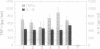Abstract
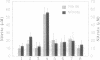
Human monocytes (M phi) require stimulation with substances such as bacterial endotoxin [LPS (lipopolysaccharide)] to produce angiogenic activity. In this study, we report that stimulation of M phi with LPS (5 micrograms/ml) in the absence of L-arginine greatly reduced their production of angiogenic activity, as assessed in vivo in rat corneas and in vitro by chemotaxis of human umbilical vein endothelial cells (HU-VECs). D-Arginine did not substitute for L-arginine in the production of angiogenic activity. The nitric oxide synthase (NO synthase, EC 1.14-13.39) inhibitors NG-monomethyl-L-arginine (L-NMMA) and NG-nitro-L-arginine methyl ester (L-NAME) both inhibited the production of angiogenic activity by LPS-stimulated M phi in the presence of L-arginine, suggesting the involvement of this enzyme in the pathway that generates angiogenic activity. Neither of these substances directly inhibited the M phi-derived angiogenic activity. LPS-induced production of the cytokines tumor necrosis factor alpha (TNF-alpha) and interleukin 8 (IL-8) was not significantly reduced when M phi were incubated in the absence of L-arginine. Similarly, L-NMMA and L-NAME did not significantly reduce the LPS-induced production of these cytokines by M phi in the presence of L-arginine. These results suggest that the LPS-stimulation-dependent generation of angiogenic activity by M phi requires an L-arginine-dependent NO-synthase effector mechanism that may be independent of the generation of TNF-alpha and IL-8.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Bartholomew B. A rapid method for the assay of nitrate in urine using the nitrate reductase enzyme of Escherichia coli. Food Chem Toxicol. 1984 Jul;22(7):541–543. doi: 10.1016/0278-6915(84)90224-2. [DOI] [PubMed] [Google Scholar]
- Efron D. T., Kirk S. J., Regan M. C., Wasserkrug H. L., Barbul A. Nitric oxide generation from L-arginine is required for optimal human peripheral blood lymphocyte DNA synthesis. Surgery. 1991 Aug;110(2):327–334. [PubMed] [Google Scholar]
- Fràter-Schröder M., Risau W., Hallmann R., Gautschi P., Böhlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Gilliam M. B., Sherman M. P., Griscavage J. M., Ignarro L. J. A spectrophotometric assay for nitrate using NADPH oxidation by Aspergillus nitrate reductase. Anal Biochem. 1993 Aug 1;212(2):359–365. doi: 10.1006/abio.1993.1341. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Leapman S. B., Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst. 1974 Feb;52(2):413–427. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- Good D. J., Polverini P. J., Rastinejad F., Le Beau M. M., Lemons R. S., Frazier W. A., Bouck N. P. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Haskill S., Johnson C., Eierman D., Becker S., Warren K. Adherence induces selective mRNA expression of monocyte mediators and proto-oncogenes. J Immunol. 1988 Mar 1;140(5):1690–1694. [PubMed] [Google Scholar]
- Hunt N. C., Goldin R. D. Nitric oxide production by monocytes in alcoholic liver disease. J Hepatol. 1992 Mar;14(2-3):146–150. doi: 10.1016/0168-8278(92)90150-n. [DOI] [PubMed] [Google Scholar]
- Hunt T. K., Knighton D. R., Thakral K. K., Goodson W. H., 3rd, Andrews W. S. Studies on inflammation and wound healing: angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery. 1984 Jul;96(1):48–54. [PubMed] [Google Scholar]
- Ialenti A., Ianaro A., Moncada S., Di Rosa M. Modulation of acute inflammation by endogenous nitric oxide. Eur J Pharmacol. 1992 Feb 11;211(2):177–182. doi: 10.1016/0014-2999(92)90526-a. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol. 1991 Feb 15;41(4):485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Hunt T. K., Scheuenstuhl H., Halliday B. J., Werb Z., Banda M. J. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983 Sep 23;221(4617):1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Phillips G. D., Fiegel V. D. Wound healing angiogenesis: indirect stimulation by basic fibroblast growth factor. J Trauma. 1990 Dec;30(12 Suppl):S134–S144. [PubMed] [Google Scholar]
- Koch A. E., Burrows J. C., Polverini P. J., Cho M., Leibovich S. J. Thiol-containing compounds inhibit the production of monocyte/macrophage-derived angiogenic activity. Agents Actions. 1991 Nov;34(3-4):350–357. doi: 10.1007/BF01988728. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Cho M., Burrows J. C., Polverini P. J., Leibovich S. J. Inhibition of production of monocyte/macrophage-derived angiogenic activity by oxygen free-radical scavengers. Cell Biol Int Rep. 1992 May;16(5):415–425. doi: 10.1016/s0309-1651(06)80061-5. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Cho M., Burrows J., Leibovich S. J., Polverini P. J. Inhibition of production of macrophage-derived angiogenic activity by the anti-rheumatic agents gold sodium thiomalate and auranofin. Biochem Biophys Res Commun. 1988 Jul 15;154(1):205–212. doi: 10.1016/0006-291x(88)90671-7. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Kunkel S. L., Harlow L. A., DiPietro L. A., Elner V. M., Elner S. G., Strieter R. M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992 Dec 11;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Leibovich S. J. Induction of neovascularization by activated human monocytes. J Leukoc Biol. 1986 Feb;39(2):233–238. doi: 10.1002/jlb.39.2.233. [DOI] [PubMed] [Google Scholar]
- Kohno T., Brewer M. T., Baker S. L., Schwartz P. E., King M. W., Hale K. K., Squires C. H., Thompson R. C., Vannice J. L. A second tumor necrosis factor receptor gene product can shed a naturally occurring tumor necrosis factor inhibitor. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8331–8335. doi: 10.1073/pnas.87.21.8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon N. S., Nathan C. F., Stuehr D. J. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989 Dec 5;264(34):20496–20501. [PubMed] [Google Scholar]
- Leibovich S. J., Polverini P. J., Shepard H. M., Wiseman D. M., Shively V., Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987 Oct 15;329(6140):630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- Mace K. F., Ehrke M. J., Hori K., Maccubbin D. L., Mihich E. Role of tumor necrosis factor in macrophage activation and tumoricidal activity. Cancer Res. 1988 Oct 1;48(19):5427–5432. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moroco J. R., Solt D. B., Polverini P. J. Sequential loss of suppressor genes for three specific functions during in vivo carcinogenesis. Lab Invest. 1990 Sep;63(3):298–306. [PubMed] [Google Scholar]
- Muñoz-Fernández M. A., Fernández M. A., Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-alpha and IFN-gamma through a nitric oxide-dependent mechanism. Immunol Lett. 1992 Jun;33(1):35–40. doi: 10.1016/0165-2478(92)90090-b. [DOI] [PubMed] [Google Scholar]
- Philip R., Epstein L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages--induction by multiple nonphagocytic stimuli. Cell Immunol. 1981 Apr;59(2):301–318. doi: 10.1016/0008-8749(81)90411-1. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran R. S., Sholley M. M. Endothelial proliferation in the delayed hypersensitivity reaction: an autoradiographic study. J Immunol. 1977 Feb;118(2):529–532. [PubMed] [Google Scholar]
- Polverini P. J., Leibovich S. J. Induction of neovascularization and nonlymphoid mesenchymal cell proliferation by macrophage cell lines. J Leukoc Biol. 1985 Mar;37(3):279–288. doi: 10.1002/jlb.37.3.279. [DOI] [PubMed] [Google Scholar]
- Rastinejad F., Polverini P. J., Bouck N. P. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989 Feb 10;56(3):345–355. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Salvemini D., de Nucci G., Gryglewski R. J., Vane J. R. Human neutrophils and mononuclear cells inhibit platelet aggregation by releasing a nitric oxide-like factor. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6328–6332. doi: 10.1073/pnas.86.16.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckinger P., Vey E., Turcatti G., Wingfield P., Dayer J. M. Tumor necrosis factor inhibitor: purification, NH2-terminal amino acid sequence and evidence for anti-inflammatory and immunomodulatory activities. Eur J Immunol. 1990 May;20(5):1167–1174. doi: 10.1002/eji.1830200533. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Jaraki O., Osborne J., Simon D. I., Keaney J., Vita J., Singel D., Valeri C. R., Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Rolfe M. W., Evanoff H. L., Allen R. M., Strieter R. M. Regulation of human alveolar macrophage- and blood monocyte-derived interleukin-8 by prostaglandin E2 and dexamethasone. Am J Respir Cell Mol Biol. 1992 Jan;6(1):75–81. doi: 10.1165/ajrcmb/6.1.75. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Cho H. J., Kwon N. S., Weise M. F., Nathan C. F. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderkötter C., Goebeler M., Schulze-Osthoff K., Bhardwaj R., Sorg C. Macrophage-derived angiogenesis factors. Pharmacol Ther. 1991;51(2):195–216. doi: 10.1016/0163-7258(91)90077-y. [DOI] [PubMed] [Google Scholar]
- Wiseman D. M., Polverini P. J., Kamp D. W., Leibovich S. J. Transforming growth factor-beta (TGF beta) is chemotactic for human monocytes and induces their expression of angiogenic activity. Biochem Biophys Res Commun. 1988 Dec 15;157(2):793–800. doi: 10.1016/s0006-291x(88)80319-x. [DOI] [PubMed] [Google Scholar]
- Wright C. E., Rees D. D., Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992 Jan;26(1):48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Yu S. F., Koerner T. J., Adams D. O. Gene regulation in macrophage activation: differential regulation of genes encoding for tumor necrosis factor, interleukin-1, JE, and KC by interferon-gamma and lipopolysaccharide. J Leukoc Biol. 1990 Nov;48(5):412–419. doi: 10.1002/jlb.48.5.412. [DOI] [PubMed] [Google Scholar]
- Yu S. F., Koerner T. J., Adams D. O. Gene regulation in macrophage activation: differential regulation of genes encoding for tumor necrosis factor, interleukin-1, JE, and KC by interferon-gamma and lipopolysaccharide. J Leukoc Biol. 1990 Nov;48(5):412–419. doi: 10.1002/jlb.48.5.412. [DOI] [PubMed] [Google Scholar]