Abstract
Purpose of review
Neutrophils rapidly migrate to sites of injury and infection. Egress of neutrophils from the circulation into tissues is a highly regulated process involving several distinct steps. Cell–cell interactions mediated by selectins and integrins and reorganization of the actin cytoskeleton are key mechanisms facilitating appropriate neutrophil recruitment. Neutrophil function is impaired in inherited and acquired disorders, such as leukocyte adhesion deficiency and myelodysplasia. Since the discovery that deletion of all or part of chromosome 5 is the most common genetic aberration in myelodysplasia, the roles of several of the deleted genes have been investigated in hematopoiesis. Several genes encoding proteins of the serum response factor (SRF) pathway are located on 5q. This review focuses, in particular, on the role of SRF in myeloid maturation and neutrophil function.
Recent findings
SRF and its pathway fulfill multiple complex roles in the regulation of the innate and adaptive immune system. Loss of SRF leads to defects in B-cell and T-cell development. SRF-deficient macrophages fail to spread, transmigrate, and phagocytose bacteria, and SRF-deficient neutrophils show defective chemotaxis in vitro and in vivo with failure of inside-out activation and trafficking of the Mac1 integrin complex. Loss of the formin mammalian Diaphanous 1, a regulator of linear actin polymerization and mediator of Ras homolog family member A signaling to SRF, results in aberrant myeloid differentiation and hyperactivity of the immune system.
Summary
SRF is an essential transcription factor in hematopoiesis and mature myeloid cell function. SRF regulates neutrophil migration, integrin activation, and trafficking. Disruption of the SRF pathway results in myelodysplasia and immune dysfunction.
Keywords: actin polymerization, innate immune function, integrin trafficking, migration, myelodysplasia, polarization
INTRODUCTION
Neutrophils are part of the innate immune system and migrate rapidly to sites of injury and inflammation and thus represent the first line of host defense. Inherited and acquired disorders of neutrophil function, such as leukocyte adhesion deficiency (LAD) and myelodysplasia (MDS), lead to significant morbidity and mortality secondary to bacterial and fungal infections [1,2]. Recruitment of neutrophils to sites of inflammation requires a complex chain of events. Neutrophil migration is initiated by release of chemoattractants at the site of injury or infection, which activate G protein-coupled receptors on neutrophils. Ligand binding induces GTPase-mediated downstream signaling with induction of cytoskeletal changes required for chemotaxis [3,4]. Neutrophils initially roll along the endothelial lining of the microvasculature, then slow to allow firm adhesion, followed by intraluminal crawling, and paracellular and transcellular migration (reviewed in [5]). Although both selectins and the integrin leukocyte function-associated antigen 1 (LFA-1) mediate initial rolling attachments, engagement of the integrin MAC-1 (CD11b/CD18) results in firm adhesion and mediates migration of neutrophils to the points of transendothelial migration ([6–10] and reviewed in [11,12]). The actin cytoskeleton is a key component of the migratory apparatus of the cell with formation of protrusions from the leading edge by the outward extensions of actin filaments, formation of focal adhesions, forward movement of the cell by actomyosin-based contractions, and release of adhesions at the rear of the cell (reviewed in [13,14]). Tight control of these processes is essential for appropriate inflammatory responses.
SERUM RESPONSE FACTOR IS ESSENTIAL IN HEMATOPOIETIC DEVELOPMENT AND CELLULAR FUNCTION
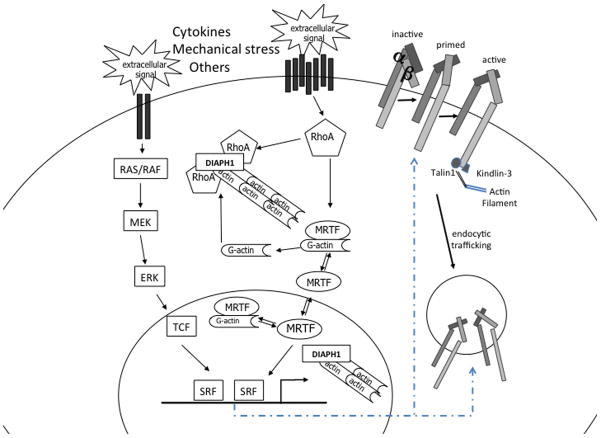
Serum response factor (SRF), a ubiquitously expressed member of the MADS box family of transcription factors, is regarded as the master regulator of the actin cytoskeleton [15]. It performs critical functions in multiple cellular processes, such as migration, cell contractility, cell growth, and cell differentiation in numerous tissues and cell types. SRF represents the convergence point for serum-induced Rho-actin and Ras-extracellular signal-regulated kinase (ERK) signals via recruitment of the myocardin-related transcription factors (MRTFs) and the ternary complex factors (TCFs) family of ETS-binding proteins, respectively (reviewed in [16] and [17]). MRTFs are bound by G-actin and thus respond to Rho GTPase signaling-induced fluctuations in cytoplasmic G-actin. G-actin is reduced upon actin polymerization, whereby MRTFs become localized to the nucleus upon release from G-actin (Fig. 1). Recently, a similar regulatory mechanism has been identified in the nucleus, whereby mammalian Diaphanous (mDIA)-induced nuclear actin polymerization results in nuclear MRTF accumulation and activation of SRF-dependent transcription [18▪▪]. TCF-mediated regulation of SRF transcriptional activity is controlled by Ras-ERK signaling [19,20]. MRTF and TCF binding to SRF is mutually exclusive, thus binding competition may represent an additional layer of regulation. In addition to regulation by gene-specific recruitment of MRTF or TCF cofactors, cell and context-specific SRF-mediated transcriptional regulation is achieved by SRF binding to gene enhancers facilitated by lineage-specific transcription factors. Genome-wide location analysis of SRF in macrophages reveals enrichment of SRF at promoters of ubiquitously expressed genes, whereas binding of SRF at intergenic and intragenic enhancers of cell type-restricted target genes is accompanied by the lineage-specific ETS transcription factor PU.1 [21].
FIGURE 1.
Serum response factor pathway. Extracellular signals activate the MAPK/RAS/ERK or the RhoA signaling pathways. TCFs or MRTFs are recruited to SRF and modulate SRF-mediated transcriptional activity. SRF regulates β2 integrin inside-out activation and trafficking. DIAPH1, Diaphanous 1; ERK, extracellular signal-regulated kinase; MKL1, megakaryoblastic leukemia 1; MRTF, myocardin-related transcription factor; RAS/RAF, rat sarcoma virus oncogene; RhoA, Ras homolog family member A; SRF, serum response factor; TCF, ternary complex factor.
SRF is widely expressed in the hematopoietic system and key roles for SRF-mediated transcriptional regulation in hematopoietic stem cells [22], megakaryopoiesis and platelet function [23,24], lymphocyte development [25], and macrophage [21] and neutrophil function [26▪▪] have been identified. Additional roles of SRF in hematopoiesis are evident by the fact that Vav-Cre-mediated deletion of SRF in the hematopoietic stem cell leads to early death in the newborn period resulting from bleeding diathesis and severe anemia. In the adult adoptive transplant setting, complete deletion of SRF in the stem cell results in eventual loss of all hematopoietic elements and bone marrow failure (A. Taylor, S. Halene, unpublished observation). Thus, SRF is an essential transcription factor in hematopoiesis affecting all lineages with distinct roles in development and function.
SERUM RESPONSE FACTOR CONTROLS INTEGRIN ACTIVITY AND TRAFFICKING IN NEUTROPHILS
Neutrophil functions depend greatly on the β2 (CD18, ITB2) class of integrins. This class consists of four αβ2 heterodimers including LFA-1 (CD11a/CD18, αLβ2), MAC-1 (CD11b/CD18, αMβ2), the inactivated-C3b (iC3b) receptor 4 (CR4; CD11c/CD18, αXβ2), and CD11d/CD18 (αDβ2). Integrins act as bidirectional transducers that function to relay signals from the outside of the cell to the inside and vice versa. Outside-in signaling by integrins is effected via a variety of mechanisms, such as recruitment of focal adhesion kinases to transmit signals to the migratory and proliferative machinery of the cell or via modulation of receptor–ligand interactions (reviewed in [27]). The β2 integrins usually reside in an inactive conformation with low ligand-affinity. Activation of neutrophils through binding of G protein-coupled and other receptors triggers inside-out signaling that activates integrins via induction of a high-affinity configuration. Binding of two FERM (4.1, ezrin, radixin, moesin) domain-containing proteins, TALIN and KINDLIN-3, to the tail of the cytoplasmic domain of the β2 integrin induces a conformational change of the extracellular domain, increasing its ability to bind ligand [28–31]. Although mutations in ITGB2, encoding the integrin β2 chain, cause LAD-I with markedly reduced expression of the α2β2 heterodimers, mutations in FERMT3, the gene encoding KINDLIN-3, cause LAD-III. Loss of KINDLIN-3 in mice is sufficient to reproduce the findings in LAD-III, with abrogation of firm adhesion of neutrophils on activated endothelium [27,32–34].
Taylor et al. [26▪▪] have recently shown that SRF is essential for neutrophil function. SRF-deficient neutrophils fail to migrate to sites of inflammation in vivo. When mice are challenged with lipopolysaccharide (LPS) via nebulization to mimic Gram-negative infection of the lungs or are injected with monosodium urate crystals into the peritoneal space mimicking peritonitis, SRF-deficient neutrophils fail to migrate to the site of inflammation even in the presence of an otherwise intact immune system. This lack of neutrophil recruitment to sites of inflammation is explained by failure of SRF-deficient neutrophils to chemotax in vitro as well as markedly reduced polarization and cellular adhesion. Also, F-actin polymerization is markedly reduced, which is consistent with the essential role of SRF in the regulation of the actin cytoskeleton and actin polymerization. However, a novel role for SRF emerged in these studies. SRF-deficient neutrophils exhibit increased cell surface expression of one of the two major neutrophil integrin complexes, MAC-1, the CD11b/CD18 heterodimer. Nevertheless, intercellular adhesion molecule 1 binding in response to activation with fMLP was markedly reduced. This suggests defective inside-out signaling-mediated induction of the high-affinity conformation of the MAC-1 complex. Studies by Halene et al. [23] found a similar phenomenon in platelets lacking expression of SRF. Mice with deletion of SRF specifically in megakaryocytes and platelets exhibited prolonged bleeding times. Megakaryocytes showed markedly decreased adhesion to fibronectin and platelet activation was significantly reduced. Expression of actin cytoskeletal proteins, FILAMIN A (FLNA), CORONIN 1a (CORO1a), and CALPONIN 2 (CNN2), was significantly downregulated. Interestingly, FLNA is one of the most abundant and widely expressed actin cross-linking proteins that connect multiple transmembrane and signaling proteins, including the integrin complex to the cytoskeleton [35–37]. More importantly, although expression of the integrin complex αIIbβ3 (gpIIb/IIIa) on SRF-deleted platelets was similar to wild-type platelets, acquisition of its active conformation was markedly reduced. This suggests a global role for SRF in integrin activation.
Once integrins are activated on neutrophils, they tightly bind to their ligands, such as intercellular adhesion molecule 1, on the endothelial surface. In order for subsequent cell migration to occur, ligand–integrin binding must be terminated and tight adhesions released. Integrin regulation not only occurs via conformational changes altering ligand-binding affinities, but also by spatial regulation via clustering of integrins in the membrane and endocytic trafficking. It has long been known that the actin cytoskeleton is actively involved in the surface presentation of CD11b/CD18 in response to fMLP stimulation [38]. Anderson et al. [39] showed that modulation of the cytoskeleton actively regulates the binding conformation of the CD11b/CD18 integrins as well as their mobility in the membrane, and that these processes are essential to initial neutrophil immobilization (rolling, adhesion) and subsequent release (migration). Kumar et al. [40▪▪] established an important link between CD11b clustering at the uropod and maintenance of neutrophil polarization by stabilization of microtubules.
Integrin trafficking is one of the key mechanisms effecting focal adhesion disassembly, matrix turnover, and localized integrin redistribution to new adhesion sites such as to the leading edge of the cell. Integrins are internalized via clathrin-dependent and clathrin-independent mechanisms. A given integrin heterodimer can be internalized by more than one mechanism. Integrins are either transported to the lysosomes for degradation or recycled back to the cell surface in which they can undergo renewed activation and ligand binding ([41] and reviewed in [27,34]). Integrins are recruited to clathrin-coated vesicles or caveolae by a variety of adaptor proteins, such as AP2 or the signal transducer DAB1, which bind to the cytoplasmic tail of the integrin. Although it has been shown that LFA-1 is actively recycled in a cholesterol-sensitive caveolae-dependent manner, the mechanism for MAC-1 endocytosis and recycling is not known [42]. It has recently been shown that the junctional adhesion molecule A, expressed by leukocytes, mediates intracellular signaling pathways that control integrin, specifically integrin β1, internalization and recycling, enabling the leukocyte uropod to release its substrate thereby facilitating directional motility [43]. HCLS1-associated protein X1, mutated in a subset of patients with severe congenital neutropenia who do not carry mutations in ELANE, the gene encoding neutrophil elastase, is a widely expressed protein that, together with cortactin, is involved in clathrin-mediated endocytosis [44]. Its downregulation leads to loss of activation of Ras homolog family member A (RhoA) and increased integrin-mediated adhesion resulting in impaired neutrophil migration [45]. Detailed understanding of the mechanisms of endocytic recycling of key neutrophil integrins will shed further light on diseases such as and LAD and severe congenital neutropenia.
Interestingly, SRF not only is essential to integrin activation, but Taylor et al. [26▪▪] also highlight its importance in integrin trafficking. When localization of the MAC-1 integrin was assessed in resting and activated neutrophils, Srf KO neutrophils lacked polarized localization of MAC-1 complexes to the leading and trailing edges. This suggests that in addition to inside-out activation, SRF is essential for CD11b/CD18 trafficking. Unlike wild-type neutrophils, Srf-deficient neutrophils lack polarization of clathrin, but not of talin or kindlin, to the leading and trailing edges. Considering that clathrin-mediated endocytosis is highly dependent on cytoskeletal proteins, SRF may be essential to clathrin-dependent endocytic pathways, thereby affecting CD11b/CD18 trafficking.
A SERUM RESPONSE FACTOR NETWORK EXPRESSED ON CHROMOSOME 5Q IS ESSENTIAL FOR NORMAL MYELOPOIESIS
Heterozygous loss of chromosome 5 is one of the most common chromosomal abnormalities in MDS. Several genes that are part of the SRF pathway are located on chromosome 5, such as the diaphanous-related formin mDIA (DIAPH1), α-catenin (CTNNA1), early growth response gene 1 (EGR1), and the miRNAs 143 and 145. The small GTPase RhoA controls activity of SRF by inducing changes in actin dynamics via its effector mDIA [46]. α-Catenin induces SRF-dependent transcriptional activity in a partly RhoA/ROCK-dependent manner [47]. Although DIAPH1 and CTNNA1 lie upstream of SRF, EGR-1 and miRNAs 143 and 145 are downstream targets of SRF and their expression is regulated by SRF-mediated transcriptional activation [48,49].
The formin protein mDIA1, encoded by DIAPH1 on 5q31, controls SRF activity through its effects on linear actin polymerization [50]. mDIA1 is required for neutrophil polarization, migration, and activation [51]. When mDIA1 is knocked out, mice develop hematologic abnormalities reminiscent of myeloproliferative/myelodysplastic disorders with accumulation of immature myeloid cells as well as of activated CD11b+ and CD14+ cells [52]. Interestingly, some of these CD14+ cells are neutrophils that aberrantly overexpress CD14 and are responsible for a hypersensitive innate immune response to LPS stimulation through CD14/toll-like receptor (TLR) 4 signaling. Chronic stimulation with LPS accelerates the development of MDS in mDIA heterozygous or knockout mice [53▪▪]. This effect is at least partially mitigated by treatment with lenalidomide, which not only carries anti-inflammatory functions, but also activates the RhoGTPase RHOA thereby inducing F-actin polymerization and increased cell migration [54].
The miRNAs 143 and 145 are significantly repressed in acute myeloid leukemia cells compared with mature neutrophils, and their depletion inhibits differentiation of acute promyelocytic leukemia blasts to mature neutrophils [55]. EGR-1 expression is regulated via binding of SRF to serum response elements in its promoter and upregulated upon stimulation with G-CSF via recruitment of the ETS transcription factor FLI-1 [49]. Interestingly, in myelopoiesis EGR-1 is also part of a network of counter antagonistic repressors, regulating macrophage/neutrophil lineage decisions in response to PU.1. EGR-1 and EGR-2 redundantly activate macrophage genes and repress a neutrophil program [56]. It will thus be particularly interesting to understand how the combined haploinsufficiency of mDIA, CTNNA1, the miRNAs 143 and 145, and EGR-1 affect SRF-mediated transcriptional activation in MDS with 5q–. Complete loss of SRF results in eventual loss of the hematopoietic stem cell and bone marrow failure, whereas haploinsufficiency of mDIA results in accumulation of immature precursors and neutrophil dysfunction with aberrant activation of the innate immune system via CD14/TLR4 signaling [52,53▪▪]. The mechanism of aberrant CD14 expression on mDIA-deficient neutrophils is not known. However, CD11b/CD18 is part of the TLR activation cluster and contributes to LPS-mediated internalization of TLR4 as well as CD14 [57,58▪▪]. Loss of SRF in the hematopoietic stem cell results in decreased expression of integrins [22], whereas in neutrophils it results in increased expression of nonfunctional CD11b/CD18 on the cell surface, most likely secondary to a defect in inside-out signaling and endocytic trafficking [26▪▪]. It would be intriguing to assess whether expression of CD14 and TLR signaling is regulated by SRF and whether CD11b/CD18 activation and trafficking are affected by loss of mDIA, further explaining the defect in neutrophil function in MDS (Fig. 2). SRF transcriptional activation is regulated by the MAPK/RAS/ERK or the RHOA pathways via recruitment of the ternary complex factors or the myocardin-related transcription factors, respectively. Further studies are necessary to define the role for these two pathways in SRF-mediated neutrophil function.
FIGURE 2.
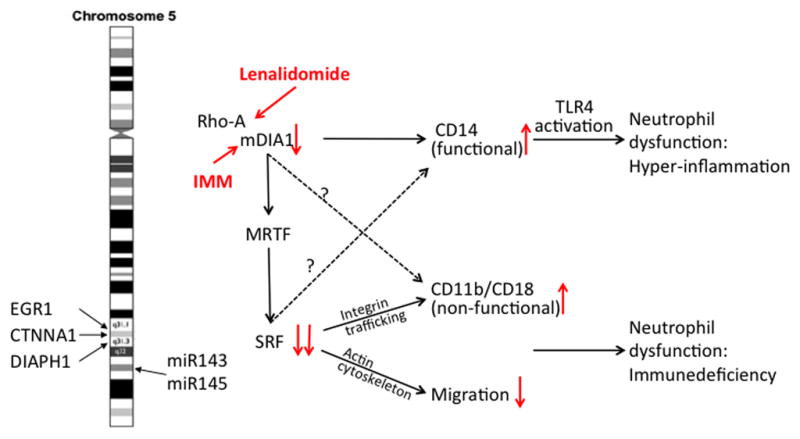
The serum response factor pathway in myelopoiesis and myelodysplasia with 5q abnormality. DIAPH1, CTNNA1, EGR1, miR143, and miR145 are localized on chromosome 5q and are either upstream or downstream of SRF. RhoA signaling and mDIA can be stimulated with lenalidomide or IMMs, respectively. Loss of mDIA leads to aberrant CD14/TLR4 signaling, whereas loss of SRF leads to aberrant CD11b regulation and loss of migration. CTNNA1, α-catenin; DIAPH1, Diaphanous 1; EGR1, early growth response gene 1; IMM, intramimic; mDIA, mammalian Diaphanous; MRTF, myocardin-related transcription factor; SRF, serum response factor; TLR, toll-like receptor.
Defects in MAC-1 and LFA-1 integrin function result in LAD. Further understanding of the regulation of specific integrin functions, such as inside-out signaling-mediated conformational changes, and integrin trafficking will be helpful to further understand these diseases. Detailed assessment of clathrin-mediated endocytosis in SRF-deficient cells may further elucidate essential ‘players’ in this fundamental process.
Targeting of the SRF pathway in MDS with 5q abnormalities may provide a novel treatment approach. Taking advantage of the fact that 5q abnormalities invariably lead to haploinsufficiency of 5q genes, targeting of SRF via partial upstream inhibition of mDIA, may lead to loss of the diseased hematopoietic stem cell due to complete abrogation of SRF-mediated transcriptional activation while sparing remnant normal hematopoiesis. Alternatively, activation of mDIA1 may restore neutrophil function and correct the aberrant innate immune signaling, thus restoring normal hematopoiesis even without abrogation of the 5q– clone.
So called ‘intramimics’ that may re-establish formin-directed cytoskeletal remodeling in diseases affected by formin dysfunction are in development [59▪▪]. These may lead the way to novel therapeutics for MDS with 5q abnormalities and other diseases of neutrophil dysfunction based on disruption of the SRF pathway.
CONCLUSION
Neutrophil migration and response to inflammation is a meticulously orchestrated process essential to maintenance of homeostasis in an environment of constant inflammatory challenge. SRF is a key transcription factor in the hematopoietic system, from the stem cell to the mature lineage cells, and master regulator of the actin cytoskeleton. The actin cytoskeleton not only functions in ‘mechanic’ forward propulsion of the cell, but also as a sensor of extracellular stimuli and regulator of transcriptional activation of structural and regulatory effectors of actin dynamics. As such, the SRF pathway controls the innate immune response by regulation of cell surface expression of regulatory receptors, such as CD14, integrin activation, and trafficking, and ultimately cell polarization and migration. Elements of the SRF pathway are disrupted in MDS with 5q abnormalities and targeting of the SRF pathway may provide novel treatment strategies.
KEY POINTS.
SRF regulates CD11b/CD18 (MAC-1) activation and trafficking.
SRF is essential for neutrophil migration to sites of inflammation.
The formin mDIA, which lies upstream of SRF activation, directs linear actin polymerization. Loss of mDIA results in aberrant expression of CD14 and activation of TLR-4 signaling in neutrophils, thereby contributing to MDS via aberrant innate immune signaling.
Acknowledgments
The authors thank Dr Diane Krause, Yale University School of Medicine, and Dr Peter Gaines, University of Massachusetts, Lowell, for the careful review of the manuscript, and Dr Elenoe Smith, Harvard Medical School, for her artwork.
Financial support and sponsorship
This work was funded by a career development award from the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342) (S.H.) and the Yale Comprehensive Cancer Center.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Boogaerts MA, Nelissen V, Roelant C, et al. Blood neutrophil function in primary myelodysplastic syndromes. Br J Haematol. 1983;55:217–227. doi: 10.1111/j.1365-2141.1983.tb01241.x. [DOI] [PubMed] [Google Scholar]
- 2.Bunting M, Harris ES, McIntyre TM, et al. Leukocyte adhesion deficiency syndromes: adhesion and tethering defects involving beta 2 integrins and selectin ligands. Curr Opin Hematol. 2002;9:30–35. doi: 10.1097/00062752-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi Y. Neutrophil infiltration and chemokines. Crit Rev Immunol. 2006;26:307–316. doi: 10.1615/critrevimmunol.v26.i4.20. [DOI] [PubMed] [Google Scholar]
- 4.Niggli V. Signaling to migration in neutrophils: importance of localized pathways. Int J Biochem Cell Biol. 2003;35:1619–1638. doi: 10.1016/s1357-2725(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 5.Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 6.Phillipson M, Heit B, Colarusso P, et al. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarbock A, Abram CL, Hundt M, et al. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling. J Exp Med. 2008;205:2339–2347. doi: 10.1084/jem.20072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes BJ, Hollers JC, Crockett-Torabi E, et al. Recruitment of CD11b/CD18 to the neutrophil surface and adherence-dependent cell locomotion. J Clin Invest. 1992;90:1687–1696. doi: 10.1172/JCI116041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheikh S, Nash GB. Continuous activation and deactivation of integrin CD11b/CD18 during de novo expression enables rolling neutrophils to immobilize on platelets. Blood. 1996;87:5040–5050. [PubMed] [Google Scholar]
- 10.Diacovo TG, Roth SJ, Buccola JM, et al. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood. 1996;88:146–157. [PubMed] [Google Scholar]
- 11.Pick R, Brechtefeld D, Walzog B. Intraluminal crawling versus interstitial neutrophil migration during inflammation. Mol Immunol. 2013;55:70–75. doi: 10.1016/j.molimm.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Wiesner S, Legate KR, Fassler R. Integrin–actin interactions. Cell Mol Life Sci. 2005;62:1081–1099. doi: 10.1007/s00018-005-4522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell. 2009;17:310–322. doi: 10.1016/j.devcel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Stephens L, Milne L, Hawkins P. Moving towards a better understanding of chemotaxis. Curr Biol. 2008;18:R485–R494. doi: 10.1016/j.cub.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 15.Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 2007;292:C70–C81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- 16.Posern G, Treisman R. Actin’ together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–596. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪▪.Baarlink C, Wang H, Grosse R. Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science. 2013;340:864–867. doi: 10.1126/science.1235038. Formins rapidly polymerize actin inside the mammalian nucleus in response to serum stimulation to drive serum-dependent MAL-SRF activity. [DOI] [PubMed] [Google Scholar]
- 19.Janknecht R, Ernst WH, Pingoud V, et al. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993;12:5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchwalter G, Gross C, Wasylyk B. Ets ternary complex transcription factors. Gene. 2004;324:1–14. doi: 10.1016/j.gene.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan AL, Benner C, Heinz S, et al. Serum response factor utilizes distinct promoter- and enhancer-based mechanisms to regulate cytoskeletal gene expression in macrophages. Mol Cell Biol. 2011;31:861–875. doi: 10.1128/MCB.00836-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ragu C, Elain G, Mylonas E, et al. The transcription factor Srf regulates hematopoietic stem cell adhesion. Blood. 2010;116:4464–4473. doi: 10.1182/blood-2009-11-251587. [DOI] [PubMed] [Google Scholar]
- 23.Halene S, Gao Y, Hahn K, et al. Serum response factor is an essential transcription factor in megakaryocytic maturation. Blood. 2010;116:1942–1950. doi: 10.1182/blood-2010-01-261743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ragu C, Boukour S, Elain G, et al. The serum response factor (SRF)/megakaryocytic acute leukemia (MAL) network participates in megakaryocyte development. Leukemia. 2010;24:1227–1230. doi: 10.1038/leu.2010.80. [DOI] [PubMed] [Google Scholar]
- 25.Fleige A, Alberti S, Grobe L, et al. Serum response factor contributes selectively to lymphocyte development. J Biol Chem. 2007;282:24320–24328. doi: 10.1074/jbc.M703119200. [DOI] [PubMed] [Google Scholar]
- 26▪▪.Taylor A, Tang W, Bruscia EM, et al. SRF is required for neutrophil migration in response to inflammation. Blood. 2014;123:3027–3036. doi: 10.1182/blood-2013-06-507582. SRF regulates adhesion and migration in neutrophils. CD11b/CD18 inside-out activation and trafficking require intact SRF-mediated transcription. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 28.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 29.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brahme NN, Calderwood DA. Cell adhesion: a FERM grasp of the tail sorts out integrins. Curr Biol. 2012;22:R692–R694. doi: 10.1016/j.cub.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moser M, Bauer M, Schmid S, et al. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med. 2009;15:300–305. doi: 10.1038/nm.1921. [DOI] [PubMed] [Google Scholar]
- 33.Svensson L, Howarth K, McDowall A, et al. Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat Med. 2009;15:306–312. doi: 10.1038/nm.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margadant C, Monsuur HN, Norman JC, et al. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol. 2011;23:607–614. doi: 10.1016/j.ceb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Kiema T, Lad Y, Jiang P, et al. The molecular basis of filamin binding to integrins and competition with talin. Mol Cell. 2006;21:337–347. doi: 10.1016/j.molcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Legate KR, Fassler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122(Pt 2):187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- 37.Falet H. New insights into the versatile roles of platelet FlnA. Platelets. 2013;24:1–5. doi: 10.3109/09537104.2011.654004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheikh S, Gratzer WB, Pinder JC, et al. Actin polymerisation regulates integrin-mediated adhesion as well as rigidity of neutrophils. Biochem Biophys Res Commun. 1997;238:910–915. doi: 10.1006/bbrc.1997.7407. [DOI] [PubMed] [Google Scholar]
- 39.Anderson SI, Hotchin NA, Nash GB. Role of the cytoskeleton in rapid activation of CD11b/CD18 function and its subsequent downregulation in neutrophils. J Cell Sci. 2000;113(Pt 15):2737–2745. doi: 10.1242/jcs.113.15.2737. [DOI] [PubMed] [Google Scholar]
- 40▪▪.Kumar S, Xu J, Kumar RS, et al. The small GTPase Rap1b negatively regulates neutrophil chemotaxis and transcellular diapedesis by inhibiting Akt activation. J Exp Med. 2014;211:1741–1758. doi: 10.1084/jem.20131706. Rap1b deficiency promotes transcellular migration of neutrophils through endothelial cells via enhanced PI3K-Akt activation and formation of invadopodia-like protrusions leading to enhanced inflammatory response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ezratty EJ, Bertaux C, Marcantonio EE, et al. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–747. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fabbri M, Di Meglio S, Gagliani MC, et al. Dynamic partitioning into lipid rafts controls the endo-exocytic cycle of the alphaL/beta2 integrin, LFA-1, during leukocyte chemotaxis. Mol Biol Cell. 2005;16:5793–5803. doi: 10.1091/mbc.E05-05-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cera MR, Fabbri M, Molendini C, et al. JAM-A promotes neutrophil chemotaxis by controlling integrin internalization and recycling. J Cell Sci. 2009;122(Pt 2):268–277. doi: 10.1242/jcs.037127. [DOI] [PubMed] [Google Scholar]
- 44.Ortiz DF, Moseley J, Calderon G, et al. Identification of HAX-1 as a protein that binds bile salt export protein and regulates its abundance in the apical membrane of Madin-Darby canine kidney cells. J Biol Chem. 2004;279:32761–32770. doi: 10.1074/jbc.M404337200. [DOI] [PubMed] [Google Scholar]
- 45.Cavnar PJ, Berthier E, Beebe DJ, et al. Hax1 regulates neutrophil adhesion and motility through RhoA. J Cell Biol. 2011;193:465–473. doi: 10.1083/jcb.201010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geneste O, Copeland JW, Treisman R. LIM kinase and Diaphanous cooperate to regulate serum response factor and actin dynamics. J Cell Biol. 2002;157:831–838. doi: 10.1083/jcb.200203126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merdek KD, Jaffe AB, Dutt P, et al. Alpha(E)-Catenin induces SRF-dependent transcriptional activity through its C-terminal region and is partly RhoA/ROCK-dependent. Biochem Biophys Res Commun. 2008;366:717–723. doi: 10.1016/j.bbrc.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xin M, Small EM, Sutherland LB, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gineitis D, Treisman R. Differential usage of signal transduction pathways defines two types of serum response factor target gene. J Biol Chem. 2001;276:24531–24539. doi: 10.1074/jbc.M102678200. [DOI] [PubMed] [Google Scholar]
- 50.Copeland JW, Treisman R. The diaphanous-related formin mDia1 controls serum response factor activity through its effects on actin polymerization. Mol Biol Cell. 2002;13:4088–4099. doi: 10.1091/mbc.02-06-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi Y, Zhang J, Mullin M, et al. The mDial formin is required for neutrophil polarization, migration, and activation of the LARG/RhoA/ROCK signaling axis during chemotaxis. J Immunol. 2009;182:3837–3845. doi: 10.4049/jimmunol.0803838. [DOI] [PubMed] [Google Scholar]
- 52.Peng J, Kitchen SM, West RA, et al. Myeloproliferative defects following targeting of the Drf1 gene encoding the mammalian diaphanous related formin mDia1. Cancer Res. 2007;67:7565–7571. doi: 10.1158/0008-5472.CAN-07-1467. [DOI] [PubMed] [Google Scholar]
- 53▪▪.Keerthivasan G, Mei Y, Zhao B, et al. Aberrant overexpression of CD14 on granulocytes sensitizes the innate immune response in mDia1 heterozygous del(5q) MDS. Blood. 2014;124:780–790. doi: 10.1182/blood-2014-01-552463. Neutrophils from mDIA1 heterozygous and knockout mice aberrantly overexpress CD14 leading to a hypersensitive innate immune response to LPS stimuli through CD14/toll-like receptor 4 signaling. Chronic stimulation with LPS accelerates the development of MDS in mDIA1 heterozygous and knockout mice that can be rescued by lenalidomide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Y, Li J, Ferguson GD, et al. Immunomodulatory drugs reorganize cytoskeleton by modulating Rho GTPases. Blood. 2009;114:338–345. doi: 10.1182/blood-2009-02-200543. [DOI] [PubMed] [Google Scholar]
- 55.Batliner J, Buehrer E, Fey MF, et al. Inhibition of the miR-143/145 cluster attenuated neutrophil differentiation of APL cells. Leuk Res. 2012;36:237–240. doi: 10.1016/j.leukres.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Laslo P, Spooner CJ, Warmflash A, et al. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell. 2006;126:755–766. doi: 10.1016/j.cell.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 57.Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23:301–304. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- 58▪▪.Ling GS, Bennett J, Woollard KJ, et al. Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nat Commun. 2014;5:3039. doi: 10.1038/ncomms4039. CD11b positively regulates LPS-induced signaling pathways selectively in myeloid dendritic cells but not in macrophages. In dendritic cells CD11b facilitates LPS-induced TLR4 endocytosis and is required for the subsequent signaling in the endosomes. By modulating the trafficking and signaling functions of TLR4 in a cell-type-specific manner CD11b fine tunes the balance between adaptive and innate immune responses initiated by LPS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59▪▪.Lash LL, Wallar BJ, Turner JD, et al. Small-molecule intramimics of formin autoinhibition: a new strategy to target the cytoskeletal remodeling machinery in cancer cells. Cancer Res. 2013;73:6793–6803. doi: 10.1158/0008-5472.CAN-13-1593. Intramimics (IMMs) are a novel class of small-molecule agonists of the mDIA-related formins, which act downstream of Rho GTPases to assemble actin filaments, and their organization with microfilaments to establish and maintain cell polarity during migration and asymmetric division. Two molecules, IMM-01 and IMM-02, were sufficient to trigger actin assembly and microtubule stabilization, SRF-mediated gene expression, cell cycle arrest, and apoptosis. This work establishes the use of intramimics and mDIA-related formins as a new general strategy for therapeutic targeting of the cytoskeletal remodeling machinery of cancer cells. [DOI] [PubMed] [Google Scholar]