Abstract
In muscle responses of proprioceptive origin, including the stretch/tendon reflex (T-reflex), the corresponding reciprocal excitation and irradiation to distant muscles have been described from newborn infants to older adults. However, the functioning of other responses mediated primarily by Ia-afferents has not been investigated in infants. Understanding the typical development of these multiple pathways is critical to determining potential problems in their development in populations affected by neurological disease, such as spina bifida or cerebral palsy. Hence, the goal of the present study was to quantify the excitability of Ia-mediated responses in lower limb muscles of infants with typical development. These responses were elicited by mechanical stimulation applied to the distal tendons of the gastrocnemius-soleus (GS), tibialis anterior (TA) and quadriceps (QAD) muscles of both legs in twelve 2- to 10-month-old infants and recorded simultaneously in antagonist muscle pairs by surface EMG. Tendon taps alone elicited responses in either, both or neither muscle. The homonymous response (T-reflex) was less frequent in the TA than the GS or QAD muscle. An 80 Hz vibration superimposed on tendon taps induced primarily an inhibition of monosynaptic responses; however, facilitation also occurred in either muscle of the recorded pair. These responses were not influenced significantly by age or gender. Vibration alone produced a tonic reflex response in the vibrated muscle (TVR) and/or the antagonist muscle (AVR). However, for the TA muscle the TVR was more frequently elicited in older than younger infants. High variability was common to all responses. Overall, the random distribution and inconsistency of muscle responses suggests that the gain of Ia-mediated feedback is unstable. We propose that during infancy the central nervous system needs to learn to set stable feedback gain, or destination of proprioceptive assistance, based on their use during functional movements. This will tailor the neuromuscular connectivity to support adaptive motor behaviors.
Keywords: T-reflex, Tonic vibration reflex, Development, Proprioception
Introduction
Muscle proprioceptive information transmitted from stretch receptors is known to supply feedback to homonymous muscle motoneurons via two pathways: the Ia monosynaptic pathway, mediating the stretch reflex, and polysynaptic pathways, contributing to the tonic vibration reflex (Desmedt and Godaux 1978; Lackner et al. 1992; Martin and Park 1997; Park and Martin 1993; Romaiguere et al. 1991, 1993) in conjunction with the monosynaptic pathway (Romaiguere et al. 1991). In addition, the monosynaptic pathway is also modulated peripherally by an autogenic presynaptic inhibition (see for review Schieppati 1987), as illustrated by vibration-induced inhibition of the monosynaptic reflex in human adults (Lance 1968; Desmedt and Godaux 1978; Martin et al. 1984, 1986; Bove et al. 2003), reflex hyperactivity in spasticity (Ashby et al. 1974; Faist et al. 1994; Nielsen et al. 1995; Morita et al. 2001) and an homosynaptic depression of transmitter release by Ia-afferents immediately following their activation by muscle stretch, also called post-activation depression (Nielsen et al. 1995; Hultborn et al. 1996). The stability of these Ia-mediated responses as well as the context dependency of their organization, such as reversal of the monosynaptic reflex (Lacquaniti et al. 1991) modulation of the heteronymous monosynaptic Ia facilitation (Hultborn et al. 1987; Katz et al. 1988; Morita et al. 1995) and redirection of the TVR expression into an antagonist vibration response named AVR (Roll et al. 1980a; Feldman and Latash 1982; Calvin-Figuiere et al. 1999) are clearly defined in adults.
Investigations of the myotatic response as a function of development in young infants from a few days old (Mayer and Mosser 1969; Myklebust and Gottlieb 1993; Prechtl et al. 1967) to several months and years of age (O'Sullivan et al. 1991, 1998) have pointed out the versatility of the stretch reflex. In leg muscles, large variability in homonymous monosynaptic responses as well as heteronymous responses such as “reciprocal excitation” of antagonist muscles, possibly via Ia monosynaptic projections (Myklebust et al. 1986), and irradiation to muscles of the adjacent limb segment excitations are commonly observed (Myklebust et al. 1986; O'Sullivan et al. 1991; Myklebust and Gottlieb 1993; Leonard et al. 1995). Myklebust (1990) suggested that “several monosynaptic and oligosynaptic pathways for stretch reflexes may coexist at the time of birth; different circuits…may be activated by successive tendon taps” (p. 193). Then, the heterogeneity of Ia excitatory projections “become restricted and focused during the first four postnatal years” (Myklebust 1990; O'Sullivan et al. 1991, 1998), except in patients affected by neurological diseases such as spasticity (Lance and Degail 1965; Myklebust 1990; O'Sullivan et al. 1998).
Other destinations of Ia messages, including the homonymous and heteronymous Ia polysynaptic pathways, mediating tonic vibration-induced reflex responses, and the presynaptic auto-inhibition of the myotatic reflex, contributing to vibration-induced modulation of the T-reflex have not been considered in infants during development. Hence, the diversity of multiple excitatory and/or inhibitory Ia pathways has not been fully investigated in young infants and the emergence of this finely responsive underlying mechanistic system is not described. Furthermore, the quantification of the stretch reflex in infants was derived from an exclusive use of hand-held percussive devices, including most commonly a reflex hammer, although a hand-held electromagnetic hammer was used by one group of investigators (O'Sullivan et al. 1991). Hence, previous methods included an inherent source of variability/instability of the mechanical stimulation since muscle stretch responses are known to be sensitive to angle of stimulus application, force, velocity, and location on the tendon, which are particularly difficult to control in infants and young children. Further, few studies have systematically examined both limbs, multiple muscles and their antagonist within each limb, across the first year post-birth. The ratio of responses obtained relative to the number of attempts to elicit a response, that is, the consistency or stability of responses is not typically reported either. Finally, the patterns of responses indicating whether responses in antagonist muscles are exclusive of each other, simultaneously present or absent are neither described nor analyzed.
The current state of the literature suggests that the responses associated with the multiple Ia pathways need to be assessed in a more controlled manner in young infants with typical development (TD). A description of the initial distribution of Ia information is expected to lead to a better understanding of reflex mechanisms organization and possibly determine the effects of neurological diseases on peripheral proprioceptive loops. Hence, the aims of the present study were to determine the behavior of Ia-based feedbacks and gain a deeper understanding of radiation and organization of proprioceptive feedbacks in infants with typical development, prior to walking onset. A testing apparatus was designed to deliver standardized stimuli in order to assess the multiple responses in the thigh and shank muscles of both legs. Responses recorded simultaneously in antagonistic muscle pairs were quantified in terms of magnitude (peak-to-peak amplitude), ratios (number of responses obtained relative to the number of stimulations) and patterns (response in agonist only, or in both muscle…).
Method
Subjects
Twelve infants with typical development (TD) ranging in age from 2 to 10 months participated in the study. All participants were born full term and with no known cognitive or motor impairments. Infants were recruited from the city area through newspaper advertisements, websites, fliers and word-of-mouth communication. The study was approved by the University of Michigan Institutional Review Board, and all parents gave informed consent before inclusion of the infants into the study. Participants were divided into a younger (1 male, 5 females; age 2–5 months) and older (3 males, 3 females; age 7–10 months) group. These age groups represent distinct periods related to observed levels of leg control. Between 2 and 5 months of age, few motor milestones for leg function are achieved while 7–10 months spans a period during which infants learn to sit independently, creep and crawl (Bayley 2005).
Experimental setup
The infants were tested in a quiet room. The monosynaptic tendon reflex (T-reflex), the vibration-induced modulation of the tendon reflex (VIM-T-reflex) and the tonic vibration-induced reflex (VIR: TVR for the stimulated muscle and AVR for its antagonist) were tested in the left and right gas-trocnemius-soleus (GS), tibialis anterior (TA) and quadriceps (QAD) muscles. Infants were seated in a comfortable position on a custom-designed padded seat. The inclination of the seat back support and height of the seat pan and its depth were adjustable to accommodate differences in anthropometry and posture of infants as a function of the muscle tested (Fig. 1).
Fig. 1.
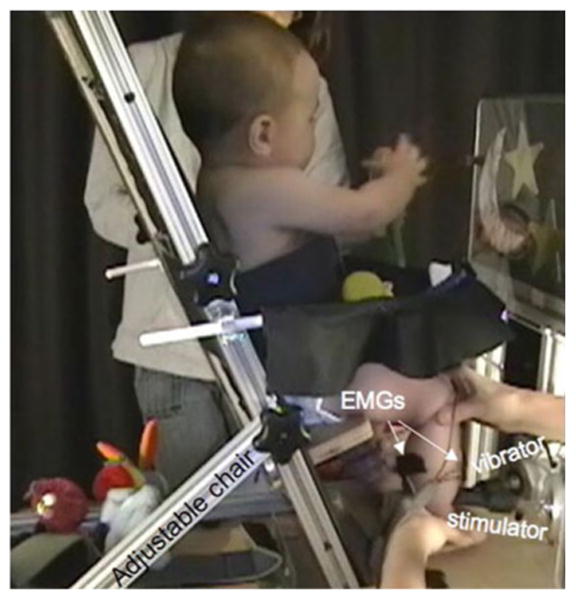
Illustration of the infant reflex testing chair and stimulus apparatus. The configuration corresponds to the stimulation of the tibialis anterior muscle
Electromyographic activity was recorded by preamplified pediatric bipolar surface electrodes (Noraxon™ 272, 1.7 cm center to center) placed on the belly of each pair of muscles (QAD/Hamstring (HS), GS/TA and TA/GS), as each was tested, to identify radiation/distribution of responses between the stimulated muscle and its antagonist. Electromagnetic stimulators equipped with smooth hammer heads made of polycarbonate plastic (see Fig. 1) delivered the tendon taps and vibratory stimuli (Roll et al. 1980b; Martin et al. 1984, 1986; Martin and Park 1997). As opposed to manual hammers commonly used for clinical assessments, the use of an electromagnetic hammer allows the production of standardized and calibrated tendon taps, which minimizes inter-stimuli variations that would other-wise contribute to large reflex-response variations. In all configurations, the mechanical stimuli were applied perpendicularly to the distal tendon of the muscle tested. The stimulator heads were adjusted to be placed in contact with the tendon, and the foot was held by one of the experimenters to keep the tendon and joint in appropriate alignment. For testing responses associated with GS stimulation, the seat back was inclined 30° from vertical, hip was slightly flexed, knee was extended and ankle flexed to 90° with probes positioned over the Achilles tendon. For the TA and QAD, the seat back was reclined only 10° from vertical, with hip and knee flexed close to 90° and ankle extended to approximately 140° for TA testing. For QAD stimulation, the probe position was just inferior to the patella. For GS stimulation, the probe was positioned over the Achilles' tendon just above the calcaneus. For TA stimulation, the probe was positioned at the level of (or slightly above) the medial malleolus. Care was taken to adjust the azimuth orientation of the vibrator to avoid a direct contact with the tibia. The foot was held only immediately before and during the application of stimulations to ensure a stable posture and adequate contact with the stimulator heads.
Stimulation control and data recording
A computer connected to the power amplifiers driving the stimulators controlled tendon taps and vibratory stimuli. A custom-designed Labview™ virtual instrument was used to generate the stimuli. The stimulus controlling the tendon tap consisted of a 1 V, 5 ms pulse; the mechanical amplitude, adjusted by the power amplifier corresponded to a range of 2–3 mm. The vibratory stimulus was a 1 V, 80 Hz sine wave whose mechanical amplitude, adjusted by the power amplifier, corresponded to a 40–60 μm range.
The EMG electrodes were attached to preamplifiers connected to a Noraxon™ multi-channel system. EMG signals were amplified (gain = 1,000), low pass filtered (cut off frequency = 500 Hz), digitized (sampling rate = 1,000 Hz) and recorded simultaneously with the stimuli signals. Antagonist muscle pairs were recorded simultaneously to identify “radiation” (Myklebust and Gottlieb 1993).
Procedure
Muscles were tested in a random order; however, all stimulation series corresponding to each test situation (T-reflex, VIM-T-reflex, VIR) were completed before testing another muscle using the same order. Rest periods were included between each individual stimulation and stimulation series. The T-reflex was elicited first, then VIM-T-reflex, followed by VIR. This order was chosen to avoid post-vibration effects that might have interfered with the monosynaptic responses. Any test period was suspended momentarily if the infant became agitated or too active. The T-reflex and VIM-T-reflex were evaluated from the responses elicited by 8–20 stimulations delivered with a minimal interval of 8 s. Each response was quantified from a .5 s recording triggered by the stimulation. VIR activities were quantified from 2 recordings, each including a 20-s vibration period preceded by a muscle-relaxed period and a post-vibration period of 10 s each used as references (see Fig. 4). To reduce the inherent gain variations of the tested sensorimotor pathways, stimulations were applied when the infant was calm and the leg muscles relaxed, as assessed by the experimenter observing the overall behavior of the infant while holding the tested leg in the appropriate posture. On a 1–6 scale (where 1 = asleep; 2 = drowsy, 3 = awake and quiet; 4 = awake and moving arms; 5 = fussy, mild crying; 6 = crying), the average arousal score for all babies was in the 3.4–3.8 range for all testing conditions.
Fig. 4.
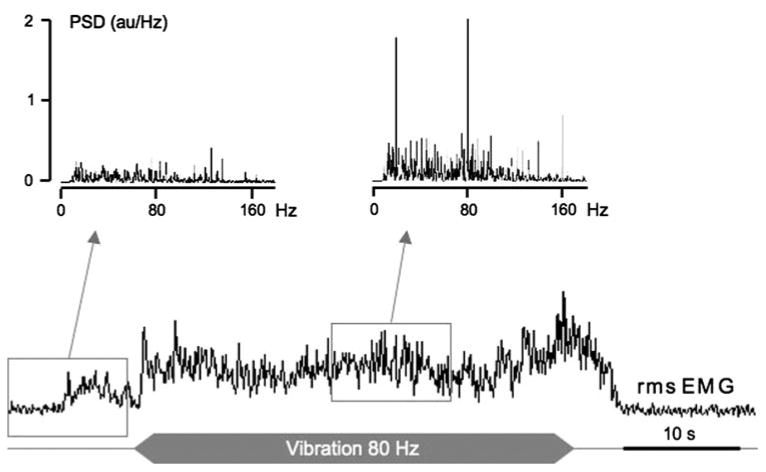
Example of a tonic vibration reflex response (TVR) elicited in the GS muscle by homonymous tendon vibration. In this example, the EMG activity increases in the vibrated muscle and the power spectrum indicates the appearance of muscle activity synchronized with the vibration frequency, which ascertains that the increase in EMG magnitude is not related to a voluntary command. The power spectra (PSD), obtained from the raw EMG signal, correspond to 10-s periods indicated by the windows
Control of mechanical spread of stimulus and response cross-talk
Despite the fact that the force of the tendon tap and placement of vibratory stimulus were controlled for each individual infant, the pattern of the antagonist muscle pairs responses differ across multiple stimulus applications during the T-reflex testing, as shown on Fig. 5a–c. For example, one tendon tap could elicit a response in the stimulated muscle (agonist) alone, while the following identical stimulus could produce the reverse pattern (activation of the antagonist without activating the agonist). In the same way, when vibration was applied (VIR responses), the power spectrum analysis showed sometimes no increase at the vibration frequency (80 Hz), sometimes a large increase in the peak at 80 Hz in the agonist muscle with no effect in the antagonist, and sometimes the peak increased only in the antagonist (see Fig. 5g–i). This shows that, even if mechanical spread to the reciprocal muscle could not be totally excluded, it also could not be accepted as a major contribution to the subsequent pattern of results. Furthermore, the randomness of response patterns, more particularly in the antagonist (non-stimulated muscle) demonstrated that responses could not be attributed to cross-talk.
Fig. 5.
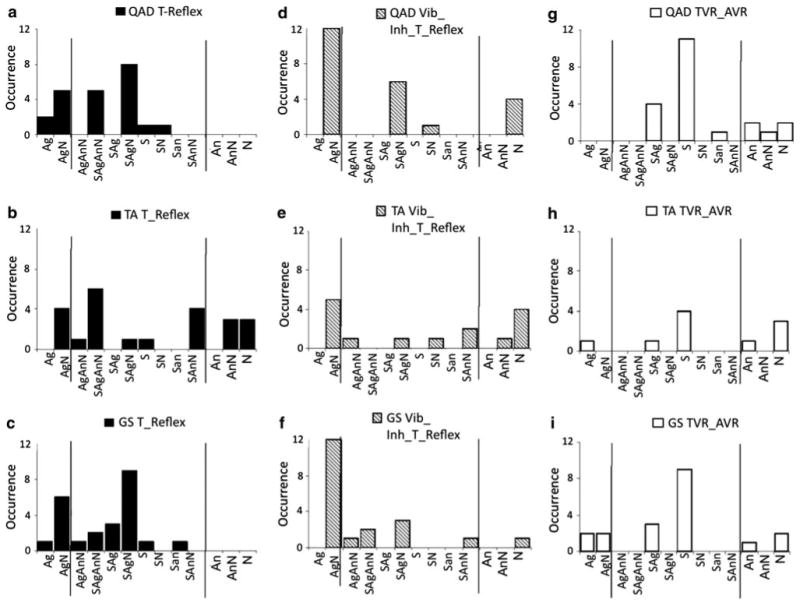
Response pattern occurrence for each stimulated muscle and stimulation type. Quadriceps (a, d and g), the tibialis anterior (b, e, h) and the gastrocnemius-soleus (c, f, i) for stimulation corresponding to T-reflex (left graphs), vibration-induced modulation of the T-reflex condition (middle graphs) and TVR/AVR (right graphs). The x axis is split in three parts that correspond to the patterns of responses elicited only in the stimulated muscle for a series of successive stimulations (i.e., Ag or AgN), responses elicited in both the antagonist muscle pair (middle) and responses evoked in the antagonist only (i.e., An, AnN). The y axis represents the number of pattern occurrence for a series of stimulations
Data processing
Initially, the raw EMG data were viewed to identify responses concurrent with or immediately following a burst of muscle contraction generated by the infant. These responses were not quantified to avoid inclusion of responses that possibly contained activity-induced variations along with superimposed facilitatory or inhibitory mechanisms. Responses were evaluated for each muscle of each antagonistic muscle pair.
T-reflex
The peak-to-peak amplitude and latency of the first peak of individual reflex responses were computed automatically using a custom-designed Labview™ virtual instrument. The reflex response was characterized by the average of individual response amplitudes and latencies, and the response ratio (number of responses observed over the total number of stimulations).
Vibration-induced modulation of T-reflex
The decrease in response magnitude and the response ratio were used to quantify the inhibitory effect resulting from vibration exposure.
Tonic vibration-induced reflexes (TVR/AVR)
The tonic vibration-induced reflexes in the stimulated muscle (TVR) and/or in its antagonist (AVR) were quantified as the increase in rms (root mean square) EMG value concomitant to a peak at the vibration frequency or an harmonic in the power spectrum of the raw EMG (see Park and Martin 1993 for procedure details). For each age group and each muscle, the percentage of response elicitation was determined by the ratio of responses over the total number of trials for the tendon of interest [24 = 6 infants × 2 legs × 2 trials].
Patterns of response between stimulated muscles and their antagonists
Four patterns of response could occur: a response in the stimulated muscle alone (Ag), a response in the antagonist muscle alone (An), a simultaneous response in both muscles (S) or no response at all (N). As the pattern of responses within an infant may vary across repeated application of the same stimulus, each child's overall response pattern to a particular stimulus was categorized by the distribution of responses observed. For example, the pattern corresponding to responses elicited only in the agonist muscle was labeled Ag, while a pattern for which responses were elicited sometimes in the agonist alone (Ag) and sometimes simultaneously in both muscles (S) was assigned an AgS response pattern.
Data analysis
Normality of dependent variables was first determined by a Kolmogorov–Smirnov test. For parametric data distributions, analyses of variance (ANOVA) were conducted to determine: (a) the main effects of age (younger, older), gender (male, female) and/or leg (right, left) for each muscle; (b) the main effects associated with muscles (GS, TA, QAD), vibration (no vibration, vibration) and their interaction on T-reflex-dependent variables, and (c) the main effects associated with age, muscle and their interaction on TVR/AVR ratios. Post hoc tests (Tukey Honestly Significant Differences—HSD—for multiple comparisons) were conducted to compare responses between muscles. When normality was violated, significance of comparisons was based on a Mann–Whitney U (MWU) test and results were expressed as median and interquartile range. For categorical data, chi-square statistics were used to analyze changes with age and vibration/no vibration, in the distribution of patterns of response (i.e., Ag, AgS). Pearson chi-square values were reported. Statistical significance was set at P ≤ .05.
Results
The ANOVAs performed for each muscle indicated that the main effects of age, gender or leg had no significant influence on T-reflex latency, or alteration by vibration (P > .1). Similarly, MWU tests show that T-reflex-response ratios are not influenced by age (P > .05). Hence, data were collapsed to quantify the respective dependent variables tested.
Quantification and qualification of responses
T-reflex
Latency
A one-way ANOVA indicated a significant difference (F(2, 63) = 17.16, P = .0001) in response latency between stimulated muscles. The mean reflex response latency for each muscle was 17.7 ms (QAD), 22.3 ms (GS) and 24.2 ms (TA). The latency was significantly shorter for the upper leg (QAD) than the lower leg (GS, TA) muscles (Tukey HSD, P < .01). Repeated-measure ANOVAs were run to test for significant differences in latencies between responses in each antagonistic muscle pair. For the QAD/HS, the latency was significantly shorter [F(1,16) = 13.95, P < .01] in the QAD [16.4 ms (SD 3.4)] than in the HS [19.8 ms (SD 4.8)]. For the GS/TA, a significant muscle pair effect was found [F(1,14) = 8.753, P < .01], with the response emerging earlier in the GS [21.3 ms (SD 1.4)] than the TA [24.1 ms (SD 2.8)]. For the TA/GS, the difference in latency between the response in the stimulated TA [24.3 ms (SD 3.3)] and response in the antagonist GS [25.7 ms (SD 4.4)] also tended to be shorter for the stimulated muscle but was not significant.
Response ratio
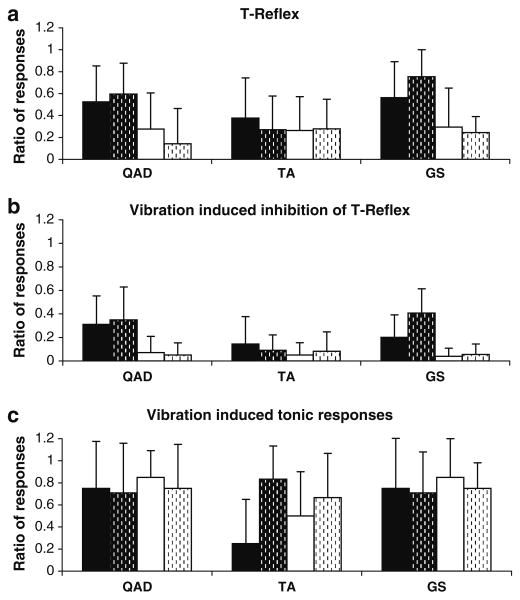
The one-way ANOVA indicated a significant difference (F(2, 63) = 7.47, P = .0012) in response ratios between stimulated muscles. The response ratios in antagonist muscle pairs were: (a) 56% (SD 29) for the QAD and 20.9% (SD 25) for the HS, (b) 66% (SD 29) for the GS and 26% (SD 30) for the TA and (c) 32% (SD 34) for the TA muscle and 27% (SD 28) for the GS. The response ratio in the stimulated muscle was significantly lower for the TA than the GS or QAD only (Tukey HSD, P < .05), as presented in Fig. 2a.
Fig. 2.
Reflex-response ratios. a T-reflex ratio between the number of responses obtained over the number of stimulations in the quadriceps (QAD), tibialis anterior (TA) and gastrocnemius-soleus (GS); b vibration-induced modulation of T-reflex; c tonic vibration-induced reflexes (TVR/AVR). The ratios are calculated for the stimulated (Agonist in black) and corresponding antagonist (in white) muscles, and for the younger (no dashes) and older (dashes) infants
Vibration-induced modulation of T-reflex
Reflex magnitude
The two-way ANOVA indicated a significant effect (P = .0001) of vibration on reflex magnitude and no statistical difference in vibration-induced effects among stimulated muscles (P = .059). Vibration induced either an inhibition or a facilitation of the reflex responses. Overall, one-way ANOVAs indicated that the average decrease in magnitude of the T-reflex responses in the stimulated muscles was statistically significant in the QAD (45.7%; F(1,45) = 30.98, P = .0001) and TA (43.2%; F(1,38) = 22.93, P = .0001) muscles and did not reach significance (21.4%; F(1,45) = 3.40, P = .07) in the GS muscle. Responses in the antagonist (non-stimulated muscles) were not significantly affected by vibration (P > .05). As observed in the stimulated muscle, vibration had a predominant inhibitory effect, but also induced facilitation in the antagonist muscle in some trials.
Response Ratio
MWU tests showed that the decrease in agonist response ratio was significant in the GS (P = .003) but not in the QAD and TA (P > .05) when vibration was applied. MWU tests also revealed that vibration had a significant inhibitory effect on antagonist muscle response ratio (P < .01 for each muscles pair; see Fig. 2b).
These results indicate that vibration mainly induced an inhibition of the T-reflex. However, variability was quite large (Fig. 3) as alternative outcomes (inhibition or facilitation) were frequently observed in the same leg or inhibition effects on one leg and facilitation in the other within the same infant.
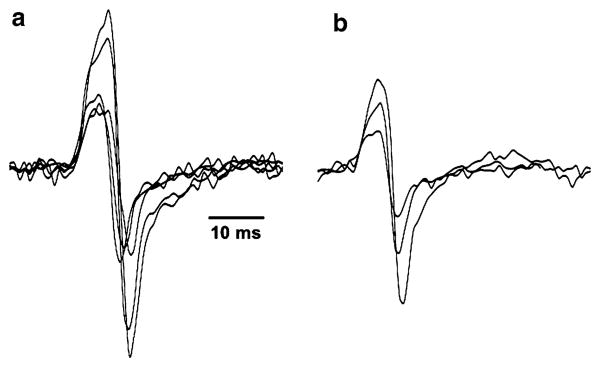
Fig. 3.
Examples of T-reflex responses obtained from the stimulation of the GS muscle of one subject in the control (a) and vibration (b) conditions. When compared to the control condition, fewer responses of smaller amplitude are generally obtained during vibration
Tonic vibration-induced reflexes (TVR/AVR)
Vibration-induced tonic activity was observed in all muscles for most infants, as illustrated in Fig. 4. The magnitude of TVR (or AVR) responses varied as a function of time during the stimulation. Increases in muscle activity during the vibration period were considered as TVR (or AVR) components only when the power spectrum of the corresponding period exhibited a large peak at the stimulation frequency or at a harmonic frequency. Therefore, spontaneous voluntary activities were eliminated from the analysis.
Overall, due to unavoidable interferences from voluntary muscle activations, it was not easy to elicit the TVR as systematically as the T-reflex and the VIM-T-reflex since infants generally do not remain stationary for 30 consecutive seconds. Nevertheless, a vibration-induced response was easier to elicit in older than younger infants for the TA muscle when the TA was the stimulated or the antagonist muscle, as indicated by a significant difference between age groups for that muscle only (MWU, P = .003) and inspection of mean values (Fig. 2c).
MWU tests on muscle (Ag vs. An) were run to determine whether the TVR occurred more frequently than the AVR. No significant difference between the two response ratios was observed when the QAD and the TA were stimulated (P > .1). When the GS was stimulated, the TVR and the AVR were as frequent in each age group (P > .1 for each); however, the number of responses increased with age (see Fig. 2c).
Patterns of response between stimulated muscles and their antagonists
For each muscle (QAD, TA and GS), a chi-square analysis was run to determine the relationship between age and the distribution of the patterns of responses for the T-reflex, VIM-T-reflex and TVR/AVR. No significant differences in the distribution of response patterns were found between the younger and the older groups for any of the muscles tested (P > .1).
T-reflex
For all infants tested, many different patterns of responses to a tendon tap stimulation were identified (Fig. 5). The variability ‘within infants’ (response patterns like SAgAnN) and ‘between infants’ (number of patterns elicited) was high for all stimulated muscles. A single pattern of EMG responses was seldom observed over a series of identical stimuli (i.e., only an Ag, S, An, or N pattern). Furthermore, the response patterns differed between legs as well. Figure 5 (panels A, B, C) shows that all possible patterns of responses were observed; indeed, when a muscle is stimulated, a response may appear in that muscle and/or its antagonist, or an absence of response(s) may occur as well. Table 1 summarizes the details illustrated in Fig. 2 by collapsing across ages and participants the percentage of responses in the agonist only (Ag), in the antagonist only (An), in both muscles (simultaneous, S) and percentage of no response (N) for each condition.
Table 1. Percentage of simple response patterns: Ag (agonist only), An (antagonist only), S (agonist and antagonist), N (no response).
| % of Ag | % of An | % of S | % of N | |
|---|---|---|---|---|
| QAD | ||||
| T-reflex | 36.4 (28.0) | 1.4 (.30) | 19.6 (25.2) | 43.2 (28.6) |
| VIM-T-reflex | 28.0 (20.1) | .0 (.00) | 6.5 (12.2) | 66.1 (24.4) |
| TVR/AVR | 9.2 (20.3) | 16.3 (33.0) | 63.8 (45.6) | 10.8 (27.4) |
| TA | ||||
| T-reflex | 16.0 (21.3) | 10.7 (13.7) | 16.3 (22.8) | 57.6 (34.7) |
| VIM-T-reflex | 14.7 (19.6) | 7.1 (10.9) | 3.9 (6.4) | 74.3 (22.1) |
| TVR/AVR | 8.3 (20.4) | 12.5 (25.1) | 45.8 (50.8) | 33.3 (49.2) |
| GS | ||||
| T-reflex | 42.3 (24.8) | 3.3 (9.7) | 23.6 (30.3) | 30.8 (29.6) |
| VIM-T-reflex | 27.2 (19.6) | 1.5 (3.6) | 3.2 (5.8) | 68.0 (18.9) |
| TVR/AVR | 19.8 (28.7) | 9.4 (18.6) | 52.5 (45.5) | 13.6 (20.5) |
Values correspond to mean (SD), n = 24 data points per muscle, T-reflex tendon (stretch)-reflex, VIM vibration-induced modulation, TVR tonic vibration reflex, AVR antagonist vibration reflex
VIM-T-reflex
Chi-square test was used to determine whether the vibration induced a change in the pattern of T-reflex responses in the antagonist muscle pairs. When vibration was applied to the QAD, EMG patterns exhibited were primarily composed of Ag or N responses. This differs from the “SAgAnN” pattern most frequently observed in the absence of vibration [χ2(6, N = 45) = 15.1, P = .019]. That is, the pattern shifted from multiple responses to patterns restricted to single type of response or no response across most trials. When vibrations were superimposed on the tendon tap at the TA and GS, the pattern of responses did not change significantly (P > .1). This lack of significance seems to be associated with a broader distribution among the possible patterns. However, a decrease in frequency of concurrent activation of the antagonist muscle pairs (S pattern) was observed during tendon vibration (Fig. 5, panels D, E, F), expressing the most common, but not exclusive, inhibitory effect of vibration.
Tonic vibration-induced reflexes (TVR/AVR)
For each muscle, a chi-square analysis was run to determine the difference in patterns of responses between the T-reflex and the VIR. For each muscle: QAD [χ2(11, N = 44) = 40.3, P < .01]; TA [χ2(11, N = 34) = 22.9, P < .01]; GS [χ2(9, N = 43) = 24.4, P < .01], the VIR induced significantly more simultaneous Ag–An responses than the T-reflex (see Fig. 2g–i). The results show that reciprocal excitation is more frequent for polysynaptic than monosynaptic responses. All response patterns were also observed, as indicated in Table 1.
Discussion
This study brings a new perspective to the understanding of neonatal Ia pathways functioning as the results reveal five major characteristics of associated motor responses in infants of typical development between 2 and 10 months of age. First, this study showed for the first time that the Ia-presynaptic inhibition pathway and the Ia-mediated polysynaptic pathway are present in infants as young as 2 months of age, but with an immature functionality (i.e., unstable gain) across the first 10 months of life. This reinforces the knowledge so far limited to the stretch reflex organization (Myklebust et al. 1986; O'Sullivan et al. 1991; Myklebust and Gottlieb 1993; Leonard et al. 1995). Second, the Vibration-induced modulation of T-reflex differs between infants and adults since facilitation could be observed as an alternative to the strict inhibition usually seen in adults. Third, the Tonic vibration-induced reflexes (TVR/AVR) also differ from the adult responses since the AVR was elicited as often as the TVR, in an apparently random manner in infants. Fourth, the functioning of Ia-mediated pathways was not influenced significantly by age or gender between 2 and 10 months of age, while motor behaviors progressed significantly during that period. Finally, significant variability (gain variations) was common to all Ia-originating responses. For each reflex pathway tested, excitation and/or inhibition of the responses in each stimulated muscle and its antagonist occurred randomly, as opposed to stable patterns observed in individuals older than 4 years.
In the following discussion, it is understood that the gain of a sensorimotor pathway includes both the excitability of the motoneurons and the strength of the sensory feedback. Reflex responses are modulated by multiple mechanisms and affected by many factors. Within the context of this experiment and results, however, some mechanisms may not be critical to the possible/likely role in the modulation of the reflex gains and will not be discussed.
Ia pathways behaviors/structures
Stretch reflex
Results from this study are in line with the large variability in response magnitude previously observed (Myklebust et al. 1986; O'Sullivan et al. 1991; Myklebust and Gottlieb 1993; Leonard et al. 1995). Our results on the latency of reflex responses, longer for two antagonists than the corresponding stimulated muscles (QAD-HS, GS-TA, but not TA-GS), may be associated with amplitude variability, as calculation was based on the time of peak amplitude. This interpretation is more likely than the proposition of an interneuron in the pathway to the antagonist muscles, as the GS (stimulated)/TA (antagonist) condition shows a latency difference while the TA (stimulated)/GS (antagonist) does not. The role of oligosynaptic pathways may not be excluded (see Lacquaniti et al. 1991). Also to be considered is the antigravity role of the GS muscle which, in adults, requires different heteronymous projections (Meunier et al. 1993).
Vibration-induced modulation of T-reflex
Unlike the strict inhibition observed in adults (Van Boxtel 1979; Roll et al. 1980b; Martin et al. 1986), our results show that vibration could also induce facilitation in infants. Thus, it is unlikely that in the present context, a major role is played by the homosynaptic depression of neurotransmitter release by Ia-afferents following their activation (Nielsen et al. 1995; Hultborn et al. 1996). Rather, the vibration-induced modulation of the tendon reflex suggests, for the first time, that the autogenic presynaptic inhibition of Ia-afferents is functioning at least by 2-months post-birth. The expression of a facilitation of the stretch reflex may simply result from an imbalance between the strength of the Ia-presynaptic inhibition and the strength of the Ia polysynaptic excitation that depolarizes the α-motoneurons. The net result is an increase in motoneuron excitability, as further emphasized in the context of tonic vibration-induced reflex responses. Hence, a decrease in strength of the presynaptic inhibition is a factor to consider in vibration-induced facilitation of the stretch reflex in infants, as opposed to the context-induced gating of alternative pathways observed in adults (Lackner and Taublieb 1983; Carson et al. 1990; Martin et al. 1990; Lackner et al. 2000). However, alternation of inhibition and facilitation of the T-reflex appears random, although skewed toward the prevalence of inhibition corresponding to a resultant low gain setting.
Tonic vibration-induced reflexes (TVR/AVR)
In adults, the tonic muscle contraction induced by tendon vibration is primarily mediated by spinal and supraspinal polysynaptic pathways and, to a lesser extent by the monosynaptic pathway (Desmedt and Godaux 1978; Romaiguere et al. 1991). Our results highlight the presence of the TVR in infants, which indicates that polysynaptic pathways are functioning by 2-months post-birth. It also supports the assumption that these pathways can modulate the expression of the stretch reflex. Furthermore, as indicated in the method section, the presence of a large peak at the vibration frequency in the power spectral density of the vibrated muscle EMG indicates a synchronization of muscle fiber activity (Lebedev and Poliakov 1991; Martin and Park 1997) with Ia vibration-induced firing frequency and ascertains the reflex contribution of Ia-afferents to the response (Martin and Park 1997). This confirms that the increase in EMG amplitude is not solely associated with a sudden change in descending motor command. Nevertheless, in the tested infants, the reflex tonic contractions were rarely sustained during the vibration period, which suggests large temporal variability in these responses as well.
Also, the AVR was expressed as often as the TVR, either independently or simultaneously. This latter pattern has not been observed in adults, for which the two responses (TVR, AVR) are alternatives and the activation of homonymous muscles is usually the “default” setting, while the AVR is expressed only under specific conditions (Burke and Schiller 1976; Calvin-Figuière et al. 2000; Feldman and Latash 1982; Hagbarth et al. 1976; Martin et al. 1990; Martin and Park 1997; Roll et al. 1980b).
Variability and organization of Ia-originating motor responses
Variability in reflex responses, in the three tested situations, was manifested by large changes in amplitude between consecutive responses and their random absence, which are common in young infants, as observed only for the stretch reflex in previous studies (Myklebust et al. 1986; Myklebust 1990; Leonard et al. 1995) or re-direction of responses to the antagonist of the stimulated muscle (radiation).
In the present study, the pattern analysis of several Ia-mediated responses leads to the suggestion that these “variabilities” stem from a single phenomenon. First, when the stretch reflex is tested, the majority of infants exhibit 3 or 4 of the 4 possible response patterns for each stimulated muscle (simultaneous, agonist alone, antagonist alone, or No Response). Unstructured succession of these patterns, from one stimulation to the next, suggests instability of the pathway gain setting, and thus, instability of the control of feedback assistance to leg muscles. Indeed, in adults the stretch reflex contributes to a significant portion of the soleus muscle contraction during walking (e.g., af Klint et al. 2008; Nielsen and Sinkjaer 2002; Yang et al. 1991) and this contribution is phase-dependent. Second, the occurrence of facilitation as well as inhibition of stretch reflex responses induced by tendon vibration indicates that the gain of this circuit is adjustable and not yet stereotypic, unlike in adults (Martin et al. 1984, 1986), even by 10-month post-birth. Hence, T-reflex facilitation may be associated with a switch to a large gain of the polysynaptic pathway that could be concomitant with a reduction in the gain of the Ia-presynaptic inhibition. This suggests that in young infants, the Ia-presynaptic inhibition may be ineffective. Furthermore, the presence of facilitation in one leg muscle and inhibition in the homologous contralateral muscle or facilitation for some muscles and inhibition for other muscles in the same infant support an emergent and plastic gain in reflex control. On the one hand, this gain control hypothesis is supported by the central modulation of the Ia poly-synaptic pathway demonstrated in adults by the switch from the vibration-induced tonic activation of agonist and synergistic muscles, to the activation of antagonist muscles (Roll et al. 1980a; Feldman and Latash 1982). On the other hand, the non-negligible contribution of the monosynaptic pathway to the TVR (Romaiguere et al. 1991), the large range of reflex inhibition as a function of vibration frequency (Martin et al. 1984, 1986) and post-vibration facilitation of the stretch reflex (Shinohara et al. 2005) demonstrate the plasticity of that mechanism.
Despite the fact that reflex responses were elicited while infants were calm, changes in individual response magnitudes may result primarily from the significant sensitivity of the pathway gain to central influences (see for review Schieppati 1987), which is likely to fluctuate rapidly with changes in efferent controls as they modify α-motoneuron excitability, as well as the sensitivity of stretch receptors via the γ-fusimotor drive. For example, stretch reflex excitability could fluctuate as a function of changes in the visual environment when attention of the baby was attracted by the experimenters or the parent; this is consistent with changes in motor cortex and motoneuronal excitability during action viewing (Borroni et al. 2005; Montagna et al. 2005). Since modulation of the monosynaptic reflex response is also affected by changes in Ia-presynaptic inhibition during motor tasks (Hultborn et al. 1987; Nielsen and Kagamihara 1993; Faist et al. 1996; Perez et al. 2005), skill acquisition (Perez et al. 2005), specific training (Casabona et al. 1990; Nielsen et al. 1993) or short-term conditioning (Wolpaw 1997), we hypothesize that in infants the gain of the Ia-presynaptic inhibition lacks “restriction” and thus contributes to fluctuations of reflex responses. This gain instability and explanation of limited refinement is reasonable given the observed lack of control of other Ia-peripheral pathway gains, allowing reflex radiation to antagonist muscles and irradiation to distant muscles.
A variation in the mechanical stretch of the tendon cannot be excluded; however, the stimuli were delivered via a computer-controlled electrodynamic stimulator and close attention was paid to keep leg position and the probe-tendon contact constant for each stimulation. In addition, radiation of responses to antagonist muscles supports the consistency of the mechanical stimuli when a response was not observed in the stimulated muscle.
We propose that the randomness of response variations may primarily reflect a general indetermination of pathway settings in young infants that precedes their restriction with age and self-initiated experience moving and controlling their limbs. In other words, the extreme plasticity of peripheral destination of sensory information suggests that most or all of the possible feedback pathways exist very early in life.
A learning/adaptive process
In comparison with our results, restriction of reflex organization with age, as illustrated by the more systematic reciprocal inhibition in infants older than 4 years of age (O'Sullivan et al. 1991) and adults (Baldissera et al. 1987; Crone and Nielsen 1989; Sabatino et al. 1994; Marchand-Pauvert et al. 2002), as well as the almost exclusive expression of the homonymous TVR (Burke and Schiller 1976; Hagbarth et al. 1976; Roll et al. 1980a; Feldman and Latash 1982; Martin and Park 1997), suggest that adequate gating or setting of sensory feedback gains result from a learning/adaptive process that requires a few years of trial and errors. It is assumed that the ultimate goal of this process is to improve the stability of functional adaptive motor behaviors. This interpretation is in agreement with the plasticity of the central nervous system (see for review Rossini and Pauri 2000), as reflected by functional adaptations (Aimonetti et al. 1999).
Furthermore, studies in adults show the importance of the perceptual context in the determination of motor responses (Roll et al. 1980a; Feldman and Latash 1982; Lackner and Taublieb 1983; Carson et al. 1990; Martin et al. 1990; Lackner et al. 2000). This supports the learning process and demonstrates that maturation does not suppress neural connections but rather results from the development of a plastic organization of feedback destinations. This feature is demonstrated by the transient reversal of the stretch reflex to assist ball catching movements (Lacquaniti et al. 1991). It is assumed that maturation alone does not seem to be sufficient to explain the shift hypothesized. Structural changes such as increase in numbers of synapses or cortico-spinal tract changes, while particularly rapid during the first 2 years, at the general systemic level tend to bring increased speed of processing in a continuous and relatively linear way over the first 2 years and beyond (Koh and Eyre 1988; Paus et al. 1999). In the present study, a lack of change in muscle responses over the first year of life was observed. It is assumed that a shift will occur during the second year of life (Leonard et al. 1995). Thus, a nonlinear change near the end of the first year, in conjunction with walking onset, would not be predicted on the basis of this maturational mechanism alone. Instead, the plasticity of the infant nervous system would allow the learning process modify its structures, in the same way excitability of the cerebral cortex changes following periods of motor skill training in adults (Perez et al. 2004).
The observed period of pathway gain instability corresponds to a period of high levels of muscle co-contraction and highly variable muscle activation patterns during functional movements of both upper and lower limbs in young infants during motor skills learning (Berger et al. 1984, 1985, 1990; Chang et al. 2006; Forssberg 1985; Gatev 1972; Hadders-Algra et al. 1992). In the developing rat pup, restriction of the antagonist projections has been associated with a modification of co-contraction to alternating agonist/antagonist contraction patterns (Seebach and Ziskind-Conhaim 1994). In human infants, the present study clearly shows that this restriction is not present, during the first 10 months of life post-birth, as suggested by Myklebust (1990), but perhaps emerges during the second year of life when children begin to walk (Leonard et al. 1995). Reciprocal excitations, and thus the common occurrence of a high gain setting for the corresponding pathway, tend to persist until at least 4 to 6 years of age, while adults show a more systematic reciprocal inhibition (Baldissera et al. 1987; Crone and Nielsen 1989; Marchand-Pauvert et al. 2002; Sabatino et al. 1994) as well as the almost exclusive expression of the homonymous TVR (Burke and Schiller 1976; Feldman and Latash 1982; Hagbarth et al. 1976; Martin and Park 1997).
Based on the extant literature and our current data, we propose that the human neuromotor system needs experience to learn how to set the gain of the lower limb sensory feedback pathways appropriately. We hypothesize that with maturation and experience, repetitions of trials and errors in the service of acquiring functional motor goals, the nervous system learns to respond effectively to stimuli and utilize adequately sensory information in order to improve the stability of motor responses. When the central nervous system (CNS) has learned how to set the gain appropriately, adapted switching from reciprocal inhibition to reciprocal excitation is based on the perceptual conditions, as demonstrated by Lacquaniti et al. (1991). The role of a not adequately developed CNS has been shown by multiple studies (Leonard et al. 1991; Myklebust and Gottlieb 1993; O'Sullivan et al. 1998), in which children or adults with early brain damage, as occurs in cerebral palsy, are unable to tune their system and thus produce immature responses such as those resulting from conflicting reciprocal excitation. Similarly, motor performance in adults with Parkinson's disease seems to be disturbed by inappropriate supraspinal modulation of the spinal reflex pathways (Morita et al. 2005). Animal studies show some evidence that the normal development of synaptic input to α-motoneurons depends upon the integrity of descending motor pathways and spinal afferent input (Commissiong et al. 1991; Goldberger 1988; Lowrie et al. 1987; McCouch et al. 1958; Nacimiento et al. 1993; O'Hanlon and Lowrie 1996).
Overall, during the first year of post-natal life, the immaturity of the CNS and the lack of exposure to specific stimuli and tasks may explain the long duration involved in learning to set appropriate gains of assistive sensorimotor pathways in humans. The clear delineation of those disparate pathway responses during early months of life is vital to understanding more deeply infant neuromotor development and to building a database of ‘normal responses’. Promising studies (Chen et al. 2006; Hodapp et al. 2009; Morita et al. 2005; Perez et al. 2007) have highlighted the ability of those reflexes to be trained or re-trained to improve or stabilize motor performance and may lead to new early therapeutic intervention for paediatric population with deficient Ia reflex responses.
Acknowledgments
This work was supported by a grant from the National Institute of Health (1 R01 HD047567). The authors are grateful to C. Woolley and E. Claxton for their technical contribution to the design of the seat and stimulators supports. We also thank S. Y. Yu for his help in designing the software and B. Smith and M. Daneshvar for their contribution to data collection and data processing, respectively.
Contributor Information
Caroline Teulier, Email: Caroline.Teulier@staffmail.ul.ie, Department of Physical Education and Sport Sciences, University of Limerick, Limerick, Ireland.
Beverly D. Ulrich, Department of Movement Science, School of Kinesiology, University of Michigan, 401 Washtenaw Avenue, Room 4745H, Ann Arbor, MI 48109-2214, USA
Bernard Martin, Department of Industrial Operations Engineering, University of Michigan, 1205 beal Avenue, Ann Arbor, MI 48109-2117, USA.
References
- af Klint R, Nielsen JB, Cole J, Sinkjaer T, Grey MJ. Within-step modulation of leg muscle activity by afferent feedback in human walking. J Physiol. 2008;586(Pt 19):4643–4648. doi: 10.1113/jphysiol.2008.155002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimonetti JM, Morin D, Schmied A, Vedel JP, Pagni S. Proprioceptive control of wrist extensor motor units in humans: dependence on handedness. Somatosens Mot Res. 1999;16:11–29. doi: 10.1080/08990229970618. [DOI] [PubMed] [Google Scholar]
- Ashby P, Verrier M, Lightfoot E. Segmental reflex pathways in spinal shock and spinal spasticity in man. J Neurol Neurosurg Psychiatry. 1974;37:1352–1360. doi: 10.1136/jnnp.37.12.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Cavallari P, Fournier E, Pierrot-Deseilligny E, Shindo M. Evidence for mutual inhibition of opposite Ia interneurones in the human upper limb. Exp Brain Res. 1987;66:106–114. doi: 10.1007/BF00236207. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of infant and toddler development. Pearson; San Antonio: 2005. [Google Scholar]
- Berger W, Altenmueller E, Dietz V. Normal and impaired development of children's gait. Hum Neurobiol. 1984;3(3):163–170. [PubMed] [Google Scholar]
- Berger W, Quintern J, Dietz V. Stance and gait perturbations in children: developmental aspects of compensatory mechanisms. Electroencephalogr Clin Neurophysiol. 1985;61(5):385–395. doi: 10.1016/0013-4694(85)91030-2. [DOI] [PubMed] [Google Scholar]
- Berger W, Horstmann GA, Dietz V. Interlimb coordination of stance in children: divergent modulation of spinal reflex responses and cerebral evoked potentials in terms of age. Neurosci Lett. 1990;116(1–2):118–122. doi: 10.1016/0304-3940(90)90396-q. [DOI] [PubMed] [Google Scholar]
- Borroni P, Montagna M, Cerri G, Baldissera F. Cyclic time course of motor excitability modulation during the observation of a cyclic hand movement. Brain Res. 2005;1065:115–124. doi: 10.1016/j.brainres.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Bove M, Nardone A, Schieppati M. Effects of leg muscle tendon vibration on group Ia and group II reflex responses to stance perturbation in humans. J Physiol. 2003;550:617–630. doi: 10.1113/jphysiol.2003.043331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Schiller HH. Discharge pattern of single motor units in the tonic vibration reflex of human triceps surae. J Neurol Neurosurg Psychiatry. 1976;39:729–741. doi: 10.1136/jnnp.39.8.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin-Figuiere S, Romaiguere P, Gilhodes JC, Roll JP. Antagonist motor responses correlate with kinesthetic illusions induced by tendon vibration. Exp Brain Res. 1999;124(3):342–350. doi: 10.1007/s002210050631. [DOI] [PubMed] [Google Scholar]
- Calvin-Figuière S, Romaiguère P, Roll J. Relations between the directions of vibration-induced kinesthetic illusions and the pattern of activation of antagonist muscles. Brain Res. 2000;881(2):128–138. doi: 10.1016/s0006-8993(00)02604-4. [DOI] [PubMed] [Google Scholar]
- Carson RG, Elliott D, Goodman D, Dickinson J. Manual asymmetries in the reproduction of a 3-dimensional spatial location. Neuropsychologia. 1990;28:99–103. doi: 10.1016/0028-3932(90)90090-b. [DOI] [PubMed] [Google Scholar]
- Casabona A, Polizzi MC, Perciavalle V. Differences in H-reflex between athletes trained for explosive contractions and non-trained subjects. Eur J Appl Physiol Occup Physiol. 1990;61:26–32. doi: 10.1007/BF00236689. [DOI] [PubMed] [Google Scholar]
- Chang CL, Kubo M, Buzzi U, Ulrich B. Early changes in muscle activation patterns of toddlers during walking. Infant Behav Dev. 2006;29(2):175–188. doi: 10.1016/j.infbeh.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT, Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci. 2006;26(48):12537–12543. doi: 10.1523/JNEUROSCI.2198-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commissiong JW, Sauve Y, Csonka K, Karoum F, Toffano G. Recovery of function in spinalized, neonatal rats. Brain Res Bull. 1991;27(1):1–4. doi: 10.1016/0361-9230(91)90272-l. [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Spinal mechanisms in man contributing to reciprocal inhibition during voluntary dorsiflexion of the foot. J Physiol. 1989;416:255–272. doi: 10.1113/jphysiol.1989.sp017759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt JE, Godaux E. Mechanism of the vibration paradox: excitatory and inhibitory effects of tendon vibration on single soleus muscle motor units in man. J Physiol. 1978;285:197–207. doi: 10.1113/jphysiol.1978.sp012567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faist M, Mazevet D, Dietz V, Pierrot-Deseilligny E. A quantitative assessment of presynaptic inhibition of Ia afferents in spas-tics. Differences in hemiplegics and paraplegics. Brain. 1994;117(Pt 6):1449–1455. doi: 10.1093/brain/117.6.1449. [DOI] [PubMed] [Google Scholar]
- Faist M, Dietz V, Pierrot-Deseilligny E. Modulation, probably presynaptic in origin, of monosynaptic Ia excitation during human gait. Exp Brain Res. 1996;109:441–449. doi: 10.1007/BF00229628. [DOI] [PubMed] [Google Scholar]
- Feldman AG, Latash ML. Inversions of vibration-induced senso-motor events caused by supraspinal influences in man. Neurosci Lett. 1982;31:147–151. doi: 10.1016/0304-3940(82)90107-0. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Ontogeny of human locomotor control. I. Infant stepping, supported locomotion and transition to independent locomotion. Exp Brain Res. 1985;57(3):480–493. doi: 10.1007/BF00237835. [DOI] [PubMed] [Google Scholar]
- Gatev V. Role of inhibition in the development of motor co-ordination in early childhood. Dev Med Child Neurol. 1972;14(3):336–341. doi: 10.1111/j.1469-8749.1972.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Goldberger ME. Spared-root deafferentation of a cat's hindlimb: hierarchical regulation of pathways mediating recovery of motor behavior. Exp Brain Res. 1988;73(2):329–342. doi: 10.1007/BF00248225. [DOI] [PubMed] [Google Scholar]
- Hadders-Algra M, Van Eykern LA, Klip-Van den Nieuwendijk AW, Prechtl HF. Developmental course of general movements in early infancy. II. EMG correlates. Early Hum Dev. 1992;28(3):231–251. doi: 10.1016/0378-3782(92)90170-l. [DOI] [PubMed] [Google Scholar]
- Hagbarth K, Burke D, Wallin G, Lofstedt L. Single unit spindle responses to muscle vibration in man. Prog Brain Res. 1976;44:281–289. [PubMed] [Google Scholar]
- Hodapp M, Vry J, Mall V, Faist M. Changes in soleus H-reflex modulation after treadmill training in children with cerebral palsy. Brain. 2009;132(Pt 1):37–44. doi: 10.1093/brain/awn287. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol. 1987;389:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Katz R, Meunier S, Pierrot-Deseilligny E. Changes in presynaptic inhibition of Ia fibres in man while standing. Brain. 1988;111(Pt 2):417–437. doi: 10.1093/brain/111.2.417. [DOI] [PubMed] [Google Scholar]
- Koh TH, Eyre JA. Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex. Arch Dis Child. 1988;63:1347–1352. doi: 10.1136/adc.63.11.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JR, Taublieb AB. Reciprocal interactions between the position sense representations of the two forearms. J Neurosci. 1983;3:2280–2285. doi: 10.1523/JNEUROSCI.03-11-02280.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JR, DiZio P, Fisk J. Tonic vibration reflexes and background force level. Acta Astronaut. 1992;26(2):133–136. doi: 10.1016/0094-5765(92)90055-n. [DOI] [PubMed] [Google Scholar]
- Lackner JR, Rabin E, DiZio P. Fingertip contact suppresses the destabilizing influence of leg muscle vibration. J Neurophysiol. 2000;84:2217–2224. doi: 10.1152/jn.2000.84.5.2217. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Borghese NA, Carrozzo M. Transient reversal of the stretch reflex in human arm muscles. J Neurophysiol. 1991;66:939–954. doi: 10.1152/jn.1991.66.3.939. [DOI] [PubMed] [Google Scholar]
- Lance JW. The effect of vibration on afferent nerve conduction and spinal reflex mechanisms. Electroencephalogr Clin Neuro-physiol. 1968;25:407–408. [PubMed] [Google Scholar]
- Lance JW, Degail P. Spread of phasic muscle reflexes in normal and spastic subjects. J Neurol Neurosurg Psychiatry. 1965;28:328–334. doi: 10.1136/jnnp.28.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev MA, Poliakov AV. Analysis of the interference electromyogram of human soleus muscle after exposure to vibration. Neirofiziologiia. 1991;23:57–65. [PubMed] [Google Scholar]
- Leonard CT, Hirschfeld H, Moritani T, Forssberg H. Myotatic reflex development in normal children and children with cerebral palsy. Exp Neurol. 1991;111(3):379–382. doi: 10.1016/0014-4886(91)90106-m. [DOI] [PubMed] [Google Scholar]
- Leonard CT, Matsumoto T, Diedrich P. Human myotatic reflex development of the lower extremities. Early Hum Dev. 1995;43:75–93. doi: 10.1016/0378-3782(95)01669-t. [DOI] [PubMed] [Google Scholar]
- Lowrie MB, Krishnan S, Vrbova G. Permanent changes in muscle and motoneurones induced by nerve injury during a critical period of development of the rat. Brain Res. 1987;428(1):91–101. doi: 10.1016/0165-3806(87)90086-1. [DOI] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Nicolas G, Burke D, Pierrot-Deseilligny E. Suppression of the H reflex in humans by disynaptic auto-genetic inhibitory pathways activated by the test volley. J Physiol. 2002;542:963–976. doi: 10.1113/jphysiol.2002.021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BJ, Park HS. Analysis of the tonic vibration reflex: influence of vibration variables on motor unit synchronization and fatigue. Eur J Appl Physiol Occup Physiol. 1997;75:504–511. doi: 10.1007/s004210050196. [DOI] [PubMed] [Google Scholar]
- Martin BJ, Roll JP, Gauthier GM. Spinal reflex alterations as a function of intensity and frequency of vibration applied to the feet of seated subjects. Aviat Space Environ Med. 1984;55:8–12. [PubMed] [Google Scholar]
- Martin BJ, Roll JP, Gauthier GM. Inhibitory effects of combined agonist and antagonist muscle vibration on H-reflex in man. Aviat Space Environ Med. 1986;57:681–687. [PubMed] [Google Scholar]
- Martin BJ, Roll JP, Hugon M. Modulation of cutaneous flexor responses induced in man by vibration-elicited proprioceptive or exteroceptive inputs. Aviat Space Environ Med. 1990;61:921–928. [PubMed] [Google Scholar]
- Mayer RF, Mosser RS. Excitability of motoneurons in infants. Neurology. 1969;19(10):932–945. doi: 10.1212/wnl.19.10.932. [DOI] [PubMed] [Google Scholar]
- McCouch GP, Austin GM, Liu CN, Liu CY. Sprouting as a cause of spasticity. J Neurophysiol. 1958;21(3):205–216. doi: 10.1152/jn.1958.21.3.205. [DOI] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E, Simonetta M. Pattern of monosynaptic heteronymous Ia connections in the human lower limb. Exp Brain Res. 1993;96(3):534–544. doi: 10.1007/BF00234121. [DOI] [PubMed] [Google Scholar]
- Montagna M, Cerri G, Borroni P, Baldissera F. Excitability changes in human corticospinal projections to muscles moving hand and fingers while viewing a reaching and grasping action. Eur J Neurosci. 2005;22:1513–1520. doi: 10.1111/j.1460-9568.2005.04336.x. [DOI] [PubMed] [Google Scholar]
- Morita H, Shindo M, Yanagawa S, Yoshida T, Momoi H, Yanagisawa N. Progressive decrease in heteronymous monosynaptic Ia facilitation with human ageing. Exp Brain Res. 1995;104:167–170. doi: 10.1007/BF00229867. [DOI] [PubMed] [Google Scholar]
- Morita H, Crone C, Christenhuis D, Petersen NT, Nielsen JB. Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain. 2001;124:826–837. doi: 10.1093/brain/124.4.826. [DOI] [PubMed] [Google Scholar]
- Morita H, Shindo M, Ikeda S. Paradoxical modulation of tendon tap reflex during voluntary contraction in Parkinson's disease. Clin Neurophysiol. 2005;116(4):769–774. doi: 10.1016/j.clinph.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Myklebust BM. A review of myotatic reflexes and the development of motor control and gait in infants and children: a special communication. Phys Ther. 1990;70:188–203. doi: 10.1093/ptj/70.3.188. [DOI] [PubMed] [Google Scholar]
- Myklebust BM, Gottlieb GL. Development of the stretch reflex in the newborn: reciprocal excitation and reflex irradiation. Child Dev. 1993;64:1036–1045. [PubMed] [Google Scholar]
- Myklebust BM, Gottlieb GL, Agarwal GC. Stretch reflexes of the normal infant. Dev Med Child Neurol. 1986;28:440–449. doi: 10.1111/j.1469-8749.1986.tb14281.x. [DOI] [PubMed] [Google Scholar]
- Nacimiento W, Mautes A, Topper R, Oestreicher AB, Gispen WH, Nacimiento AC, et al. B-50 (GAP-43) in the spinal cord caudal to hemisection: indication for lack of intraspinal sprouting in dorsal root axons. J Neurosci Res. 1993;35(6):603–617. doi: 10.1002/jnr.490350604. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol. 1993;464:575–593. doi: 10.1113/jphysiol.1993.sp019652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB, Sinkjaer T. Afferent feedback in the control of human gait. J Electromyogr Kinesiol. 2002;12(3):213–217. doi: 10.1016/s1050-6411(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Crone C, Hultborn H. H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur J Appl Physiol Occup Physiol. 1993;66:116–121. doi: 10.1007/BF01427051. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Crone C. Changes in transmission across synapses of Ia afferents in spastic patients. Brain. 1995;118(Pt 4):995–1004. doi: 10.1093/brain/118.4.995. [DOI] [PubMed] [Google Scholar]
- O'Hanlon GM, Lowrie MB. The effects of neonatal dorsal root section on the survival and dendritic development of lumbar motoneurons in the rat. Eur J Neurosci. 1996;8(6):1072–1077. doi: 10.1111/j.1460-9568.1996.tb01274.x. [DOI] [PubMed] [Google Scholar]
- O'Sullivan MC, Eyre JA, Miller S. Radiation of phasic stretch reflex in biceps brachii to muscles of the arm in man and its restriction during development. J Physiol. 1991;439:529–543. doi: 10.1113/jphysiol.1991.sp018680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan MC, Miller S, Ramesh V, Conway E, Gilfillan K, McDonough S, Eyre JA. Abnormal development of biceps brachii phasic stretch reflex and persistence of short latency heteronymous reflexes from biceps to triceps brachii in spastic cerebral palsy. Brain. 1998;121(Pt 12):2381–2395. doi: 10.1093/brain/121.12.2381. [DOI] [PubMed] [Google Scholar]
- Park HS, Martin BJ. Contribution of the tonic vibration reflex to muscle stress and muscle fatigue. Scand J Work Environ Health. 1993;19(1):35–42. doi: 10.5271/sjweh.1506. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159(2):197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nielsen JB. Presynaptic control of group Ia afferents in relation to acquisition of a visuo-motor skill in healthy humans. J Physiol. 2005;568:343–354. doi: 10.1113/jphysiol.2005.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Lundbye-Jensen J, Nielsen JB. Task-specific depression of the soleus H-reflex after cocontraction training of antagonistic ankle muscles. J Neurophysiol. 2007;98(6):3677–3687. doi: 10.1152/jn.00988.2007. [DOI] [PubMed] [Google Scholar]
- Prechtl HF, Vlach V, Lenard HG, Grant DK. Exteroceptive and tendon reflexes in various behavioural states in the newborn infant. Biol Neonat. 1967;11(3):159–175. doi: 10.1159/000240063. [DOI] [PubMed] [Google Scholar]
- Roll JP, Gilhodes JC, Tardy-Gervet MF. Effects of vision on tonic vibration response of a muscle or its antagonists in normal man (author's transl) Experientia. 1980a;36(1):70–72. doi: 10.1007/BF02003980. [DOI] [PubMed] [Google Scholar]
- Roll JP, Martin B, Gauthier GM, Mussa Ivaldi F. Effects of whole-body vibration on spinal reflexes in man. Aviat Space Environ Med. 1980b;51(11):1227–1233. [PubMed] [Google Scholar]
- Romaiguere P, Vedel JP, Azulay JP, Pagni S. Differential activation of motor units in the wrist extensor muscles during the tonic vibration reflex in man. J Physiol. 1991;444:645–667. doi: 10.1113/jphysiol.1991.sp018899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaiguere P, Vedel JP, Pagni S. Effects of tonic vibration reflex on motor unit recruitment in human wrist extensor muscles. Brain Res. 1993;602(1):32–40. doi: 10.1016/0006-8993(93)90237-h. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Pauri F. Neuromagnetic integrated methods tracking human brain mechanisms of sensorimotor areas ‘plastic’ reorganisation. Brain Res Brain Res Rev. 2000;33:131–154. doi: 10.1016/s0169-328x(00)00090-5. [DOI] [PubMed] [Google Scholar]
- Sabatino M, Sardo P, Ferraro G, Caravaglios G, La Grutta V. Bilateral reciprocal organisation in man: focus on IA interneurone. J Neural Transm Gen Sect. 1994;96:31–39. doi: 10.1007/BF01277926. [DOI] [PubMed] [Google Scholar]
- Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol. 1987;28:345–376. doi: 10.1016/0301-0082(87)90007-4. [DOI] [PubMed] [Google Scholar]
- Seebach BS, Ziskind-Conhaim L. Formation of transient inappropriate sensorimotor synapses in developing rat spinal cords. J Neurosci. 1994;14(7):4520–4528. doi: 10.1523/JNEUROSCI.14-07-04520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Moritz CT, Pascoe MA, Enoka RM. Prolonged muscle vibration increases stretch reflex amplitude, motor unit discharge rate, and force fluctuations in a hand muscle. J Appl Physiol. 2005;99:1835–1842. doi: 10.1152/japplphysiol.00312.2005. [DOI] [PubMed] [Google Scholar]
- Van Boxtel A. Selective effects of vibration on monosynaptic and late EMG responses in human soleus muscle after stimulation of the posterior tibial nerve or a tendon tap. J Neurol Neurosurg Psychiatry. 1979;42:995–1004. doi: 10.1136/jnnp.42.11.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR. The complex structure of a simple memory. Trends Neurosci. 1997;20:588–594. doi: 10.1016/s0166-2236(97)01133-8. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB, James KB. Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Exp Brain Res. 1991;87(3):679–687. doi: 10.1007/BF00227094. [DOI] [PubMed] [Google Scholar]