Abstract
Neural oscillations are ubiquitous in olfactory systems of mammals, insects and molluscs. Neurophysiological and computational investigations point to common mechanisms for gamma or odor associated oscillations across phyla (40–100 Hz in mammals, 20–30 Hz in insects, 0.5–1.5 Hz in molluscs), engaging the reciprocal dendrodendritic synapse between excitatory principle neurons and inhibitory interneurons in the olfactory bulb, antennal lobe, or procerebrum. Recent studies suggest important mechanisms that may modulate gamma oscillations, including neuromodulators and centrifugal input to the olfactory bulb and antennal lobe. Beta (20 Hz) and theta (2–12 Hz) oscillations coordinate activity within and across brain regions. Olfactory beta oscillations are associated with odor learning and depend on centrifugal olfactory bulb input, while theta oscillations are strongly associated with respiration.
Introduction
Oscillations abound in cortical circuits. If excitatory and inhibitory neurons get together in large, interconnected groups, oscillations happen. Oscillations that have become the hallmark of olfactory areas in all vertebrate species so far examined have analogous counterparts in arthropods and molluscs. The olfactory circuit has evolved separately across phyla [1], which suggests that oscillations may be a very good solution to a neural processing problem.
This review addresses circuitry and other mechanisms that support neural oscillations within and across three. Most research has been focused on the mammalian system, but there are important lessons to be learned from reaching across the taxonomic aisles. In fact, this reach has so far enabled a deeper mechanistic and functional understanding of odor-evoked gamma oscillations.
Local sensory processing and coordination of neurons
Olfactory bulb (OB) gamma oscillations, which initiate on the transition from inhalation to exhalation, were the first cortical oscillations described in detail (Fig. 1). Lord Adrian detailed these oscillations recorded from hedgehogs, cats and rabbits with frequencies around 40 Hz. He noted both induced (by odors) and evoked (spontaneous) waves recorded from electrodes on the surface of the olfactory bulb [2]. The gamma oscillation circuitry was first described for pyriform cortex (PC) by Freeman [3], and a few years later for the OB by Rall and Shepherd [4]. We now know that fast (gamma) oscillations in the mammalian OB range from 40 to 100 Hz or more and that the frequency varies across species [5]. In cortical systems olfactory oscillations arise in isolated frequency bands out of the 1/f (log(power)/log(frequency)) background cortical activity (Fig. 1a; [6]). OB gamma oscillations are supported by the reciprocal dendrodendritic synapse between glutamatergic mitral or tufted (MT) cells and GABAergic granule cells (GCs). MT cells’ firing probability matches gamma oscillations, so the oscillation represents relative precision among mitral cells (reviewed in [7]).
Figure 1. Rhythms in the mammalian olfactory bulb.
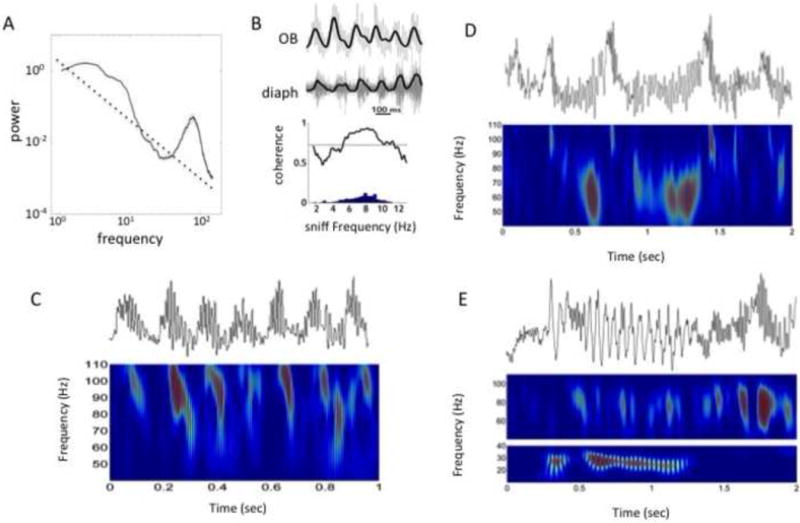
A. 1/f power spectrum with deviations for theta beta and gamma rhythms from rat OB. B. Gamma and theta rhythms recorded from the rat OB. Seven respiratory cycles are shown by the low frequency high amplitude wave in which the peak is the end of inhalation. The gamma rhythm begins at the peak with a high frequency oscillation giving way to a lower frequency one, sweeping form above 90 Hz to near 70 Hz. Top panel is the LFP trace and bottom panel is a wavelet spectrogram of the gamma band. C. LFP theta rhythms are coherent with respiratory drive at all waking respiratory frequencies. Left: OB LFP with theta filtered signal overlaid. The diaphragm EMG signal is rectified and smoothed, which shows the similarity with the simultaneously recorded OB theta rhythm. Right: Coherence between the two signals from one rat (histogram of respiratory frequencies on bottom of plot); horizontal line is the significance floor for coherence. Figure modified from [47] with permission. D. Gamma2 example from the rat OB. During slower respiration in alert rats, a low frequency high amplitude bursts in the 50–60 Hz range are seen. Wavelet spectrogram is shown and the frequency of bursts is lower than the end of the gamma sweep in A. E. Beta rhythm example recorded from the mouse OB in response to sniffing a highly volatile odor as in [55]. Wavelet spectrograms in the gamma and beta bands below (color scale in top is 1/10 that of the bottom to emphasize bursts).
Odor induced oscillations occur in non-mammalian vertebrate OBs (frogs, zebrafish, turtles, salamanders), in the insect antennal lobe (AL; locusts, bees, moths, drosophila) and mollusc procerebrum (terrestrial slugs and snails). Frequencies vary widely across species (~20 Hz in insects [8], ~30 Hz in zebrafish [9], 15 Hz in salamanders [10], 7–15 Hz in turtles [11,12], ~10 Hz in frogs [13], ~0.7 Hz in Limax [14,15]; Fig. 2). These oscillations are analogous for two reasons: 1) they are elicited by exposure to odorants, and 2) they are supported primarily by interactions between the principal excitatory neurons (MT cells in vertebrates, projection neurons in insects) and inhibitory neurons (granule cells and other OB GABAergic cells, GABAergic local neurons in insects). The spike patterns of the principal neurons conform to a restricted range of phases, around 90° before the peak of the LFP oscillation, and do not change phase in response to odor exposure or any other measured behavioral event. Frequencies associated with a given species are an intrinsic feature, the result of biophysical properties of the neurons and synapses involved. Freeman’s classic book, Mass Action in the Nervous System [6], describes influences by both negative and positive feedback and deep computational insight on oscillations and their frequencies.
Figure 2. Olfactory systems from 3 phyla.
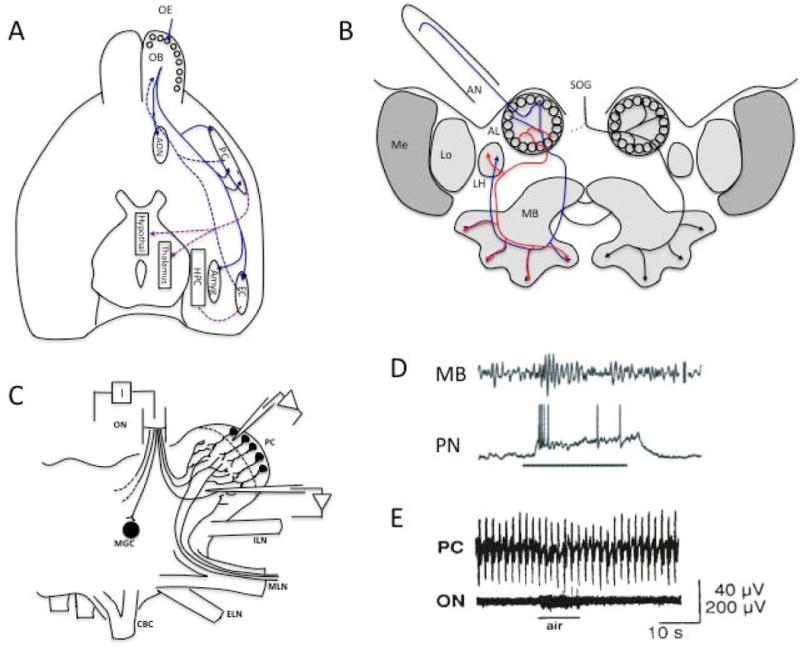
A. The mammalian central olfactory system begins with the OB, which receives olfactory nerve input from the olfactory epithelium (OE) in the glomeruli around the periphery of the bulb. Primary olfactory cortex is primarily represented by the anterior olfactory nucleus (AON) [64]. The pyriform cortex (PC) is a higher order sensory association cortex [65,66], but it is often referred to as primary olfactory cortex. The OB also projects to the multimodal entorhinal cortex (EC), which sends fibers into the hippocampus (HPC), and the amygdala (amyg) among other limbic and subcortical areas. The PC projects to the EC, hypothalamus and thalamus, as well as other higher order areas. Most OB connections to other brain regions are bidirectional. Centrifugal projections to the OB synapse primarily onto GABAergic GCs in the deep layers, except for the AON, which targets superficial juxtaglomerular cells and mitral cells [67]. (Figure adapted from [68].) B. The honeybee central olfactory system begins with antennal nerve (AN) input to the antennal lobe (AL) projection neurons in peripheral glomeruli. AL neurons project to the mushroom body (MB), a higher order multimodal area, and the lateral horn (LH). Descending modulatory input associated with appetitive state comes from the VUM-mx optopaminergic neuron in the subesophogeal ganglion (SOG). (Figure adapted from [69]). C. The limax cerebral ganglion receives olfactory nerve (ON) input to glomeruli in the procerebrum (PC). The PC also receives input from the medial lip nerve (MLN) in the inferior nose. (Figure adapted from [15] with permission.) D. Odor evoked oscillations (~20 Hz) are recorded in the locust mushroom body (MB) but are produced by axon terminals from the antennal lobe (AL) projection neurons (PN). Simultaneously recorded PN shows depolarization, odor evoked spikes and subthreshold oscillations. (Figure adapted from [26], with permission). E. Procerebral lobe oscillations in limax showing the <1Hz oscillation typical of this species. (Figure adapted from [15] with permission.)
Physiological analyses of brain slices are powerful tools for studying gamma oscillation circuits in the mammalian OB. Several studies have focused on the role of NMDA and AMPA receptors in setting GABA release kinetics at the dendrodendritic synapse [16,17] and intrinsic oscillatory properties of MT cells independent of GC spiking [18]. There is disagreement as to whether AMPA or NMDA receptors dominate GABA release at the reciprocal synapse, which has important implications for inhibition timing. When input fibers from the olfactory nerve are stimulated with a single shock, GC NMDA receptors dominate GABA release [19]; with 4 Hz stimulation simulating sniffing, AMPA receptors dominate [16]. AMPA receptors depolarize GC dendrites very quickly allowing short latency and duration GABA release. MT cells can produce gamma oscillations even in the absence of GC bodies meaning that reciprocal inhibition does not require GC spiking [20]. This feature, coupled with the fast action of GC AMPA receptors during sniffing, may support gamma oscillations in waking rats and mice [21].
Invertebrate studies provide rich insight into oscillations and odor responses. Odor induced oscillations produced in the insect AL are measured from the mushroom body where AL axon terminals produce coherent excitatory drive (Fig. 2b,d). Much of the early work was done in locusts, but this has been expanded to other species, including drosophila [22,23]. Within the oscillation, individual neurons fire on identified cycles and are constrained to a defined phase within the cycle, similar to MTs in anesthetized rabbits [24,25]. Thus, odors are not represented by firing relative to the phase of the fast oscillation but rather by what neurons fire on which cycles. When oscillations are disrupted by applying picrotoxin to the AL disabling fast GABAergic transmission, the neurons that read these uncoordinated spikes in the beta lobe respond more promiscuously to odors [26].
The molluscan system has a much lower oscillatory frequency (<1Hz), but the circuit that produces the oscillation is analogous to the mammalian and insect systems with reversed action of glutamate and GABA [27]. Olfactory nerve input targets projection neurons in the procerebral glomeruli, and these neurons interact with local inhibitory circuits to produce the oscillations (Figs. 2c,e). Oscillation frequency can vary dependent on odors, learning and neurotransmitter levels [15,27,28].
Multiple gammas
As described above, gamma oscillations can vary widely in frequency across species. However, within a species or group of species variations in fast oscillations can be informative. For example, in rats and mice there are two main bands of gamma oscillations. Gamma1 oscillations are those above 60 Hz and are what we commonly think of as evoked or induced gamma initiating at the transition from inhalation to exhalation. High amplitude, narrow band gamma2 oscillations (50–55 Hz) occur in behavioral states such as alert immobility are strongly coherent with pyriform cortex [29]. They do not occur in β3 knockout mice that lack GABAA receptors on OB GCs.
A further subdivision of gamma1 oscillations can be made. Gamma1 oscillations slow down after the first part of exhalation. Within the OB, mitral and tufted cells fire at different phases of the respiratory cycle. Tufted cells get direct olfactory nerve input [30] and fire at the peak of inhalation with little variation [31]; mitral cells receive indirect nerve input and fire in the early expiratory phase with significant variability driven by local inhibition. These different activation times may correspond to the two types of gamma1 oscillations, with faster oscillations driven by tufted cells and slower oscillations by mitral cells [32]. These two sets of neurons project to different pathways, so the two types of gamma1 oscillations may represent targeting of different neural pathways.
Functional role and modulation of gamma oscillations
Several studies have addressed and solidified the functional role of gamma oscillations, and here again is where we cross phylogenetic lines [33–35]. When oscillations are blocked in the honeybee AL, projection neurons maintain their slow temporal activation patterns in response to odors, but fine temporal coordination is lost [33]. Behaviorally, these honeybees are deficient in discriminating closely related but not more different odors. In the β3 knockout mouse the β3 subunit of the GABAA receptor is knocked out, which in the OB selectively deletes the functional GABAA receptors on GCs. These mice have abnormally large gamma oscillations without increased firing rates among MT cells, which means that they are much more precise in their timing relative to each other and the LFP oscillation [34]. These mice are deficient in generalizing among similar odors. Finally, when rats are challenged with difficult discriminations, gamma oscillations in the OB increase dramatically associated with performance increases [35].
We can view gamma oscillations as a simple negative feedback relationship between excitatory and inhibitory units, but stopping at this point would miss the exquisite circuitry that modifies these oscillations in behavioral contexts and provides converging evidence to support gamma oscillations’ functional properties (Fig. 3). First, centrifugal inputs to OB GABAergic deep short axon cells [36,37] and GCs desynchronize gamma oscillations. Ablating feedback to the OB greatly increases the amplitude of gamma oscillations and tightens MT cell firing precision [38,39]. Another source of gamma desynchronization is inhibition of GCs via GABAA receptors which can come from many sources, both intrinsic to the OB and from the basal forebrain [36,40,41].
Figure 3. A. Olfactory bulb circuitry associated with oscillations.
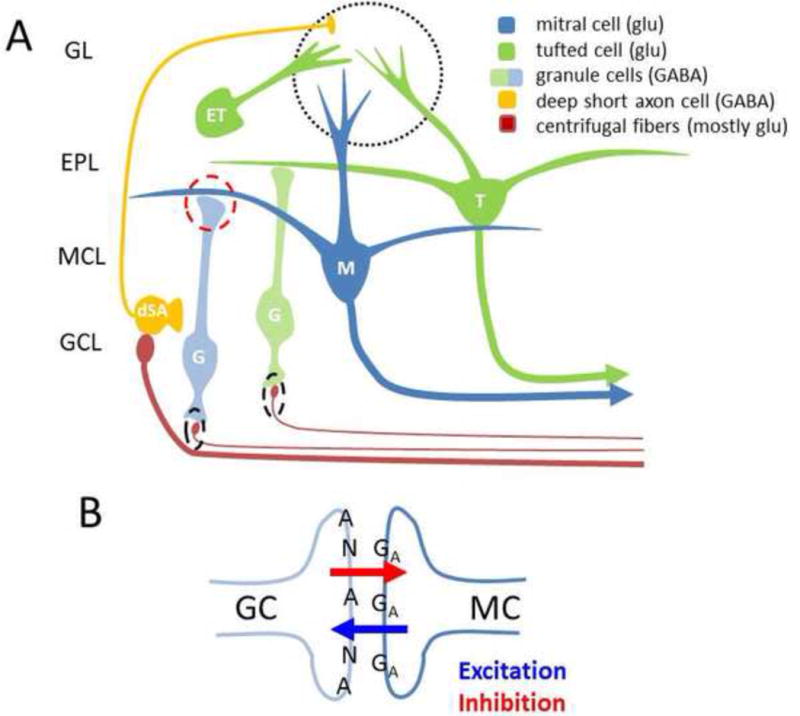
Over the past few years, the canonical picture of OB circuitry has changed and some of the changes may have implications on mechanism and modification of OB oscillations: 1) Olfactory nerve input (not shown) targets external tufted (ET) cells directly (and possibly all tufted cells within glomeruli [31]) and then MT cells via excitatory inputs from ETs and inhibitory relays from ETs to periglomerular (PG) cells (not shown) to MT cells. ETs fire in bursts that can match the respiratory rhythm and may support theta oscillations [70]. One population of GABAergic deep short axon cells (dSAC) target PG cells [36]. Pyriform cortex input targets dSACs [37]. B. Reciprocal synapse as shown in the red dashed circle in A is on the distal dendrites of GCs and may support local graded inhibition through AMPA receptors with ~4.2 ms rise times [17]. This mechanism may support gamma oscillations independent of GC spikes [21]. NMDA and AMPA receptors are present on GCs at most synapses. About 25% are NMDA silent and a very small number are AMPA silent. GABAA receptors mediate inhibition of MTs. Synapses proximal to the GC soma from centrifugal axon fibers also have NMDA and AMPA receptors at most of these synapses (dashed black ovals). Activation of GCs by stimulating these fibers produces GABA release at the reciprocal synapse with faster rise times (1.3 ms). Additional abbreviations: GL- glomerular layer, EPL- external plexiform layer, MCL- mitral cell layer, GCL-granule cell layer.
Neuromodulators can also play a large role in adjusting the power of gamma oscillations; many of them act either at the OB reciprocal synapse or at the synapses from centrifugal input. Acetylcholine in the frog OB increases gamma power; in models of the pyriform cortex it is predicted to decrease gamma power [13,42]. In rats, drugs that increase cholinergic action at either muscarinic or nicotinic receptors decrease odor generalization, and those that decrease cholinergic action decrease odor discrimination [43]. In other cortical systems, acetylcholine is assigned to attention modulation signified by increased gamma power [44]. While this may be an oversimplification, it addresses neural mechanisms of attention.
Gamma oscillations are large and interesting, and we want them to be important, but there can be too much of a good thing. β3 mice discriminate too much; this can be bad for survival if they can’t generalize to similar odors when foraging or detecting a predator. A recent study combining slice physiology and modeling addresses the impact of heterogeneity in MT cells and shows that differences in phase resetting among neurons accounts for the asynchrony of neurons and oscillations [45]. Heterogeneity then provides what the authors refer to as a plastic substrate, allowing cells to flexibly enter and exit correlated assemblies of neurons, as Freeman hypothesized some time ago [46].
Processes that organize activity and link olfactory areas
Gamma oscillations are relatively local phenomena that represent neural firing precision in the OB. Slower cortical rhythms, theta (2–12 Hz) and beta (15–30 Hz) oscillations, may organize local activity and link brain areas.
In terrestrial mammals, breathing and olfaction are linked by sniffing, which an individual uses to gather odor stimuli from the environment and present them to the olfactory epithelium and receptor neurons. Most research on sniffing has been carried out in rats and mice. Humans do not perform rhythmic repetition of sniffs, so I will concentrate on rhythmic properties associated with respiratory rhythm in rodents.
The rat OB theta rhythm tracks the respiratory cycle at every frequency, which incorporates the motor signal within OB sensory processing [47]. MT cells were shown some time ago to fire at preferred phases all along the respiratory cycle which can change with learning [48,49]. The phase preference was recently reconfirmed with more technical power [50]. However, when rats sniff at high rates, as they do during odor discrimination, MT cells uncouple from respiration [48,49,51], which makes respiratory phase coding somewhat unreliable except in conditions of slow breathing associated with odor habituation. However, the respiratory cycle does, under many circumstances, group spikes into sniffs, and these cycles are intermittently coherent with the same rhythm in pyriform cortex and hippocampus [52–55]. Insects may have their own sniff cycle associated with sensorimotor processes. The moth AL and olfactory nerve responds optimally to odor pulses that cover their wing beat range of 18–20 Hz [56].
Of all the olfactory oscillation bands, beta oscillations are most coherent across areas [55,57–59] and occupy a very narrow frequency band centered about 20 Hz in mammals [60]. We know very little about the mechanisms of olfactory system beta oscillations, except that they require centrifugal input to the OB [39]. Beta oscillations are associated with operant odor learning and vary in power dependent on odor volatility [55,57,60]. In anesthetized rats, beta oscillations have a defined relationship with the respiratory rhythm, occurring during late exhalation [61]. It is not known whether this respiratory phase relationship persists during waking states.
Because beta oscillations depend on centrifugal feedback to the OB, it might be assumed that they do not have an analog in invertebrate olfactory systems. However, there is abundant centrifugal input to the insect AL, and odor responses in the mushroom body can precede the final sorting out of responses in the AL [62]. A recent study in terrestrial snails shows that the normal oscillation associated with odors slows down by as much as 40% in response to aversive odors following tentacle withdrawal [63]. Might these be molluscan beta oscillations?
Conclusion
Seven decades of research using many approaches at many levels in three different phyla have contributed to a deep and nuanced understanding of olfactory system gamma oscillations, even though there is still more to learn. Oscillations that organize and link activity within and across brain areas, theta and beta oscillations, are less well understood and will require broad and convergent studies across phyla to understand their mechanisms and functional roles.
What we have learned from gamma oscillations is to focus on the circuit, the behavior and the functional relationships to define them, not the frequency. As the scope of our knowledge about these enigmatic processes expands, we may want to consider revising definitions of these oscillations to focus not on frequency but rather on the function and the circuit.
Highlights.
Odor evoked oscillations rely on similar mechanisms across several phyla.
Slower oscillations help group activity within the olfactory bulb and link brain areas.
Olfactory bulb or antennal lobe centrifugal input may adjust inhibition modes and frequencies.
Acknowledgments
The author thanks Donald Frederick for help with MATLAB programming for Figure 1. Funding: NIDCD R01 DC014367 to LMK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Nothing to declare
References
- 1.Eisthen HL. Why are olfactory systems of different animals so similar? [Internet] Brain Behav Evol. 2002;59:273–293. doi: 10.1159/000063564. [DOI] [PubMed] [Google Scholar]
- 2.Adrian ED. The electrical activity of the mammalian olfactory bulb. EEG Clin Neurophysiol. 1950;2:377–388. doi: 10.1016/0013-4694(50)90075-7. [DOI] [PubMed] [Google Scholar]
- 3.Freeman WJ. Linear distributed feedback model for prepyriform cortex. Exp Neurol. 1964;10:525–547. doi: 10.1016/0014-4886(64)90049-4. [DOI] [PubMed] [Google Scholar]
- 4.Rall W, Shepherd GM. Theoretical reconstruction dendrodendritic of rield potentials and in olfactory bulb synaptic interactions. J Neurophysiol. 1968;31:884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- 5.Bressler SL, Freeman WJ. Frequency analysis of olfactory system EEG in cat, rabbit, and rat [Internet] Electroencephalogr Clin Neurophysiol. 1980;50:19–24. doi: 10.1016/0013-4694(80)90319-3. [DOI] [PubMed] [Google Scholar]
- 6.Freeman WJ. Mass Action in the Nervous System. Academic Press; 1975. [Google Scholar]
- 7.Rojas-Líbano D, Kay LM. Olfactory system gamma oscillations: the physiological dissection of a cognitive neural system. Cogn Neurodyn. 2008;2:179–194. doi: 10.1007/s11571-008-9053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurent G, Davidowitz H. Encoding of olfactory information with oscillating neural assemblies. Science (80-) 1994;265:1872–1875. doi: 10.1126/science.265.5180.1872. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich RW, Laurent G. Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science (80-) 2001;291:889–894. doi: 10.1126/science.291.5505.889. [DOI] [PubMed] [Google Scholar]
- 10.Dorries KM, Kauer JS. Relationships between odor-elicited oscillations in the salamander olfactory epithelium and olfactory bulb [Internet] J Neurophysiol. 2000;83:754–765. doi: 10.1152/jn.2000.83.2.754. [DOI] [PubMed] [Google Scholar]
- 11.Boudreau JC, Freeman WJ. Olfactory bulb response in turtle. Nature. 1962;193:782–783. [Google Scholar]
- 12.Lam Y-W, Cohen LB, Wachowiak M, Zochowski MR. Odors elicit three different oscillations in the turtle olfactory bulb [Internet] J Neurosci. 2000;20:749–762. doi: 10.1523/JNEUROSCI.20-02-00749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall B, Delaney K. Cholinergic modulation of odor-evoked oscillations in the frog olfactory bulb [Internet] Biol Bull. 2001;201:276–277. doi: 10.2307/1543363. [DOI] [PubMed] [Google Scholar]
- 14.Kleinfeld D, Delaney KR, Fee MS, Flores JA, Tank DW, Gelperin A. Dynamics of propagating waves in the olfactory network of a terrestrial mollusk: an electrical and optical study [Internet] J Neurophysiol. 1994;72:1402–1419. doi: 10.1152/jn.1994.72.3.1402. [DOI] [PubMed] [Google Scholar]
- 15.Gelperin A, Tank DW. Odour-modulated collective network oscillations of olfactory interneurons in a terrestrial mollusc. Nature. 1990;345:437–440. doi: 10.1038/345437a0. [DOI] [PubMed] [Google Scholar]
- 16.Schoppa NE. AMPA/Kainate receptors drive rapid output and precise synchrony in olfactory bulb granule cells [Internet] J Neurosci. 2006;26:12996–13006. doi: 10.1523/JNEUROSCI.3503-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balu R, Pressler RT, Strowbridge BW. Multiple modes of synaptic excitation of olfactory bulb granule cells [Internet] J Neurosci. 2007;27:5621–5632. doi: 10.1523/JNEUROSCI.4630-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagier S, Carleton A, Lledo P-M. Interplay between local GABAergic interneurons and relay neurons generates gamma oscillations in the rat olfactory bulb [Internet] J Neurosci. 2004;24:4382–4392. doi: 10.1523/JNEUROSCI.5570-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoppa NE, Kinzie JM, Sahara Y, Segerson TP, Westbrook GL. Dendrodendritic inhibition in the olfactory bulb is driven by NMDA receptors [Internet] J Neurosci. 1998;18:6790–6802. doi: 10.1523/JNEUROSCI.18-17-06790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bathellier B, Lagier S, Faure P, Lledo P-M. Circuit properties generating gamma oscillations in a network model of the olfactory bulb [Internet] J Neurophysiol. 2006;95:2678–2691. doi: 10.1152/jn.01141.2005. [DOI] [PubMed] [Google Scholar]
- 21.Brea JN, Kay LM, Kopell NJ. Biophysical model for gamma rhythms in the olfactory bulb via subthreshold oscillations [Internet] Proc Natl Acad Sci U S A. 2009;106:21954–21959. doi: 10.1073/pnas.0910964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kay LM, Stopfer M. Information processing in the olfactory systems of insects and vertebrates [Internet] Semin Cell Dev Biol. 2006;17:433–442. doi: 10.1016/j.semcdb.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka NK, Ito K, Stopfer M. Odor-evoked neural oscillations in Drosophila are mediated by widely branching interneurons [Internet] J Neurosci. 2009;29:8595–8603. doi: 10.1523/JNEUROSCI.1455-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashiwadani H, Sasaki YF, Uchida N, Mori K. Synchronized oscillatory discharges of mitral/tufted cells with different molecular receptive ranges in the rabbit olfactory bulb [Internet] J Neurophysiol. 1999;82:1786–1792. doi: 10.1152/jn.1999.82.4.1786. [DOI] [PubMed] [Google Scholar]
- 25.Wehr M, Laurent G. Odour encoding by temporal sequences of firing in oscillating neural assemblies. Nature. 1996;384:162–166. doi: 10.1038/384162a0. [DOI] [PubMed] [Google Scholar]
- 26.MacLeod K, Laurent G. Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science (80-) 1996;274:976–979. doi: 10.1126/science.274.5289.976. [DOI] [PubMed] [Google Scholar]
- 27*.Kobayashi S, Matsuo R, Sadamoto H, Watanabe S, Ito E. Excitatory effects of GABA on procerebrum neurons in a slug [Internet] J Neurophysiol. 2012;108:989–998. doi: 10.1152/jn.01137.2010. In insects neurotransmitters often have different actions than they do in vertebrate systems. This paper shows that GABA has excitatory effects on the system via GABA-B receptors. This suggests that evolutionary pressure occurs at the level of the circuit activity, not the individual receptor types. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe S, Kirino Y, Gelperin A. Neural and molecular mechanisms of microcognition in Limax [Internet] Learn Mem. 2008;15:633–642. doi: 10.1101/lm920908. [DOI] [PubMed] [Google Scholar]
- 29.Kay LM. Two species of gamma oscillations in the olfactory bulb: dependence on behavioral state and synaptic interactions. J Integr Neurosci. 2003;2:31–44. doi: 10.1142/s0219635203000196. [DOI] [PubMed] [Google Scholar]
- 30**.Gire DH, Franks KM, Zak JD, Tanaka KF, Whitesell JD, Mulligan AA, Hen R, Schoppa NE. Mitral cells in the olfactory bulb are mainly excited through a multistep signaling path [Internet] J Neurosci. 2012;32:2964–2975. doi: 10.1523/JNEUROSCI.5580-11.2012. We always thought that the olfactory nerve excites mitral cells directly. It turns out that instead the nerve fibers contact primarily tufted cells, which then excite mitral cells via glutamatergic processes. Mitral cells are typically activated much later than tufted cells. Together with [43] we now know that the olfactory nerve excites external tufted cells and GABAergic periglomerular cells. This paper also provides a mechanism for the different timing of mitral and tufted cell activation on the sniff cycle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Fukunaga I, Berning M, Kollo M, Schmaltz A, Schaefer AT. Two distinct channels of olfactory bulb output [Internet] Neuron. 2012;75:320–329. doi: 10.1016/j.neuron.2012.05.017. We have known for some time that MT cells are coordinated by respiration and that their timing on the sniff cycle can be meaningful for associative behavior. This paper uses intracellular recordings to show that tufted cells in awake mice are activated early and mitral cells later during the sniff cycle. Furthermore, the movement of mitral cell timing along the exhalation phase of respiration depends on local inhibition. Tufted cells do not change their phases. [DOI] [PubMed] [Google Scholar]
- 32*.Manabe H, Mori K. Sniff rhythm-paced fast and slow gamma-oscillations in the olfactory bulb: relation to tufted and mitral cells and behavioral states [Internet] J Neurophysiol. 2013;110:1593–1599. doi: 10.1152/jn.00379.2013. Gamma oscillations start fast at the peak of inhalation and then slow down as exhalation initiates. This paper proposes that the fast part of the gamma oscillation is driven by tufted cells, which project to the anterior olfactory nucleus and to the olfactory tubercle. Mitral cells are proposed to support the slower gamma oscillations, and their projections extend to the piriform cortex and other limbic areas. This theory forms good, testable hypotheses concerning the functional roles of these different oscillations. [DOI] [PubMed] [Google Scholar]
- 33.Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies [Internet] Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- 34.Nusser Z, Kay LM, Laurent G, Mody I, Homanics GE. Disruption of GABA(A) receptors on GABAergic interneurons leads to increased oscillatory power in the olfactory bulb network [Internet] J Neurophysiol. 2001;86:2823–2833. doi: 10.1152/jn.2001.86.6.2823. [DOI] [PubMed] [Google Scholar]
- 35.Beshel J, Kopell N, Kay LM. Olfactory bulb gamma oscillations are enhanced with task demands [Internet] J Neurosci. 2007;27:8358–8365. doi: 10.1523/JNEUROSCI.1199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eyre MD, Antal M, Nusser Z. Distinct deep short-axon cell subtypes of the main olfactory bulb provide novel intrabulbar and extrabulbar GABAergic connections. J Neurosci. 2008;28:8217–8229. doi: 10.1523/JNEUROSCI.2490-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Boyd AM, Sturgill JF, Poo C, Isaacson JS. Cortical feedback control of olfactory bulb circuits [Internet] Neuron. 2012;76:1161–1174. doi: 10.1016/j.neuron.2012.10.020. Common knowledge is that centrifugal fibers target granule cells preferentially, but this paper shows that centrifugal inputs from pyriform cortex target deep short axon cells that provide feed forward inhibition to granule cells. This makes interpretation of olfactory system dynamics more complex, but also more interesting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray CM, Skinner JE. Centrifugal regulation of neuronal activity in the olfactory bulb of the waking rabbit as revealed by reversible cryogenic blockade. Exp Brain Res. 1988;69:378–386. doi: 10.1007/BF00247583. [DOI] [PubMed] [Google Scholar]
- 39.Martin C, Gervais R, Messaoudi B, Ravel N. Learning-induced oscillatory activities correlated to odour recognition: a network activity. Eur J Neurosci. 2006;23:1801–1810. doi: 10.1111/j.1460-9568.2006.04711.x. [DOI] [PubMed] [Google Scholar]
- 40.Manns ID, Alonso A, Jones BE. Rhythmically discharging basal forebrain units comprise cholinergic, GABAergic, and putative glutamatergic cells. J Neurophysiol. 2003;89:1057–1066. doi: 10.1152/jn.00938.2002. [DOI] [PubMed] [Google Scholar]
- 41.Pressler RT, Strowbridge BW. Blanes cells mediate persistent feedforward inhibition onto granule cells in the olfactory bulb [Internet] Neuron. 2006;49:889–904. doi: 10.1016/j.neuron.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Liljenstrom H, Hasselmo ME, Liljenström H. Cholinergic modulation of cortical oscillatory dynamics [Internet] J Neurophysiol. 1995;74:288–297. doi: 10.1152/jn.1995.74.1.288. [DOI] [PubMed] [Google Scholar]
- 43.Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci. 2006;24:3234–3244. doi: 10.1111/j.1460-9568.2006.05212.x. [DOI] [PubMed] [Google Scholar]
- 44.Deco G, Thiele A. Attention: oscillations and neuropharmacology [Internet] Eur J Neurosci. 2009;30:347–354. doi: 10.1111/j.1460-9568.2009.06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burton S, Ermentrout G, Urban N. Intrinsic heterogeneity in oscillatory dynamics limits correlation-induced neural synchronization. J Neurophysiol. 2012;108:2115–2133. doi: 10.1152/jn.00362.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skarda CA, Freeman WJ. Brains make chaos to make sense of the world. Behav Brain Sci. 1987;10:161–173. [Google Scholar]
- 47*.Rojas-Libano D, Frederick DE, Egana JI, Kay LM. The olfactory bulb theta rhythm follows all frequencies of diaphragmatic respiration in the freely behaving rat [Internet] Front Behav Neurosci. 2014;8:214. doi: 10.3389/fnbeh.2014.00214. This paper confirms what has been commonly claimed, that the theta rhythm in the rat olfactory bulb (and likely in other mammals) represents the respiratory rhythm at every sniffing frequency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pager J. Unit responses changing with behavioral outcome in the olfactory bulb of unrestrained rats. Brain Res. 1983;289:87–98. doi: 10.1016/0006-8993(83)90009-4. [DOI] [PubMed] [Google Scholar]
- 49.Bhalla US, Bower JM. Multiday recordings from olfactory bulb neurons in awake freely moving rats: spatially and temporally organized variability in odorant response properties [Internet] J Comput Neurosci. 1997;4:221–256. doi: 10.1023/a:1008819818970. [DOI] [PubMed] [Google Scholar]
- 50.Shusterman R, Smear MC, Koulakov AA, Rinberg D. Precise olfactory responses tile the sniff cycle [Internet] Nat Neurosci. 2011;14:1039–1044. doi: 10.1038/nn.2877. [DOI] [PubMed] [Google Scholar]
- 51.Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci. 1999;2:1003–1009. doi: 10.1038/14801. [DOI] [PubMed] [Google Scholar]
- 52.Fontanini A, Bower JM. Variable coupling between olfactory system activity and respiration in ketamine/xylazine anesthetized rats [Internet] J Neurophysiol. 2005;93:3573–3581. doi: 10.1152/jn.01320.2004. [DOI] [PubMed] [Google Scholar]
- 53.Wilson DA, Yan XD. Sleep-Like States Modulate Functional Connectivity in the Rat Olfactory System [Internet] J Neurophysiol. 2010;104:3231–3239. doi: 10.1152/jn.00711.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kay LM. Theta oscillations and sensorimotor performance [Internet] Proc Natl Acad Sci U S A. 2005;102:3863–3868. doi: 10.1073/pnas.0407920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lowry CA, Kay LM. Chemical factors determine olfactory system beta oscillations in waking rats [Internet] J Neurophysiol. 2007;98:394–404. doi: 10.1152/jn.00124.2007. [DOI] [PubMed] [Google Scholar]
- 56*.Tripathy SJ, Peters OJ, Staudacher EM, Kalwar FR, Hatfield MN, Daly KC. Odors pulsed at wing beat frequencies are tracked by primary olfactory networks and enhance odor detection [Internet] Front Cell Neurosci. 2010;4:1. doi: 10.3389/neuro.03.001.2010. This paper is one of the few to show that the temporal dynamics of a natural behavior selectively enhance neural representations of a sensory system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin C, Beshel J, Kay LM. An olfacto-hippocampal network is dynamically involved in odor-discrimination learning [Internet] J Neurophysiol. 2007;98:2196–2205. doi: 10.1152/jn.00524.2007. [DOI] [PubMed] [Google Scholar]
- 58.Kay LM, Beshel J. A beta oscillation network in the rat olfactory system during a 2-alternative choice odor discrimination task [Internet] J Neurophysiol. 2010;104:829–839. doi: 10.1152/jn.00166.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hermer-Vazquez R, Hermer-Vazquez L, Srinivasan S, Chapin JK. Beta- and gamma-frequency coupling between olfactory and motor brain regions prior to skilled, olfactory-driven reaching [Internet] Exp Brain Res. 2007;180:217–235. doi: 10.1007/s00221-007-0850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin C, Gervais R, Hugues E, Messaoudi B, Ravel N. Learning modulation of odor-induced oscillatory responses in the rat olfactory bulb: A correlate of odor recognition? [Internet] J Neurosci. 2004;24:389–397. doi: 10.1523/JNEUROSCI.3433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cenier T, David F, Litaudon P, Garcia S, Amat C, Buonviso N. Respiration-gated formation of gamma and beta neural assemblies in the mammalian olfactory bulb [Internet] Eur J Neurosci. 2009;29:921–930. doi: 10.1111/j.1460-9568.2009.06651.x. [DOI] [PubMed] [Google Scholar]
- 62*.Strube-Bloss MF, Herrera-Valdez MA, Smith BH. Ensemble response in mushroom body output neurons of the honey bee outpaces spatiotemporal odor processing two synapses earlier in the antennal lobe [Internet] PLoS One. 2012;7:e50322. doi: 10.1371/journal.pone.0050322. This paper is one of the very few to address centrifugal projections to the insect antennal lobe. The mushroom body neurons show odor-specific patterns before the completion of pattern separation in the antennal lobe. The authors suggest that there may be very fast neurons in the antennal lobe that deliver information to the mushroom body, which then feeds back to the antennal lobe to assist in pattern separation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samarova E, Balaban P. Changes in frequency of spontaneous oscillations in procerebrum correlate to behavioural choice in terrestrial snails [Internet] Front Cell Neurosci. 2009;3:8. doi: 10.3389/neuro.03.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayar A, Karnup S, Ennis M, Shipley MT. External tufted cells: a major excitatory element that coordinates glomerular activity [Internet] J Neurosci. 2004;24:6676–6685. doi: 10.1523/JNEUROSCI.1367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kay RB, Meyer EA, Illig KR, Brunjes PC. Spatial distribution of neural activity in the anterior olfactory nucleus evoked by odor and electrical stimulation [Internet] J Comp Neurol. 2011;519:277–289. doi: 10.1002/cne.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haberly LB, Bower JM. Olfactory Cortex – Model Circuit for Study of Associative Memory. Trends Neurosci. 1989;12:258–264. doi: 10.1016/0166-2236(89)90025-8. [DOI] [PubMed] [Google Scholar]
- 67.Kay LM, Sherman SM. An argument for an olfactory thalamus. Trends Neurosci. 2007;30:47–53. doi: 10.1016/j.tins.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 68*.Illig K. Corticofugal projections from the anterior olfactory nucleus target olfactory bulb principal cells. Nat Preced. 2011 This is another study challenging our common knowledge about olfactory system circuitry. We formerly believed that centrifugal cortical projections target only inhibitory neurons in the OB. This study shows that anterior olfactory nucleus (AON) pyramidal cells target mitral cells in the OB. This argues that the AON is very much like other primary sensory cortices, and further supports the argument that the pyriform cortex is a higher order association cortex instead of primary olfactory cortex. [Google Scholar]
- 69.De Castro F. Wiring Olfaction: The Cellular and Molecular Mechanisms that Guide the Development of Synaptic Connections from the Nose to the Cortex [Internet] Front Neurosci. 2009;3:52. doi: 10.3389/neuro.22.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sandoz JC. Behavioral and neurophysiological study of olfactory perception and learning in honeybees [Internet] Front Syst Neurosci. 2011;5:98. doi: 10.3389/fnsys.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayar A, Karnup S, Shipley MT, Ennis M. Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input. J Neurosci. 2004;24:1190–1199. doi: 10.1523/JNEUROSCI.4714-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


