Abstract
Background
Walking-related disability is the most frequent reason for inpatient stroke rehabilitation. Task-related practice is a critical component for improving patient outcomes.
Objective
To test the feasibility of providing quantitative feedback about daily walking performance and motivating greater skills practice via remote sensing.
Methods
In this phase III randomized, single blind clinical trial, patients participated in conventional therapies while wearing wireless sensors (tri-axial accelerometers) at both ankles. Activity-recognition algorithms calculated the speed, distance, and duration of walking bouts. Three times a week, therapists provided either feedback about performance on a 10-meter walk (speed-only) or walking speed feedback plus a review of walking activity recorded by the sensors (augmented). Primary outcomes at discharge included total daily walking time, derived from the sensors, and a timed 15-meter walk.
Results
Sixteen rehabilitation centers in 11 countries enrolled 135 participants over 15 months. Sensors recorded more than 1800 days of therapy, 37,000 individual walking bouts, and 2.5 million steps. No significant differences were found between the two feedback groups in daily walking time (15.1±13.1min vs. 16.6±14.3min, p=0.54) or 15-meter walking speed (0.93±0.47m/s vs. 0.91±0.53m/s, p=0.96). Remarkably, 30% of participants decreased their total daily walking time over their rehabilitation stay.
Conclusions
In this first trial of remote monitoring of inpatient stroke rehabilitation, augmented feedback beyond speed alone did not increase the time spent practicing or improve walking outcomes. Remarkably modest time was spent walking. Wireless sensing, however, allowed clinicians to audit skills practice and provided ground truth regarding changes in clinically important, mobility-related activities.
Keywords: stroke, rehabilitation, telemedicine, wireless technology, accelerometer, walking
INTRODUCTION
Mobility outcomes after disabling stroke are highly dependent on the intensity of rehabilitation training and practice.1–3 Surveys using a diary or videotaping of physical and occupational therapy sessions, however, describe modest amounts of supervised skills practice during inpatient stroke rehabilitation.4–6 Given the sense that perhaps too little treatment of sufficient cardiovascular intensity is provided,7 sporadic efforts have been made to increase the type and quantity of skills practice.8–10
Past interventions to increase patient participation in therapies have been limited by the lack of an objective, inexpensive method to continuously measure the quantity and quality of patients’ movements, i.e., parameters that go beyond a step counter in the case of walking. Wireless health technologies, including unobtrusive physiologic sensors and advanced activity-recognition algorithms, offer the possibility of daily patient monitoring.11–13 When tested for several days in patients with chronic hemiparetic stroke,14 these wireless sensors returned accurate, reliable activity summaries even for patients who walked slowly (i.e. <0.5 m/s).
The Stroke Inpatient Rehabilitation Reinforcement of ACTivity (SIRRACT) trial is the first international, multi-center trial to deploy a wireless, Internet-based remote sensing strategy in patients disabled by stroke. It was designed as a follow-up to the multicenter Stroke Inpatient Rehabilitation With Reinforcement of Walking Speed (SIRROWS) trial that found that feedback about walking speed, from a timed 10-meter walk three times a week, led to significantly higher speeds at discharge from inpatient stroke rehabilitation as compared to no such feedback.8 For SIRRACT, investigators employed remote wireless sensing to monitor all lower extremity movements performed by patients during the course of daily activities. Activity summaries derived from sensor data were used to provide an augmented feedback intervention that was compared to feedback about walking speed alone.
Our aim for this trial was to motivate patients and their therapists to engage in greater skills practice to obtain improved walking-related outcomes. We endeavored to demonstrate the feasibility of deploying sensors in inpatient rehabilitation centers regardless of culture, language, or familiarity with clinical research. Finally, we anticipated using the rich data set generated by the wireless sensors during inpatient therapy to characterize changes in a variety of walking-related parameters.
METHODS
Study design and setting
This phase III, single blind, parallel group, randomized control trial was carried out at 12 international and 4 American inpatient rehabilitation centers. Investigators at the UCLA Wireless Health Institute designed and managed all aspects of the trial. Since no funding was provided to individual sites, the protocol was designed for implementation within each site’s usual rehabilitation practices and structure. Instructions about procedures were provided from an online manual of operations with videos (http://www.sirract.ucla.edu). Training webinars, email responses to questions, and automated assessment reminders enabled trial management via the Internet. All demographic information and blinded study outcomes were entered into a secure online clinical database with separate logins and webpage views for the treating clinicians and blinded assessors.
Selection criteria
Sites were recruited from the membership of the World Federation of NeuroRehabilitation and the American Society of NeuroRehabilitation. Inclusion criteria included stroke of any type with residual hemiparesis, the ability to walk 5 steps within 10 days of admission for rehabilitation, and admission to the facility within 35 days of stroke. Exclusion criteria included aphasia limiting the ability to follow 2-step commands and ongoing medical disease limiting participation in physical therapy. Patients who had suffered a prior stroke were eligible for participation if they had experienced full motor recovery.
Randomization and baseline data collection
After eligibility criteria were entered into the central database, participants were assigned to a speed-only feedback (SF) or augmented feedback (AF) trial arm by a computer using a concealed allocation sequence. A block randomization design was employed to achieve equal group numbers at each study site.
Baseline demographic data including age, gender, stroke type, hemiparetic side, and NIH Stroke Scale score were entered into the clinical database by site investigators. A blinded observer at each location collected a stopwatch-timed 15-meter walk, the distance walked in 3 minutes, and Functional Ambulation Category (FAC) score.
Wireless sensor system
The inertial sensor system and activity-recognition algorithms were previously described and tested for short-term reliability.14 Three sets of tri-axial accelerometers (Gulf Coast Data Concepts, Waveland, MS) were mailed to each site’s coordinator. Therapists placed one sensor on each ankle before participants got out of bed each morning and removed them once they were in bed at the end of the day; sensor use during weekends was optional. A soft snap band secured each sensor proximal to the medial malleolus, flush against the bony tibia. Every night, sensors were plugged into a local computer to recharge while accelerometer data were uploaded to the central server at UCLA for secure storage and processing.
Sensor calibration and data processing
In recognition of the variations in gait speed and stand and swing symmetry that occur in patients who need inpatient rehabilitation after stroke, we chose to generate individual templates of each participant’s gait from a pair of standardized walks. On study entry participants performed two stopwatch-timed 10-meter walks at self-selected casual and safest fast walking speeds. A hybrid classifier employing dynamic time warping and Naïve Bayes algorithms generated statistical models of each participant’s gait based on the two walks. Repeat walks were performed and the templates updated weekly for the remainder of each participant’s rehabilitation stay to account for expected changes in gait parameters.
New activity data that were uploaded to the central server at UCLA underwent preprocessing followed by classification to identify periods of a participant’s daily activity that matched his or her walking template. For this trial, a bout of walking was defined as lasting at least 5 seconds. Bouts separated by 5 seconds or more were labeled as separate walking episodes. After classification the system calculated gait parameters for each identified walking bout (speed, duration), as well as for each day’s total walking activity (number of walking bouts, average walking speed, total time spent walking, total distance, total steps). Personalized bar graphs summarizing daily step count, average and maximum walking speed, and distance walked were updated and made available to therapists for the AF intervention.
Feedback intervention
Three times a week after performing a stopwatch-timed 10-meter walk at their fastest safe walking speed, all participants received standardized verbal feedback from their therapists similar to that used in SIRROWS. For example, “Very good! You walked that in (number of) seconds.” Then, (a) “Keep working to improve further” or (b) “Keep up the good work and continue to practice” or (c) “I believe you will soon be walking faster with more practice.”
In addition to receiving immediate verbal feedback about walking speed, participants in the AF group also reviewed the results of their summary activity graphs with the therapists. Using a scripted statement, therapists encouraged these patients to meet or exceed their prior activity levels. For example, (a) “You are showing some improvement” or (b) “You have not yet increased your (walking speed, distance, steps).” Then, “Let’s see if you can make further improvements today.”
Study outcomes
Primary outcomes were the average daily time spent walking recorded by the sensors and a stopwatch-timed fastest safe 15-meter walking speed collected by a blinded observer prior to patient discharge from the rehabilitation unit. The blinded observer also collected FAC scores and 3-minute walking distances at the time of discharge to serve as secondary outcomes. Participants’ perceptions of their function were collected using the Stroke Impact Scale (SIS-16). Sensor ease of use was assessed using Likert-style questionnaires.
Power analysis
We aimed to increase the time spent walking by 30%. Our power calculation was based upon admission-to-discharge changes in walking speed reported in the SIRROWS trial.8 An effect size of 0.4 could be detected with a two-sided significance level of 0.05 and a power of 0.8 if 92 subjects were recruited to each group, assuming no more than 10% attrition. An interim futility analysis to be performed by a statistician blinded to intervention assignment was planned after the first 100 subjects had completed participation in the trial.
Statistical analyses
Baseline comparisons between the SF and AF groups were performed using t-tests, chi-squared tests, or Wilcoxon rank-sum tests as appropriate. Primary outcomes for all participants who received the intervention were analyzed using t-tests. Linear mixed effects models were constructed to evaluate for differences in the rate of change in walking time between feedback groups. Functional walking groups were identified from within baseline 15-meter walking speeds by post hoc quartile analysis. Two-way ANOVAs were used to compare primary outcomes between functional sub-groups. Statistical analyses were performed using SAS (SAS Institute Inc., Cary, NC).
Protocol approvals, registrations, and patient consent
Investigators at UCLA provided a standard IRB template that was approved by each site’s local institutional review board after any necessary modifications. All subjects provided written informed consent prior to participation. The study was registered at ClinicalTrials.gov (NCT01246882). A Data Safety Monitoring Committee was not formed for this trial given the low perceived risk to participants and the simplicity of the trial’s design.
RESULTS
Recruitment and baseline demographics
Study sites reported screening 156 patients who were highly likely to meet entry criteria between March 2011 and October 2012 (Figure 1). Interim outcomes analysis performed after the first 100 participants completed the study did not identify any significant difference between groups and the decision was made to halt the trial after a total of 125 subjects had completed participation. On closure of the study, 135 patients had received the intervention and 125 completed the trial (83% of all randomized participants).
Figure 1.
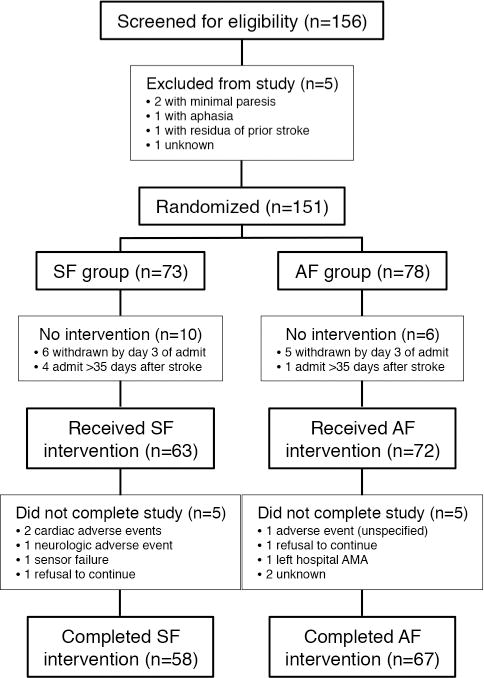
At baseline, subjects in the two intervention groups were found to be equally matched for age, gender, stroke location, and disability (Table 1). Patients with a range of walking disabilities at entry were included. There was no significant difference in either the length of stay in rehabilitation or in the median number of days of trial participation between the two intervention groups (Table 2).
Table 1.
Baseline clinical demographics.
| SF (n=73) | AF (n=78) | p-value | |
|---|---|---|---|
| Age, years | 65.0 ± 13.2 | 61.8 ± 15.7 | 0.18 |
| Women, n (%) | 28 (40.0) | 31 (40.3) | 0.98 |
| Stroke type, n (%) | 0.45 | ||
| large vessel ischemic | 41 (56.2) | 45 (57.7) | |
| lacunar | 11 (15.0) | 18 (23.0) | |
| hemorrhagic | 14 (19.2) | 13 (16.7) | |
| unknown | 7 (9.6) | 2 (2.6) | |
| Hemiparetic side, n (%) | 0.73 | ||
| right | 42 (59.2) | 44 (56.4) | |
| left | 29 (40.8) | 34 (43.6) | |
| Second stroke, n (%) | 4 (5.5) | 3 (3.9) | 0.63 |
| Time from stroke to rehabilitation, days | 8.5 [4.2, 14.8] | 8 [5, 16] | 0.70 |
| NIHSS score | 6 [4,9] | 6 [4,7] | 0.50 |
| FAC score, n (%) | 0.68 | ||
| 0: non-functional ambulation | 6 (9.0) | 6 (8.2) | |
| 1: manual assistance, heavy | 21 (31.2) | 28 (38.4) | |
| 2: manual assistance, light | 20 (30.0) | 19 (26.0) | |
| 3: stand-by assistance | 9 (13.4) | 8 (11.0) | |
| 4: assistance for stairs | 9 (13.4) | 7 (9.6) | |
| 5: independent | 2 (3.0) | 5 (6.8) | |
| 15-meter walking speed, m/s | 0.52 ± 0.47 | 0.52 ± 0.45 | 0.96 |
| 3-minute walking distance, m | 79.7 ± 68.5 | 80.9 ± 67.5 | 0.92 |
Abbreviations: SF: speed-only feedback, AF, augmented feedback; NIHSS, NIH Stroke Scale; FAC, Functional Ambulation Category. Values are presented as mean ± SD, median [interquartile range], or n (%).
Table 2.
Sensor and secondary clinical outcomes data at discharge from inpatient rehabilitation.
| SF (n=63) | AF (n=72) | p-value | |
|---|---|---|---|
| Rehabilitation length of stay, days | 25 [18, 36.5] | 25 [17, 36] | 0.64 |
| Participation in trial, days | 20 [14, 33] | 22.5 [13.8, 31] | 0.92 |
| Days with processed sensor data | 10 [7, 18.5] | 13 [9, 18.8] | 0.14 |
| Daily activity monitoring, hours | 8.7 [7.8, 10.8] | 8.7 [7.9, 9.9] | 0.78 |
| FAC ≥ 4 | 34 (58.6) | 41 (61.2) | 0.39 |
| 3-minute walking distance, m | 137.4 ± 72.8 | 137.1 ± 69.9 | 0.98 |
| SIS-16 score | 72.9 ± 21.5 | 71.4 ± 18.9 | 0.68 |
Abbreviations: SF: speed-only feedback, AF, augmented feedback; FAC, Functional Ambulation Category; SIS, Stroke Impact Scale. Values are presented as mean ± SD, median [interquartile range], or n (%).
Wireless sensor usage and algorithm outputs
We recorded 2117 days of rehabilitation from the 135 participants. Accounting for weekends and holidays, sensor data were obtained for 84.4% of all study days. Technical problems including sensor desynchronization, hardware failure, and incorrectly collected walking templates prevented the machine-learning algorithms from classifying activity for 226 therapy days. We obtained 18,579 hours of fully processed data from 1891 therapy days. The algorithms identified over 37,000 discrete walking episodes and 2.5 million steps. On average, the sensors were actively recording data for over 8 hours a day.
Outcomes
We found no significant difference between groups in the average daily time spent walking over the duration of the trial (SF: 15.1 ± 13.1 min; AF: 16.6 ± 14.3 min; p=0.54). Between study entry and discharge from rehabilitation, there was no difference between groups in the rate of change in time spent walking (p=0.32). No significant difference in final 15-meter walking speed was found between groups after accounting for baseline demographic variables (SF: 0.93 ± 0.47 m/s; AF: 0.91 ± 0.53 m/s; p=0.96). The intervention groups did not differ on secondary or patient-reported outcomes including the FAC, 3-min walking distance, and SIS-16 scores (Table 2). Questionnaires completed by study participants at discharge revealed that the sensors had been comfortable to wear (87%) and did not interfere with movement (97.6%) either some or all of the time.
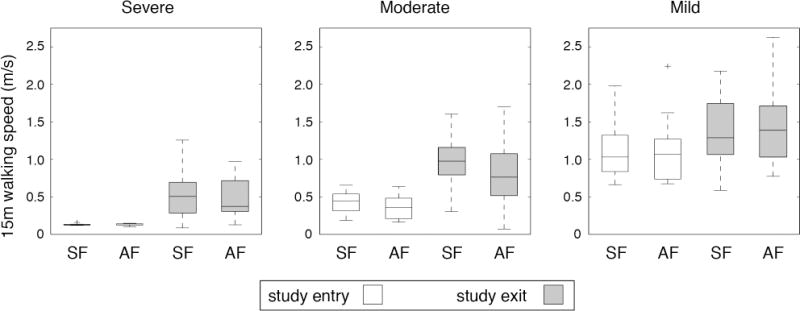
By post-hoc analysis, we identified three functional impairment groups based on mean baseline 15-m walking speeds of 0.13m/s (severely affected), 0.38m/s (moderately affected), and 1.12m/s (mildly affected). The SF and AF interventions did not differ in the distribution of participants between functional groups (p=0.15). As shown in Figures 2 and 3, both the time spent walking and 15-m walking speed differed between functional groups (time: F=13.48, p<0.001; speed: F=45.31, p<0.001). Remarkably, the mean time spent in walking practice was less than 8 min a day in the severe group and 12 min daily in the moderate group. Within each functional group, the SF and AF interventions did not result in significantly different primary outcomes (time: F=0.48, p=0.62; speed: F=0.29, p=0.75).
Figure 2.
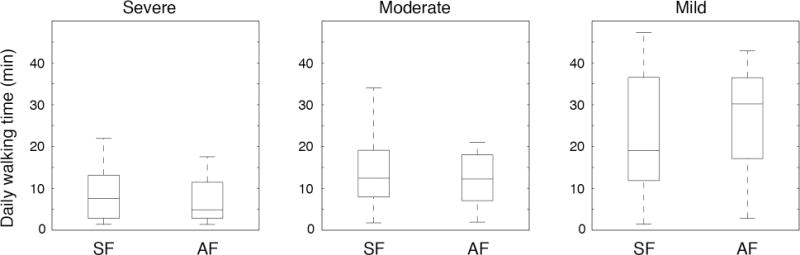
Figure 3.
Unique sensor-derived outcomes
Daily remote monitoring with sensors provided a unique opportunity to evaluate walking performance and patterns of activity during inpatient rehabilitation. As seen in Figure 4, participants employed a range of speeds when walking, with the greatest variation in speeds present during shorter walking bouts. Across all study participants, the majority of continuous daily walking bouts lasted between 10 and 30 seconds. 124 participants had at least one therapy day during which they engaged in continuous walking for 2 or more minutes, and on average 62% of these participants’ days included longer walking bouts. AF was not associated with a greater amount of time spent in longer (≥ 2 minutes) walking bouts (SF:7.5 ± 6 min; AF:9.0 ± 8 min; p=0.33). Remarkably, when evaluating for changes in total time spent walking over the course of rehabilitation we found that 30% of participants decreased their total daily walking time.
Figure 4.
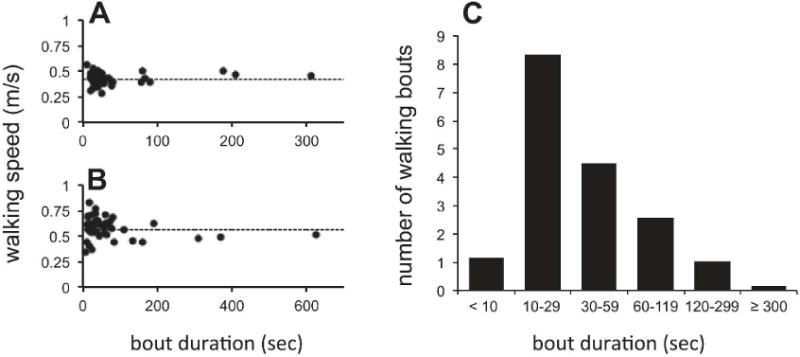
A and B: Speed and duration of every walking bout for one day of rehabilitation in two participants. Dotted lines represent the casual 10-meter walking speeds of template walks for that respective week of rehabilitation. C: Median number of daily walking bouts across all study participants sorted by bout duration.
Over the duration of the study, sensor-derived average daily walking speeds closely paralleled those of the weekly casual 10-meter template walks (r=0.977, p<0.001). A weaker positive correlation was present between the maximum daily speed calculated from the sensors and 15-meter walking speeds collected by the blinded observer (r=0.647, p<0.001). These findings support the reliability, validity, and sensitivity to change of hybrid motion classification algorithms using bilateral inertial sensor recordings.
DISCUSSION
The SIRRACT investigators completed the first large, multicenter randomized clinical trial that demonstrates the feasibility, validity, and sensitivity of continuous remote activity monitoring to facilitate a walking-related intervention. International study sites of varied cultures and languages, some of which do not routinely participate in clinical research, were able to obtain laboratory quality data on a daily basis. Data were successfully collected, transferred, and processed solely by Internet-based protocols in a timely fashion, which greatly lowered the cost of the trial. Therapists and patients were highly compliant with sensor use and the devices were reported to be an unobtrusive means of recording walking activities during inpatient rehabilitation.
Across all participants, the augmented feedback intervention was not associated with a greater amount of time spent walking. Disability did not appear to be the primary factor limiting participation, as post-hoc analysis demonstrated that even patients who walked at potential community ambulation speeds (i.e. ≥0.8 m/s)15 on study entry did not increase their practice time with augmented feedback. Two patterns in the daily activity data suggest explanations for why additional feedback did not result in more practice. First, only a fraction of all walking bouts were of sufficient length that one could assume they represented practice directed at skilled walking and not the performance of activities of daily living. Second, almost a third of all participants decreased the time spent walking over the course of their stay in rehabilitation. These patterns likely reflect constraints inherent to the acute rehabilitation environment (e.g. hallway length, practice time used to reduce gait pattern deviations and learn to employ an assistive device or orthotic, scheduled therapist time, patient fatigue) and the time allocated to manage other rehabilitation priorities. Given that the design of this trial was not meant to change how rehabilitative therapies were delivered, these constraints may have limited participants’ opportunities to ambulate more frequently regardless of walking ability. Clearly, walking was not used specifically to improve cardiovascular fitness.
Although there were no between-group differences in 15-meter walking speed, distance walked in 3 minutes, or FAC scores, both intervention groups significantly increased their average 15-meter walking speed between study entry and discharge, surpassing the criterion for a meaningful change in gait speed.16 A fair correlation was found between daily walking speeds and those on the 15-meter outcome test, suggesting that, although participants increased their ability to walk faster over the course of inpatient rehabilitation, this was not reflected as much in their daily self-selected walking speed. The magnitude of the walking speed achieved by the SF and AF groups at discharge was similar to that reported for the 179 participants in SIRROWS8 (0.91m/s for feedback vs. 0.73m/s for no feedback about walking speed). Indeed, given the degree of improvement in SIRROWS, the investigators were uncertain that further improvements would be achieved in SIRRACT through additional feedback. The changes in walking speed seen in both studies (0.45m/s in SIRROWS and 0.39m/s in SIRRACT) were greater than those reported for more intensive interventions, including robotic step training17 and outpatient body weight-supported treadmill with over-ground training and home-based exercise.18 A direct comparison with these interventions is not possible, however, due to study differences including time from stroke onset and range of initial walking speeds. SIRRACT does confirm the positive effect of regular verbal feedback on walking speed, a simple intervention that has not yet been incorporated into most inpatient rehabilitation practices.
Limitations of this trial were primarily related to the absence of funding. The site PIs and coordinators carried out all tasks voluntarily, so they could not screen all admissions for eligibility, possibly allowing for bias in subject recruitment. The requirement for participants to provide two 10-meter walks for sensor calibration may have served as an inadvertent additional selection criterion leading to the recruitment of less affected patients, although the protocol allowed a timed walk over a shorter distance if necessary. In addition, the central UCLA site could not monitor how consistently feedback was delivered to the AF group at study sites, potentially confounding the feedback’s effect on participants and preventing the determination of whether therapists were introducing bias. Information regarding the content and dose of therapies provided at each study site was not collected, precluding a comparison of participants’ walking performed during versus outside of formal therapy times. As mentioned above, the allocation of time spent across a range of rehabilitation activities may have constrained the ability of therapists to increase the amount of time devoted to physical practice in the inpatient setting, limiting the potential effectiveness of the AF intervention. Finally most sites were unable to follow up their patients after discharge. The study protocol asked them to try to obtain a 30-day and 90-day follow-up, but this was not practical due to transportation and funding constraints. Thus, we could not identify delayed effects from the two interventions.
Wireless sensors and classification algorithms enabled an appraisal of skills practice that was novel for a stroke rehabilitation trial. For example, participants’ engagement in the practice of skills other than walking was identified from within the continuous data streams. We collected templates and monitored the frequency with which repetitive movements including cycling, knee extensions, and leg lifts were performed (not shown, due to wide variations across sites). Most important for the conduct and outcomes of this study, the system was able to recognize and quantify walking performed at speeds below 0.5m/s, a recognized limitation of pedometers19 and other commercial inertial sensor systems.20 We have also begun to examine the data for more complex daily and weekly motor skill changes over the course of rehabilitation, such as stride-to-stride variations, limb asymmetries in stance and swing times, and smoothness of lower extremity swing accelerations. The acquisition of such data usually requires a formal gait laboratory assessment.
Our findings argue for the feasibility and utility of continuous activity monitoring to accurately assess skills practice as it is actually performed by patients during formal and self-directed rehabilitative activities. Related technologies have assessed gait speed in community dwelling elderly21 and quantified changes in gait22 as well as aspects of the intensity of physical activity7 for patients participating in acute inpatient rehabilitation at a single site. Additional advancements in reducing sensor size, increasing energy efficiency, and developing inexpensive communications standards will make possible real-time feedback based upon the continuous collection of data from the home and community. Wireless health technologies, in combination with telemedicine infrastructure, may become valued neurologic tools for clinical trials or daily care that monitor compliance with skills training and exercise for secondary prevention, as well as provide feedback and ratio-scale, ecologically sound outcome measurements.23
Acknowledgments
Team members at each study site volunteered their time as a function of their interest in participating in clinical trials under the aegis of the World Federation of NeuroRehabilitation and the American Society of NeuroRehabilitation. The UCLA Wireless Health Institute provided the wireless sensors and data processing algorithms. We thank Chi-hong Tseng, PhD in Biomathematics at UCLA for assistance with statistical analyses.
SOURCES OF FUNDING
Data analysis and support for study coordination at UCLA was partially funded by NIH/NICHD R01 HD07809 to Dr. Dobkin and by NIH/NCATS grant UL1TR000124.
Footnotes
AUTHOR’S NOTE
The following is a list of the SIRRACT investigators. Ain Shams University, Cairo, Egypt: Tamer Emara, MD. Burke Rehabilitation Hospital, White Plains, NY: Dylan Edwards, PhD, PT and Pasquale Fonzetti, MD. Burwood Hospital, Christchurch, New Zealand: John Maasch, MD. Chonnam National University Medical School and Hospital, Gwanju, South Korea: Sam-Gyu Lee, MD, PhD. College of Medicine, UCH, University of Ibadan, Ibadan, Nigeria: Mayowa O. Owolabi, MBBS, MSC, DMed and Talhatu K. Hamzat, PhD. Fairlawn Rehabilitation Hospital, Worcester, MA: Corey J. LeBlanc, MSPT/DPT and Regina Morse, DPT. Father Muller Medical College, Mangalore, India: Narasimman Swaminathan, MPTh. Gazi University, Ankara, Turkey: Gulcin Kaymak Karatas, MD. Hospital de l’Esperanca, Barcelona, Spain: Roser Boza, MD. Mayo Clinic, Rochester, MN: Allen W. Brown, MD. Morinomiya Hospital, Osaka, Japan: Ichiro Miyai, MD, PhD and Teiji Kawano, MD. National Taiwan University Hospital, Taipei, Taiwan: Ssu-Yuan Chen, MD, PhD. Princess Margaret Hospital, Christchurch, New Zealand: H. Carl Hanger, MBChB, FRACP. San Camillo Hospital, Venice, Italy: Carla Zucconi, DPT. San Raffaele Hospital, Milan, Italy: Silvia Mammi, MD and Chiara Ghislanzoni, PT. University Hospital of Vigo, Vigo, Spain: Francisco Juan, MD, PhD. Washington University School of Medicine, St. Louis, MO: Catherine E. Lang, PT, PhD.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377:1693–1702. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- 2.Duncan PW, Sullivan KJ, Behrman AL, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364:2026–36. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 4.De Wit L, Putman K, Lincoln N, et al. Stroke rehabilitation in Europe: what do physiotherapists and occupational therapists actually do? Stroke. 2006;37:1483–9. doi: 10.1161/01.STR.0000221709.23293.c2. [DOI] [PubMed] [Google Scholar]
- 5.Lang CE, Macdonald JR, Reisman DS, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009;90:1692–8. doi: 10.1016/j.apmr.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latham NK, Jette DU, Slavin M, et al. Physical therapy during stroke rehabilitation for people with different walking abilities. Arch Phys Med Rehabil. 2005;86:S41–S50. doi: 10.1016/j.apmr.2005.08.128. [DOI] [PubMed] [Google Scholar]
- 7.Prajapati SK, Mansfield A, Gage WH, Brooks D, McIlroy WE. Cardiovascular responses associated with daily walking in subacute stroke. Stroke Res Treat. 2013;2013:612458. doi: 10.1155/2013/612458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobkin BH, Plummer-D’Amato P, Elashoff R, et al. International randomized clinical trial, Stroke Inpatient Rehabilitation With Reinforcement of Walking Speed (SIRROWS), improves outcomes. Neurorehabil Neural Repair. 2010;24:235–42. doi: 10.1177/1545968309357558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooke EV, Mares K, Clark A, Tallis RC, Pomeroy VM. The effects of increased dose of exercise-based therapies to enhance motor recovery after stroke: a systematic reviews and meta-analysis. BMC Med. 2010;8:60. doi: 10.1186/1741-7015-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Wijk R, Cumming T, Churilov L, Donnan G, Bernhardt J. An early mobilization protocol successfully delivers more and earlier therapy to acute stroke patients: further results from Phase II of AVERT. Neurorehabil Neural Repair. 2012;26:20–6. doi: 10.1177/1545968311407779. [DOI] [PubMed] [Google Scholar]
- 11.Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788–98. doi: 10.1177/1545968311425908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clifford GD, Clifton D. Wireless technology in disease management and medicine. Annu Rev Med. 2012;63:479–92. doi: 10.1146/annurev-med-051210-114650. [DOI] [PubMed] [Google Scholar]
- 13.Patel S, Park H, Bonato P, Chan L, Rodgers M. A review of wearable sensors and systems with application in rehabilitation. J Neuroeng Rehabil. 2012;9:21. doi: 10.1186/1743-0003-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobkin BH, Xu X, Batalin M, Thomas S, Kaiser W. Reliability and validity of bilateral ankle accelerometer algorithms for activity recognition and walking speed after stroke. Stroke. 2011;42:2246–50. doi: 10.1161/STROKEAHA.110.611095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–9. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 16.Tilson JK, Sullivan KJ, Cen SY, et al. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther. 2010;90:196–208. doi: 10.2522/ptj.20090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hidler J, Nichols D, Pelliccio M, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair. 2009;23:5–13. doi: 10.1177/1545968308326632. [DOI] [PubMed] [Google Scholar]
- 18.Nadeau SE, Wu SS, Dobkin BH, et al. Effects of task-specific and impairment-based training compared with usual care on functional walking ability after inpatient stroke rehabilitation: LEAPS trial. Neurorehabil Neural Repair. 2013;27:370–80. doi: 10.1177/1545968313481284. [DOI] [PubMed] [Google Scholar]
- 19.Carroll SL, Greig CA, Lewis SJ, et al. The use of pedometers in stroke survivors: are they feasible and how well do they detect steps? Arch Phys Med Rehabil. 2012;93:466–70. doi: 10.1016/j.apmr.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 20.Yang S, Zhang JT, Novak AC, Brouwer B, Li Q. Estimation of spatio-temporal parameters for post-stroke hemiparetic gait using inertial sensors. Gait Posture. 2013;37:354–8. doi: 10.1016/j.gaitpost.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 21.Wang F, Stone E, Skubic M, Keller JM, Abbott C, Rantz M. Toward a passive low-cost in-home gait assessment system for older adults. IEEE J Biomed Health Inform. 2013;17:346–55. doi: 10.1109/JBHI.2012.2233745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prajapati SK, Gage WH, Brooks D, Black SE, McIlroy WE. A novel approach to ambulatory monitoring: investigation into the quantity and control of everyday walking in patients with subacute stroke. Neurorehab Neural Repair. 2011;25:6–14. doi: 10.1177/1545968310374189. [DOI] [PubMed] [Google Scholar]
- 23.Dobkin BH. Wearable motion sensors to continuously measure real-world physical activities. Curr Opin Neurol. 2013;26:602–8. doi: 10.1097/WCO.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]


