Abstract
Introduction:
We investigated the value of the preoperative neutrophil-lymphocyte ratio (NLR) in predicting recurrence and progression of high-grade pT1 non-muscle-invasive tumour in patients with bladder cancer during a 5-year follow-up period.
Methods:
We retrospectively reviewed data of 1100 patients with bladder cancer; these patients underwent transurethral resection and were monitored at multiple centres from 2008 to 2013. In total, 166 consecutive and newly diagnosed patients with high-grade pT1 tumours were included in this study. The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count.
Results:
Of the 166 patients, 152 were male. The patients were evaluated as two separate groups in terms of recurrence and progression. The mean follow-up period was 24.2 months (interquartile range 13.8–36.6 months). A statistically significant difference was found between recurrence and tumour size (p = 0.001), number of tumours (p < 0.001), NLR (p < 0.001), and smoking (p = 0.007). No statistically significant correlation was found between NLR and progression. According to receiver operating characteristic (ROC) analysis, the optimum cut-off value for the NLR was ≥2.43 (74% sensitivity, 60% specificity, p < 0.001; area under the curve [AUC] 0.687, 95% confidence interval [CI] 0.607–0.767). Multivariate logistic regression analysis determined that the following factors were independent predictors of recurrence in patients with high-grade pT1 non-muscle-invasive bladder cancer: tumour number (OR 5.32, 95% CI 2.10–12.90), NLR of ≥2.43 (OR 2.587; 95% CI 1.156–5.789), and smoking (OR 4.17, 95% CI 1.31–13.21).
Conclusion:
A high preoperative NLR may play an important role in predicting recurrence of superficial transitional cell type high-grade pT1 bladder tumours. Prospective studies are required to validate the role of NLR as a prognostic marker in high-grade pT1 bladder tumours.
Introduction
At diagnosis, about 75% of bladder cancers are non-muscle-invasive tumours (Ta, T1, and carcinoma in situ).1 Of these, 20% are pT1 tumours, with 5-year recurrence and progression rates of 30% to 80% and 1% to 50%, respectively.2–4 Recurrence and progression of these tumours are predicted based on the scores and risk tables of the European Organization for Research and Treatment of Cancer (EORTC); no biochemical indicators are currently used for this purpose. It is particularly difficult to predict the behaviour of high-grade pT1 superficial bladder tumours over time. Such tumours either do not show recurrence or progress to muscle-invasive and metastatic stages. This complicates the clinician’s decision-making process regarding follow-up and treatment.5
Many studies have shown that inflammation plays a role in tumour response to systemic treatment, tumour metastasis, and angiogenesis in the formation and progression stages of most cancer types.6 The neutrophil-lymphocyte ratio (NLR) is an indicator of systemic inflammation and is convenient and inexpensive to measure. Recent studies have demonstrated that a high NLR is an important marker of recurrence and poor prognosis in colorectal, gastric, ovarian, and thyroid cancers, as well as in renal and hepatocellular carcinoma and malignant mesothelioma.7–13
Our aim in this study was to determine the value of the preoperative NLR in predicting recurrence and progression of high-grade pT1 non-muscle-invasive bladder tumours during a 5-year follow-up period.
Methods
Patient selection
We retrospectively evaluated the data of 1100 patients with bladder cancer who underwent transurethral resection and were monitored at 4 medical centres from 2008 to 2013. Approval for this study was obtained from the ethics committee at our university. The tumours were graded according to the World Health Organization-International Society of Urologic Pathology 2004 guidelines and the TNM 2002 staging system. All pertinent laboratory and pathology results (tumour size and number, presence of carcinoma in situ, retransurethral resection [TUR], postoperative immunotherapy and chemotherapy), and medical data were obtained from the hospital databases. The recurrence and progression status was determined for each patient based on these data. Recurrence was defined as the relapse of transitional cell carcinoma of the bladder at any pathological stage after initial surgery. Re-TUR was defined as the TUR which was performed 2 to 6 weeks after the initial transurethral resection. Patients with non-transitional cell bladder cancers, carcinoma in situ, acute inflammatory disease, bleeding disorders, hematological disorders, autoimmune diseases, any malignancies other than bladder tumour, preoperative active infection, or a history of splenectomy as well as patients on maintenance intravesical chemotherapy after transurethral resection were excluded from the study. Of the 1100 examined patients, we consecutively selected 166 patients with pathologically newly diagnosed high-grade pT1 tumours after initial surgery. When we were designing our study, we thought that the systemic inflammatory effects of pTa and low-grade superficial bladder tumors would be very low. Among these tumours, pT1 G3 tumours would be at a greater risk of recurrence and progression; therefore, we included only patients with pT1 G3 tumours. The follow-up period was calculated from the date of surgery, to either the last follow-up or death.
Blood tests
Data obtained from the patients’ routine preoperative test results included the neutrophil count, lymphocyte count, red cell distribution width, and mean platelet volume. The NLR was determined by dividing the absolute neutrophil count by the absolute lymphocyte count.
Statistical methods
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY). Continuous variables were tested for normality by the Kolmogorov–Smirnov test. Normally distributed data were presented as means ± standard deviation. The rates and proportions of discrete variables were determined using the chi-squared test. The median with data range (minimum to maximum) was used for non-normally distributed data. The independent samples t-test and Mann–Whitney U test were used for parametric and nonparametric groups, respectively. Correlations between tumour size and recurrence were evaluated using Spearman’s rank correlation coefficient. Potential predictors of recurrence and progression in individual patients with superficial transitional cell cancer of the bladder were initially compared, and variables that showed a p value of <0.05 were included in a logistic regression model. Results were expressed as odds ratio (OR) and 95% confidence interval (CI). The two-sided p value of <0.05 was considered statistically significant.
Results
In total, 166 patients were enrolled in the study (152 male, 14 female). The patients were evaluated as two separate groups in terms of recurrence and progression. The mean follow-up period was 24.2 months (interquartile range 13.8–36.6 months).
Spearman’s correlation test showed a statistically significant positive correlation between tumour size and recurrence (r = 0.368, p < 0.001). In the recurrence group, the tumour size was ≤3 cm in 20 patients (31.2%) and >3 cm in 45 patients (69.2%). A statistically significant difference was found between recurrence and tumour size (p < 0.001). A statistically significant positive correlation was also found between tumour size and progression (r = 0.171, p = 0.028). In the progression group, the tumour size was ≤3 cm in 6 patients (31.6%) and >3 cm in 13 patients (68.4%). However, there was no statistically significant difference between progression and tumour size (p = 0.097) (Table 1).
Table 1.
Comparison of patients according to the presence of recurrence and progression
| Recurrence | Progression | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
|
Absent N = 101 |
Present N = 65 |
p value |
Absent N = 147 |
Present N = 19 |
p value | ||
| Sex (F/M) | 6/95 | 8/57 | 0.15 | 11/136 | 3/16 | 0.22 | |
| Age (year)* | 67.3 ± 11 | 67.7 ± 9.4 | 0.84 | 67.5 ± 10.5 | 67.5 ± 10.2 | 1 | |
| MPV* | 8.2 ± 1.2 | 8.5 ± 1.3 | 0.09 | 8.4 ± 1.3 | 8.2 ± 1.2 | 0.57 | |
| RDW* | 15.6 ± 1.6 | 15.5 ± 1.3 | 0.67 | 15.6 ± 1.5 | 15.4 ± 1.3 | 0.46 | |
| NLR¥ | 2.2 (0.7–8.2) | 2.8 (1.4–16.8) | <0.001 | 2.5 (0.7–16.3) | 2.6 (1.9–16.8) | 0.34 | |
| BCG usage | 36 (35.6%) | 17 (26.2%) | 0.2 | 50 (34.0%) | 3 (15.8%) | 0.11 | |
| Single dose mitomicyn | 20 (19.6%) | 14 (21.9%) | 0.725 | 30 (20.3%) | 4 (22.2%) | 0.846 | |
| Tumour size (cm) | ≤3 | 65 (64.4%) | 20 (30.8%) | 0.001 | 79 (53.7%) | 6 (31.6%) | 0.047 |
| >3 | 36 (35.6%) | 45 (69.2%) | 68 (46.3%) | 13 (68.4%) | |||
| No. tumours | Single | 78 (76.5%) | 26 (40.6%) | <0.001 | 98 (66.2%) | 6 (33.3%) | 0.006 |
| Multiple | 24 (23.5%) | 38 (59.4%) | 50 (33.8%) | 12 (66.7%) | |||
| Smoking | 48 (64%) | 39 (86.7%) | 0.007 | 77 (70.6%) | 10 (90.9%) | 0.15 | |
mean ± standard deviation;
median(minimum-maximum); BCG: Bacillus Calmette-Guerin; MPV: mean platelet volume; NLR: neutrophil-lymphocyte ratio; RDW: red cell distribution.
After Spearman’s correlation analysis there was positive correlation between tumour number and recurrence (r = 0.361, p < 0.001). Recurrence rates were significantly higher in patients with multiple tumours than in patients with a single tumour (59.4% vs. 40.6%, respectively, p < 0.001). There was also a positive correlation between tumour numbers and progression (r = 0.211, p = 0.006). Progression rates were significantly higher in patients with multiple tumours than in patients with a single tumour (66.7% vs. 33.3%, respectively, p = 0.006) (Table 1). Similarly, there was a positive correlation between re-TUR and recurrence (r = 0.25, p = 0.001); recurrence rates were significantly higher in patients with re-TUR than in patients who were treated once (41.3% vs. 18.4%, respectively, p = 0.001). There was no statistically significant relation between progression rates and re-TUR (p = 0.080).
The Spearman’s correlation test revealed a statistically significant relation between smoking and reccurence (r = 0.246 p = 0.007). Among the patients in the smoking group, 48 (64%) had reccurence, while 36 (86.7%) did not. Consequently, there was a statistically significant relation between smoking and reccurence (p = 0.007).
The NLR was 2.8 ± 2.7 (range: 1.4–16.8) in the recurrence group and 2.2 ± 1.2 (range: 0.7–8.2) in the non-recurrence group. A significant correlation was found between recurrence and the NLR. According to receiver operating characteristic (ROC) analysis, the optimum cut-off value for the NLR was ≥2.43 (74% sensitivity, 60% specificity, p < 0.001; area under the curve [AUC] 0.687, 95% CI, 0.607–0.767) (Fig. 1). The NLR was 2.6 ± 3.4 (range: 1.9–16.8) in the progression group and 2.5 ± 1.7 (range: 0.7–16.3) in the non-progression group. No significant correlation was found between progression and the NLR (p = 0.34) (Table 1).
Fig. 1.
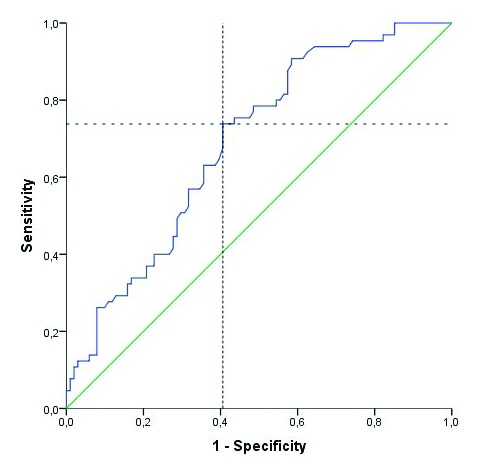
Assesment of cut off value of neutrophil-to-lymphocyte ratio to predict recurrence.
In univariate analyses, tumour size greater than 3 cm, multiple tumors, and NLR of ≥2.43 were risk factors for recurrence. Multivariate logistic regression analysis determined that the following factors were independent predictors of recurrence in patients with high-grade pT1 non-muscle-invasive bladder cancer: tumour number (OR 5.32, 95% CI 2.10–12.90), NLR of ≥2.43 (OR 2.587; 95% CI 1.156–5.789), and smoking (OR 4.17, 95% CI 1.31–13.21) (Table 2). Since there was a significant correlation between tumour size and number, only tumour number was used in the multivariate analysis.
Table 2.
Univariate and multivariate logistic regression analysis of independent predictive factors for recurrence in high–grade pT1 bladder cancer
| Reference | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| OR | 95% CI | p value | OR | 95% CI | p value | ||
| Age | ≥65 | 0.95 | 0.50–1.81 | 0.87 | 1.09 | 0.42–2.85 | 0.84 |
| Sex | Female | 2.29 | 0.75–6.93 | 0.13 | 2.58 | 0.40–16.64 | 0.31 |
| BCG Usage | 0.66 | 0.33–1.32 | 0.24 | 0.75 | 0.33–2.23 | 0.75 | |
| No. tumours | Multiple | 4.75 | 2.41–9.35 | <0.001 | 5.32 | 2.1–12.9 | <0001 |
| NLR | ≥2.43 | 4.29 | 2.15–8.54 | <0.001 | 3.81 | 1.50–9.67 | 0.005 |
| Smoking | 3.66 | 1.37–9.74 | 0.007 | 4.17 | 1.31–13.210 | 0.015 | |
OR: odds ratio; CI: confidence interval; BCG: Bacillus Calmette–Guerin; NLR: neutrophil–lymphocyte ratio.
Multivariate logistic regression analysis determined only tumour number as an independent predictive factor to determine progression in patients with high-grade pT1 non-muscle-invasive bladder cancer (OR 3.29; 95% CI 1.08–10.06) (Table 3).
Table 3.
Univariate and multivariate logistic regression analysis of independent predictive factors for progression in high-grade pT1 bladder cancer
| Reference | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| OR | 95% CI | p value | OR | 95% CI | p value | ||
| Age | ≥65 | 0.9 | 0.33–2.47 | 0.842 | 0.73 | 0.25–2.15 | 0.570 |
| Sex | Female | 2.5 | 0.62–9.93 | 0.183 | 1.97 | 0.43–9.03 | 0.383 |
| BCG Usage | 0.39 | 0.11–1.42 | 0.141 | 0.47 | 0.13–1.77 | 0.265 | |
| No. tumours | Multiple | 3.92 | 1.4–11.06 | 0.006 | 3.29 | 1.08–10.06 | 0.036 |
| NLR | ≥2.43 | 1.79 | 0.64–5.04 | 0.262 | 1.47 | 0.49–4.38 | 0.490 |
| Smoking | 1.52 | 0.57–4.1 | 0.403 | 1.08 | 0.37–3.18 | 0.891 | |
OR: odds ratio; CI: confidence interval; BCG: Bacillus Calmette-Guerin; NLR: neutrophil-lymphocyte ratio.
Discussion
Many studies have demonstrated a link between systemic inflammation and cancer development and prognosis.6,14–16 The NLR indicates the presence of systemic inflammation. The correlation between a high NLR and poor prognosis and recurrence in patients with cancer has been confirmed.9,13,17–22 In the present study, the optimal cut-off value of the NLR as an indicator of recurrence and progression in patients with bladder cancer was ≥2.43. Patients with an NLR above this value exhibited significantly higher recurrence, but no progression, highlighting the potential role of the NLR in pT1 superficial bladder tumours. There is limited information on this topic in the literature. To our knowledge, this is the first clinical study investigating the correlation between the NLR and recurrence in patients with only high-grade pT1 bladder tumours.
The neutrophil and lymphocyte counts play important roles in systemic inflammation. The neutrophil count is increased by anti-apoptotic markers that affect tumour growth and progression (NF-κB), growth factors, and proangiogenic factors (VEGF).23–25 The lymphocytic response is the main component in the control of cancer progression. Lymphocytopaenia leads to a decrease in the cellular immune response. While some studies have reported that decreased T-cell activity inside the tumour speeds up primary tumour progression, other studies have shown a link between lymphocytes and the cell-mediated response with respect to tumour infiltration;. Additionally, a low level of lymphocytic infiltration at tumour margins indicates a poor prognosis.17,26,27 The fact that the NLR indicates only part of the inflammatory response somewhat limits its value as a marker. However, the NLR is still a convenient, inexpensive, and reproducible parameter that demonstrates the link between inflammation and tumour development.
Few studies have examined the link between an increased NLR and muscle-invasive bladder cancers. Some of these studies have shown that the risk of invasion significantly increases beyond a certain cut-off NLR value and that a high NLR is significantly correlated with the pathologic stage.28–30 In a recent study that evaluates the NRL in terms of reccurence and progression in non-invasive bladder cancer, the NLR cut-off point for reccurence and progression was 2.43 and 2.41, respectively. A statistically significant increase in terms of reccurence and progression has been found among individuals with NLR levels over the cut-off limit. A positive correlation between tumour grade and higher levels of NRL has been found as well.31 In our study, while the cut-off limit for NLR was able to establish a significant difference in reccurence, we could not find a significant difference regarding progression. In the study mentioned above patients with pTa and pT1 tumours were evaluated;31 in our study, however, we evaluated only high-grade pT1 tumours. Therefore we considered our study more significant regarding case homogenity and number.
Several recent studies also examined the link between the preoperative NLR and recurrence of other types of cancer. Motomura and colleagues determined a NLR cut-off value of ≥4 for recurrence of hepatocellular carcinoma.19 Mallappa and colleagues found that the stage of colorectal cancer and a preoperative NLR of >5 were important independent risk factors for recurrence.21 In a study of recurrence of clear-cell renal carcinoma, Ohno and colleagues reported a significant difference in the 10-year survival rate between patients with an NLR of ≥2.7 and those with an NLR of <2.7.22 All of these findings are consistent with the results obtained in our present study regarding the NLR cut-off value beyond which recurrence significantly increased.
In a study carried out with patients on sunitinib for meta-static renal cell carcinoma, Keizman and colleagues found that NLR values lower than 3 prior to treatment correlated with overall survival and progression-free survival.32 In a recent meta-analysis evaluating the role of NLR in solid tumours, overall survival, progression-free survival and recurrence-free survival negatively correlated with NRL values over the cut-off point.33 Contrary to previous studies, in our study, no statistically correlation was found between NLR and progression.
Conclusion
Our results indicate that a high preoperative NLR can play an important role in determining recurrence of superficial transitional cell type high-grade pT1 bladder tumours. However, it did not accurately predict progression in this study, possibly because of the short follow-up period and small sample size. Prospective studies are required to validate the role of NLR as a prognostic marker in high-grade pT1 bladder cancers.
Footnotes
Competıng interests: Authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–41. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology. 2005;66:4–34. doi: 10.1016/j.urology.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Oosterlinck W, Sylvester R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008;54:303–14. doi: 10.1016/j.eururo.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 4.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–5. doi: 10.1016/j.eururo.2005.12.031. discussion 465–7. [DOI] [PubMed] [Google Scholar]
- 5.Bertz S, Otto W, Denzinger S, et al. Combination of CK20 and Ki-67 immunostaining analysis predicts recurrence, progression, and cancer-specific survival in pT1 urothelial bladder cancer. Eur Urol. 2014;65:218–26. doi: 10.1016/j.eururo.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 7.Kishi Y, Kopetz S, Chun YS, et al. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16:614–22. doi: 10.1245/s10434-008-0267-6. [DOI] [PubMed] [Google Scholar]
- 8.Yamanaka T, Matsumoto S, Teramukai S, et al. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–20. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 9.Cho H, Hur HW, Kim SW, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohno Y, Nakashima J, Ohori M, et al. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of recurrence in patients with nonmetastatic renal cell carcinoma. J Urol. 2010;184:873–8. doi: 10.1016/j.juro.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Lang BH, Ng CP, Au KB, et al. Does preoperative neutrophil lymphocyte ratio predict risk of recurrence and occult central nodal metastasis in papillary thyroid carcinoma? World J Surg. 2014;38:2605–12. doi: 10.1007/s00268-014-2630-z. [DOI] [PubMed] [Google Scholar]
- 12.Liao W, Zhang J, Zhu Q, et al. Preoperative neutrophil-to-lymphocyte ratio as a new prognostic marker in hepatocellular carcinoma after curative resection. Transl Oncol. 2014;7:248–55. doi: 10.1016/j.tranon.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao SC, Pavlakis N, Harvie R, et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res. 2010;16:5805–13. doi: 10.1158/1078-0432.CCR-10-2245. [DOI] [PubMed] [Google Scholar]
- 14.Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24:1997–2005. doi: 10.1200/JCO.2005.03.9594. [DOI] [PubMed] [Google Scholar]
- 15.Ilie M, Hofman V, Ortholan C, et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophilto-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012;118:1726–37. doi: 10.1002/cncr.26456. [DOI] [PubMed] [Google Scholar]
- 16.Jensen TO, Schmidt H, Moller HJ, et al. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer. 2012;118:2476–85. doi: 10.1002/cncr.26511. [DOI] [PubMed] [Google Scholar]
- 17.Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–4. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 18.Ubukata H, Motohashi G, Tabuchi T, et al. Evaluations of interferon-gamma/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol. 2010;102:742–7. doi: 10.1002/jso.21725. [DOI] [PubMed] [Google Scholar]
- 19.Motomura T, Shirabe K, Mano Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58:58–64. doi: 10.1016/j.jhep.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Sejima T, Iwamoto H, Morizane S, et al. The significant immunological characteristics of peripheral blood neutrophil-to-lymphocyte ratio and Fas ligand expression incidence in nephrectomized tumor in late recurrence from renal cell carcinoma. Urol Oncol. 2013;31:1343–9. doi: 10.1016/j.urolonc.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Mallappa S, Sinha A, Gupta S, et al. Preoperative neutrophil to lymphocyte ratio >5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis. 2013;15:323–8. doi: 10.1111/codi.12008. [DOI] [PubMed] [Google Scholar]
- 22.Ohno Y, Nakashima J, Ohori M, et al. Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol. 2012;187:411–7. doi: 10.1016/j.juro.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short-and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–24. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 24.Halazun KJ, Hardy MA, Rana AA, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141–51. doi: 10.1097/SLA.0b013e3181a77e59. [DOI] [PubMed] [Google Scholar]
- 25.Jung MR, Park YK, Jeong O, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;104:504–10. doi: 10.1002/jso.21986. [DOI] [PubMed] [Google Scholar]
- 26.Gomez D, Farid S, Malik HZ, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–62. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- 27.Ali AA, McMillan DC, Matalka II, et al. Tumour T-lymphocyte subset infiltration and tumour recurrence following curative resection for colorectal cancer. Eur J Surg Oncol. 2004;30:292–5. doi: 10.1016/j.ejso.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Can C, Baseskioglu B, Yilmaz M, et al. Pretreatment parameters obtained from peripheral blood sample predicts invasiveness of bladder carcinoma. Urologia Int. 2012;89:468–72. doi: 10.1159/000343278. [DOI] [PubMed] [Google Scholar]
- 29.Ceylan C, Doluoglu OG, Keles I, et al. Importance of the neutrophil-to-lymphocyte ratio in muscle-invasive and non-muscle invasive bladder tumors. Urologia. 2014;81:120–4. doi: 10.5301/uro.5000031. [DOI] [PubMed] [Google Scholar]
- 30.Kaynar M, Yildirim ME, Badem H, et al. Bladder cancer invasion predictability based on preoperative neutrophil-lymphocyte ratio. Tumour Biol 2014. 2014;35:6601–5. doi: 10.1007/s13277-014-1889-x. . Epub 2014 Apr 3. [DOI] [PubMed] [Google Scholar]
- 31.Mano R, Baniel J, Shoshany O, et al. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancer. Urol Oncol. 2014. [In press]; [DOI] [PubMed]
- 32.Keizman D, Ish-Shalom M, Huang P, et al. The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer. 2012;48:202–8. doi: 10.1016/j.ejca.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Instit. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]


