Abstract
Objective
The present investigation examined whether higher-functioning children with autism would demonstrate impaired response inhibition performance in an emotional Go/No-Go task, and whether severity of ADHD or autism symptoms correlated with performance.
Method
Forty-four children (21 meeting criteria for autism; 23 typically developing controls (TDC)) completed an emotional Go/No-Go task where an emotional facial expression (angry, fearful, happy, or sad) was the Go stimulus and a neutral facial expression was the No-Go stimulus, and vice versa.
Results
The autism group was faster than the TDC group on all emotional Go trials. Moreover, the children in the autism group who had the fastest RTs on emotional Go trials were rated as having the greatest number of symptoms (ADOS Social+Communication score); even after accounting for the association with ADHD symptoms. The autism group also made more impulsive responses (i.e., lower d′, more false alarms) than the TDC group on all No-Go trials. As d′ decreased or false alarms increased so did ADHD symptoms. Hyperactivity/Impulsivity symptoms were significantly correlated with false alarms, but Inattention symptoms were not. There was not a significant relationship between No-Go false alarms and autism symptoms, and even after partialling out associations with autism symptoms the significant correlation between ADHD symptoms and No-Go false alarms remained. Conclusions: The present findings support a comorbidity model that argues for shared and independent risk factors, because ADHD and autism symptoms related to independent aspects of emotional Go/No-Go performance.
Keywords: Autism, Attention Deficit Hyperactivity Disorder, cognitive control, response inhibition, emotion
The ability to coordinate cognitive control processes to accomplish a goal has been identified as impaired in individuals with autism spectrum disorder (ASD) for over 25 years (Rumsey, 1985). Cognitive control deficits have also been proposed as a core deficit in attention deficit/hyperactivity disorder (ADHD) (Barkley, 1997; Nigg & Casey, 2005; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). As our knowledge of the high levels of comorbidity of ADHD and ASD has increased (Leyfer et al., 2006), so has our appreciation of the influences that ADHD symptoms may exert on cognitive control performance in ASD (Corbett, Constantine, Hendren, Rocke, & Ozonoff, 2009; Rommelse, Geurts, Franke, Buitelaar, & Hartman, 2011; Sinzig, Morsch, Bruning, Schmidt, & Lehmkuhl, 2008; Sinzig, Morsch, & Lehmkuhl, 2008; Yerys et al., 2009). Thus, examining whether ASD and ADHD symptoms make independent contributions to cognitive control performance in social contexts is an important and relatively underexplored area in children with autism.
Response inhibition is a cognitive control process that involves withholding an inappropriate response in a specific context (i.e., an impulsive response). It has been reliably indexed as an impaired cognitive control process in ADHD (Castellanos & Tannock, 2002; Doyle et al., 2005; Pennington & Ozonoff, 1996), while the evidence in ASD is mixed (Christ, Holt, White, & Green, 2007; Christ, Kester, Bodner, & Miles, 2011; Corbett et al., 2009; Geurts, Begeer, & Stockmann, 2009; Kenworthy, Yerys, Anthony, & Wallace, 2008; Luna, Doll, Hegedus, Minshew, & Sweeney, 2007; Ozonoff & Strayer, 1997). In a study using an emotional Go/No-Go task that measured response inhibition with social-emotional stimuli, children with ASD responded with similar levels of accuracy and speed as matched controls. Children were instructed to respond as quickly as possible to the presentation of faces with happy expressions (Go response) and to withhold responses when faces with angry expressions were presented (No-Go response) (Geurts et al., 2009). When letters and symbols were the stimuli in non-emotional Go/No-Go tasks, children with ASD have demonstrated both impaired (Christ et al., 2007) and intact performance (Ozonoff, Strayer, McMahon, & Filloux, 1994; Ozonoff & Strayer, 1997). One common explanation for different findings across studies employing highly similar (or identical) paradigms is heterogeneity of the ASD phenotype. A recent study in ASD children showed that ADHD symptoms played a significant role in a continuous performance task that also assesses response inhibition (Corbett et al., 2009), suggesting that ADHD symptoms may be a potential source of phenotypic variability in ASD.
An additional factor in the study of cognitive control in ASD is its common intersection with processing social-emotional stimuli. This is critical from a real world perspective, because everyday situations require inhibiting responses to certain types of social cues (e.g., inhibiting an approach to someone who stares angrily at you). In the laboratory, this type of social and cognitive control intersection has been modeled with the use of facial expressions with or without an emotional expression, such as the emotional Go/No-Go task described above (Geurts et al., 2009) or the use of interference suppression tasks with face stimuli (Dichter & Belger, 2007; Vaidya et al., 2011). Specifically, interference suppression tasks have required ignoring the eye gaze of a neutral facial expression that “looks” to the correct or incorrect direction. Across these three studies, children and adults with ASD performed similarly to their matched control groups in the conditions containing social-emotional stimuli. The lack of group differences with social-emotional stimuli suggests that individuals with ASD can stop or suppress an inappropriate response embedded in a social context. What remains unclear is whether the good performance of children with ASD is supported by low-level task factors. For example, the use of extreme facial expression stimuli (i.e., happy is ‘Go’ and angry is ‘No-Go’) that differ on salient, low-level features like spatial frequency and luminance could have supported good performance for children with ASD. Furthermore, the use of happy facial expressions as the Go response is easier for individuals with ASD to recognize because of the upturned mouth relative to negative emotions like anger, fear and sadness (Harms, Martin, & Wallace, 2010). The upturned mouth may have been the single visual feature to drive good performance. Thus, in order to confirm that inhibition with socio-emotional stimuli is intact in ASD, it is important to probe for group × emotion interactions. This requires a common baseline condition of a neutral facial expression as the Go/No-Go response in comparison to an emotional facial expression. Therefore, the present study will assess cognitive control with socio-emotional stimuli by including neutral facial expression stimuli opposite each emotional facial expression. This will allow a comparison across emotions, and provide a better test of whether children with ASD are able to appropriately inhibit responses to specific emotional facial expressions.
The present investigation has two goals: (1) Examine whether children meeting full criteria for autism (autism group) demonstrate intact response inhibition performance in an emotional Go/No-Go task that requires discriminating four emotional facial expressions (angry, fearful, happy, and sad) from neutral ones (Ladouceur et al., 2006); (2) Examine whether ADHD and ASD symptoms distinctly correlate with performance in the autism group. With respect to Go trial performance, prior autism studies with social-emotional cognitive control tasks would predict no response time (RT) differences between the autism group and a typically developing control (TDC) group. However, emotion recognition studies suggest that children with autism should be slower to identify emotions from facial expressions (Bal et al., 2010). For No-Go trial performance (i.e., response inhibition), we predict that the autism group will have more difficulty withholding responses. We further predict that this failure will correlate with ADHD symptoms, and that this will help explain the mixed response inhibition findings in the literature. We did not predict a relationship between task performance and ASD symptoms in the present study.
Method
Participants
Twenty-eight children with autism and 23 TDC children were enrolled in this study. Four children in the autism group were excluded due to failure to complete the study and three did not meet the IQ requirement described below. Only children meeting a Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.) diagnosis of autism were included. This ensured a more homogeneous group of children presenting with classic autism symptoms. Children were recruited through the local community via advertisements and a hospital's outpatient clinic specializing in ASD and neuropsychological assessment. All children were between the ages of 7 and 14 years, and were required to have a Full Scale IQ≥90, as measured by a Wechsler Intelligence scale (Wechsler Intelligence Scale for Children–4th Edition, or Wechsler Abbreviated Scale of Intelligence; (Wechsler, 1999, 2003)). This Full Scale IQ requirement ensured a similar IQ range between groups (See Table 1), and thus preventing the influence of relatively low IQ scores on performance in the autism group. The Institutional Review Board at Children's National Medical Center approved the study. Written informed consent from parents of children, and assent from children were obtained according to Institutional Review Board guidelines.
Table 1. Participant demographics.
| Total N | TDC 23 | Autism 21 | p-value |
|---|---|---|---|
| Chronological Age (Years) | |||
| M(SD) | 10.62 (1.55) | 10.22 (1.81) | .64 |
| Range | 7.30 – 12.92 | 7.00 – 12.98 | |
| Full Scale IQ1 | |||
| M(SD) | 118.96 (12.86) | 111.43 (12.74) | .08 |
| Range | 98 – 143 | 91 – 134 | |
| Gender (male/female) | 15/8 | 19/3 | .10 |
| ADHD Rating Scale | |||
| Hyperactivity/Impulsivity | Raw Score | ||
| M(SD) | 2.17 (2.90) | 10.24 (5.21) | <.001 |
| Range | 0 – 12 | 2 – 21 | |
| Inattentive Raw Score | |||
| M(SD) | 3.30 (2.99) | 14.67 (6.15) | <.001 |
| Range | 0 – 10 | 0 – 26 | |
| Total Raw Score | |||
| M(SD) | 5.48 (5.08) | 24.91 (9.22) | <.001 |
| Range | 0 – 22 | 2 – 45 | |
| ADI-R | |||
| Social | |||
| M(SD) | 20.15 (4.72) | ||
| Range | 10 – 28 | ||
| Communication | |||
| M(SD) | 15.75 (4.59) | ||
| Range | 8 – 26 | ||
| Repetitive Behaviors | |||
| M(SD) | 5.40 (1.70) | ||
| Range | 3 – 9 | ||
| ADOS | |||
| Social+Communication | |||
| M(SD) | 13.25 (3.55) | ||
| Range | 10 - 21 |
Wechsler Intelligence Scale for Children–4th Edition or Wechsler Abbreviated Scale of Intelligence
The autism group received a clinical diagnosis based on Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.) criteria (American Psychiatric Association, 2000). They all met the criteria for autism on the Autism Diagnostic Interview-Revised (Lord, Rutter, & Le Couteur, 1994) and the Social+Communication score from module 3 of the Autism Diagnostic Observation Schedule (Lord et al., 2000). The autism group was screened through a parent phone interview and excluded if they had any history of known genetic or neurological disorders (e.g., Fragile X syndrome or Tourette's syndrome). Stimulant medications were withheld at least 24 hours prior to testing (n=2), and one child was also prescribed an anti-psychotic, an anti-depressant, and an anti-cholinergic, which were not withheld. Two other children were prescribed melatonin. The TDC group was screened and excluded if they or a first-degree relative had an identified neurogenetic, language, learning, neurological, or psychiatric disorder, or if the child utilized psychiatric medication or met the clinical criteria for a childhood disorder on the Child Symptom Inventory – Fourth Edition (CSI-IV) or Child and Adolescent Symptom Inventory (CASI) (Gadow & Sprafkin, 2000, 2010). This screening process required TDCs to score below clinical threshold on the ADHD, autism, and Asperger scales of the CSI-IV/CASI. Participant demographics are shown in Table 1.
Measures
Emotional Go/No-Go
The Emotional Go/No-Go task was a modified variant of a probability Go/No-Go task in which cognitive control was measured in the context of emotional information, and the study implemented the identical paradigm to Ladouceur and colleagues (2006). This task was obtained from Dr. Casey's website at the Sackler Institute for Developmental Psychobiology (http://www.sacklerinstitute.org/cornell/assays_and_tools/Face_Go_No-Go.zip). The task required participants to press the ‘Space’ bar in response to an emotional facial expression and withhold a response to a neutral facial expression, or vice versa. There were four conditions: Angry/Neutral, Fearful/Neutral, Happy/Neutral, Sad/Neutral. Experimenters instructed children to ‘respond as fast as you can without making mistakes’. All stimuli were digitized black-and-white photographs from the Ekman faces (Ekman & Friesen, 1976), which included pictures of 10 adults (5 males and 5 females). Stimuli were displayed centrally on a white background at a viewing distance of approximately 60 cm on a 17-inch monitor using E-Prime version 1.1 (Psychology Software Tools Inc., Pittsburgh, PA).
The task consisted of 240 trials with 8 different block types with 30 trials per block: Angry Go/Neutral No-Go, Fearful Go/Neutral No-Go, Happy Go/Neutral No-Go, Sad Go/Neutral No-Go, Neutral Go/Angry No-Go, Neutral Go/Fearful No-Go, Neutral Go/Happy No-Go, Neutral Go/Sad No-Go. Block order was randomized across participants and trial order was randomized within participants. On each trial the stimulus was presented for 500ms with an interstimulus interval of 1000ms. The key dependent variables were response accuracy (d′= z(Correct Go trials) – z(False Alarms on No-Go trials)), Go response time (RT) from correct trials, and False Alarm (FA) percentage.
Attention Deficit Hyperactivity Disorder Rating Scale – Parent Edition (ADHD Rating Scale)
The ADHD Rating Scale (DuPaul, Power, Anastopoulos, & Reid, 1998) assessed severity in inattention and hyperactivity/impulsivity symptoms. This 18-question scale yields two domains: inattention and hyperactivity/impulsivity. For each question, parents use a 0 –3 scale to rate the participant. A higher score indicated greater symptom severity, and the total score (0-54) was the dependent variable of interest.
Autism Diagnostic Observation Schedule – Module 3 (ADOS)
The ADOS (Lord et al., 2000), a semi-structured play and conversational interview, assessed social, communication, and restricted repetitive behavior and interest symptoms through a series of social presses and opportunities.
Statistical Analysis Plan
We trimmed our data to exclude trials with response times shorter than 100 ms and greater than 1000ms; trimmed data comprised less than 1% of all ‘Go’ trials and was commensurate with prior investigations (Ladouceur et al., 2006; Waters & Valvoi, 2009). We first examined the influence of emotional facial expressions on Go/No-Go task performance by conducting a repeated measures analysis of variance (ANOVA) on d′. The model was a 2 (Group: autism vs. TDC) × 4 (Emotion: Angry, Fearful, Happy, Sad) × 2 (Go Stimuli: Emotional Facial Expression vs. Neutral Facial Expression) design where Group was the between-subjects variable, and Emotion and Go Stimuli were the within-subjects variables. We employed the same repeated measures ANOVA design with RT. As part of a secondary analysis, we also examined the percentage of FAs with a repeated measures ANOVA and correlations between FA and RT (i.e., speed-accuracy tradeoffs) to capture impulsive responses. Significant within-group analyses were followed up with simple contrasts. For all repeated measures ANOVAs, a Greenhouse-Geisser correction was applied when appropriate and effect sizes are reported in partial Eta Squared (η2p). The groups trended towards a significant difference in Full Scale IQ, and there are varying opinions about the appropriateness of covarying scores that reflect core aspects of a neurodevelopmental disorder (Dennis et al., 2009). We conducted analyses with and without Full Scale IQ entered as a covariate (repeated measures ANCOVA). Pearson's r, Spearman's rho and partial correlations were conducted with ASD and ADHD symptoms and dependent variables of interest that differed between groups (e.g., Go RT and FA percentage with emotional No-Go stimuli). All analyses were two-tailed tests, and because of our a priori hypotheses alpha was set at p<0.05.
Results
Primary Analyses of d′ and RT
There was a significant main effect of Emotion on d′, F(3,40)=10.07, p <.05, η2p=0.75, a main effect of Go stimuli, F(1,42)=125.17, p<.05, η2p=0.75, and a main effect of Group F(1,42)=5.31, p<.05, η2p=0.11 (see Table 2). The main effects are qualified by a Go Stimuli × Group interaction F(1,42)=4.81, p <.05). All other interactions were not significant (all Fs<1.72, all ps>18). Collapsing across groups, pairwise analyses showed that d′ was significantly higher for Fearful and Happy trials compared to Angry and Sad trials (ps<05). Moreover, both groups had a higher d′ when the emotional facial expression was the Go stimulus. While the autism group had a lower d′ overall, there was a significantly larger difference when the Go stimulus was a neutral facial expression (p<05). After covarying the effects of IQ, there were no significant main effects of Go stimuli or Emotion (all Fs<2.35, all ps>13 η2s<0.06), but the main effect of group, F(1,41)=6.32, p<.05, η2=0.13, and the Group × Go stimuli interaction, F(1,41)=3.92, p=.05, η2p=0.09, remained significant. This repeated measures ANCOVA also revealed a Go stimuli × Emotion interaction, F(3,39)=3.89, p<0.05, η2p=0.23, with the lowest d′ occurring when Sad facial expressions are the No-Go stimulus.
Table 2.
Performance on d′ (z(Correct Hits) – z(False Alarms)), Response Time (RT), and false alarm (FA) percentage for each diagnostic group and emotion condition.
| Dependent Variable | TDC M(SD) | Autism M(SD) |
|---|---|---|
| d′ Emotional Go/Neutral No-Go | ||
| Angry | 2.35 (0.50) | 2.20 (0.84) |
| Fear | 2.61 (0.81) | 2.67 (1.02) |
| Happy | 3.18 (1.13) | 2.87 (1.02) |
| Sad | 2.36 (0.65) | 2.11 (0.63) |
| d′ Neutral Go/Emotional No-Go | ||
| Angry | 1.60 (0.82) | 1.13 (0.87) |
| Fear | 2.04 (1.22) | 1.51 (1.18) |
| Happy | 2.01 (1.32) | 1.18 (0.86) |
| Sad | 1.35 (0.62) | 0.84 (0.86) |
| Emotional Go RT (ms) | ||
| Angry | 610.15 (92.23) | 550.21 (92.81) |
| Fear | 604.61 (118.61) | 536.07 (75.80) |
| Happy | 591.76 (116.80) | 526.87 (83.00) |
| Sad | 618.90 (92.79) | 562.82 (128.08) |
| FA Percentage | ||
| Emotional No-Go | 32.50 (21.78) | 46.20 (23.22) |
| Neutral No-Go | 5.20 (4.11) | 8.70 (5.80) |
For RT, there was a trend towards a main effect of emotion, F(2.67,114.71)=2.49, p=.07, η2p=0.06, a significant main effect of group, F(1,43)=4.16, p <.05, η2p=0.09, but no emotion by group interaction, F(2.67,114.71)=0.16, p=90, η2p=0.00. Responses to the Sad trials were significantly slower than Angry trials (p<05), and the autism group was faster than TDCs across all emotion conditions (see Table 2). The significant main effect of group became a trend with a medium effect size when IQ was entered as a covariate F(1,42)=2.89, p=.10, η2p=0.06.
This set of analyses demonstrated that the autism group was less accurate overall, and most inaccurate compared to the TDC group when they had to respond to neutral facial expressions and withhold responses to emotional facial expressions. Both groups were more accurate for Fearful and Happy emotional expressions. Furthermore, the autism group was faster in all conditions, and that both groups were slower during the Sad trials compared to the Angry trials.
Secondary Analyses for Impulsive Responding
The repeated measures ANOVA with FA revealed main effects of Emotion, F(3,40)=12.79, p <.05, η2p=0.49, Go stimuli, F(1,42)=184.49, p <.05, η2p=0.82 and Group, F(1,42)=10.05, p <.05, η2p=0.19. There was also a significant Go stimuli × Group interaction, F(1, 41)=6.65, p <.05, η2p=0.14. Collapsing across groups, pairwise analyses showed that the more FAs were made during Sad trials, then Angry and Happy trials, with the fewest FAs occurring during Fear trials (all ps≤.05). Both groups made more FAs when neutral facial expressions were the Go stimulus and emotional facial expressions were the No-Go stimuli. The autism group made more FAs than TDCs. The interaction showed that the autism group made significantly more FAs than TDCs when the Go stimuli were neutral facial expressions and the No-Go stimuli were emotional facial expressions (see Table 2). The main effects of Go stimuli, F(1,41)=8.90, p <.05, η2p=0.18, and Group remained significant when IQ was entered as a covariate, F(1,41)=6.50, p <.05, η2p=0.13; however, the main effect of Emotion was no longer significant, F(3,39)=0.06, p=98, η2p=0.00. The Go stimuli × Group interaction also remained significant with IQ as a covariate, F(1,41)=4.30, p <.05, η2p=0.10.
In order to examine potential speed-accuracy tradeoffs, we correlated RT with FAs for the combined sample, and within each group. We found a significant relationship between RT and FAs when the No-Go stimuli were emotional facial expressions: combined group, r(44)=-.68, p<.05; autism, r(21)= -.58, p<.05; TDC, r(23)=-.68, p<.05. The same relationships were observed when the No-Go stimuli were neutral facial expressions: combined group, r(44)=-.53, p<.05; autism, r(21)=-.47, p<.05; TDC, r(23)=-.48, p<.05.
This secondary set of analyses demonstrated that the autism group made more FAs than the TDC group overall, but the discrepancy was greater when the No-Go stimuli were emotional facial expressions. Both groups made the most FAs to Sad trials and the fewest during Fear trials. These findings do support the possibility of a speed-accuracy trade-off in so much as they demonstrate that both groups show an increased FA percentage as RT decreases.
Correlations of Task Performance with Autism and Attention Deficit and Hyperactivity Disorder Symptoms
Because the four emotional facial expressions did not interact significantly with group in any analysis, RT and FA rates were collapsed across all emotion conditions in order to reduce the number of comparisons. In the autism group, the ADOS Social+Communication score had no significant relationship with d′ for neutral or emotional Go stimuli (see Table 3) but the ADHD total score was significantly negatively correlated with d′ for neutral Go and emotional No-Go stimuli only. This relationship survived partial correlations for IQ or autism symptoms. The mean emotional Go RT in the autism group was significantly negatively correlated with the ADOS Social+Communication scale (Figure 1a), but not significantly related to the total ADHD rating scale score (Figure 1b). Moreover, the emotional Go RT-ADOS Social+Communication negative correlation remained significant even after controlling for Full Scale IQ or ADHD symptoms. The neutral Go RT revealed a marginally significant negative correlation with the total ADHD rating scale score (Figure 1b). The mean emotional No-Go FA percentage in the autism group had a significantly positive correlation with the total ADHD rating scale score (Figure 1d), but was not significantly related to the ADOS Social+Communication scale (Figure 1c). The neutral No-Go FA had a marginally significant positive correlation with the total ADHD rating scale score. The emotional No-Go FA percentage-ADHD rating scale positive correlation remained significant even after controlling for Full Scale IQ or ADOS Social+Communication scale scores. A secondary analysis revealed that significant or marginally significant relationships between ADHD symptoms and performance variables were larger with Hyperactivity/Impulsivity symptoms than Inattention symptoms (See Table 4).
Table 3.
Pearson's r, Spearman's rho, and partial (controlling for IQ, ADOS, or ADHD symptoms) correlations between task performance and both ASD and ADHD symptoms in the autism group.
| Task Performance | ADOS Social+Communication (n=20) | ADHD Total (n =21) |
|---|---|---|
| Emotional go d′a | ||
| r | .01 | -.22 |
| rho | -.18 | -.28 |
| Partial rb | .01 | -.27 |
| Partial rc | -- | -.24 |
| Neutral go d′ | ||
| r | -.06 | -.50* |
| rho | -.01 | -.50* |
| Partial rb | -.07 | -.62* |
| Partial rd | -- | -.52* |
| Go RT | ||
| R | -.54* | .26 |
| Rho | -.49* | .23 |
| Partial rb | -.53* | -.23 |
| Partial rc | -.55* | -- |
| No-Go FA | ||
| r | .25 | .53* |
| Rho | .23 | .53* |
| Partial rb | .22 | .47* |
| Partial rd | -- | .50* |
Note. ADOS = Autism Diagnostic Observation Schedule–Module 3; ADHD = attention-deficit/hyperactivity disorder; ASD = autism spectrum disorder; RT = reaction time; FA = False Alarm.
Because group did not significantly interact with emotional facial expression, conditions were collapsed to generate a single (Emotional go and Neutral go d′, RT and FA, respectively) mean value.
After controlling for Full Scale IQ.
After controlling for ADOS Social+Communication Symptoms score.
After controlling for Total ADHD Symptoms score.
p<0.05
Figure 1.
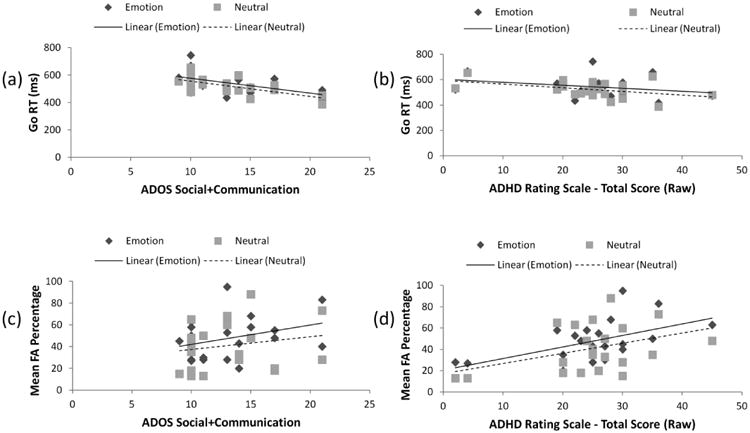
These scatterplots show the relationship between task performance and autism or ADHD symptoms for the autism group. (a) The mean Emotional and Neutral Go RTs and the ADOS Social+Communication scores. (b) The relationship between the mean Emotional and Neutral Go RTs and the ADHD rating scale total raw score. (c) The relationship between the mean Emotional and Neutral No-Go FA and the ADOS Social+Communication scores. (d) The relationship between the mean Emotional and Neutral No-Go FA and the ADHD rating scale total raw score.
Table 4.
Pearson's r, Spearman's rho, and partial (controlling for IQ or ADOS symptoms) correlations between task performance and both ADHD symptom dimensions in the autism group.
| Task Performance | Inattention | Hyperactivity/Impulsivity |
|---|---|---|
| Emotional Go d′a | ||
| r | .14 | .09 |
| Rho | .07 | .16 |
| Partial rb | -.24 | -.22 |
| Partial rc | -.17 | -.23 |
| Neutral Go d′ | ||
| r | -.28 | -.57* |
| Rho | -.29 | -.48* |
| Partial rb | -.47* | -.58* |
| Partial rc | -.31 | -.57* |
| Emotional Go RT | ||
| r | .20 | -.53* |
| Rho | -.07 | -.54* |
| Partial rb | -.16 | -.53* |
| Partial rc | -.12 | -.48* |
| Neutral Go RT | ||
| r | .37 | .53* |
| Rho | .30 | .56* |
| Partial rb | .26 | .56* |
| Partial rc | .33 | .51* |
| Emotional No-Go FA | ||
| r | .08 | .64* |
| Rho | .00 | .58* |
| Partial rb | .12 | .64* |
| Partial rc | .07 | .61* |
| Neutral No-Go FA | ||
| r | -.28 | -.57* |
| Rho | -.29 | -.48* |
| Partial rb | -.47* | -.58* |
| Partial rc | -.31 | -.57* |
Group did not significantly interact with emotional facial expression, so conditions were collapsed to generate a single (d′, RT and FA, respectively) “mean emotion” value.
After controlling for Total ADHD Symptoms Score
After controlling for ADOS Social+Communication Symptoms Score
p<0.05
This set of analyses demonstrated significant relationships between task performance and ASD or ADHD symptoms in the autism group. Those children who responded fastest to Go stimuli had more ASD symptoms, and this relationship was not explained by variability related to IQ or ADHD symptoms. Those children who made the most errors had higher parent ratings of ADHD symptoms, and this relationship was not explained by variability related to IQ or ASD symptoms.
Discussion
The present investigation examined whether higher-functioning children with autism would demonstrate impaired response inhibition performance in an emotional Go/No-Go task, and whether ADHD or ASD symptoms correlated with performance. The autism group was faster than the TDC group on all emotional trials. Moreover, within the autism group faster responses during emotional Go trials were correlated with a greater number of ASD symptoms. There was not a significant relationship between ADHD symptoms and emotional Go RT; however, there was a significant relationship between ADHD symptoms and accuracy during the condition where neutral expressions were Go trials and emotional expressions were No-Go responses. The autism group also showed a positive correlation between ADHD symptoms and impulsive responses to emotional and neutral No-Go stimuli. There was not a significant relationship between impulsive responses and ASD symptoms. Finally, ADHD symptoms did not explain the relationship between response speed and ASD symptoms, and ASD symptoms did not explain the relationship between impulsive responses or accuracy with ADHD symptoms. Controlling for Full Scale IQ either had minimal or no effect on group differences analyses or correlations. Taken as a whole, these findings indicate that the autism group's impairments on a social-emotional response inhibition task can be separated into social and cognitive control components, where each component correlates with ASD symptoms or ADHD symptoms, respectively.
Our finding of faster RTs in the autism group's than the TDC group differs from previous cognitive control investigations that combined inhibition with social-emotional stimuli (Dichter & Belger, 2007; Geurts et al., 2009; Vaidya et al., 2011); furthermore the negative correlation of Go RT and ASD symptoms, even when controlling for ADHD symptoms, is a novel contribution of the present study. There are at least three potential explanations for why the present study differs from prior investigations. One possible explanation relates to the social motivation hypothesis of autism (Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012; Dawson, Webb, & McPartland, 2005; Schultz, 2005). According to this account, a lack of motivation for social input may lead children with autism to disengage from, and thus respond more quickly to, social stimuli than TDCs. While speculative, the social motivation hypothesis also offers a common mechanism to explain the relationship between autism symptoms and RT performance – those with the lowest social motivation may exhibit the greatest number of autism symptoms and are least engaged by the social stimuli. It is also possible that the social motivation hypothesis could have explained decreased RT performance in the autism group, because they may be less motivated to respond. One future direction is to examine the relationship of social motivation to processing emotional facial expressions. A second potential explanation is that the autism group, particularly higher-functioning individuals, receive intense intervention around face and emotion identification, and the present study depicted fully expressed emotions with minimal complexity and subtlety that would be experienced in most real world interactions. While our paradigm attempted to increase ecological validity by contrasting multiple emotional faces with neutral faces, it may have also tapped into a highly trained skill in these school-age higher-functioning children. Thus the present sample may have an over-learned discrimination response supporting task performance. Indeed, the issue of face training influencing task performance has been raised previously regarding face emotion recognition in ASD (Harms et al., 2010). This explanation may extend to the correlation with symptoms by suggesting that those with the greatest number of autism symptoms may also receive the most intervention; however, it is unknown whether face emotion recognition is a top intervention priority, or whether children with the greatest number of ASD symptoms would definitively receive, and profit from, more intervention than others on the autism spectrum. Finally, a theoretically less interesting explanation for the reduced RT in the autism group is that they ignored facial stimuli and responded as quickly as possible to end the task and that as ASD symptom severity increased so did their drive to complete the task quickly. However, the main effect of emotion suggests that the autism and the TDC group were influenced by facial expression to a similar degree (i.e., slowest RT to angry and sad facial expressions). Future investigations may seek to address the first two potential explanations by examining the role of social motivation and face identification/emotion recognition training in emotional Go/No-Go performance (or other combined social-emotional and cognitive control paradigms).
Our results showed that as ADHD symptoms increased so did the FAs and d′ for neutral Go trials-emotional NoGo trials in the autism group, supporting the hypothesis that comorbid ADHD might relate to inhibition performance in autism. Moreover, there was a significant speed-accuracy trade-off observed in the autism group, as emotional facial expression FAs and neutral Go RTs or neutral facial expression FAs and emotional Go RTs were negatively correlated. Neutral and emotional FAs and neutral Go RT correlated with total ADHD symptoms, suggesting that children's impulsive responses were related to attention impairments. These correlations converge with recent evidence that ADHD symptoms in children with ASD lead to increased impairments in: inhibition tasks such as Go/No-Go (Sinzig, Morsch, Bruning, et al., 2008); standardized neuropsychological measures of interference suppression and response inhibition (Corbett et al., 2009); and everyday measures of inhibition (Yerys et al., 2009). This ADHD symptom-performance relationship is not specific to ASD, and is also present in children with ADHD without ASD (Nikolas & Nigg, 2012), and other disorders with a high comorbidity rate of ADHD like Fragile X syndrome (Knox et al., 2012). Two studies have shown statistically non-significant differences in lab-based inhibition performance among children with ASD and elevated ADHD symptoms compared to children with ASD alone (Sinzig, Bruning, Morsch, & Lehmkuhl, 2008; Yerys et al., 2009). However, medium effect sizes were reported in both studies indicating that these studies were underpowered to detect group differences. Taken together, this line of research suggests that ADHD symptoms are likely playing a role in the presentation of inhibition deficits for children on the autism spectrum.
Perhaps the most compelling findings are the potentially independent contributions of ASD and ADHD symptoms to emotional Go/No-Go performance. These findings in the same cohort contribute to an emerging debate about the nature of ASD and ADHD comorbidity. A comprehensive review by Rommelse and colleagues (2011) on ASD and ADHD comorbidity narrowed 10 potential explanatory models down to two plausible accounts. One model is pleiotropic where ASD and ADHD share overlapping risk genes and endophenotypes as well as independent risk genes and endophenotypes; the shared genes influence both ASD and ADHD. The other model argues for ADHD and ASD as different manifestations within a single overarching developmental disorder, such that ADHD without social communication problems represents the mildest presentation of the disorder and ADHD+ASD as the most severe. The correlations of ASD symptoms with emotional Go RT and ADHD symptoms with FAs without cross-correlations (i.e., ASD symptoms with FAs or ADHD symptoms with emotional Go RT) combined with the significant partial correlations that control for variance related to symptoms of the other disorder, suggests that processing social-emotional information and withholding prepotent responses may represent independent dimensions of behavior (i.e., endophenotypes). This pattern of findings supports the pleiotropic model where ADHD and ASD may have overlapping and independent risk genes and endophenotypes that converge in children presenting with comorbid symptoms. Furthermore, a secondary analysis reveals that Hyperactivity/Impulsivity symptoms were more related to emotional Go/No-Go performance than Inattention symptoms, and this provides new insight into our understanding of the overlap between ASD and ADHD. The stated symptoms of each disorder in the DSM do not appear to have significant overlap, for example “acts as if driven by a motor” in ADHD vs. “lack of social emotional reciprocity” in ASD. However, it is hard to ignore that many ADHD symptoms are rooted in social contexts, such as interrupting others, can't wait his/her turn, gets up from his/her seat when expected to remain seated, and often does not seem to listen when spoken to directly. Thus, it is possible that many of these ADHD symptoms may relate to impairments in understanding social demands rather than inattention and hyperactivity/impulsivity deficits. With that said, prior findings of impulsive responding on traditional Go/No-Go tasks (Christ et al., 2007; Corbett et al., 2009; Johnson et al., 2007) and our findings suggest that these impairments are not simply accounted for by social deficits but are linked specifically to Hyperactivity/Impulsivity symptoms of ADHD.
In summary, the present study provides evidence that a higher-functioning autism group responds faster to social-emotional stimuli in the context of cognitive control demands and this relates to social-communication symptoms, while their excessive impulsive responses (i.e., FAs) relate to ADHD symptoms. The significant partial correlations suggest independent influence of the symptoms from each disorder on performance. A challenge to our findings is that the study did not include an emotion recognition task to demonstrate equal performance in emotion identification; however, the study's main goal was to examine social-emotional processing in the context of cognitive control demands. To that end, only extreme facial expressions were included to optimize accuracy. Prior research suggests that higher-functioning autism groups are more likely to exhibit intact face emotion recognition than lower functioning children (Harms et al., 2010). The FA rate in the conditions where emotional facial expressions were the No-Go response may appear higher in both groups than what we would have expected given prior Go/No-Go findings; however, our mean FA is within 1 standard deviation of at least one prior study (Christ et al., 2007). Finally, the present study included only children meeting stringent DSM-IV autism criteria rather than the broader autism spectrum; the goal of including such a rigorously identified sample was to alleviate potential concerns that our clinical group included mildly affected children that might be considered by some to have a primary diagnosis of ADHD with mild social impairments. Future research should extend the present findings by continuing to probe the intersection of cognitive control and social-emotional processing to further our understanding of how core social-communication impairments contribute to self-regulatory behavior. For example, more work is needed to examine the influence of ADHD symptoms on the cognitive control profile in children on the autism spectrum, and mirroring this approach, future investigations in ADHD can also examine whether social communication impairments influence the ADHD cognitive control profile. These avenues of research can assist in elucidating common and independent endophenotypes for ASD and ADHD.
Acknowledgments
We thank all the families for volunteering their time and effort to participate in our studies. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Mental Health, Isadore and Bertha Gudelsky Foundation, the Elizabeth and Frederick Singer Foundation, and the National Institute of Mental Health - Award Numbers: K23MH086111 (PI: BEY), R21MH092615 (PI: BEY). We thank Rachel Weinblatt and Jennifer Sokoloff from the Center for Autism Spectrum Disorders at Children's National Medical Center for their assistance in data collection. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR Fourth Edition. 4th. American Psychiatric Publishing, Inc; 2000. [Google Scholar]
- Bal E, Harden E, Lamb D, Van Hecke AV, Denver JW, Porges SW. Emotion recognition in children with autism spectrum disorders: relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders. 2010;40(3):358–370. doi: 10.1007/s10803-009-0884-3. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Reviews Neuroscience. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012 doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ SE, Holt DD, White DA, Green L. Inhibitory control in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2007;37(6):1155–1165. doi: 10.1007/s10803-006-0259-y. [DOI] [PubMed] [Google Scholar]
- Christ SE, Kester LE, Bodner KE, Miles JH. Evidence for selective inhibitory impairment in individuals with autism spectrum disorder. Neuropsychology. 2011 doi: 10.1037/a0024256. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Research. 2009;166(2-3):210–22. doi: 10.1016/j.psychres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15(3):331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Belger A. Social stimuli interfere with cognitive control in autism. NeuroImage. 2007;35(3):1219–30. doi: 10.1016/j.neuroimage.2006.12.038. doi:S1053-8119(06)01242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AE, Faraone SV, Seidman LJ, Willcutt EG, Nigg JT, Waldman ID, et al. Biederman J. Are endophenotypes based on measures of executive functions useful for molecular genetic studies of ADHD? Journal of Child Psychology and Psychiatry. 2005;46(7):774–803. doi: 10.1111/j.1469-7610.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklists, norms, and clinical interpretation. Guilford Press; New York: 1998. [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto, SF: Consulting Psychologist Press; 1976. [Google Scholar]
- Gadow KD, Sprafkin J. Childhood Symptom Inventory - Fourth Edition. Stony Brook, NY: Checkmate Plus; 2000. [Google Scholar]
- Gadow KD, Sprafkin J. Child & Adolescent Symptom Inventory - Fourth Edition Revised. Stony Brook, NY: Checkmate Plus; 2010. [Google Scholar]
- Geurts HM, Begeer S, Stockmann L. Brief report: inhibitory control of socially relevant stimuli in children with high functioning autism. Journal of Autism and Developmental Disorders. 2009;39(11):1603–1607. doi: 10.1007/s10803-009-0786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MB, Martin A, Wallace GL. Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychology Review. 2010;20:290–322. doi: 10.1007/s11065-010-9138-6. [DOI] [PubMed] [Google Scholar]
- Kenworthy L, Yerys BE, Anthony LG, Wallace GL. Understanding executive control in autism spectrum disorders in the lab and in the real world. Neuropsychology Review. 2008;18(4):320–38. doi: 10.1007/s11065-008-9077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox A, Schneider A, Abucayan F, Hervey C, Tran C, Hessl D, Berry-Kravis E. Feasibility, reliability, and clinical validity of the Test of Attentional Performance for Children (KiTAP) in Fragile X syndrome (FXS) Journal of neurodevelopmental disorders. 2012;4(1):2. doi: 10.1186/1866-1955-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Axelson DA, Ryan ND, Casey BJ. Processing emotional facial expressions influences performance on a Go/NoGo task in pediatric anxiety and depression. Journal of Child Psychology and Psychiatry. 2006;47(11):1107–1115. doi: 10.1111/j.1469-7610.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, et al. Lainhart JE. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. Journal of Autism and Developmental Disorders. 2006;36(7):849–61. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–23. doi:11055457. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–85. doi: 10.1007/BF02172145. doi:7814313. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological Psychiatry. 2007;61(4):474–81. doi: 10.1016/j.biopsych.2006.02.030. doi:S0006-3223(06)00381-7. [DOI] [PubMed] [Google Scholar]
- Nigg J, Casey B. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Nikolas MA, Nigg JT. Neuropsychological performance and attention-deficit hyperactivity disorder subtypes and symptom dimensions. Neuropsychology. 2012 doi: 10.1037/a0030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL. Inhibitory function in nonretarded children with autism. Journal of Autism and Developmental Disorders. 1997;27(1):59–77. doi: 10.1023/a:1025821222046. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL, McMahon WM, Filloux F. Executive function abilities in autism and Tourette syndrome: an information processing approach. Journal of Child Psychology and Psychiatry. 1994;35(6):1015–32. doi: 10.1111/j.1469-7610.1994.tb01807.x. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Rommelse NNJ, Geurts HM, Franke B, Buitelaar JK, Hartman CA. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neuroscience and Biobehavioral Reviews. 2011 doi: 10.1016/j.neubiorev.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Rumsey JM. Conceptual problem-solving in highly verbal, nonretarded autistic men. Journal of Autism and Developmental Disorders. 1985;15(1):23–36. doi: 10.1007/BF01837896. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23(2-3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Bruning N, Morsch D, Lehmkuhl G. Attention profiles in autistic children with and without comorbid hyperactivity and attention problems. Acta Neuropsychiatrica. 2008;20(4):207–215. doi: 10.1111/j.1601-5215.2008.00292.x. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Morsch D, Bruning N, Schmidt MH, Lehmkuhl G. Inhibition, flexibility, working memory and planning in autism spectrum disorders with and without comorbid ADHD-symptoms. Child and Adolescent Psychiatry and Mental Health. 2008;2(1):4. doi: 10.1186/1753-2000-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinzig J, Morsch D, Lehmkuhl G. Do hyperactivity, impulsivity and inattention have an impact on the ability of facial affect recognition in children with autism and ADHD? European Child & Adolescent Psychiatry. 2008;17(2):63–72. doi: 10.1007/s00787-007-0637-9. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Foss-Feig J, Shook D, Kaplan L, Kenworthy L, Gaillard WD. Controlling attention to gaze and arrows in childhood: an fMRI study of typical development and Autism Spectrum Disorders. Developmental Science. 2011;14(4):911–924. doi: 10.1111/j.1467-7687.2011.01041.x. [DOI] [PubMed] [Google Scholar]
- Waters AM, Valvoi JS. Attentional bias for emotional faces in paediatric anxiety disorders: An investigation using the emotional go/no go task. Journal of Behavior Therapy and Experimental Psychiatry. 2009;40(2):306–316. doi: 10.1016/j.jbtep.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wechsler D. Wechsler Scales of Intelligence - Fourth Edition. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57(11):1336–46. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Yerys BE, Wallace GL, Sokoloff JL, Shook DA, James JD, Kenworthy L. Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Research. 2009;2(6):322–333. doi: 10.1002/aur.103. [DOI] [PMC free article] [PubMed] [Google Scholar]


