Abstract
We herein describe a novel procedure for dentin regeneration that mimics the biological processes of tooth development in nature. The canonical Wnt signaling pathway is an important regulator of the Dentin sialophosphoprotein (Dspp) expression. Our approach mimics the biological processes underlying tooth development in nature and focuses on the activation of canonical Wnt signaling to trigger the natural process of dentinogenesis. The coronal portion of the dentin and the underlying pulp was removed from the first molars. We applied lithium chloride (LiCl), an activator of canonical Wnt signaling, on the amputated pulp surface to achieve transdifferentiation toward odontoblasts from the surrounding pulpal cells. MicroCT and microscopic analyses demonstrated that the topical application of LiCl induced dentin repair, including the formation of a complete dentin bridge. LiCl-induced dentin is a tubular dentin in which the pulp cells are not embedded within the matrix, as in primary dentin. In contrast, a dentin bridge was not induced in the control group treated with pulp capping with material carriers alone, although osteodentin without tubular formation was induced at a comparatively deeper position from the pulp exposure site. We also evaluated the influence of LiCl on differentiation toward odontoblasts in vitro. In the mDP odontoblast cell line, LiCl activated the mRNA expression of Dspp, Axin2 and Kallikrein 4 (Klk4) and downregulated the Osteopontin (Osp) expression. These results provide a scientific basis for the biomimetic regeneration of dentin using LiCl as a new capping material to activate dentine regeneration.
Introduction
The use of a stem/progenitor cell-based approach in regenerative medicine is well documented in various tissues and/or organs. However, an efficient tooth regeneration approach has not been achieved in the clinical setting or in in vivo studies. Dentin is the major component of the tooth and covers the dental pulp, which produces the inner structure of the dentin matrix. The dental pulp also contains multipotent precursor cell populations and has a regenerative capacity, and reparative dentin is observed beneath sites of decayed and/or fractured dentin in the pulp chamber [1]. When the tooth crown becomes damaged due to caries and/or fractures, the underlying pulp is partially exposed. Direct pulp capping is a current treatment choice for preserving pulp vitality and activating the self-healing capacity of dentin repair [2]. Pulp capping is the placement of a protective dressing over the exposed pulp to allow the endogenous formation of reparative dentin. In the clinical protocol, calcium hydroxide and mineral trioxide aggregate (MTA) is often used as a capping material, and various clinical and radiographic investigations support its use [3]. However, there remains controversy regarding the clinical efficacy of this material, and practical applications are limited [4,5], as, at least in part, these dressing materials do not have direct effects in inducing dentin regeneration. Indeed, if the tooth is further damaged, with critical pulp exposure, the current protocol cannot be used to heal defective conditions in the pulp via dentin self-regeneration [4].
Dentin is similar to bone in terms of its matrix protein composition and abundance of molecular cascades participating in odontoblast differentiation that are shared with osteoblast differentiation [6]. On the other hand, dentin does not undergo remodeling, and odontoblasts secrete dentin throughout their lifespan [6]. In addition, odontoblasts characteristically extend their cellular processes in well-aligned dentin tubules and exhibit cellular polarity [6]. Dentin sialophosphoprotein (DSPP) is specifically abundant in dentin and much less so in bone tissues [7–9]. In humans, mutations in DSPP result in defective dentin mineralization [10,11]. In mice, Dspp null mutant molars display a reduced dentin thickness and hypomineralization [12]. Odontoblasts in Dspp mutants also lack the cellular polarity and organization typically observed in wild-type odontoblasts, and aberrant odontoblasts can become trapped in mineralized tissue [12]. Such defective phenotypes are evident only in dentin, not in bone. These findings clearly indicate that Dspp provides dentin-specific features in odontoblasts.
Regarding the molecular regulatory mechanisms associated with the Dspp expression, the odontoblast layer demonstrates a high TOPGAL and Axin2 reporter activity, suggesting that the canonical Wnt pathway is involved in both dentinogenesis and in dentin regeneration [13–16]. In addition, we previously found that the Dspp expression is activated by WNT10a in vitro [17]. We also demonstrated that the double null mutation of Sulf1 and Sulf2 genes, both of which encode endosulfatase and modify the affinity of the cell surface HSPGs for Wnt ligands, exhibited a defective dentin matrix formation with downregulated Wnt canonical signaling pathway [18]. In humans, mutations in Axin2 and Wnt10a cause tooth agenesis and/or hypodontia [19–21]. Recent studies, including ours, have provided direct evidence that Wnt canonical signaling is involved in the promotion of Dspp mRNA expression. The canonical Wnt signaling pathway can be activated by LiCl, an inhibitor of glycogen synthase kinase-3 [22]. Mimicking Wnt signaling with LiCl treatment resulted in the upregulation of Dspp expression in odontoblast-cell lines, thus indicating the functional importance of Wnt signaling in dentinogenesis in vitro [18,23]. However, the in vivo effect of LiCl still remains to be elucidated in dentinogenesis.
Dental pulp cells can differentiate into both osteogenic and odontogenic cells [24]. Calcified tissue is formed in response to pulp capping, termed osteodentin [25]. Although osteodentin originates from the pulp, this tissue has the characteristics of bone, rather than dentin, in terms of its overall morphology. Typically, osteodentin lacks a tubular structure, and the forming cells are not trapped within the matrix. Furthermore, osteodentin often contains tunnel defects that fail to seal areas of underlying pulp exposure [5,26]. Previous efforts have emphasized the efficient formation of mineralized tissue, rather than the induction of real dentin.
In this study, we describe a novel pulp capping material that mimics the biological processes of tooth development in nature. Dental pulp itself contains stem/progenitor cell compartments [27], and our approach was to utilize bioactive that mediate the organ-specific transdifferentiation of pulp cells by activating the canonical Wnt pathway. In the present study, we topically applied LiCl, an activator of canonical Wnt signaling [22], as a novel capping material in order to activate reparative dentin formation.
Materials and Methods
Animals
Forty-five male Sprague Dawley rats that were all 5 weeks of age were randomly divided into an experimental group (n = 30) and a sham-treated control group (n = 15). The animal experiments were performed under the research protocol approved by the Animal Research Committee at Okayama University (OKU-2013284). To minimize animal suffering, the number of animals used was based on the minimum required to obtain statistically valid results.
Dentin and pulp removal, and the capping procedure
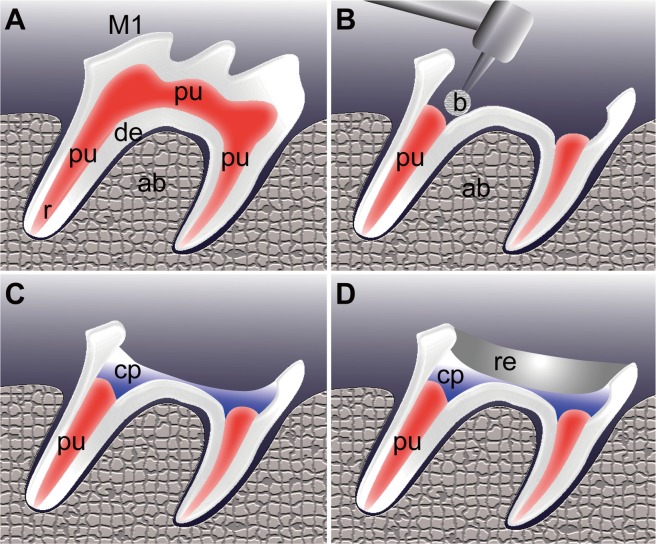
All rats were acclimatized for 1 week before beginning the experiments. At 6 weeks of age, the dentin removal and pulpotomy were performed unilaterally in the maxillary first upper molars (Fig. 1A). The coronal portion of the dentin and the underlying pulp was removed through the occlusal surface using a #1/4 round bur with a low-speed handpiece and sterile saline (Fig. 1B). Bleeding was controlled with light pressure using sterile cotton pellets and paper points before placing the capping materials. In the experimental group of 29 animals, LiCl (Sigma-Aldrich, Poole, UK) was topically applied on the exposed pulp surface (Fig. 1C). LiCl was dissolved in material carriers (macrogol and propylene glycol, 1:1), with a final concentration of LiCl of 10 mM [28]. A mixture of macrogol and propylene glycol is widely applied as a carrier to dissolve mixed antibiotics both in clinical settings and in vivo [29,30], because this mixture is viscous, but it has a low surface tension which thus allows it to demonstrate a better diffusion of the medicament [31]. The pulp in the remaining 11 animals was capped with a similar volume of material carrier and employed as a control. Thereafter, the cavity was coated with a bonding agent (Clearfil Mega Bond, Kuraray, Japan) and light cured for 20 seconds. All the cavity was further sealed with light-cured composite resin (UniFil LoFlo, GC, Japan) (Fig. 1D).
Fig 1. Pulpotomy procedure.
The coronal portion of the pulp was removed in the first molars of the 6-week-old Wistar rats. (A) Before pulpotomy. (B) Pulpotomy was performed using a small round bur (b). (C) The wound surface of the sectioned pulps was treated with a LiCl-containing pulp capping agent (cp). A non-LiCl agent was used as a negative control. (D) The cavities were subsequently filled with composite resin (re). Abbreviations: M1, first molar; de, dentin; pu, pulp; ab, alveolar bone; b, bur; cp, capping material; re, resin.
The experiments were performed under sodium pentobarbital anesthesia (40 mg/kg, ip), with supplemental doses given to maintain animal anesthesia throughout the experiment. The rats were maintained under a controlled temperature (22±2°C), light dark periods of 12 hours with free access to chow and water ad libitum. After dental treatment, any abnormal animal behaviors such as grooming, locomotion and feeding were not observed.
Microcomputed tomography (Micro-CT)
The animals were killed four weeks after pulp capping by cervical dislocation. Three-dimensional images of the tooth structure were analyzed using an inspeXio SMX-90CT Microfocus X-ray CT system (Shimadzu, Kyoto, Japan). Briefly, image acquisition was performed at 90 kV and 110 mA. Image reconstruction was carried out using appropriate cross-sections at a spatial resolution of 17 μm, and the resultant images were processed using the three-dimensional reconstruction software program VG Studio MAX 2.0 (Volume Graphics, Heidelberg, Germany).
Tissue preparation and histology
The tooth and surrounding bone were fixed in 4% paraformaldehyde or tissue fixative at 4°C overnight. The tissues were decalcified in 12.5% EDTA for three weeks, after which they were dehydrated, embedded in paraffin and serially sectioned at 7 μm for hematoxylin and eosin staining. Frozen cross-sections (10 μm) were also prepared for in situ hybridization.
In situ hybridization
In situ hybridization using digoxigenin-labeled RNA probes was performed as previously described [18]. The preparation of Dspp RNA probes has been also described previously [17].
Cell culture and real-time RT-PCR analysis
The mDP odontoblast-like cell line derived from the embryonic mouse mesenchyme [32] was used. The mDP cells were plated in 6-well plates in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) until they reached confluence. The mDP cells were then cultured with or without 10 mm of LiCl for seven days before being processed for the real-time RT-PCR analysis [28].
Total RNA was extracted from the mDP cells using Isogen (Nippon Gene, Tokyo, Japan), according to the manufacturer's protocol. Total RNA was isolated and reverse-transcribed to cDNA using oligo (dT) primer (Takara, Shiga, Japan). For the real-time RT-PCR analysis, cDNA was amplified with Blend-Taq Plus (Toyobo, Osaka, Japan) using a regular thermal cycler. Quantitative real-time PCR was performed in triplicate employing three independent sets of samples. The relative quantity of transcripts was determined using a standard curve and normalized in comparison with the expression of glyceraldehyde-3-phosphate dehydrogenase gene (Gapdh) mRNA. The sets of synthetic primers used for the amplification were as follows: Gapdh, 5´-GTCCCGTAGACAAAATGGTG-3´ (sense) and 5´-CAATGAAGGGGTCGTTGATG-3´ (antisense); mouse Dspp, 5´-AGCCGTGGAGATGCTTCTTA-3´ (sense) and 5´-TCACTCTCGCTGTCACCATC-3´ (antisense); mouse kallikrein 4 (Klk4), 5´-TTGCAAACGATCTCATGCTC-3´ (sense) and 5´-TGAGGTGGTACACAGGGTCA-3´ (antisense); mouse Axin2, 5´-CCTTGCCAAAACGGAATG-3´ (sense) and 5´-TTTCGTGGCTGTTGCGTA-3´ (antisense); mouse alkaline phosphatase (Alp), 5´-GCTGATCAT TCCCACGTTTT-3´ (sense) and 5´-CTGGGCCTGGTAGTTGTTGT-3´ (antisense); mouse osteopontin (Opn), 5´-CTGGCTGAATTCTGAGGGACT-3´ (sense) and 5´-CTTCTGAGATGGGTCAGGCA-3´ (antisense).
Each PCR cycle was carried out as previously described [18,24]. Each amplification reaction was performed and checked to ensure the absence of nonspecific PCR products according to a melting curve analysis using the LightCyclerTM system (Roche Applied Science, Mannheim, Germany). The relative cDNA copy numbers were computed using data obtained from serial dilutions of representative samples for each target gene.
Comparison of quantitative variables in two groups was performed by the Mann-Whitney U test. P values less than 0.05 were considered significant. Significance values were calculated using statistical analysis software (JMP version 5, SAS Institute Inc., Japan).
Results
Pulp capping experiments
At four weeks after pulpotomy, two mice were died and excluded from the analysis. Ten teeth in the LiCl group and six teeth in the control group were excluded from the study as the resin sealing dropped off, leaving 19 and eight teeth in total for the LiCl and control groups, respectively.
Micro-CT analysis
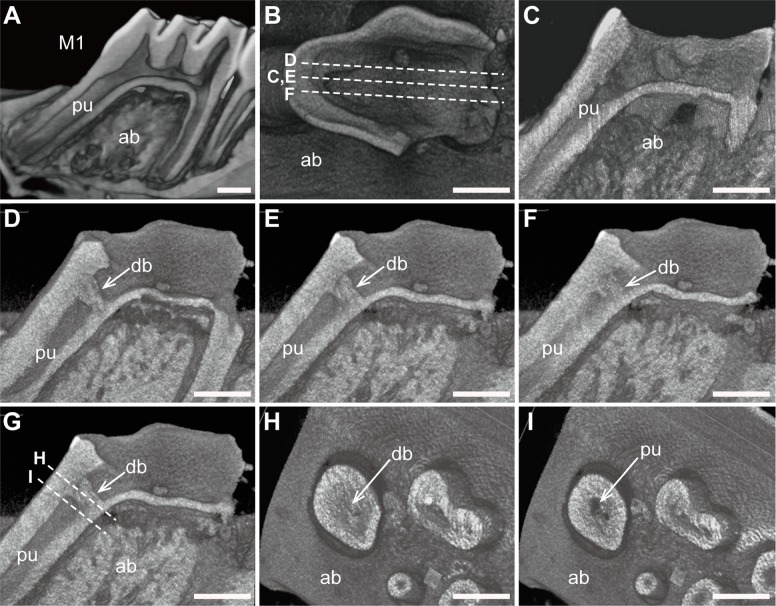
Image reconstruction was conducted to analyze whether dentin bridges were formed over the exposed pulp at four weeks after pulp capping. Following image reconstruction (Fig. 2A), two-dimensional virtual slices were acquired in the sagittal (Fig. 2B-G) and coronal (Fig. 2H,I) planes.
Fig 2. Reconstructed micro-CT images and two-dimensional virtual slices.
(A) Three-dimensional reconstructed image of the intact lower first molars in a 10-week-old rat. (B) Virtual sagittal slices were obtained in the control (C) and experimental groups (D-F). (C) No dentin bridges were observed in any of the control mice. (D-F) Series of reconstructed images showing that LiCl treatment induced dentin bridge formation over the exposed pulp surface. (G) Virtual coronal slices were obtained in the experimental group. (H,I) Reconstructed coronal images also illustrated deposition of reparative tissues (H). Abbreviations: M1, first molars; ab, alveolar bone; pu, pulp; db, dentin bridge. Scale bars, 1 mm.
The control group did not show any dentin bridges on the reconstructed sagittal micro-CT images (Fig. 2B). In contrast, 11 of the 19 samples in the LiCl group exhibited complete dentin bridges over the exposed pulp surface (Fig. 2D). Serial reconstructed sagittal images revealed that the dentin bridges were uniform in radiopacity (Fig. 2D-F). Serial sections (Fig. 2G,H) obtained perpendicular to the root axis also demonstrated that the dentin bridges occupied the entire cross-sections of the root chamber (Fig. 2H).
Histological analysis
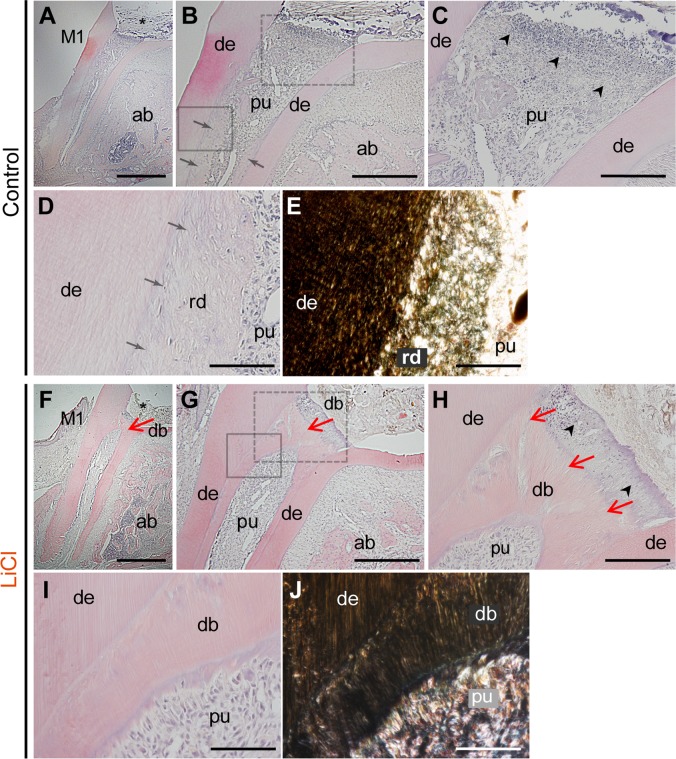
Reparative dentin formation was also evaluated histologically in serial sagittal sections obtained four weeks after pulp capping. None of the control group molars capped with material carriers alone showed reparative dentin beneath the region of pulp exposure (Fig. 3A-C). Typically, necrotic and/or disintegrated pulp tissue was observed beneath the area of pulp exposure, with a few particles of the rounded matrix (Fig. 3B,C). Reparative hard tissue was noted along the inner surface of the root with an irregular surface (arrows in Fig. 3B,D). A tubular structure was hardly detected in the reparative tissues, as observed on bright and dark field micrographs (Fig. 3B,D), and some cells were entrapped in the regenerated matrix (Fig. 3D).
Fig 3. Histological micrographs (H-E) of sagittal sections of the first upper molars.
Reparative dentin formation was evaluated on the serial sections in the control (A-E) and LiCl (F-J) groups at four weeks after pulpotomy. (A,F) Pulpotomy was performed in the first molars (M1), and the coronal part of the pulp was removed (asterisk). (B) In the control molars, no reparative dentin bridges were observed beneath the pulp exposure site. However, hard reparative tissue was detected along the residual root dentin surface (arrows). (C) Higher magnification view of the dot box in panel B. Necrotic and disintegrated tissue was observed just beneath the pulp exposure site (arrow heads). (D,E) Bright (D) and dark (E) field images at higher magnification of the solid box in panel B. Dentin tubules were observed in the residual dentin (de), whereas a tubular structure was hardly detected in the deposited reparative tissue (rd). (F-G) The LiCl group exhibited dentin bridges beneath the exposed surface, and the reparative tissue further expanded in the direction of the apex (red arrows). (H) Higher magnification view of the dot box in panel G. A tubular structure was evident in the dentin bridges (red arrows), with only a few cells entrapped in the matrix. (I,J) Bright (I) and dark (J) field images at higher magnification of the solid box in panel G. The reparative matrix in the LiCl group was more condensed, with a well-developed tubular structure, than that observed in the control group. The figure represented the similar results from independent samples. Abbreviations: M1, the first upper molar; mr, mesial root; ab, alveolar bone; de, dentin; pu, pulp; rd, regenerative dentin, db, dentin bridge. Scale bars, 100 μm in A,F; 50 μm in B,G; 20 μm in C,H; 10 μm in D,E,I,J.
In contrast, the LiCl group demonstrated dentin bridges at the exposed surface, and the reparative tissue further expanded along the root surface (red arrows in Fig. 3F,G). At high magnification, a tubular structure was evident in the dentin bridges, with only a few cells entrapped in the matrix (Fig. 3G,H). Furthermore, the reparative matrix in the LiCl group was more condensed, with a well-developed tubular structure, than that seen in the control group, as revealed on bright and dark field micrographs (Fig. 3I,J). These findings indicate that the LiCl-induced reparative hard tissues possessed characteristics of the dentin rather than bone.
Dspp mRNA expression in vivo
We evaluated the mRNA expression of Dspp during regenerative matrix formation in vivo. In the LiCl group, the Dspp mRNA expression was confirmed in the pulp cells located beneath the dentin bridges (Fig. 4A,B).
Fig 4. In situ hybridization for Dspp mRNA in the pulp in the LiCl group.
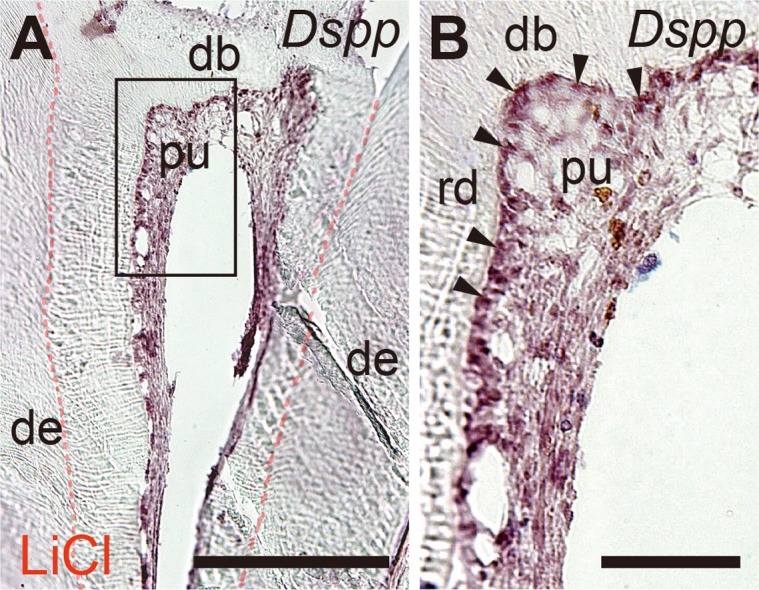
(A) The Dspp mRNA expression was confirmed in the pulp cells beneath the dentin bridges. (B) Higher magnification image of the solid box in panel A showing the Dspp mRNA expression in the pulp cells. Abbreviations: de, dentin; pu, pulp; rd, regenerative dentin, db, dentin bridge. Arrowheads indicate Dspp transcripts. Scale bars, 50 μm in A; 10 μm in B.
In vitro evaluation
We then attempted to investigate the mechanisms underlying regenerative dentin formation in vitro. mDP were harvested until they reached confluence. The cells were then cultured with or without 10 mM of LiCl for seven days before being processed for the real-time RT-PCR analysis. Figure demonstrated microscopic view of the mDP cells before and after LiCl treatment and LiCl treatment did not affect the appearance of mDP cells (Fig. 5A,B).
Fig 5. Effects of LiCl on the expression of osteogenic and odontogenic markers.
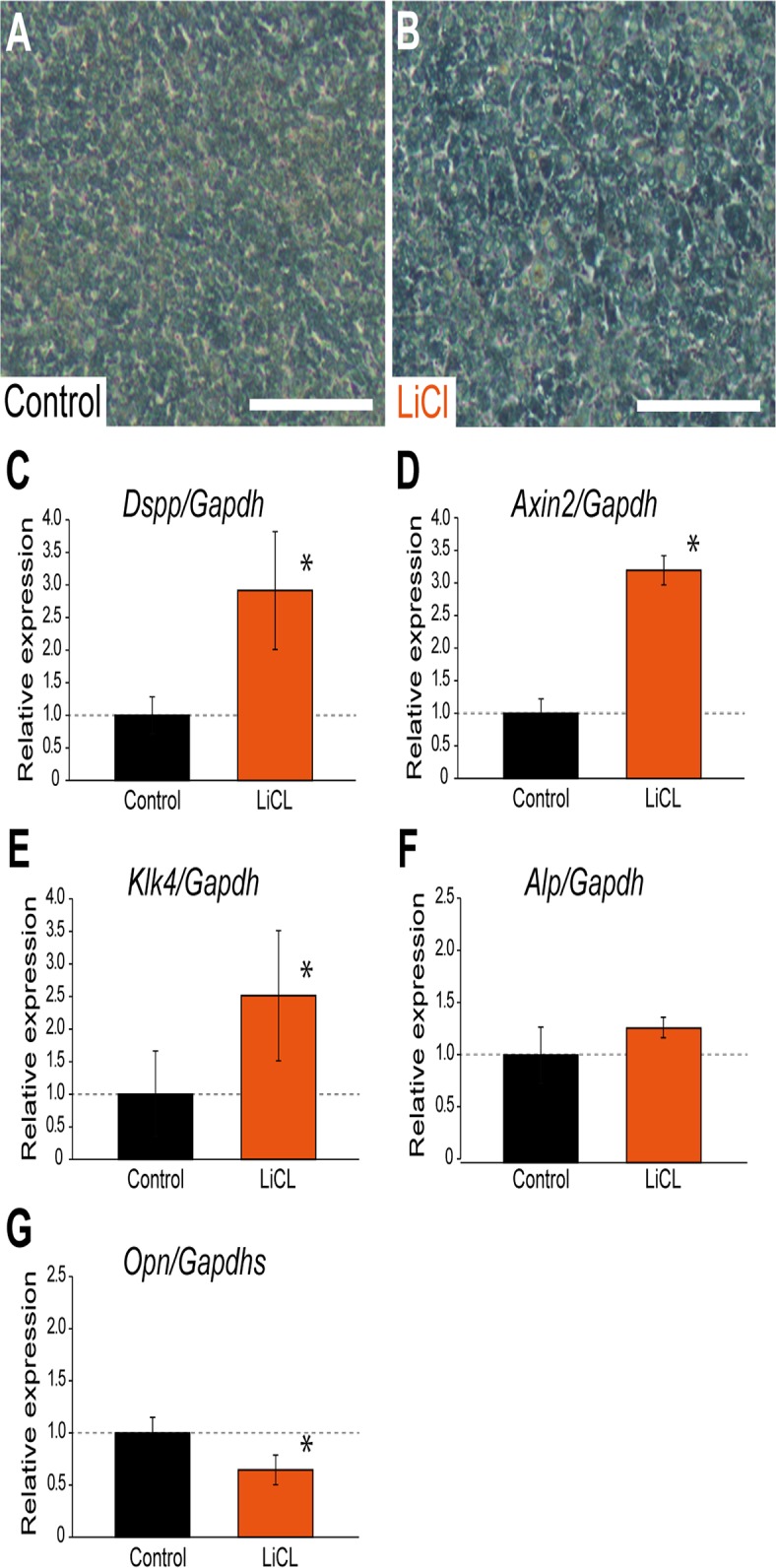
(A and B) mDP were harvested until they reached confluence. The cells were then cultured with (A) or without (B) 10 mM of LiCl for seven days before being processed for the real-time RT-PCR analysis. (C-G) Effects of LiCl on the expression of osteogenic and odontogenic markers on quantitative RT-PCR. Values are the average ± SD of triplicate wells, and are representative of three independent experiments performed. (C-E) LiCl treatment significantly upregulated the expression of the odontogenic markers Dspp (*, p<0.05; C) and Klk4 (*, p<0.05; E) and the Wnt canonical marker Axin2 (*, p<0.05; D). (F) The Alp expression was not affected. (G) In contrast, LiCl treatment significantly downregulated the expression of the osteogenic markers Opn (*, p<0.05). Scale bars, 100 μm in A and B.
Real-time PCR demonstrated that LiCl significantly upregulated the Dspp and Axin2 mRNA expression at a dose of 10 mM (p<0.05; Fig. 5C,D), as shown in previous study [18]. LiCl application also activated the Klk4 mRNA expression significantly (p<0.05; Fig. 5E). Klk4 is a serine proteinase characteristically expressed by odontoblasts and ameloblasts in a stage-specific manner [33,34]. In contrast, LiCl did not change the Alp mRNA expression levels (Fig. 5F) and LiCl significantly downregulated the mRNA expression of the osteogenic marker Opn (p<0.05; Fig. 5G).
Discussion
This study employed a biomimicry approach using the topical application of LiCl on the amputated pulp surface to achieve transdifferentiation toward odontoblasts from surrounding progenitor cells and subsequent dentin regeneration. This work highlights the potential of this novel pulp capping approach, which mimics and activates the molecular mechanisms of dentine regeneration.
Our approach mimics the biological processes underlying tooth development in nature and focuses on the activation of canonical Wnt signaling to trigger the natural process of dentinogenesis. It has been established that Dspp is a key molecule for dentinogenesis [11,12], and the Dspp expression is regulated, at least in part, via the Wnt canonical pathway [17,18] in vitro. The importance of Wnt signaling is also supported by genetic studies of human agenesis and/or hypodontia caused by mutations in Axin2 and Wnt10a [19,20]. The present capping procedure using LiCl treatment efficiently induced the formation of dentin bridges just beneath the treated regions. Furthermore, the regenerated tissues exhibited dentin characteristics with well-developed tubular formation. These results provide in vivo evidence that LiCl may be used a new tool for efficiently achieving dentin regeneration as a novel treatment approach.
The disadvantages of the previous capping procedure is that the technique resulted in the regeneration of hard tissue, not dentin-like tissue, although osteodentin has a bone-like structure that is more fragile than that of dentin [35]. Such findings are supported by a recent in vitro study that clearly showed that traumatic pulp exposure activates the osteogenic potential, while downregulating the odontogenic capacity, in vitro [28]. In a previous study, BMPs, such as BMP2, BMP7 and GDF11, were evaluated as possible bioinductive materials activating the self-healing response in the pulp [36]. Such application typically induces the formation of mineralized tissue on the exposed pulp surface; however, the induced matrix is not like that of dentin, but rather that of a bone-like tissue called osteodentin. Since these BMPs activate the Runx2 expression, it is conceivable that the use of BMPs induces osteogenic commitment during the self-regeneration process and consequent osteodentin formation. Interestingly, the odontoblast-specific overexpression of Runx2 results in defective phenotypes of dentin formation [37], and the Runx2 mRNA expression is remarkably downregulated in tooth development, with differentiation to mature odontoblasts [38]. These findings suggest that Runx2 is involved in the cell fate determination of pulpal mesenchymal progenitors regarding whether to commit to the osteogenic lineage or odontogenic lineage and that the downregulation of Runx2 is important for odontogenic commitment.
A recent study demonstrated that the expression of Axin2, a marker of the Wnt canonical pathway, has counteracting effects on the Runx2 expression in the cranial sutures [39]. In the present study, although we found that activation of the Wnt pathway downregulated the osteogenic marker of Opn in the odontoblast cell line, the odontoblast line of mDP cells did not express Runx2 (Osf2). Further studies are therefore needed to elucidate the possible associations between the Runx2 expression and activation of the canonical Wnt signaling pathway in the process of odontoblast differentiation.
The use of a stem/progenitor cell-based approach in regenerative medicine is well documented [40]. The efficacy of autologous transplantation of stem cells has been investigated with respect to dentin regeneration [41]; however, the dental pulp itself contains stem/progenitor cells [27,42], which serve as available donor sources for stem/progenitor cell engraftment into host tissues. Hence, an endogenous cell homing approach, which stimulates self-healing mechanisms using cells already present in the tissues, has the potential to provide novel therapeutic options for dentin regeneration. Regarding efficient dentin regenerations, LiCl has great potential as a bioactive mediating organ-specific transdifferentiation.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by Grant-in-Aid for Scientific Research (KAKENHI) 19390529 and 24249093 from the Japan Society for the Promotion of Science (JSPS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28: 77–92. [DOI] [PubMed] [Google Scholar]
- 2. Huang GT. Dental pulp and dentin tissue engineering and regeneration: advancement and challenge. Front Biosci (Elite Ed) 2011;3: 788–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36: 400–413. 10.1016/j.joen.2009.09.009 [DOI] [PubMed] [Google Scholar]
- 4. Aguilar P, Linsuwanont P. Vital pulp therapy in vital permanent teeth with cariously exposed pulp: a systematic review. J Endod. 2011;37: 581–587. 10.1016/j.joen.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 5. Cox CF, Subay RK, Ostro E, Suzuki S, Suzuki SH. Tunnel defects in dentin bridges: their formation following direct pulp capping. Oper Dent. 1996;21: 4–11. [PubMed] [Google Scholar]
- 6. Linde A, Goldberg M. Dentinogenesis. Crit Rev Oral Biol Med. 1993;4: 679–728. [DOI] [PubMed] [Google Scholar]
- 7. D'Souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB, et al. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res. 1997;12: 2040–2049. [DOI] [PubMed] [Google Scholar]
- 8. Feng JQ, Luan X, Wallace J, Jing D, Ohshima T, Kulkarni AB, et al. Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. J Biol Chem. 1998;273: 9457–9464. [DOI] [PubMed] [Google Scholar]
- 9. Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, et al. (2002) The expression of dentin sialophosphoprotein gene in bone. J Dent Res 81: 392–394. [DOI] [PubMed] [Google Scholar]
- 10. Xiao S, Yu C, Chou X, Yuan W, Wang Y, Bu L, et al. Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nat Genet. 2001;27: 201–204. [DOI] [PubMed] [Google Scholar]
- 11. Zhang X, Zhao J, Li C, Gao S, Qiu C, Liu P, et al. DSPP mutation in dentinogenesis imperfecta Shields type II. Nat Genet. 2001;27: 151–152. [DOI] [PubMed] [Google Scholar]
- 12. Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, et al. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278: 24874–24880. [DOI] [PubMed] [Google Scholar]
- 13. Lim WH, Liu B, Cheng D, Hunter DJ, Zhong Z, Ramos DM, et al. Wnt signaling regulates pulp volume and dentin thickness. J Bone Miner Res. 2014;29: 892–901. 10.1002/jbmr.2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lohi M, Tucker AS, Sharpe PT. Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre- and postnatal tooth development. Dev Dyn. 2010;239: 160–167. 10.1002/dvdy.22047 [DOI] [PubMed] [Google Scholar]
- 15. Nakatomi M, Ida-Yonemochi H, Ohshima H. Lymphoid enhancer-binding factor 1 expression precedes dentin sialophosphoprotein expression during rat odontoblast differentiation and regeneration. J Endod. 2013;39: 612–618. 10.1016/j.joen.2012.12.016 [DOI] [PubMed] [Google Scholar]
- 16. Suomalainen M, Thesleff I. Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/beta-catenin signaling in the epithelial stem cells. Dev Dyn. 2010;239: 364–372. 10.1002/dvdy.22106 [DOI] [PubMed] [Google Scholar]
- 17. Yamashiro T, Zheng L, Shitaku Y, Saito M, Tsubakimoto T, Takada K, et al. Wnt10a regulates dentin sialophosphoprotein mRNA expression and possibly links odontoblast differentiation and tooth morphogenesis. Differentiation 2007;75: 452–462. [DOI] [PubMed] [Google Scholar]
- 18. Hayano S, Kurosaka H, Yanagita T, Kalus I, Milz F, Ishihara Y, et al. Roles of heparan sulfate sulfation in dentinogenesis. J Biol Chem. 2012;287: 12217–12229. 10.1074/jbc.M111.332924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adaimy L, Chouery E, Megarbane H, Mroueh S, Delague V, Nicolas E, et al. Mutation in WNT10A is associated with an autosomal recessive ectodermal dysplasia: the odonto-onycho-dermal dysplasia. Am J Hum Genet. 2007;81: 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lammi L, Arte S, Somer M, Jarvinen H, Lahermo P, Thesleff I, et al. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet. 2004;74: 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nawaz S, Klar J, Wajid M, Aslam M, Tariq M, Schuster J, et al. WNT10A missense mutation associated with a complete odonto-onycho-dermal dysplasia syndrome. Eur J Hum Genet. 2009;17: 1600–1605. 10.1038/ejhg.2009.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93: 8455–8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han N, Zheng Y, Li R, Li X, Zhou M, Niu Y, et al. Beta-catenin enhances odontoblastic differentiation of dental pulp cells through activation of Runx2. PLoS One 2014;9: e88890 10.1371/journal.pone.0088890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawanabe N, Murata S, Fukushima H, Ishihara Y, Yanagita T, Yanagita E, et al. Stage-specific embryonic antigen-4 identifies human dental pulp stem cells. Exp Cell Res. 2012;318: 453–463. 10.1016/j.yexcr.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 25. Tziafas D. The future role of a molecular approach to pulp-dentinal regeneration. Caries Res. 2004;38: 314–320. [DOI] [PubMed] [Google Scholar]
- 26. Goldberg M, Farges JC, Lacerda-Pinheiro S, Six N, Jegat N, Decup F, et al. Inflammatory and immunological aspects of dental pulp repair. Pharmacol Res. 2008;58: 137–147. 10.1016/j.phrs.2008.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97: 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Yan M, Wang Z, Wu J, Wang Z, Zheng Y, et al. Dental pulp stem cells from traumatically exposed pulps exhibited an enhanced osteogenic potential and weakened odontogenic capacity. Arch Oral Biol 2013;58: 1709–1717. 10.1016/j.archoralbio.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 29. Gomes-Filho JE, Duarte PC, de Oliveira CB, Watanabe S, Lodi CS, Cintra LT, et al. Tissue reaction to a triantibiotic paste used for endodontic tissue self-regeneration of nonvital immature permanent teeth. J Endod. 2012;38: 91–94. 10.1016/j.joen.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 30. Takushige T, Cruz EV, Asgor Moral A, Hoshino E. Endodontic treatment of primary teeth using a combination of antibacterial drugs. Int Endod J. 2004;37: 132–138. [DOI] [PubMed] [Google Scholar]
- 31. Cruz EV, Kota K, Huque J, Iwaku M, Hoshino E. Penetration of propylene glycol into dentine. Int Endod J. 2002;35: 330–336. [DOI] [PubMed] [Google Scholar]
- 32. Wu N, Iwamoto T, Sugawara Y, Futaki M, Yoshizaki K, Yamamoto S, et al. PDGFs regulate tooth germ proliferation and ameloblast differentiation. Arch Oral Biol. 2010;55: 426–434. 10.1016/j.archoralbio.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 33. Hu JC, Sun X, Zhang C, Liu S, Bartlett JD, Simmer JP. Enamelysin and kallikrein-4 mRNA expression in developing mouse molars. Eur J Oral Sci. 2002;110: 307–315. [DOI] [PubMed] [Google Scholar]
- 34. Nagano T, Oida S, Ando H, Gomi K, Arai T, Fukae M. Relative levels of mRNA encoding enamel proteins in enamel organ epithelia and odontoblasts. J Dent Res. 2003;82: 982–986. [DOI] [PubMed] [Google Scholar]
- 35. Hosoya A, H. N, Akahane S, Yoshiba K, Yoshiba N, Ninomiya T, et al. Immunohistochemical study of osteodentin in the unerupted rat incisor. J Oral Biosci 2006;48: 132–137. [Google Scholar]
- 36. Goldberg M, Six N, Decup F, Lasfargues JJ, Salih E, Tompkins K, et al. Bioactive molecules and the future of pulp therapy. Am J Dent. 2003;16: 66–76. [PubMed] [Google Scholar]
- 37. Li S, Kong H, Yao N, Yu Q, Wang P, Lin Y, et al. The role of runt-related transcription factor 2 (Runx2) in the late stage of odontoblast differentiation and dentin formation. Biochem Biophys Res Commun. 2011;410: 698–704. 10.1016/j.bbrc.2011.06.065 [DOI] [PubMed] [Google Scholar]
- 38. Yamashiro T, Aberg T, Levanon D, Groner Y, Thesleff I. Expression of Runx1, -2 and -3 during tooth, palate and craniofacial bone development. Mech Dev. 2002;119 Suppl 1: S107–110. [DOI] [PubMed] [Google Scholar]
- 39. McGee-Lawrence ME, Li X, Bledsoe KL, Wu H, Hawse JR, Subramaniam M, et al. Runx2 protein represses Axin2 expression in osteoblasts and is required for craniosynostosis in Axin2-deficient mice. J Biol Chem. 2013;288: 5291–5302. 10.1074/jbc.M112.414995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dong F, Caplan AI. Cell transplantation as an initiator of endogenous stem cell-based tissue repair. Curr Opin Organ Transplant. 2012;17: 670–674. 10.1097/MOT.0b013e328359a617 [DOI] [PubMed] [Google Scholar]
- 41. Ji YM, Jeon SH, Park JY, Chung JH, Choung YH, Choung PH. Dental stem cell therapy with calcium hydroxide in dental pulp capping. Tissue Eng Part A. 2010;16: 1823–1833. 10.1089/ten.TEA.2009.0054 [DOI] [PubMed] [Google Scholar]
- 42. Nakashima M, Iohara K. Regeneration of dental pulp by stem cells. Adv Dent Res. 2011;23: 313–319. 10.1177/0022034511405323 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.