Abstract
Background & Aims
Chronic intestinal pseudo-obstruction (CIPO) is characterized by severe intestinal dysmotility that mimicks a mechanical sub-occlusion with no evidence of gut obstruction. We searched for genetic variants associated with CIPO to increase our understanding of its pathogenesis and indentify potential biomarkers.
Methods
We performed whole-exome sequencing of genomic DNA from patients with familial CIPO syndrome. Blood and lymphoblastoid cells were collected from patients and controls (individuals without CIPO); levels of mRNA and proteins were analyzed by quantitative reverse transcription PCR, immunoblot, and mobility shift assays. cDNAs were transfected into HEK293 cells. Expression of rad21 was suppressed in zebrafish embryos using a splice-blocking morpholino (rad21a MO). Gut tissues were collected and analyzed.
Results
We identified a homozygous mutation (p.622, encodes Ala>Thr) in RAD21 in patients from a consanguineous family with CIPO. Expression of RUNX1, a target of RAD21, was reduced in cells from patients with CIPO compared with controls. In zebrafish, suppression of rad21a reduced expression of runx1; this phenotype was corrected by injection of human RAD21 mRNA, but not with the mRNA from the mutated p.622 allele. rad21a MO zebrafish had delayed intestinal transit and greatly reduced numbers of enteric neurons, similar to patients with CIPO. This defect was greater in zebrafish with suppressed expression of ret and rad21, indicating their interaction in regulation of gut neurogenesis. The promoter region of APOB bound RAD21 but not RAD21 p.622 Ala>Thr; expression of wild-type RAD21 in HEK293 cells repressed expression of APOB, compared with control vector. The gut-specific isoform of APOB (APOB48) is overexpressed in sera from patients with CIPO who carry the RAD21 mutation. APOB48 is also overexpressed in sporadic CIPO in sera and gut biopsies.
Conclusions
Some patients with CIPO carry mutations in RAD21 that disrupt the ability of its product to regulate genes such as RUNX1 and APOB. Reduced expression of rad21 in zebrafish, and dysregulation of these target genes, disrupts intestinal transit and development of enteric neurons.
Keywords: sporadic and familial chronic intestinal pseudo-obstruction, intestinal motility, animal model, genetic analysis
Introduction
Chronic intestinal pseudo-obstruction (CIPO), a rare and potentially life-threatening disorder with unknown prevalence and incidence,1–3 is viewed typically as an insufficiency of the intestinal peristalsis that mimicks a sub-occlusive disease in the absence of mechanical obstructions.4–6 The severity of the clinical presentation and the limited understanding of the disorder contribute to poor quality of life and increased mortality.2,4–7 In addition, there are no specific biochemical or molecular biomarkers of CIPO, hindering further a correct diagnosis. From a genetic perspective, most CIPO patients are sporadic, although X-linked, autosomal dominant and recessive forms have been identified with mutations in filamin A (FLNA)8,9, actin G2 (ACTG2)10, thymidine phosphorylase (TYMP)11 / polymerase gamma (POLG1)12 and more recently in SGOL1.13 However, the underlying genetic alterations and molecular mechanisms remain unknown in most CIPO cases.
We previously mapped a locus in a large consanguineous family segregating an autosomal recessive form of CIPO.14,15In the affected family members, the major clinical feature was represented by CIPO, in addition to megaduodenum, long-segment Barrett esophagus, and cardiac abnormalities of variable severity (OMIM 611376; Mungan syndrome, MGS). Here, we intersected mapping data with WES to identify RAD21 as a causal locus for CIPO. Our combined genetic and functional data suggest a loss of function mechanism that disrupts the structure and function of enteric innervation. Moreover, based on our previous observations that identified Apolipoprotein B (APOB) as a target of RET signaling,16 we explored the role of this protein in CIPO etiopathology in the context of RAD21 mutations. Here we report a key role for APOB48, a gut-specific isoform17 as a transcriptional target of RAD21, and thus a contributor to CIPO, with a potential utility as a biomarker.
Methods
Patients and controls
The clinical characteristics of the patients with syndromic CIPO are indicated in the Supplementary Material. An additional 21 Italian and 12 Swedish sporadic patients with idiopathic CIPO were included in the study (eight males and 25 females; mean age: 38.6+/−16.6 years). In Table 1 the major clinical characteristics of these patients are described. 500 Turkish controls were recruited at the Universities of Ankara and Istanbul; 240 controls of European ancestry were recruited at the University of Bologna. All data from patients and controls, including the informed consents, were handled in accordance with local ethical committee’s approved protocols and in compliance with the Helsinki declaration.
Table 1.
Clinical characteristics of idiopathic CIPO patients included in the RAD21 mutation screening.
| Patient reference code |
Age | Sex | Feeding | Intestinal Manometry | Histopathology |
|---|---|---|---|---|---|
| SWE CIPO004 | 60 | F | Oral | Severe hypomotility | Inflammatory neuropathy; severe depletion of ICC |
| SWE CIPO010 | 44 | F | Oral+PPN | BUPA and absence of feeding activity |
Inflammatory neuropathy |
| SWE CIPO035 | 60 | F | Oral+PPN | Abnormally propagated AFs |
Inclusion neuropathy |
| SWE CIPO042 | 56 | F | TPN | --- | Inflammatory neuropathy |
| SWE CIPO094 | 61 | F | Oral | Abnormally configurated and propagated AFs; BUPA |
Inclusion neuropathy |
| SWE CIPO095 | 49 | F | Oral | BUPA | Inflammatory neuropathy |
| SWE CIPO096 | 56 | F | Oral | BUPA; SPUPA; abnormally configurated and propagated AFs |
Degenerative neuropathy; vacuolar myopathy |
| SWE CIPO097 | 41 | F | EN+PPN | BUPA; absence of feeding activity |
Inflammatory neuropathy |
| SWE CIPO099 | 50 | F | Oral+PPN | BUPA; SPUPA | Degenerative neuropathy |
| SWE CIPO149 | 37 | M | TPN | Bowel dilatation | Inflammatory neuropathy |
| SWE CIPO245 | 41 | F | Oral+PPN | Bowel dilatation | Degenerative myopathy; atrophic desmosis |
| SWE CIPO247 | 20 | F | TPN | Bowel dilatation | Degenerative myopathy |
| ITA CIPO08 | 17 | M | EN | Bowel dilatation | Degenerative myopathy |
| ITA CIPO27 | 39 | F | Modified oral | Abnormally configurated and propagated AFs; BUPA |
Intrinsic neuropathy |
| ITA CIPO15 | 38 | F | Modified oral | Abnormally propagated AFs |
Intrinsic neuropathy |
| ITA CIPO18 | 40 | F | Modified oral | --- | Extrinsic neuropathy |
| ITA CIPO22 | 26 | M | EN/oral | Abnormally configurated and propagated AFs; BUPA |
Intrinsic neuropathy |
| ITA CIPO10 | 56 | M | EN | --- | --- |
| ITA CIPO37 | 21 | F | EN/liquid diet/ integrators |
Abnormally configurated and propagated AFs |
Intrinsic neuropathy |
| ITA CIPO23 | 22 | M | EN/oral | Abnormally configurated and propagated AFs; BUPA |
Intrinsic neuropathy |
| ITA CIPO38 | 44 | F | Oral | Abnormally configurated and propagated AFs |
Severe depletion of ICC |
| ITA CIPO13 | 25 | F | Oral | BUPA; clustered contractions during fasting |
--- |
| ITA CIPO36 | 18 | F | Oral | --- | Degenerative myopathy |
| ITA CIPO29 | 1 | M | Oral | --- | Extrinsic neuropathy |
| ITA CIPO39 | 16 | F | EN/oral | --- | --- |
| ITA CIPO26 | 18 | F | Oral | Abnormally configurated and propagated AFs; numerous BUPA |
Intrinsic neuropathy |
| ITA CIPO25 | 40 | M | EN/oral | Abnormally configurated and propagated AFs; numerous BUPA |
Inflammatory neuropathy |
| ITA CIPO24 | 35 | F | EN/oral | Abnormally configurated and propagated AFs; numerous BUPA |
Degenerative myopathy |
| ITA CIPO16 | 67 | F | EN/oral | --- | --- |
| ITA CIPO14 | 40 | F | EN | Irregularly propagated clustered contractions |
Degenerative myopathy |
| ITA CIPO33 | 20 | M | EN/oral | Abnormally configurated and propagated AFs; |
--- |
| ITA CIPO17 | 54 | F | EN | Lack of AFs; postprandial clustered contractions; BUPA;, propagated clustered contractions |
Intrinsic neuropathy |
| ITA CIPO30 | 46 | F | Modified oral | --- | Extrinsic neuropathy |
Abbreviations: AF, activity front(s); BUPA, bursts of uncoordinated phasic activity; EN, enteral nutrition; ICC, interstitial cells of Cajal; PPN, partial parenteral nutrition; TPN, total parenteral nutrition; SPUPA, sustained periods of uncoordinated phasic activity.
High-Throughput SNP genotyping and Whole Exome Sequencing Analysis
A detailed description of the SNP genotyping and whole exome sequencing analyses is reported in Supplementary materials. Variant detection and genotyping were annotated with the SeattleSeq137 Annotation Server.
RAD21 mutation screening in idiopathic CIPO cases
Genomic DNA extracted from peripheral blood was amplified as reported in Supplementary materials.
RAD21 cDNA transfection into HEK293 cells
3×105 HEK293 cells were plated for transfection of the different plasmids using liposomes as described in Supplementary materials.
Gene expression analysis
Total RNA from 1.5 ml fresh blood was extracted with the QIAGEN Blood Total RNA kit (QIAGEN, Venlo, Limburg, Netherlands). Total RNA from lymphoblastoid or transfected cells was extracted with RNeasy kit (QIAGEN). Real-time quantitative RT-PCR was performed as reported in Supplementary materials.
Zebrafish functional assays
To determine the effect of rad21a suppression in zebrafish embryos, a splice blocking morpholino was designed as described in Supplementary materials. A published ret MO was used.18 To measure rad21a MO efficiency, total mRNA was extracted from control and MO injected embryos, reverse-transcribed and the site targeted by the MO was PCR amplified (Supplementary Figure 1). runx1 expression analysis and enteric nervous system characterization are reported in Supplementary materials.
Microgavage
Control and rad21 MO injected embryos were developed to 5dpf. Zebrafish larvae were anesthetized in Tricaine (Sigma), mounted in 3% methylcellulose and injected with fluorescent beads into the mouth as described.19
Electromobility shift assay (EMSA)
2×106 LCLs were processed for nuclear extract preparation as described in Supplementary materials.
Immunoprecipitation and western blotting
2×106 LCLs were used for immunoprecipitation assays. Crude sera of patients were diluted in PBS. Serial dilutions for cases and controls were performed (1:5, 1:10, 1:100, Supplementary Figure 2A). Immunoprecipitation and western blotting were performed as reported in Supplementary materials.
Immunohistochemistry
Immunohistochemistry was performed as reported in Supplementary materials. Incubations with the corresponding blocking peptides or with the secondary antibodies only were performed as negative controls (Supplementary Figure 2B, C).
Quantitative evaluation of ganglion cells
Quantitative evaluation of neuron number in myenteric and submucosal ganglia was performed according to20 (see Supplementary materials).
Statistical analysis
Case-control association study for SNP rs72105712 was performed using Haploview 4.0 (http://www.broadinstitute.org/scientific-community/science/programs/medical-and-population-genetics/haploview/). Statistical analysis of quantitative differences was performed using the Student’s t-test from the GraphPad package (http://graphpad.com/quickcalcs/). Fluorescent cell count was performed with ImageJ (http://rsbweb.nih.gov/ij); χ2 tests were calculated using the dedicated option from GraphPad.
Results
Identification of a novel RAD21 mutation in CIPO
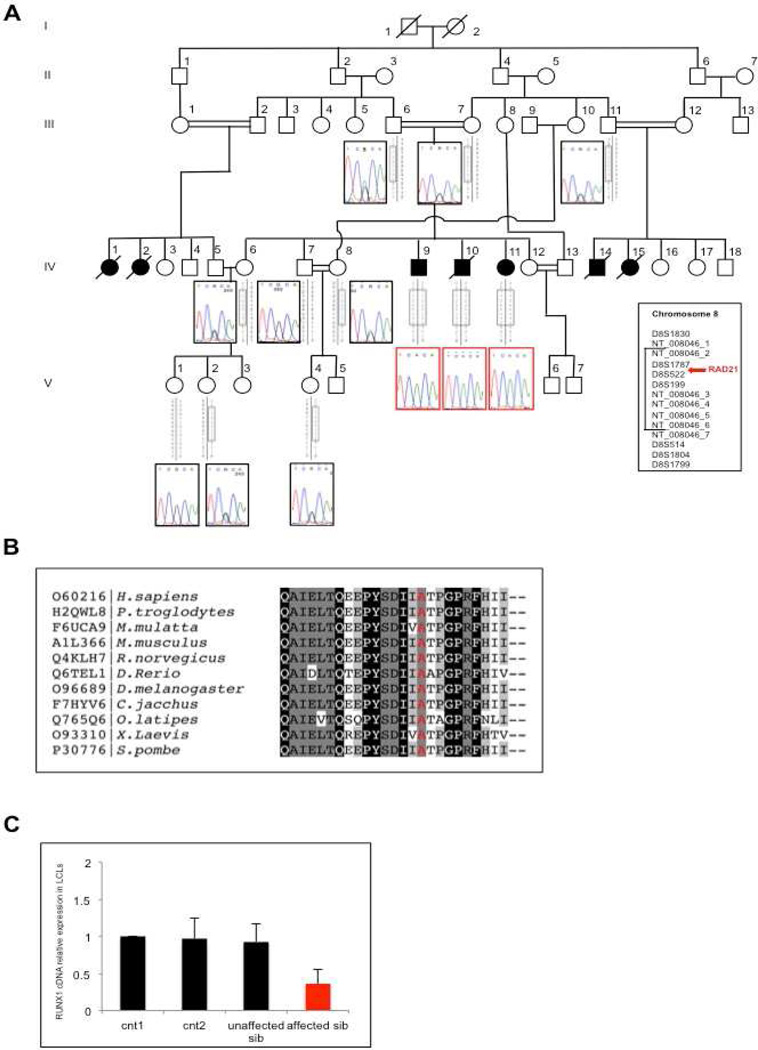
We performed a combined single nucleotide polymorphism (SNP)-genotyping/next generation-sequencing approach in a consanguineous CIPO pedigree of Turkish origin (Figure 1A), where we previously mapped a linkage locus with a multipoint LOD score=5.019. 14 High-throughput SNP-genotyping in the family and detection of Runs of Homozygosity (ROH) by PLINK (http://ngu.mgh.harvard.edu/~purcell/plink/) confirmed the locus,14 by identifying two regions of extended homozygosity: 91,878,147-113,307,176; 116,713,296-124,956,205 (Supplementary Table 1). We then performed WES on genomic DNA from two affected individuals (IV-9 and IV-11, Figure 1A). We filtered data for variants that were a) homozygous in our patients; b) rare (MAF <1%) in public databases (EVS, dbSNP); and c) predicted computationally to be pathogenic. We found a single variant fulfilling all these conditions inside our linkage interval on chromosome 8 (Figure 1; Supplementary Figure 3A). This homozygous allele affects the coding change c. 1864 G>A in RAD21 (NM_006265.2), and is predicted to generate a missense substitution p.622 Ala>Thr (Figure 1B). Mutation Taster (http://www.mutationtaster.org/) and PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/)21 analysis predicted this change to be "disease causing" and "damaging", respectively (Mutation Taster p= 0.9999; PolyPhen-2 HumDiv score: 0.999, sensitivity: 0.14; specificity: 0.99; HumVar score: 0.993; sensitivity: 0.47; specificity: 0.96), and the variant was absent from public databases (dbSN: www.ncbi.nlm.nih.gov/SNP/, 1000 Genomes: www.1000genomes.org/, ESP: http://evs.gs.washington.edu/EVS/) and from 1000 control chromosomes of Turkish origin analyzed by our group. To test the candidacy of this allele, we performed segregation analysis on all available family members: all three affected sibs carried the change in a homozygous state and all carriers of the risk haplotype (including the parents) were heterozygous (Figure 1A).
Figure 1. Identification of a novel homozygous mutation in RAD21.
(A) Pedigree of the Turkish consanguineous family showing the segregation of the RAD21 mutation in the available members. For the homozygous patients, electropherograms are boxed in red. Grey boxes represent the haplotypes derived from microsatellite analysis. (B) RAD21 conservation across species surrounding the position p.622Ala (highlighted in red). (C) RUNX1 expression in controls and patient's LCLs (IV-9).
To evaluate whether RAD21 mutations were also prevalent in other idiopathic CIPO cases, we screened 21 Italian and 12 Swedish individuals with pseudo-obstruction, defined by clinical, manometric and radiological examination (Table 1). We did not identify any mutation in RAD21 coding region, although a 1bp-indel was detected with high frequency in the upstream region (m.a.f= 0.364; g.11788122-11788123 ins(C)); rs72105712 (dbSNP137)). Since no allele frequencies were known for this SNP, we investigated the frequency in a control group of European ancestry (N= 240); we found no differences between cases and controls (m.a.f.= 0.36 in cases; 0.34 in controls; χ2= 0.08, p-value= 0.77).
Mutant RAD21 p.622 Ala>Thr alters RUNX1 expression
To test the candidacy of RAD21 further, we evaluated its expression in blood and lymphoblastoid cell lines (LCLs) obtained from one homozygous patient and several controls, including one unaffected wild-type brother. Real-time quantitative RT-PCR (qRT-PCR) showed that RAD21 expression in the affected individual was comparable to that in controls, either in blood (Supplementary Figure 3B) or in LCL cDNA (Supplementary Figure 3C). The mutant protein was also expressed in LCLs in amounts comparable to wild-type cells (Supplementary Figure 3D; upper panel). Likewise, testing the interaction of RAD21 with SMC1, one of its known partners,22 co-immunoprecipitation experiments showed that the mutant protein still retains SMC1-binding activity (Supplementary Figure 3D, middle panel).
Since one of the main target genes activated by RAD21 is RUNX123,24 we investigated whether mutated RAD21 could hamper its transcription activity: indeed, the affected individual’s LCLs showed significantly reduced RUNX1 expression compared to controls (Figure 1C). Similarly, transfection of mutant RAD21 cDNA in HEK293 cells and subsequent RT-qPCR revealed a significant decrease in RUNX1 expression (p=0.0028, Student’s t-test) compared to cells transfected with wild-type RAD21 cDNA (Supplementary Figure 3E).
Rad21a suppression causes downregulation of runx1 expression and loss of enteric neurons in vivo
To test the hypothesis that RAD21 is necessary for enteric development and to assay the pathogenic potential of the newly discovered allele in a physiologically relevant in vivo system, we investigated RAD21 in zebrafish development.
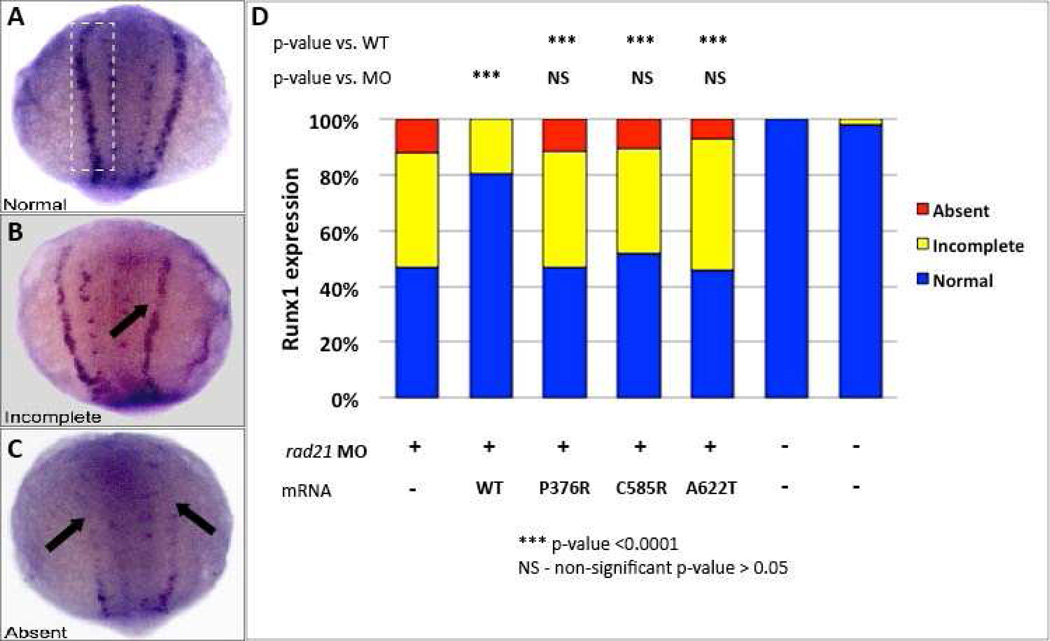
Given our in vitro observations on the role of RAD21 on RUNX1 expression and its downregulation in patient's cells, we asked whether RAD21 can replicate this defect in vivo and whether the patient’s mutation has the expected effect. We identified (by reciprocal BLAST) the sole ortholog of RAD21 and we injected zebrafish embryos with a rad21a splice blocking morpholino (MO) (at 1–2 cell stage, Supplementary Figure 2A,B). Embryos were fixed at 14hpf (hours post fertilization) and stained with a runx1 probe. During zebrafish development, runx1 is expressed in the posterior lateral plate mesoderm (PLM). We observed defects in runx1 expression patterns recapitulating prior studies,23,24 with runx1 expression either partially or completely absent in rad21a morphants (Figures 2A–C). Runx1 expression was rescued by co-injection of MO with human WT RAD21 mRNA. In contrast, co-injection of MO and mRNA encoding the p.622 Thr allele was not able to rescue runx1 expression, indicating that the RAD21 mutation has a loss-of-function effect in vivo (Figure 2D).
Figure 2. (A–D) RAD21 A622T affects runx1 expression in zebrafish embryos.
runx1 expression in the PLM in rad21a morpholino injected embryos rescued with wild type or mutants RAD21. Morpholino injected embryos were scored according to runx1 expression in the PLM as normal (A), partial (B), or absent (C). rad21a morphants could be rescued by wild type human RAD21 mRNA, while mutations previously reported in patients with “cohesinopathy” (P376A, C585R) and A622T can not rescue this phenotype.
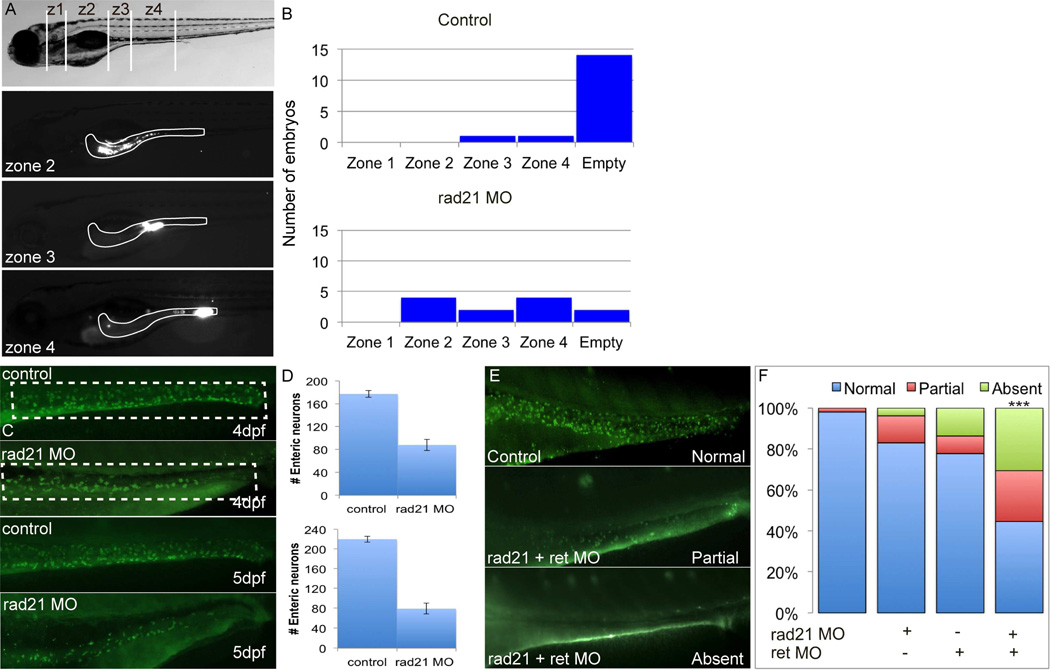
To test whether we could recapitulate the CIPO phenotype seen in RAD21 mutant patients, including a severe impairment of gut motility and marked hypoganglionosis,14 we characterized the zebrafish gut. Subsequent to MO injection, embryos were allowed to develop to 5dpf, at which time the digestive system has developed.25 Notably, we saw no appreciable runx1 expression in the gut at this stage of development (data not shown), suggesting an earlier onset of the enteric phenotype. Control and MO embryos were fed fluorescent beads through microgavage, a technique that allows to determine the rate of intestinal motility as a function of time.19 After eight hours post bead injection (hpi), embryos were divided into 1–4 zones based on anatomical landmarks (Figure 3A) and the presence of fluorescence in each segment was scored. Consistent with the CIPO phenotype, rad21a morphants showed delayed food transit along the gut (Figure 3B). Moreover, staining of enteric neurons along the gut with antibodies against HuC/D showed a significant depletion of enteric neurons (Figure 3C). Quantitative analysis of the zebrafish gut (Figure 3D) at 4 (upper panel) and 5 (lower panel) dpf stages revealed that rad21 morphants had a marked reduction of HuC/D-immunolabeled enteric neurons compared to controls, suggesting a neurogenic cause of the observed motility defects.
Figure 3. Gut dysmotility is caused by enteric neuronal loss in zebrafish embryos.
To assess gut motility in zebrafish larvae, we injected fluorescent beads into the mouth and recorded the rate of gut motility versus time. (A) Lateral view of 5dpf zebrafish larvae. Representative images of injected larvae show (after eight hours post injection, hpi) fluorescent beads in different gut compartments (zone 1–4). (B) In control embryos, most of the fluorescent beads have exited the gut by 8hpi, while rad21 MO injected embryos have reduced gut motility. (C-D) Compared to control larvae, rad21 morphants have a significant reduction of enteric HuC/D immunoreactive neurons at 4dpf (D; upper panel) and 5dpf (D; lower panel). (E) rad21 and ret interaction during the ENS development. Combination of suboptimal doses of rad21 and ret MOs causes a significant decrease in HuC/D enteric neurons, while embryos injected with suboptimal doses of rad21 MO or ret MO causes loss of HuC/D enteric neurons, not statistically significant (F).
Notably, previous reports on rad21a morphants and rad21nz171 mutants have shown significantly reduced expression of ascl1a,26 a neuronal marker. Ascl1 is also a transcription factor required for the development of serotonergic neurons.26 An ASCL1 mutation has been reported previously in Haddad syndrome, a condition encompassing congenital central hypoventilation syndrome and Hirschsprung disease (MIM 209880).
These data, the similarity of rad21a morphants to the hypoganglionic phenotype observed in a mouse model for the ret mutation C620R27,28raised the possibility that RAD21 and RET might act synergistically during gut neurogenesis. We therefore performed epistasis analysis by co-injecting rad21a and ret MOs at subeffective doses, at which each MO alone was indistinguishable from control embryos. We observed strong epistasis on the innervation of the gut; co-injection of the two genes phenocopied the phenotype of high dose rad21 MOs (Figures 3E, F). At the same time, overexpression of RET did not rescue rad21 morphants, or vice versa, suggesting that the two genes act on the same process but not directly in the same pathway. This observation is consistent with previous studies that showed RET to be induced by NGF in a runx1-independent manner.29
The mutant RAD21 p.622 Ala>Thr does not bind to RAD21-binding elements in Apolipoprotein B promoter
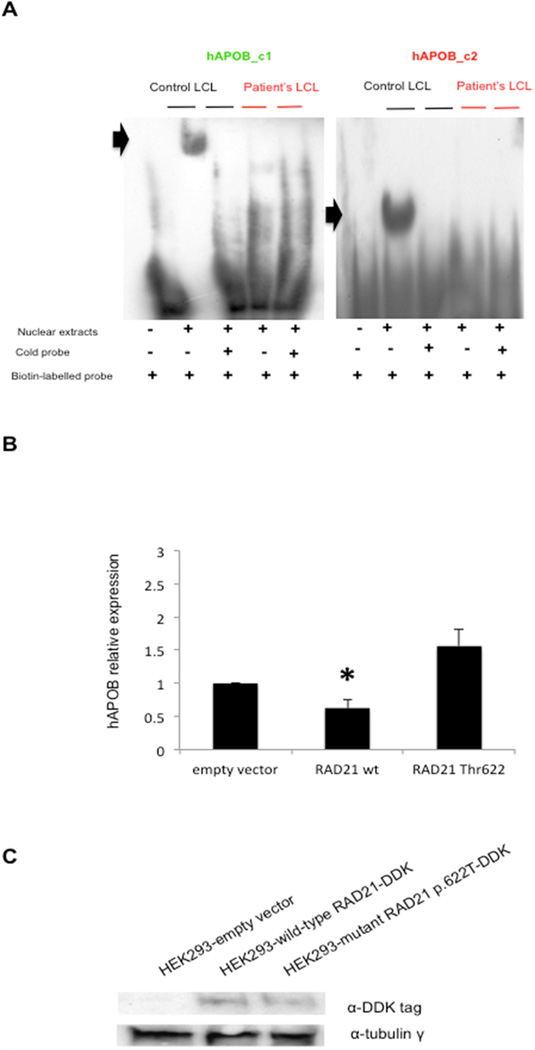
Recent data have shown that RAD21/CTCF binding sites are present in the apolipoprotein A1/C3/A4/A5 gene cluster on chromosome 11 and that altered binding of these factors to these sites dysregulates apolipoprotein expression.30 We therefore performed electromobility shift assay (EMSA) experiments to test whether the mutant RAD21 protein could retain its binding affinity for the above-mentioned sites, in particular for the AC2 site. 30 We observed no differences between the nuclear extracts from wild-type and mutant LCLs in binding to this site (Supplementary Figure 3F). Next, we investigated if the nuclear extracts from wild-type and mutant LCLs could show differences in binding to human APOB promoter 31. In silico analysis with MatInspector identified two binding sites for RAD21/CTCF (hAPOB_c1, chr2:21,267,137-21,267,173, matrix score: 0.862; hAPOB_c2, chr2:21,266,910-21,266,945, matrix score: 0.807) which maps to the two regulatory regions of the proximal APOB promoter31. EMSA assays performed with probes corresponding to the two sites showed a specific shift only in the presence of wild-type nuclear extracts (Figure 4A). Moreover, specific supershifts with an anti-RAD21 antibody were detectable only in wild-type, but not in mutant, nuclear extracts (Supplementary Figure 4A). Transfection of the plasmids carrying either wild-type or mutant RAD21 cDNAs in frame with the DDK-epitope, in HEK293 cells, and subsequent RT-qPCR analysis of APOB expression revealed that wild-type RAD21 overexpression reduced APOB levels compared to the empty vector (p=0.0098; Student’s t-test). In contrast, overexpression of the mutant protein had no effect, similarly to the empty-vector transfections (Figures 4B,C). These data suggest that RAD21 might act as a repressor of APOB.
Figure 4. RAD21 regulates APOB overexpression.
(A) EMSA on human APOB promoter containing two putative binding sites for RAD21: hAPOB_c1 (green), hAPOB_c2 (red). EMSA analysis for hAPOB_c1 and _c2 regions biotin-labelled probes: only the nuclear extracts from wild-type (control) LCL) show a specific gel-shift (black arrows). (B): RT-qPCR for APOB expression in HEK293 cells transfected either with empty, RAD21 wild-type, RAD21 mutant p.622 Thr vectors. Data represent the mean values of three independent transfection experiments. Bars represent the standard deviation; black asterisk indicates the significant p-value (see in text). (C) Western blotting analysis showing the expression of recombinant wild-type and mutant RAD21 in frame with DDK tag in transfected HEK293.
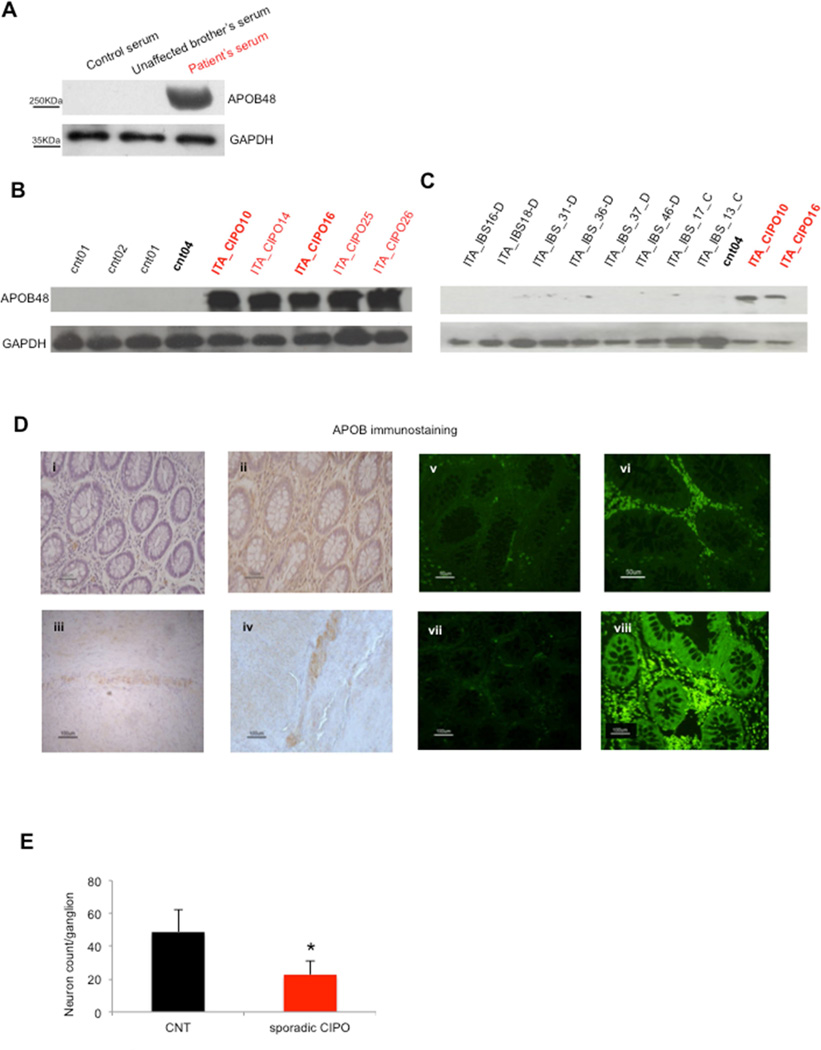
Apolipoprotein B (APOB) is overexpressed in CIPO patients
APOB transcript generates two different proteins, APOB48 and APOB100 isoforms, both present in serum.17 Since our data suggested a RAD21-regulated expression of APOB, we analyzed sera from the CIPO patient homozygous for RAD21 mutation (IV-9), and from wild-type controls. We detected an elevated expression of APOB48 in the patient, whereas no significant differences could be appreciated for APOB100 (Figure 5A and Supplementary Figure 5A). To understand whether APOB overexpression was unique to this patient or whether it might represent a more generalized phenomenon, we evaluated sera from RAD21 mutation-negative idiopathic CIPO patients and from control subjects. APOB48 showed an increase in CIPO patients (Figure 5B and Supplementary Figure 5B). Moreover, compared to CIPO, sera derived from 12 patients with functional bowel disease, namely seven diarrhea-predominant IBS (IBS-D), four constipation predominant IBS (IBS-C) and one with alternating bowel IBS (IBS-C/D), did not show APOB48 overexpression (Figure 5C and Supplementary Figure 5C). Furthermore, western blot analysis on the sera of patients with other gastrointestinal disorders, i.e. anorexia nervosa and mechanical intestinal obstruction, did not identify any APOB48 increase (F. Bianco et al., data not shown).
Figure 5. APOB overexpression is specific for CIPO patients.
(A)Western blotting analysis showing APOB48 expression in the patient carrying the homozygous RAD21 mutation (third lane), compared to control sera (upper panel); GAPDH was used as internal loading control (lower panel). (B) Western blotting analysis showing APOB overexpression in the sera of CIPO patients compared to controls (upper panel). (C) Western blotting analysis for APOB48 in IBS samples vs controls (in black) and CIPO patients (in red). Samples loaded in duplicates/triplicates are marked in bold (as index of reproducible blotting). IBS-C: constipation predominant irritable bowel syndrome; IBS-D: diarrhea predominant irritable bowel syndrome.
(D) Immunohistochemistry and immunofluorescence staining for APOB in controls (D-i, iii, v), IBS cases (D-vii) and CIPO patients (D-ii, iv, vi). Representative figures (D-i, ii) illustrate immunostaining of immunocyte-like cells distributed throughout the mucosa, including the lamina propria and the myenteric plexus (D-iii, iv). Note that the density of APOB immunoreactive immunocyte-like cells in the lamina propria was higher in CIPO patients (D-vi) vs. controls or IBS cases.
(E) Histogram showing the significant decrease in the number of neurons per ganglion in the myenteric plexus observed in CIPO, compared to control tissue biopsies.
Immunohistochemical analysis of gut biopsies (mainly ileum) of sporadic CIPO patients revealed APOB48 expression in myenteric neurons and in cells of the lamina propria, reminiscent of immunocytes (Figure 5). Consistent with the results obtained in the sera, the APOB48 signal was increased markedly in the biopsies of CIPO cases compared to controls and IBS cases (Figures 5D i-iv-vii). The quantitative analysis of immunolabeled cells in the lamina propria of eight CIPO patients (five Italian and three Swedish) indicated a significant increase in the number of APOB48-positive cells compared to the other individuals (controls n=3;IBS patients n=3) (32.9±9.2% vs 7.2±2.5% cases vs controls; p=0.0012, Student’ t test; 32.9±9.2% vs 5.6±1.5% CIPO cases vs IBS cases; p=0.0008; CIPO cases (8) vs all (6), p=0.0001; Figures 5Dv-vi-vii). In addition, quantitative analysis performed in the gut biopsies of sporadic CIPO patients with a marked increased in APOB48 revealed a significant reduction in the number of neuron specific enolase (NSE)-labeled myenteric ganglion cell bodies / gaglion compared to control specimens (CIPO cases 22.71± 8.10 vs controls 48.65±13.80; p-value=0.0039, Student’s t-test; Figure 5E). No significant differences were observed for neurons in the submucosal plexus between CIPO cases and controls (CIPO cases 5.61 ± 0.39 vs controls 8.36 ±2.96; p-value=0.1079, Student’s t-test). We observed RAD21 staining in multiple tissues and throughout different components of the gut, as shown previously (www.proteinatlas.org), although no differences were appreciated between CIPO and controls (Supplementary Figure 6A i-iv). Expression of APOBEC1, the gut-specific RNA editing enzyme responsible of the formation of APOB48 isoform, was also investigated; we observed similar immunostaining in control and CIPO tissue biopsies (Supplementary Figure 6B i-ii).
Discussion
This study provides in vitro and in vivo evidence that a novel homozygous mutation in RAD21 is associated with a syndromic form of CIPO. RAD21 is part of the cohesin complex, forms a physical link between SMC1/SMC3 and STAG subunits, and controls sister chromatid pairing and unpairing during cell replication. 32 RAD21 is also a transcriptional regulator that binds to many sites in the genome. 33 In concordance with the key role(s) of RAD21 in regulating cell division, altered expression and somatic loss-of-function mutations have been reported in different cancers.34,35 Furthermore, heterozygous germline mutations in cohesin subunits, i.e. RAD21, SMC1, STAG, or in regulators of cohesin, e.g. NIPBL, cause a broad spectrum of disorders referred to as cohesinopathies (namely Cornelia de Lange syndrome, CdLS, OMIM 122470), characterized by facial dysmorphisms, growth retardation, developmental delay and/or intellectual disability, and multiorgan involvement, including musculoskeletal malformations ranging from brachyclinodactyly to severe reduction defects.36–39
We observed a similar loss-of-function phenotype for two missense CdL mutations (p.376Pro>Arg, p.585Cys>Arg) and for our CIPO variant in RAD21 (p.622Ala>Thr). However, in the consanguineous family studied herein the heterozygous carriers of the RAD21 mutation did not show sign of CdLS.14 Previously described patients with CdLS and different RAD21 heterozygous mutations did not show gastrointestinal abnormalities such as CIPO.32,40 It is worth noting that the mutations observed in CdLS and in our CIPO patients map to different RAD21 domains, potentially suggesting that specific mutational mechanisms in RAD21 can lead to different clinical entities in humans. Consistent with this notion, the CIPO-causing mutations do not appear to abolish the ability of RAD21 to bind to SMC1, whereas it does attenuate its transcriptional repressive role of other targets, such as APOB. Recently, however, a mutation in SGOL1, another cohesin protein, has been associated to severe gut and cardiac dysrhythmia in the absence of other congenital abnormalities or cohesinopathies.13
RAD21 plays an important role in the development, survival and maintenance of epithelial cells and neurons of the gastrointestinal tract and mice heterozygous for a Rad21 null mutation exhibit gastrointestinal defects following X-ray irradiation.41 Our zebrafish studies suggest that rad21 is essential for enteric neuron development: rad21a morphants recapitulate the CIPO phenotype, as they show a delayed transit along the gut and a significant depletion of enteric neurons. This latter finding is reminiscent of an enteric neuronal hypoganglionic phenotype observed in heterozygous retC620R/+ mice16 and shares similarities with the histopathology of some CIPO patients.14,27,28 The similarity of the rad21 suppression phenotype in zebrafish to the neuronal defects of heterozygous retC620R/+ mice prompted us to test whether those two genes might interact genetically. We showed that RET and RAD21 interact epistatically during differentiation or maintenance of enteric neurons. However, the failure of the two genes to rescue the gut innervation phenotype suggests that they activate different molecular pathways.
Our previous studies identified Apolipoprotein B (Apob) as a target of RET signaling in Neuro2a cells, a murine model of enteric nervous system development. Moreover, in ret+/C620R mice that have an enteric neuronal hypoganglionic phenotype16 reminiscent of the histopathology observed in some CIPO patients,27,29 Apob was markedly overexpressed. APOB is a major constituent of the plasma lipoprotein, and in mammals is synthesized in two different tissues, i.e. liver and intestine. Two different proteins derive from the APOB transcript by a RNA-editing enzyme, APOBEC1: the full-lenght APOB100, a large protein of 512 kDa synthesized by the liver (which does not express APOBEC1), essential for triglyceride-rich VLDL formation; and the gut-specific isoform APOB48, formed via the RNA-editing process and co-linear with the N-terminal half of APOB100. APOB48 has a key role in chylomicron assembly and transport in the intestine.17
APOB expression is regulated by a strong promoter in the proximal upstream region, containing several positive regulatory elements, including one within the noncoding exon 1, but also a negative regulatory element between bases −261 and −129.31 In this study we identified two RAD21-binding sites in APOB proximal promoter, i.e. one overlapping the negative element, and the other partially overlapping the exon 1 positive regulatory element. Only nuclear extracts from wild-type cells form a specific complex with either region, whereas no complex is observed in the presence of mutant RAD21. This suggests that RAD21 may act as a repressor of APOB transcription, associating to its negative regulatory element and competing with the transcriptional activators that bind to positive regulatory element.
Finally, we found that, compared to controls, gut-specific APOB4817 levels were increased in the serum of the RAD21-mutated CIPO patient. Interestingly, sera from the sporadic CIPO patients, negative for RAD21 mutations, also showed consistently elevated APOB48 levels as compared to either IBS patients or healthy controls. We observed a variable expression of APOB100 in both controls and CIPO patients, in line with the fact that its regulation depends on different factors, including cholesterol and insulin levels.42 Notably, CIPO patients did not show evidence of altered lipid metabolism, i.e. total cholesterol and HDL levels were within the normal range (Supplementary Table 2).
APOB48 overexpression was further corroborated by the data on gut biopsies of CIPO patients: compared to controls, APOB48 immunoreactivity was significantly increased in cells (with morphological features of immunocytes) distributed throughout the lamina propria and in myenteric neurons of CIPO patients. Interestingly, a recent study identified a specific neuron-macrophage crosstalk in regulating gut motility.43 These data bear implications to the pathogenesis of functional bowel disorders, such as IBS. However, our study did not show any change in APOB expression in patients with IBS, suggesting that other molecular pathways can be also involved in patients with a more prominent gut dysfunction such as those with CIPO.
Furthermore, we found a significant decrease in the mean number of myenteric neurons /ganglion in CIPO tissues exhibiting a high APOB immunoreactivity. This finding is reminiscent of severe hypoganglionosis14 in the patients who were found to carry the RAD21 p.622Ala>Thr homozygous mutation in this study.
Based on our data, APOB48 expression (at serum and tissue level) was homogeneously increased in sporadic and familial CIPO and therefore making a possible correlation between this marker and the degree of neuronal loss / symptom severity undetectable. Further studies are eagerly awaited to clarify whether the correlation between APOB expression levels and neuronal / clinical CIPO phenotype exists.
It is currently unclear the pathophysiological significance of the generalized APOB overexpression observed in sporadic CIPO, where no mutation of RAD21 were identified. Our previous studies suggested a role for APOB in the molecular pathways required for enteric neuron development and survival.16 Therefore, APOB48 increase may be activated as a compensatory effect to an abnormal / defective enteric nervous system occurring in CIPO. We still do not know the precise temporal origin of the defect that leads to CIPO in patients with RAD21 mutations. The observed transcriptional downregulation of runx1, which is likely relevant in early neuronal progenitors, possibly in the crest, argues for a migratory defect. At the same time, the observed loss of APOB suppression might be more relevant to neuronal progenitors in the gut itself (Figure 6). Further studies of patients and conditional mouse mutants will be required to understand the potential relative contribution of different sites to CIPO pathology. Likewise, further research will be required to elucidate the factors contributing to the specific APOB48 overexpression and its clinical value in the management of CIPO patients. Nonetheless, our data inform the molecular aspects underlying the pathogenesis of CIPO and lead to the identification of a candidate biomarker, namely APOB48 overexpression, for this severe, disabling gut dysmotility disorder.
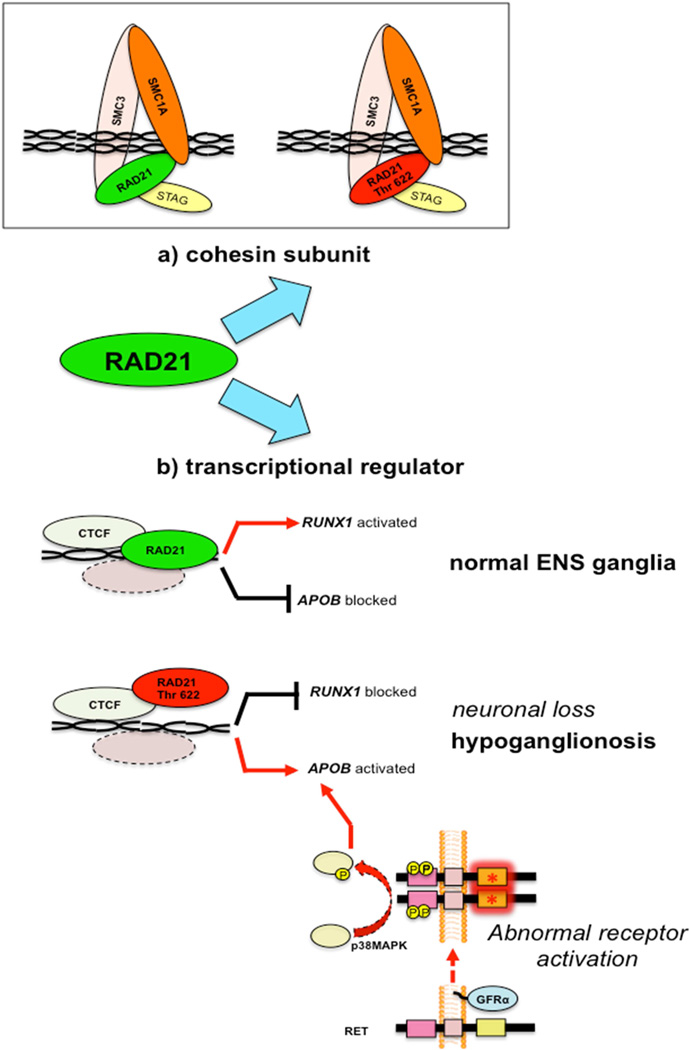
Figure 6. Model of RAD21 functioning as cohesin complex (A) or as transcription factor (B).
(A) RAD21 belongs to the cohesin complex regulating chromosomal replication. RAD21 p.622Thr does not alter SMC1A subunit-binding. (B) Wild-type RAD21 promotes RUNX1 and represses APOB expression. RAD21 p.622Thr downregulates RUNX1 expression, de-represses APOB expression and leads to ENS neuron loss with resultant hypoganglionosis. Alternative pathways, including RET abnormal activation (e.g. the p.620 Cys>Arg mutation [red asterisk] in heterozygous mice) can lead to APOB overexpression, which is phenotypically associated with hypoganglionosis.
Supplementary Material
Acknowledgements
We thank Dr. M. Vargiolu for helpful suggestions and critical reading. N. K. is a Distinguished Brumley Professor.
Grant support: This work was supported by FP7-EU grant n° 223692 “CHERISH” to G. R., by NIH grant n° 1U54HG006493 to M. B. and P50DK096415 to N.K., by Ricerca Finalizzata RER2009 (Ita-MNGIE), Ministry of Health, and the Italian Ministry of University and Research (PRIN/COFIN 2009MFSXNZ_002) to R. De G. R. De G. is also the recipient of grants from ‘Fondazione del Monte di Bologna e Ravenna’, Bologna, Italy.
Abbreviations used in this paper
- CIPO
Chronic intestinal pseudo-obstruction
- APOB48
Apolipoprotein B48
- WES
Whole exome sequencing
- EMSA
electromobility shift assays
- FLNA
filamin A
- ACTG
actin G2
- TYMP
thymidine phosphorylase
- POLG1
polymerase gamma
- MGS
Mungan syndrome
- IBS
Irritable Bowel Disease
- ROH
Runs Of Homozygosity
- BWA
Burrows-Wheeler Aligner; v0.6.2
- GATK
Genome Analysis Toolkit
- dpf
days post fertilization
- SNP
single nucleotide polymorphism
- LCLs
lymphoblastoid cell lines
- qRT-PCR
Real-time quantitative RT-PCR
- MO
morpholino
- hpf
hours post fertilization
- hpi
post bead injection
- nNOS
nitric oxide synthase
- IBS-D
diarrhea-predominant IBS
- IBS-C
constipation predominant IBS
- CdLS
Cornelia de Lange syndrome
- AF
activity front(s)
- BUPA
bursts of uncoordinated phasic activity
- EN
enteral nutrition
- ICC
interstitial cells of Cajal
- PPN
partial parenteral nutrition
- TPN
total parenteral nutrition
- SPUPA
sustained periods of uncoordinated phasic activity
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflict of interest
Author contributions: Study concept and design: EB, RDG, NK; acquisition of data: FB, LC, DD, LF, AG, RC, GL, CG; analysis and interpretation of data: EB, MDA, TP, MB, RDG; drafting of the manuscript: EB, RDG, NK, GR; critical revision of the manuscript for important intellectual content: MDA, NK, EB, RDG, GR, GL, MS, VS, MB; statistical analysis: EB, FB, DD, LF; obtained funding: RDG, NK, MB, GR; material support: GL, ZM, KC, TO, AG, SP, SO, UV, GC, GB; study supervision: RDG, EB, GR, NK.
References
- 1.Amiot A, Joly F, Alves A, et al. Long-term outcome of chronic intestinal pseudo-obstruction adult patients requiring home parenteral nutrition. Am J Gastroenterol. 2009;104:1262–1270. doi: 10.1038/ajg.2009.58. [DOI] [PubMed] [Google Scholar]
- 2.Lindberg G, Iwarzon M, Tornblom H. Clinical features and long-term survival in chronic intestinal pseudo-obstruction and enteric dysmotility. Scand J Gastroenterol. 2009;44:692–699. doi: 10.1080/00365520902839642. [DOI] [PubMed] [Google Scholar]
- 3.Stanghellini V, Cogliandro R, De Giorgio R, et al. Natural history of chronic idiopathic intestinal pseudo-obstruction in adults: a single center study. Clin Gastroenterol Hepatol. 2005;3:449–458. doi: 10.1016/s1542-3565(04)00675-5. [DOI] [PubMed] [Google Scholar]
- 4.De Giorgio R, Sarnelli G, Corinaldesi R, et al. Advances in our understanding of the pathology of chronic intestinal pseudo-obstruction. Gut. 2004;53:1549–1552. doi: 10.1136/gut.2004.043968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Giorgio R, Seri M, van Eys G. Deciphering chronic intestinal pseudo-obstruction: do mice help to solve the riddle? Gastroenterology. 2007;133:2052–2055. doi: 10.1053/j.gastro.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 6.Stanghellini V, Cogliandro RF, De Giorgio R, et al. Chronic intestinal pseudo-obstruction: manifestations, natural history and management. Neurogastroenterol Motil. 2007;19:440–452. doi: 10.1111/j.1365-2982.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 7.Mann SD, Debinski HS, Kamm MA. Clinical characteristics of chronic idiopathic intestinal pseudo-obstruction in adults. Gut. 1997;41:675–681. doi: 10.1136/gut.41.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton-Smith J, Walters S, Hobson E, et al. Xq28 duplication presenting with intestinal and bladder dysfunction and a distinctive facial appearance. Eur J Hum Genet. 2009;17:434–443. doi: 10.1038/ejhg.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gargiulo A, Auricchio R, Barone MV, et al. Filamin A is mutated in X-linked chronic idiopathic intestinal pseudo-obstruction with central nervous system involvement. Am J Hum Genet. 2007;80:751–758. doi: 10.1086/513321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehtonen HJ, Sipponen T, Tojkander S, et al. Segregation of a missense variant in enteric smooth muscle actin gamma-2 with autosomal dominant familial visceral myopathy. Gastroenterology. 2012;143:1482–1491. doi: 10.1053/j.gastro.2012.08.045. e3. [DOI] [PubMed] [Google Scholar]
- 11.Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science. 1999;283:689–692. doi: 10.1126/science.283.5402.689. [DOI] [PubMed] [Google Scholar]
- 12.Giordano C, Powell H, Leopizzi M, et al. Fatal congenital myopathy and gastrointestinal pseudo-obstruction due to POLG1 mutations. Neurology. 2009;72:1103–1105. doi: 10.1212/01.wnl.0000345002.47396.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chetaille P, Preuss C, Burkhard S, et al. Mutations in SGOL1 cause a novel cohesinopathy affecting heart and gut rhythm. Nat Genet. 2014;46:1245–1249. doi: 10.1038/ng.3113. [DOI] [PubMed] [Google Scholar]
- 14.Deglincerti A, De Giorgio R, Cefle K, et al. A novel locus for syndromic chronic idiopathic intestinal pseudo-obstruction maps to chromosome 8q23-q24. Eur J Hum Genet. 2007;15:889–897. doi: 10.1038/sj.ejhg.5201844. [DOI] [PubMed] [Google Scholar]
- 15.Mungan Z, Akyuz F, Bugra Z, et al. Familial visceral myopathy with pseudo-obstruction, megaduodenum, Barrett's esophagus, and cardiac abnormalities. Am J Gastroenterol. 2003;98:2556–2560. doi: 10.1111/j.1572-0241.2003.08707.x. [DOI] [PubMed] [Google Scholar]
- 16.Evangelisti C, Bianco F, Pradella LM, et al. Apolipoprotein B is a new target of the GDNF/RET and ET-3/EDNRB signalling pathways. Neurogastroenterol Motil. 2012;24:e497–e508. doi: 10.1111/j.1365-2982.2012.01998.x. [DOI] [PubMed] [Google Scholar]
- 17.Black DD. Development and physiological regulation of intestinal lipid absorption. I. Development of intestinal lipid absorption: cellular events in chylomicron assembly and secretion. Am J Physiol Gastrointest Liver Physiol. 2007;293:G519–G524. doi: 10.1152/ajpgi.00189.2007. [DOI] [PubMed] [Google Scholar]
- 18.Heanue TA, Pachnis V. Ret isoform function and marker gene expression in the enteric nervous system is conserved across diverse vertebrate species. Mech Dev. 2008;125:687–699. doi: 10.1016/j.mod.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Cocchiaro JL, Rawls JF. Microgavage of zebrafish larvae. J Vis Exp. 2013;72:e4434. doi: 10.3791/4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganns D, Schrodl F, Neuhuber W, et al. Investigation of general and cytoskeletal markers to estimate numbers and proportions of neurons in the human intestine. Histol Histopathol. 2006;21:41–51. doi: 10.14670/HH-21.41. [DOI] [PubMed] [Google Scholar]
- 21.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panigrahi AK, Zhang N, Otta SK, et al. A cohesin-RAD21 interactome. Biochem J. 2012;442:661–670. doi: 10.1042/BJ20111745. [DOI] [PubMed] [Google Scholar]
- 23.Horsfield JA, Anagnostou SH, Hu JK, et al. Cohesin-dependent regulation of Runx genes. Development. 2007;134:2639–2649. doi: 10.1242/dev.002485. [DOI] [PubMed] [Google Scholar]
- 24.Marsman J, O'Neill AC, Kao BR, et al. Cohesin and CTCF differentially regulate spatiotemporal runx1 expression during zebrafish development. Biochim Biophys Acta. 2014;1839:50–61. doi: 10.1016/j.bbagrm.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Ng AN, de Jong-Curtain TA, Mawdsley DJ, et al. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev Biol. 2005;286:114–135. doi: 10.1016/j.ydbio.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Pattyn A, Simplicio N, van Doorninck JH, et al. Ascl1/Mash1 is required for the development of central serotonergic neurons. Nat Neurosci. 2004;7:589–595. doi: 10.1038/nn1247. [DOI] [PubMed] [Google Scholar]
- 27.Carniti C, Belluco S, Riccardi E, et al. The Ret(C620R) mutation affects renal and enteric development in a mouse model of Hirschsprung's disease. Am J Pathol. 2006;168:1262–1275. doi: 10.2353/ajpath.2006.050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin L, Puliti A, Bonora E, et al. C620R mutation of the murine ret proto-oncogene: loss of function effect in homozygotes and possible gain of function effect in heterozygotes. Int J Cancer. 2007;121:292–300. doi: 10.1002/ijc.22378. [DOI] [PubMed] [Google Scholar]
- 29.Luo W, Wickramasinghe SR, Savitt JM, et al. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron. 2007;54:739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Mishiro T, Ishihara K, Hino S, et al. Architectural roles of multiple chromatin insulators at the human apolipoprotein gene cluster. Embo J. 2009;28:1234–1245. doi: 10.1038/emboj.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlsson P, Bjursell G. Negative and positive promoter elements contribute to tissue specificity of apolipoprotein B expression. Gene. 1989;77:113–121. doi: 10.1016/0378-1119(89)90365-x. [DOI] [PubMed] [Google Scholar]
- 32.Deardorff MA, Wilde JJ, Albrecht M, et al. RAD21 mutations cause a human cohesinopathy. Am J Hum Genet. 2012;90:1014–1027. doi: 10.1016/j.ajhg.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parelho V, Hadjur S, Spivakov M, et al. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell. 2008;132:422–433. doi: 10.1016/j.cell.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Cuadrado A, Remeseiro S, Gomez-Lopez G, et al. The specific contributions of cohesin-SA1 to cohesion and gene expression: implications for cancer and development. Cell Cycle. 2012;11:2233–2238. doi: 10.4161/cc.20318. [DOI] [PubMed] [Google Scholar]
- 35.Kon A, Shin LY, Minamino M, et al. Recurrent mutations in multiple components of the cohesin complex in myeloid neoplasms. Nat Genet. 2013;45:1232–1237. doi: 10.1038/ng.2731. [DOI] [PubMed] [Google Scholar]
- 36.de Lange C. Sur un type nouveau de degenerescence (typus Amstelodamensis) Arch. Med. Enfants. 1933;36:713–719. [Google Scholar]
- 37.Ireland M, Donnai D, Burn J. Brachmann-de Lange syndrome. Delineation of the clinical phenotype. Am J Med Genet. 1993;47:959–964. doi: 10.1002/ajmg.1320470705. [DOI] [PubMed] [Google Scholar]
- 38.Jackson L, Kline AD, Barr MA, et al. de Lange syndrome: a clinical review of 310 individuals. Am J Med Genet. 1993;47:940–6. doi: 10.1002/ajmg.1320470703. [DOI] [PubMed] [Google Scholar]
- 39.Opitz JM. The Brachmann-de Lange syndrome. Am J Med Genet. 1985;22:89–102. doi: 10.1002/ajmg.1320220110. [DOI] [PubMed] [Google Scholar]
- 40.Minor A, Shinawi M, Hogue JS, et al. Two novel RAD21 mutations in patients with mild Cornelia de Lange syndrome-like presentation and report of the first familial case. Gene. 2014;537:279–284. doi: 10.1016/j.gene.2013.12.045. [DOI] [PubMed] [Google Scholar]
- 41.Xu H, Balakrishnan K, Malaterre J, et al. Rad21-cohesin haploinsufficiency impedes DNA repair and enhances gastrointestinal radiosensitivity in mice. PLoS One. 2010;5:e12112. doi: 10.1371/journal.pone.0012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watts GF, Ooi EM, Chan DC. Therapeutic regulation of apoB100 metabolism in insulin resistance in vivo. Pharmacol Ther. 2009;123:281–291. doi: 10.1016/j.pharmthera.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Muller PA, Koscsó B, Rajani GM, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.