, since the L-succinimide intermediate can undergo conversion to D-succinimide (Fig. 1A, step D), followed by hydrolysis to either D-Asp (Fig. 1A, step E) or D-isoAsp (Fig.1A, step F) (Fujii et al., 2012; Geiger and Clarke, 1987). The new Asp and isoAsp moieties introduce destabilizing negative charges, and production of racemized D-isoAsp and D-Asp would be expected to further perturb protein structure. Asp residues can also undergo similar succinimide ring formation and conversion to D-Asp, L-isoAsp, and D-isoAsp. A competing reaction with deamidation is backbone cleavage at Asn (Fig. 1B) further discussed below.
Fig. 1.
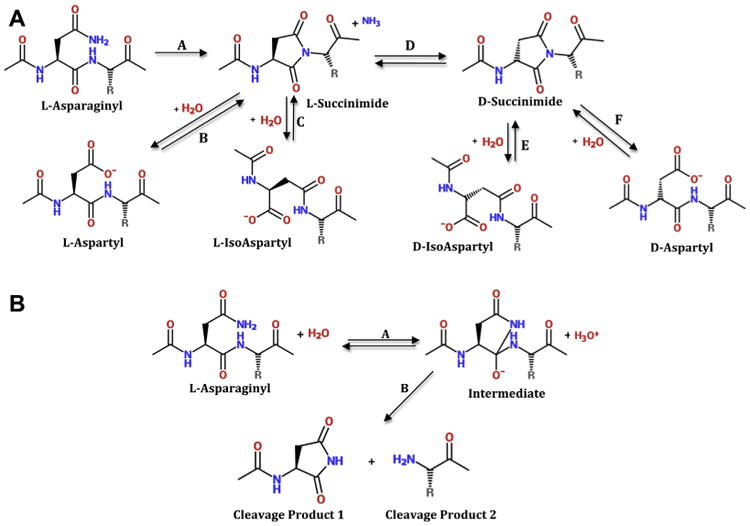
A. Reactions of the labile Asn/Asp residues in proteins from aged and cataractous lenses. Step A, formation of a succinimide ring structure and loss of ammonia. Step B, hydrolysis of succinimide ring and formation of L-Asp. Note: L-asp residues can also undergo the reverse reaction to form the succinimide ring. Step C, alternate hydrolysis of succinimide ring to form L-isoAsp. Step D, racemization of L-succinimide ring to the D-succinimide ring form. Steps E and F, hydrolysis of D- succinimde ring to either D-isoAsp or D-Asp, respectively. B. Backbone cleavage at Asn/Asp (shown for Asn). The cleavage reaction is in competition with formation of the succinimide ring shown in Fig. 1A.
The mechanism of Gln deamidation in lens is less well-understood, but may occur via a similar mechanism involving a slower-forming glutarimide intermediate (Robinson and Robinson, 2004b), or through simple hydrolysis of Gln amides during the long lifespan of lens proteins. Gln residues can also undergo enzymatic deamidation due to transglutaminase, activity, which is capable of causing deamidation of Gln residues in βB2- and βB3-crystallins (Boros et al., 2008). Regardless of the mechanism, deamidation at Gln occurs less frequently than at Asn in lens crystallins (Hains and Truscott, 2010; Wilmarth et al., 2006).
Another feature of deamidation is that it occurs more readily at certain Asn and Gln residues than others (Hooi et al., 2012b; Lapko et al., 2002; Robinson and Robinson, 2004a; Wright,1991; Xie et al., 2000). This may be due to both adjacent amino acids residues (Robinson and Robinson, 2004b) and the location of amides in unstructured regions where the succinimide or glutarimide ring structures can form more readily, or on the surface of crystallins where hydrolysis could occur more ready.
3. Deamidation in aged human lens
Crystallins are extensively modified during normal aging and cataracts (Hains and Truscott, 2007, 2010; Harrington et al., 2004; Hooi et al., 2012a; Lampi et al., 1998; Lund et al., 1996; Ma et al., 1998; Miesbauer et al., 1994; Srivastava and Srivastava, 2003; Takemoto, 1998b; Wilmarth et al., 2006; Zhang et al., 2003). The use of mass spectrometry has unambiguously identified deamidation as the cause for the increase in acidic crystallins in early reports from the human lens (de Jong et al., 1988; Groenen et al., 1990; Van Kleef et al., 1975; Voorter et al., 1988, 1987). Many of these studies targeted the analysis of deamidation in individual purified crystallins or globally at individual amides within crystallins (Hanson et al., 2000; Lampi et al., 1998; Lapko et al., 2002; Lund et al., 1996; Miesbauer et al., 1994; Srivastava and Srivastava, 2003; Takemoto and Boyle, 2000). When lenses are homogenized, increasing proportions of the proteins isolated from the inner-most “nuclear” region become insoluble in water with increasing age (Liu and Liang, 2007). The long-standing hypothesis is that the accumulation of post-translational modification to crystallins is the cause of this insolubilization. This does not, however, exclude the possibility that the modifications occurred after the aggregation event.
More recently, global approaches have been used to study lens deamidation. Wilmarth et al. (2006) reported that deamidation of Asn and Gln were the major modifications identified in several human cataractous and aged lenses, totaling 66% of the total modifications tabulated when water-soluble and water-insoluble fractions were analyzed by 2D LC/MS and the relative extent of modifications estimated by spectral counting. This suggested that deamidation is one of the major, if not the major modification in aged lens. Of the potential deamidation sites in the β-crystallins, about half were detected in vivo, with relative levels ranging from 5 to 70%. Unfortunately, due to insufficient spectral counts from single peptides in data from individual lenses, there was no comparison between aged normal and cataractous lenses in these studies. However, data summed from groups of lenses demonstrated that the extent of deamidation in the water-insoluble crystallins was greater than in the water-soluble fractions. This supported the hypothesis that deamidation is associated with precipitation of crystallins. Of the deamidations detected in the insoluble protein fraction, over half were in β-crystallins and in particular the β-subunits βB1 and βA3 (Wilmarth et al., 2006).
Reports Hains and Truscott (2007, 2010)) also showed deamidation to be the most prevalent modification in the adult lens. Using high-resolution mass spectrometry and software that automatically extracted and matched chromatographic peaks for peptides between 2D LC/MS runs, they found no generalized increase in deamidation in cataractous versus normal lenses. Similar results were also reported in a study of deamidation at Gln 92 and Gln170 residues in γS-crystallin (Hooi et al., 2012a). This is in contrast to other researchers who have reported specific sites of deamidation that are increased in cataractous lenses (Hooi et al., 2012b; Lapko et al., 2002; Takemoto and Boyle, 1999, 2000; Takemoto et al., 1990). These results suggest that many amides deamidate to similar extent in normal aged and cataractous lenses, while others may undergo greater deamidation in cataract.
4. Challenges of detecting and quantifying deamidation
Deamidation in β-crystallins and other proteins has been most successfully detected using liquid chromatography-mass spectrometry (LC-MS). In a typical “bottom-up” LC-MS experiment, a mixture of lens proteins is digested using trypsin, the peptides separated by reverse phase chromatography, and peptides identified by the mass spectrometer. The alternative “top-down” approach is where proteins are directly analyzed by generating fragments within the mass spectrometer instead of using proteolytic digestion. While top-down experiments can measure deamidation in relatively young lenses (Robinson et al., 2005, 2006b), the technique is less useful in studying deamidation in aged and cataractous lenses. This is because crystallins from very aged lens are extremely heterogeneous in mass, making isolation of all crystallin forms difficult. This complicates quantitative measurements since only an unknown portion of the crystallin forms would be analyzed. Similarly, chromatographic (Hanson et al., 2000) or electrophoretic (Lampi et al., 1998; Harrington et al., 2004) purification of crystallins from aged lenses prior to their digestion in a bottom-up experiment could yield biased quantitative data. This is why recent studies aimed at the global analysis of lens crystallin modifications have used digests of the entire complement of crystallins prior to MS analysis (Hains and Truscott, 2007, 2010; MacCoss et al., 2002; Wilmarth et al., 2006).
While there have been dramatic improvements in mass spectrometry that have profound implications in studies of deamidation (Hebert et al., 2014; Robinson et al., 2005), the analysis of the resulting data can still be challenging. Conversion of an amide to a carboxylic acid causes a 0.984 Da mass increase, which is almost identical to the mass of the non-deamidated peptide having an extra neutron from naturally occurring heavy isotopes of the constitute elements. Since mass spectrometers can accurately measure the monoistopic masses of peptides, it is easy to distinguish between non-deamidated and deamidated forms if the resulting chromatographic peaks of the two peptides forms are chromatographically resolved from one another (Dasari et al., 2007). Unfortunately, this is not always possible. While synthetic peptides differing by a single L-Asn, L-Asp, D-Asp, L-isoAsp, or D-isoAsp can be at least partially resolved from one another during reverse phase chromatography (Hooi et al., 2012b), actual peptides from aged crystallins often contain multiple Asn, Gln, and Asp residues, each of which may exist in multiple states. This leads to very complex peptide elution profiles during reverse phase chromatography (Dasari et al., 2009) making it difficult to resolve single peak features in chromatograms that would allow their accurate relative quantitation (Hains and Truscott, 2010).
An alternative approach to quantify relative extent of deamidation in lens is to use spectral counting of the numbers of assigned MS/MS spectra to particular peptide forms to measure the relative extent of deamidation (Wilmarth et al., 2006). This method is limited because the numbers of spectral counts from individual peptides from single lens digests are often too low to accurately measure the extent of deamidation at all sites of interest.
Typically, quantitative information from mass spectrometers is determined by integrating the area under the curve of the chromatogram of a specific m/z value. This assumes that chromatographic peaks have well-behaved shapes and that m/z values are distinctive. However, aged lens peptides have complex chromatograms and the deamidated mass is nearly identical to the first isotopic peak of the non-deamidated peptide resulting in complicated, mixed chromatograms. Fortunately, the analysis can be inverted and equivalent ion current information produced by extracting m/z spectra associated with ranges of elution times encompassing the complex chromatograms. A single m/z spectrum containing the mixture of isotope peaks for the various peptide forms can be decomposed into the non-deamidated peptide and its forms containing one or more deamidations by a mixture model fitting of calculated isotopic distributions so that they best reproduce the observed m/z spectrum. This allows accurate measurements of relative deamidation in peptides without the need to chromatographically resolve all forms of the peptide. This method has been used successfully to accurately measure the extent of deamidation in a few selected sites in crystallins (Dasari et al., 2009) (Fig. 2). Higher resolution instruments now available may simplify this method and allow quantification of deamidation at more sites and in larger numbers of lenses.
Fig. 2.
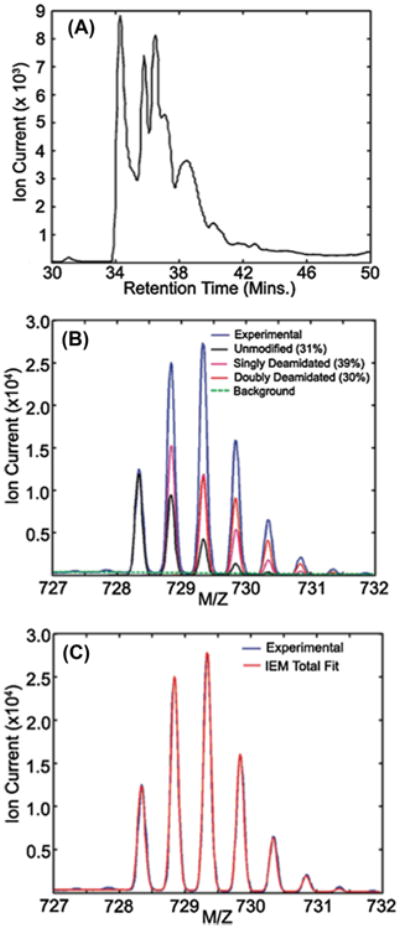
Calculation of percent single and double deamidation in peptide ITIYD-QENFQGK (αA-crystallin 33–44) from the water-insoluble fraction of a 93-year-old human lens by mixture model fitting. (A) The combined ion trace for the non-deamidated, singly-deamidated, and doubly deamidated forms of the peptide exhibiting a complex chromatogram due to deamidation, racemization, and isomerization. (B) MS scans from 34 to 42 min were averaged (experimental) and overlaid with fits to the calculated isotopic distributions for the unmodified, singly deamidated, and doubly deamidated peptide forms with their corresponding relative areas shown. (C) The composite fit resulting from the mixture modeling is shown overlaid with the experimental averaged mass spectrum to illustrate the quality of the fit (used with permission from Dasari et al., 2009).
5. Racemization and isomerization in aged human lens
As described above in Fig. 1, deamidation of Asn residues and similar succinimide ring formation at Asp residues results in several isomeric products: L-Asp, D-Asp, L-isoAsp, and D-isoAsp. Asp and isoAsp are isobaric making them difficult to distinguish using mass spectrometry. There are weak reporter ions unique to isoAsp that can be liberated if alternative peptide fragmentation methods are employed (Yang and Zubarev, 2010). Chiral isomers are also very difficult to distinguish with conventional procedures.
Peptides differing only in the presence of these residues can be at least partially resolved by reverse phase chromatography. This has enabled studies quantifying the relative extent of these modifications in aged human lenses using synthetic standards to establish retention times for the respective forms of the modified peptides (Fujii et al., 2012; Hooi et al., 2012b). This is a simpler protocol than previous studies requiring isolation of isomerized and racemized peptides from lens followed by amino acids analysis to determine their racemization/isomerization status (Takemoto et al., 2001).
These studies, and ones where unfractionated crystallins were subjected to amino acid analysis (Hooi and Truscott, 2011) have demonstrated high abundances of both isomerized and racemized Asp residues in aged and cataractous lenses. For example, Asn76 of γS-crystallin from cataractous lenses was approximately 60% deamidated, a value double that found in aged normal lenses (Hooi et al., 2012b). Furthermore, over 50% of this Asp76 had been converted to isoAsp in cataractous lenses. Measurements of isomerization and racemization in six Asp residues found in αA- and αB-crystallin from normal aged human lenses found that over 30% of Asp58 in αA-crystallin and Asp151 in αA-crystallin had been converted to D-isoAsp (Fujii et al., 2012). Recent evidence also suggests that racemization in aged human lens proteins is not restricted to Asn and Asp residues. Amino acid hydrolysis found that 4.5% of all Ser residues had become racemized to D-Ser by 70 years of age (Hooi and Truscott, 2011). There is only one study investigating isomerization and racemization in a β-crystallin (Fujii et al., 2011). Asp4 of βB2 crystallin underwent isomerization to isoAsp in aged human lenses and became stereoinverted so that there was a higher concentration of D-isoAsp than L-isoAsp.
Large-scale studies measuring relative conversion of the Asn, Asp, and Ser residues to their racemized forms in lens are problematic due to the cost of synthesizing synthetic standards to establish retention times for the various modified peptides. While electron capture and electron transfer dissociation fragmentation of peptides can be used to distinguish between Asp and IsoAsp containing peptides (Wang et al., 2010), this type of analysis has never been used to detect isoAsp containing peptides on a global scale with complex lens digests. It is also difficult to perform structural studies of crystallins containing isomerized and racemized amino acids, since simple in vitro mutagenesis cannot introduce these residues into proteins. However, introduction of racemized and isomerized residues into proteins would be expected to significantly alter their structure and stability.
6. Normal β-crystallin structure
Crystallins are the major proteins in the lens of the eye and maintain the transparency of the lens by their short-range interactions (Delaye and Tardieu, 1983). Of the three classes of crystallins, α, β, and γ, the β-crystallins have remained the most elusive in their structural significance in the lens. This is in part due to their higher ordered oligomer arrangements, not seen with the γ-crystallins and their lack of a specific non-refractive function, as with the α-crystallins. However, β-crystallins comprise roughly 40% of the crystallin content of the young human lens (Lampi et al., 1997; Robinson et al., 2006a) and become highly modified with aging (Hains and Truscott, 2007, 2010; Lampi et al., 1998; Wilmarth et al., 2006). Therefore, they likely play a significant role in aging and cataract.
Despite the difference in size, shape, hardness, composition and longevity of the lens between different species, the conservation of the βγ-crystallins in ancestral vertebrates (Kappe et al., 2010) supports their critical role in lens function. The βγ-crystallin family is derived from a single ancestral gene coding for a two domain protein, where each domain contains four β-strands in a “Greek key” structural motif (Bax et al., 1990; Nalini et al., 1994), as shown in the schematic structures for βB2 and βB1 in Figs. 3A and B. This double Greek key structure is unlike other proteins containing two β-sheets that pack to form a β-sandwich. Most notably, each linear 4-stranded Greek key motif exchanges its third β–strand with a β-sheet belonging to its partner motif. Residues within each domain are tightly packed as can be seen in the cpk model of βB2 in Fig. 3C, which is important for their overall stability and function in the lens (Bloemendal et al., 2004).
Fig. 3.
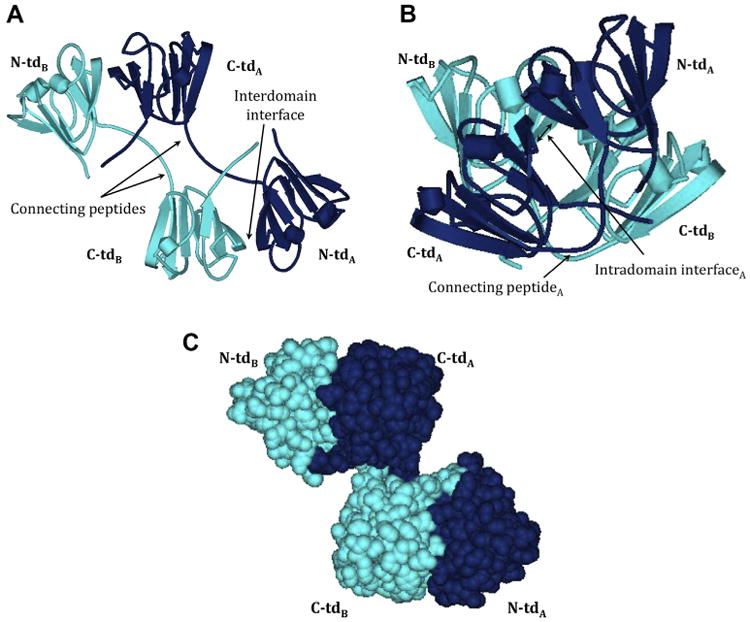
Schematic models of (A) βB2 homodimer (PBD:1YTQ), (B) truncated (tr) βB1 homodimer (PBD:1OKI), and (C) CPK model of βB2 homodimer (PBD:1YTQ). Dark blue, polypeptide chain A; light blue, polypeptide chain B; N- and C-td, N- and C-terminal domains.
There are 7 common β-crystallin subunits in mammalian lenses: 3 basic ones (βB1, βB2, βB3) and 4 acidic ones (βA1, βA2, βA3, βA4), ranging in molecular weight from approximately 23–28 kDa βA1 and βA3 are actually encoded by the same gene and are produced by alternate in-frame start codons, yielding βA1 that is 17 amino acids shorter at its N-terminus than βA3 (Werten et al., 1999); Berbers et al., 1982). A similar process also produces a minor form of βA4-crystalin in human lens containing 12 extra residues at its N-terminus (Wilmarth et al., 2009). The relative abundance of β-subunits varies greatly between various species. For example, βA2 and βB3 are not readily observed on 2D electrophoresis gels of young human lenses (Lampi et al., 1998), but can be detected in minor quantities using shotgun proteomics techniques (Wilmarth et al., 2009, 2006). In contrast, βA2 and βB3 are major proteins in rat, mouse, guinea pig, and bovine lenses (Lampi et al., 2002b; Shih et al., 1998; Simpanya et al., 2008; Ueda et al., 2002).
The β-crystallins are distinct from the γ-crystallins in that they form higher-ordered oligomers. These can be readily detected when lens proteins are extracted and separated by gel filtration to yield β-crystallin oligomers containing from 2 to 8 subunits (Harms et al., 2004; Ma et al., 1998; Bateman and Slingsby, 1992; Slingsby and Bateman, 1990). The first elucidated β-crystallin oligomeric structure was for the domain swapped βB2-dimer (Bax et al., 1990). This dumb-bell shaped structure contained an extended connecting peptide between intramolecular N- and C-terminal domains, and swapped intermolecular N- and C-terminal domains (Fig. 3A). The βB2 tetrameric structure in the crystal lattice uses an additional dimer/dimer interface (Nalini et al., 1994). Subsequent crystallographic data for a dimeric truncated βB1-crystallin indicated that other β-crystallins use an assembly quite different from βB2-crystallin (Van Montfort et al., 2003) (Fig. 3B). In truncated βB1-crystallin, the intramolecular N-td and C-td pair as in γ-crystallins (Fig. 3B), and the intermolecular interface resembled one-half of the interfaces used in dimer/dimer assembly in βB2 tetramer. Crystallographic structures for βB3- and βA4-crystallin have been solved and within their lattices they are homodimers that share a similar structure to truncated βB1-crystallin (Slingsby et al., 2013) (PDB #3QK3 and 3LWK).
Hetero-oligomeric β-crystallin structures likely predominate in vivo. β-crystallin hetero-oligomers readily assemble from purified β-crystallin homodimers (Bateman et al., 2003; Slingsby and Bateman, 1990), and βA4-crystallin is insoluble, unless co-expressed with βB2-crystallin (Marin-Vinader et al., 2006). However, crystallization of β-crystallin hetero-oligomers has been largely unsuccessful, presumably due to polydispersity caused by variations in subunit assembly and interface selection. For example, there is evidence that the structure of βA3-crystallin homodimer is in a dynamic equilibrium between monomeric and dimeric states in solution (Sergeev et al., 2000). Such behavior is essential for the observed subunit exchange in β-crystallins oligomers. That crystallins exhibit polydispersity is not surprising, given that this property would also prevent their in vivo crystallization in the lens.
Another distinguishing feature of the β-crystallins is their N-terminal extensions, which range from 10 to 57 residues in length and are absent in γ-crystallins (Bloemendal et al., 2004). These extensions are not resolved in crystallographic structures, and NMR studies indicate they remain highly flexible (Carver, 1999). As discussed in a later section, these extensions likely play a role in β-crystallin subunit assembly (Hope et al., 1994; Kroone et al., 1994), and alter the physical properties of these proteins in the lens cytosol.
7. The effect of deamidation on β-crystallin structure and stability
Deamidation has been shown to a) decrease β-crystallin stability, b) enhance protein aggregation, c) alter crystalline–crystallin interactions, d) saturate the protective α-crystallin chaperone, and e) disrupt structure.
7.1. Deamidation decreases β-crystallin stability
Deamidation at critical structural regions decreases stability of β-crystallins (Kim et al., 2002; Lampi et al., 2006, 2002a; Takata et al., 2007; Takata et al., 2008). Deamidation was mimicked by mutating the Gln to Glu residues at the interface between interacting domains in β-crystallin subunits. These residues are extremely conserved in β-crystallins, where the paired domain interaction is either intermolecular as in βB2 (Fig. 4A, Gln70 and Gln162) or intramolecular as in βB1 (Fig. 4B, Gln204) and in γ-crystallins (Van Montfort et al., 2003). The structure of βA3 is not known, but the homologous residues are highlighted in the structure of βA4 (Fig. 4C, Gln85 and Gln180). This strict conservation implies a critical structural role, which is probably related to dimer stability. Mimicking deamidation at these conserved interface Gln residues destabilized β-crystallin subunits during unfolding in urea (Kim et al., 2002; Lampi et al., 2006; Takata et al., 2007) (Table 1) and enhanced solution turbidity and their precipitation upon heating (Lampi et al., 2002a; Takata et al., 2007) (Fig. 5, compare βB1 wild type to βB1 Q204E). The urea-refolding curve of βB1 Q204E suggested that the partially unfolded intermediate was prone to aggregation (Kim et al., 2002). Deamidations at both conserved Gln residues in βB2 and βA3 further decreased protein stability (Lampi et al., 2006; Takata et al., 2007) (Table 1).
Fig. 4.
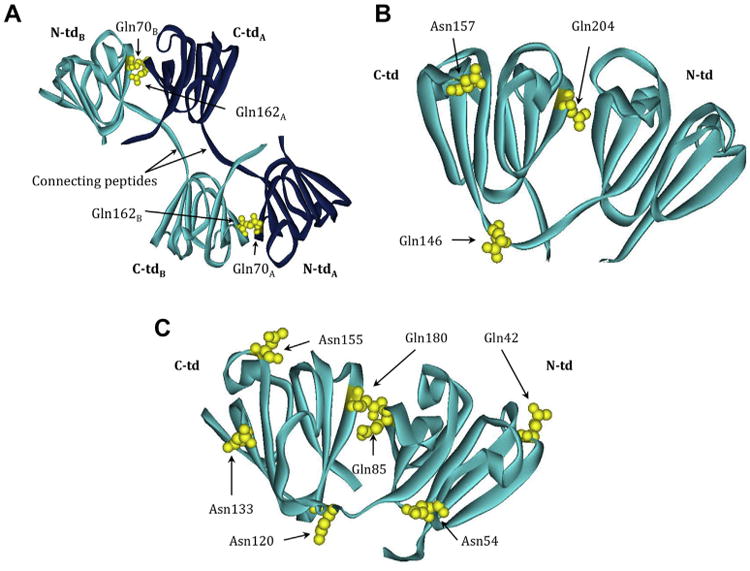
β-crystallin structures highlighting labile Asn/Gln that are deamidated in vivo. Ribbon model of (A) βB2 homodimer (PBD: 1YTQ) showing Gln70, Gln162, and Gln184. The light and dark blue color scheme represents the two homologous monomers. Ribbon model of (B) truncated (tr) βB1 homodimer (PBD:1OKI) showing Gln146, Gln157, and Gln204. Ribbon model of (C) βA4 monomer (PBD:3WLK) with residues homologous to Gln42, Asn54, Gln85, Asn120, Asn133, Asn155 and Gln180 found in βA3 highlighted. Dark blue, polypeptide chain A; light blue, polypeptide chain B; N- and C-td, N- and C-terminal domains. Deamidated residues shown in yellow. The numbering system is based on the amino acid sequence with the N-terminal methionine retained in βA3, but post-translationally removed in βB1 and βB2.
Table 1.
Stability of β-crystallins with deamidations at the domain interfaces.
| βB1a | βA3b | βB2c | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| CMd | CM | ΔGD°e | CM | ΔGD1°f | ΔGD2°g | |||
| Wild-type | 5.9 | Wild-type | 4.5 | 14 | Wild-type | 2.3 | 7 | 8 |
| Q85E | 4.2 | 10 | Q70E | 1.8 | 3 | 4 | ||
| Q204E | 4.1 | Q180E | 4.1 | 10 | Q162E | 1.8 | 3 | 8 |
| Q85E/Q180E | 3.7 | 4 | Q85E/Q180E | 1.3 | 4 | 5 | ||
The molarity of urea at midpoints of apparent equilibrium transitions during unfolding.
Apparent Gibbs free energy, GD° (kcal/mol), from urea unfolding curves.
Native to intermediate transition.
Intermediate to unfolded transition.
Fig. 5.
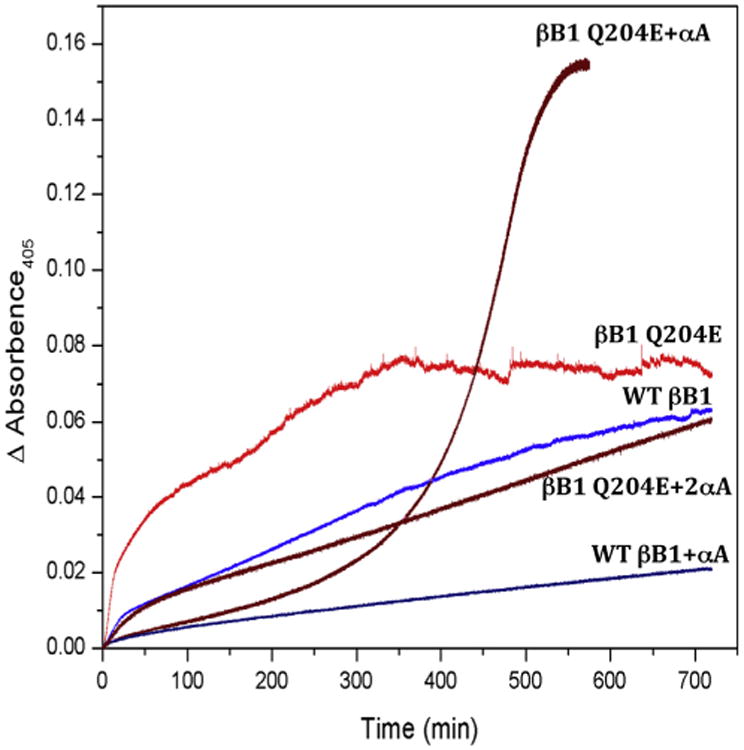
Thermal denaturation at 55 °C of WTand Q204E βB1 adapted with permission from Lampi et al., 2002a, b. (A) WT βB1 (blue curve) and Q204E βB1 (red curve) were heated with an equal molar amount of αA (dark blue and dark red, respectively). Additionally, Q204E βB1 was heated with αA in a molar ratio of 1:2 (brown).
King and coworkers have reported similar findings for γ-crystallins (Flaugh et al., 2006). By introducing a negative charge into the hydrophobic sticky region at the interface between domains or subunits, deamidation has been shown to decrease the stability of βB1, βB2, βA3, and γD subunits of the βγ-crystallin family.
Deamidation decreases stability and enhances the aggregation pathway. Combining differential scanning calorimetry (DSC) as a measure of unfolding, dynamic light scattering (DLS) as a measure of aggregation, and small angle X-ray scattering (SAXS) as a measure of precipitation, unfolding of deamidated βB2 was followed quickly by aggregation and precipitation (Michiel et al., 2010). The βB2 remaining in solution after heating retained its native structure, suggesting that it was not necessary for 100% of the protein to precipitate to obtain a large increase in light scattering (Michiel et al., 2010). These data support a model that accumulation of age-related modifications that build up over the lifespan of an individual may reach a threshold at which precipitation occurs rapidly.
Not all deamidation sites are detrimental. For example, deamidation at Gln146 in the peptide connecting the N- and C-terminal domains was compared to deamidation at Asn157 in an outside loop region of βB1 (Harms et al., 2004) (Fig. 4B). These two sites are detected in vivo in adult lenses at comparable levels of 17% and 13%, respectively (Harms et al., 2004). Deamidation at Gln146 led to the formation of higher-ordered oligomers ranging from 200 to 400 kDa and to lower stability not observed when deamidation was mimicked at Asn157 (Harms et al., 2004). Deamidation in this case may be an example of a deamidation programmed to control oligomer size during development.
7.2. Deamidation enhances β-crystallin aggregation
Deamidation enhances aggregation of soluble β-crystallins, which may be a required event for insolubilization. Deamidation at proposed exposed sites on βA3 at Gln42, Asn120, and Asn133 and at the partially buried sites Asn155 and Asn54 (Takata et al., 2008 and Fig. 4C) ranged from 15 to 35% in the insoluble proteins, more than double the amount found in the soluble proteins (Wilmarth et al., 2006). Sedimentation velocity measurements were used to characterize soluble βA3 oligomers containing deamidations at these sites in βA3 (Takata et al., 2008). The results for the deamidated proteins indicated that they contained aggregates (15–20%) that were elongated and ranged from 250 to 500 kDa in size, not detected in the wild type βA3. While these aggregates are not large enough to scatter light, deamidations at many sites, which is expected to occur during aging, may lead to additional aggregation and induce light scattering.
7.3. Deamidation alters β-crystallin interactions
Members of the β-crystallin family form complex hetero-oligomers of dimers, tetramers, and other higher-ordered assemblies when isolated from lenses (Bateman and Slingsby, 1992; Berbers et al., 1982). During aging, the higher-ordered oligomer fraction decreases with a relative increase in an intermediate tetramer fraction, which is associated with numerous modifications, including deamidation (Lampi et al., 1998; Ma et al., 1998). Also during aging, a high molecular weight fraction is produced containing many modified β- and β-crystallins (Harrington et al., 2004).
In vitro, the acidic and basic subunits of β-crystallins readily form complexes (Bateman et al., 2003; Liu and Liang, 2007; Slingsby and Bateman, 1990). βB2 preferentially forms hetero-dimers with other β-crystallins subunits and this has been proposed to be due to a strategically placed destabilizing Cys at the tight interface between domains creating more flexibility in the interface than observed in other β-crystallin subunits (MacDonald et al., 2005). The heterodimers of βB2/βA3 are more resistant to heat-denaturation than βA3 by itself, supporting the role of β-crystallin hetero-oligomer interactions promoting stability of crystallin complexes (Takata et al., 2009).
Mimicking deamidations at the domain interface Gln residues and at Asn54 and Asn155 in βA3 decreased the rate of formation of hetero-oligomers with either βB1 or βB2 (Takata et al., 2008, 2009). However, once the hetero-dimer forms between the deamidated βA3 and wild type βB2 or (especially) βB1, the heterodimer is more resistant to heat denaturation than the βA3 homodimer alone (Takata et al., 2009). While heterodimer interactions appear to have a stabilizing affect, this effect can be modulated by deamidations identified in vivo.
7.4. Deamidation decreases ability of β-crystallins to be chaperoned by the protective α-crystallin
The chaperone properties of α-crystallin have been well established in vitro (Horwitz, 1992) and its ability to chaperone modified β-crystallins is a potential protective mechanism in the lens. When β-crystallins are partially unfolded during urea-denaturation (McHaourab et al., 2007; Moreau and King, 2012; Sathish et al., 2004) or thermal-denaturation (Lampi et al., 2012; McHaourab et al., 2007; Moreau and King, 2012; Sathish et al., 2004; Takata et al., 2009), they form a complex with α-crystallin, similar to what has been reported between α-crystallin and γ-crystallins (Acosta-Sampson and King, 2010). However, α-crystallin is not able to completely rescue deamidated β-crystallins from heat-induced precipitation (Lampi et al., 2002a; Michiel et al., 2010) (Fig. 5). Both the deamidated β-crystallin and the chaperone precipitated, with the deamidated β-crystallin potentially saturating the α-chaperone (Lampi et al., 2002a) (Fig. 5, compare βB1 Q204E to βB1 Q204E with αA). Increasing amounts of αA are required to prevent precipitation of the β-substrate (Fig. 5, compare βB1 Q204E to βB1 Q204E with 2× αA). Saturation of the α-chaperone could contribute to cataracts where light scattering from large, insoluble α-crystallin and modified βγ-crystallin aggregates form (Acosta-Sampson and King, 2010; Evans et al., 2008).
7.5. Deamidation disrupts β-crystallin structure
The effects of deamidation on stability described above can be explained by subtle changes in crystallin structure. Homology modeling was used to explore the effect of replacing a Gln with a charged Glu residue at the interacting interfaces between domains in β-crystallins (Takata et al., 2008). The Glu disrupted a hydrogen bond at the interface, using either the βB1 or the βB2 crystal structure as the model. The difference in apparent free energies of unfolding between the wild type and deamidated β-subunits is consistent with loss of a hydrogen bond predicted by the model.
Deamidation at the interacting domain interface altered the secondary and tertiary structures of β-crystallins, as detected by circular dichroism, and this alteration was most noted when both interface Gln residues were mutated (Lampi et al., 2006; Takata et al., 2007). For the doubly deamidated mutant of βB2, Q70E/Q162E, there was a shift in maximum intensity during tryptophan fluorescence emission in the absence of urea, further suggesting the unperturbed protein was already slightly unfolded (Lampi et al., 2006). However, these changes were not enough to disrupt the homodimers detected by multi-angle laser light scattering, nor the overall shape and radius of hydration detected by Quasi-elastic laser light scattering.
In order to further examine the subtle structural changes due to deamidation, a series of hydrogen/deuterium (H/D) exchange reactions with high-resolution mass spectrometry were performed with the βB2 homodimer. Overall, the results suggested a compact βB2. There was only 20–30% deuterium uptake in the N- and C-terminal domain regions, with the C-terminal domain being the less solvent accessible of the two domains. The dimer interface was also buried with only 7–14% deuterium uptake after 60 min (Takata et al., 2010). The cpk model of the human βB2 structure illustrates this point well, where the two partner subunits appear to be in a “tango embrace” (as described above in Fig. 3C). The greater accessibility of the N-terminal domain corresponds to its lower stability and greater unfolding in urea compared to the C-terminal domain (Wieligmann et al., 1999). Mimicking deamidation at the homologous Gln70 residues at the buried interface between subunits in the βB2 homodimer increased accessibility at the interface to deuterium uptake and led to small, but significant changes distant to the deamidation site (Takata et al., 2010) (Fig. 6). Additionally, deamidation at the interface in both βB2 and βA3 increased its susceptibility to trypsin digestion (Lampi et al., 2006; Takata et al., 2007). This is consistent with an increased exposure of protein regions creating a “space” that would then be accessible to digestion, similar to what was reported when an Asp replaced a Phe in γB (Palme et al., 1997).
Fig. 6.
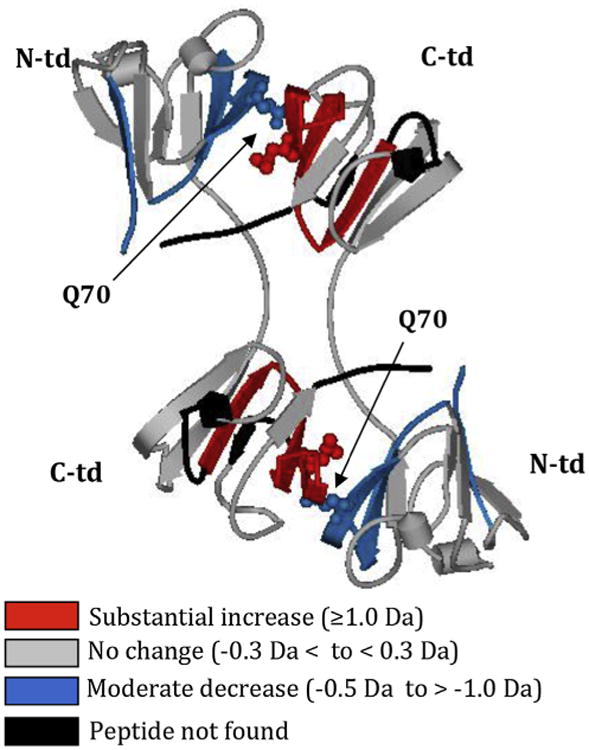
Conformational changes in WT βB2 due to deamidation at Gln70 adapted with permission from Takata et al., 2010. Differences in relative deuterium levels between WT βB2 and Q70E βB2 mutant peptides were overlaid onto the human βB2 crystal structure (PDB:1YTQ). The color of the peptides indicates the differential trend between the mutant and WT. Arrows indicate Gln70 shown in cpk model that was mutated to mimic deamidation. Gln70 in the N-td is oriented towards the C-td, disrupting the C-td and the interface. N- and C-td, N- and C-terminal domain.
In summary, the compact structure of the β-crystallins gives these proteins their extremely stable structures throughout the lifespan of an individual. Deamidation at critical structural regions, such as the buried interface, induces structural changes significant enough to disrupt the stability of the β-crystallins and lead to their aggregation in vitro. Deamidations at the surface disrupt interactions with other crystallins, possibly due to charge repulsion from the added charge. Therefore deamidation, depending on the site, can alter the stability and solubility of β-crystallins. This may lead to their precipitation in vivo.
8. Truncation of β-crystallin N-terminal extensions
The unstructured N-terminal extensions of human β-crystallins become progressively truncated with increasing age. For example, forms of βB1-crystallin missing 15, 33, 34, 35, 36, 39, 40, and 41 amino acids were detected in lenses by 29 years of age (David et al., 1996). The first cleavage removing 15 residues from the N-terminus of bB1-crystallin can be observed in newborn human lenses (Lampi et al., 1997). βA3 crystallin also undergoes cleavage in human lenses and is missing 22 residues from its N-terminus at birth. Both these early cleavages in βB1- and βA3-crystallin occur at a peptide bond between Asn-Pro residues. These fragments were first thought to occur due to endopeptidase cleavage. However, a non-enzymatic process is more likely, with subsequent trimming by lens exopeptidases producing the ragged termini observed in older lenses. Deamidation and backbone cleavage at Asn are competing reactions during the formation of the intermediate described above and in Fig.1B. For some protein sequences, especially -Asn/Asp-Pro-, this cleavage reaction is highly favored (Robinson and Robinson, 2004a). Protein structure can either suppress or enhance rates of deamidation and backbone cleavage. Known cleavage sites in the lens that may occur due to this mechanism include: Asn101 in αA-crystallin (Takemoto, 1999; Takemoto and Boyle, 1999; Takemoto, 1998a) Asn15, Asn246 and Asn259 in Aquaporin 0 (Ball et al., 2004), Asn15 in βB1, and Asn22 in βA3-crystallins (David et al., 1996; Lampi et al., 1997; Zhang et al., 2003). The cleavage sites in the β-crystallins are in the flexible N-terminal extension. At Asn246 and Asn259 in Aquaporin 0, the cleavage sites are in the exposed cytosolic C-terminal tail. Cleavage at these sites would not be hindered by tertiary structure.
During development, the protein concentration in the lens increases (Ringens et al., 1982), while the water content in the central nucleus of the lens decreases relative to the outer cortical region (Heys and Truscott, 2008; Lahm et al., 1985). The structure of the β-crystallin assemblies in these different compartments is not known, but has been proposed to involve processing of the N-terminal extensions. The highly charged N-terminal extensions have been suggested to act as spacers contributing to the defined distance between β-crystallins (Werten et al., 1996). Several studies have suggested that the long N-terminal extension of βB1 is needed for the initial formation of the βH-assembly comprised of hetero-oligomers (Ajaz et al., 1997; Trinkl et al., 1994). Likewise, the extensions have been proposed to facilitate ordering of interactions in the β-crystallin assemblies at the high concentrations found in the lens (Hope et al., 1994; Kroone et al., 1994; Nalini et al., 1994). The charged extensions most likely are involved in charge–charge interactions. However, once the initial β-assembly is formed, the extensions may no longer be needed. In fact, removal of the N-terminal extensions may increase their stability, and allow further packing of the ordered crystallins as the lens develops and concentrates its proteins in the nucleus.
9. Potential model of cataract
An emerging theme from congenital cataracts is that only minimal structural changes from single amino acid substitutions are needed to cause lens opacity. In congenital cataracts, characterized by mutations P23T, R26S, or R58H, the mutated γD structure was either indistinguishable from the native protein or had only small differences (Basak et al., 2003; Ji et al., 2012, 2013a, 2013b). These cataracts may have been due to changes in surface interactions, disrupting the short-range order of the crystallins. Similarly, deamidations on the surface of β-crystallins may disrupt crystallin interactions. Furthermore, deamidation at critical structural regions, such as the buried interface, may disrupt the structure of the protein and lead to partial unfolding. At the high protein concentrations found in vivo, only small surface changes or structural changes in βγ-crystallins may be needed to disrupt their highly ordered state.
Numerous post-translational modifications have been identified in the aged normal lens with similar modification sites detected in age-matched cataractous lenses. A distinguishing factor in cataracts may be an increased abundance of these modifications. An accumulation of many deamidations at critical structural regions, even at low levels, is likely to decrease the stability of crystallins in the lens and ultimately lead to their aggregation. Additionally, aggregation and light scatter may be enhanced, since the α-crystallin chaperone is unable to completely prevent deamidated β-crystallins from entering the aggregation pathway (Fig. 7). It is also possible that aggregating β-crystallin can bind α-crystallin and precipitate together.
Fig. 7.
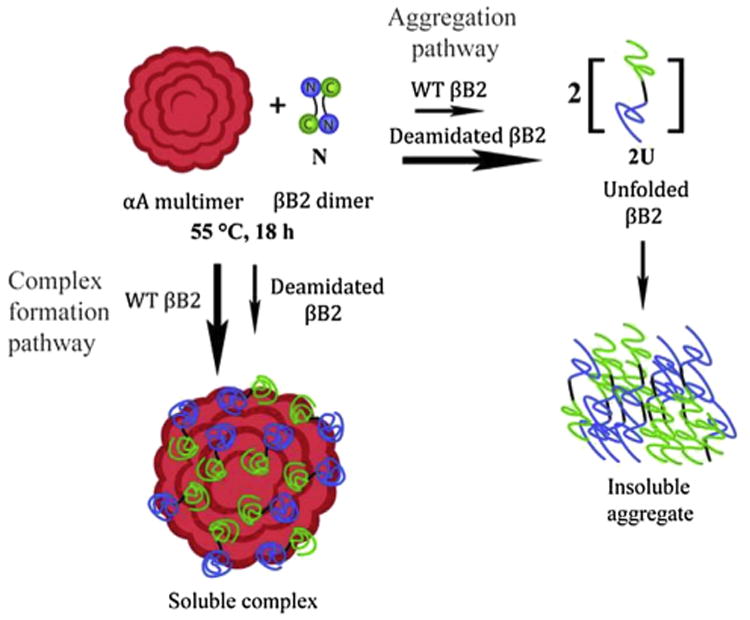
Proposed model for cataract formation adapted with permission from Lampi et al., 2012. During heat denaturation, both the N-terminal domain and C-terminal domain of βB2 partially unfold. The αA-chaperone forms a complex with the partially unfolded βB2, rescuing βB2 from precipitation. In contrast, deamidated βB2 precipitates under the same conditions due to a faster conversion to the more severely unfolded state and is unable to be rescued by α-crystallin.
10. Future prospects
Mutagenesis studies, where individual amides are substituted for Asp or Glu residues as described above to determine the significance of deamidation, are hindered by lack of data on the most prevalent deamidation sites associated with cataractous lenses. Measurements are needed of deamidation rates at all Asn and Gln residues within crystallins from aged and cataractous lenses. In addition to the challenges of quantifying deamidation mentioned above, proteolytic digestion of crystallins prior to LC/MS analyses often produce peptides containing more than one Asn or Gln residue. For example, 8 out of 12 amide containing tryptic peptides from βB2-crystallin contain 2 or more amides. Independent measurement of the relative extent of deamidation in peptides containing more than one amide will require collection of high-resolution MS/MS data to examine the isotopic distributions of peptide fragment ions to assign relative amounts of deamidation at individual sites. Similar methods have been used to measure H/D exchange rates at individual amides in proteins (Abzalimov et al., 2013) and the relative extent of histone acetylation in peptides containing multiple lysines (Smith et al., 2003). To the best of our knowledge, these types of measurements have not been made in deamidated crystallin peptides containing multiple amides. However, they are critical in order to target specific amides in structural studies that have relevance in aged and cataractous lenses.
Surprisingly, there have been no experiments using absolute quantitation of modified peptides in lens utilizing heavy labeled peptide standards (Kirkpatrick et al., 2005). These experiments would likely provide improved data quantifying differences in the extent of modifications in normal versus cataractous lenses. There is very limited data on the extent of racemization and isomerization of amino acids in β-crystallins in aged human lenses. These modifications may potentially perturb the structure of these important lens proteins more greatly than deamidation alone. Quantitative data is greatly needed to investigate the importance of these major age-related modifications in cataract formation.
Finally, very little is known about the structure of the higher-ordered β-crystallin assemblies. Studies are currently underway using H/D exchange and chemical cross-linking to probe these structures. Future studies may also use mass spectrometry to measure the masses of native β-crystallin hetero-oligomers. Such studies have provided important information concerning the structure of another highly polydisperse protein, α-crystallin (Aquilina et al., 2005). These studies are critical to understanding the more complex β-crystallin interactions found in vivo. Methods aimed to slow the progression of protein aggregation and light scatter in cataracts will certainly be improved if a thorough understanding of the structure and interactions of lens crystallins emerges.
Acknowledgments
Authors wish to acknowledge Jackson Wong for his expert assistance with figures. Financial support was provided by NIH RO1 EY012239 (KL), RO1 EY007755 (LD), and core grant P30 EY010572.
References
- Abzalimov RR, Bobst CE, Kaltashov IA. A new approach to measuring protein backbone protection with high spatial resolution using H/D exchange and electron capture dissociation. Anal Chem. 2013;85:9173–9180. doi: 10.1021/ac401868b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Sampson L, King J. Partially folded aggregation intermediates of human gammaD-, gammaC-, and gammaS-crystallin are recognized and bound by human alphaB-crystallin chaperone. J Mol Biol. 2010;401:134–152. doi: 10.1016/j.jmb.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajaz MS, Ma Z, Smith DL, Smith JB. Size of human lens beta-crystallin aggregates are distinguished by N-terminal truncation of betaB1. J Biol Chem. 1997;272:11250–11255. doi: 10.1074/jbc.272.17.11250. [DOI] [PubMed] [Google Scholar]
- Aquilina JA, Benesch JL, Ding LL, Yaron O, Horwitz J, Robinson CV. Subunit exchange of polydisperse proteins: mass spectrometry reveals consequences of alphaA-crystallin truncation. J Biol Chem. 2005;280:14485–14491. doi: 10.1074/jbc.M500135200. [DOI] [PubMed] [Google Scholar]
- Ball LE, Garland DL, Crouch RK, Schey KL. Post-translational modifications of aquaporin 0 (AQP0) in the normal human lens: spatial and temporal occurrence. Biochemistry. 2004;43:9856–9865. doi: 10.1021/bi0496034. [DOI] [PubMed] [Google Scholar]
- Basak A, Bateman O, Slingsby C, Pande A, Asherie N, Ogun O, Benedek GB, Pande J. High-resolution X-ray crystal structures of human gammaD crystallin (1.25 A) and the R58H mutant (1.15 A) associated with aculeiform cataract. J Mol Biol. 2003;328:1137–1147. doi: 10.1016/s0022-2836(03)00375-9. [DOI] [PubMed] [Google Scholar]
- Bateman OA, Sarra R, van Genesen ST, Kappe G, Lubsen NH, Slingsby C. The stability of human acidic beta-crystallin oligomers and hetero-oligomers. Exp Eye Res. 2003;77:409–422. doi: 10.1016/s0014-4835(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Bateman OA, Slingsby C. Structural studies on beta H-crystallin from bovine eye lens. Exp Eye Res. 1992;55:127–133. doi: 10.1016/0014-4835(92)90100-7. [DOI] [PubMed] [Google Scholar]
- Bax B, Lapatto R, Nalini V, Driessen H, Lindley PF, Mahadevan D, Blundell TL, Slingsby C. X-ray analysis of beta B2-crystallin and evolution of oligomeric lens proteins. Nature. 1990;347:776–780. doi: 10.1038/347776a0. [DOI] [PubMed] [Google Scholar]
- Berbers GA, Boerman OC, Bloemendal H, de Jong WW. Primary gene products of bovine beta-crystallin and reassociation behavior of its aggregates. Eur J Biochem. 1982;128:495–502. doi: 10.1111/j.1432-1033.1982.tb06992.x. [DOI] [PubMed] [Google Scholar]
- Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Boros S, Wilmarth PA, Kamps B, de Jong WW, Bloemendal H, Lampi K, Boelens WC. Tissue transglutaminase catalyzes the deamidation of glutamines in lens betaB(2)- and betaB(3)-crystallins. Exp Eye Res. 2008;86:383–393. doi: 10.1016/j.exer.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Carver JA. Probing the structure and interactions of crystallin proteins by NMR spectroscopy. Prog Retin Eye Res. 1999;18:431–462. doi: 10.1016/s1350-9462(98)00027-5. [DOI] [PubMed] [Google Scholar]
- Dasari S, Wilmarth PA, Reddy AP, Robertson LJ, Nagalla SR, David LL. Quantification of isotopically overlapping deamidated and 18o-labeled peptides using isotopic envelope mixture modeling. J Proteom Res. 2009;8:1263–1270. doi: 10.1021/pr801054w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Wilmarth PA, Rustvold DL, Riviere MA, Nagalla SR, David LL. Reliable detection of deamidated peptides from lens crystallin proteins using changes in reversed-phase elution times and parent ion masses. J Proteom Res. 2007;6:3819–3826. doi: 10.1021/pr070182x. [DOI] [PubMed] [Google Scholar]
- David LL, Lampi KJ, Lund AL, Smith JB. The sequence of human betaB1-crystallin cDNA allows mass spectrometric detection of betaB1 protein missing portions of its N-terminal extension. J Biol Chem. 1996;271:4273–4279. doi: 10.1074/jbc.271.8.4273. [DOI] [PubMed] [Google Scholar]
- de Jong WW, Mulders JW, Voorter CE, Berbers GA, Hoekman WA, Bloemendal H. Post-translational modifications of eye lens crystallins: crosslinking, phosphorylation and deamidation. Adv Exp Med Biol. 1988;231:95–108. doi: 10.1007/978-1-4684-9042-8_8. [DOI] [PubMed] [Google Scholar]
- Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Evans P, Slingsby C, Wallace BA. Association of partially folded lens betaB2-crystallins with the alpha-crystallin molecular chaperone. Biochem J. 2008;409:691–699. doi: 10.1042/BJ20070993. [DOI] [PubMed] [Google Scholar]
- Flaugh SL, Mills IA, King J. Glutamine deamidation destabilizes human gammaD-crystallin and lowers the kinetic barrier to unfolding. J Biol Chem. 2006;281:30782–30793. doi: 10.1074/jbc.M603882200. [DOI] [PubMed] [Google Scholar]
- Fujii N, Kawaguchi T, Sasaki H, Fujii N. Simultaneous stereoinversion and isomerization at the Asp-4 residue in betaB2-crystallin from the aged human eye lenses. Biochemistry. 2011;50:8628–8635. doi: 10.1021/bi200983g. [DOI] [PubMed] [Google Scholar]
- Fujii N, Sakaue H, Sasaki H, Fujii N. A rapid, comprehensive liquid chromatography-mass spectrometry (LC-MS)-based survey of the Asp isomers in crystallins from human cataract lenses. J Biol Chem. 2012;287:39992–40002. doi: 10.1074/jbc.M112.399972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T, Clarke S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem. 1987;262:785–794. [PubMed] [Google Scholar]
- Groenen PJ, van den Ijssel PR, Voorter CE, Bloemendal H, de Jong WW. Site-specific racemization in aging alpha A-crystallin. FEBS Lett. 1990;269:109–112. doi: 10.1016/0014-5793(90)81131-7. [DOI] [PubMed] [Google Scholar]
- Hains PG, Truscott RJ. Post-translational modifications in the nuclear region of young, aged, and cataract human lenses. J Proteom Res. 2007;6:3935–3943. doi: 10.1021/pr070138h. [DOI] [PubMed] [Google Scholar]
- Hains PG, Truscott RJ. Age-dependent deamidation of lifelong proteins in the human lens. Invest Ophthalmol Vis Sci. 2010;51:3107–3114. doi: 10.1167/iovs.09-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SR, Hasan A, Smith DL, Smith JB. The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Exp Eye Res. 2000;71:195–207. doi: 10.1006/exer.2000.0868. [DOI] [PubMed] [Google Scholar]
- Harms MJ, Wilmarth PA, Kapfer DM, Steel EA, David LL, Bachinger HP, Lampi KJ. Laser light-scattering evidence for an altered association of beta B1-crystallin deamidated in the connecting peptide. Protein Sci. 2004;13:678–686. doi: 10.1110/ps.03427504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington V, McCall S, Huynh S, Srivastava K, Srivastava OP. Crystallins in water soluble-high molecular weight protein fractions and water insoluble protein fractions in aging and cataractous human lenses. Mol Vis. 2004;10:476–489. [PubMed] [Google Scholar]
- Hebert AS, Richards AL, Bailey DJ, Ulbrich A, Coughlin EE, Westphall MS, Coon JJ. The one hour yeast proteome. Mol Cell Proteomics. 2014 Jan;13(1):339–347. doi: 10.1074/mcp.M113.034769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heys KR, Truscott RJ. The stiffness of human cataract lenses is a function of both age and the type of cataract. Exp Eye Res. 2008;86:701–703. doi: 10.1016/j.exer.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Hooi MY, Raftery MJ, Truscott RJ. Age-dependent deamidation of glutamine residues in human gammaS crystallin: deamidation and unstructured regions. Protein Sci. 2012a;21:1074–1079. doi: 10.1002/pro.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooi MY, Raftery MJ, Truscott RJ. Racemization of two proteins over our lifespan: deamidation of asparagine 76 in gammaS crystallin is greater in cataract than in normal lenses across the age range. Invest Ophthalmol Vis Sci. 2012b;53:3554–3561. doi: 10.1167/iovs.11-9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooi MY, Truscott RJ. Racemisation and human cataract. D-Ser, D-Asp/Asn and D-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. Age (Dordr) 2011;33:131–141. doi: 10.1007/s11357-010-9171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope JN, Chen HC, Hejtmancik JF. Beta A3/A1-crystallin association: role of the N-terminal arm. Protein Eng. 1994;7:445–451. doi: 10.1093/protein/7.3.445. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji F, Jung J, Gronenborn AM. Structural and biochemical characterization of the childhood cataract-associated R76S mutant of human gammaD-crystallin. Biochemistry. 2012;51:2588–2596. doi: 10.1021/bi300199d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji F, Jung J, Koharudin LM, Gronenborn AM. The human W42R gammaD-crystallin mutant structure provides a link between congenital and age-related cataracts. J Biol Chem. 2013a;288:99–109. doi: 10.1074/jbc.M112.416354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji F, Koharudin LM, Jung J, Gronenborn AM. Crystal structure of the cataract-causing P23T gammaD-crystallin mutant. Proteins. 2013b;81:1493–1498. doi: 10.1002/prot.24321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappe G, Purkiss AG, van Genesen ST, Slingsby C, Lubsen NH. Explosive expansion of betagamma-crystallin genes in the ancestral vertebrate. J Mol Evol. 2010;71:219–230. doi: 10.1007/s00239-010-9379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Kapfer DM, Boekhorst J, Lubsen NH, Bachinger HP, Shearer TR, David LL, Feix JB, Lampi KJ. Deamidation, but not truncation, decreases the urea stability of a lens structural protein, betaB1-crystallin. Biochemistry. 2002;41:14076–14084. doi: 10.1021/bi026288h. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick DS, Gerber SA, Gygi SP. The absolute quantification strategy: a general procedure for the quantification of proteins and post-translational modifications. Methods. 2005;35:265–273. doi: 10.1016/j.ymeth.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Kroone RC, Elliott GS, Ferszt A, Slingsby C, Lubsen NH, Schoenmakers JG. The role of the sequence extensions in beta-crystallin assembly. Protein Eng. 1994;7:1395–1399. doi: 10.1093/protein/7.11.1395. [DOI] [PubMed] [Google Scholar]
- Lahm D, Lee LK, Bettelheim FA. Age dependence of freezable and non-freezable water content of normal human lenses. Invest Ophthalmol Vis Sci. 1985;26:1162–1165. [PubMed] [Google Scholar]
- Lampi KJ, Amyx KK, Ahmann P, Steel EA. Deamidation in human lens betaB2-crystallin destabilizes the dimer. Biochemistry. 2006;45:3146–3153. doi: 10.1021/bi052051k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi KJ, Fox CB, David LL. Changes in solvent accessibility of wild-type and deamidated betaB2-crystallin following complex formation with alphaA-crystallin. Exp Eye Res. 2012;104:48–58. doi: 10.1016/j.exer.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi KJ, Kim YH, Bachinger HP, Boswell BA, Lindner RA, Carver JA, Shearer TR, David LL, Kapfer DM. Decreased heat stability and increased chaperone requirement of modified human betaB1-crystallins. Mol Vis. 2002a;8:359–366. [PubMed] [Google Scholar]
- Lampi KJ, Ma Z, Hanson SR, Azuma M, Shih M, Shearer TR, Smith DL, Smith JB, David LL. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp Eye Res. 1998;67:31–43. doi: 10.1006/exer.1998.0481. [DOI] [PubMed] [Google Scholar]
- Lampi KJ, Ma Z, Shih M, Shearer TR, Smith JB, Smith DL, David LL. Sequence analysis of betaA3, betaB3, and betaA4 crystallins completes the identification of the major proteins in young human lens. J Biol Chem. 1997;272:2268–2275. doi: 10.1074/jbc.272.4.2268. [DOI] [PubMed] [Google Scholar]
- Lampi KJ, Shih M, Ueda Y, Shearer TR, David LL. Lens proteomics: analysis of rat crystallin sequences and two-dimensional electrophoresis map. Invest Ophthalmol Vis Sci. 2002b;43:216–224. [PubMed] [Google Scholar]
- Lapko VN, Purkiss AG, Smith DL, Smith JB. Deamidation in human gamma S-crystallin from cataractous lenses is influenced by surface exposure. Biochemistry. 2002;41:8638–8648. doi: 10.1021/bi015924t. [DOI] [PubMed] [Google Scholar]
- Liu BF, Liang JJ. Protein-protein interactions among human lens acidic and basic beta-crystallins. FEBS Lett. 2007;581:3936–3942. doi: 10.1016/j.febslet.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AL, Smith JB, Smith DL. Modifications of the water-insoluble human lens alpha-crystallins. Exp Eye Res. 1996;63:661–672. doi: 10.1006/exer.1996.0160. [DOI] [PubMed] [Google Scholar]
- Ma Z, Hanson SR, Lampi KJ, David LL, Smith DL, Smith JB. Age-related changes in human lens crystallins identified by HPLC and mass spectrometry. Exp Eye Res. 1998;67:21–30. doi: 10.1006/exer.1998.0482. [DOI] [PubMed] [Google Scholar]
- MacCoss MJ, McDonald WH, Saraf A, Sadygov R, Clark JM, Tasto JJ, Gould KL, Wolters D, Washburn M, Weiss A, Clark JI, Yates JR., 3rd Shotgun identification of protein modifications from protein complexes and lens tissue. Proc Natl Acad Sci U S A. 2002;99:7900–7905. doi: 10.1073/pnas.122231399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JT, Purkiss AG, Smith MA, Evans P, Goodfellow JM, Slingsby C. Unfolding crystallins: the destabilizing role of a beta-hairpin cysteine in betaB2-crystallin by simulation and experiment. Protein Sci. 2005;14:1282–1292. doi: 10.1110/ps.041227805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Vinader L, Onnekink C, van Genesen ST, Slingsby C, Lubsen NH. In vivo heteromer formation. Expression of soluble betaA4-crystallin requires coexpression of a heteromeric partner. FEBS J. 2006;273:3172–3182. doi: 10.1111/j.1742-4658.2006.05326.x. [DOI] [PubMed] [Google Scholar]
- McHaourab HS, Kumar MS, Koteiche HA. Specificity of alphaA-crystallin binding to destabilized mutants of betaB1-crystallin. FEBS Lett. 2007;581:1939–1943. doi: 10.1016/j.febslet.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiel M, Duprat E, Skouri-Panet F, Lampi JA, Tardieu A, Lampi KJ, Finet S. Aggregation of deamidated human betaB2-crystallin and incomplete rescue by alpha-crystallin chaperone. Exp Eye Res. 2010;90:688–698. doi: 10.1016/j.exer.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesbauer LR, Zhou X, Yang Z, Yang Z, Sun Y, Smith DL, Smith JB. Post-translational modifications of water-soluble human lens crystallins from young adults. J Biol Chem. 1994;269:12494–12502. [PubMed] [Google Scholar]
- Moreau KL, King JA. Cataract-causing defect of a mutant gamma-crystallin proceeds through an aggregation pathway which bypasses recognition by the alpha-crystallin chaperone. PLoS One. 2012;7:e37256. doi: 10.1371/journal.pone.0037256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalini V, Bax B, Driessen H, Moss DS, Lindley PF, Slingsby C. Close packing of an oligomeric eye lens beta-crystallin induces loss of symmetry and ordering of sequence extensions. J Mol Biol. 1994;236:1250–1258. doi: 10.1016/0022-2836(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Palme S, Slingsby C, Jaenicke R. Mutational analysis of hydrophobic domain interactions in gamma B-crystallin from bovine eye lens. Protein Sci. 1997;6:1529–1536. doi: 10.1002/pro.5560060717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringens PJ, Hoenders HJ, Bloemendal H. Protein distribution and characterization in the prenatal and postnatal human lens. Exp Eye Res. 1982;34:815–823. doi: 10.1016/s0014-4835(82)80041-9. [DOI] [PubMed] [Google Scholar]
- Robinson NE, Lampi KJ, McIver RT, Williams RH, Muster WC, Kruppa G, Robinson AB. Quantitative measurement of deamidation in lens betaB2-crystallin and peptides by direct electrospray injection and fragmentation in a Fourier transform mass spectrometer. Mol Vis. 2005;11:1211–1219. [PMC free article] [PubMed] [Google Scholar]
- Robinson NE, Lampi KJ, Speir JP, Kruppa G, Easterling M, Robinson AB. Quantitative measurement of young human eye lens crystallins by direct injection Fourier transform ion cyclotron resonance mass spectrometry. Mol Vis. 2006a;12:704–711. [PubMed] [Google Scholar]
- Robinson NE, Robinson AB. Molecular Clocks: Deamidation of Asparaginyl and Glutaminyl Residues in Peptides and Proteins. Althouse Press; 2004a. [Google Scholar]
- Robinson NE, Robinson AB. Prediction of primary structure deamidation rates of asparaginyl and glutaminyl peptides through steric and catalytic effects. J Pept Res. 2004b;63:437–448. doi: 10.1111/j.1399-3011.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- Robinson NE, Zabrouskov V, Zhang J, Lampi KJ, Robinson AB. Measurement of deamidation of intact proteins by isotopic envelope and mass defect with ion cyclotron resonance Fourier transform mass spectrometry. Rapid Commun Mass Spectrom. 2006b;20:3535–3541. doi: 10.1002/rcm.2767. [DOI] [PubMed] [Google Scholar]
- Sathish HA, Koteiche HA, McHaourab HS. Binding of destabilized betaB2-crystallin mutants to alpha-crystallin: the role of a folding intermediate. J Biol Chem. 2004;279:16425–16432. doi: 10.1074/jbc.M313402200. [DOI] [PubMed] [Google Scholar]
- Sergeev YV, Wingfield PT, Hejtmancik JF. Monomer-dimer equilibrium of normal and modified beta A3-crystallins: experimental determination and molecular modeling. Biochemistry. 2000;39:15799–15806. doi: 10.1021/bi001882h. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Santhoshkumar P. Lens aging: effects of crystallins. Biochim Biophys Acta. 2009;1790:1095–1108. doi: 10.1016/j.bbagen.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih M, Lampi KJ, Shearer TR, David LL. Cleavage of beta crystallins during maturation of bovine lens. Mol Vis. 1998;4:4. [PubMed] [Google Scholar]
- Simpanya MF, Wistow G, Gao J, David LL, Giblin FJ, Mitton KP. Expressed sequence tag analysis of guinea pig (Cavia porcellus) eye tissues for NEIBank. Mol Vis. 2008;14:2413–2427. [PMC free article] [PubMed] [Google Scholar]
- Slingsby C, Bateman OA. Quaternary interactions in eye lens beta-crystallins: basic and acidic subunits of beta-crystallins favor heterologous association. Biochemistry. 1990;29:6592–6599. doi: 10.1021/bi00480a007. [DOI] [PubMed] [Google Scholar]
- Slingsby C, Wistow GJ, Clark AR. Evolution of crystallins for a role in the vertebrate eye lens. Protein Sci. 2013;22:367–380. doi: 10.1002/pro.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM, Gafken PR, Zhang Z, Gottschling DE, Smith JB, Smith DL. Mass spectrometric quantification of acetylation at specific lysines within the amino-terminal tail of histone H4. Anal Biochem. 2003;316:23–33. doi: 10.1016/s0003-2697(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Srivastava OP, Srivastava K. Existence of deamidated alphaB-crystallin fragments in normal and cataractous human lenses. Mol Vis. 2003;9:110–118. [PubMed] [Google Scholar]
- Takata T, Oxford JT, Brandon TR, Lampi KJ. Deamidation alters the structure and decreases the stability of human lens betaA3-crystallin. Biochemistry. 2007;46:8861–8871. doi: 10.1021/bi700487q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata T, Oxford JT, Demeler B, Lampi KJ. Deamidation destabilizes and triggers aggregation of a lens protein, betaA3-crystallin. Protein Sci. 2008;17:1565–1575. doi: 10.1110/ps.035410.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata T, Smith JP, Arbogast B, David LL, Lampi KJ. Solvent accessibility of betaB2-crystallin and local structural changes due to deamidation at the dimer interface. Exp Eye Res. 2010;91:336–346. doi: 10.1016/j.exer.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata T, Woodbury LG, Lampi KJ. Deamidation alters interactions of beta-crystallins in hetero-oligomers. Mol Vis. 2009;15:241–249. [PMC free article] [PubMed] [Google Scholar]
- Takemoto L. Increased deamidation of asparagine-101 from alpha-A crystallin in the high molecular weight aggregate of the normal human lens. Exp Eye Res. 1999;68:641–645. doi: 10.1006/exer.1999.0668. [DOI] [PubMed] [Google Scholar]
- Takemoto L, Boyle D. Deamidation of alpha-A crystallin from nuclei of cataractous and normal human lenses. Mol Vis. 1999;5:2. [PubMed] [Google Scholar]
- Takemoto L, Boyle D. Increased deamidation of asparagine during human senile cataractogenesis. Mol Vis. 2000;6:164–168. [PubMed] [Google Scholar]
- Takemoto L, Emmons T, Granstrom D. The sequences of two peptides from cataract lenses suggest they arise by deamidation. Curr Eye Res. 1990;9:793–797. doi: 10.3109/02713689008999575. [DOI] [PubMed] [Google Scholar]
- Takemoto L, Fujii N, Boyle D. Mechanism of asparagine deamidation during human senile cataractogenesis. Exp Eye Res. 2001;72:559–563. doi: 10.1006/exer.2001.0983. [DOI] [PubMed] [Google Scholar]
- Takemoto LJ. Quantitation of asparagine-101 deamidation from alpha-A crystallin during aging of the human lens. Curr Eye Res. 1998a;17:247–250. doi: 10.1076/ceyr.17.3.247.5218. [DOI] [PubMed] [Google Scholar]
- Takemoto LJ. Quantitation of specific cleavage sites at the C-terminal region of alpha-A crystallin from human lenses of different age. Exp Eye Res. 1998b;66:263–266. doi: 10.1006/exer.1997.0411. [DOI] [PubMed] [Google Scholar]
- Trinkl S, Glockshuber R, Jaenicke R. Dimerization of beta B2-crystallin: the role of the linker peptide and the N- and C-terminal extensions. Protein Sci. 1994;3:1392–1400. doi: 10.1002/pro.5560030905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Duncan MK, David LL. Lens proteomics: the accumulation of crystallin modifications in the mouse lens with age. Invest Ophthalmol Vis Sci. 2002;43:205–215. [PubMed] [Google Scholar]
- Van Kleef FS, De Jong WW, Hoenders HJ. Stepwise degradations and deamidation of the eye lens protein alpha-crystallin in ageing. Nature. 1975;258:264–266. doi: 10.1038/258264a0. [DOI] [PubMed] [Google Scholar]
- Van Montfort RL, Bateman OA, Lubsen NH, Slingsby C. Crystal structure of truncated human betaB1-crystallin. Protein Sci. 2003;12:2606–2612. doi: 10.1110/ps.03265903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorter CE, de Haard-Hoekman WA, van den Oetelaar PJ, Bloemendal H, de Jong WW. Spontaneous peptide bond cleavage in aging alpha-crystallin through a succinimide intermediate. J Biol Chem. 1988;263:19020–19023. [PubMed] [Google Scholar]
- Voorter CE, Roersma ES, Bloemendal H, de Jong WW. Age-dependent deamidation of chicken alpha A-crystallin. FEBS Lett. 1987;221:249–252. doi: 10.1016/0014-5793(87)80935-3. [DOI] [PubMed] [Google Scholar]
- Wang Z, Obidike JE, Schey KL. Posttranslational modifications of the bovine lens beaded filament proteins filensin and CP49. Invest Ophthalmol Vis Sci. 2010;51:1565–1574. doi: 10.1167/iovs.09-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werten PJ, Carver JA, Jaenicke R, de Jong WW. The elusive role of the N-terminal extension of beta A3- and beta A1-crystallin. Protein Eng. 1996;9:1021–1028. doi: 10.1093/protein/9.11.1021. [DOI] [PubMed] [Google Scholar]
- Werten PJ, Stege GJ, de Jong WW. The short 5′ untranslated region of the betaA3/A1-crystallin mRNA is responsible for leaky ribosomal scanning. Mol Biol Rep. 1999;26:201–205. doi: 10.1023/a:1007046926233. [DOI] [PubMed] [Google Scholar]
- Wieligmann K, Mayr EM, Jaenicke R. Folding and self-assembly of the domains of betaB2-crystallin from rat eye lens. J Mol Biol. 1999;286:989–994. doi: 10.1006/jmbi.1999.2554. [DOI] [PubMed] [Google Scholar]
- Wilmarth P, David L, Lampi K. Normal age-related changes: crystallin modifications, lens hardening. In: Dartt DA, Besharse JC, Dana R, editors. Encyclopedia of the Eye. Academic Press; Italy: 2010. p. 161. [Google Scholar]
- Wilmarth PA, Riviere MA, David LL. Techniques for accurate protein identification in shotgun proteomic studies of human, mouse, bovine, and chicken lenses. J Ocul Biol Dis Inf. 2009;2:223–234. doi: 10.1007/s12177-009-9042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA, David LL. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteom Res. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright HT. Sequence and structure determinants of the nonenzymatic deamidation of asparagine and glutamine residues in proteins. Protein Eng. 1991;4:283–294. doi: 10.1093/protein/4.3.283. [DOI] [PubMed] [Google Scholar]
- Xie H, Becker JM, Gibbs RA, Naider F. Structure, biological activity and membrane partitioning of analogs of the isoprenylated a-factor mating peptide of Saccharomyces cerevisiae. J Pept Res. 2000;55:372–383. doi: 10.1034/j.1399-3011.2000.00705.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Zubarev RA. Mass spectrometric analysis of asparagine deamidation and aspartate isomerization in polypeptides. Electrophoresis. 2010;31:1764–1772. doi: 10.1002/elps.201000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Smith DL, Smith JB. Human beta-crystallins modified by backbone cleavage, deamidation and oxidation are prone to associate. Exp Eye Res. 2003;77:259–272. doi: 10.1016/s0014-4835(03)00159-3. [DOI] [PubMed] [Google Scholar]


