Abstract
Introduction
Methamphetamine (MA) is one of the most commonly used illicit drugs in pregnancy, yet studies on MA-exposed pregnancy outcomes have been limited because of retrospective measures of drug use, lack of control for confounding factors: other drug use, including tobacco; poverty; poor diet; and lack of prenatal care. This study presents prospective collected data on MA use and birth outcomes, controlling for most confounders.
Materials and Methods
This is a retrospective cohort study of women obtaining prenatal care from a clinic treating women with substance use disorders, on whom there are prospectively obtained data on MA and other drug use, including tobacco. MA-exposed pregnancies were compared with non-MA exposed pregnancies as well as non-drug exposed pregnancies, using univariate and multivariate analysis to control for confounders.
Results
One hundred forty-four infants were exposed to MA during pregnancy, 50 had first trimester exposure only, 45 had continuous use until the second trimester, 29 had continuous use until the third trimester, but were negative at delivery and 20 had positive toxicology at delivery. There were 107 non MA-exposed infants and 59 infants with no drug exposure. Mean birth weights were the same for MA-exposed and non-exposed infants (3159 g vs. 3168 g p=0.9), though smaller than those without any drug exposure (3159 vs. 3321 p=0.04), Infants with positive toxicology at birth (meconium or urine) were smaller than infants with first trimester exposure only (2932 g vs. 3300 g p=0.01). Gestation was significantly shorter among the MA-exposed infants compared to non-exposed infants (38.5 vs. 39.1 weeks p=0.045) and those with no drug exposure (38.5 vs. 39.5 p=0.0011), The infants with positive toxicology at birth had a clinically relevant shortening of gestation (37.3 weeks vs. 39.1 p=0.0002).
Conclusions
MA use during pregnancy is associated with shorter gestational ages and lower birth weight, especially if used continuously during pregnancy. Stopping MA use at any time during pregnancy improves birth outcomes, thus resources should be directed towards providing treatment and prenatal care.
Keywords: methamphetamine, pregnancy, birth outcomes, preterm labor, small for gestational age
Introduction
Methamphetamine (MA) is one of the most commonly abused drugs during pregnancy, with prevalence estimates ranging from 0.7% to 4.8% in highly endemic areas (Arria et al. 2006, Derauf et al. 2007). Its use continues to grow world wide (United Nations Office on Drugs and Crime 2013), yet what is known about the effects of use during pregnancy is limited by studies using retrospectively gathered data on drug use and insufficient controlling for confounding factors, such as poverty, poor diet, lack of prenatal care and other drug and tobacco use.
MA acts as a competitive inhibitor of the neurotransmitter transporters, specifically serotonin, norepinephrine, and dopamine (Amara and Kuhar 1993, Rudnick and Clark 1993). Among these three targets, the serotonin and norepinephrine transporters are expressed abundantly in the placenta (Ganapathy et al. 1999). These transporters are thought to play an important role in homeostasis of the amniotic fluid and fetal circulation (Ganapathy 1993), as well as control vasoconstriction of the placental vascular bed, which may contribute to the development of preeclampsia (Bottalico et al. 2004), intrauterine growth restriction, abruption and preterm labor (Ganapathy 2011).
The studies looking at pregnancy outcomes with MA use have been conflicting. No consistent teratological effects of in utero MA exposure on the developing human fetus have been identified (Nora et al. 1965, Nora et al. 1970, Levin 1971, Saxen 1975, Dixon and Bejar 1989, Bays 1991, Hansen et al. 1993, Thomas 1995, Stewart and Meeker 1997, Forrester and Merz 2006). Given that women with substance use disorders suffer from chaotic lifestyles, research on drug use during pregnancy is fraught with difficulties. Studies of MA-exposed infants suffer from methodological problems such as poor compliance, small sample size and multiple other confounding variables, such as the effects of poverty, poor diet, and tobacco use. In studies of other drug use during pregnancy, these factors have been shown to be as harmful or more harmful than the drug use itself (Schempf 2007). There are some data on the effects of MA use on maternal complications during pregnancy (Eriksson et al. 1981, Oro and Dixon 1987, Little et al. 1988, Albertson et al. 1999, Cox et al. 2008), birth weight and gestational age (Oro and Dixon 1987, Little et al. 1988, Smith et al. 2003, Smith 2004) and neurodevelopment (Oro and Dixon 1987, Little et al. 1988, Gillogley et al. 1990). The IDEAL study, which is the largest study to date on meth use during pregnancy (Nguyen et al. 2010, Shah et al. 2012, Zabaneh et al. 2012) has demonstrated an increased risk of small for gestational age, decreased head circumference and length, and NICU admissions, but no increased risk of pre-eclampsia, abruption, fetal distress, chronic hypertension, or placenta previa.
Of the data on the effects of MA use on maternal complications during pregnancy, two large database studies showed increased complications of pregnancy, controlling for confounders with the use of regression techniques, though neither collected data on drug use prospectively. Cox et al (Cox et al. 2008) showed increased risks of hypertension complicating pregnancy, premature rupture of membranes, placenta previa, placental abruption, premature delivery, precipitate labor, infection of amniotic cavity, intrauterine death, and poor fetal growth among MA-using women when compared with non-substance using women, but when compared with cocaine-using women, these risks were all lower, with the exception of hypertension complicating pregnancy, which was increased over cocaine. Gorman et al. (Gorman et al. 2014) retrospectively used paired maternal and infant data from the state of California and showed increased risk of gestational hypertension, preeclampsia, IUFD, abruption, preterm birth, neonatal death, and infant death, but didn’t compare with other drug use. With the exception of the IDEAL study and the Cox study, previous studies have been small and lacking in controls for other confounding variables such as other drug and tobacco use. Even in the IDEAL study recruitment was done at delivery and thus no prospective data on drug use and pregnancy outcome were collected.
The current study reports data on women collected prospectively during pregnancy, including dates and amounts of MA and other drug use, tobacco and alcohol use, housing and psychosocial factors, pre-existing medical and psychiatric co-morbidities, compliance with prenatal care; and correlates these factors with maternal and infant outcomes.
Methods
The Path clinic was founded in 2007 in Honolulu, Hawaii to provide prenatal care for women with addictions. MA is the most common illicit substance used by the women with addictions obtaining care at the clinic. Details of the clinic model and implementation process have been previously reported (Wright et al. 2012). Briefly, the clinic provides prenatal and postpartum care for the women, as well as social services, addiction counseling and referral to treatment, childcare, assistance with transportation, group classes, and tobacco cessation services. Deliveries are done at two local hospitals by the residents and faculty of the University of Hawaii.
This study analyzes data prospectively collected for quality assurance purposes throughout and after the pregnancy. The current cohort being analyzed obtained care from April 2007 through December 2013. From April 2007 through April 2011, the clinic was run as a faculty practice through the University of Hawaii. During that time, women who obtained care at the clinic had either current or past drug use and/or addiction diagnosis. In May 2011, the clinic became part of a larger Federally Qualified Community Health Center and the mission changed to include all women in the catchment area or who were homeless or at risk of becoming homeless, regardless of addiction history.
MA-exposed pregnancies were compared with non MA-exposed pregnancies. The non MA-exposed pregnancies were either women who had a history of MA use prior to pregnancy, used tobacco only, used drugs other than MA, or who did not use illicit drugs but obtained care from the clinic and thus were from the same catchment area and socio-economic status. Screening for MA use was done by a combination of validated screening tools (4Ps and 4Ps Plus) on all patients, as well as questioning on recent drug use on patients with a history of addiction at each visit. Random toxicology was done throughout pregnancy and as indicated by clinical or social concerns (i.e. missed appointments). MA use was noted in the database in a semi-quantitative fashion, using patient self-report of use of amount and frequency (daily, twice weekly, monthly). Last reported use was noted in the chart. The majority of women with a history of addiction had toxicology done at the time of birth (urine, meconium or both). Positive toxicology at birth was considered either a positive maternal or neonatal urine toxicology, as meconium can theoretically reflect maternal use many months before delivery. Non-MA exposed women were those who denied any drug use on validated screening tools or those with a past history of drug use and negative toxicology. The authors input all data into the database directly from the medical chart, including medical and psychiatric co-morbidities, number of prenatal visits, substance use, referral sources, housing situation, and pregnancy complications. Birth outcomes were obtained shortly after delivery by abstraction from the electronic medical records of the two delivery hospitals. The University of Hawaii Committee on Human Studies reviewed the project and found it to be exempt from consent requirements in order to report clinical outcomes.
Sample size calculations were performed using birth weight as the primary outcome variable. To detect a 250 g difference in birth weight, using a power of 80% and two-tailed α of 0.05, 36 infants in each group were required. Data were summarized by descriptive statistics. Dichotomous data were compared using Chi-squared tests and continuous data were compared using student’s t-tests.
The association between MA and two primary outcomes of interest, preterm delivery and SGA, were then evaluated using multiple logistic regression, adjusted for important covariates. The covariates that were identified to be associated with the outcomes variables with a p-value <0.05 on univariate analysis were used in the multivariable model. Adjusted odds ratios and their 95% Confidence Intervals were obtained.
Results
There were 251 live births among the cohort that obtained care between April 2007 and December 2013. There were five sets of twins, two in the meth-exposed and three in the non-meth exposed groups. There were 4 third trimester intrauterine fetal deaths (IUFD) during this time (3 meth-exposed, 2 positive for CMV exposure and 1 with Down’s syndrome, cardiac defect and duodenal atresia and 1 non meth-exposed without other risk factors other than advanced maternal age). The IUFDs were removed from further analysis. Of the 251 live births, 107 had no meth exposure, 50 had first trimester exposure only, 45 had continuous use until the second trimester, 29 had continuous use until the third trimester, but were negative at delivery and 20 had positive toxicology at delivery. Demographics are presented in table 1.
Table 1.
Summary of demographics
| All women in study (n=251) | Women with no other drug use (n=119) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any MA use during pregnancy n=144 |
No MA use during pregnancy n=107 |
Only MA use during pregnancy n=60 |
No drug use during pregnancy n=59 |
|||||||
| Mean ± SD | Range | Mean ± SD | Range | p-value | Mean ± SD | Range | Mean ± SD | Range | p-value | |
| Maternal Age (years) | 28.6 ± 6.1 | (16–45) | 28.4 ± 6.1 | (14–41) | 0.65 | 28.4 ± 6.7 | (16–45) | 29.1 ± 5.5 | (18–41) | 0.52 |
| Gravidity | 4.9 ± 3 | (1–12) | 3.5 ± 2.5 | (1–15) | <0.0001 | 4.8 ± 3.5 | (1–15) | 3.6 ± 2.5 | (1–15) | 0.02 |
| Parity | 2.5 ± 2.1 | (0–9) | 1.5 ± 1.6 | (0–6) | <0.0001 | 2.2 ± 2.2 | (0–8) | 1.7 ± 1.7 | (0–6) | 0.16 |
| Aborta | 1.4 ± 2.1 | (0–12) | 1.1 ± 1.7 | (0–11) | 0.12 | 1.6 ± 2.3 | (0–12) | 0.9 ± 1.1 | (0–11) | 0.08 |
| Primary Ethnicity n=239 * | n | % | n | % | n | % | n | % | p-value | |
| Caucasian | 27 | 20.1 | 44 | 41.9 | 0.0004 | 14 | 25.0 | 18 | 31.6 | 0.57 |
| NH/OPI | 76 | 56.7 | 34 | 32.3 | 0.0003 | 30 | 53.6 | 22 | 38.6 | 0.16 |
| Asian | 10 | 7.5 | 15 | 14.2 | 0.17 | 4 | 7.1 | 9 | 15.8 | 0.15 |
| Filipina | 8 | 6.0 | 4 | 3.8 | 0.65 | 3 | 5.4 | 3 | 5.3 | 1.00 |
| African American | 4 | 3.0 | 3 | 2.9 | 1.00 | 2 | 3.6 | 2 | 3.5 | 1.00 |
| Hispanic | 7 | 5.0 | 5 | 4.8 | 1.00 | 2 | 3.6 | 3 | 5.3 | 1.00 |
| Other substance use | n | % | n | % | p-value | n | % | n | % | p-value |
| Smoker (any during pregnancy) n=241 | 124 | 89.9 | 80 | 77.7 | 0.01 | 54 | 91.5 | 39 | 72.2 | 0.01 |
| Alcohol n=233 | 18 | 14.0 | 13 | 12.5 | 0.75 | 0 | 0 | 0 | 0 | NA |
| Cocaine n=238 | 6 | 4.5 | 5 | 4.8 | 0.93 | 0 | 0 | 0 | 0 | NA |
| Heroin n=251 | 6 | 4.2 | 2 | 1.9 | 0.47 | 0 | 0 | 0 | 0 | NA |
| Marijuana n=239 | 44 | 32.6 | 16 | 15.4 | 0.002 | 0 | 0 | 0 | 0 | NA |
| Other opioid use n=233 | 9 | 6.3. | 21 | 19.4 | 0.002 | 0 | 0 | 0 | 0 | NA |
| Co-occurring mental health disorders | n | % | n | % | p-value | n | % | n | % | p-value |
| Mood disorder n=246 | 64 | 45.1 | 41 | 39.1 | 0.38 | 32 | 53.3 | 21 | 36.2 | 0.06 |
| Schizophrenia/Schizoaffective n=245 | 8 | 5.6 | 0 | 0 | 0.02 | 3 | 5.0 | 0 | 0 | 0.24 |
| PTSD n=245 | 26 | 18.3 | 11 | 10.68 | 0.09 | 15 | 25.0 | 6 | 10.5 | 0.04 |
| Any co-occurring disorder n=246 | 72 | 50.7 | 44 | 42.3 | 0.19 | 35 | 59.3 | 25 | 42.4 | 0.06 |
NH/OPI=native Hawaiian/other Pacific Islander
50% of women in the study were more than one ethnicity.
Mood disorder=depression, anxiety, bipolar disorder.
PTSD=post-traumatic stress disorder
n and percentages may differ because of missing values
Women who used MA had higher gravidity and parity and were more likely to smoke cigarettes and use marijuana during pregnancy. Cocaine and alcohol use was similar between the two groups. The non MA-using group was more likely to use other opioids and be Caucasian. Interestingly the group who didn’t use any drugs at all during their pregnancy more closely resembled the non-MA group. Heroin use was low in both groups reflecting the low prevalence of heroin use in Hawaii. As noted in previous studies (Wright and Tam 2010), Native Hawaiian and other Pacific Islander (NH/OPI) were overrepresented in the MA-using group. Schizophrenia/schizoaffective disorders and PTSD were more common among the MA-using women.
Univariate analyses of birth outcomes are presented in Table 2. MA-using women presented significantly later for prenatal care and had fewer prenatal visits. There was no difference in birth weight between the MA-using group and the non MA-using group, though the gestational age at delivery was slightly younger (6/10 of a week). The non-drug using group had a significantly longer gestational age (1 week) and was 176 g heavier than the MA-using group and the group that used other drugs. They had a bigger head circumference and were longer. There was no difference in the incidence of preterm delivery, preterm premature rupture of membranes, abruption, non-reassuring heart rate, chorioamnionitis, asthma, diabetes, low-birth weight, sepsis, intraventricular hemorrhage, necrotizing enterocolitis, or NICU admission between the MA-exposed newborns and the non-MA exposed newborns. There was a significant increase in chronic hypertension and cesarean delivery associated with MA use and a non-significant increase in the incidence of preeclampsia. The majority of cesarean sections were repeat.
Table 2.
Birth Outcomes of MA-exposed pregnancies compared with non-MA exposed pregnancies
| All women (n=251)
|
Women with no other drug use (n=119)
|
|||||
|---|---|---|---|---|---|---|
| Any MA use during pregnancy n=144 |
No MA use during pregnancy n=107 |
Only MA during pregnancy n=60 |
No drug use during pregnancy n=59 |
|||
|
| ||||||
| Outcome | Mean ± SD | Mean ± SD | p-value | Mean ± SD | Mean ± SD | p-value |
|
| ||||||
| Gestational age (weeks) | 38.5 ± 2.0 | 39.1 ± 2.1 | 0.048 | 38.8 ± 2.1 | 39.5 ± 1.6 | 0.043 |
| Birth Weight (grams) | 3159 ± 561 | 3168 ± 533 | 0.9 | 3103 ± 537 | 3321 ± 451 | 0.019 |
| Head Circumference (cm) | 33.5 ± 3.2 | 33.9 ± 2.9 | 0.42 | 33.2 ± 3.4 | 34.6 ± 1.5 | 0.01 |
| Length (cm) | 50.3 ± 3.0 | 50.6 ± 3.4 | 0.52 | 49.8 ± 3.4 | 51.3 ± 2.5 | 0.009 |
| Cord pH | 7.25 ± 0.1 | 7.27 ± 0.1 | 0.18 | 7.25 ± 0.1 | 7.27 ± 0.1 | 0.26 |
| Maternal LOS (days) | 2.7 ± 1.3 | 2.4 ± 1.2 | 0.12 | 2.52 ± 0.9 | 2.2 ± 0.8 | 0.02 |
| Infant LOS (days) | 3.9 ± 7.0 | 3.5 ± 4.7 | 0.62 | 4.3 ± 7.8 | 2.5 ± 1.9 | 0.1 |
| First prenatal visit (weeks) | 23.3 ± 9.5 | 17.7 ± 9.5 | <0.0001 | 24.2 ± 9.4 | 17.2 ± 10.4 | 0.0009 |
| Number of prenatal visits | 7 ± 4.3 | 8.4 ± 3.9 | 0.018 | 7.5 ± 4.4 | 8.6 ±4.2 | 0.22 |
Figures 1 and 2 show gestational age and birth weight stratified by trimester of last use of MA. Significantly only women who continued to use throughout pregnancy delivered early and had smaller babies. This was also true when compared with women who didn’t use any drugs during their pregnancies. In addition, women who continued to use MA throughout their pregnancies were significantly more likely to have inadequate prenatal care. (68% vs. 18% p<0.0001).
Figure 1.
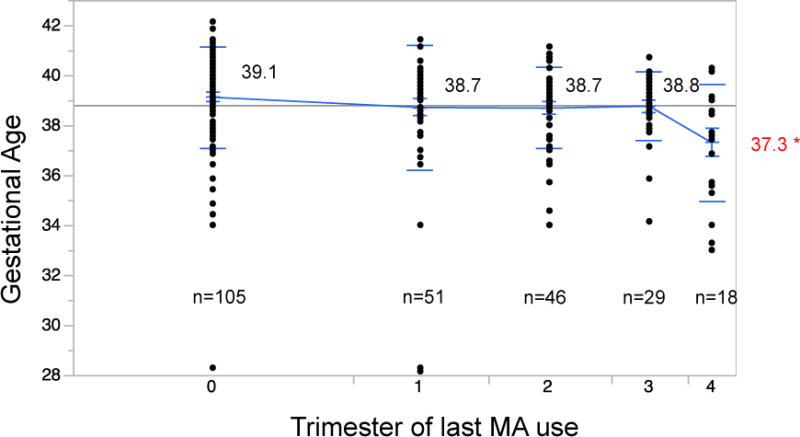
Comparison of mean gestational age with trimester of last use of methamphetamines. As the data points show, the amount of women using MA decreased throughout pregnancy. Mean gestational ages were not different between any use and use in trimesters 1–3. Only use at the time of delivery was associated with shorter gestation (p=0.0145*).
Figure 2.
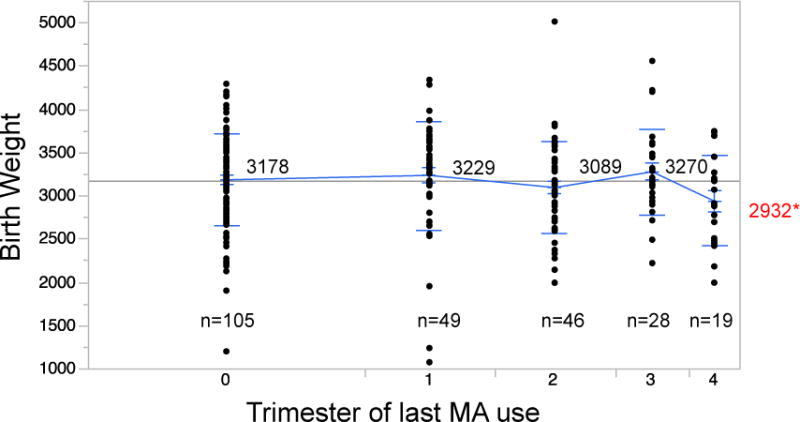
Comparison of mean birth weight by last trimester of methamphetamine use. Women with positive toxicology at birth had lower unadjusted birth weights than those that stopped in 1st or 3rd trimester (p=0.04*).
There were 5 major birth defects among the 251 births (2%). Of these 3 were MA exposed (cardiac defect, portal vein anomaly, and cystic hygroma) and 2 were non-MA exposed (bilateral ventriculomegaly and laryngiomalacia). There were 3 minor birth defects (1 MA exposed and 2 non-MA exposed).
Multivariate analyses are presented in tables 3–4. In the multivariate logistic model, using insufficient prenatal care (<5 visits), chronic hypertension, preeclampsia and diabetes, trimester of last MA use, and other drug use (defined as any other illicit drug use besides MA) as covariates, only persistent MA use (positive toxicology at birth) and other drug use were associated with preterm delivery. Persistent MA use was associated with 3.5-fold increase in preterm delivery and other drug use with a 2.4-fold increase. Interestingly smoking was not associated with preterm delivery in this model on univariate or multivariate analysis. Each week of delaying prenatal care increased the odds of delivering preterm by 1.07 (1.01–1.15) p=0.043.
Table 3.
Unadjusted associations between Pregnancy complications and MA use
| All women (n=251)
|
Women with no other drug use (n=119)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Any MA use during pregnancy n=144 |
No MA use during pregnancy n=107 |
Only MA during pregnancy n=60 |
No drug use during pregnancy n=59 |
|||||
|
| ||||||||
| Pregnancy Complications | n (percent) | n (percent) | OR (95%CI) | p-value | n (percent) | n (percent) | OR (95%CI) | p-value |
|
| ||||||||
| Preterm delivery | 18 (12.6) | 13 (12.0) | 1.05 (0.5–2.3) | 1.00 | 8 (13) | 3 (5) | 2.8 (0.7–11.2) | 0.2 |
| Low birth weight | 15 (10.7) | 10 (9.4) | 1.2 (0.5–2.7) | 0.83 | 8 (13) | 2 (3.4) | 4.3 (0.9–21.2) | 0.09 |
| Chronic Hypertension | 11 (7.7) | 2 (1.9) | 4.4 (0.9–20.2) | 0.035 | 3 (4.9) | 0 (0) | NA | 0.24 |
| Preeclampsia | 10 (7.0) | 4 (3.7) | 1.94 (0.6–6.3) | 0.28 | 4 (6.6) | 2 (3.4) | 2.0 (0.4–11.5) | 0.68 |
| Cesarean Delivery | 46 (32.2) | 15 (12.0) | 2.9 (1.5–5.6) | 0.0006 | 12 (19.7) | 4 (6.8) | 3.4 (1.0–11.1) | 0.058 |
| NICU Admission | 10 (7.3) | 10 (9.6) | 0.74 (0.3–1.9) | 0.63 | 4 (6.8) | 2 (3.6) | 1.9 (0.3–11.0) | 0.68 |
| Small for gestational age | 15 (10.5) | 15 (14.0) | 0.72 (0.3–1.5) | 0.43 | 8 (13) | 6 (10.3) | 1.3 (0.4–4.0) | 0.78 |
NICU=newborn intensive care unit, OR=odds ratio, CI=confidence interval.
n and percentages may differ by column due to lack of data on outcome
Table 4.
Multiple logistic regression of preterm delivery (<37 weeks)
| Variable | aOR (95% CI) | p-value |
|---|---|---|
| Insufficient prenatal care (<5 visits) | 2.11 (0.77–5.49) | 0.14 |
| Chronic Hypertension | 3.53 (0.68–16.40) | 0.13 |
| Pre-eclampsia | 2.30 (0.38–10.64) | 0.33 |
| Diabetes | 2.27 (0.60–7.32) | 0.21 |
| Other drugs | 2.40 (1.01–6.00) | 0.048 |
| MA-positive at delivery | 3.54 (1.02–11.66) | 0.046 |
| Delayed prenatal care (per week) | 1.07 (1.01–1.15) | 0.043 |
aOR=adjusted odds ratio, CI=confidence interval.
Persistent smoking, but not MA use, nor other drug use, was associated with small for gestational age (SGA), defined as a baby measuring <10% for gestational age using Alexander’s algorithm (Alexander et al. 1996).
Discussion
This is the largest cohort study of methamphetamine-exposed pregnancies to date where information on MA and other drug use was collected prospectively. In addition, the groups are similar in the presence of other confounding factors, including tobacco use (90% vs. 78% vs. state average 12%), other drug use, poverty levels (98% of women in the study were on State Medicaid) and housing status (the great majority of women (>90%) in each group were either in residential drug treatment, homeless or at-risk homeless, or incarcerated). All the non MA-using women either had a past history of addiction or were either from the same catchment area as the meth-using women, and homeless or at-risk for becoming homeless. Given the similarities in these factors, we showed that continuous MA and other drug use are associated with lower gestational age and birth weight, but that any MA use during pregnancy is not associated with adverse pregnancy outcomes other than chronic hypertension and cesarean delivery. Women who continually used MA throughout pregnancy did have a higher risk of delivering preterm. We did show that women who stop using MA at any time during pregnancy have improved birth outcomes as far as birth weight and gestational age, and these do not differ from women who do not use MA during pregnancy. Reassuringly MA use was not associated with any increase in birth defects above baseline.
The IDEAL study has a larger enrollment, as it is a multi-center study (Arria et al. 2006, Smith et al. 2006). However enrollment in that study was done at birth and data on MA use was collected retrospectively. In addition, the control group was not matched for socio-economic status. In contrast to the IDEAL study (Smith et al. 2006), we did not show an increase in small for gestational age (SGA) in the MA-exposed infants. The MA-exposed infants were smaller, but not once controlled for the earlier gestational age. We did show an increase risk of maternal chronic hypertension with MA use, which is consistent with other studies that show a multitude of cardiovascular effects from chronic MA use (Carvalho et al. 2012). It could be that this is the mechanism causing SGA in the IDEAL study.
The increase in cesarean deliveries could be secondary to the increased gravidity and parity of the MA-using women as the majority of cesarean deliveries were for the indication of prior cesarean. Before the establishment of the clinic, many of these women did not get prenatal care and often ended up at the hospital with complications necessitating cesarean delivery that could’ve been prevented with adequate prenatal care, (e.g. breech presentation where external cephalic version could be offered or better blood pressure control during pregnancy so that late preterm delivery would not be necessary for uncontrolled hypertension). Even in this study, women who used MA entered prenatal care later and had fewer prenatal visits, and women who persisted using MA were much more likely to have inadequate prenatal care, which will increase the rate of pregnancy complications. Even in patients with drug use throughout pregnancy, prenatal care of at least 4 visits has been shown to improve pregnancy outcomes (El-Mohandes et al, 2009). Presenting late to care also makes it less likely the pregnancy will be accurately dated, which may inadvertently increase the preterm delivery rate, as dating depends on early ultrasound or clinical exam. For example, if a woman presents at 30 weeks for care, her pregnancy dating ultrasound may be off by up to 3 weeks. If she then goes on to deliver at 36 weeks by that dating, she would be considered preterm, but in actuality may be 39 weeks and full-term. Inversely if she were considered 39 weeks, but was actually only 36 weeks, she may iatrogenically be delivered preterm.
This study has many limitations. The women in the clinic were self-selected and often motivated to quit using MA, which most likely improved compliance with prenatal care and other self-care practices. This could be reflected in the fact that women who continued to use until delivery had worse pregnancy outcomes. There is somewhat limited generalizability to other communities, given low rates of heroin usage and less exposure to multiple drugs other than marijuana and tobacco. In addition, we didn’t have extremely accurate assessment of alcohol usage and no information on weight gain was collected, which can influence the incidence of SGA. In addition, strict measurements of socio-economic status (SES) were not collected, thus Medicaid-eligibility was used as a proxy measure. This information will be collected going forward. In addition, further studies on infant development should be done.
Conclusion
Continuous methamphetamine use during pregnancy is associated with preterm delivery and low-birth weight, both of which contribute to neonatal morbidity and mortality. The majority of women in the study stopped using MA (86%), which is extremely reassuring. The women that did stop engaged in prenatal care more often and had normal birth outcomes. Stopping MA use at any time during pregnancy improves birth outcomes, thus resources should be aimed at treatment of addiction and promotion of prenatal care.
Supplementary Material
Table 5.
Multiple logistic regression of small for gestational age (<10%)
| Variable | aOR (95% CI) | p-value |
|---|---|---|
| Persistent Smoker | 4.58 (1.90–12.80) | 0.0004 |
| MA-positive at delivery | 0.34 (0.01–1.83) | 0.24 |
| Other drugs | 1.69 (0.77–3.80) | 0.19 |
aOR=adjusted odds ratio, CI=confidence interval
Acknowledgments
Funding for the establishment for the Path clinic was given by the Hawaii State Legislature (Acts 248–2006 and 147–2007). Funding for clinical outcomes studies was provided in part by NIH grant 5U54RR014607. Funding for statistical review was provided by NIH grants U54MD007584 and G12MD007601. Philanthropic support was provided by the Office of Hawaiian Affairs, The Hawaii Community Foundation, Healthy Mothers, Healthy Babies-Hawaii, March of Dimes Hawaii and other community members. The Salvation Army Family Treatment Center has promoted our peaceful coexistence these many years. Thank you to Jennifer Elia and Dr. John Chen for reviewing the paper draft and thank you to the many clinic staff and volunteers, without whom the clinic would not have such outcomes, especially Bernadette Scanlan-Hodges Julia Yoshimoto, and Rachel Dorr, Cynthia Nguyen, Christina Post, Shyna Estubio, Kelly Meyers and Porsha Arnold.
Footnotes
The authors report no relevant conflicts of interest
Data from this study was presented in abstract form at the following meetings:
III Global Congress of Maternal and Infant Health, Buenos Aires, Argentina, November 2013
American Society of Addiction Medicine, Orlando, FL April 2014
American College of Obstetrics and Gynecology Annual Clinical Meeting. Chicago, IL April, 2014
References
- Albertson TE, Derlet RW, Van Hoozen BE. Methamphetamine and the expanding complications of amphetamines. West J Med. 1999;170(4):214–219. [PMC free article] [PubMed] [Google Scholar]
- Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan MA. United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Arria AM, Derauf C, Lagasse LL, et al. Methamphetamine and other substance use during pregnancy: preliminary estimates from the Infant Development, Environment, and Lifestyle (IDEAL) study. Matern Child Health J. 2006;10(3):293–302. doi: 10.1007/s10995-005-0052-0. [DOI] [PubMed] [Google Scholar]
- Bays J. Fetal vascular disruption with prenatal exposure to cocaine or methamphetamine. Pediatrics. 1991;87(3):416–418. [PubMed] [Google Scholar]
- Bottalico B, Larsson I, Brodszki J, Hernandez-Andrade E, Casslen B, Marsal K, Hansson SR. Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre-eclamptic and normotensive pregnancies. Placenta. 2004;25(6):518–529. doi: 10.1016/j.placenta.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Carvalho M, Carmo H, Costa VM, et al. Toxicity of amphetamines: an update. Arch Toxicol. 2012;86(8):1167–1231. doi: 10.1007/s00204-012-0815-5. [DOI] [PubMed] [Google Scholar]
- Cox S, Johnson CH, Meikle S, Jamieson DJ, Posner SF. Trends in rates of hospitalization with a diagnosis of substance abuse among reproductive-age women, 1998 to 2003. Womens Health Issues. 2007;17(2):75–83. doi: 10.1016/j.whi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Cox S, Posner SF, Kourtis AP, Jamieson DJ. Hospitalizations with amphetamine abuse among pregnant women. Obstet Gynecol. 2008;111(2):341–347. doi: 10.1097/01.AOG.000300377.82722.ad. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report. 2013 Available at http://www.unodc.org/wdr/. Accessed Accessed June 16, 2014.
- Derauf C, LaGasse LL, Smith LM, et al. Demographic and psychosocial characteristics of mothers using methamphetamine during pregnancy: preliminary results of the infant development, environment, and lifestyle study (IDEAL) Am J Drug Alcohol Abuse. 2007;33(2):281–289. doi: 10.1080/00952990601175029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SD, Bejar R. Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: incidence and clinical correlates. J Pediatr. 1989;115(5 Pt 1):770–778. doi: 10.1016/s0022-3476(89)80661-4. [DOI] [PubMed] [Google Scholar]
- El-Mohandes A, Herman AA, Nabil El-Khorazaty M, Katta PS, White D, Grylack L. Prenatal care reduces the impact of illicit drug use on perinatal outcomes. J Perinatol. 2009;23(5):354–360. doi: 10.1038/sj.jp.7210933. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Larsson G, Zetterstrom R. Amphetamine addiction and pregnancy. II. Pregnancy, delivery and the neonatal period. Socio-medical aspects. Acta Obstet Gynecol Scand. 1981;60(3):253–259. doi: 10.3109/00016348109158127. [DOI] [PubMed] [Google Scholar]
- Forrester MB, Merz RD. Comparison of trends in gastroschisis and prenatal illicit drug use rates. J Toxicol Environ Health A. 2006;69(13):1253–1259. doi: 10.1080/15287390500361750. [DOI] [PubMed] [Google Scholar]
- Ganapathy V. Drugs of abuse and human placenta. Life Sci. 2011;88(21–22):926–930. doi: 10.1016/j.lfs.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V, Ramamoorthy S, Leibach FH. Transport and metabolism of monoamines in the human placenta. Trophoblast Res. 1993;7:35–51. [Google Scholar]
- Ganapathy VV, Prasad PD, Ganapathy ME, Leibach FH. Drugs of abuse and placental transport. Adv Drug Deliv Rev. 1999;38(1):99–110. doi: 10.1016/s0169-409x(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Gillogley KM, Evans AT, Hansen RL, Samuels SJ, Batra KK. The perinatal impact of cocaine, amphetamine, and opiate use detected by universal intrapartum screening. Am J Obstet Gynecol. 1990;163(5 Pt 1):1535–1542. doi: 10.1016/0002-9378(90)90621-d. [DOI] [PubMed] [Google Scholar]
- Gorman MC, Orme KS, Nguyen NT, Kent EJ, Caughey AB. Outcomes in Pregnancies Complicated by Methamphetamine Use. Am J Obstet Gynecol. 2014 doi: 10.1016/j.ajog.2014.06.005. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hansen RL, Struthers JM, Gospe SM. Visual evoked potentials and visual processing in stimulant drug-exposed infants. Dev Med Child Neurol. 1993;35(9):798–805. doi: 10.1111/j.1469-8749.1993.tb11731.x. [DOI] [PubMed] [Google Scholar]
- Levin JN. Amphetamine ingestion with biliary atresia. J Pediatr. 1971;79(1):130–131. doi: 10.1016/s0022-3476(71)80075-6. [DOI] [PubMed] [Google Scholar]
- Little BB, Snell LM, Gilstrap LC. Methamphetamine abuse during pregnancy: outcome and fetal effects. Obstet Gynecol. 1988;72(4):541–544. [PubMed] [Google Scholar]
- Nguyen DL, Smith M, Lagasse LL, et al. Intrauterine growth of infants exposed to prenatal methamphetamine: results from the infant development, environment, and lifestyle study. J Pediatr. 2010;157(2):337–339. doi: 10.1016/j.jpeds.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora JJ, Trasler DG, Fraser FC. Malformations in mice induced by dexamphetamine sulphate. Lancet. 1965;2(7420):1021–1022. doi: 10.1016/s0140-6736(65)92892-8. [DOI] [PubMed] [Google Scholar]
- Nora JJ, Vargo TA, Nora AH, Love KE, McNamara DG. Dexamphetamine: a possible environmental trigger in cardiovascular malformations. Lancet. 1970;1(7659):1290–1291. doi: 10.1016/s0140-6736(70)91765-4. [DOI] [PubMed] [Google Scholar]
- Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. J Pediatr. 1987;111(4):571–578. doi: 10.1016/s0022-3476(87)80125-7. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993;1144(3):249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- Saxen I. Associations between oral clefts and drugs taken during pregnancy. Int J Epidemiol. 1975;4(1):37–44. doi: 10.1093/ije/4.1.37. [DOI] [PubMed] [Google Scholar]
- Schempf A. Illicit drug use and neonatal outcomes: a critical review. Obstet Gynecol Surv. 2007 Nov;62(11):749–757. doi: 10.1097/01.ogx.0000286562.31774.76. [DOI] [PubMed] [Google Scholar]
- Shah R, Diaz SD, Arria A, et al. Prenatal methamphetamine exposure and short-term maternal and infant medical outcomes. Am J Perinatol. 2012;29(5):391–400. doi: 10.1055/s-0032-1304818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, LaGasse L, Derauf C, Lester B. Intrauterine Growth of Infants Exposed to Prenatal Methamphetamine: Preliminary Results from the Infant Development, Environment, and Lifestyle Study (IDEAL) Pediatric Research. 2004;55:72A. doi: 10.1016/j.jpeds.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Yonekura ML, Wallace T, Berman N, Kuo J, Berkowitz C. Effects of prenatal methamphetamine exposure on fetal growth and drug withdrawal symptoms in infants born at term. J Dev Behav Pediatr. 2003;24(1):17–23. doi: 10.1097/00004703-200302000-00006. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, et al. The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118(3):1149–1156. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Meeker JE. Fetal and infant deaths associated with maternal methamphetamine abuse. J Anal Toxicol. 1997;21(6):515–517. doi: 10.1093/jat/21.6.515. [DOI] [PubMed] [Google Scholar]
- Thomas DB. Cleft palate, mortality and morbidity in infants of substance abusing mothers. J Paediatr Child Health. 1995;31(5):457–460. doi: 10.1111/j.1440-1754.1995.tb00857.x. [DOI] [PubMed] [Google Scholar]
- Wright TE, Schuetter R, Fombonne E, Stephenson J, Haning WF. Implementation and evaluation of a harm-reduction model for clinical care of substance using pregnant women. Harm Reduct J. 2012;9(1):5. doi: 10.1186/1477-7517-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TE, Tam E. Disparate rates of persistent smoking and drug use during pregnancy of women of Hawaiian ancestry. Ethnicity and Disease. 2010;20(1 Suppl 1):S1–215–218. [PubMed] [Google Scholar]
- Zabaneh R, Smith LM, LaGasse LL, et al. The effects of prenatal methamphetamine exposure on childhood growth patterns from birth to 3 years of age. Am J Perinatol. 2012;29(3):203–210. doi: 10.1055/s-0031-1285094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


