Abstract
Objective
To compare maternal characteristics, prenatal care and newborn outcomes in a cohort of opioid-dependent pregnant women treated with methadone vs. buprenorphine.
Methods
Retrospective cohort study. 609 pregnant, opioid-dependent women were treated with methadone (n=248) or buprenorphine (n=361) between 2000–2012 at a single institution.
Results
Mothers treated with buprenorphine were more likely to start medication prior to or earlier in pregnancy, had longer gestation and larger infants. Newborns of buprenorphine- vs. methadone-maintained mothers required treatment for neonatal abstinence significantly less often and for a shorter duration.
Conclusions
These data suggest pregnancy outcomes following buprenorphine to treat opioid dependence during pregnancy in clinical practice are as good and often better than outcomes with methadone. These results are consistent with efficacy data from randomized clinical trials and further support the use of buprenorphine for the treatment of opioid-dependence during pregnancy.
Keywords: pregnancy, opioid dependence, buprenorphine, methadone
INTRODUCTION
Opioid dependence during pregnancy is often compounded by multiple risk factors contributing to adverse maternal and newborn consequences (Kandall et al 1977; Lifschitz et al 1985; Hulse et al 1997; Lester et al, 2004; Messinger et al 2004; Winklbaur et al 2008). For over four decades methadone has been the recommended standard of care for treating opioid-dependent pregnant patients. In the context of comprehensive care, methadone maintenance improves maternal and newborn outcomes relative to no treatment or medication-assisted-withdrawal (Kaltenbach et al, 1998, Jones et al 2008, Winklbaur et al 2008).
Despite numerous small studies that have examined the safety and efficacy of buprenorphine for the treatment of opioid dependence during pregnancy (Jones et al 2012), only a few studies compared treatment with methadone to buprenorphine (Jones et al 2005; Fischer et al 2006; Lejeune et al 2006; Binder et al 2008; Kakko 2008; Bakstad et al 2009; Jones et al 2010; Lacroix 2011). Most data suggest that newborns exposed to buprenorphine in utero demonstrate milder, more limited neonatal abstinence symptoms compared to methadone. The most rigorous comparison conducted was the MOTHER study, a multi-site randomized controlled trial in which maternal participants received study medication in a double-blind, double-dummy manner (Jones et al 2010). The rigorous trial requirements of the MOTHER study specifically addressed the efficacy of buprenorphine versus methadone for the treatment of opioid dependence during pregnancy.
In everyday practice, however, treatment allocation is not randomized. Development of a treatment plan for substance abuse includes addressing myriad of social factors, including housing, family drug use, and often prior treatment failures. In this regard, the tightly controlled environment of randomized trials may not provide sufficient information for general use.
The goal of this study is to compare maternal characteristics, prenatal care and newborn outcomes in women treated with buprenorphine versus methadone for opioid-dependence during pregnancy in a setting in which treatment allocation was clinically determined. To our knowledge, this is the largest study to date comparing the effectiveness of these two opioid agonist therapies (OAT) during pregnancy as prescribed in clinical practice.
METHODS
Participants
Study subjects were identified retrospectively from a database of newborns with a known in utero opioid exposure maintained for quality assurance purposes. A total of 806 mother-newborn dyads were identified in the database for potential study inclusion. Mother-newborn dyads were excluded from the study if the mother was treated with an OAT for reasons other than opioid dependence (n=47), the mother was treated as part of the MOTHER study (n=25), the mother had a known opioid dependence but was not prescribed an OAT (n=78), the mother delivered at an outside institution (n=40), or the newborn had an APGAR score of 0 (n=7). 609 mother-newborn dyads treated for opioid dependence during pregnancy with methadone or buprenorphine and delivered at a single institution between August 2000–June 2012 were included in this study. The study was reviewed and approved by the Committees on Human Research at the University of Vermont.
Obstetric care
Women were treated at the tertiary care obstetric clinic with an on-site social worker and a single provider for both obstetrics and buprenorphine maintenance or at community obstetric practices. Ultrasound for viability was performed before initiation of medication for opioid dependence. Subsequently, routine prenatal care was provided. Decisions regarding pregnancy management, analgesia, timing and mode of delivery, and postpartum care were per standard obstetric practice. For mothers who were not on OAT at time of conception, estimated gestational age (EGA) at initiation of treatment was recorded.
Newborn outcomes included gender (male/female), EGA at delivery, preterm delivery (EGA less than 37 weeks), birth weight in grams, birth weight standardized for gender and EGA (z-score), birth weight less than 5th percentile (yes/no), head circumference in centimeters, head circumference standardized for gender and EGA (z-score), non-preterm newborn length of hospital stay in days, newborn receiving any breast milk at discharge (yes/no) and if the newborn was discharged in the care of the mother or family (yes/no). In addition, newborns were classified as neonatal abstinence syndrome (NAS) positive if the newborn required pharmacologic treatment for withdrawal symptoms. For NAS positive newborns, NAS treatment duration in days was also measured.
Opioid medication management
Opioid agonist therapy medication management was determined by individual assessment, which included success with prior or ongoing treatment, treatment availability, acuity, transportation needs, and patient choice (SAMHSA, 2004). Pregnant women without insurance were eligible for Medicaid, which covered either medication over the course of the pregnancy. In general, women that conceived while participating successfully in an opioid maintenance program were maintained on that medication throughout pregnancy. All were informed that methadone was the treatment of choice during pregnancy and offered a medication change if desired. For those in buprenorphine treatment, monotherapy (i.e., Subutex) was prescribed once pregnancy viability was established.
Pregnant women self-reporting opioid dependence (regular use of an opioid agonist with withdrawal symptoms upon cessation) but not receiving treatment were offered medical social work assessment and admission to confirm physical dependence on opioids. Medication assisted therapy was initiated in the hospital setting over 24–36 hours, with regular assessment for signs of opioid withdrawal using the Clinical Institute Narcotic Assessment (CINA) scale (Peachy et al, 1988) and medication initiated when mild-moderate symptoms were observed (score of 10). Medication was initiated at a low dose (methadone 10 mg to maximum 30 mg in 24 hours; buprenorphine (i.e., Subutex) 2 mg to maximum 4 mg in 24 hours) based on symptom relief; on rare occasions, higher doses were used for severe symptoms. After initiation, patients were discharged and seen every 24 hours (methadone) or 24–72 hours (buprenorphine) until dose was stable and withdrawal symptoms improved.
Methadone was the only medication initiated during pregnancy until 2004, when use of buprenorphine began. Buprenorphine use increased over time until 2008, at which time the rates of use of methadone and buprenorphine stabilized. After initiation, methadone maintenance was initially provided through the pharmacy (n=34) and later at an opioid treatment center (n=193; missing data n=21). In each setting, methadone was administered daily. Mothers treated through the pharmacy were referred for counseling in the community. Mothers treated at the opioid treatment program had counseling and mental health assessment within the program. Buprenorphine maintenance was provided by an obstetric provider (n=190), a treatment center (n=24), or community provider (n=134) (undocumented, n=13). Counseling was recommended to all women receiving buprenorphine, with social work visits to assist in coordination.
After initial stabilization on selected medication, the medication dose was increased incrementally during pregnancy based on reported withdrawal symptoms and clinical assessment. Urine drug screens were performed weekly at the place of the opioid agonist medication provider and used for clinical decision-making but were not available for analysis. Once medication was initiated, there was no medication change without a specific indication. During hospitalization for delivery and postpartum, women remained on their opioid maintenance therapy.
Newborn care
All mothers receiving OAT were referred to the Neonatal Medical Follow-up Clinic during pregnancy for consultation. Upon delivery, newborns were hospitalized for assessment of NAS. Breastfeeding was encouraged, except for women actively using cocaine (no patients were HIV positive). While hospitalized, NAS assessments were conducted every 3–4 hours for at least the first 4 days postnatal (96h) using a 19-item modified Finnegan Scale (Jansson, 2009). Pharmacologic treatment for two consecutive total scores ≥ 9 or one score > 13. The majority of newborns requiring pharmacological treatment for neonatal abstinence symptoms at our university-affiliated hospital are treated in an outpatient setting using methadone, once stabilized in the inpatient setting.
Variables
The 609 mother-newborn dyads included in the study were classified as either methadone or buprenorphine according to the mother’s OAT type at delivery. Variables regarding maternal characteristics, prenatal care and newborn outcomes were measured and compared for these two study groups.
Maternal characteristics included age at delivery (in years), hepatitis C positive (yes/no), nulliparous (yes/no), smoking during pregnancy (yes/no) and number of cigarettes smoked per day during third trimester.
Prenatal care characteristics included estimated gestational age (EGA) in weeks at initial prenatal care visit, prenatal care initiated in first trimester (yes/no), adequate prenatal care received (yes/no) according to Kotelchuck index (Kotelchuck et al, 1994), maternal body mass index (BMI) at initial prenatal care visit, maternal weight change in pounds during pregnancy, delivery method (cesarean-section vs. other). Information regarding OAT initiation was measured, categorizing initiation as prior to conception (yes/no).
Statistical analysis
All analyses were conducted using Stata 12.0 (StatCorp. College station, TX). Descriptive statistics were used to describe the characteristics of the mother-newborn dyads included in the study cohort. Both parametric and non-parametric univariate analyses were performed to identify significant differences (P<0.05) between the methadone and buprenorphine treated groups. Additional multivariate analyses were conducted using variables that were identified as significant in the univariate analyses to determine if they remained significant in the multivariate setting.
RESULTS
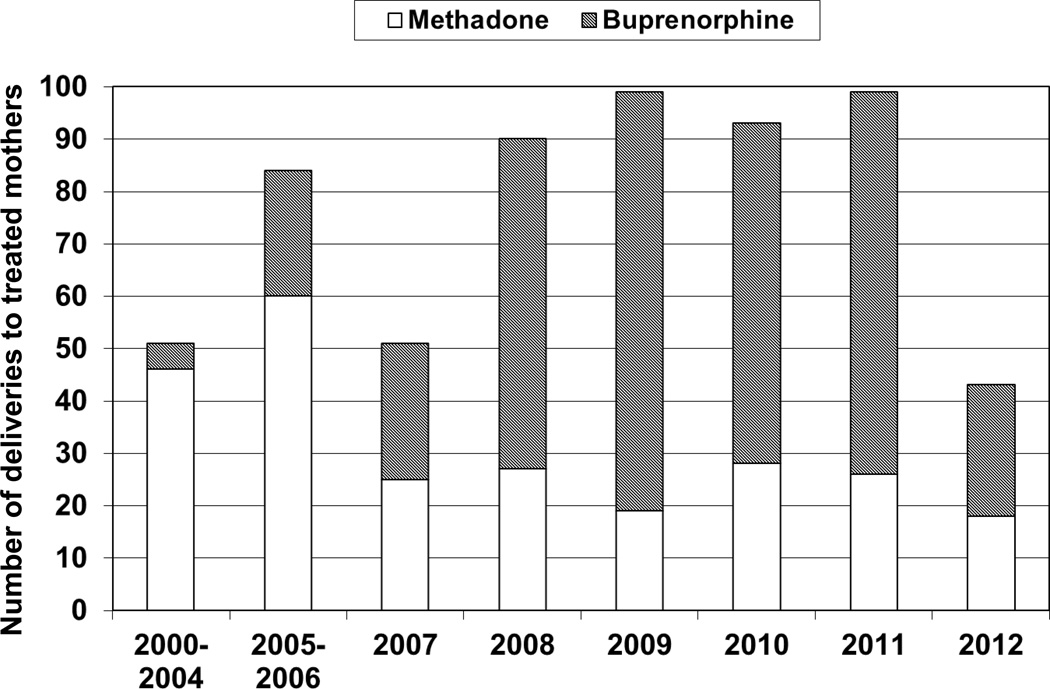
This study included 609 mother-newborn dyads treated for opioid-dependence during pregnancy using methadone or buprenorphine. The distribution of women on OAT at delivery and treatment from 2000–2012 are reflected in Figure 1. Maternal characteristics are outlined in Table 1. Nulliparous mothers were more often treated with buprenorphine; hepatitis C was more prevalent in mothers treated with methadone. The prevalence of smoking was greater than 80% in both groups. Differences in nulliparity and hepatitis C status remained significant in the multivariate analysis controlling for year of birth, maternal age and smoking during pregnancy.
Figure 1. Distribution of women treated with methadone or buprenorphine during pregnancy from August 2000–June 2012.
A: Percent of women receiving opiate agonist therapy prior to pregnancy. The open bar represents women treated with methadone (n=95/225 (39.7%)); the hatched bar represents women treated with buprenorphine (n=196/342 (60.3%)). B: Gestational age at which opiate agonist therapy was initiated in women starting treatment during pregnancy; circles represent the mean gestational age (weeks) and lines represent the standard deviation. The open circle represents women treated with methadone (n=124: 18.9±9.1 weeks; n=6 missing data); the hatched circle represents women treated with buprenorphine (n=137: 15.9±8.1 weeks; n=9 missing data).
Table 1.
Maternal Characteristics
| Methadone (N=248) |
Buprenorphine (N=361) |
P | |||
|---|---|---|---|---|---|
| N | m (sd) or n (%) | N | m (sd) or n (%) | ||
| Age at delivery (years) | 248 | 26.3 (4.5) | 361 | 26.0 (4.0) | .456 |
| Hepatitis C positive | 245 | 100 (41%) | 343 | 88 (26%) | <.001 |
| Nulliparous | 248 | 58 (23%) | 361 | 131 (36%) | .001 |
| Smoker, during pregnancy | 211 | 184 (87%) | 353 | 292 (83%) | .156 |
| 3rd trimester, number of cigarettes per day | 211 | 10.0 (9.3) | 353 | 8.6 (9.0) | .085 |
m, Mean; sd, Standard Deviation
Indices of prenatal care are presented in Table 2; entry into prenatal care was similar between the two groups with more than 65% of mothers initiating prenatal care in the first trimester. Mothers treated with buprenorphine were more likely to receive adequate prenatal care, as defined by Kotelchuck, et al. (Kotelchuck et al, 1994). This significant difference persisted in the multivariate analysis while controlling for EGA at initial visit, body mass index (BMI), pregnancy weight change, gestational age OAT initiated, year of birth, maternal age and smoking during pregnancy. Body Mass Index and pregnancy weight change were similar between the two groups. Delivery methods were also similar between the two groups, with approximately 30% of women delivering via cesarean section.
Table 2.
Prenatal Characteristics
| Methadone (N=248) |
Buprenorphine (N=361) |
P | |||
|---|---|---|---|---|---|
| N | m (sd) or n (%) | N | m (sd) or n (%) | ||
| EGA at initial visit (wks) | 244 | 12.2 (6.4) | 357 | 11.7 (6.3) | .296 |
| Initial visit in 1st trimester | 244 | 160 (66%) | 357 | 254 (71%) | .344 |
| Adequate prenatal care (Kotelchuck) | 236 | 212 (90%) | 349 | 329 (94%) | .046 |
| Body Mass Index at initial visit | 190 | 25.1 (5.3) | 342 | 24.6 (5.6) | .331 |
| Pregnancy weight change (lbs) | 189 | 26.3 (17.4) | 339 | 26.4 (16.9) | .913 |
| Cesarean-section delivery | 248 | 80 (32%) | 361 | 104 (29%) | .362 |
| Maternal OAT prior to conception | 225 | 95 (39.7%) | 342 | 196 (60.3%) | <0.0001 |
| Gestational age OAT initiated (weeks)* | 124 | 18.9±19.1 | 137 | 15.9±8.1 | 0.006 |
| Medication dose at delivery (mg) | 237 | 87.4 (49.9) | 354 | 15.4 (6.4) | N/A |
EGA, Estimated gestational age; m, Mean; sd, Standard Deviation; OAT, Opiate agonist therapy;
includes only patients initiated during pregnancy
Women treated with buprenorphine were more likely to be on an OAT at time of conception. For those women not in treatment prior to pregnancy, OAT with buprenorphine was initiated at an earlier gestational age compared to methadone.
Nineteen women changed their OAT type during pregnancy from buprenorphine to methadone. Eleven of these women were transitioned to the more intensive and structured methadone program due to illicit use of other substances (n=7) or noncompliance with appointments or counseling (n=4). Five women requested the change due to suboptimal control of symptoms or other medication related effects (n=3) or because they could not be certain that buprenorphine would be available for longer than the immediate postpartum period (n=2). Three women were switched for unknown reasons. We identified 4 women that were treated with buprenorphine early in pregnancy but not in treatment at delivery (and not included in the study). Of these women, one weaned (self-directed); one was not compliant with office-based treatment, declined alternative treatment, and left the practice; and 2 were lost to follow-up after buprenorphine initiation. No women were transitioned from methadone to buprenorphine.
Newborn outcomes are shown in Table 3. Maternal treatment with buprenorphine, when compared with methadone, was associated with significantly longer gestational age, reduced incidence of preterm birth, larger birth weight and head circumference. Differences in gestational age and preterm birth remained significant in the multivariate analysis. Standardized birth weight and head circumference (z-scores) were similar between the two groups in the univariate analysis.
Table 3.
Newborn Outcomes
| Methadone (N=248) |
Buprenorphine (N=361) |
P | |||
|---|---|---|---|---|---|
| Infant Characteristics | N | m (sd) or n (%) | N | m (sd) or n (%) | |
| Male | 248 | 111 (45%) | 361 | 177 (49%) | .299 |
| EGA at delivery (wks) | 248 | 38.2 (2.5) | 361 | 39.2 (2.2) | <.001 |
| Preterm (EGA<37 wks) | 248 | 43 (17%) | 361 | 36 (10%) | <.001 |
| Birth weight (grams) | 248 | 2899.7 (583.1) | 361 | 3143.3 (578.9) | <.001 |
| Standardized (z-score) | 248 | −0.59 (.93) | 361 | −0.46 (.98) | .089 |
| < 5th percentile | 248 | 32 (13%) | 361 | 40 (11%) | .494 |
| Head circumference (cm) | 209 | 33.0 (2.0) | 279 | 33.6 (2.1) | <.001 |
| Standardized (z-score) | 209 | −0.50 (.80) | 279 | −0.46 (.98) | .669 |
| Infants treated for NAS | 245 | 106 (42%) | 358 | 82 (23%) | <0.001 |
| Duration of treatment for NAS (days) | 106 | 133±83 | 79 | 83±60 | <0.001 |
| Length of stay, EGA ≥ 37 wks, (days) | 205 | 5.6 (2.8) | 325 | 4.2 (12.6) | .107 |
| Breast milk at discharge | 247 | 156 (63%) | 358 | 267 (75%) | .003 |
| Discharged in care of mother/family | 248 | 237 (96%) | 360 | 351 (98%) | .189 |
EGA, Estimated gestational age; m, Mean; sd, Standard Deviation; NAS, Neonatal abstinence syndrome
Buprenorphine exposed newborns required medication for NAS less frequently and for a shorter duration compared to methadone-exposed newborns. These differences were significant in both the univariate and multivariate setting controlling for EGA at initial prenatal visit, adequacy of prenatal care, nulliparity, maternal BMI, maternal pregnancy weight change, gestational age maternal OAT initiated, year of birth, maternal age and smoking during pregnancy. Buprenorphine exposed infants had higher rates of breastfeeding at discharge. Over 95% of newborns in both groups were discharged to the care of the mother and/or family members. Two infants had congenital malformations at birth: one infant born to a mother that conceived while being treated with methadone had an absent hand; another infant born to a mother that conceived while being treated with buprenorphine had an isolated cleft palate.
Although not included in the study it is interesting to note, that 7 fetuses born to six mothers were stillborn. Of the 5 stillbirths born to 4 mothers (one twin) treated with methadone, 1 mother had severe pneumonia with septic shock at 26 weeks, 1 mother presented for an ultrasound and was noted to have a fetal demise at 38 weeks with intrauterine growth restriction, 1 mother experienced uterine rupture at 32 weeks following a motor vehicle accident resulting in the birth of stillborn twins. One mother presented in labor with a fetal demise at 26 weeks (she had been evaluated at another institution for a motor vehicle accident 24 hours prior to presentation). Of the 2 stillbirths born to buprenorphine treated mothers, 1 mother had a complete abruption at 39 weeks and the other had preterm premature rupture of membranes at 21 weeks followed by induction of labor.
DISCUSSION
Women treated with buprenorphine during pregnancy were more likely to 1) initiate OAT treatment prior to pregnancy or begin earlier in pregnancy, 2) deliver at term and have a longer duration of gestation, 3) deliver larger newborns with larger head circumferences, and 4) have newborns that did not requirement treatment for NAS, or required treatment for NAS for a shorter duration of time compared to newborns exposed to methadone in utero. Overall, these results suggest treatment with buprenorphine during pregnancy in a clinical context (as opposed to a structured randomized trial), provides outcomes that are at least as favorable as methadone. The significance of this cohort is the finding that office based treatment during pregnancy can provide comparable pregnancy outcomes outside the confines of a tightly controlled clinical trial.
Comparisons of outcomes from randomized and nonrandomized trials often yield similar outcomes, but larger effects sizes are frequently observed in the nonrandomized studies (Ioannidis et al 2001). Consistent with this pattern, the present nonrandomized study observed significant differences between medication conditions on a number of outcomes that trended toward significance in the randomized MOTHER study including significantly longer gestation, lower rate of preterm delivery, and lower proportion of neonates requiring treatment for NAS among buprenorphine- as compare to methadone-treated women (Jones et al 2010). One trend in the MOTHER study that was not borne out in the present study was a tendency for more women randomized to buprenorphine to drop out of the study prematurely, largely due to reports of dissatisfaction with the medication during the induction period. In our non-randomized cohort, few women switched from buprenorphine to methadone due to dissatisfaction with buprenorphine; of those women inducted onto buprenorphine during pregnancy, only 3/137 (2.2%) requested a change to methadone due to dissatisfaction with buprenorphine. We did not have any patient leave methadone treatment during pregnancy. These findings suggest that when women are considered candidates for office based treatment based on the general SAMHSA guidelines, there is a high chance of successful induction and treatment with buprenorphine during pregnancy. Our observations are quite reassuring for those that want to consider a stepped approach to the treatment of opioid dependence during pregnancy: identify patients that may be candidates for office based treatment and switch only if needed for improved symptom control or more structured program (Kakko et al, 2007).
Our findings are limited by the lack of randomization and retrospective nature of the data collection. In the practice of treatment, however, multiple issues are considered and multiple outcomes are of importance regarding patient outcomes from a chosen therapy. In this regard, a cohort study that allows for patient treatment selection, assessment of a wide range of outcomes (difficult in tightly controlled settings), and medication effectiveness are quite useful. We recognize the limitations of assessing causality in this study, which is optimally addressed by a randomized trial design (Ioannidis et al, 2001).
Our database may have missed women that initiated treatment but were lost to follow-up prior to entry into the database. We identified 3 women in whom buprenorphine was initiated but were lost to follow-up. Nonetheless, our findings are reflective of the approach many communities may have in the treatment of opioid dependent women during pregnancy. Overall, our data demonstrated a high degree of compliance with prenatal care and continuation of treatment for women initiated or maintained on buprenorphine during pregnancy. It is of note that a large majority of our neonates were discharged into the care of a family member at birth. This is possible in part due to close follow-up of all families for at least a year after birth in a state supported program.
The present study is the largest cohort comparing pregnancy outcomes in women treated with methadone vs. buprenorphine in the United States. Our findings are reassuring for opioid treatment in general, as both groups demonstrated good compliance with prenatal care and over 95% of infants were discharged to the care of the mother or family. Because of the cohort size and reassuring outcomes, these data add significantly to the literature examining the efficacy and clinical effectiveness of buprenorphine for the treatment of opioid dependence during pregnancy and strongly support its use based on standard clinical criteria for office based treatment of opioid dependence.
Conclusions
Pregnancy outcomes of office based treatment for opioid dependence during pregnancy with buprenorphine are at least comparable to outcomes observed with daily dosing with methadone. Neonatal abstinence is reduced in the patients treated with buprenorphine in this cohort.
Acknowledgements
We thank Maureen Matthews RN and Jerilyn Metayer RN, who kindly provided the data necessary for our analysis by maintaining the Quality Assurance database used in this study.
Funding Source: Preparation of this manuscript was supported in part by R34Da030534 and R01 DA031928 from the National Institute on Drug Abuse. Support for maintenance of the Quality Assurance data was supported by the Vermont Child Health Improvement Program. Neither NIH nor VCHIP had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Contributor Information
Marjorie C. Meyer, Department of Obstetrics, Gynecology & Reproductive Sciences, University of Vermont, Burlington, VT, United States.
Anne M. Johnston, Department of Pediatrics, University of Vermont, Burlington, VT, United States.
Abigail M. Crocker, Department of Mathematics and Statistics, University of Vermont, Burlington, VT, United States.
Sarah H. Heil, Departments of Psychiatry and Psychology, University of Vermont, Burlington, VT, United States.
REFERENCES
- Bakstad B, Sarfi M, Welle-Strand GK, Ravndal E. Opioid maintenance treatment during pregnancy: occurrence and severity of neonatal abstinence syndrome. A national prospective study. Eur Addict Res. 2009;15(3):128–134. doi: 10.1159/000210042. [DOI] [PubMed] [Google Scholar]
- Binder T, Vavrinkova B. Prospective randomized comparative study of the effect of buprenorphine, methadone and heroin on the course of pregnancy, birthweight of newborns, early postpartum adaptation and course of the neonatal abstinence syndrome (NAS) in women followed up in the outpatient department. Neuro Endocrinol Lett. 2008;29(1):80–86. [PubMed] [Google Scholar]
- Fischer G, Ortner R, Rohrmeister K, Jagsch R, Baewert A, Langer M, Aschauer H. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275–281. doi: 10.1111/j.1360-0443.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- Hulse GK, Milne E, English DR, Holman CD. The relationship between maternal use of heroin and methadone and infant birth weight. Addiction. 1997;92(11):1571–1579. [PubMed] [Google Scholar]
- Ioannidis JP, Haidich AB, Pappa M, Pantazis N, Kokori SI, Tektonidou MG, Contopoulos-Ioannidis DG, Lau J. Comparison of evidence of treatment effects in randomized and nonrandomized studies. JAMA. 2001;286(7):821–830. doi: 10.1001/jama.286.7.821. [DOI] [PubMed] [Google Scholar]
- Jansson LM, Velez M, Harrow C. The opioid-exposed newborn: assessment and pharmacologic management. J Opioid Manag. 2009;5(1):47–55. [PMC free article] [PubMed] [Google Scholar]
- Jones HE, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79(1):1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Jones HE, O'Grady KE, Malfi D, Tuten M. Methadone maintenance vs. methadone taper during pregnancy: maternal and neonatal outcomes. Am J Addict. 2008;17(5):372–386. doi: 10.1080/10550490802266276. [DOI] [PubMed] [Google Scholar]
- Jones HE, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, et al. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107(Suppl 1):5–27. doi: 10.1111/j.1360-0443.2012.04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakko J, et al. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trial. Am J Psychiatry. 2007;164(5):797–803. doi: 10.1176/ajp.2007.164.5.797. [DOI] [PubMed] [Google Scholar]
- Kakko J, Heilig M, Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008;96(1–2):69–78. doi: 10.1016/j.drugalcdep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139–151. doi: 10.1016/s0889-8545(05)70362-4. [DOI] [PubMed] [Google Scholar]
- Kandall SR, Albin S, Gartner LM, Lee KS, Eidelman A, Lowinson J. The narcotic-dependent mother: fetal and neonatal consequences. Early Hum Dev. 1977;1(2):159–169. doi: 10.1016/0378-3782(77)90017-2. [DOI] [PubMed] [Google Scholar]
- Kotelchuck M. The Adequacy of Prenatal Care Utilization Index: its US distribution and association with low birthweight. Am J Public Health. 1994;84(9):1486–1489. doi: 10.2105/ajph.84.9.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix I, et al. Buprenorphine versus methadone in pregnant opioid-dependent women: a prospective multicenter study. Eur J Clin Pharmacol. 2011;67(10):1053–1059. doi: 10.1007/s00228-011-1049-9. [DOI] [PubMed] [Google Scholar]
- Lejeune C, Simmat-Durand L, Gourarier L, Aubisson S Groupe d'Etudes Grossesse et Addictions (GEGA) Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenorphine substitution. Drug Alcohol Depend. 2006;82(3):250–257. doi: 10.1016/j.drugalcdep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Lester BM, Andreozzi L, Appiah L. Substance use during pregnancy: time for policy to catch up with research. Harm Reduct J. 2004;1(1):5. doi: 10.1186/1477-7517-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschitz MH, Wilson GS, Smith EO, Desmond MM. Factors affecting head growth and intellectual function in children of drug addicts. Pediatrics. 1985;75(2):269–274. [PubMed] [Google Scholar]
- Messinger DS, et al. The maternal lifestyle study: cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics. 2004;113(6):1677–1685. doi: 10.1542/peds.113.6.1677. [DOI] [PubMed] [Google Scholar]
- Peachey JE, Lei H. Assessment of opioid dependence with naloxone. Br J Addict. 1988;83(2):193–201. doi: 10.1111/j.1360-0443.1988.tb03981.x. [DOI] [PubMed] [Google Scholar]
- SAMHSA: Substance Abuse and Mental Health Services Administration. Clinical Guidelines for the use of buprenorphine in the treatment of opioid addiction. A Treatment Improvement Protocol (TIP) 2004;40 [PubMed] [Google Scholar]
- Winklbaur B, Kopf N, Ebner N, Jung E, Thau K, Fischer G. Treating pregnant women dependent on opioids is not the same as treating pregnancy and opioid dependence: a knowledge synthesis for better treatment for women and neonates. Addiction. 2008;103(9):1429–1440. doi: 10.1111/j.1360-0443.2008.02283.x. [DOI] [PubMed] [Google Scholar]