Summary
Secreted Wnt morphogens are essential for embryogenesis and homeostasis, and require a lipid/palmitoleoylate modification for receptor binding and activity. Notum is a secreted Wnt antagonist that belongs to the α/β hydrolase superfamily, but its mechanism of action and roles in vertebrate embryogenesis are not fully understood. Here we report that Notum hydrolyzes the Wnt palmitoleoylate adduct extracellularly, resulting in inactivated Wnt proteins that form oxidized oligomers incapable of receptor binding. Thus Notum is a Wnt deacylase, and palmitoleoylation is obligatory for the Wnt structure that maintains its active monomeric conformation. Notum is expressed in naïve ectoderm and neural plate in Xenopus and is required for neural and head induction. These findings suggest that distinct mechanisms of Wnt inactivation by the Tiki protease in the Organizer and the Notum deacylase in presumptive neuroectoderm orchestrate vertebrate brain development.
Introduction
The Wnt family of secreted lipoproteins controls animal development including axial patterning and cell fate specification, and governs tissue homeostasis and stem cell renewal (Clevers and Nusse, 2012; MacDonald et al., 2009). Anomalies in Wnt signaling cause human diseases including birth defects, cancer and osteoporosis (Clevers and Nusse, 2012; MacDonald et al., 2009). Wnt proteins engage multiple transmembrane receptors, including the Frizzled (Fz) serpentine receptors and LDL receptor-related proteins 5 and 6 (LRP5/6), which induce stabilization of the transcription co-activator β-catenin (MacDonald and He, 2012). Wnt proteins act locally near the source of their secretion in many contexts, and they also behave as morphogens with long range signaling properties (Hausmann et al., 2007; Strigini and Cohen, 2000; Zecca et al., 1996; but see Alexandre et al., 2014). Critical for these versatile signaling properties is a lipid modification of Wnt proteins referred to as O-palmitoleoylation (Takada et al., 2006; Willert et al., 2003). This form of O-acylation, which has been best demonstrated for the mouse Wnt3a, conjugates a mono-unsaturated palmitoleic acid onto the hydroxyl group of a conserved serine residue (serine 209 of Wnt3a), likely through the action of a Wnt-specific O-acyltransferase called Porcupine in the endoplasmic reticulum (Rios-Esteves et al., 2014; Takada et al., 2006). Wnt palmitoleoylation serves two essential functions. Firstly, palmitoleoylation appears to be obligatory for Wnt secretion, as the Wnt3a(S209A) mutant, which has serine 209 substituted by an alanine and thus lacks palmitoleoylation, is not secreted (Takada et al., 2006) possibly as a result of failure to bind to Wntless, a Wnt chaperone in the secretory pathway (Coombs et al., 2010; Herr and Basler, 2012; Tang et al., 2012). Secondly, the lipid modification is required for secreted Wnt ligands to signal, as the palmitoleate-adduct inserts into a hydrophobic cleft of the Fz receptor to form one of the two Wnt-Fz binding interfaces (Janda et al., 2012).
Canonical Wnt signaling plays multiple roles including axial patterning and germ layer specification in vertebrate embryogenesis (De Robertis and Kuroda, 2004; Hikasa and Sokol, 2013; Stern, 2005). In Xenopus embryos maternal Wnt/β-catenin signaling promotes the dorsal Spemann-Mangold Organizer and dorso-ventral (DV) axis formation (Harland and Gerhart, 1997). During gastrulation, a gradient of Wnt/β-catenin signaling occurs along the anterio-posterior (AP) axis, with higher levels posteriorly (Kiecker and Niehrs, 2001). The Organizer promotes head development via secreting Wnt antagonists such as sFRPs and Dickkopf-1 (Dkk1), which bind to and inhibit Wnt/Fz and LRP6, respectively (Cruciat and Niehrs, 2013; De Robertis and Kuroda, 2004). We recently identified another Organizer-specific and membrane-tethered Wnt antagonist, Tiki, which is a prototypic Wnt inactivating protease and is required for head formation (Zhang et al., 2012). The Organizer is also essential for neural induction. This has been primarily attributed to Organizer-secreted BMP (bone morphogenetic protein) antagonists such as Chordin and Noggin, which shield the naïve ectoderm from the influence of BMPs that promote epidermal differentiation, thereby permitting “default” neuralization (De Robertis and Kuroda, 2004; Ozair et al., 2013; Stern, 2005). Evidence suggests that inhibition of Wnt signaling and active FGF (fibroblast growth factor) signaling are also required for neural induction in Xenopus and chick embryos (Delaune et al., 2005; Fuentealba et al., 2007; Heeg-Truesdell and LaBonne, 2006; Kengaku and Okamoto, 1995; Lamb and Harland, 1995; Marchal et al., 2009; Pera et al., 2003; Stern, 2005; Wilson et al., 2001). But how regulation of Wnt signaling is achieved and contributes to neural induction by the Organizer remains unknown.
Notum (or Wingful) is a secreted antagonist of Wingless (Wg, Drosophila Wnt1) (Gerlitz and Basler, 2002; Giraldez et al., 2002). Sequence analysis places Notum in the so-called α/β hydrolase superfamily that includes various hydrolytic enzymes (Nardini and Dijkstra, 1999). Notum appears to regulate Wg extracellular distribution during wing development (Gerlitz and Basler, 2002; Giraldez et al., 2002). As heparan sulfate proteoglycans, in particular glypicans Dally and Dally-like protein (Dlp), are involved in modulating the Wg gradient (Han et al., 2005; Yan and Lin, 2009), and as Notum is in sequence most similar to plant pectin acetylesterases, Notum was first proposed to modify glycosaminoglycans (long unbranched polysaccharides) in Dally and Dlp (Giraldez et al., 2002). Subsequent experiments led to a revised model that Notum cleaves the glycosylphosphatidylinositol (GPI) anchor of Dlp, thereby switching Dlp from a membrane-anchored activator into a secreted antagonist (Kreuger et al., 2004). But because Dlp and Dally participate in functions of most or all morphogen families and play both positive and negative roles (Beckett et al., 2008; Filmus et al., 2008; Yan and Lin, 2009), the model that Notum antagonizes Wg signaling via modifying Dlp is controversial. Notum is conserved from invertebrates to human (Giraldez et al., 2002). In planarians, Notum and Wnt govern head and tail regeneration, respectively (Petersen and Reddien, 2011), reflecting a common theme of Wnt antagonists versus Wnt in AP patterning. In zebrafish, Notum has a role in DV patterning of the neural tube (Flowers et al., 2012). But the roles of Notum in early vertebrate embryogenesis are unknown. Here we report that Notum is a Wnt-inactivating deacylase and is critical for neural and head induction by acting within the presumptive neuroectoderm.
Results
Notum is a specific Wnt antagonist in vertebrate embryos and mammalian cells
The mouse or human genome harbors a single Notum gene. Expression of the mouse Notum in human embryonic kidney 293T (HEK293T) cells produced secreted Notum in the conditioned medium (CM) (see below), and inhibited a prototypic Wnt-responsive TOPFLASH reporter induced by either co-expression of mouse Wnt3a or treatment with recombinant human WNT3A proteins (Figure 1A and 1B). Thus Notum inhibits Wnt3a extracellularly. Notum shares with other α/β hydrolase proteins a conserved motif GxSxG, in which the serine mediates the hydrolytic reaction (Nardini and Dijkstra, 1999). Notum(S239A), in which the serine was replaced by an alanine, was unable to inhibit Wnt3a signaling (Figure 1C), consistent with results that an analogous Drosophila Notum mutant fails to inhibit Wg (Giraldez et al., 2002). Thus the enzymatic activity of Notum appears to be obligatory for Wg/Wnt antagonism.
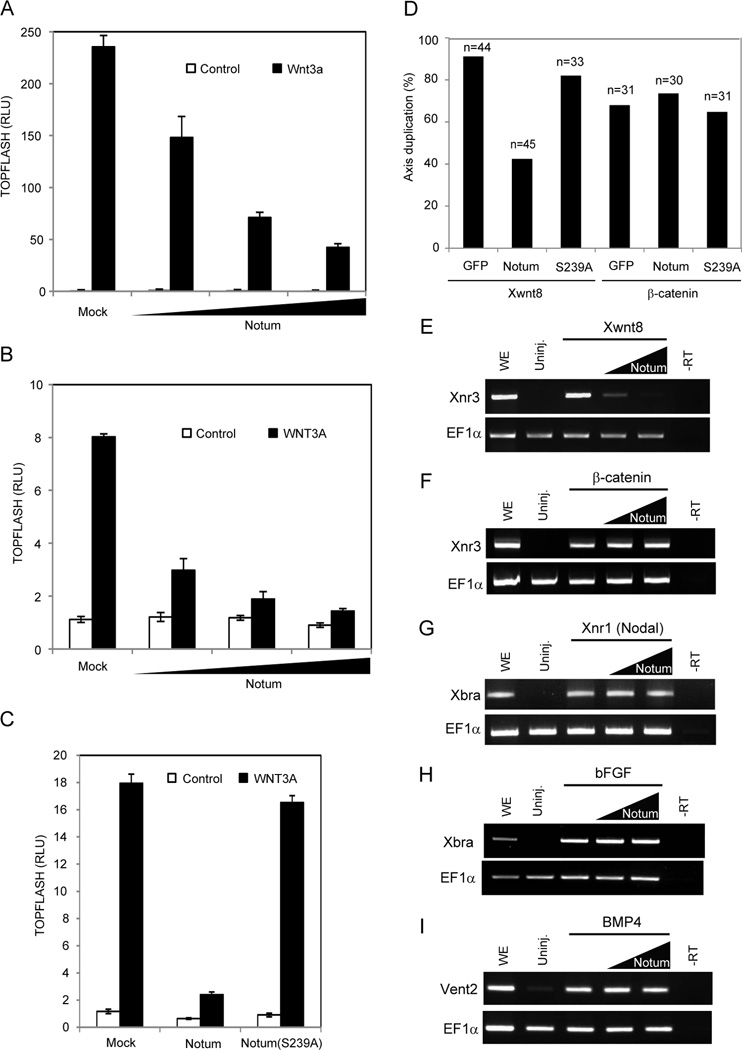
Figure 1. Notum antagonizes Wnt signaling in mammalian cells and Xenopus embryos.
(A) Notum inhibited TOPFLASH induced by Wnt3a in HEK293T cells. Increasing doses of a Notum expression vector were co-transfected with an expression vector for Wnt3a.
(B) Notum inhibited TOPFLASH induced by the recombinant WNT3A protein. HEK293T cells transfected with increasing doses of the Notum expression vector were incubated with the WNT3A protein.
(C) Notum(S239A) lacked the ability to inhibit TOPFLASH induced by the WNT3A protein.
(D) Notum inhibited axis duplication induced by Xenopus Wnt8 but not β-catenin, while Notum(S239A) did not inhibit axis duplication induced by either Wnt8 or β-catenin. n: embryos examined.
(E to I) Notum in animal pole explants inhibited expression of Xnr3 induced by Wnt8 (E), but not by β-catenin (F), nor did it inhibit Xbra expression induced by Nodal/Xnr1 (G) or bFGF (H), or Vent2 expression induced by BMP4 (I). EF1α, a loading control; uninj., uninjected embryos; WE, whole embryos; -RT, whole embryos without the reverse transcriptase. Two doses of Notum mRNAs were injected.
Error bars (A to C) represent SD of triplicated experiments.
See also Figure S1.
Notum inhibited Wnt signaling in Xenopus laevis embryos. Ventral injection of Xenopus Wnt8 or β-catenin mRNAs into 4-cell embryos resulted in axis duplication, and co-injection of mouse Notum mRNA, but not Notum(S239A) mRNA, inhibited axis duplication by Wnt8 (Figure 1D). Notum did not inhibit axis duplication by β-catenin (Figure 1D), consistent with Notum acting extracellularly. In animal cap explants, Notum inhibited Wnt8, but not β-catenin, induced expression of Xnr3, a β-catenin target gene and dorsal marker (Figure 1E and F). Importantly, Notum showed no effect on induction of Xbra, a mesodermal marker, by Nodal/Xnr1 (a transforming growth factor-β member) or bFGF (basic FGF), or induction of Vent2, a ventral marker, by BMP4 (Figure 1G–I). These data demonstrate that Notum is a specific Wnt antagonist, consistent with phenotypic analyses in invertebrates (Gerlitz and Basler, 2002; Giraldez et al., 2002; Petersen and Reddien, 2011) and zebrafish (Flowers et al., 2012).
Notum modifies and inactivates Wnt proteins
Notum has been suggested to cleave the GPI anchor of Dlp in flies (Kreuger et al., 2004). Of the six Glypicans encoded in the mouse/human genome, Glypican 3 (GPC3) and GPC4 have been implicated in Wnt pathways (Capurro et al., 2014; Filmus et al., 2008; Sakane et al., 2012). However in HEK293T cells the mouse Notum exhibited minimal GPI-cleaving activity on GPC3 or GPC4 when compared to a positive control, phosphatidylinositol-specific phospholipase C (PI-PLC), which cleaved the GPI of both Glypicans, shedding them into CM (Figure S1A and S1B).
We previously identified Tiki as a prototypic Wnt modifying and inactivating enzyme (Zhang et al., 2012). While the Wnt3a protein is hydrophobic because of its lipid modification (Willert et al., 2003), Wnt3a modified by Tiki is hydrophilic, as we demonstrated in a Triton X-114 detergent-aqueous phase separation assay (Zhang et al., 2012). This observation led to the suspicion that Tiki might have Wnt deacylase activity, but this was ruled out by metabolic labeling showing that Wnt3a palmitoleoylation was unaltered by Tiki (Zhang et al., 2012). Notum shares the distinction with Tiki as the only established Wnt antagonists with enzymatic motifs, raising the possibility that Notum may modify Wnt proteins. Indeed Wnt3a when co-expressed with Notum, like when co-expressed with Tiki, became predominantly hydrophilic as demonstrated by the Triton X-114 phase separation assay (Figure 2A). Correlating with the inability to inhibit Wnt3a signaling (Figure 1C), Notum(S239A) did not alter Wnt3a hydrophobicity (Figure 2A). None of several other secreted or secretory pathway enzymes that exhibit depalmitoylation activities including palmitoyl protein thioesterases (Zeidman et al., 2009), or are genetically implicated in Wnt-regulated events such as secreted phospholipase A2 (Cormier et al., 1997), was able to alter Wnt3a hydrophobicity (data not shown). Therefore, the ability of Notum to modify Wnt3a hydrophobicity, like that of Tiki, is specific and dependent on its hydrolase motif.
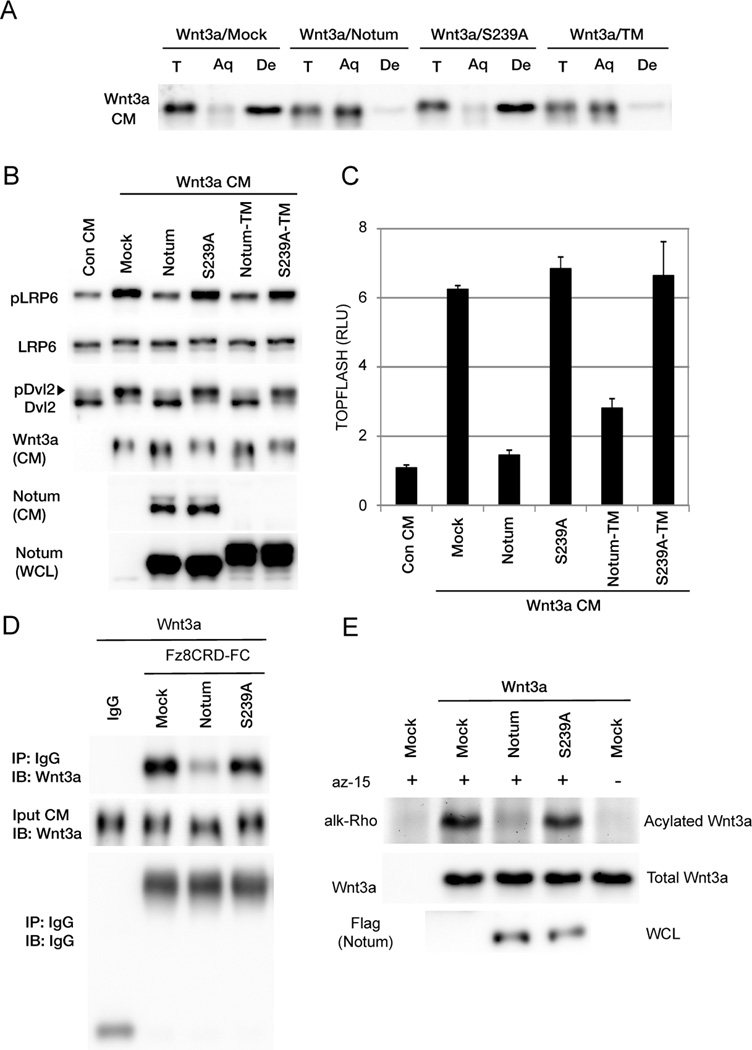
Figure 2. Notum causes Wnt deacylation and inactivation.
(A) The Wnt3a protein modified by Notum became hydrophilic in the Triton X-114 detergent-aqueous phase separation assay. Wnt3a from mock or Notum(S239A)-expressing cells was hydrophobic and partitioned in the detergent (De) phase, but Wnt3a from Notum- or Notum-TM-expressing cells partitioned in the aqueous (Aq) phase. T, total input.
(B) Wnt3a CM from Notum- or Notum-TM-expressing cells was inactive and induced minimal phosphorylation of LRP6 and Dvl2 in mouse embryonic fibroblast cells, whereas Wnt3a CM from mock, Notum(S239A)- or Notum(S239A)-TM-expressing cells induced phosphorylation of LRP6 and Dvl2. Note that Wnt3a from Notum- or Notum-TM-expressing cells exhibited slightly faster migration. WCL: whole cell lysates.
(C) Wnt3a CM from Notum or Notum-TM-expressing cells induced minimal TOPFLASH in HEK293T cells, whereas Wnt3a CM from mock, Notum(S239A)- or Notum(S239A)-TM-expressing cells induced TOPFLASH. Error bars represent SD of triplicated experiments.
(D) Wnt3a secreted from Notum-expressing cells exhibited minimal binding to mFz8CRD-IgG, whereas Wnt3a secreted from mock or Notum(S239A)-expressing cells exhibited binding.
(E) Notum but not Notum(S239A) reduced Wnt3a acylation when they were coexpressed in HEK293T cells.
See also Figure S2.
Wnt-induced phosphorylation of the LRP6 coreceptor and Dishevelled (Dvl), a component downstream of Fz, indicates activation of transmembrane signaling (MacDonald and He, 2012). Wnt3a CM from control HEK293T cells or cells expressing Notum(S239A) induced phosphorylation of LRP6 and Dvl2 and the TOPFLASH reporter, but Wnt3a CM from cells that co-expressed Notum failed to do so (Figure 2B and 2C), implying Wnt3a inactivation by Notum. But under this condition secreted Notum and Wnt3a proteins co-existed in the CM (Figure 2B). To this end we generated Notum-TM, which fuses Notum to a transmembrane domain at the carboxyl terminus and is anchored on the plasma membrane. Notum-TM was undetectable in CM (Figure 2B), but diminished Wnt3a hydrophobicity and inhibited Wnt3a-induced TOPFLASH as Notum did (Figures 2A and S2A). Wnt3a CM from cells co-expressing Notum-TM, in contrast to Wnt3a CM from cells co-expressing Notum(S239A)-TM, induced neither phosphorylation events nor TOPFLASH (Figure 2B and 2C), suggesting that Wnt3a had been inactivated by Notum or Notum-TM. Wnt3a from Notum-expressing cells exhibited poor binding to Fz, as examined using pulldowns by Fz8CRD-Fc (Fz8 extracellular cysteine-rich domain fused with IgG-Fc), whereas Wnt3a from Notum(S239A) cells showed normal Fz binding (Figure 2D). Therefore Wnt3a modified by Notum, like that modified by Tiki (Zhang et al., 2012), is not competent to bind to the Wnt receptor.
Notum causes Wnt depalmitoleoylation
Notum, like Tiki, had no appreciable effect on Wnt3a secretion, but resulted in the Wnt3a protein exhibiting faster mobility during gel electrophoresis (Figure 2B) (Zhang et al., 2012), consistent with Wnt3a being modified by Notum. Tiki cleavage of eight residues at Wnt3a amino terminus causes the change in Wnt3a hydrophobicity and electrophoretic mobility (Zhang et al., 2012). However unlike Tiki, Notum did not exhibit amino terminal cleavage activity towards Wnt3a (Figure S2B). Thus despite the fact that Wnt3a modified by Notum and Tiki behaved similarly, Notum is unlikely to be a Wnt protease, and therefore modifies Wnt3a in a fundamentally different manner.
Mass spectrometry of Wnt3a and the Wnt8 crystal structure suggest that O-palmitoleoylation and N-glycosylation are the predominant modifications of an active Wnt protein (Janda et al., 2012; Takada et al., 2006; Zhang et al., 2012). Others and we have shown that glycosylation/de-glycosylation does not alter Wnt3a hydrophobicity (Komekado et al., 2007; Zhang et al., 2012). These considerations raise the possibility that Notum may be a Wnt deacylase that removes palmitoleic acid through hydrolysis, thereby inactivating Wnt. We performed metabolic labeling of Wnt3a with a palmitic acid analog (az-15) (Zhang et al., 2012), which can be desaturated and incorporated into Wnt3a in the cell (Hannoush, 2012; Rios-Esteves and Resh, 2013; Zhang et al., 2012). The resulting lipidation of Wnt3a can be detected using a fluorescent dye through click chemistry (Zhang et al., 2012). Indeed Wnt3a CM from control or Notum(S239A)-expressing cells exhibited strong lipid labeling indicating palmitoleoylation, but Wnt3a CM from Notum-expressing cells lacked lipid labeling (Figure 2E). Therefore Notum through its hydrolase motif causes Wnt3a depalmitoleoylation.
Notum is likely a Wnt deacylase and acts extracellularly
Wnt palmitoleoylation appears to be essential for Wnt secretion, as Wnt3a(S209A), which lacks the palmitoleoylated serine, is not secreted, nor are any of the Wnt proteins that are expressed in cells/embryos deficient for Porcupine (Barrott et al., 2011; Biechele et al., 2011; Proffitt and Virshup, 2012; Takada et al., 2006). Notum does not affect Wnt3a secretion (Figure 2B), suggesting that depalmitoleoylation occurs after Wnt3a secretion. Indeed in contrast to secreted Wnt3a (Figure 2A), hydrophobicity of Wnt3a from cell lysates was less affected by Notum (Figure 3A), suggesting that Wnt3a in the secretory pathway is insensitive to Notum. Importantly, incubation of recombinant WNT3A in Notum CM in culture resulted in loss of Wnt3a hydrophobicity (Figure 3B). This result is consistent with Notum inhibition of recombinant WNT3A proteins added to responding cells (Figure 1B). We purified, from respective CM, secreted Notum, Notum(S239A), and Wnt3a that had been metabolically labeled by the palmitic acid analog, and reconstituted the depalmitoleoylation reaction in vitro. Notum, but not Notum(S239A), caused Wnt3a deacylation (Figure 3C). The simplest interpretation of these results is that Notum is a Wnt deacylase.
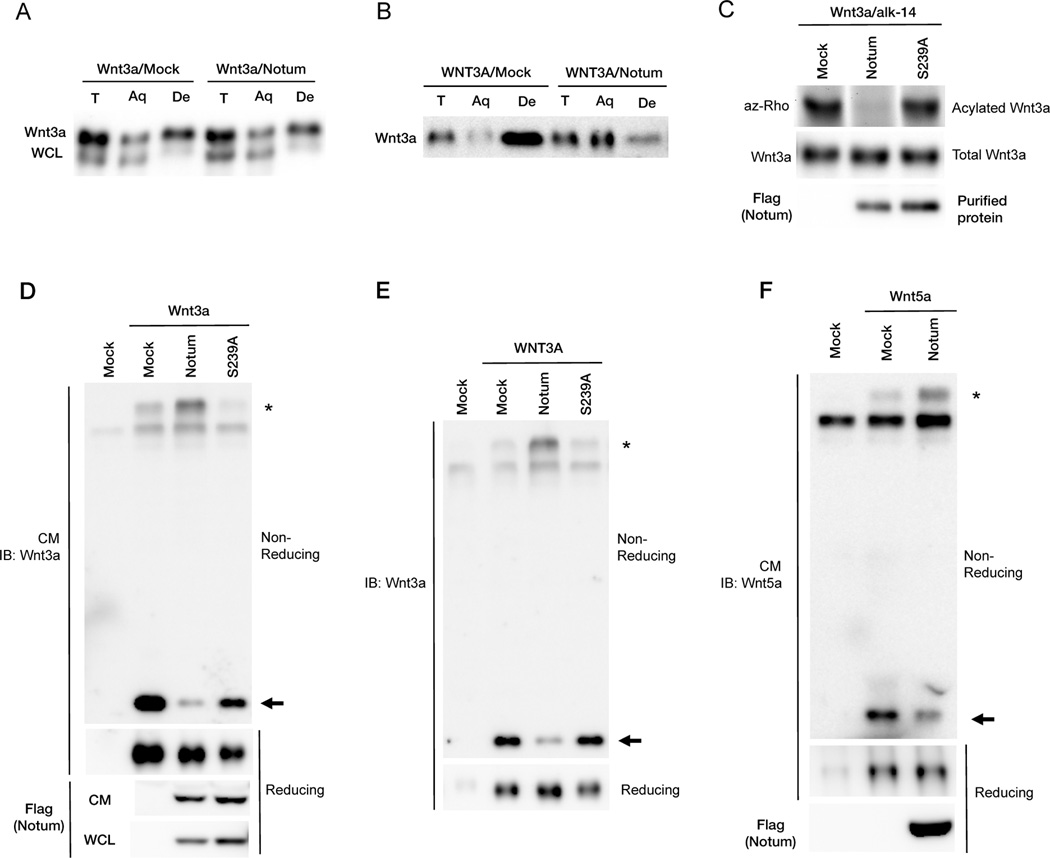
Figure 3. Notum is likely a Wnt deacylase acting extracellularly and causes Wnt3a and Wnt5a to form oxidized oligomers.
(A) Notum had minimal effects on hydrophobicity of Wnt3a from WCL in the detergent-aqueous phase separation assay.
(B) Recombinant WNT3A proteins lost hydrophobicity after incubation with Notum-expressing cells.
(C) Notum reduced Wnt3a acylation in vitro. Purified metabolically labeled Wnt3a proteins were incubated with mock or purified Notum or Notum(S239A) proteins in vitro.
(D) Wnt3a secreted from Notum- but not Notum(S239A)-expressing cells formed oxidized oligomer. Wnt3a CM from mock, Notum- or Notum(S239A)-expressing cells was analyzed by non-reducing or reducing SDS-PAGE. Wnt3a monomers and oxidized oligomers were labeled by an arrow and asterisk, respectively.
(E) Recombinant WNT3A proteins formed oxidized oligomers upon incubation with Notum-, but not mock or Notum(S239A)-expressing cells.
(F) Wnt5a proteins secreted from Notum-expressing cells formed oxidized oligomers.
See also Figure S2.
Palmitoleoylation is required for the Wnt structure and active monomeric state
Wnt3a cleaved by Tiki retains normal palmitoleoylation, but is hydrophilic (Zhang et al., 2012). This paradox appears to be explained by our finding that Tiki-cleaved Wnt3a (or an engineered Wnt3aΔN that lacks the 8 amino terminal residues removed by Tiki) forms large but soluble Wnt3a oligomers that are linked by inter-Wnt3a disulfide bonds (Zhang et al., 2012). These oxidized Wnt3a oligomers behave in a strictly hydrophilic manner in the Triton X-114 phase separation assay and thus may have buried the lipid adduct inside the oligomer (Zhang et al., 2012). It is worth noting that Wnt3a amino terminal residues cleaved by Tiki do not contain a cysteine, yet their absence profoundly alters Wnt3a structural integrity and disulfide bond patterns (Zhang et al., 2012). Unexpectedly, Wnt3a modified by Notum behaved in a similar manner: it formed soluble and large Wnt3a oligomers that were linked by inter-Wnt3a disulfide bonds and detected under non-reducing conditions (Figure 3D). Wnt3a CM from control or Notum(S239A)-expressing cells yielded mostly monomeric proteins under the same non-reducing condition (Figure 3D). This conversion of the active/monomeric form into inactive/oxidized oligomeric forms by Notum occurred extracellularly, because recombinant WNT3A, which is active/monomeric, became oxidized oligomers upon incubation in vitro with Notum, but not with Notum(S239A), CM (Figure 3E). Such a conversion after depalmitoleoylation by Notum was not a unique property of Wnt3a: Wnt5a appeared to be deacylated and lose its hydrophobicity upon co-expression with Notum (Figure S2C), and was converted concomitantly from monomers to oxidized oligomers (Figure 3F). Therefore palmitoleoylation is essential for the Wnt structure that ensures its active monomeric state.
Notum is expressed dynamically during Xenopus embryogenesis
Notum functions in vertebrates have been studied in zebrafish, which has three Notum genes, Notum1a, Notum1b, and Notum2 (Cantu et al., 2013; Flowers et al., 2012). Notum 1a and 1b are paralogs due to teleost genome duplication and are orthologs of the mammalian/Drosophila Notum, and indeed Notum1a antagonizes Wnt/β-catenin signaling (Flowers et al., 2012). Depletion of Notum1a causes neural tube defects in DV patterning (Flowers et al., 2012). Notum2 is a distant relative of mammalian/Drosophila Notum and does not appear to have Wnt antagonist activity (Cantu et al., 2013). Notum2 is strictly expressed in muscle pioneer cells in larvae and has a role in axon guidance during primary motor neurogenesis (Cantu et al., 2013), which is unique to teleosts and amphibians (to endow larvae with escape responses from predators, for example). The Xenopus laevis (allotetraploid) genome contains Notum and Notum’, which are pseudo-alleles due to genome duplication, and Notum2 (Figure S3). Notum and Notum’ are 94% identical in nucleotide (and corresponding amino acid) sequences and are orthologs of the mammalian/Drosophila Notum and fish Notum1a, whereas Notum2 is the ortholog of fish Notum2 (Figure S3). The Xenopus tropicalis (diploid) genome contains a single Notum gene, and Notum2 (Figure S3).
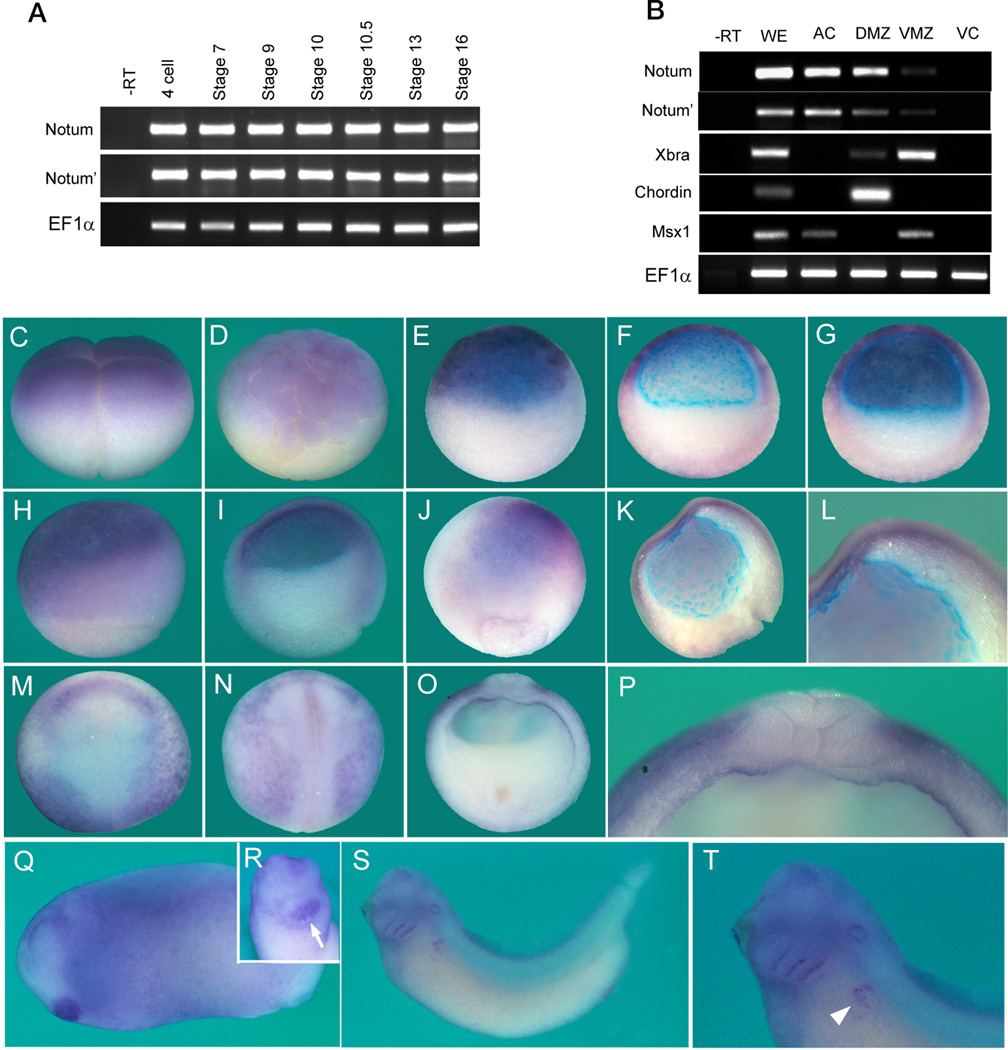
Notum and Notum’ mRNAs are expressed in the egg (and are enriched in the animal half, data not shown) and through cleavage to gastrulation stages (Figure 4A), and are enriched in the animal (prospective ectoderm) and dorsal regions in early gastrula (Figure 4B). Whole mount in situ hybridization showed expression of Notum and Notum’ (as the two pseudo-alleles were likely cross-hybridized by the probe) in animal blastomeres at 4-cell and 32-cell stages (Figure 4C and 4D). At stages 8.5 and 9.5 (blastula), Notum mRNA was detected broadly in the animal region (Figure 4E–G). At stage 10 (early gastrula) Notum mRNA remains broadly expressed animally but was also detected in the dorsal marginal zone (the Organizer), with lower expression in the ventral marginal zone (Figure 4H and I). At stages 11 Notum mRNA was found in the forming neural plate in a noticeable A-P (high to low) gradient, with additional weaker expression in the head mesoderm (Figure 4J–L). Notum mRNA remains detectable but becomes faint in the neural plate at stage 13 (data not shown). By stage 15 Notum mRNA was detected at the anterior border of the neural plate and in ventro-lateral epidermis excluding the neural plate (Figure 4M and N). Cross-section of a stage 15 embryo showed Notum expression in the lateral surface of the epidermis and the lateral plate mesoderm (Figure 4O and P). Later Notum was detected in the cement gland (an anterior organ) at tailbud stages (stage 25, Figure 4Q and R), in branchial arches, the otic vesicle, and developing pronephros, with diffused expression in the head (stage 35, Figure 4S and T). Thus Notum mRNA exhibits dynamic expression during Xenopus embryogenesis, in particular during neural induction and AP patterning.
Figure 4. Notum expression patterns during Xenopus embryogenesis.
(A and B) RT-PCR revealed that Notum is maternally and zygotically expressed throughout Xenopus embryogenesis (A), and is enriched animally and dorsally at the stage 10.5. AC: animal caps; DMZ: dorsal marginal zone; VMZ: ventral marginal zone; VC, vegetal caps; Xbra: a pan-mesodermal marker; Chordin: a dorsal marker; Msx1: an animal and ventral marker.
(C to T) Whole mount in situ hybridization for Notum/Notum’ expression, showing lateral view at 4-cell and stage 6.5 (C and D); lateral and bisected view at stage 8.5 (E and F); bisected view at stage 9.5 (G); lateral and bisected view at stage 10.5, with dorsal on right (H and I); dorsal and bisected view at the stage 11 (J and K) with an enlarged anterior view showing stronger expression anteriorly (L); dorso-anterior (M), dorsal (N, anterior on top), and cross-section (O, dorsal on top) views at stage 15 with an enlarged dorsal view (P); lateral view at the tailbud stage (Q), with an enlarged anterior view indicating expression in the cement gland (R, arrow); lateral view at the early tadpole stage (S), with an enlarged view indicating expression in the pronephros region (T, arrowhead). The blue color at the blastocoel surface in bisections was non-specific.
See also Figures S3.
Notum is required for head formation
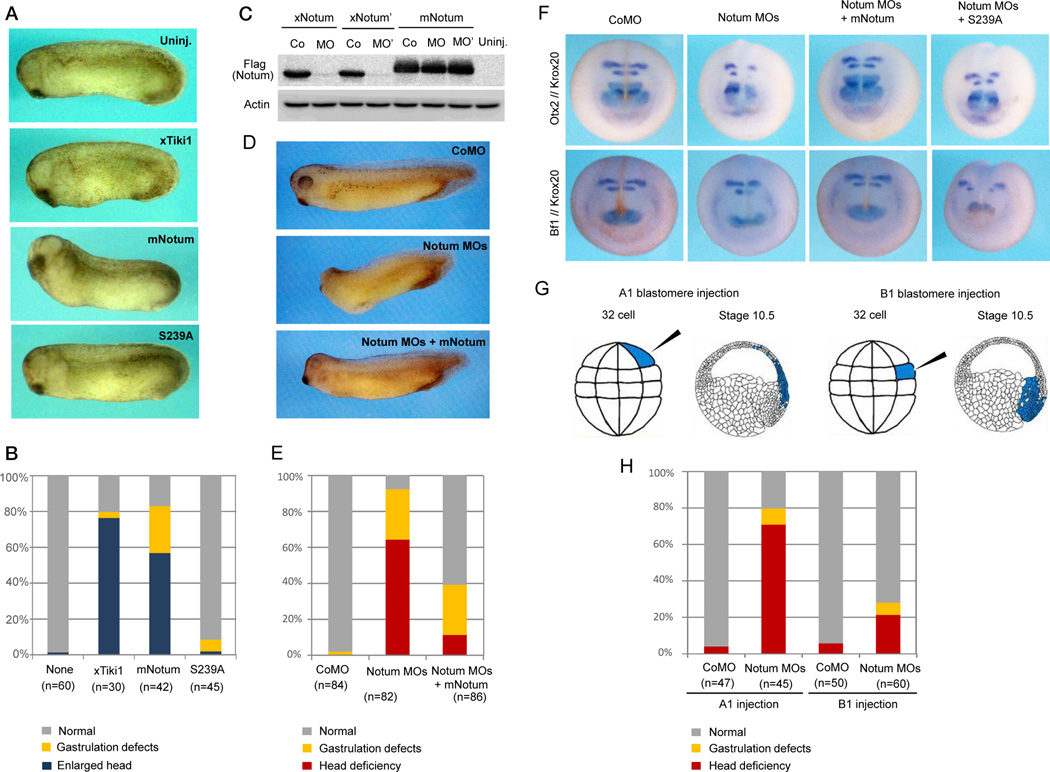
Xenopus Notum and Notum’ behaved identically as the mouse Notum in Wnt deacylation and inactivation (Figure S4A to 4D). Dorsal injection of synthetic mRNAs for the mouse Notum, but not Notum(S239A), induced an enlarged head as seen for Tiki1 or Dkk1 mRNA injection (Figure 5A and 5B) (Zhang et al., 2012), consistent with Wnt inhibition in embryos. We designed two morpholino antisense oligonucleotides (MOs) against the 5’ region surrounding the ATG initiation codon of Notum and Notum’. These two MOs blocked protein synthesis from mRNAs for Xenopus Notum and Notum’, respectively, but not the mouse Notum (Figure 5C) that does not have the MO-targeting sequence, demonstrating the specificity of these MOs. Dorso-animal injection of the two MOs together, but not of a control MO, at the 8-cell stage caused severe deficiency in head formation, resulting in embryos lacking the forebrain, eyes, and the cement gland (Figure 5D and 5E). These anterior defects were rescued when the MOs were co-injected with mouse Notum mRNA (Figure 5C and 5E), illustrating that the phenotypes were a result of lacking Notum. These anterior defects by the Notum MOs were also rescued/over-rescued by co-injection of Dkk1 mRNA (Figure S5A), and by co-injection of a β-catenin MO that depletes the β-catenin protein (Heasman et al., 2000) (Figure S5B and S5C), supporting the notion that the defects were due to excessive Wnt/β-catenin signaling. The overt anterior deficiencies by the Notum MOs were accompanied by, at stage 17, diminished expression of anterior markers Otx2, Bf1, Pax6 (forebrain) and XAG (the cement gland), and of a hindbrain marker Krox20, each of which was rescued by co-injection of the mouse Notum, but not Notum(S239A), mRNA (Figure 5F, S5D, S5E, Table S1 and S2). The expression of the spinal cord marker Hbox6 was unaffected by the Notum MOs (Figure S5E). Thus like Tiki1, Notum is required for Xenopus head patterning.
Figure 5. Notum is required for anterior development in Xenopus embryos.
(A and B) Dorsal injection of Notum mRNA, but not Notum(S239A), induced an enlarged head similar to that induced by Tiki1 mRNA, and resulting phenotypes were tabulated.
(C) The Notum MO and the Notum’ MO inhibited protein synthesis from Xenopus Notum (xNotum) or Notum’ (xNotum’) but not mouse Notum (mNotum) mRNAs. Co, MO, and MO’ indicate control MO and MOs against xNotum and xNotum’, respectively.
(D and E) Dorso-animal injection of the two Notum MOs together caused anterior defects that were rescued by mNotum mRNA, and resulting phenotypes were tabulated.
(F) The Notum MOs suppressed expression of forebrain markers Otx2 and Bf1 and a hindbrain marker Krox20 at stage 17. See also Table S1.
(G) Illustration of the MO-injected A1 and B1 blastomeres at 32-cell stage and their descendent tissues at stage 10.5.
(H) The Notum MOs injected into the A1 blastomeres caused anterior defects in more embryos (69%) than those injected into the B1 blastomeres (22%).
See also Figure S4, S5, Table S2 and S3.
Notum functions in prospective ectoderm
Tiki1 is specifically expressed in the Organizer, in particular the so-called head organizer that is composed of endomesodermal tissues underlying the future forebrain (Zhang et al., 2012). In Tiki1-depleted embryos, the head organizer function is significantly compromised as evidenced by diminished expression of all head organizer markers examined, including Dkk1, Chordin, Lim1, Goosecoid, and Otx2, while the expression of other dorsal markers such as Xnr3 and Xnot1 is unaffected (Zhang et al., 2012). However in Notum-depleted embryos, the expression of head organizer markers Chordin and Goosecoid, like that of Xnr3 and Xnot1, was unchanged (Figure S5F and Table S3). Thus unlike Tiki1, Notum function appears to minimally impact the head organizer integrity, and may therefore participate in head patterning by acting within the prospective ectoderm, in which Notum is expressed during blastula and gastrula stages (Figure 4E to 4L).
To examine this issue further, we injected, at the 32-cell stage, Notum MOs into the dorsal A1 blastomeres, which give rise mostly to the future head and neuro-ectoderm, or the dorsal B1 blastomeres, which are fated primarily to become the dorsal Organizer (Dale and Slack, 1987; Moody, 1987) (Figure 5G). Notum depletion in A1 and B1 progenies showed drastically different outcomes and resulted in head deficiencies in 69% and 22% of embryos, respectively (Figure 5H), supporting the notion that Notum is required primarily in the prospective ectoderm for anterior neural development.
Notum is required for neural induction
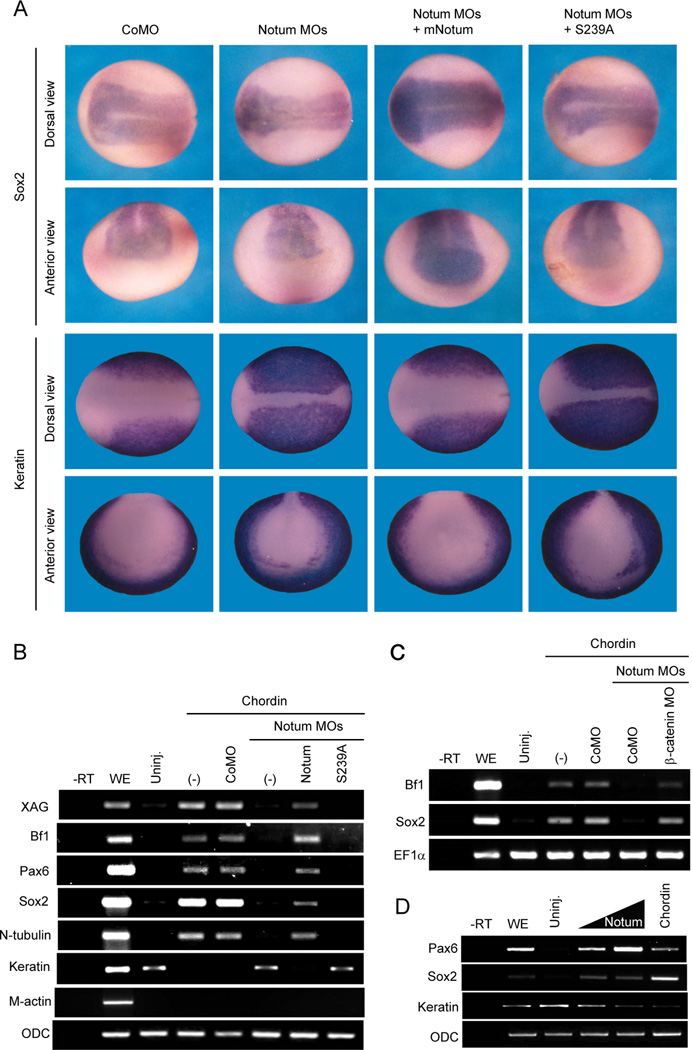
Inhibition of Wnt signaling has been suggested to be critical for neural induction in Xenopus (Fuentealba et al., 2007; Heeg-Truesdell and LaBonne, 2006), but the profound effect of Wnt signaling on dorsal Organizer, which induces neural tissues through secreting BMP antagonists, complicates studies on this issue (Stern, 2005). Interestingly, embryos injected with the Notum MOs, but not the control MO, showed a significant reduction of a pan-neural marker Sox2 but an expansion of the epidermal marker cytokeratin, and these reciprocal changes were rescued by co-injection of the mouse Notum, but not Notum(S239A), mRNA (Figure 6A and Table S4). Because Organizer markers were unaffected (Figure S5E), these results suggest that Notum acts within prospective ectoderm for neural specification.
Figure 6. Notum is required for neural induction in embryos and animal explants.
(A) The Notum MOs reduced the expression domain of Sox2, a pan-neural marker, and expanded the expression domain of cytokeratin, an epidermal marker, and the reciprocal changes were rescued by mNotum, but not mNutum(S239A), mRNA. See also Table S4.
(B) The Notum MOs suppressed expression of neural markers induced by injection of the Chordin mRNA, and restored cytokeratin expression that was inhibited by Chordin. The effect of Notum MOs was rescued by mNotum, but not mNotum(S239A), mRNA. XAG, a cement gland marker; Bf1 and Pax6, anterior neural markers; Sox2, a pan-neural marker; N-tubulin, a neuronal marker; Keratin, an epidermal marker; M-Actin, a mesodermal marker; ODC, a loading control.
(C) Chordin induced Bf1 and Sox2 expression when the Notum MOs and a β-catenin MO were co-injected.
(D) Injection of Notum mRNA, like that of Chordin mRNA, induced expression of neural markers Sox2 and Pax6 and suppressed that of an epidermal marker, cytokeratin, in animal cap explants.
See also Figure S6.
To examine this issue directly, we employed a classical neural induction assay using animal cap explants. Chordin or Noggin induced pan and anterior neural markers and suppressed the epidermal marker in explants from control embryos, but failed to do so in explants from Notum-depleted embryos, and this neural induction defect was rescued by the mouse Notum, but not Notum(S239A), mRNA (Figure 6B and Figure S6A). Importantly, the ability of Chordin to induce neural tissue was restored when the β-catenin MO was co-injected with the Notum MOs (Figure 6C), suggesting the failure of neural induction upon Notum depletion as a result of excessive Wnt/β-catenin signaling. To rule out the possibility that Notum is required for the BMP antagonists to function properly, we showed that Chordin or Noggin inhibited BMP4 induction of the ventral Vent2 regardless of Notum depletion (Figure S6B). Thus Notum suppression of Wnt signaling is a prerequisite for neural induction by BMP antagonists. Indeed overexpression of Notum, like that of Dkk1 (Fuentealba et al., 2007; Glinka et al., 1998), induced pan and anterior neural markers in animal explants (Figure 6D).
Discussion
Notum as a Wnt deacylase: comparisons with the Wnt protease Tiki
Genetic analyses in planarian and zebrafish show that Notum inhibits signaling by Wnt, but not by Hedgehog or other growth factors (Flowers et al., 2012; Petersen and Reddien, 2011). We show that the mouse or Xenopus Notum is a Wnt3a antagonist in mammalian cells, and inhibits target gene activation by Wnt8, but not BMP, Nodal, or FGF in Xenopus animal explants (Figure 1). Notum belongs to the α/β hydrolase superfamily that includes peptidases, lipases, esterases, and other hydrolytic enzymes (Nardini and Dijkstra, 1999). It was proposed that Notum inhibits Wg signaling via hydrolyzing the GPI anchor of the Dlp glypican (Kreuger et al., 2004). But this model is strongly challenged by the fact that Dlp, Dally, and their homologues participate in most or all morphogen signaling pathways (Filmus et al., 2008). Compared to PI-PLC we detected minimal Notum cleavage of the GPI anchor of GPC3 or GPC4 (Figure S1), two mammalian Glypicans that have been implicated in Wnt pathways (Capurro et al., 2014; Filmus et al., 2008; Sakane et al., 2012).
Tiki is an archetypal Wnt antagonist that modifies/cleaves the Wnt ligand (Zhang et al., 2012). Tiki and Notum represent a unique group of extracellular Wnt inhibitors with catalytic capacities. We show that Notum is also a Wnt-inactivating enzyme and shares several commonalities with Tiki in modifying Wnt substrates (Table 1A): neither affects Wnt secretion; and either causes Wnt to exhibit (i) faster electrophoretic mobility, (ii) loss of hydrophobicity, (iii) oxidized oligomer formation, and importantly (iv) loss of receptor-binding and signaling activity (Figures 2, 3, S2 and S4). Despite these similarities and that both Tiki and Notum are hydrolytic enzymes, the natures of their Wnt-inactivating modifications are different: Tiki cleaves the amino terminal residues of Wnt proteins as a protease (Zhang et al., 2012) whereas Notum cleaves the palmitoleoylate adduct of Wnt proteins as a deacylase (Figures 2 and 3). Therefore distinct Wnt-inactivating enzymes operate in modulating Wnt signaling, with secreted Notum being potentially diffusible whereas the membrane-tethered Tiki affecting a cell autonomously or adjacent cells (Zhang et al., 2012). The recently determined structure of Notum reveals a predicted α/β hydrolase fold with a large hydrophobic cavity, into which docking of a palmitoleoylate positions the acyl-oxyester bond in proximity to the GxSxG catalytic center, providing a structural basis for Notum deacylase function (Kakugawa et al., 2015). Notum deacylates Wnt3a and possibly Wnt5a, but Notum specificity towards Wnt proteins and different Wnt signaling pathways remain to be studied.
Table 1.
Comparisons of Tiki and Notum
| A. Comparisons of Wnt3a modifications by Tiki and Notum | |||
|---|---|---|---|
| Wnt3a | Wnt3a + Tiki | Wnt3a + Notum | |
| Secretion | Normal | Normal | Normal |
| Hydrophobicity | Hydrophobic | Hydrophilic | Hydrophilic |
| Mobility in gel | Normal | Faster | Faster |
| Oxidized oligomer | No | Yes | Yes |
| Signaling activity | Yes | No | No |
| Binding to receptor | Yes | No | No |
| Nature of modification | N-terminal cleavage | Deacylation | |
| B. Comparisons of Tiki1 and Notum expression and functions in Xenopus embryos | ||
|---|---|---|
| Tiki1 | Notum | |
| Expression stages | From early gastrulation | From egg throughout embryogenesis |
| Early expression patterns | Organizer | Ectoderm, forming neural plate, and anterior neural plate border |
| Depletion phenotypes | Headless | Headless and neural plate reduction |
| Action sites (tissues) | Head organizer | Ectoderm |
| Extracellular features | Membrane tethered (non-diffusible) | Secreted (Diffusible?) |
Wnt lipidation as a requirement for its structure and active monomeric conformation
Wnt palmitoleoylation have two critical functions: it may be recognized by Wntless, a Wnt chaperone in the secretory pathway (Coombs et al., 2010; Herr and Basler, 2012; Tang et al., 2012), and it is essential for Wnt binding to Fz (Janda et al., 2012). We show that Wnt depalmitoleoylation by Notum occurs extracellularly, likely accounting for normal secretion but lack of receptor binding by deacylated Wnt3a (Figures 1 to 3). But unexpectedly Wnt3a and Wnt5a depalmitoleoylated by Notum, similar to Wnt3a that is amino-terminally cleaved by Tiki (Zhang et al., 2012), form large yet soluble Wnt oligomers that are covalently linked by inter-Wnt disulfide bonds (Figure 3). These oxidized Wnt oligomers generated by Notum or Tiki appear to be similar, and are recapitulated by the Wnt3ΔAN mutant that lacks the amino terminal residues cleaved by Tiki (Zhang et al., 2012). Therefore the Wnt oxidized oligomeric state (or states), which is prevalent during Wnt biogenesis (Zhang et al., 2012), may be a default state of the Wnt protein. Wnt palmitoleoylation therefore appears to have a third critical role, i.e., for maintaining Wnt structural integrity to ensure its active monomeric conformation. The intact Wnt amino terminus, which is rich in hydrophobic residues, plays a similar role in maintaining the Wnt fold (Zhang et al., 2012). The structural basis for the requirement of the lipid, and for the Wnt amino terminus, is not yet obvious from the Wnt crystal structure (Janda et al., 2012). Given that lipid modifications of proteins are common in signaling, a general role for lipid adducts in protein conformation deserves investigation.
Notum in vertebrate neural and head induction: comparisons with the role of Tiki
While Tiki1 is expressed in the Organizer and its descendent head organizer during gastrulation (Zhang et al., 2012), Notum (including Notum’) is maternally expressed and is enriched in naïve ectoderm (Figure 4). Overexpression of Notum or Tiki1 dorsally results in head enlargement; depletion of Notum or Tiki1 causes deficiency in head formation (Figure 5) (Zhang et al., 2012). Thus these two Wnt-inactivating enzymes are each required for anterior development and not redundant. The action sites of Notum and Tiki1 are distinct and complementary (Table 1B): Tiki1 acts within the head organizer (Zhang et al., 2012), which induces overlying ectoderm to form anterior neural tissues, whereas Notum primarily acts within ectoderm to endow it with competence to become anterior brain, and its depletion does not affect Organizer formation (Figures 5, S5 and Table 1B).
The action of Notum within ectoderm appears to explain its significant but unique role among Wnt antagonists in neural induction. Upon Notum depletion, the expression of anterior and pan neural markers is reduced, while that of an epidermal marker is expanded (Figure 6), suggesting neural to epidermal fate conversion. Naïve ectoderm with Notum depletion loses its competence to respond to neural inducers/BMP antagonists (Figure 6 and S6). Conversely, overexpression of Notum, like that of Dkk1 (Fuentealba et al., 2007; Glinka et al., 1998), induces anterior neural tissues (Figure 6). Thus Wnt inactivation by Notum within ectoderm is a critical part of the “default” neural fate (De Robertis and Kuroda, 2004; Ozair et al., 2013; Stern, 2005). This requirement of Wnt inhibition by Notum, in addition to that of BMP inhibition (and of FGF activation), for neuralization in Xenopus seems to mirror neural induction mechanisms described in the chick (Stern, 2005; Streit et al., 2000; Streit et al., 1998; Wilson et al., 2000; Wilson et al., 2001). The dependence on Wnt and BMP co-inhibition (and FGF activation) for neural induction may act through parallel signaling pathways and/or through crosstalks by these pathways (Fuentealba et al., 2007; Pera et al., 2003).
Notum exhibits dynamic expression in naïve ectoderm in blastula, in the forming neural plate in early gastrula, and at the anterior border of (and exclusion from) the neural plate in late gastrula (Figure 4). Interestingly its ectodermal expression encompasses the so-called BCNE (Blastula Chordin and Noggin Expression) center, which represents the dorsal ectoderm giving rise to future fore-, mid-, and hindbrain (Kuroda et al., 2004). The transient ectodermal Chordin and Noggin expression in blastula is required for dorsal ectoderm to become anterior neural tissues that are reinforced by Chordin and Noggin from the Organizer during gastrulation (Kuroda et al., 2004). Similarly we propose that ectodermal Notum expression in blastula primes the dorsal ectoderm for sustained neuralization during gastrulation by Organizer-generated BMP and Wnt antagonists, thereby underlying its requirement for neural and anterior induction. Subsequent Notum expression in the anterior neural plate border may further reinforce Wnt inactivation for patterning forebrain and surrounding regions such as pre-placodal ectoderm, which is induced to form placodes through Wnt inhibition (Litsiou et al., 2005). Experiments will be required to dissect the temporal order and potentially multiple roles of Notum during neural induction and patterning. Our results demonstrate that distinct Wnt inactivation mechanisms by Notum in naïve ectoderm and Tiki in Organizer coordinate early brain formation, and that Notum function in head formation is conserved from planarians to vertebrates. Notum as a Wnt inactivating enzyme represents a potential therapeutic target.
EXPERIMENTAL PROCEDURES
Plasmids
The mouse Notum cDNA in pCAGGS contains the full coding region fused with a 3xFLAG tag and was from Drs. S. Park and S. Sockanathan. GPC3 and GPC4 expressing vectors were from A. Kikuchi. Notum coding region fused with a 1xFLAG tag was subcloned into pCS2+. Notum(S239A) was generated by PCR-based mutagenesis method based on pCS2+/Notum-FLAG. Notum-TM and Notum(S239A)-TM were constructed by inserting a cDNA fragment encoding the transmembrane domain of EGFR (IATGMVGALLLLLVVALGIGLFM) between Notum and FLAG tag. Xenopus laevis Notum and Notum’ cDNAs were amplified from embryo cDNAs via RT-PCR and cloned into pCS2+. Details of plasmids are available upon request.
Triton X-114 phase separation
Triton X-114 phase separation was performed as described (Zhang et al., 2012). Briefly, for CM separation, Wnt3a or Wnt5a CM was mixed with equal volume of Triton X-114 buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 4.5% Triton X-114); for WCL separation, WCL containing 1% Triton X-114 was mixed with equal volume of 3.5% Triton X-114 buffer. The mixtures were incubated on ice for 5 min and then at 37°C for 5 min. After centrifugation at 2000g for 5 min, the top aqueous phase, the bottom detergent phase, and the original mixture (total) were analyzed by SDS-PAGE and Western blotting.
Metabolic labeling and click-chemistry
Metabolic labeling and click-chemistry assays were performed as described (Zhang et al., 2012). Briefly, HEK293T cells or HEK293T cells stably expressing HA-Wnt3a (Zhang et al., 2012) in 60 mm tissue culture dishes were transfected with indicated plasmids. 24 hrs after transfection, cells were washed with serum-free DMEM once and then cultured in the labeling medium (DMEM containing 5% dialyzed FBS, 40 µM az-15 or alk-14) for 12 hrs. CM was collected and cleared by centrifugation. HA-Wnt3a was enriched from CM by anti-HA beads. The beads were washed and re-suspended in 20 µl of PBS plus 2.25 µl freshly premixed click-chemistry reaction mixture (alk-Rho or az-Rho, 100 µM, TCEP, 1 mM, Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA), 100 µM, and CuSO4·5H2O, 1 mM) for 1 hr at room temperature. The samples were denatured and separated by SDS-PAGE, and the gels were fixed and scanned on a GE Healthcare Typhoon 9400 variable-mode imager for Rhodamine-associated signal at excitation 532 nm/emission 580 nm. The same samples were also subjected to SDS-PAGE and Western blotting to detect total Wnt3a protein.
Protein purification and in vitro deacylation of Wnt3a
The CM from HEK293T cells expressing Notum-FLAG or Notum(S239A)-FLAG was mixed with anti-FLAG beads and incubated at 4°C overnight. The beads were washed with PBS/0.1% Triton X-100 and the FLAG fusion proteins were eluted with elution buffer (50 mM HEPES (pH 7.4), 100 mM NaCl, 0.1% Triton X-100, 50 µg/ml 3xFLAG peptide). For in vitro deacylation, alk-14 labeled Wnt3a in CM was immunoprecipitated with anti-HA agarose beads and incubated with purified Notum or Notum(S239A) protein at room temperature overnight followed with click-chemistry reaction. The reaction mixtures were denatured and separated by SDS-PAGE. The fluorescent signal and total Wnt3a proteins were detected by in-gel scanning and Western blotting.
Xenopus embryo manipulations
Procedures for embryo manipulation, reverse transcription PCR and in situ hybridization were performed as previously described (Zhang et al., 2012). The full length Xenopus laevis Notum’ cDNA was used to make in situ probe.
mRNA and Morpholino injection
Axis duplication and animal cap assays were performed as described (Zhang et al., 2012). For axis duplication assays, Xwnt8 (1 pg) or β-catenin (50 pg) mRNA was injected alone or together with Notum (200 pg) mRNA into the ventral marginal zone at the 4- or 8-cell stage and the phenotype was scored at the tadpole stage. GFP was used as a negative control. For animal cap assays, indicated mRNAs (Notum, 100 and 200 pg; Xwnt8, 5 pg; β-catenin 30 pg; Xnr1, 250 pg; BMP4, 100 pg; Chordin, 200pg) and morpholinos (20ng) were injected into the animal pole at 4-cell stage, and animal caps were dissected at stage 9 (in the case of Xwnt8, Xnr1, BMP4, and Chordin), or at stage 8.5 and treated with recombinant proteins (bFGF, 100ng/ml; Noggin, 500ng/ml) until stage 10 (for FGF) or stage 18 (for Noggin or Chordin) before RT-PCR. To examine Notum and Notum’ MO specificity, 500 pg of xNotum, xNotum’ or mNotum mRNA was injected with control MO, Notum or Notum’ MO (20 ng) into the animal pole at the 2-cell stage, and cultured until the stage 10 for Western blotting. To knockdown the endogenous xNotum and xNotum’, 20 ng of control MO or Notum and Notum’ MOs were injected into two dorso-animal blastomeres at the 8-cell stage, or two dorsal A1 or B1 cells at 32-cell stage, and the phenotype was scored at stage 35. To rescue the MOs (xNotum and xNotum’) phenotype, 200 pg of mNotum, 20 pg of DKK1 mRNA or 10 ng of β-catenin MO was injected together with MOs.
Supplementary Material
ACKNOWLEDGEMENTS
We thank S. Park, S. Sockanathan, and A. Kikuchi for plasmids, J-P. Saint-Jeannet for comments, and J-P Vincent for discussion. X.Z. and S-M.C. were in part supported by a grant from Harvard William Randolph Hearst Fund and a fellowship from National Research Foundation of Korea, respectively. N.G.A. is in part supported by a postdoctoral fellowship from Science Without Borders program/CNPq of Brazil. M.Z. held an IEF Marie Curie fellowship. J. G. A. and A. H. R. are supported by CNPq and FAPERJ of Brazil. E.Y.J. acknowledges support by Cancer Research UK (A10976) and by the Wellcome Trust Centre for Human Genetics (090532/Z/09/Z). X.H. acknowledges support by NIH (RO1-GM057603) and by Boston Children's Hospital Intellectual and Developmental Disabilities Research Center (P30 HD-18655). X.H. is an American Cancer Society Research Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ACCESSION NUMBERS
Sequences of Xenopus laevis Notum, Notum’ and Notum2 have been deposited in GenBank under accession numbers KP781855, KP781856, and KP781857, respectively.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures, six figures and four tables, and can be found with this article online at
AUTHOR CONTRIBUTIONS
X. Z. performed all biochemical experiments. S-M. C. performed most Xenopus experiments. N. G. A., A. H. R., and J. G. A. performed additional Xenopus experiments. B. T. M. contributed to experiments in mammalian cells and performed bioinformatic analysis. X. Z., S-M. C., J. G. A., M. Z., E. Y. J., and X. H. contributed to experimental designs, X. Z., S-M. C., and X. H. wrote the manuscript, and all co-authors edited the manuscript.
REFERENCES
- Alexandre C, Baena-Lopez A, Vincent JP. Patterning and growth control by membrane-tethered Wingless. Nature. 2014;505:180–185. doi: 10.1038/nature12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrott JJ, Cash GM, Smith AP, Barrow JR, Murtaugh LC. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett K, Franch-Marro X, Vincent JP. Glypican-mediated endocytosis of Hedgehog has opposite effects in flies and mice. Trends in cell biology. 2008;18:360–363. doi: 10.1016/j.tcb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Biechele S, Cox BJ, Rossant J. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Developmental biology. 2011;355:275–285. doi: 10.1016/j.ydbio.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Cantu JA, Flowers GP, Topczewski J. Notum homolog plays a novel role in primary motor innervation. The Journal of neuroscience. 2013;33:2177–2187. doi: 10.1523/JNEUROSCI.3694-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro M, Martin T, Shi W, Filmus J. Glypican-3 binds to Frizzled and plays a direct role in the stimulation of canonical Wnt signaling. Journal of cell science. 2014;127:1565–1575. doi: 10.1242/jcs.140871. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Coombs GS, Yu J, Canning CA, Veltri CA, Covey TM, Cheong JK, Utomo V, Banerjee N, Zhang ZH, Jadulco RC, et al. WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. Journal of cell science. 2010;123:3357–3367. doi: 10.1242/jcs.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier RT, Hong KH, Halberg RB, Hawkins TL, Richardson P, Mulherkar R, Dove WF, Lander ES. Secretory phospholipase Pla2g2a confers resistance to intestinal tumorigenesis. Nature genetics. 1997;17:88–91. doi: 10.1038/ng0997-88. [DOI] [PubMed] [Google Scholar]
- Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harbor perspectives in biology. 2013;5:a015081. doi: 10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale L, Slack JM. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987;99:527–551. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annual review of cell and developmental biology. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]
- Filmus J, Capurro M, Rast J. Glypicans. Genome biology. 2008;9:224. doi: 10.1186/gb-2008-9-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers GP, Topczewska JM, Topczewski J. A zebrafish Notum homolog specifically blocks the Wnt/beta-catenin signaling pathway. Development. 2012;139:2416–2425. doi: 10.1242/dev.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlitz O, Basler K. Wingful, an extracellular feedback inhibitor of Wingless. Genes & development. 2002;16:1055–1059. doi: 10.1101/gad.991802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez AJ, Copley RR, Cohen SM. HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Developmental cell. 2002;2:667–676. doi: 10.1016/s1534-5807(02)00180-6. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Han C, Yan D, Belenkaya TY, Lin X. Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development. 2005;132:667–679. doi: 10.1242/dev.01636. [DOI] [PubMed] [Google Scholar]
- Hannoush RN. Profiling cellular myristoylation and palmitoylation using omega-alkynyl fatty acids. Methods Mol Biol. 2012;800:85–94. doi: 10.1007/978-1-61779-349-3_7. [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann's organizer. Annual review of cell and developmental biology. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Hausmann G, Banziger C, Basler K. Helping Wingless take flight: how WNT proteins are secreted. Nature reviews Molecular cell biology. 2007;8:331–336. doi: 10.1038/nrm2141. [DOI] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Developmental biology. 2000;222:124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- Heeg-Truesdell E, LaBonne C. Neural induction in Xenopus requires inhibition of Wnt-beta-catenin signaling. Developmental biology. 2006;298:71–86. doi: 10.1016/j.ydbio.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Herr P, Basler K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Developmental biology. 2012;361:392–402. doi: 10.1016/j.ydbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Hikasa H, Sokol SY. Wnt signaling in vertebrate axis specification. Cold Spring Harbor perspectives in biology. 2013;5:a007955. doi: 10.1101/cshperspect.a007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakugawa S, Langton PF, Zebisch M, Howell SA, Chang TH, Liu Y, Feizi T, Bineva G, O’Reilly N, Snijders AP, et al. Notum deacylates Wnt proteins to suppress signalling activity. [published online 25 February 2015];Nature. 2015 doi: 10.1038/nature14259. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kengaku M, Okamoto H. bFGF as a possible morphogen for the anteroposterior axis of the central nervous system in Xenopus. Development. 1995;121:3121–3130. doi: 10.1242/dev.121.9.3121. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- Komekado H, Yamamoto H, Chiba T, Kikuchi A. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Genes to cells : devoted to molecular & cellular mechanisms. 2007;12:521–534. doi: 10.1111/j.1365-2443.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Kreuger J, Perez L, Giraldez AJ, Cohen SM. Opposing activities of Dally-like glypican at high and low levels of Wingless morphogen activity. Developmental cell. 2004;7:503–512. doi: 10.1016/j.devcel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Wessely O, De Robertis EM. Neural induction in Xenopus: requirement for ectodermal and endomesodermal signals via Chordin, Noggin, beta-Catenin, and Cerberus. PLoS biology. 2004;2:E92. doi: 10.1371/journal.pbio.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TM, Harland RM. Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior-posterior neural pattern. Development. 1995;121:3627–3636. doi: 10.1242/dev.121.11.3627. [DOI] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–4062. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/beta-catenin signaling. Cold Spring Harbor perspectives in biology. 2012:4. doi: 10.1101/cshperspect.a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal L, Luxardi G, Thome V, Kodjabachian L. BMP inhibition initiates neural induction via FGF signaling and Zic genes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17437–17442. doi: 10.1073/pnas.0906352106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody SA. Fates of the blastomeres of the 32-cell-stage Xenopus embryo. Developmental biology. 1987;122:300–319. doi: 10.1016/0012-1606(87)90296-x. [DOI] [PubMed] [Google Scholar]
- Nardini M, Dijkstra BW. Alpha/beta hydrolase fold enzymes: the family keeps growing. Current opinion in structural biology. 1999;9:732–737. doi: 10.1016/s0959-440x(99)00037-8. [DOI] [PubMed] [Google Scholar]
- Ozair MZ, Kintner C, Brivanlou AH. Neural induction and early patterning in vertebrates. Wiley interdisciplinary reviews Developmental biology. 2013;2:479–498. doi: 10.1002/wdev.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera EM, Ikeda A, Eivers E, De Robertis EM. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes & development. 2003;17:3023–3028. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW. Polarized notum activation at wounds inhibits Wnt function to promote planarian head regeneration. Science. 2011;332:852–855. doi: 10.1126/science.1202143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proffitt KD, Virshup DM. Precise regulation of porcupine activity is required for physiological Wnt signaling. The Journal of biological chemistry. 2012;287:34167–34178. doi: 10.1074/jbc.M112.381970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Esteves J, Haugen B, Resh MD. Identification of Key Residues and Regions Important for Porcupine-mediated Wnt Acylation. The Journal of biological chemistry. 2014;289:17009–17019. doi: 10.1074/jbc.M114.561209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Esteves J, Resh MD. Stearoyl CoA desaturase is required to produce active, lipid-modified Wnt proteins. Cell reports. 2013;4:1072–1081. doi: 10.1016/j.celrep.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane H, Yamamoto H, Matsumoto S, Sato A, Kikuchi A. Localization of glypican-4 in different membrane microdomains is involved in the regulation of Wnt signaling. Journal of cell science. 2012;125:449–460. doi: 10.1242/jcs.091876. [DOI] [PubMed] [Google Scholar]
- Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–78. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- Streit A, Lee KJ, Woo I, Roberts C, Jessell TM, Stern CD. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development. 1998;125:507–519. doi: 10.1242/dev.125.3.507. [DOI] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Current biology : CB. 2000;10:293–300. doi: 10.1016/s0960-9822(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Developmental cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Tang X, Wu Y, Belenkaya TY, Huang Q, Ray L, Qu J, Lin X. Roles of N-glycosylation and lipidation in Wg secretion and signaling. Developmental biology. 2012;364:32–41. doi: 10.1016/j.ydbio.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Graziano E, Harland R, Jessell TM, Edlund T. An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Current biology : CB. 2000;10:421–429. doi: 10.1016/s0960-9822(00)00431-0. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Rydstrom A, Trimborn T, Willert K, Nusse R, Jessell TM, Edlund T. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–330. doi: 10.1038/35077115. [DOI] [PubMed] [Google Scholar]
- Yan D, Lin X. Shaping morphogen gradients by proteoglycans. Cold Spring Harbor perspectives in biology. 2009;1:a002493. doi: 10.1101/cshperspect.a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]
- Zeidman R, Jackson CS, Magee AI. Protein acyl thioesterases (Review) Molecular membrane biology. 2009;26:32–41. doi: 10.1080/09687680802629329. [DOI] [PubMed] [Google Scholar]
- Zhang X, Abreu JG, Yokota C, MacDonald BT, Singh S, Coburn KL, Cheong SM, Zhang MM, Ye QZ, Hang HC, et al. Tiki1 is required for head formation via Wnt cleavage-oxidation and inactivation. Cell. 2012;149:1565–1577. doi: 10.1016/j.cell.2012.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.