Abstract
In this study, the first mechanism-based monoclonal antibodies have been produced that recognize and differentiate diethoxy- and monoethoxyphosphorylated serine residues. Haptens were synthesized as the stable phosphonate form of phosphoserine esters to improve the immunoresponse. Following condensation with a glutaric anhydride to link the phosphoserine moieties to carrier protein, the hapten densities attached to bovine serum albumin and keyhole limpet henocyananin were determined by partial trypsin digestion and MALDI mass spectrometry, and confirmed using a fluorescent assay (FITC) to quantify unmodified lysine residues. The conjugation reactions were pH optimized to improve hapten density. Screening of subclones led to the identification of two monoclonal antibodies: (a) N257/25.11 that specifically recognizes (EtO)2P(O)-Ser as the phosphylated or inhibited form, and (b) N262/16 that recognizes (EtO)(HO)P(O)-Ser as the ‘aged’ form. Analysis of blood samples treated with paraoxon (EtO)2P(O)-OPhNO2 showed a concentration dependent recognition of the phosphylated form.
Keywords: organophosphates, haptens, OP-modified serine, antibody, MALDI
1. Introduction
Chronic, low-level exposure to organophosphate (OP) insecticides has been associated with a number of minor conditions, including fatigue, headache, and common cold or flu-like symptoms, but also other, more concerning morbidities such as acute cholinergic poisoning, ataxia, delayed neuropathy, intermediate syndrome, pulmonary toxicity, genotoxicity, Parkinson's disease and vision loss [1-13]. The diversity of conditions and illnesses suggests that OPs react with numerous protein targets [14, 15] other than acetylcholinesterase (AChE) and early detection of a wider range of OP-modified targets could indicate courses of treatment and aid in disease prevention.
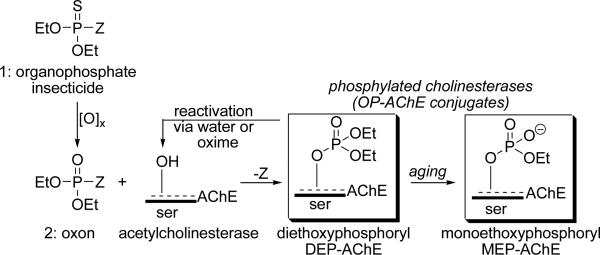
Most commercial OP insecticides share a common structural motif: a diethoxyphosphorothionate 1 that vary in the leaving group Z (Scheme 1). However, it is widely known that the myriad of cholinergic and non-cholinergic effects following OP exposure are due to the highly reactive oxon form 2 of the insecticide from oxidative desulfurization that acts as an indiscriminate phosphylating agent with chemical properties similar to nerve agents. For example, diethoxy OP oxons react readily with the target enzyme AChE to form DEP-AChE adducts (Scheme 1) that trigger cholinergic toxicity.
Scheme 1.
Structure of organophosphate insecticides, conversion to oxons and reaction with acetylcholinesterase.
Formation of the OP-AChE conjugate can be reversed by water or oxime antidotes to partially restore the enzymatic activity [16-18]. Subsequent to the inhibition, a process known as ‘aging’ can also occur that results in the loss of a phosphoester group and formation of the oxyanion, or monoethoxyphosphoryl (MEP) AChE conjugate (Scheme 1).
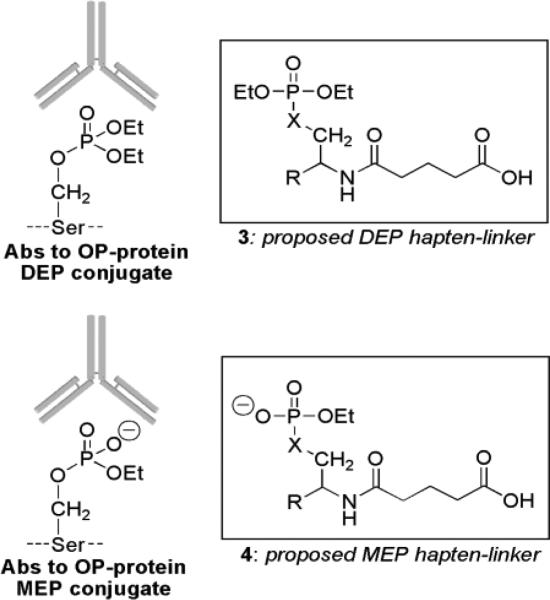
Oxons also react to afford other OP-modified proteins [14, 15, 19, 20]. However, OP oxons are too reactive to quantify in vivo, and researchers have focused their attention on OP-modified proteins or OP-biomarkers since they offer a more reliable alternative for confirming low-level exposures. Identification of insecticide-based biomarkers should be straightforward since a majority of OP insecticides form the aforementioned diethoxy- and monoethoxyphosphoryl conjugates at serine (OP-tyrosine adducts are also known, see: [21-24]) thereby reducing the biomarker panel to the DEP-serine and MEP-serine subtypes. Detection of DEP- and/or MEP-serine conjugates would provide accurate, mechanistically precise indicators of OP insecticide exposure. Of key importance is that those two general biomarkers of OP insecticide exposure are chemically distinct as neutral (DEP) or charged (MEP) conjugates that can be differentiated by antibodies (Scheme 2) [25]. Moreover, a dual-analyte approach introduces the possibility of identifying each conjugate, which may distinguish between acute and chronic exposure and thus may prove important for directing proper therapeutic intervention.
Scheme 2.
Proposed haptens leading to antibodies that recognize distinctive OP-protein conjugates.
Although antibodies for detecting organophosphorus compounds in vitro are known [26-30], antibodies to OP-adducted proteins have not been widely reported [30-32]. Indirectly, immunoprecipitation of OP-protein targets using antibodies to butyrylcholinesterase followed by digestion and mass spectral characterization of the OP-modified peptide has been applied to address the problem [20, 33-37].
As noted, insecticide oxons are similar to chemical nerve gas agents in their reactivity and selectivity toward protein residues such as serine. As a result, DEP- or MEP-modified serines 3 and 4 (Scheme 2) represent chemically precise, small molecule representations of insecticide oxon biomarkers. Antibodies thus derived from DEP-serine and MEP-serine would be expected to selectively recognize proteins modified at serine by insecticide oxons. Therefore, this study seeks to prepare and characterize DEP- and MEP-serine moieties as haptens (Scheme 2) and produce antibodies that selectively recognize those structures.
2. Materials and Methods
2.1. General
Chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Bovine serum albumin (BSA) was obtained from Sigma-Aldrich (St. Louis, MO), keyhole limpet hemocyanin (KLH) from Calbiochem (La Jolla, CA), and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDCI) and N-hydroxysuccinimide (NHS) from Thermo Scientific (Rockford, IL). Sequencing grade modified trypsin was obtained from Promega Corporation (Madison, WI). The protein conjugates were obtained by conjugation of the amino groups on BSA or KLH to the hapten carboxylic acid group using EDCI/NHS activation [38, 39]. Common anhydrous reagents and/or solvents were employed as received.
Flash chromatography on silica gel (200-300 mesh) was conducted using various solvent combinations. Thin-layer chromatography (TLC) was conducted on aluminum-backed plates and visualized by UV and/or staining by ninhydrin or iodine.
The 1H NMR spectra were recorded on a Varian 400-MHz spectrometer. Chemical shifts are reported in parts per million relative to tetramethylsilane (Me4Si, δ = 0.00 ppm) with CDCl3 as solvent. 31P NMR spectra were recorded at 202 MHz and chemical shifts reported in parts per million relative to external 85% phosphoric acid (δ = 0.0 ppm). High resolution mass spectrometry was conducted using aMicromass LCT - Waters 2795 HPLC with 2487 UV Detector (Milford, MA) with caffeine as a molecular weight standard.
2.2.1. Synthesis of DEP-hapten linker 3 (X = CH2, R =CN); 4-(3-(diethoxyphosphono)-1-cyanopropylcarbamoyl)butanoic acid
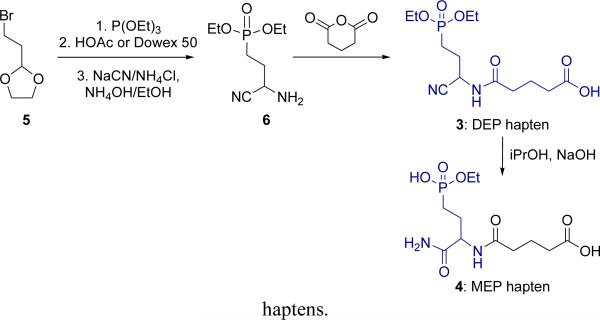
3-Amino-3-cyanopropylphosphonic acid diethyl ester was prepared from 2-(2-bromoethyl)-1,3-dioxolane in 52% overall yield by stepwise reaction with triethylphosphite, deprotection of the aldehyde, and Strecker reaction [40-42]. To the resultant aminonitrile 6 (212 mg, 0.96 mmol, 1 equiv) in CH2Cl2 (5 mL) was added glutaric anhydride (165 mg, 1.44 mmol, 1.5 equiv). The reaction mixture was stirred at rt for 12 h, concentrated under reduced pressure, and the residue purified by column chromatography over silica gel (EtOAc, 100%; then EtOAc/MeOH, 9:1), affording the DEP-hapten 3 as an oil (320 mg). 1H NMR (400 MHz, CDCl3) δ 7.77 (s, 1H), 4.93 (q, J = 6.0 Hz, 1H), 4.08-4.18 (m, 4H), 2.40 (t, J = 7.0 Hz, 2H), 2.37 (t, J = 7.0 Hz, 2H), 2.10-2.30 (m, 2H), 1.92-2.05 (m, 4H), 1.33 (dt, J = 7.0 Hz, J = 2.4 Hz, 6H); 31P NMR (400 MHz, CDCl3) δ 31.39; MS (ES+) Calcd for: C13H23N2O6P 334.3067; Found: 335.3370 [(M+H)+].
2.2.2. Synthesis of MEP-hapten linker 4 (X = CH2, R = C(O)NH2; 4-(3-(ethoxyphosphono)-1-carbamoylpropyl carbamoyl)butanoic acid
The DEP-hapten 3 (200 mg, 0.6 mmol, 1 equiv) was dissolved in i-PrOH (10 mL), followed by the addition of a 1.0 N NaOH (3 mL). The mixture was stirred at 70 °C for 12 h. After cooling to rt, the solution mixture was diluted with water and extracted (EtOAc, 5 mL). The aqueous layer was adjusted to pH 1 with 1.0 N HCl, and to this was added i-PrOH:CHCl3 (3:1, 15 mL). The product was extracted, dried over Na2SO4, filtered and concentrated under reduced pressure to afford the bis-hydrolyzed phosphorus monoester carboxamide MEP-hapten 4 as an oil (180 mg; 93%).1H NMR (400 MHz, D2O) δ 4.26-4.29 (m, 1H), 3.84-3.89 (m, 2H), 2.21-2.31 (m, 4H), 2.02-1.96 (m, 1H), 1.80-1.86 (m, 1H), 1.74-1.79 (m, 2H), 1.64-1.74 (m, 2H), 1.12 (t, J = 7.0 Hz, 3H); 31P NMR (400 MHz, D2O) δ 25.11; MS (ES+) Calcd for chemical formula C11H21N2O7P 324.2687; Found: 348.2703 [(M+H+Na)+].
2.2.3. Synthesis of BSA-3 and KLH-3 conjugates
To a solution of diethoxy phosphonate hapten 3 (2 mg) in 200 μL of solvent at rt was added EDC (3.3 mg), the reaction mixture vortexed, then stirred for 10 min. N-hydroxysuccinimide (1.4 mg) in 100 μL of acetonitrile was added, vortexed again, and stirred for 20 min. A solution of BSA (2 mg) or KLH (2 mg) in 500 μL of 0.1 M MES/0.15 M NaCl (BSA) or 0.1 M MES/0.9 M NaCl (KLH) at pH 4.7 was added to the activated hapten 3 and the reaction mixture stirred at rt for 2 h. The hapten density was determined by MALDI-TOF mass spectrometry following trypsin digestion (see: Section 2.2.5.).
2.2.4. Synthesis of BSA-4 and KLH-4 conjugates
The monoethoxy phosphonate hapten 4 (2 mg) was conjugated to BSA and KLH in the same manner as hapten 3 except 500 μL of 0.1 M phosphate buffer pH 7.2 was used in place of MES. The hapten density was determined by MALDI-TOF mass spectrometry following trypsin digestion (see: Section 2.2.5.).
2.2.5. Partial trypsin digestion and MALDI-TOF MS analysis
To determine the hapten density per protein, the protein was digested with trypsin prior to and after conjugation with the hapten. The mass spectra of the untreated tryptic peptides was compared to the mass spectra of haptenized tryptic peptides to determine the hapten density. Untreated BSA or KLH protein samples were trypsin-digested (1:10 trypsin:protein) in 50 mM ammonium bicarbonate (pH 8.0) at 25 °C for 24 h. The hapten-conjugated protein samples (BSA or KLH) were isolated after Sephadex G-25 separation, and then trypsin-digested at a 1:10 ratio of trypsin:protein in 50 mM ammonium bicarbonate (pH 8.0) at 25 °C for 24 h. The digests were then desalted by C4 Micro SpinColumn (Harvard Apparatus, Holliston, MA), spotted on the MALDI plate with sinapinic acid as the matrix, and analyzed by the MALDITOF mass spectrometer (ABI Voyager DE STR, Applied Biosystems, Foster City, CA) in linear mode. The hapten density (Dh) was calculated by the following equation:
where Tc is the m/z of the protein-hapten conjugate; Tu the m/z of the carrier protein; Mh the molecular weight of the hapten (mw ~ 334.3); Mw the molecular weight of water; z is the sample ion charge assigned as 1 (mainly single-charged ions are produced by MALDI).
2.2.6. Spectrophotometric analysis of BSA- and KLH-hapten conjugates
The hapten density was assessed by conjugation of remaining lysine residues to a fluorescent probe. The resultant measured fluorescence correlates with the number of available residues. The BSA- and KLH-conjugates were diluted in 0.1 M Na2CO3 to a concentration of 1 mg/mL and reacted with 0.005% fluorescein isothiocyanate (FITC; w/v) at RT in the dark for 3 h. The reaction was terminated by the addition of ammonium chloride (0.5 M; 100 μL) and incubated at RT in the dark for 1 h. Excess FITC was removed by separation on Sephadex G-25, and the samples measured by spectrophotometry. The degree of conjugation (Dc) was calculated by the equation:
where Ap1 is the absorbance of the BSA or KLH at 280 nm; Af1 the absorbance of the BSA or KLH at 498 nm; Ap2 the absorbance of the BSA or KLH-hapten conjugate at 280 nm; Af2 the absorbance of the BSA- or KLH-hapten conjugate at 498 nm; 0.32 is a correction factor FITC absorption at 280 nm.
2.2.7. Monoclonal Antibodies
Two monoclonal antibodies raised against the diethoxyphosphonyl-serine 3 (N257/25) and monoethoxyphosphonyl-serine 4 (N262/16; four subclones) haptens were used this study. Briefly, mice were immunized with either of the KLH-conjugated haptens 3 or 4 in the presence of Freund's adjuvants. Antigen injection was repeated until the anti-mouse AChE antibody titer reached a plateau in the plasma as determined by ELISA. Antibodies were generated by UC Davis/NeuroMab by hybridoma fusion. The hybridomas were screened for antibody production in our lab using common immunological assays and the cross-reactivity validated by NeuroMab. Antibodies N257/25 and N262/16 are available (neuromab.ucdavis.edu).
2.2.8. ELISA Assays
BSA ELISA. BSA-hapten conjugates were diluted in 50 mM sodium carbonate (pH 9.6) to the concentration of 10 μg/mL and added to ELISA plates. After incubation at 4°C overnight, the plates were washed with phosphate buffered saline with Tween (PBST, 0.05% Tween-20) and the remaining adsorption sites were blocked with 3% BSA/PBST at RT for 1 h. The primary antibodies were diluted in 1% BSA/PBST, added to the plates and incubated at rt for 2 h then the secondary antibody (donkey anti-mouse IgG HRP conjugate, 1:5,000 dilution in 1% BSA/PBST, Jackson ImmunoResearch, West Grove, PA) was added and incubated at rt for 2 h. For detection, 1-Step ABTS (Pierce, Rockford, IL) was added and the plates were analyzed by the VERSAmax tunable microplate reader (Molecular Devices, Sunnyvale, CA).
Rat blood ELISA. Whole rat blood (male Sprague-Dawley; 300-400 g) was via the lateral tail vein or following decapitation, placed directly into heparin-treated vacuum tubes, and stored at 5 °C until used. Rat blood samples (50 μL) were brought to room temperature and treated with paraoxon (1 mM to 10 nM), the treated blood separated into serum and erythrocytes, and ELISA's conducted on each fraction as indicated above for BSA-haptens.
3. Results and Discussion
3.1. Hapten Synthesis and Conjugation
The hapten structures, diethyl phosphate ester 3 (neutral) and ethyl phosphate monoester 4 (monoacid; oxyanion at physiologic pH), represent the expected conjugates formed at serine following AChE inhibition by a diethyl insecticide oxon and should be distinguishable by antibodies. However, serine-O-phosphylated groups (Scheme 2; X = O) are relatively unstable in vivo due to hydrolysis by phospho[di]esterases, carboxyesterases, and other non-specific hydrolytic mechanisms. Indeed, incubation of either the phosphoryl haptens 3 and 4 when X = O with whole blood, PBS (pH 7.6), or elevated basic conditions led to significant loss of the phosphate group(s). To increase the in vivo lifetime required of the proposed haptens for effective immunization and antibody response, the corresponding phosphonates were chosen (Scheme 2; X = CH2) as haptens.
The key intermediate, 4-diethoxyphosphonyl)amino butyrylnitrile 6, was previously prepared in three steps from a 3-bromo-diethoxyacetal [40-42]. A similar process using commercially available, 2-bromoethyl-1,3-dioxolane 5 (Scheme 3) afforded the aminonitrile phosphonate diester 6 in 52% overall yield.
Scheme 3.
Synthesis of the diethoxy-and monoethoxy-phosphonyl
Due to the instability of 6 (cyclization), glutaric anhydride (1.5 eq.) was added immediately following isolation (i.e., without purification) to form the 5-carbon linker and overall hapten structure 3. Using iPrOH/NaOH at elevated temperature, dual hydrolysis occurred to yield the ethyl phosphonate monoester and carboxamide 4. Selective hydrolysis to the carboxamide was not possible so hapten 3 was used as the nitrile form.
Conjugation to the carrier proteins was accomplished by activation of the carboxylic acid terminus of haptens 3 and 4 using NHS/EDC. However, the resulting low titers of antibodies suggested that the conjugation reactions may have been ineffectual. Indeed, MALDI-TOF MS analyses of the protein conjugates obtained under standard conjugation conditions (EDC/NHS; pH 4.7) showed a low number of haptens (3-5) per protein (see: Section 3.2.). Two changes to the conjugation experiment improved the hapten-protein ratio. Thus, the use of acetonitrile as co-solvent and an increase in pH led to surprisingly favorable results. At pH 6.5, the amount of hapten 3 conjugated to KLH or BSA improved to a ratio of 8:1 and at pH 8.0 approximately 30 haptens per protein were observed. For hapten 4, alteration in the reaction pH led to less impressive results but still afforded almost 16 haptens per protein conjugated at pH 8.0. Hapten 4 differs from 3 due to the phosphonic acid ionization, which may have played a role in the conjugation reaction outcomes.
3.2. Determination of hapten density by MALDI-TOF MS
Initial failures to achieve sufficient hapten density required that improved conjugation conditions be devised. To determine the best conditions, a method to analyze the hapten density was developed. Here we present an efficient method to determine the hapten density of KLH and BSA conjugates using partial trypsin digestion and matrix-assisted laser desorption ionization (MALDI) time-of-flight mass spectrometry. Determination of hapten density on BSA conjugates by MALDI-MS has been reported previously [43].
Haptens 3 and 4 were conjugated to both BSA and KLH using EDCI/NHS coupling conditions. Excess reagents were separated from the protein conjugates prior to mass spectrometric and spectrophotometric analysis. Hapten density by MALDI-TOF MS were determined from protein conjugates that were trypsin digested. . Due to the known complexity of MS analysis of KLH [44], partial trypsin digestion of KLH-hapten conjugates was required in order to obtain protein fragments within the range of MS detection (10,000 – 100,000 m/z) .
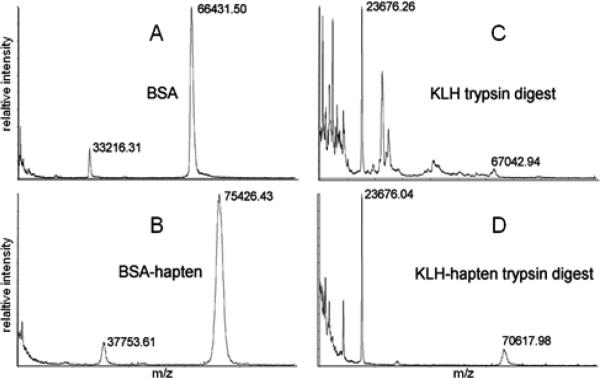
In general, the MALDI MS spectra of the conjugated protein differed from the unreacted protein consistent with successful hapten conjugation (Fig. 1). For BSA, a shift from 66431.50 to 75426.43 m/z was observed by MALDI MS with hapten 3, which corresponds with a hapten to protein ratio of 28.4:1 (Fig. 1, left). When KLH was reacted with hapten 3 no clear molecular ion and no obvious change to the molecular ion resulting from the addition of hapten was observed in the MALDI MS (data not shown). However, when unconjugated and hapten-conjugated KLH were subjected to partial trypsin digest as described (Fig. 1 right) the MALDI MS spectra reveal a consistent trypsinized peak at 23676 m/z. Moreover, a reduced number of peaks were observed for the KLH-hapten partial trypsin digest as compared to that of KLH trypsin indicating that a number of hapten moieties occupy lysine residues of KLH thereby diminishing the number of possible trypsin digest locations. A consistent and reproducible shift from 67042.94 to 70617.98 m/z was also observed for the hapten-conjugated KLH that calculates to a hapten-protein ratio of 11.3 for the KLH conjugates.
Figure 1.
MALDI-TOF MS analysis of: (A) BSA; (B) BSA-hapten 3 conjugate; (C) KLH trypsin digest; and (D) KLH-hapten 3 trypsin digest. Relative intensity (% intensity) plotted versus m/z (10,000 – 100,000).
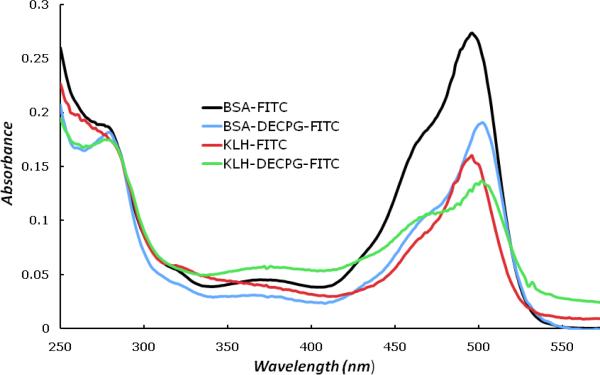
To validate the partial trypsin digest method of hapten density, conventional colorimetric assays were conducted. However, we were unable to accurately measure the degree of hapten conjugation on BSA or KLH using the TNBS assay [45-47] (data not shown). This may have been due, in part, to functional groups present on haptens 3 or 4. As an alternative, fluorescein isothiocyanate (FITC) was employed to derivatize the remaining free lysine groups of BSA after hapten conjugation (Fig. 2) [48, 49]. Based on a total of 60 possible lysine residues available for conjugation, FITC analysis of the BSA-hapten conjugates showed Dc of 45.0% correlating to 27 haptens per BSA molecule, which is close to the calculated Dh of 28.4 haptens/BSA for the MALDI analyses.
Figure 2.
Absorbance spectra of the FITC assay of BSA- and KLH-hapten conjugates.
Results of the FITC assay show that the Dc of KLH-hapten is 17.0%. To evaluate the BSA- and KLH-hapten conjugate molecules observed by MS, the specific hapten density (Dhs) is derived by the equation Dhs = Dh / Mu × 1000 × 100%, where Mu the molecular weight of the unconjugated fragment or carrier protein. The Dhs of BSA-hapten conjugate was 42.3% corresponding well with the Dc (45.0%). Likewise, the Dhs of KLH-hapten conjugate fragment is 16.9%, which correlates with the Dc (17.0%). Therefore, the specific hapten density values obtained from the MS analysis are confirmed by the FITC assay. Moreover, the MS and spectrophotometric data indicate that KLH contains less available amine residues for hapten conjugation than BSA.
3.3. Assessment of Antibody Recognition and Sensitivity
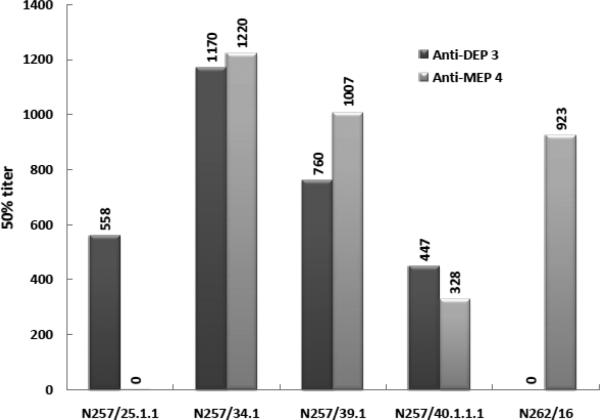
The KLH-conjugated haptens 3 and 4 were submitted to NeuroMab (Davis, CA) for monoclonal antibody production. mAb screening resulted in four hybridoma supernatants (N257/ 25.1.1, 34.1, 39.1, and 40.1.1.1) from 3 and one hybridoma supernatant (N262/16) from 4. The supernatants were further characterized with respect to their cross-reactivity (Figure 3). In the N257 series, subclone 25.1.1 produced a highly selective antibody to the diethoxyphosphylated hapten, whereas subclones N257/34.1, 257/39.1 and 257/40.1.1.1 were poorly selective with near equal recognition of both diethoxyphosphylated- and monoethoxyphosphylated BSA-hapten.
Figure 3.
Antibody titers of hapten conjugates 3 and 4.
This may be due to partial hydrolysis of the hapten in vivo to afford the monoester, which in turn affords a mixture of antibodies. Importantly, the presence of the nitrile group showed no adverse effect on the antibody production. The subclone N262/16 raised against the hapten 4 showed no cross-reactivity with diethoxyphosphylated BSA indicating useful selectivity toward this particular OP-protein modification. The reason for the limited number of subclones recognizing the MEP hapten is unclear but may be due to hydrolysis of the monoethyl ester to afford a phosphoserine (dianionic) whose antibodies are not reactive toward the monoester. Given these results, N257/25.1.1 and N262/16 represent antibodies capable of selectively recognizing diethoxyphosphylated and monoethoxyphosphylated serine residues, respectively. Those antibodies will be tested for their ability to recognize and differentiate OP-modified proteins in future studies.
3.4. N257/25.1.1 Recognition of Paraoxon-Modified AChE in rat blood
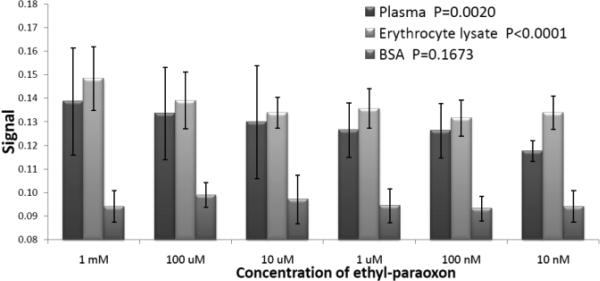
To assess the utility of N257/25.1.1, we explored the possibility of directly detecting OP-modified proteins in biological samples. Whole rat blood was treated with paraoxon (EtO)2P(O)-OArNO2 (from 10 mM to 1 nM), the blood separated into serum and erythrocytes and ELISA's conducted on the putative OP-protein conjugates formed using the diethoxy monoclonal antibody (Fig 4). The diethoxy OP-conjugate monoclonal antibody showed no reactivity toward the control BSA with increasing concentration, and no reactivity toward untreated blood, whereas both the serum and erythrocytes showed a dose-dependent recognition of OP-modified proteins from 100 μM to 100 nM suggesting possible utility as a diagnostic antibody for organophosphate exposures.
Figure 4.
ELISA of paraoxon-treated rat blood and untreated BSA using concentrated N257/25.1.1 supernatant.
Highlights.
phosphonate analogs of phosphoserine were prepared.
diethoxyphosphoserine and monoethoxyphosphoserine antibodies were prepared.
a new mass spectrometry method was devised to assess hapten density.
antibodies recognize paraoxon-modified rat blood.
Acknowledgements
Supported by the CounterACT Program, Office of the Director, National Institutes of Health (OD) and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number U44 NS058229 (ATERIS Technologies LLC), and NIH grant R43 ES016392 (ATERIS Technologies LLC). The authors thank the UC Davis/NIH NeuroMab Facility for production of the monoclonal antibodies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. Conflict of interest
Some authors are employees of ATERIS Technologies, Inc.
References
- 1.Ames RG, Brown SK, Mengle DC, Kahn E, Stratton JW, Jackson RJ. Protecting agricultural applicators from over-exposure to cholinesterase-inhibiting pesticides: perspectives from the California programme. J Soc Occup Med. 1989;39:85–92. doi: 10.1093/occmed/39.3.85. [DOI] [PubMed] [Google Scholar]
- 2.Ames RG, Steenland K, Jenkins B, Chrislip D, Russo J. Chronic neurologic sequelae to cholinesterase inhibition among agricultural pesticide applicators. Arch Environ Health. 1995;50:440–444. doi: 10.1080/00039896.1995.9935980. [DOI] [PubMed] [Google Scholar]
- 3.Arima H, Sobue K, So M, Morishima T, Ando H, Katsuya H. Transient and reversible parkinsonism after acute organophosphate poisoning. J Toxicol Clin Toxicol. 2003;41:67–70. doi: 10.1081/clt-120018273. [DOI] [PubMed] [Google Scholar]
- 4.Ballantyne B, Marrs T. Clinical and experimental toxicology of organophosphates and carbamates. Butterworth Heinemann; Boston: 1992. [Google Scholar]
- 5.Dahlgren JG, Takhar HS, Ruffalo CA, Zwass M. Health effects of diazinon on a family. J Toxicol Clin Toxicol. 2004;42:579–591. doi: 10.1081/clt-200026979. [DOI] [PubMed] [Google Scholar]
- 6.Dementi B. Ocular effects of organophosphates: a historical perspective of Saku disease. J Appl Toxicol. 1994;14:119–129. doi: 10.1002/jat.2550140214. [DOI] [PubMed] [Google Scholar]
- 7.Imamura T, Gandy J. Pulmonary toxicity of phosphorothioate impurities found in organophosphate insecticides. Pharmacol Ther. 1988;38:419–427. doi: 10.1016/0163-7258(88)90012-5. [DOI] [PubMed] [Google Scholar]
- 8.Jayawardane P, Dawson AH, Weerasinghe V, Karalliedde L, Buckley NA, Senanayake N. The spectrum of intermediate syndrome following acute organophosphate poisoning: a prospective cohort study from Sri Lanka. PLoS Med. 2008;5:e147. doi: 10.1371/journal.pmed.0050147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotti M, Moretto A. Organophosphate-induced delayed polyneuropathy. Toxicol Rev. 2005;24:37–49. doi: 10.2165/00139709-200524010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Moretto A. Experimental and clinical toxicology of anticholinesterase agents. Toxicol Lett. 1998;102-103:509–513. doi: 10.1016/s0378-4274(98)00245-8. [DOI] [PubMed] [Google Scholar]
- 11.Niven AS, Roop SA. Inhalational exposure to nerve agents. Respir Care Clin N Am. 2004;10:59–74. doi: 10.1016/S1078-5337(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 12.Meinert R, Schuz J, Kaletsch U, Kaatsch P, Michaelis J. Leukemia and non-Hodgkin's lymphoma in childhood and exposure to pesticides: results of a register-based case-control study in Germany. Am J Epidemiol. 2000;151:639–646. doi: 10.1093/oxfordjournals.aje.a010256. discussion 647-650. [DOI] [PubMed] [Google Scholar]
- 13.Stephens R, Spurgeon A, Calvert IA, Beach J, Levy LS, Berry H, Harrington JM. Neuropsychological effects of long-term exposure to organophosphates in sheep dip. Lancet. 1995;345:1135–1139. doi: 10.1016/s0140-6736(95)90976-1. [DOI] [PubMed] [Google Scholar]
- 14.Thompson CM, Prins JM, George KM. Mass spectrometric analyses of organophosphate insecticide oxon protein adducts. Environ Health Perspect. 2010;118:11–19. doi: 10.1289/ehp.0900824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casida JE, Quistad GB. Serine hydrolase targets of organophosphorus toxicants. Chem Biol Interact. 2005;157-158:277–283. doi: 10.1016/j.cbi.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 16.Fest C, Schmidt KJ. The chemistry of organophosphorus pesticides; reactivity, synthesis, mode of action, toxicology. Springer Verlag; Berlin, New York: 1973. [Google Scholar]
- 17.Fukuto TR. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Perspect. 1990;87:245–254. doi: 10.1289/ehp.9087245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallo MA, Lawryk NJ. Organic Phosphorus Pesticides. Academic Press; San Diego: 1991. [Google Scholar]
- 19.Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chemical research in toxicology. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- 20.Marsillach J, Richter RJ, Kim JH, Stevens RC, MacCoss MJ, Tomazela D, Suzuki SM, Schopfer LM, Lockridge O, Furlong CE. Biomarkers of organophosphorus (OP) exposures in humans. Neurotoxicology. 2011;32:656–660. doi: 10.1016/j.neuro.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding SJ, Carr J, Carlson JE, Tong L, Xue W, Li Y, Schopfer LM, Li B, Nachon F, Asojo O, Thompson CM, Hinrichs SH, Masson P, Lockridge O. Five tyrosines and two serines in human albumin are labeled by the organophosphorus agent FP-biotin. Chem Res Toxicol. 2008;21:1787–1794. doi: 10.1021/tx800144z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grigoryan H, Schopfer LM, Peeples ES, Duysen EG, Grigoryan M, Thompson CM, Lockridge O. Mass spectrometry identifies multiple organophosphorylated sites on tubulin. Toxicol Appl Pharmacol. 2009;240:149–158. doi: 10.1016/j.taap.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Schopfer LM, Grigoryan H, Thompson CM, Hinrichs SH, Masson P, Lockridge O. Tyrosines of human and mouse transferrin covalently labeled by organophosphorus agents: a new motif for binding to proteins that have no active site serine. Toxicol Sci. 2009;107:144–155. doi: 10.1093/toxsci/kfn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schopfer LM, Champion MM, Tamblyn N, Thompson CM, Lockridge O. Characteristic mass spectral fragments of the organophosphorus agent FP-biotin and FP-biotinylated peptides from trypsin and bovine albumin (Tyr410) Anal Biochem. 2005;345:122–132. doi: 10.1016/j.ab.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Nagy JO, Thompson CM. Pesticide Biomarker. A.T. LLC; US: 2007. [Google Scholar]
- 26.Edward SL, Skerritt JH, Hill AS, McAdam DP. An improved immunoassay for chlorpyriphos methyl in grain. Food and Agricultural Immunology. 1993;5:129–144. [Google Scholar]
- 27.McAdam DP, Hill AS, Beasley HL, Skerritt JH. Mono- and polyclonal antibodes to the organophosphate fenitrothion. I. Approaches to hapten-protein conjugation. J. Agric. Food Chem. 1992;40:1466–1470. [Google Scholar]
- 28.McAdam DP, Skerritt JH. Synthesis of organothiophosphate antigens for the development of specific immunoassays. Aust. J. Chem. 1993;46:959–967. [Google Scholar]
- 29.Skerritt JH, Lee N. Approaches to the synthesis of haptens for immunoassay of organophosphate and synthetic pyrethroid insecticides. Immunoassays for residue analysis: food safety. ACS Monograph. 1996:124–129. [Google Scholar]
- 30.Johnson JK, Cerasoli DM, Lenz DE. Role of immunogen design in induction of soman-specific monoclonal antibodies. Immunol Lett. 2005;96:121–127. doi: 10.1016/j.imlet.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 31.VanDine R, Babu UM, Condon P, Mendez A, Sambursky R. A 10-minute point-of-care assay for detection of blood protein adducts resulting from low level exposure to organophosphate nerve agents. Chem Biol Interact. 2013;203:108–112. doi: 10.1016/j.cbi.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Li B, Duysen EG, Froment MT, Masson P, Nachon F, Jiang W, Schopfer LM, Thiele GM, Klassen LW, Cashman J, Williams GR, Lockridge O. Polyclonal antibody to soman-tyrosine. Chem Res Toxicol. 2013;26:584–592. doi: 10.1021/tx400027n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter MD, Crow BS, Pantazides BG, Watson CM, Decastro BR, Thomas JD, Blake TA, Johnson RC. Profiling Cholinesterase Adduction: A High-Throughput Prioritization Method for Organophosphate Exposure Samples. J Biomol Screen. 2013 doi: 10.1177/1087057113497799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter MD, Crow BS, Pantazides BG, Watson CM, Thomas JD, Blake TA, Johnson RC. Direct Quantitation of Methyl Phosphonate Adducts to Human Serum Butyrylcholinesterase by Immunomagnetic-UHPLC-MS/MS. Anal Chem. 2013;85:11106–11111. doi: 10.1021/ac4029714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peeples ES, Schopfer LM, Duysen EG, Spaulding R, Voelker T, Thompson CM, Lockridge O. Albumin, a new biomarker of organophosphorus toxicant exposure, identified by mass spectrometry. Toxicol Sci. 2005;83:303–312. doi: 10.1093/toxsci/kfi023. [DOI] [PubMed] [Google Scholar]
- 36.Marsillach J, Costa LG, Furlong CE. Protein adducts as biomarkers of exposure to organophosphorus compounds. Toxicology. 2013;307:46–54. doi: 10.1016/j.tox.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsillach J, Hsieh EJ, Richter RJ, MacCoss MJ, Furlong CE. Proteomic analysis of adducted butyrylcholinesterase for biomonitoring organophosphorus exposures. Chem Biol Interact. 2013;203:85–90. doi: 10.1016/j.cbi.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan EC, Ho PC. Preparation and characterization of immunogens for antibody production against metanephrine and normetanephrine. J Immunol Methods. 2002;266:143–154. doi: 10.1016/s0022-1759(02)00143-6. [DOI] [PubMed] [Google Scholar]
- 39.Yamada H, Imoto T, Fujita K, Okazaki K, Motomura M. Selective modification of aspartic acid-101 in lysozyme by carbodiimide reaction. Biochemistry. 1981;20:4836–4842. doi: 10.1021/bi00520a005. [DOI] [PubMed] [Google Scholar]
- 40.Kurz T, Geffken D, Widyan K. Synthesis and reactivity of 3-aralkoxy-4-imino-imidazolidin-2-ones: a novel class of 4-iminohydantoins. Tetrahedron. 2004;60:2409–2416. [Google Scholar]
- 41.Kurz T, Widyan K. A convenient synthesis of 3-amino-4-imino(thioxo)-imidazolidin-2-ones. Tetrahedron Letters. 2004;45:7049–7051. [Google Scholar]
- 42.Varlet JM, Fabre G, Sauveur F, Collignon N, Savignac P. Préparation et conversion D' ω-formylalkylphosphonates en acides aminocarboxyalkylphosphoniques. Tetrahedron. 1981;37:1377–1384. [Google Scholar]
- 43.Singh KV, Kaur J, Varshney GC, Raje M, Suri CR. Synthesis and Characterization of Hapten−Protein Conjugates for Antibody Production against Small Molecules. Bioconjugate Chemistry. 2004;15:168–173. doi: 10.1021/bc034158v. [DOI] [PubMed] [Google Scholar]
- 44.Geyer H, Wuhrer M, Kurokawa T, Geyer R. Characterization of keyhole limpet hemocyanin (KLH) glycans sharing a carbohydrate epitope with Schistosoma mansoni glycoconjugates. Micron. 2004;35:105–106. doi: 10.1016/j.micron.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 45.Hermanson GT. Bioconjugate Techniques. 2nd Edition. Academic Press; San Diego: 2008. 2nd Ed. [Google Scholar]
- 46.Eklund A. On the determination of available lysine in casein and rapeseed protein concentrates using 2,4,6-trinitrobenzenesulphonic acid (TNBS) as a reagent for free epsilon amino group of lysine. Anal Biochem. 1976;70:434–439. doi: 10.1016/0003-2697(76)90467-x. [DOI] [PubMed] [Google Scholar]
- 47.Sashidhar RB, Capoor AK, Ramana D. Quantitation of epsilon-amino group using amino acids as reference standards by trinitrobenzene sulfonic acid. A simple spectrophotometric method for the estimation of hapten to carrier protein ratio. J Immunol Methods. 1994;167:121–127. doi: 10.1016/0022-1759(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 48.Jobbagy A, Jobbagy GM. Examination of FITC preparations. II. Measurements of isothiocyanate content of fluorescein isothiocyanate preparations. J Immunol Methods. 1973;2:169–181. doi: 10.1016/0022-1759(73)90014-8. [DOI] [PubMed] [Google Scholar]
- 49.Jobbagy A, Jobbagy GM. Examination of FITC preparations. I. Measurements of the dye content of fluorescein isothiocyanate preparations. J Immunol Methods. 1973;2:159–168. doi: 10.1016/0022-1759(73)90013-6. [DOI] [PubMed] [Google Scholar]